Abstract
The synergistic relationship between structure and the bulk properties of polyelectrolyte multilayer (PEM) films has generated tremendous interest in their application for loading and release of bioactive species. Layer‐by‐layer assembly is the simplest, cost effective process for fabrication of such PEMs films, leading to one of the most widely accepted platforms for incorporating biological molecules with nanometre precision. The bulk reservoir properties of PEM films render them a potential candidate for applications such as biosensing, drug delivery and tissue engineering. Various biomolecules such as proteins, DNA, RNA or other desired molecules can be incorporated into the PEM stack via electrostatic interactions and various other secondary interactions such as hydrophobic interactions. The location and availability of the biological molecules within the PEM stack mediates its applicability in various fields of biomedical engineering such as programmed drug delivery. The development of advanced technologies for biomedical applications using PEM films has seen rapid progress recently. This review briefly summarises the recent successes of PEM being utilised for diverse bio‐applications.
Inspec keywords: polymer electrolytes, multilayers, polymer films, molecular biophysics, biomedical materials, biochemistry
Other keywords: bioapplications, polyelectrolyte multilayer films, bioactive species, layer‐by‐layer assembly, biological molecules, biosensing, drug delivery, tissue engineering, biomolecules, proteins, DNA, RNA, electrostatic interactions, secondary interactions, hydrophobic interactions, biomedical engineering, programmed drug delivery, biomedical applications, PEM films
1 Introduction
Over the past decade, polyelectrolyte multilayer (PEM) coatings are amongst the most widely explored and popular tools to modulate surface properties of biomaterials for inducing specific tissue responses, controlling fouling behaviour and functioning as reservoirs for active therapeutic cargo [1, 2]. These PEMs are deposited by layer‐by‐layer (LbL) process [3], where layer build‐up takes place mainly by electrostatic interactions along with various other short‐range interactions [4, 5, 6, 7, 8, 9]. Fig. 1 shows the schematic depiction of (LbL) process, where deposition is guided by reversal of surface charges at each deposition step when substrate is allowed to dip in the solution of oppositely charged polyelectrolyte with an intermediate rinse step to remove the loosely bound charges. The most fascinating aspect of this electrostatic assembly is the ability to control materials structure on the length scale of a few nanometres by varying assembly or post assembly conditions [10, 11].
Fig. 1.
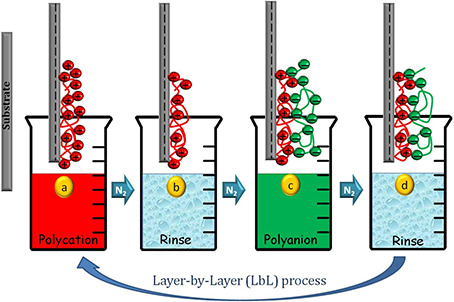
Schematic depicting of LbL process for deposition of PEM films
The key feature that makes LbL assembly a preferred choice for bio‐applications is the easy inclusion of biomolecules such as proteins, tissue growth factors, DNA, RNA and so on into the assembly process [12]. Several recent examples demonstrate PEM to be employed as efficient reservoirs for diverse cargo, including multiple bioactive agents [13] such as DNA and gene cargo for the sustained and targeted release [14]. Such excellent loading behaviours of these PEM have also been employed for the advancement of various biomedical applications including the creation of drug eluting stents, delivery of antibiotic and anti‐inflammatory drugs, guided differentiation of stem cells in tissue engineering and transcutaneous surface mediated delivery of vaccines and adjuvants [15]. Fig. 2 shows a schematic depicting the wide applicability of PEM for various bio‐applications.
Fig. 2.
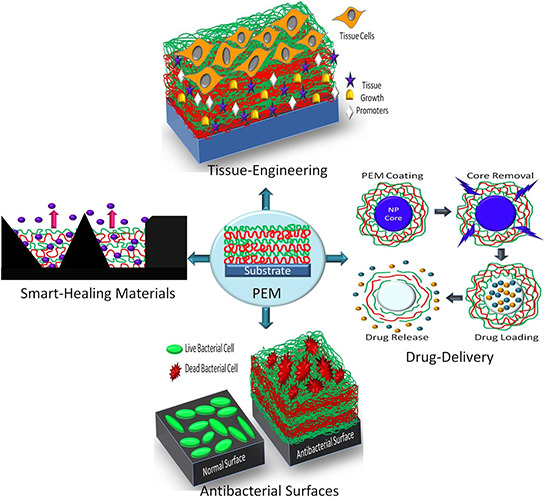
Schematic depicting the wide applicability of PEM for various bio‐applications
2 PEM as bio‐molecular delivery vehicle
LbL is an extremely robust and reproducible technique to create highly ordered polymeric stacks on any substrate irrespective of its shape, size and geometry. The tenability of bulk chemical properties of these biocompatible and biodegradable polymer assemblies has been used for loading and unloading the drugs [16]. Also, the technological ability to control layer interdiffusion and manipulation of layer stratification is of great importance for sustained and controlled release of drugs using PEM. In an effort to develop such engineered surfaces, substrates such as PEMs have pioneered the implementation of substrate‐mediated delivery in biomedical applications [17]. Originated from planar surfaces, the multilayers topologically organised in the shape of hollow spheres became attractive systems for drug‐delivery applications for their employment as drug carriers [18, 19]. In addition to this, PEM offers a wide spectrum of permeability for different species ranging from small ions to large drug molecules by stimulating the physical and chemical parameter of the surroundings [20, 21, 22]. Fig. 3 enlists the physico‐chemical parameters utilised for fabricating highly responsive PEM stack for the desired bio‐molecule delivery into the surroundings [22].
