Abstract
In the present work cassava starch/agar Ag and ZnO nanocomposite films were prepared by the solution casting method. The structural, physical and antimicrobial properties of the nanocomposite films were studied as a function of the concentration of Ag and ZnO nanoparticles. The results of the thermogravimetric analysis showed 8–15% degradation of both the nanocomposite films at 150°C endorsing the thermal stability of the films. Scanning electron microscopic analysis reveals the uniform blending of Ag and ZnO nanoparticles with a starch/agar matrix with tiny waves like appearance on the surface. The incorporation of Ag and ZnO nanoparticles in the film was found to reduce the moisture content, water solubility and water vapour permeability with increase in the concentration of Ag and ZnO nanoparticles. The growth kinetics study of Pseudomonas aeruginosa and Staphylococcus aureus in the presence of Ag and ZnO blended nanocomposite films showed promising results especially against Gram‐negative P. aeruginosa. Thus, the film synthesised in the present study bears the potential to be used as active packaging material to prevent food from bacterial contamination and spoilage.
Inspec keywords: casting, microorganisms, scanning electron microscopy, nanoparticles, food preservation, solubility, thermal analysis, zinc compounds, food processing industry, food products, thermal stability, permeability, antibacterial activity, food packaging, contamination
Other keywords: water vapour permeability, food packaging, solution casting method, structural properties, physical properties, antimicrobial properties, water solubility, agar nanocomposite film, starch nanocomposite film, Pseudomonas aeruginosa, Staphylococcus aureus, bacterial contamination prevention, spoilage prevention, scanning electron microscopic analysis, thermogravimetric analysis, temperature 150.0 degC, Ag, ZnO
1 Introduction
The recent conundrums created by the usage of petroleum‐based packaging materials have triggered the development of eco‐friendly techniques for combating environmental issues [1, 2]. The synthetic packaging materials are employed in various industries due to their cheap costs and excellent mechanical properties, but they tend to become non‐biodegradable wastes after usage [3]. With the recent advances in research, multifarious varieties of bio‐polymer‐based packaging materials have been developed and employed in the packaging industry. Few among these naturally derived bio‐polymer packaging materials are polysaccharides, proteins, lipids which have shown good biodegradable properties [2].
Polysaccharides such as starch, agar [4], cellulose and cellulose derivatives [5], chitin and chitosan [6], which have been used as packaging materials, have shown excellent properties like biodegradability, biocompatibility and good barrier properties against low to intermediate level relative humidity. However, due to its poor mechanical and antimicrobial activity, it is required to incorporate these films with additional antimicrobials, antifungals, colours and other such bioactive components to reinforce its effects.
Agar has been used as a chief compound in the making of such food packaging films due to its meteoric film‐forming properties, gelling properties, as well as high compatibility with other bio‐polymers. It is produced by the extraction process from seaweed families Gelidium and Gracilaria . Banana [7], grape fruit seed extract [4], soy protein [8], chitosan [9] and metallic nanoparticles [10] are some of the versatile materials which have been used along‐side with agar to improve mechanical strength, antimicrobial capability, water resistance of the films.
Cassava, which is cultivated globally in over 90 countries, has been used as the major source of starch; its production has already increased manifolds over the course of time (doubled over the past three decades). It is already used as a source of starch for animal feed, human consumption and in the pharmaceuticals as well as the food sector. Hence, the use of powdered cassava or tapioca (Manihot esculenta) starch as a film constituent is elucidated in this paper due to its reliability for incessant supply. The beneficial roots are cultivated in tropical countries on large scale and have been viewed as a means of food security and food availability [11]. The crude protein content in cassava foliage ranges from 19 to 23% of dry weight and the starch content is ∼80% [12]. On the other hand, starches have some setbacks, such as poor melting processability, high water solubility (WS), the difficulty of processing and brittleness, causing the need for a plasticiser [13]. Glycerol, which is used along with cassava films, as a plasticiser strengthens the mechanical properties and affects the moisture adsorption capacity of the films [14].
