Abstract
Nanoparticle‐based treatment has become a potential therapeutic approach. The nanosize of these particles provides them with unique physicochemical properties and enhances their interaction with the biological system. Nanomaterials have the potential to overcome some of the major issues in the clinical world which may include cancer treatment and may be utilised to resolve the major problem of drug resistance in infection control. These particles are being used to improve present therapeutics by virtue of their shape, size and diverse intrinsic as well as chemical properties. The authors have discussed the use of nanoparticles in cancer treatment, infections caused by multidrug‐resistant microbial strains and biofilm inhibition along with the detailed description of the current status of nanomaterials in the field of medicine.
Inspec keywords: drugs, molecular biophysics, cellular biophysics, nanomedicine, cancer, nanoparticles, microorganisms, biomedical materials, drug delivery systems, nanofabrication, antibacterial activity
Other keywords: anticancer, antimicrobial treatment, nanoparticle‐based treatment, potential therapeutic approach, nanosize, unique physicochemical properties, biological system, nanomaterials, clinical world, cancer treatment, drug resistance, infection control, therapeutics, diverse intrinsic, chemical properties, multidrug‐resistant microbial strains, biofilm inhibition, nanomedicine
1 Introduction
Nanotechnology has emerged as a new frontier for the development of novel therapeutics. It is an interdisciplinary field which entails different fields of science to form nanoscale materials [1]. In its rigid definition, nanotechnology entails the synthesis and manipulation of structures in the size range of 1–100 nm in at least one dimension [2]. However, the definition of a nanomaterial is more intricate than simply size. The particles in this size range have unique capabilities based on their physicochemical properties, which are very different from particles of macroscopic, microscopic or atomic size. These physicochemical properties of nanoparticles influence their interaction when they come in contact with the biological system [3]. Furthermore, many biological phenomena such as entry across the biological barrier and immune recognition are governed by size consideration [4]. The advantages of nanotechnology are because of these properties and interactions. Hence, particles that are >100 nm in size but which can exhibit these unique properties are also being considered as nanomaterials.
Conventionally used therapeutic treatments have limited cellular penetration and poor retention. At present, there is a need for the advance treatment method to overcome the cell membrane barrier as well as to deliver drug and retain it at the target site for the required time period. Drugs designed on the nanoscale may confer all these pharmacological advantages as compared to a conventional agent. The amalgamation of knowledge of nanoparticles with recent understanding of molecular and cellular function may lead to the development of novel and superior ‘nanodrugs’. These nanoparticles have the competence to encapsulate, incorporate, or conjugate an array of drug molecules for target‐specific delivery of drug [5, 6].
This review will summarise nanotechnology‐based approaches to help in the cure of cancer and multidrug resistance related to a tumour, control of biofilms and to overcome infectious bacterial diseases. It will present an idea how nanomedicine is rising as a startling platform for the advanced therapeutic approach.
2 Nanoparticles interaction with the biological system
There are arrays of researches going on the applications of nanoparticles and significant strides have been made in the formulation of different kinds of nanoparticles having applications in therapeutics. Several types of nanoparticles have been reported for the treatment and the impediment of diseases [7, 8, 9]. Basic definitions of various nanoparticles being used in biomedical applications are summarised in Table 1 and pictorial representation of these nanoparticles having a specific shape and properties is presented in Fig. 1.
Table 1.
Definition of nanoparticles generally used in the biomedical system
| Nanoparticles | Definition |
|---|---|
| liposome | vesicular nanosized structures made up of one or more phospholipid bilayer membranes surrounding an aqueous core [10] |
| dendrimer | synthetic nanosized structure made up of multiple branched monomeric unit radiating from central core [11] |
| micelle | nanosized structure made up of the shell and a core (made up of water‐soluble and hydrophobic polymers) [12] |
| nanocapsule | a nanosized structure consisting of a shell surrounding a space within which drugs are placed [13] |
| polymeric nanoparticles | polymeric matrix with therapeutic agent attached to its surface or encapsulated within its interior [14] |
| polymeric‐drug conjugate | side chain grafting of the drug to a polymeric chain of nanosize [15] |
| polymerosome | like liposome but are composed of synthetic polymer/polypeptide amphiphiles and self‐assemble to form polymer shell vesicles of nanoscale [16] |
| quantum dots and rods | semiconductor nanocrystal having the shape of rod and dots [17] |
| solid lipid nanoparticles | nanoparticles with solid lipid matrix and diameter in nanometre range [18] |
| inorganic nanoparticles | metal‐based particle of nanosize (Ag‐NPs, gold nanoparticles) [19] |
| nanocomposite | hybrid material formed by combination of two or more nanoscale material to improve their individual properties (graphene based, carbon nanotubes) [20] |
Fig. 1.
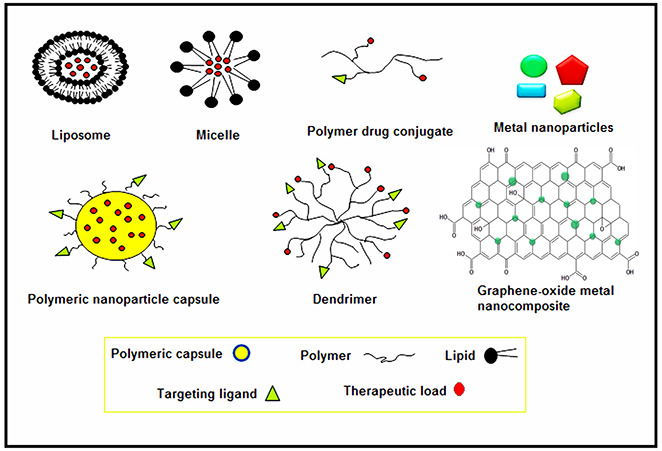
Pictorial representation of different types of nanoparticles
Ensuring that nanoparticles should reach its specific site unaltered and in its functional form is a huge challenge for researchers. There are several biological barriers which hamper their delivery into the target site. After entering the body, nanoparticles initially interact with macromolecules and then they are distributed to various organs. During their passage from the site of administration to the place of their activity, they come across several biological barriers [4]. These barriers are enzymatic degradation and poor stability, epithelial endothelial barrier, immunological barriers. Immunological barriers include opsonisation and uptake by a reticuloendothelial system which leads to aggregation of nanoparticles and activation of defence mechanisms [21, 22, 23]. Along with that there are cellular and extracellular barriers which inhibit nanoparticles to traverse cellular membrane and penetrate mucin and extracellular matrix, respectively [21]. There are also some intracellular barriers which lead to entrapment of nanoparticles within endosomes and their ejection by efflux pumps [24].
These biological barriers may overcome by improving and optimising the surface chemistry of nanoparticles which may result in enhanced and desired physicochemical properties. Different aspects have been studied by workers to make nanoparticles a helpful pharmaceutical which include biocompatibility, intracellular delivery, cellular targeting and site‐specific drug release.
2.1 Biocompatibility
To reduce non‐specific cytotoxic effects to healthy tissue and maximise drug effect on the target tissue, biocompatibility is the main feature to take into consideration. Biocompatible nanoparticles have been made from a variety of materials, including polymethacrylic acid (PMA), polyethylene glycol (PEG), poly(lactide‐co‐glycolide) (PLGA), polylactic acid (PLA), natural polymers such as chitosan, gelatine or alginate and other material such as lipid and silica [6]
PLGA exhibits good biodegradability, biocompatibility, appropriate mechanical properties and degradation kinetics; moreover, they are easy to process. PLGA has been considered as a promising material for the formulation of nanoparticles for drug delivery systems, and enormous research is going on in this area [25]. PLGA has already been permitted by the Food and Drug Administration for numerous biomedical applications including implants, surgical suture as well as prosthetic devices [26]. To protect the nanoparticles in the blood stream from rapid elimination by the mononuclear phagocytic system, PEG has been used as a coating agent. Coating of PEG enhances circulation time, which may increase the therapeutic capacity of nanoparticles [27, 28]. Chitosan and alginate which are the natural sources derived polymers may offer friendly conditions by avoiding the use of an organic solvent for the encapsulation or incorporation of DNA or peptides [29].
Lipid nanocapsule and liposome have also been investigated as a promising nanodelivery system. Aminoglycosides (such as gentamicin) encapsulated in liposome have shown a higher therapeutic ability than conventional aminoglycoside preparations in the liver and spleen using a murine Salmonella typhi infection model [30]. Jung et al. have reported that lipid encapsulated amphotericin B (an antifungal) when given to mice with an Aspergillus fumigates infection the mice survived longer as compared to the mice treated with other amphotericin B formulations. Moreover, liposome‐treated mice showed reduced renal toxicity and longer circulation time [31]. Lipid nanocapsules have pegylated surfactant membrane covered oily core. Promises have been shown by these lipid nanocapsules in the encapsulation of anticancer drugs (paclitaxel, etoposidein doxorubicin) in vitro and in vivo [32].