Fig. 3.
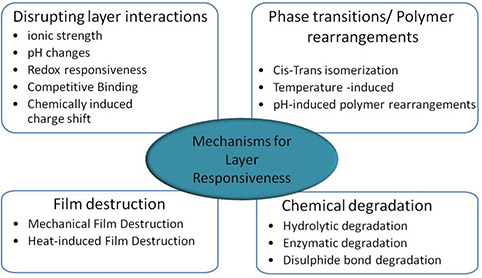
Mechanisms resulting PEM‐layer responsiveness and considered for biomolecule‐release as mentioned in [22]
Recently, a surface mediated drug delivery system has been developed which is comprised of hyaluronic acid covalently functionalised liposomes (HALNPs) embedded into PEM [23]. PEM platform used here is made of poly‐L lysine and poly(sodium styrene sulfonate) (PLL/SPS), anionic HALNPs were allowed to adsorb into the PEM matrix. This is an excellent example of a surface mediated and dual (hydrophillic and hydrophobic) drug nanocarrier. The successful embedment of a translatable lipid‐based nanocarrier (HALNPs) into a substrate allowed the delivery of the chemotherapeutic drug doxorubicin. Therefore, PEM or drug eluting polymer films are prompting a new era in surface mediated drug delivery applications.
A novel programmed and extended drug releasing PEM platform has been reported which is capable of early osteogenic differentiation of human mesenchymal stem cells [24]. The advancement in designing this engineered PEM stack offers controlled release of dexamethasone (dex) to mesenchymal cells. The PEM stack is comprised of biocompatible poly(methacrylic acid) (PMAA)/poly(acrylamide) (PAAm)/PMAA/poly(ethylene oxide)‐block ‐poly(ɛ‐caprolactone) (PEO‐b ‐PCL) micelles arranged in LbL films. This is the one of the best performing PEM stack exhibiting a highly programmed release profile. There are several examples of drug‐loaded polyelectrolyte coated microcapsules being employed for the increased efficacy of targeted delivery of anticancer drugs by sustaining the release of drugs [25, 26, 27].
These polyelectrolyte coated microcapsules are fabricated by depositing polyelectrolytes onto the template core that can be chemically dissolved or removed after the desired PEM stack build‐up and core is utilised to encapsulate or deliver a wide range of macromolecular agents or drugs by modulating the chemistry. The release of these encapsulated species is guided by the various external environmental stimuli and the rate is determined by its ability to diffuse through the PEM stack [28]. A brief look through the literature reflects a significant increase in such successful examples of PEM films being employed for drug delivery and other bio‐related applications. Some of the recent examples of this applied research have been enlisted in Table 1, which reflects that there will be further increase in the number of such reports in the near future.
Table 1.
Recent examples of PEM used for various bio‐applications
| PEM | Constituents | Objective | Applications | Ref. |
|---|---|---|---|---|
| PLL/SPS | Hyaluronic acid liposomes (HALNPs) and doxorubicin (DOX) and cholesterol | Local substrate‐mediated hydrophilic and hydrophobic cargo delivery | HALNP embedded PEM system efficient for local therapeutic release | [23] |
| PAA/GS | BMP‐2 | Early release of antibiotic GS and sustained release of BMP‐2 | Antibacterial against S. aureus. Better Osteogenic efficacy for MC3T3‐E1 cells | [29] |
| CHI/DS |
Ciprofloxacin or ceftriaxone (antibiotics) |
Efficient and targeted release of drug ciprofloxcin | Treatment of intraphagosomal infections caused by Salmonella typhimurium | [30] |
| HA/CHI | Antibacterial CHI | Increased surface density of primary amino groups of CHI | Biocidal against Staphylococcus aureus and Pseudomonas aeruginosa | [31] |
| BPEI/PAA | Urushiol particles | Better durability with antibacterial effect | Antimicrobial against Gram + ve and Gram −ve bacteria, Wettability control and durable nanocoatings | [32] |
| CHI‐SH/gelatin | AgNPs | Antibacterial Ti implants | Antibacterial for Bacillus subtitles and Escherichia coli | [33] |
| PAA/CHI | Tobramycin | pH responsive antibacterial coatings | Antibacterial against Staphylococcus aureus | [34] |
PLL: poly‐L‐lysine; SPS: poly(sodium styrene sulfonate); PAA: poly(acrylic acid); CHI: chitosan; DS: dextran sulphate; HA: hyaluronanic acid; CS: chondroitin sulphate; CHI‐SH: sulfhydrylated chitosan; GS: gentamicin sulphate; BPEI: branched polyethyleneimine; PAA: poly(acrylic acid)
3 Biocompatible surface tailoring
In recent years, PEM have been utilised for surface modification applications to introduce desired surface chemistry or bio‐compatibility. PEM coating offers easy tuning of substrate physiological properties, e.g. surface charge, wettability, roughness and stiffness and so on, which affects cell‐substrate adhesion [35]. Thus, by utilising PEM coating technology, any cytophobic (cell‐resistant) surface can be turned to cryophilic (cell‐attracting) by depositing PEM until desired effect is achieved [36]. Fig. 4 shows a schematic representation of conversion of a cytophobic biomaterial's surface into a cytophilic one, in order to promote the desired cell attachment and growth.