Silver has been known to exhibit strong toxicity to a wide range of micro‐organisms [15]. Due to its strong antimicrobial effects, it has been employed as a preservative as well as an ingredient in bactericidal creams [16]. Silver nanoparticles tend to dissociate into silver ions which have the ability to create porous cell walls in the microbes resulting in their death [17]. Experimental evidence of the anti‐bacterial effects of silver ions has been proposed due to its interaction with thiol compounds present in enzymes and proteins of bacterial cells [18]. On the other hand, reactive oxygen species are formed from the inhibition of respiratory enzymes which attack their own cells. Silver is a typical soft acid which has a tendency to react with a soft base, likely phosphorus and sulphur, which is abundantly present in the nuclear material casing damage in the replication process [19].
Extensive research on signal transduction on bacteria has shown that silver affects the machinery negatively. The rationale behind selecting the above nanoparticles is that some of these are FDA approved and is non‐toxic [20]. Escherichia coli, Staphylococcus aureus and Pseudomonas putida have been widely reported [21, 22] to be sensitive to these nanoparticles.
ZnO nanoparticles have been reported to be stable at high temperatures, thus making it a suitable candidate to be used in packaging warm food materials. It further plays an important role as a micronutrient and is known to have higher antibacterial activity on a broader spectrum. ZnO nanoparticles induce the rupture of cell membranes preventing any growth on food material surfaces such as pork meat etc. [23].
In this study the films formed with cassava starch and agar blends along with the necessary plasticiser, i.e. glycerol (non‐toxic) were found to have promising features that suggest its possible use in food packaging processes replacing non‐biodegradable packaging materials. Added cassava starch provides mechanical strength, tensile properties and improved water barrier properties to the otherwise agar film [24]. Furthermore, the films have been incorporated with nanoparticles namely Ag and ZnO to provide the added benefit of antimicrobial activities. Characterisations of these films by scanning electron microscopy (SEM), X‐ray diffraction (XRD), thermogravimetric analysis (TGA) etc. have been reported apart from its antimicrobial property.
2 Experimental section
2.1 Materials
Freshly harvested cassava (M. esculenta) tubers obtained from a local farm in Kerala were used to prepare the starch. Silver nitrate (AgNO3), sodium borohydride (NaBH4), polyvinylpyrrolidone (PVP), zinc acetate dihydrate (ZnCH3 COOH·2H2 O) and all other chemicals were obtained from Sigma Aldrich. All the reagents were of analytical grade and used without further purification.
2.2 Preparation of cassava starch
Cassava starch powder was prepared according to the method of Rungsinee and Natcharee (2007) [25]. Fresh tuberous roots of cassava were taken and washed thoroughly to remove impurities. The roots were then peeled and sliced into small pieces. A 0.01% of sodium chloride solution was taken in a 500 ml beaker and the sliced root pieces were soaked in it for a period of 30 min. The solution was then drained completely and the cassava slices were removed carefully. The slices were then spread evenly on the plate of a dry air oven and dried at 70°C for 12 h. Cassava powder was finally obtained by grinding the dried cassava slices using an electric mixer and then passed through a 100‐mesh sieve to get fine particles.
2.3 Synthesis of Ag and ZnO nanoparticles
Chemical reduction process as reported by Suh and DiLella (1983) [27] was employed for the synthesis of silver nanoparticles which involves the reduction of a silver salt such as silver nitrate with a reducing agent like sodium borohydride in the presence of colloidal stabiliser [26, 27].
Briefly, a magnetic stir bar was introduced to 30 ml of 0.002 M sodium borohydride (NaBH4) and placed in an ice bath on a stir plate. After allowing the liquid to stir and cool for about 20 min, 2 ml of 0.001 M silver nitrate (AgNO3) was dripped into the stirring NaBH4 solution. Stirring is stopped when all of the AgNO3 is added. By mixing both solutions (i.e. NaBH4 and AgNO3), Ag ions were reduced and clustered to form monodispersed nanoparticles as a transparent sol in the aqueous medium. The addition of a few drops of 1.5 M sodium chloride (NaCl) solution to the above causes the suspension to turn dark yellow, then grey as the nanoparticles aggregate. The addition of a drop of 0.3% polyvinylpyrrolidone (PVP) prevents aggregation. Finally, the suspension was evaporated in a toaster oven for about 30 min [28].