The consequence of the long‐term accumulation of the nanoparticles is unknown, so it is better to use material that is fully biodegradable. Silica has also emerged as a biodegradable material which may also be used to formulate silica‐based nanoparticles that can incorporate a variety of agents for drug delivery application [33]. The benefit of using silica‐based compounds is that its degradation avoids tissue accumulation concern. As discussed here different materials show various biocompatible properties and characteristics, suited to their specific applications. It is essential to determine the properties of nanoparticles for distinct applications.
2.2 Cytosolic delivery of nanomedicine
The challenge faced in the treatment of intracellular pathogen is that it is necessary to penetrate the cellular compartment to ensure an adequate amount of the drug is reaching to the pathogen. Internalisation of nanoparticles can occur through a mechanism of endocytosis, in particular it includes receptor‐mediated and clathrin‐coated pit endocytosis [34]. Following endocytosis nanoparticles may dwell inside acidic endolysosome compartment and may cause drug degradation. Therefore, it becomes important for nanoparticles to elude this endolysosome compartment and achieve entry to the cytosol for targeted drug release. PLGA nanoparticles loaded with doxorubicin have been reported to escape the endosomal compartment by surface charge reversal. By virtue of this property, these particles interact with membrane and elude into the cytosol where they release doxorubicin [26]. In a study performed on mesoporous hybrid silica nanoparticles, it has been demonstrated that internalisation of these nanoparticles occur by receptor‐mediated endocytosis and this results in their localisation in the endocytic compartment and finally releasing the loaded material into the cytosol [35]. To release the drug into the cell by endocytic mechanism ‘Thiolated PMA hydrogel capsules’ have been prepared, which have been shown to work in a time‐dependent manner [36]. As a whole, these studies revealed that nanoparticles may be formulated which have the ability to penetrate cells and release the drug in intracellular compartments.
2.3 Targeted drug delivery
The main purpose of targeting nanodrugs to the population of specific cells is to increase the therapeutic efficacy of drug simultaneously minimising adverse effect on healthy tissues, in turn reducing or eliminating the side effects. Targeted drug delivery of nanoparticles can be achieved by conjugating a targeting ligand to their surface. A spacer arm may also be used to attach these ligands which can increase the probability of specific binding of nanoparticles to the target surface due to enhanced flexibility of arm [37]. Antibodies, receptor ligands, aptamers, peptides or small molecules may be used as targeting ligands [37, 38, 39].
Hybrid lipid nanoparticles coupled with anticarcino‐embryonic antigen half antibody has demonstrated targeting capacity to pancreatic cancer cells [39]. Poly(ethylene glycol)‐block‐poly(ɛ‐caprolactone) (PEG‐b‐PCL) micelle bearing surface epidermal growth factor has been studied to be capable of targeting cancer cells which were overexpressing the epidermal growth factor receptor in vitro and in vivo [40]. Moreover, for specifically targeting glicoma and breast tumour cells expressing GRP 78 marker, paclitaxel carrying polyester nanoparticles have been targeted to irradiating tumour cells with the help of short peptide GIRLRG, which bind specifically to GRP78 in response to radiation therapy [41]. Gu et al. have reported the conjugation of the A10 aptamer to PLGA/PEG nanoparticles, which is a specific targeting strategy. These nanoparticles bind to prostate‐specific membrane antigen on the surface of the prostate cancer cell [42]. As is clear from the examples cited here most of the work in the targeting of the nanoparticles is related to cancer targeting, although similar model can be used in case of the intracellular pathogen as well as pathogen‐infected cell.
By keeping in view all the data reported it can be said that nanomedicine should be fabricated to have all the requirements for an ‘ideal’ drug delivery system, which include a choice of nanomaterial, targeting molecule, cell penetrating peptides and incorporated drug molecule of interest (Fig. 2). However, there is a lot to be explored in this new field.
Fig. 2.
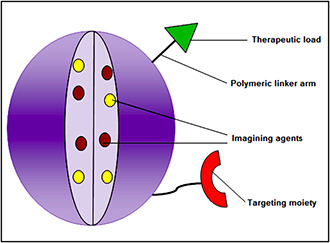
Component of nanoparticles design for an ideal drug delivery system
3 Applications
3.1 Nanoparticle‐based cancer therapies
One of the principal reasons for mortality in the modern world is cancer; millions of new cases are emerging day by day. It is well recognised that cancer is caused by multiple factors which include a complex mixture of environmental and genetic considerations [43]. Enormous advances have resulted into a deeper understanding of cancer, which has led to the new strategies and targets for cancer therapies, although more new developments are required for effective treatment of cancer. Anticancer drugs can only be efficient if they reach the target site in desired concentration and exert their pharmacological effect without injuring any healthy cells and tissues, but this is a major limitation with present therapeutic treatment [44]. The field of nanotechnology has emerged as a new horizon for the development of clinical therapeutics. The biocompatibility of nano‐sized drug carriers such as liposome and polymeric nanoparticles has led to a safer and efficient delivery of numerous drugs. Nanoscale drug delivery systems provide the benefit of improved pharmacokinetics, longer circulation half‐life and reduced side effects [45, 46]. Therapeutic nanoparticles with such advantages are establishing as a promising candidate having a potential to replace traditional chemotherapies which cause side effects and serious damage to healthy tissues. In recent times, many nanoparticle‐based drugs have emerged in the market and several are in various stages of clinical and pre‐clinical trials [46]. Noteworthy examples of nanoparticles used in cancer treatment include Doxil (a ∼100 nm liposomal formulation of doxorubicin) and Abraxane (a ∼130 nm paclitaxel‐bound protein particle), both of which are usually administered as first‐line treatments in various cancer types [47, 48]. Currently, there is lots of ongoing research in the field of cancer treatment by using nanoparticles in both targeted and non‐targeted approaches.
3.1.1 Liposome nanoparticles
Liposomes are a molecule of amphiphilic lipids that assemble to form bilayered spherical vesicles [10]. The circulation half‐life of liposome has been enhanced by coating them with PEG, which resulted in the development of PEG‐coated liposomes or pegylated liposome [47]. An example of the pegylated liposome is Doxil which has been clinically used for the treatment of numerous types of cancer and established itself as a landmark for liposomal drug delivery systems. It is made up of a packed pegylated surface (2 kDa PEG chains) and by drug diffusion method doxorubicin has been loaded in these nanoparticles [46, 47]. Nanosomes have been developed (small liposomes, <100 nm), carrying numerous drugs such as bryostatin‐1, docetaxel, vitamin D analogue and camptothecin for treatment of cancer types [49, 50]. The liposomal formulations have the ability to change surface charge properties by changes in the pH of the solution. The change in the surface charge in acidic pH leads to its fusion with endosome during endocytosis uptake, permitting the escape of the nanocarriers into the cytoplasm to deliver the drug it is carrying. Owing to their distinctive structure, liposomes can be used to load hydrophilic drugs in their aqueous core and hydrophobic drugs in their lipid bilayered membrane simultaneously [51]. By virtue of this property, the liposome may be used as a platform for combination drug delivery.
3.1.2 Multifunctional micellar nanodrug for cancer therapy
Polymeric micelles are nanosized spherical particles formed by a self‐assemblage of biocompatible amphiphilic copolymers in aqueous environments [12]. These polymeric micelles due to their unique architecture may be used to incorporate multiple functional components within single moiety. In a single platform, multiple interventions against a tumour such as stimulated release of therapeutics, delivery of imaging agents may be integrated. Multifunctional pH‐sensitive polymeric micelle loaded with doxorubicin along with conjugated folate have been proved to be more effective in treating KB cells than untargeted formulations [52]. Similarly, in another study folate conjugated polymeric micelles have been found to accumulate greater in an MCF‐7 tumour in vivo and were having better cytotoxicity as compared to folate‐free micelle [53].
3.1.3 Polymeric nanoparticles
Polymeric nanoparticles are made from a material which is biocompatible and biodegradable. These nanoparticles possess all the properties which are necessary for an ideal drug delivery system such as a sustained drug release, enhance stability, higher loading capacity, manageable physicochemical properties. Polymeric nanoparticles are made of amphiphilic di‐block copolymers that self‐assemble into nanoparticles in aqueous solutions [54, 55, 56].