Fig. 4.
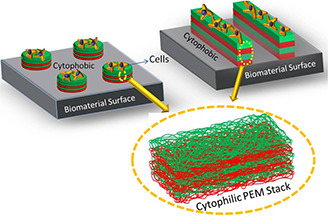
Schematic representation of PEM patterning on the cytophobic biomaterial's surface to make it cytophilic
A highly automated approach has been explored, generating PEM with variations in their physiochemical properties which ultimately decide the cell–PEM interaction [37]. With such advanced technologies, PEM can offer a platform for studying the detailed parameters deciding the cell–PEM interactions for a variety of bio‐medical applications.
Most of these surface tailoring applications are designed for improving the cell or tissue growth on the implanted biomaterial and reducing the bacterial cell attachment as well as corrosive behaviour of biomaterials [38, 39]. Development of smart nanocoatings, which self‐triggers on receiving an external stimulus, is the most advanced feature introduced to create smart antibacterial surfaces [40, 41]. Such self‐triggered PEM stack loaded with antimicrobial agents have been successfully demonstrated by utilising the bacterial growth pH as a sensing signal for the release of antimicrobial components. Once bacterial colonisation happens, it will lead to change in local pH environment as a result of which PEM matrix properties changes and antimicrobial agents diffuses out, imparting antibacterial property.
4 Anti‐bacterial surfaces
Biomedical implant rejection or failures are a major concern in the field of tissue engineering and regenerative‐medicine. Most of these implant rejections are caused by pathogen interaction on the surface of implant, which forms a biofouling layer. So, the design and development of pathogen or bacterial‐resistant coatings on the medical implants is very crucial [42, 43]. The LbL assembly of polyelectrolytes offers a convenient, reliable and cost effective technique for the preparation of biocompatible bacterial resistant films [44, 45].
Antibacterial PEM films can be made by various strategies, e.g. loading the antibiotics into the matrix. Early release of antibiotics such as gentamicin sulphate (GS) ciprofloxacin (or ceftriaxone), from the PEM stack is effective against stopping the growth of pathogenic strains of bacteria, e.g. Staphylococcus aureus and Salmonella typhimurium and lead to better host‐tissue response [29, 30]. In a similar way other bactericidal agents or nanoparticles (NPs) (AgNPs) can also be embedded into the PEM matrix to make antibacterial coatings on biomedical implants [32, 33, 34]. Thus, the idea of using biocompatible bacterial‐resistant polyelectrolyte nanocoatings on the medical implants can provide great advances in the field of tissue engineering and regenerative medicine. So far, various synthetic and biopolymers have been explored for their proven antibacterial effect against the common infections spreading pathogenic strains of bacteria [46, 47].
5 Tissue engineering applications
Amongst the various approaches used to modulate cell adhesion and proliferation, sequential deposition of polyelectrolyte appears to stand out as a very simple and widely accepted technique [48]. Tailored PEM assemblies have been utilised for governing the cellular response due to the ability to control the PEM architecture with nanometre precision. The interaction of LbL films with cells and importance of their fine control for successful tissue engineering have been extensively studied [49, 50, 51, 52]. Fig. 5 shows the list of factors which can affect cell–PEM interaction and used for surface tailoring and tissue‐engineering applications of PEM [49]. Together with the development of patterning techniques for thin films, it is believed that LbL has become a truly powerful tool for controlled adhesion and differentiation of cells [53].
Fig. 5.