Zinc oxide (ZnO) nanoparticles were synthesised using sodium hydroxide (NaOH) and zinc acetate dihydrate solution which was prepared using deionised water separately to form the liquid media of the desired concentrations. Zinc acetate dihydrate was then added dropwise to NaOH solutions under continuous stirring at room temperature of 25°C, resulting in the formation of a transparent white solution. The solution was then allowed to react at 90°C for 2 h and yield zinc oxide precipitate. The precipitate thus obtained was centrifuged at 3000 rpm for 30 min and the pellet containing ZnO nanoparticle was obtained after the removal of the supernatant. Finally, ZnO nanoparticle was dried and ground to fine powder [29].
2.4 Preparation of cassava starch/agar nanocomposite films
Cassava starch/agar binary blend films were prepared with a blending ratio of 2:2. The film‐forming solution was prepared in 150 ml of distilled water and heated at 90°C for 20 min with constant stirring. Glycerol (1.2 g) was used as a plasticiser in this method.
For the preparation of cassava starch/agar nanocomposite films, different concentrations (0.5, 1, 1.5 and 2 mM) of Ag nanoparticles and ZnO nanoparticles were prepared and added to the film casting solution. After heating at 90°C for 20 min with glycerol as a plasticiser, the film casting solution was transferred to Teflon plates. These plates were kept at 40°C in a hot air oven for 48 h to dry them completely. The dried films were peeled off from the plates and preconditioned in a constant temperature humidity chamber for 48 h at 25°C and relative humidity of 50% to normalise the moisture content (MC) prior to further analysis [30]. The films were designated as CS/A film (starch/agar binary blend film), CS/A AgNC film (cassava starch/agar silver nanocomposite film) and CS/A ZnONC film (cassava starch/agar zinc oxide nanocomposite film).
2.5 Characterisation of the starch/agar nanocomposite films
2.5.1 X‐ray diffractometer analysis
XRD pattern of the films was analysed by X‐ray diffractometer (Bruker D8 advance, Germany). Samples were prepared by placing rectangular shapes of each film (3.5 × 2.5 cm) on a glass slide and the spectra were recorded using Cu Kα, Ni filtered radiation (wavelength of 0.1541 nm).
2.5.2 MC and water solubility
MC of the food packaging films was determined by the drying oven method. Each film sample was cut into a square of 3 cm × 3 cm. The initial weight (W 0) of the sample was determined and dried at 105°C for 24 h using a hot air oven. The final weight (W l) of the film samples was measured and per cent MC of the films was calculated as follows:
The WS of the film samples was determined as the percentage of dissolved dry matter after immersion in water. Three randomly selected specimens of each type of film (3 cm × 3 cm) were first dried at 60°C for 24 h to determine the initial dry matter (W 1). Each film was immersed into 30 ml of distilled water in a 50 ml beaker with gentle stirring for 24 h. The film samples were removed after 24 h and dried in a drying oven at 105°C for 24 h to determine the undissolved final dry weight (W 2) [31].
The WS of the sample was calculated as follows:
2.5.3 Water vapour permeability (WVP)
The WVP of the synthesised nanocomposite films was gravimetrically determined following the standard method and corrected for the stagnant air gap inside the test cups [32]. Initially, the measurement cells were filled with water to build in 100% relative humidity and the air gap between the water surface and the mouth of the cell is 1.5 cm. The mouth of the cells was enclosed with the films and sealed with double‐sided adhesive tape. Cells were kept in the oven with 50% RH. The cells were weighted at regular intervals and linear regression analysis of weight loss versus time was carried out. Water vapour transmission rate (WVTR) was calculated from the constant rate of weight loss divided by film area and thus the WVP was calculated using the following equation:
where WVTR is the water vapour transmission rate and ΔP is the pressure difference across the film. The difference in vapour pressure ΔP is obtained from the equation ΔP = S (R 1 − R 1), where S denotes the saturated vapour pressure, and R 1 and R 2 are the relative humidity on the wet and dry side of the cell.