3.1.4 Dendrimers
Although liposome and polymeric nanoparticles are the most widely studied for cancer therapies, dendrimers can also be used as a promising nanomedicine. Dendrimers have an interior core made of layers of branched repeating units with functional end group on the outermost layer. The hydrophobic core contains a cavity that can encapsulate hydrophobic drugs. Hydrophilic drugs can be attached on their surface [11]. Poly (propylene‐imine) dendrimer have been prepared which have the capability to bind methotrexate (hydrophobic drug) in its core and all‐trans retinoic acid (hydrophilic drug) on its surface. The carboxyl group of the drug molecule and amine groups of dendrimers have an electrostatic interaction which helps to stabilise the loaded drug and causes pH‐dependent drug release. Under acidic conditions, carboxyl group gets deprotonated and causes a conformational change in dendritic structure which leads to the accelerated release of drug from the dendrimer particle. Much slower release kinetics have been observed in neutral and alkaline pH. The property of these dendrimers to release the drug on pH change may help in reducing systemic toxicity and may reduce premature drug leakage [57].
3.1.5 Inorganic and noble metal nanoparticles for cancer therapy
Metal nanoparticles have unique characteristics such as high surface‐to‐volume ratio, broad optical properties and simple surface chemistry which are helpful in cancer therapies. These metal nanoparticles can easily be structured with different moieties for example peptides, antibodies and/or DNA/RNA to target the desired cell specifically. They can also be functionalised with biocompatible polymers to prolong their circulation for drug delivery applications [58]. Gold nanoparticles have already been used as vehicles for the delivery of anticancer drugs, such as paclitaxel or platinum‐(Pt‐) based drugs e.g. oxaliplatin [59, 60]. Calcium phosphate nanoparticles have been utilised as a non‐toxic carrier for the delivery of different therapeutic and imaging agents in biological systems [61, 62].
3.2 Overcoming the multidrug resistance in cancer (MDR)
One of the main hindrances in the treatment of cancer is the resistance developed to multiple chemotherapeutic agents, the phenomena termed as multidrug resistance (MDR). The development of multidrug resistance is believed to be the cause of tumour survival despite invasive chemotherapeutic treatment [63]. MDR in cancer cells can be acquired by several mechanisms, among them one or more may be responsible for the multidrug resistance phenotype. The multidrug resistance is caused by various changes such as increased activity of drug efflux pump, metabolic modification or detoxification, initiation of DNA repair and transformed expression of apoptosis linked protein [64]. Of these mechanisms, overexpression of ABC transporter is most frequent. To date, there are 48 known transporters which are transmembrane protein and are classified into seven different subfamilies [65]. They transport numerous structurally related and unrelated compounds such as anticancer drug out of cells and thus decreasing the accretion of these anticancer drugs at the intracellular level. Owing to their unique physicochemical properties such as smaller size range, greater drug solubilisation, improved drug loading capacity, prolonged drug circulation, site‐specific targeting and controlled drug release nanoparticles are preferred therapeutics in cancer treatment. Nanoparticles provide a platform to co‐administer anticancer drug and efflux pump inhibitor in a single‐drug carrier for simultaneous delivery into tumour cells [66]. A new therapy to overcome this multidrug resistance is to use a co‐delivery system that employs a siRNA to silence the expression of the efflux transporter along with a suitable anticancer drug. Simultaneous delivery of doxorubicin and P‐glycoprotein siRNA loaded in mesoporous silica nanoparticles has been successfully achieved, which resulted in increased concentration of drug at the intracellular level [67]. Similar results have been quoted by different workers using nanoparticle‐based combined therapy [66].
3.3 Antibacterial property of nanoparticles
Bacterial resistance to a different antibiotic is expanding and is one of the growing problems these days. This resistance is mainly due to two reasons, firstly the bacterial genetic tolerance to antibiotics, for example in multiple drug‐resistant Staphylococcus aureus (MRSA) and secondly due to the formation of biofilms which are robustly adherent and have antibiotic resistance. Hence, it is very essential to develop novel therapeutics which may overcome these problems. Owing to their effective antimicrobial potential and distinctive mode of action, nanoparticles offer a better substitute to conventional antibiotic therapies [19, 68].
Furthermore, metal nanoparticles have been known to possess toxicity towards bacteria that mammalian cells do not have. Although the exact mechanism behind this selectivity is not yet fully described, it is generally recognised that nanoparticles may attach to the microbial cell wall, thus exert a toxic effect by disturbing cell wall permeability of the bacterial cell. The ions released by metal nanoparticles may also affect their physiological pathways [6]. Moreover, the production of toxic reactive oxygen species (ROS) such as superoxide, hydrogen peroxide and hydroxyl ions is one of the main mechanisms behind the antibacterial activity of nanoparticles [68, 69]. Based on their composition there are mainly two broad categories of nanoparticles used in antibacterial therapies.
3.3.1 Organic nanoparticles
These nanoparticles are generally polymeric and lipid based and exert their antibacterial effect by the release of entrapped or attached antibiotic, antibacterial peptides and other agents which can affect the bacterial viability [70]. As discussed earlier the unique properties of liposomes help them to carry drugs easily on their surface as well as in their core. Ampicillin loaded liposomes have been acquainted to be more effective as compared to the free antibiotic in combating infection of Salmonella typhimurium and Listeria monocytogenes [71]. Liposomal formulations having encapsulated penicillin were found to inhibit the growth of various resistant strains of bacteria [72]. Furthermore, piperacillin loaded liposomes have been reported to protect the antibiotic from hydrolysis by β‐lactamases [73]. Polymeric nanoparticles have also been used to entrap drugs for antimicrobial treatments [70]. Mohammadi et al. [74] formulated azithromycin encapsulated PLGA nanoparticles by a nanoprecipitation technique which was more effective against S. typhi as compared to free azithromycin. Cinnamaldehyde and eugenol loaded PLGA nanoparticles have been reported to show better antimicrobial activity against bacteria [75]. There are various kinds of literature on the increased efficiency of nanoparticles entrapped drugs as compared to the free formulation. Another way by which organic nanoparticles interact with bacteria is by contact killing of bacteria due to cationic surfaces of nanoparticles such as chitosan, quaternary ammonium compounds and many more. Various mechanisms have been proposed for antibacterial action of cationic groups present on nanoparticles surface. They primarily include bursting and penetration of bacterial membrane by hydrophobic chains via ion exchange between the bacterial membrane and charged surface [76]. The protonation of deacetylated amino groups of chitosan at pH lower than 6.5 provides a positive charge [77]. Perusing this positive charge chitosan has property to associate with the negatively charged surface of bacteria causing osmotic damage by increased permeability of bacterial membrane [78]. Chitosan can also bind to DNA and inhibit transcription and translation; it can chelate the metal ions and reduce the activity of metalloproteins [79]. The formulation of chitosan into nanoparticles increases its antibacterial activity. As compared to chitosan its nanoparticles have been reported to be having a good solubility in vivo; furthermore, high surface‐to‐volume ratio of nanoparticles increases the density of positive charge on its surface, hence elevating the frequency of microbial attachment to its surface [71]. Chitosan nanoparticles have been reported to possess greater efficacy against S. aureus and Escherichia coli compared to chitosan alone [77]. It has been suggested that chitosan nanoparticles might be more effective against Gram‐negative bacteria as it can displace calcium and magnesium ions which can destabilise the lipopolysaccharide membrane of Gram‐negative bacteria, thus increasing its permeability [80].
Furthermore, nanoparticles formulated using quaternary ammonium compounds have also been shown to possess antibacterial properties. They denature structural enzymes and protein by interacting with the bacterial membrane and integrating its hydrophobic tail in the bacterial hydrophobic membrane core. Beyth et al. have reported the antibacterial activity of dental composite containing quaternary ammonium polyethylenimine nanoparticles against dental pathogen Streptococcus mutans [81].
Although they have effective antibacterial properties, temperature instability is the major problem of organic nanomaterial. This instability leads to several difficulties in the preparation of these nanoparticles. Furthermore, their ability to withstand harsh conditions is also less. Inorganic nanoparticles are comparatively more stable at a higher temperature. Consequently, inorganic nanoparticles are more frequently used as antimicrobials.