List of factors which are considered for desired cell–PEM interaction [49]
The importance of substrate mechanical and chemical characteristics, which effect cell attachment and migration, has also come into the focus of research activities [54]. Substrate influence as well as different assembly and post‐assembly conditions can modulate the mechanical properties of PEM and the desired mechanical integrity of PEM for a particular cell attachment and growth can be achieved by varying the number of bilayers, surface charge, cross‐linking, pH and ionic strength and the choice of PEM combination [55]. A nanotemplating strategy has been tried for making porous PEM scaffolds with controlled loading of biomolecules. Here, NPs serve as a template core for PEM deposition, followed by chemical cross‐linking of the films to increase the mechanical integrity and finally the dissolution of NPs using chemical treatment to achieve/obtain the desired porous matrix [56, 57]. This porous matrix acts as scaffold in tissue engineering applications. The void spacing of porous matrix can be tuned by the size of NP employed as a core, which ultimately governs the cellular attachment and growth. Apart from the size of porous core, the choice of the polyelectrolyte used in the PEM stack and the deposition conditions also determines the design of the final scaffold. Similar nanotemplating has been demonstrated for achieving loading of Bone Morphogenetic Protein‐2 (BMP‐2) through the entire PEM stack and for enhancing myoblast cell attachment and proliferation as compared to non‐porous PEM where BMP‐2 loads mainly on the PEM surface [57]. Table 2 enlists the recent examples of PEM being used for various tissue‐engineering applications by modulating the physical and chemical parameters according to the desired application.
Table 2.
Recent examples of PEM used for tissue‐engineering applications
| PEM stack | Parameter controlled | Cell Lines | Applications | Ref. |
|---|---|---|---|---|
| FN/G | Thickness ∼76 µm | C2C12 myoblasts | Easy cell manipulation | [50] |
| PLL/HA |
|
C2C12 | Ti implants with better osteointegration | [51] |
|
C2C12 | Better muscle regeneration | [52] | |
|
C2C12 | Synergistic effect of the two BMPs which are osteo‐ and myo‐inductive | [53] | |
|
3T3 fibroblasts | ‘Durotaxis’ is studied | [54] | |
| PLL/PGA | Surface charge of PEM stack and serum proteins | HCS‐2/8 human chondrosarcoma cells | importance of the multilayer architecture in controlling cell attachment | [55] |
| PDADMAC/PSS | Template assisted scaffold generation | 3T3 Fibroblasts | Localised release of bio‐molecules using external stimulus | [56] |
| PAA, Poly2 | BMP‐2 and PDGF‐BB insertion | Craniomaxillofacial (CMF) reconstruction | Rapid bone repair application | [58] |
FN: fibronectin; G: gelatine; PLL: poly‐L‐lysine; HA: hyaluronanic acid; PGA: poly(L‐glutamic acid); PDADMA: poly(diallyldimethylammoniumchloride); PSS: poly(sodium 4‐styrenesulfonate); PAA: poly(acrylic acid); Poly2 = cationic polymer; BMP‐2: bone morphogenetic protein‐2 and PDGF: platelet‐derived growth factor‐BB
6 Smart healing materials
Controlling the mobility of the PEM components with respect to external stimuli is a key mechanism for designing self‐healing PEM materials. There are various investigations that discuss the factors affecting the mobility in PEM. Water content of PEM defines significant determinant of the mobility inside PEM matrix as it facilitates the diffusion inside PEM matrix by swelling. Water fraction in these PEM can be controlled by the type of polyelectrolytes selected, pH and ionic strength. The mobility of fluorescently‐tagged proteins have been visualised by FRAP (fluorescence recovery after photo bleaching) and these proteins diffuse inside matrix in a concentration‐dependent manner. Similar diffusive behaviour could be expected for targeted self‐healing agents as well [59], which can also be stimulated by external environmental factors. After receiving a particular stimulus, PEM's matrix losses its integrity and releases a self‐healing agent into the site of defect, even at nanoscale. Super hydrophobic LbL deposited PEMs with self‐healing properties and PEM on bio‐substrate (implants/stents) having self‐healing capacity has been reported in the literature [60]. The multilayers of biocompatible chitosan and heparin could promote re‐endothelialisation and fast healing process after stent implantation [61]. PEM coating has recently been used for soft‐tissue wound healing applications [62]. Here, PEM is spray coated on electrospun three‐dimensional scaffolds meant for tissue wound care. LbL deposition of PEM on these scaffolds offers better tissue interaction on the scaffold surface by facilitating unidirectional water vapour transport and reducing cell attachment over the dressing material.
7 Conclusions and future prospective
Every technology has its pros and cons. Although PEM has achieved considerable success for bio‐applications, a number of practical challenges need to be combated to make them more applicable to the large scale industrial processes. Most of the literature about LbL deposited polyelectrolyte films spans around better understanding of physicochemical conditions and kinetics of multilayer fabrication [3, 4, 5, 6, 7, 8, 9]. Fig. 6 shows the basic statistics of publications related to PEM research as well as the use of PEM for bio‐medical applications generated from Pub med. One can clearly make out the huge lag of PEM being used for biomedical applications. Further investigations into the literature reveal the actual reasons for this limited practical applicability of these PEM. Few of them are listed here.
(i) Long‐term storage or stability of PEM is a critical requirement for their in‐vivo applications.
(ii) For large‐scale industrial applications, a fast and consistent method of deposition capable of producing homogenous films on bigger substrates is required.
(iii) PEM with well‐defined biodegradation rates can be preferred choice for large‐scale drug‐delivery applications.
(iv) There is less understanding of molecular transport across the bulk of PEM matrix. Probing the molecules in PEM is quite an important parameter to consider for variety of practical applications ranging from bio‐applications to other technological areas such as membrane filtration, which are mainly dependent on location and availability of molecules across the porous architecture of PEM [63].
Fig. 6.
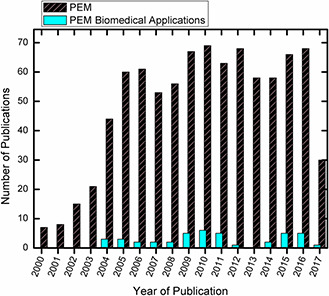
Basic statistics of total number of publications with title topic ‘PEM films’ and publications with ‘PEM films + biomedical applications’ based on Pubmed data dated 2 June 2017
With all practical challenges mentioned above, the highly versatile nature of PEM still holds the potential of their high‐performance in a variety of applications. Combined efforts from the research community to fundamentally examine and reconsideration of these challenges are leading us to design brighter multifunctional tools for healthcare industry. Recently, a highly automated method of PEM layering has been demonstrated, where Inkjet printing issued for depositing consistent PEM layers on cotton‐based substrates [64]. These inkjet printed PEM are claimed to be highly antibacterial for a period of ∼100 days, when loaded with antibiotics against bacteria. Such innovations in designing low cost and highly scalable PEM show great promise for smart wound healing applications. There are a number of such multifunctional PEM coatings, which can be fabricated by high throughput recent technological innovations [37, 65, 66].
In summary, this review enlists the most recent applications of PEM films for biomedical research. The examples presented here demonstrate highly successful approaches for various bio‐applications using PEMs films. LbL deposited PEM are not only used for loading and controlled release of drugs but also for designing model surfaces with tailored physical and chemical properties that can control the interaction with the cells for better biomaterial designs. In short, PEM films have a very bright future in biological applications and have emerged as a promising approach for the advancement of a variety of biomedical procedures and devices.
8 References
- 1. Peyratout C.S. Dähne L.: ‘Tailor‐made polyelectrolyte microcapsules: from multilayers to smart containers’, Angew. Chem. Int. Ed., 2004, 43, (29), pp. 3762 –3783 [DOI] [PubMed] [Google Scholar]
- 2. Ai H. Jones S.A. Lvov Y.M.: ‘Biomedical applications of electrostatic layer‐by‐layer nano‐assembly of polymers, enzymes, and nanoparticles’, Cell Biochem. Biophys., 2003, 39, (1), pp. 23 –44 [DOI] [PubMed] [Google Scholar]
- 3. Decher G.: ‘Fuzzy nanoassemblies: toward layered polymeric multicomposites’, Science, 1997, 277, pp. 1232 –1237 [Google Scholar]
- 4. Haynie D.T. Cho E. Waduge P.: ‘In and out diffusion hypothesis of exponential multilayer film buildup revisited’, Langmuir, 2011, 27, (9), pp. 5700 –5704 [DOI] [PubMed] [Google Scholar]
- 5. Ariga K. Hill J.P. Ji Q.: ‘Layer‐by‐layer assembly as a versatile bottom‐up nanofabrication technique for exploratory research and realistic application’, Phys. Chem. Chem. Phys., 2007, 9, (19), p. 2319 [DOI] [PubMed] [Google Scholar]
- 6. Borges J. Mano J.F.: ‘Molecular interactions driving the layer‐by‐layer assembly of multilayers’, Chem. Rev., 2014, 114, (18), pp. 8883 –8942 [DOI] [PubMed] [Google Scholar]
- 7. Castelnovo M. Joanny J.: ‘Formation of polyelectrolyte multilayers formation of polyelectrolyte multilayers’, Macromolecules, 2000, 16, (11), pp. 7524 –7532 [Google Scholar]
- 8. Kotov N.a.: ‘Layer‐by‐layer self‐assembly: the contribution of hydrophobic interactions’, Nanostructured Mater., 1999, 12, (99), pp. 789 –796 [Google Scholar]
- 9. Shafir A. Andelman D.: ‘Polyelectrolyte multilayer formation: electrostatics and short‐range interactions’, Eur. Phys. J. E, 2006, 19, (2), pp. 155 –162 [DOI] [PubMed] [Google Scholar]