3.3.2 Inorganic nanomaterials
There are several kinds of literature on use of metal and metal oxides as antibacterial material [70, 82, 83]. The mechanisms behind their antimicrobial activity highly depend on the type of metal ion present. They mainly kill or inhibit the growth of microbes by the production of ROS and by membrane disruption [80]. In the present scenario silver nanoparticles (Ag‐NPs) are gaining much attention as they have broad‐spectrum antimicrobial activity [84]. Numerous studies have proposed that the antibacterial effect of Ag‐NPs is due to the release of silver ions (Ag+) from its surface [70, 83]. These Ag+ ions may interact with thiol groups of the cell wall of bacteria and create holes in the membrane facilitating the flow of cytoplasmic material out of the cell. This may cause cell death. Moreover, these Ag+ ions may interact with DNA, inhibiting DNA replication and cell division [85]. The antibacterial efficacy of Ag‐NPs also depends on its shape and size. Nanoparticles having size < 10 nm has been reported to show greater bactericidal activity as compared to bigger nanoparticles [86]. Furthermore, the shapes of nanoparticles which may increase their surface area provide them with more antibacterial potency. ROS production has been proposed as another mechanism which imparts antibacterial property to Ag‐NPs [87]. Although Ag‐NPs show remarkable antibacterial properties against a wide range of microbes, the exact mechanism is not fully understood. There are lots of disagreements and debate on the mode of action of these nanoparticles but they are perhaps the most promising antibacterial metal nanoparticles.
Zinc oxide in its nanoparticles form is believed to be antibacterial and relatively non‐toxic, safe and biocompatible as compared to other metal nanoparticles [88]. Zinc oxide nanoparticles are being widely used as drug carriers, preservatives, in cosmetics and filling in medical materials [89]. Zinc oxide nanoparticles have been reported to inhibit the growth of methicillin‐resistant strains of S. aureus (MRSA) and Staphylococcus epidermidis [90]. There is numerous literature on antibacterial activity of these nanoparticles which include their effect on a broad range of bacteria such as E. coli, S. mutans, L. monocytogenes, S. aureus, K. pneumonia [91, 92, 93]. ZnO‐NPs in their aqueous suspension produce extensive amounts of ROS which contribute to its antibacterial potential. Among all the ROS, hydrogen peroxide (H2 O2) interactions with bacterial membrane have been suggested as a dominant antibacterial mechanism of ZnO‐NPs. Like other nanoparticles, they also release a Zn2+ metal ion which helps in its antibacterial mode of action [94].
Copper oxide nanoparticles (CuO‐NPs) show antibacterial activity against different microbes, but their antibacterial potency is much lower than silver and zinc oxide nanoparticles [95]. Thus, they are antibacterial at higher concentrations. Cu ions interact with the amine and carboxylic groups of bacterial membrane and disrupt it. Hence, bacteria that possess a higher density of these groups on their surface (Bacillus subtilis) are more prone to CuO‐NPs attack [96]. Therefore, in some bacteria, use of CuO‐NPs is much beneficial than other nanoparticles. Magnesium also has been utilised in formation of different nanoformulations which show antibacterial activity. Magnesium oxide nanoparticles are easy to synthesise and show antibacterial activity against both Gram‐negative and Gram‐positive bacteria, spores and viruses [71]. Magnesium fluoride nanoparticles have been reported to inhibit the biofilm formation in E. coli, S. aureus and S. mutans [97].
Gold‐containing nanoparticles lack antibacterial activity, but they can be used as carriers of antibacterial drugs and peptides. Brown et al. have reported that gold nanoparticles functionalised ampicillin have the potential of destroying many drug‐resistant bacterial strains such as Pseudomonas aeruginosa, E. aerogegenes and MRSA [98]. Gold nanoparticles have been suggested to enhance the photodynamic therapy‐based killing of microbes by ROS production [99].
Graphene oxide (GO) is getting much attention as a nanomaterial and as a precursor of many graphene‐related materials [100]. A number of reports have shown the antibacterial activity of graphene oxide, but its antibacterial potential is much less than other nanomaterial [101]. The lateral dimensions of graphene oxide have been found to affect its antibacterial ability. Liu et al. have suggested a three‐step antibacterial mechanism of graphene oxide, which mainly relates to the close contact of bacteria with GO surface, membrane puncture and oxidative stress [102]. These nanosheets possess many reducible groups which can be functionalised with any antimicrobial material. GO is being extensively used as a supporting material for other nanoparticles as it provides a better platform for interaction of bacteria with the attached nanoparticles [103, 104].
3.4 Biofilm inhibition
Sessile, surface adhered communities of bacteria embedded in the pool of self‐produced polymeric matrix are called biofilms [105]. Biofilm formation is a process having sequential steps which include microbial surface attachment, cell proliferation, matrix production and detachment (Fig. 3). Biofilms create an environment that enhances microbial resistance. In addition to their direct bactericidal activity nanoparticles are known to disrupt biofilms formation [69].
Fig. 3.
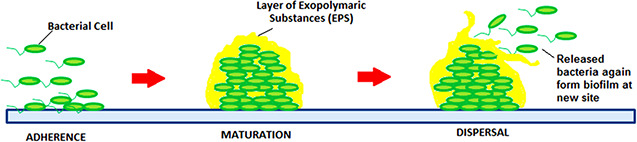
Stages of biofilm formation by bacterial cells
Nanoparticles can be exploited to eliminate preformed biofilms or they may be exploited to restrict biofilm formation. There are several independent studies going on the use of nanoparticles to inhibit biofilms, some noteworthy have been included in this review. Complete inhibition of biofilms formed by S. mutans has been shown by zerovalent bismuth nanoparticles [106]. In an investigation the magnesium fluoride nanoparticle‐coated catheters effectively restricted biofilms formation in both growth media and biologically relevant fluids [97]. Graphene/zinc oxide nanocomposites have been reported to inhibit S. mutans biofilm; moreover, it's coating has been proposed to protect dental implants against cariogenic S. mutans biofilm [20]. The novel class of ultrathin (∼1–2 nm) silver ring‐coated superparamagnetic iron oxide nanoparticles (SPIONs) with ligand gap exhibits antimicrobial characteristics against bacteria by maintaining remarkable compatibility with the cells; moreover, SPIONS have shown high therapeutic index against S. aureus and S. epidermidis infections [107]. In a recent study Kulshrestha et al. [108] have reported the use of calcium fluoride nanoparticles to inhibit the initial stages of biofilm formation in S. mutans.
Nanoparticles may also be used to enhance the photodynamic therapy, which is a novel therapeutic approach to eliminate biofilms. Gold nanoparticles have been used to enhance the methylene‐blue‐induced photodynamic therapy to inhibit Candida albicans biofilm and it has been confirmed that gold nanoparticle–methylene blue conjugate work by type I phototoxicity which is a hydroxyl free radical [99]. Another study revealed that there is significantly higher inhibition of microbial biofilms by chitosan nanoparticle‐loaded erythrosine‐induced photodynamic treatment than erythrosine in the free form [109]. Recently, Misba et al. [110] have reported the type I photodynamic therapy by using toluidine blue O‐conjugated Ag‐NPs against biofilm of S. mutans. Table 2 summarises some of the recent reports on the application of nanomaterials in their different forms as antibacterial and antibiofilm agents. From the above discussion, it may be concluded that nanoparticles are not only a potential candidate for anticancer therapies, but also gaining much attention as antibacterial and antibiofilm agents.
Table 2.
Summarisation of some recent reports on use of nanoparticles as antibacterial and antibiofilm agents
| Type of nanoparticles | Microorganism | Ref. |
|---|---|---|
| liposome loaded with ampicillin | S. typhimurium, L. monocytogenes | [71] |
| liposome encapsulated penicillin | P. aeruginosa | [73] |
| PLGA encapsulated azithromycin | S. typhi | [75] |
| chitosan nanoparticles | S. aureus, E. coli | [77] |
| quaternary ammonium polyethylenimine nanoparticles | S. mutans | [81] |
| Ag‐NPs | broad range of microbes | [86, 87] |
| zinc oxide nanoparticles | broad range of microbes | [90, 91, 92, 93] |
| CuO‐NPs | broad range of microbes | [95] |
| magnesium fluoride nanoparticles | E. coli, S. aureus, S. mutans | [97] |
| gold nanoparticles functionalised ampicillin | P. aeruginosa, E. aerogegenes | [98] |
| graphene oxide silver nanocomposite | broad range of microbes, P. aeruginosa biofilm | [103, 104] |
| zero valent bismuth nanoparticles | S. mutans biofilm | [106] |
| graphene/zinc oxide nanocomposite | S. mutans biofilm | [20] |
| silver ring‐coated SPIONs | S. aureus biofilm, S. epidermidis biofilm | [107] |
| calcium fluoride nanoparticles | S. mutans biofilm | [108] |
| gold nanoparticles enhanced photodynamic therapy | C. albicans biofilm | [99] |
| Ag‐NP‐induced photodynamic therapy | S. mutans biofilm | [110] |
| chitosan nanoparticles loaded dye‐induced photodynamic therapy | S. mutans biofilm, P. aeruginosa biofilm, C. albicans biofilm | [109] |
3.5 Safety concerns with the use of nanoparticles as an antibacterial agent
The safety concerns of inorganic nanoparticles directly as an antibacterial agent on normal mammalian cells is very important to access. Cytotoxicity assays are performed on normal human cell lines to justify that the dose of nanoparticles taken for antibacterial treatment is non‐toxic to normal mammalian cells. For example, in a study done by Kulshrestha et al. cytotoxicity assay was performed on HEK‐293 cell line (human embryonic kidney cell line) and concentrations of CaF2 ‐NPs used in the study were found non‐cytotoxic to HEK‐293 cells. Although in vivo studies are further required to estimate the toxic effect of nanoparticles as it is not necessary that the in vitro results will directly translate on a systemic level. Another potentially harmful effect is that the nanoparticles might disrupt the balance of the host gut microbiome if used orally. Although it is uncertain from the existing literature, whether these interactions occur and whether they are detrimental, positive or inconsequential. Hence, further research is required in this aspect before using nanoparticles in therapeutics [108].