- 10. Xiao F.‐X. Pagliaro M. Xu Y.‐J. et al.: ‘Layer‐by‐layer assembly of versatile nanoarchitectures with diverse dimensionality: a new perspective for rational construction of multilayer assemblies.’, Chem. Soc. Rev., 2016, 45, (11), pp. 3088 –3121 [DOI] [PubMed] [Google Scholar]
- 11. Richardson J.J. Bjornmalm M. Caruso F.: ‘Technology‐driven layer‐by‐layer assembly of nanofilms’, Science, 2015, 348, (6233), pp. aaa2491‐1 –aaa2491‐11 [DOI] [PubMed] [Google Scholar]
- 12. Jewell C. Lynn D.: ‘Multilayered polyelectrolyte assemblies as platforms for the delivery of DNA and other nucleic acid‐based therapeutics’, Adv. Drug Deliv. Rev., 2008, 60, (9), pp. 979 –999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herron M. Schurr M.J. Murphy C.J. et al.: ‘Interfacial stacks of polymeric nanofilms on soft biological surfaces that release multiple agents’, ACS Appl. Mater. Interfaces, 2016, 8, (40), pp. 26541 –26551 [DOI] [PubMed] [Google Scholar]
- 14. Yu Y. Si Y. Bechler S.L. et al.: ‘Polymer multilayers that promote the rapid release and contact transfer of DNA’, Biomacromolecules, 2015, 16, (9), pp. 2998 –3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boudou B.T. Crouzier T. Ren K. et al.: ‘Multiple functionalities of polyelectrolyte multilayer films: new biomedical applications’, Adv. Mater., 2010, 22, pp. 441 –467 [DOI] [PubMed] [Google Scholar]
- 16. De Villiers M.M. Otto D.P. Strydom S.J. et al.: ‘Introduction to nanocoatings produced by layer‐by‐layer (LbL) self‐assembly’, Adv. Drug Deliv. Rev., 2011, 63, (9), pp. 701 –715 [DOI] [PubMed] [Google Scholar]
- 17. Shchukina E.M. Shchukin D.G.: ‘Layer‐by‐layer coated emulsion microparticles as storage and delivery tool’, Curr. Opin. Colloid Interface Sci., 2012, 17, (5), pp. 281 –289 [Google Scholar]
- 18. Correa S. Dreaden E.C. Gu L. et al.: ‘Engineering nanolayered particles for modular drug delivery’, J. Control. Release, 2016, 240, pp. 364 –386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zelikin A.N.: ‘Drug releasing polymer thin films new era of surface‐mediated drug delivery’, ACS Nano, 2010, 4, (5), pp. 2494 –2509 [DOI] [PubMed] [Google Scholar]
- 20. Keeney M. Mathur M. Cheng E. et al.: ‘Effects of polymer end‐group chemistry and order of deposition on controlled protein delivery from layer‐by‐layer assembly’, Biomacromolecules, 2013, 14, (3), pp. 794 –800 [DOI] [PubMed] [Google Scholar]
- 21. Appadoo V. Carter M.C.D. Lynn D.M.: ‘Controlling the surface‐mediated release of DNA using “mixed multilayers”’, Bioeng. Transl. Med., 2016, 1, (2), pp. 181 –192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wohl B.M. Engbersen J.F.J.: ‘Responsive layer‐by‐layer materials for drug delivery’, J. Control. Release, 2012, 158, (1), pp. 2 –14 [DOI] [PubMed] [Google Scholar]
- 23. Hayward S.L. Francis D.M. Sis M.J. et al.: ‘Ionic driven embedment of hyaluronic acid coated liposomes in polyelectrolyte multilayer films for local therapeutic delivery’, Sci. Rep., 2015, 5, p. 14683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hong J. Alvarez L.M. Shah N.J. et al.: ‘Multilayer thin‐film coatings capable of extended programmable drug release: application to human mesenchymal stem cell differentiation’, Drug Deliv. Transl. Res., 2012, 2, (5), pp. 375 –383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vergaro V. Scarlino F. Bellomo C. et al.: ‘Drug‐loaded polyelectrolyte microcapsules for sustained targeting of cancer cells’, Adv. Drug Deliv. Rev., 2011, 63, (9), pp. 847 –864 [DOI] [PubMed] [Google Scholar]
- 26. Liu X.Q. Picart C.: ‘Layer‐by‐Layer assemblies for cancer treatment and diagnosis’, Adv. Mater., 2016, 28, (6), pp. 1295 –1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao X. Liu P.: ‘PH‐sensitive fluorescent hepatocyte‐targeting multilayer polyelectrolyte hollow microspheres as a smart drug delivery system’, Mol. Pharm., 2014, 11, (5), pp. 1599 –1610 [DOI] [PubMed] [Google Scholar]