4 Recent advances in the field of nanomedicine
Nanomedicine is a rapidly growing field and has a broad impact on human health. Researchers have already made progress in this field which has led to the development of a wide range of products and nanoformulations for treatment of cancer and infectious diseases. There are several nanomedicine products which have been approved by the FDA or are under clinical investigation.
The research on liposome has led to the development of the first FDA approved nanomedicine called DOXIL as well as 12 additional liposome‐based therapeutics. Furthermore, there are 30 lipid‐based nanoformulations which are under clinical investigation. DOXIL is the liposomal formulation of doxorubicin and is especially used in the treatment of cancer. After the expiration of its patent, there was a worldwide shortage of DOXIL, for that reason alternative formulation of DOXIL such as Lipodox has been approved by FDA. Lipodox has pharmacokinetics similar to DOXIL [111, 112]. ThermoDox (Celsion Corp.) is another doxorubicin liposomal platform which is under clinical trials. It is temperature sensitive liposome, which releases doxorubicin at high temperatures. The drug is being investigated for the treatment of liver metastasis and breast cancer chest wall recurrence [112]. A promising effect against haematologic malignancies has been shown by CPX‐351 (Celator Pharma) which is a dual‐agent liposomal formulation of both cytarabine and daunorubicin. It is under preclinical trials. Liposome entrapped with nucleic acid formulations such as gene therapy agents and small interfering RNAs (siRNAs) are of great interest in cancer treatment and are at various phases of preclinical and clinical investigations [113].
Protein nanoparticles as therapeutics have also been approved by the FDA, such as Abraxane, ABI‐007 (Abraxis Corporation), which is albumin‐bound formulation of paclitaxel that is without any solvent. Paclitaxel is an FDA approved chemotherapeutic drug which is used for the treatment of different solid tumours such as breast, lung, gastrointestinal and ovarian cancers. Abraxane is a formulation of 130 nm particles in the bottle, which rapidly dissociates into ∼8 nm paclitaxel‐coated albumin molecules in the plasma [112].
Drug conjugated with a polymer to form drug conjugated polymeric nanoparticles is also showing a promising effect in cancer treatment as compared to the drug alone. Many of these conjugates are under preclinical and clinical trials. Poliglumex paclitaxel is a nanoconjugate formulation of polyglutamic acid paclitaxel and is commercially available under the trade name Xyotax, later changed to Opaxio (CTI Biopharma) is under clinical investigation and has been suggested as a potential treatment of non‐small cell lung cancer and ovarian cancer [114]. Another example may include HPMA (N ‐(2‐hydroxylpropyl) methylacrylamide) copolymer doxorubicin, which has completed early phase clinical trials against metastatic solid tumour malignancies. Its commercial name is PK1 (Pfizer Inc.) [115]. Further, numerous other nanomedicines for delivery of different anticancer drugs are under different stages of clinical trials such as NK012 (Nippon Kayaku Co. Ltd.), SP1049C (Supratech Pharma Inc.), BIND‐014 (Bind Therapeutics) and many more.
Other than drug delivery several inorganic nanoformulations are being studied to be used as imagining such as SPIONs are under clinical trials as an aid in imagining of tumours and cancers [112].
Many nanomedicines are being formulated and been applied for the treatment of microbial diseases. These nanomedicine are FDA approved or under clinical trials. Clinical translation is a challenging process. It entails wide preclinical research, judiciously selected clinical results, appropriate design of clinical trials and the efficacious completion of these trials [112]. For use in the treatment of microbial diseases nanoliposomes such as AmBisome (Gilead Sciences, Inc.) and DepoCyt[e] (Pacira Pharmaceuticals, Inc.) have been approved by FDA. AmBisome is amphotericin loaded nanoliposome while DepoCyt[e] is cytarabine loaded nanoliposome. The lipid nanoparticle formulations such as Amphotec (Sequus Pharmaceuticals, Inc.), which is lipid nanoparticles loaded with amphotericin, and MEGACE ES (Par Pharmaceutical Companies, Inc.), which is nanoparticles loaded with megestrol acetate, have been approved by the FDA. The nanodendrimer (VivaGel, Starpharma Holdings Limited) formulated as a water‐based gel and delivered vaginally now has European Union regulatory approval for the topical treatment and rapid relief of bacterial vaginosis [116]. Furthermore, there are several studies on inorganic nanoparticles as antimicrobial agents which are needed to be clinically translated.
5 Future prospects
Nanoparticle‐based therapeutics having refined properties and biocompatibility are expected to be designed which may enhance human health. To achieve this goal, it is required to focus our research on reducing the toxicity of nanoparticles and formulating nanoparticles which may have the potential to work in a target‐specific manner. Nanotechnology is expected to provide a platform for the development of improved therapeutics which may revolutionise the field of medicine. These nanoparticle‐based medicines and diagnostics will open the doors of the healthy world with the human having enhanced physical abilities.
6 Acknowledgment
DST grant no. DST: SR/NM/NS‐41/2016(G) is highly acknowledged for the support of this submission.
7 References
- 1. Farokhzad O.C. Langer R.: ‘Impact of nanotechnology on drug delivery’, ACS Nano, 2009, 3, (1), pp. 16 –20 [DOI] [PubMed] [Google Scholar]
- 2. McNeil S.E.: ‘Unique benefits of nanotechnology to drug delivery and diagnostics’, in Mc Neil S.E. (Ed.): ‘Characterization of nanoparticles intended for drug delivery’ (Springer Science + Business Media, LLC, New York, USA, 2011), pp. 3 –8 [DOI] [PubMed] [Google Scholar]
- 3. Verma A. Stellacci F.: ‘Effect of surface properties on nanoparticle‐cell interactions’, Small, 2010, 6, pp. 12 –21 [DOI] [PubMed] [Google Scholar]
- 4. Blanco E. Shen H. Ferrari M.: ‘Principles of nanoparticle design for overcoming biological barriers to drug delivery’, Nat. Biotechnol., 2015, 33, (9), pp. 941 –951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parveen S. Misra R. Sahoo S.K.: ‘Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging’, Nanomedicine: N.B.M., 2012, 8, (2), pp. 147 –166 [DOI] [PubMed] [Google Scholar]
- 6. Armstead A. Li B.: ‘Nanomedicine as an emerging approach against intracellular pathogens’, Int. J. Nanomed., 2011, 6, pp. 3281 –3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu C.M. Aryal S. Zhang L.: ‘Nanoparticle‐assisted combination therapies for effective cancer treatment’, Ther. Deliv., 2010, 1, (2), pp. 323 –334 [DOI] [PubMed] [Google Scholar]
- 8. Petros R.A. DeSimone J.M.: ‘Strategies in the design of nanoparticles for therapeutic applications’, Nat. Rev. Drug Discov., 2010, 9, (8), pp. 615 –627 [DOI] [PubMed] [Google Scholar]
- 9. Zhu Y. Liao L.: ‘Applications of nanoparticles for anticancer drug delivery: a review’, J. Nanosci. Nanotechnol., 2015, 15, (7), pp. 4753 –4773 [DOI] [PubMed] [Google Scholar]
- 10. Chrai S.S. Murari R. Ahmad I.: ‘Liposomes: A review’, Pharmaceut. Tech., 2002, 26, pp. 28 –34 [Google Scholar]