- 28. Wood K.C. Chuang H.F. Batten R.D. et al.: ‘Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer‐by‐layer thin films’, Proc. Natl. Acad. Sci. USA, 2006, 103, (27), pp. 10207 –10212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Min J. Choi K.Y. Dreaden E.C. et al.: ‘Designer dual therapy nanolayered implant coatings eradicate biofilms and accelerate bone tissue repair’, ACS Nano, 2016, 10, (4), pp. 4441 –4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gnanadhas D.P. Ben Thomas M. Elango M. et al.: ‘Chitosan‐dextran sulphate nanocapsule drug delivery system as an effective therapeutic against intraphagosomal pathogen Salmonella’, J. Antimicrob. Chemother., 2013, 68, (11), pp. 2576 –2586 [DOI] [PubMed] [Google Scholar]
- 31. Hernandez‐Montelongo J. Lucchesi E.G. Gonzalez I. et al.: ‘Hyaluronan/chitosan nanofilms assembled layer‐by‐layer and their antibacterial effect: a study using Staphylococcus aureus and Pseudomonas aeruginosa ’, Colloids Surf. B Biointerfaces, 2016, 141, pp. 499 –506 [DOI] [PubMed] [Google Scholar]
- 32. Jeong H. Heo J. Son B. et al.: ‘Intrinsic hydrophobic Cairnlike multilayer films for antibacterial effect with enhanced durability’, ACS Appl. Mater. Interfaces, 2015, 7, (47), pp. 26117 –26123 [DOI] [PubMed] [Google Scholar]
- 33. Li W. Xu D. Hu Y. et al.: ‘Surface modification of titanium substrates with silver nanoparticles embedded sulfhydrylated chitosan/gelatin polyelectrolyte multilayer films for antibacterial application’, J. Mater. Sci. Mater. Med., 2014, 25, (6), pp. 1435 –1448 [DOI] [PubMed] [Google Scholar]
- 34. Lee H.‐S. Dastgheyb S.S. Hickok N.J. et al.: ‘Targeted release of Tobramycin from a pH‐responsive grafted bilayer challenged with S. aureus’, Biomacromolecules, 2015, 16, (2), pp. 650 –659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo S. Zhu X. Loh X.J.: ‘Controlling cell adhesion using layer‐by‐layer approaches for biomedical applications’, Mater. Sci. Eng. C, 2017, 70, pp. 1163 –1175 [DOI] [PubMed] [Google Scholar]
- 36. Arias C.J. Surmaitis R.L. Schlenoff J.B.: ‘Cell adhesion and proliferation on the ‘Living’ surface of a polyelectrolyte multilayer’, Langmuir, 2016, 32, (21), pp. 5412 –5421 [DOI] [PubMed] [Google Scholar]
- 37. Jaklenec A. Anselmo A.C. Hong J. et al.: ‘High throughput layer‐by‐layer films for extracting film forming parameters and modulating film interactions with cells’, ACS Appl. Mater. Interfaces, 2016, 8, (3), pp. 2255 –2261 [DOI] [PubMed] [Google Scholar]
- 38. Seon L. Lavalle P. Schaaf P. et al.: ‘Polyelectrolyte multilayers: a versatile tool for preparing antimicrobial coatings’, Langmuir, 2015, 31, (47), pp. 12856 –12872 [DOI] [PubMed] [Google Scholar]
- 39. Schmidt S. Madaboosi N. Uhlig K. et al.: ‘Control of cell adhesion by mechanical reinforcement of soft polyelectrolyte films with nanoparticles’, Langmuir, 2012, 28, (18), pp. 7249 –7257 [DOI] [PubMed] [Google Scholar]
- 40. Pavlukhina S. Lu Y. Patimetha A. et al.: ‘Polymer multilayers with pH‐triggered release of antibacterial agents’, Biomacromolecules, 2010, 11, (12), pp. 3448 –3456 [DOI] [PubMed] [Google Scholar]
- 41. Lu Y. Wu Y. Liang J. et al.: ‘Self‐defensive antibacterial layer‐by‐layer hydrogel coatings with pH‐triggered hydrophobicity’, Biomaterials, 2015, 45, pp. 64 –71 [DOI] [PubMed] [Google Scholar]
- 42. Wong S.Y. Moskowitz J.S. Veselinovic J. et al.: ‘Dual functional polyelectrolyte multilayer coatings for implants: permanent microbicidal base with controlled release of therapeutic agents’, J. Am. Chem. Soc., 2010, 132, (50), pp. 17840 –17848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goodman S.B. Yao Z. Keeney M. et al.: ‘The future of biologic coatings for orthopaedic implants’, Biomaterials, 2013, 34, (13), pp. 3174 –3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lichter J.A. Van Vlietpa K.J. Rubner M.F.: ‘Design of antibacterial surfaces and interfaces: polyelectrolyte multilayers as a multifunctional platform’, Macromolecules, 2009, 42, (22), pp. 8573 –8586 [Google Scholar]
- 45. Zhu X. Jun Loh X.: ‘Layer‐by‐layer assemblies for antibacterial applications’, Biomater. Sci., 2015, 3, (12), pp. 1505 –1518 [DOI] [PubMed] [Google Scholar]
- 46. Briones A.V. Sato T. Bigol U.G.: ‘Antibacterial activity of polyethylenimine/carrageenan multilayer against pathogenic bacteria’, Adv. Chem. Eng. Sci., 2014, 4, pp. 233 –241 [Google Scholar]