- 11. Pedziwiatr‐Werbicka E. Ferenc M. Zaborski M. et al.: ‘Characterization of complexes formed by polypropylene imine dendrimers and anti‐HIV oligonucleotides’, Colloids Surf.B., 2011, 83, pp. 360 –366 [DOI] [PubMed] [Google Scholar]
- 12. Kwon G.S. Forrest M.L.: ‘Amphiphilic block copolymer micelles for nanoscale drug delivery’, Drug. Develop. Res., 2006, 67, pp. 15 –22 [Google Scholar]
- 13. Hillaireau H. Le Doan T. Appel M. et al.: ‘Hybrid polymer nanocapsules enhance in vitro delivery of azidothymidine‐triphosphate to macrophages’, J. Control Release, 2006, 116, pp. 346 –352 [DOI] [PubMed] [Google Scholar]
- 14. Shah L.K. Amiji M.M.: ‘Intracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDS’, Pharm. Res., 2006, 23, pp. 2638 –2645 [DOI] [PubMed] [Google Scholar]
- 15. Campone M. Rademaker‐Lakhai J.M. Bennouna J. et al.: ‘Phase I and pharmacokinetic trial of AP5346, a DACH‐platinum‐polymer conjugate, administered weekly for three out of every 4 weeks to advanced solid tumor patients’, Cancer. Chemother. Pharmacol., 2007, 60, pp. 523 –533 [DOI] [PubMed] [Google Scholar]
- 16. Discher B.M. Won Y.Y. Ege D.S. et al.: ‘Polymersomes: tough vesicles made from diblock copolymers’, Science, 1999, 284, pp. 1143 –1146 [DOI] [PubMed] [Google Scholar]
- 17. Fu A. Gu W. Boussert B. et al.: ‘Semiconductor quantum rods as single molecule fluorescent biological labels’, Nano. Lett., 2006, 7, pp. 179 –182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wissing S. Kayser O. Muller R.: ‘Solid lipid nanoparticles for parenteral drug delivery’, Adv. Drug. Deliv. Rev., 2004, 56, pp. 1257 –1272 [DOI] [PubMed] [Google Scholar]
- 19. Jena P. Mohanty S. Mallick R. et al.: Toxicity and antibacterial assessment of chitosan coated silver nanoparticles on human pathogen and macrophage cell’, Int. J. Nanomed., 2012, 7, pp. 1805 –1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kulshrestha S. Khan S. Meena R. et al.: ‘A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans ’, Biofouling, 2014, 30, (10), pp. 1281 –1294 [DOI] [PubMed] [Google Scholar]
- 21. Duncan R. Gaspar R.: ‘Nanomedicine (s) under the microscope’, Mol. Pharm., 2011, 8, pp. 2101 –2141 [DOI] [PubMed] [Google Scholar]
- 22. Ferrari M.: ‘Cancer nanotechnology: opportunities and challenges’, Nat. Rev. Cancer., 2005, 5, pp. 161 –171 [DOI] [PubMed] [Google Scholar]
- 23. Karmali P.P. Simberg D.: ‘Interactions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systems’, Expert. Opin. Drug. Del., 2011, 8, pp. 1 –15 [DOI] [PubMed] [Google Scholar]
- 24. Sanhai W.R. Sakamoto J.H. Canady R. et al.: ‘Seven challenges for nanomedicine’, Nat. Nanotechnol., 2008, 3, pp. 242 –244 [DOI] [PubMed] [Google Scholar]
- 25. Danhier F. Ansorena E. Silva J.M. et al.: ‘PLGA‐based nanoparticles: an overview of biomedical applications’, J. Control. Release, 2012, 161, (2), pp. 505 –522 [DOI] [PubMed] [Google Scholar]
- 26. Panyam J. Zhou W.Z. Prabha S. et al.: ‘Rapid endolysosomal escape of poly (DL‐lactide‐co‐glycolide) nanoparticles: implications for drug and gene delivery’, FASEB J., 2002, 16, pp. 1217 –1226 [DOI] [PubMed] [Google Scholar]
- 27. Lü J.M. Wang X. Marin‐Muller C. et al.: ‘Current advances in research and clinical applications of PLGA‐based nanotechnology’, Expert Rev. Mol. Diagn., 2009, 9, (4), pp. 325 –341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao R.Z. Zeng Z.W. Zhou G.L. et al.: ‘Recent advances in PEG‐PLA block copolymer nanoparticles’, Int. J. Nanomed., 2010, 5, pp. 1057 –1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reis C.P. Neufeld R.J. Vilela S. et al.: ‘Review and current status of emulsions/dispersions technology using an internal gelation process for the design of alginate particles’, J. Microencapsul., 2006, 23, pp. 245 –257 [DOI] [PubMed] [Google Scholar]
- 30. Schiffelers R. Storm G. Bakker‐Woudenberg I.: ‘Liposome‐encapsulated aminoglycosides in pre‐clinical and clinical studies’, J. Antimicrob. Chemother., 2001, 48, pp. 333 –334 [DOI] [PubMed] [Google Scholar]
- 31. Jung S.H. Lim D.H. Jung S.H. et al.: ‘Amphotericin B‐entrapping lipid nanoparticles and their in vitro and in vivo characteristics’, Eur. J. Pharm. Sci., 2009, 37, (3), pp. 313 –320 [DOI] [PubMed] [Google Scholar]
- 32. Huynh N.T. Passirani C. Saulnier P. et al.: ‘Lipid nanocapsules: a new platform for nanomedicine’, Int. J. Pharm., 2009, 379, pp. 201 –209 [DOI] [PubMed] [Google Scholar]
- 33. Bitar A. Ahmad N.M. Fessi H. et al.: ‘Silica‐based nanoparticles for biomedical applications’, Drug Discov. Today, 2012, 17, (19), pp. 1147 –1154 [DOI] [PubMed] [Google Scholar]
- 34. Vasir J.K. Labhasetwar V.: ‘Biodegradable nanoparticles for cytosolic delivery of therapeutics’, Adv. Drug. Deliv. Rev., 2007, 59, pp. 718 –728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenholm J.M. Peuhu E. Eriksson J.E. et al.: ‘Targeted intracellular delivery of hydrophobic agents using mesoporous hybrid silica nanoparticles as carrier systems’, Nano. Lett., 2009, 9, pp. 3308 –3311 [DOI] [PubMed] [Google Scholar]
- 36. Yan Y. Johnston A.P. Dodds S.J. et al.: ‘Uptake and intracellular fate of disulfide‐bonded polymer hydrogel capsules for doxorubicin delivery to colorectal cancer cells’, ACS Nano, 2010, 4, (5), pp. 2928 –2936 [DOI] [PubMed] [Google Scholar]
- 37. Wang M. Thanou M.: ‘Targeting nanoparticles to cancer’, Pharmacol. Res., 2010, 62, pp. 90 –99 [DOI] [PubMed] [Google Scholar]
- 38. Nobs L. Buchegger F. Gurny R. et al.: ‘Current methods for attaching targeting ligands to liposomes and nanoparticles’, J. Pharm. Sci., 2007, 90, pp. 1980 –1992 [DOI] [PubMed] [Google Scholar]
- 39. Hu C.M. Kaushal S. Cao H.S. et al.: ‘Half‐antibody functionalized lipid– polymer hybrid nanoparticles for targeted drug delivery to carcinoembryonic antigen presenting pancreatic cancer cells’, Mol. pharm., 2010, 7, (3), pp. 914 –920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee H. Hu M. Reilly R.M. et al.: ‘Apoptotic epidermal growth factor (EGF)‐conjugated block copolymer micelles as a nanotechnology platform for targeted combination therapy’, Mol. Pharm., 2007, 4, (5), pp. 769 –781 [DOI] [PubMed] [Google Scholar]
- 41. Passarella R.J. Spratt D.E. van der Ende A.E. et al.: ‘Targeted nanoparticles that deliver a sustained, specific release of paclitaxel to irradiated tumors’, Cancer Res., 2010, 70, (11), pp. 4550 –4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gu F. Zhang L. Teply B.A. et al.: ‘Precise engineering of targeted nanoparticles by using self‐assembled biointegrated block copolymers’, Proc. Natl. Acad. Sci. U.S.A., 2008, 105, (7), pp. 2586 –2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wild C.P. Bucher J.R. De Jong B.W. et al.: ‘Translational cancer research: balancing prevention and treatment to combat cancer globally’, J. Natl. Cancer Inst., 2015, 107, (1), p. dju353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Balmain A. Gray J. Ponder B.: ‘The genetics and genomics of cancer’, Nat. Genet., 2003, 33, pp. 238 –244 [DOI] [PubMed] [Google Scholar]
- 45. Peer D. Karp J.M. Hong S. et al.: ‘Nanocarriers as an emerging platform for cancer therapy’, Nat. Nanotechnol., 2007, 2, pp. 751 –760 [DOI] [PubMed] [Google Scholar]
- 46. Davis M.E. Chen Z.G. Shin D.M.: ‘Nanoparticle therapeutics: an emerging treatment modality for cancer’, Nat. Rev. Drug. Discov., 2008, 7, (9), pp. 771 –782 [DOI] [PubMed] [Google Scholar]
- 47. Northfelt D.W. Dezube B.J. Thommes J.A. et al.: ‘Pegylated‐liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS‐related Kaposi's sarcoma: results of a randomized phase III clinical trial’, J. Clin. Oncol., 1998, 16, (7), pp. 2445 –2451 [DOI] [PubMed] [Google Scholar]
- 48. Harries M. Ellis P. Harper P.: ‘Nanoparticle albumin‐bound paclitaxel for metastatic breast cancer’, J. Clin. Oncol., 2005, 23, (31), pp. 7768 –7771 [DOI] [PubMed] [Google Scholar]
- 49. Ahmad A. Sheikh S. Ali S.M. et al.: ‘Nanosomal paclitaxel lipid suspension demonstrates higher response rates compared to paclitaxel in patients with metastatic breast cancer’, J. Cancer Sci. Ther., 2015, 7, pp. 116 –120 [Google Scholar]
- 50. Couvreur P. Vauthier C.: ‘Nanotechnology: intelligent design to treat complex disease’, Pharm. Res., 2006, 23, (7), pp. 1417 –1450 [DOI] [PubMed] [Google Scholar]
- 51. Castor T.P.: ‘Phospholipid nanosomes’, Curr. Drug. Deliv., 2005, 2, pp. 329 –340 [DOI] [PubMed] [Google Scholar]
- 52. Jones M. Leroux J.: ‘Polymeric micelles – a new generation of colloidal drug carriers’, Eur. J. Pharm. Biopharm., 1999, 48, pp. 101 –111 [DOI] [PubMed] [Google Scholar]
- 53. Bae Y. Jang W.D. Nishiyama N. et al.: ‘Multifunctional polymeric micelles with folate‐mediated cancer cell targeting and pH‐triggered drug releasing properties for active intracellular drug delivery’, Mol. Biosyst., 2005, 1, pp. 242 –250 [DOI] [PubMed] [Google Scholar]
- 54. Bazile D. Prudhomme C. Bassoullet M.T. et al.: ‘PEG–PLA nanoparticles avoid uptake by the mononuclear phagocytes system’, J. Pharm. Sci., 1995, 84, (4), pp. 493 –498 [DOI] [PubMed] [Google Scholar]
- 55. Lammers T. Subr V. Ulbrich K. et al.: ‘Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers’, Biomaterials, 2009, 30, (20), pp. 3466 –3475 [DOI] [PubMed] [Google Scholar]
- 56. Ortiz R. Pardos J. Melguizo C. et al.: ‘5‐Fluorouracil‐loaded poly (ɛ‐caprolactone) nanoparticles combined with phage E gene therapy as a new sratagy against colon cancer’, Int. J. Nanomed., 2012, 7, pp. 95 –107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tekade R.K. Dutta T. Gajbhiye V. et al.: ‘Exploring dendrimer towards dual drug delivery: pH responsive simultaneous drug‐release kinetics’, J. Microencap., 2009, 26, (4), pp. 287 –296 [DOI] [PubMed] [Google Scholar]
- 58. Sperling R.A. Parak W.J.: ‘Surface modification functionalization and bioconjugation of colloidal inorganic nanoparticles’, Phil. Transac. R. Soc., 2010, 368, pp. 1333 –1383 [DOI] [PubMed] [Google Scholar]
- 59. Gibson J.D. Khanal B.P. Zubarev E.R.: ‘Paclitaxel functionalized gold nanoparticles’, J. Ameri. Chem. Soc., 2007, 129, pp. 11653 –11661 [DOI] [PubMed] [Google Scholar]
- 60. Brown S.D. Nativo P. Smith J.A. et al.: ‘Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin’, J. American Chem. Soc., 2010, 132, (13), pp. 4678 –4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kester M. Heakal Y. Fox T. et al.: ‘Calcium phosphate nanocomposite particles for in vitro imaging and encapsulated chemotherapeutic drug delivery to cancer cells’, Nano Lett.., 2008, 8, (12), pp. 4116 –4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharma R. Barth B.M. Altinoğlu E.İ. et al.: ‘Bioconjugation of calcium phosphate nanoparticles for selective targeting of human breast and pancreatic cancers in vivo’, ACS Nano, 2010, 4, (3), p. 1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bradley G. Juranka P.F. Ling V.: ‘Mechanism of multidrug resitance’, Biochem. Biophys. Acta., 1988, 948, pp. 87 –128 [DOI] [PubMed] [Google Scholar]
- 64. Harris A.L. Hochhauser D.: ‘Mechanisms of multidrug resistance in cancer treatment’, Acta. Oncol., 1992, 31, pp. 205 –213 [DOI] [PubMed] [Google Scholar]
- 65. Szakács G. Varadi A. Özvegy‐Laczka C. et al.: ‘The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME–Tox)’, Drug Discov. Today, 2008, 13, (9), pp. 379 –393 [DOI] [PubMed] [Google Scholar]
- 66. Xue X. Liang X.J.: ‘Overcoming drug efflux based multidrug resistance in cancer with nanotechnology’, Chin. J. Cancer, 2012, 31, pp. 100 –107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meng H. Mai W.X. Zhang H. et al.: ‘Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo’, ACS Nano, 2013, 7, (2), pp. 994 –1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khan A.U.: ‘Medicine at nanoscale new horizon’, Int. J. Nanomed., 2012, 7, pp. 2997 –2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qayyum S. Khan A.U.: ‘Nanoparticles vs. Biofilms: a battle against another paradigm of antibiotic resistance’, Med. Chem. Comm., 2016, 7, (8), pp. 1479 –1498 [Google Scholar]
- 70. Beyth N. Houri‐Haddad Y. Domb A. et al.: ‘Alternative antimicrobial approach: nano‐antimicrobial materials’, J. Evid. Based Complementary Alter. Med., 2015, 2015, 246012. doi: 10.1155/2015/246012 p. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blecher K. Nasir A. Friedman A.: ‘The growing role of nanotechnology in combating infectious disease’, Virulence, 2011, 2, pp. 395 –401 [DOI] [PubMed] [Google Scholar]
- 72. Pinto‐Alphandary H. Andremont A. Couvreur P.: ‘Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications’, Int. J. Antimicrob. Agents, 2000, 13, pp. 155 –168 [DOI] [PubMed] [Google Scholar]
- 73. Alipour M. Suntres Z.E.: ‘Liposomal antibiotic formulations for targeting the lungs in the treatment of Pseudomonas aeruginosa ’, Ther. Deliv., 2014, 5, pp. 409 –427 [DOI] [PubMed] [Google Scholar]
- 74. Mohammadi G. Valizadeh H. Barzegar‐Jalali M. et al.: ‘Development of azithromycin‐PLGA nanoparticles: physicochemical characterization and antibacterial effect against Salmonella typhi ’, Colloids Surf. B., 2010, 80, pp. 34 –39 [DOI] [PubMed] [Google Scholar]
- 75. Gomes C. Moreira R.G. Castell‐Perez E.: ‘Poly (DL‐lactide‐co‐glycolide) (PLGA) nanoparticles with entrapped trans‐cinnamaldehyde and eugenol for antimicrobial delivery applications’, J. Food Sci., 2011, 76, pp. N16 –N24 [DOI] [PubMed] [Google Scholar]
- 76. Lichter J.A. Rubner M.F.: ‘Polyelectrolyte multilayers with intrinsic antimicrobial functionality: the importance of mobile polycations’, Langmuir, 2009, 25, pp. 7686 –7694 [DOI] [PubMed] [Google Scholar]
- 77. Friedman A.J. Phan J. Schairer D.O. et al.: ‘Antimicrobial and anti‐inflammatory activity of chitosan‐alginate nanoparticles: a targeted therapy for cutaneous pathogens’, J. Invest. Dermatol., 2013, 133, pp. 1231 –1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Huang L. Dai T. Xuan Y. et al.: ‘Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: efficacy against bacterial burn infections’, Antimicrob. Agents Chemother., 2011, 55, pp. 3432 –3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Huh A.J. Kwon Y.J.: ‘‘Nanoantibiotics’: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era’, J. Control Release, 2011, 156, pp. 128 –145 [DOI] [PubMed] [Google Scholar]
- 80. Pelgrift R.Y. Friedman A.J.: ‘Nanotechnology as a therapeutic tool to combat microbial resistance’, Adv. Drug. Deliv. Rev., 2013, 65, pp. 1803 –1815 [DOI] [PubMed] [Google Scholar]
- 81. Beyth N. Yudovin‐Farber I. Bahir R. et al.: ‘Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans ’, Biomaterials, 2006, 27, pp. 3995 –4002 [DOI] [PubMed] [Google Scholar]
- 82. Li Q. Mahendra S. Lyon D.Y. et al.: ‘Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications’, Water Res.., 2008, 42, pp. 4591 –4602 [DOI] [PubMed] [Google Scholar]