- 47. Raphel J. Holodniy M. Goodman S.B. et al.: ‘Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants’, Biomaterials, 2016, 84, pp. 301 –314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Detzel C.J. Larkin A.L. Rajagopalan P.: ‘Polyelectrolyte multilayers in tissue engineering’, Tissue Eng. B Rev., 2011, 17, (2), pp. 101 –113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gribova V. Auzely‐Velty R. Picart C.: ‘Polyelectrolyte multilayer assemblies on materials surfaces: from cell adhesion to tissue engineering’, Chem. Mater., 2012, 24, (5), pp. 854 –869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gribova V. Liu C. Nishiguchi A. et al.: ‘Biochemical and biophysical research communications construction and myogenic differentiation of 3D myoblast tissues fabricated by fibronectin‐gelatine nano film coating’, 2016, 474, pp. 515 –521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guillot R. Gilde F. Becquart P. et al.: ‘The stability of BMP loaded polyelectrolyte multilayer coatings on titanium’, Biomaterials, 2013, 34, (23), pp. 5737 –5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dalonneau F. Qiu X. Sadir R. et al.: ‘Europe PMC funders group the effect of delivering the chemokine SDF‐1α in a matrix‐bound manner on myogenesis’, 2014, 35, (15), pp. 4525 –4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Almodóvar J. Guillot R. Monge C. et al.: ‘Biomaterials spatial patterning of BMP‐2 and BMP‐7 on biopolymeric films and the guidance of muscle cell fate’, 2014, 35, (13), pp. 3975 –3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lo C.M. Wang H.B. Dembo M. et al.: ‘Cell movement is guided by the rigidity of the substrate’, Biophys. J., 2000, 79, (1), pp. 144 –152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Richert L. Lavalle P. Vautier D. et al.: ‘Cell interactions with polyelectrolyte multilayer films’, Biomacromolecules, 2002, 3, (6), pp. 1170 –1178 [DOI] [PubMed] [Google Scholar]
- 56. Paulraj T. Feoktistova N. Velk N. et al.: ‘Microporous polymeric 3D scaffolds templated by the layer‐by‐layer self‐assembly’, Macromol. Rapid Commun., 2014, 35, (16), pp. 1408 –1413 [DOI] [PubMed] [Google Scholar]
- 57. Macdonald M.L. Samuel R.E. Shah N.J. et al.: ‘Tissue integration of growth factor‐eluting layer‐by‐layer polyelectrolyte multilayer coated implants’, Biomaterials, 2011, 32, (5), pp. 1446 –1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shah N.J. Hyder M.N. Quadir M.A. et al.: ‘Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction’, Proc. Natl. Acad. Sci., 2014, 111, (35), pp. 12847 –12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Skorb E. V. Andreeva D. V.: ‘Layer‐by‐layer approaches for formation of smart self‐healing materials’, Polym. Chem., 2013, 4, (18), p. 4834 [Google Scholar]
- 60. Li Y. Li L. Sun J.: ‘Bioinspired self‐healing superhydrophobic coatings’, Angew. Chem. Int. Ed., 2010, 49, (35), pp. 6129 –6133 [DOI] [PubMed] [Google Scholar]
- 61. Meng S. Liu Z. Shen L. et al.: ‘The effect of a layer‐by‐layer chitosan‐heparin coating on the endothelialization and coagulation properties of a coronary stent system’, Biomaterials, 2009, 30, (12), pp. 2276 –2283 [DOI] [PubMed] [Google Scholar]
- 62. Reisa T.C. Castleberry s. Rego A. et al.: ‘Three‐dimensional multilayered fibrous constructs for wound healing applications’, Biomater. Sci., 2016, 26, pp. 319 –330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pahal S. Raichur A.M. Varma M.M.: ‘Subdiffraction‐resolution optical measurements of molecular transport in thin polymer films’, Langmuir, 2016, 32, (22), pp. 5460 –5467 [DOI] [PubMed] [Google Scholar]
- 64. Yang H. Peterson A.M.: ‘Inkjet printed drug‐releasing polyelectrolyte multilayers for wound dressings’, AIMS Mater. Sci., 2017, 4, (2), pp. 452 –469 [Google Scholar]
- 65. Richardson J.J. Cui J. Björnmalm M. et al.: ‘Innovation in layer‐by‐layer assembly’, Chem. Rev., 2016, 116, (23), pp. 14828 –14867 [DOI] [PubMed] [Google Scholar]
- 66. Prokopović V.Z. Vikulina A.S. Sustr D. et al.: ‘Biodegradation‐resistant multilayers coated with gold nanoparticles. Toward a tailor‐made artificial extracellular matrix’, ACS Appl. Mater. Interfaces, 2016, 8, (37), pp. 24345 –24349 [DOI] [PubMed] [Google Scholar]