- 83. Hajipour M.J. Fromm K.M. Ashkarran A.A. et al.: ‘Antibacterial properties of nanoparticles’, Trends Biotechnol., 2012, 30, pp. 499 –511 [DOI] [PubMed] [Google Scholar]
- 84. Lara H.H. Ayala‐Núnez N.V. Turrent L.D.C.I. et al.: ‘Bactericidal effect of silver nanoparticles against multidrug‐resistant bacteria’, World J. Microbiol. Biotechnol., 2010, 26, pp. 6715 –6721 [Google Scholar]
- 85. Knetsch M.L.W. Koole L.H.: ‘New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles’, Polymers. (Basel), 2011, 3, pp. 340 –366 [Google Scholar]
- 86. Pal S. Tak Y.K. Song J.M.: ‘Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram‐negative bacterium Escherichia coli ’, Appl. Environ. Microbiol., 2007, 73, pp. 1712 –1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Carlson C. Hussain S.M. Schrand A.M. et al.: ‘Unique cellular interaction of silver nanoparticles: size‐dependent generation of reactive oxygen species’, J. Phy. Chem.B., 2008, 112, pp. 13608 –13619 [DOI] [PubMed] [Google Scholar]
- 88. Raghupathi K.R. Koodali R.T. Manna A.C.: ‘Size‐dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles’, Langmuir, 2011, 27, pp. 4020 –4028 [DOI] [PubMed] [Google Scholar]
- 89. Applerot G. Lellouche J. Perkas N. et al.: ‘Zno nanoparticle‐coated surfaces inhibit bacterial biofilm formation and increase antibiotic susceptibility’, Rsc Adv., 2012, 2, pp. 2314 –2321 [Google Scholar]
- 90. Ansari M.A. Khan H.M. Khan A.A. et al.: ‘Characterization of clinical strains of MSSA, MRSA and MRSE isolated from skin and soft tissue infections and the antibacterial activity of ZnO nanoparticles’, World J. Microb. Biot., 2012, 28, pp. 1605 –1613 [DOI] [PubMed] [Google Scholar]
- 91. Jin T. Sun D. Su J.Y. et al.: ‘Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157: H7’, J. Food Sci., 2009, 74, pp. M46 –M52 [DOI] [PubMed] [Google Scholar]
- 92. Kasraei S. Sami L. Hendi S. et al.: ‘Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus ’, Restor. Dent. Endod., 2014, 39, pp. 109 –114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu Y. He L. Mustapha A. et al.: ‘Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157: H7’, J. Appl. Microbiol., 2009, 107, pp. 1193 –1201 [DOI] [PubMed] [Google Scholar]
- 94. Shi L.‐E. Li Z.‐H. Zheng W. et al.: ‘Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review’, Food Addit. Contam. Part A, 2014, 31, pp. 173 –186 [DOI] [PubMed] [Google Scholar]
- 95. Ren G. Hu D. Cheng E.W.C. et al.: ‘Characterisation of copper oxide nanoparticles for antimicrobial applications’, Int. J. Antimicrob. Agents, 2009, 33, pp. 587 –590 [DOI] [PubMed] [Google Scholar]
- 96. Ruparelia J.P. Chatterjee A.K. Duttagupta S.P. et al.: ‘Strain specificity in antimicrobial activity of silver and copper nanoparticles’, Acta Biomater., 2008, 4, pp. 707 –716 [DOI] [PubMed] [Google Scholar]
- 97. Lellouche J. Friedman A. Lahmi R. et al.: ‘Antibiofilm surface functionalization of catheters by magnesium fluoride nanoparticles’, Int. J. Nanomed., 2012, 7, p. 1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brown A.N. Smith K. Samuels T.A. et al.: ‘Nanoparticles functionalized with ampicillin destroy multiple‐antibiotic‐resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin‐resistant Staphylococcus aureus ’, Appl. Environ. Microbiol., 2012, 78, pp. 2768 –2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Khan S. Alam F. Azam A. et al.: ‘Gold nanoparticles enhance methylene blue‐induced photodynamic therapy: a novel therapeutic approach to inhibit Candida albicans biofilm’, Int. J. Nanomed., 2010, 7, p. 3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Novoselov K.S. Geim A.K.: ‘The rise of graphene’, Nat. Mater., 2007, 6, pp. 183 –191 [DOI] [PubMed] [Google Scholar]
- 101. Akhavan O. Ghaderi E.: ‘Toxicity of graphene and graphene oxide nanowalls against bacteria’, ACS Nano, 2010, 4, pp. 5731 –5736 [DOI] [PubMed] [Google Scholar]
- 102. Liu S. Zeng T.H. Hofmann M. et al.: ‘Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress’, ACS Nano, 2011, 5, pp. 6971 –6980 [DOI] [PubMed] [Google Scholar]
- 103. deFaria A.F. Martinez D.Sf.T. Meira S.M.M. et al.: ‘Anti‐adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets’, Colloids Surf. B, 2014, 113, pp. 115 –124 [DOI] [PubMed] [Google Scholar]
- 104. Tang J. Chen Q. Xu L. et al.: ‘Graphene oxide‐silver nanocomposite as a highly effective antibacterial agent with species‐specific mechanisms’, ACS Appl. Mater. Interfaces, 2013, 5, pp. 3867 –3874 [DOI] [PubMed] [Google Scholar]
- 105. Costerton J.W. Montanaro L. Arciola C.R.: ‘Biofilm in implant infections: its production and regulation’, Int. J. Artif. Org., 2005, 28, pp. 1062 –1068 [DOI] [PubMed] [Google Scholar]
- 106. Hernandez‐Delgadillo R. Velasco‐Arias D. Diaz D. et al.: ‘Zerovalent bismuth nanoparticles inhibit Streptococcus mutans growth and formation of biofilm’, Int. J. Nanomed., 2012, 7, p. e2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mahmoudi M. Serpooshan V.: ‘Silver coated engineered magnetic nanoparticles are promising for the success in the fight agaist antibacterial resistance threat’, ACS Nano, 2012, 6, pp. 2656 –2664 [DOI] [PubMed] [Google Scholar]
- 108. Kulshrestha S. Khan S. Hasan S. et al.: ‘Calcium fluoride nanoparticles induced suppression of Streptococcus mutans biofilm: an in vitro and in vivo approach’, Appl. Microbiol. Biotechnol., 2016, 100, (4), pp. 1901 –1914 [DOI] [PubMed] [Google Scholar]
- 109. Chen C.P. Chen C.T. Tsai T.: ‘Chitosan nanoparticle for antimicrobial photodynamic inactivation: characterization and in vitro investigation’, J. Photochem. Photobiol., 2012, 88, pp. 570 –576 [DOI] [PubMed] [Google Scholar]
- 110. Misba L. Kulshrestha S. Khan A.U.: ‘Antibiofilm action of a toluidine blue O‐silver nanoparticle conjugate on Streptococcus mutans: a mechanism of type I photodynamic therapy’, Biofouling, 2016, 32, (3), pp. 313 –328 [DOI] [PubMed] [Google Scholar]
- 111. Hong R.L. Tseng Y.L.: ‘Phase I and pharmacokinetic study of a stable, polyethylene‐glycolated liposomal doxorubicin in patients with solid tumors: the relation between pharmacokinetic propertyand toxicity’, Cancer, 2001, 91, pp. 1826 –1833 [PubMed] [Google Scholar]
- 112. Min Y. Caster J.M. Eblan M.J. et al.: ‘Clinical translation of nanomedicine’, Chem. Rev., 2015, 115, (19), pp. 11147 –11190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Carol H. Fan M.M.Y. Harasym T.O. et al.: ‘Efficacy of CPX351, (Cytarabine: Daunorubicin) liposome injection, against acute lymphoblastic leukemia (ALL) xenograft models of the pediatric preclinical testing program’, Pediatr. Blood Cancer., 2015, 62, pp. 65 –71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Paz‐Ares L. Ross H. O'Brien M. et al.: ‘Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second‐line treatment of non‐small‐cell lung cancer’, Br. J. Cancer, 2008, 98, pp. 1608 –1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ulbrich K. Etrych T. Chytil P. et al.: ‘HPMA copolymers with pH‐controlled release of doxorubicin‐in vitro cytotoxicity and in vivo antitumor activity’, J. Control. Release, 2003, 87, pp. 33 –47 [DOI] [PubMed] [Google Scholar]
- 116. He Z.Y. Wei X.W. Wei Y.Q.: ‘Recent advances of nanostructures in antimicrobial therapy’, Antimicrob. Nanoarchitectonics, 2017, pp. 167 –194 [Google Scholar]