2.5.4 Thermal analysis
The thermal stability of the films was determined by using a thermogravimetric analyser (SDT Q600 V 20.9 BUILD 20). Each film sample (about 5 mg) was taken in a standard aluminium cup and scanned at a heating rate of 10°C/min with temperature ranged from 30 to 600°C under a nitrogen flow of 50 cm3 /min. The empty cup was taken as a reference. The derivative of TGA (DTG) was obtained by differentials of TGA values and calculated using a central finite difference method as follows:
where Wt +Δt − Wt −Δt is the residual weight of the sample at time t + Δt and t − Δt, respectively, and t is the time interval for reading residual sample weight. The maximum decomposition temperature (T max) of films was calculated from the DTG curve and the char content and the weight loss (%) was measured by TGA curve [33, 34].
2.5.5 SEM analysis
The surface morphology of film samples was visualised using a scanning electron microscope (Hitachi S‐3400N Model) at an accelerating voltage of 20 kV.
2.6 Antimicrobial activity
Growth kinetics of Pseudomonas aeruginosa and S. aureus was studied for neat agar and starch/agar nanocomposite films. P. aeruginosa culture was aseptically inoculated in the Lysogeny broth (LB) broth and subsequently incubated at 37°C for 16 h. Inoculum of 100 µl was aseptically transferred to 50 ml LB broth containing film samples (2.5 × 2.5 cm) and incubated at 37°C for 0–16 h under continuous shaking. The inhibitory effect was estimated periodically by measuring the turbidity of the cultured medium at 600 nm using a spectrophotometer.
3 Results and discussion
3.1 Agar /cassava nanocomposite films
The synthesised starch/agar AgNC films and starch/agar ZnONC films were prepared by solution casting method and uniform independent films were obtained for different concentrations of Ag and ZnO nanoparticles. Fig. 1 shows the colour of starch/agar AgNC films which ranges from pale brown to dark brown depending on the concentration of silver nanoparticles as shown [35]. Similarly, the transparency of starch/agar ZnONC film was found to decrease with a higher concentration of nanoparticle owing to the higher scattering of nanoparticles (Fig. 2) [36].
Fig. 1.
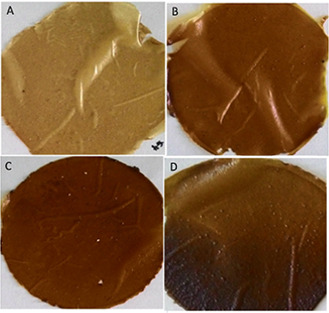
Starch/agar AgNC films with different concentration of Ag nanoparticles
(a) 0.5 mM, (b) 1 mM, (c) 1.5 mM, (d) 2 mM
Fig. 2.
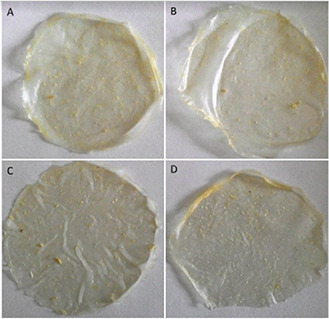
Starch/agar ZnONC filmswith different concentration of ZnO nanoparticles
(a) 0.5 mM, (b) 1 mM, (c) 1.5 mM, (d) 2 mM
3.2 Characterisation of starch/agar nanocomposite films
The crystalline structure of silver and zinc oxide nanoparticle reinforced films was established by analysing the XRD pattern (Fig. 3). The results of XRD analysis of starch/agar AgNC and starch/agar ZnONC films along with control films are provided in Fig. 3. The high‐intensity peaks at 2θ of 19.2°, 30.22°, 41.09° for control film (CS/A) and 18.76°, 29.08° and 42.86° in case of CS/A AgNC film are common for agar‐based films.
Fig. 3.
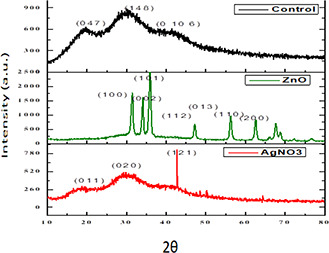
XRD analysis of starch/agar AgNC and starch/agar ZnONC films
Additionally, a sharp intense diffraction peak was noticed at a 2θ value of 42.86° and no such intense diffraction peak was seen in the case of control film. Similarly, for starch/agar ZnONC films, the sharp intense diffraction peaks appearing at about 2θ of 34.47°, 36.53°, 47.79°, 57.17°, 63.1° and 67.07° can be attributed to (100), (002), (101), (013), (110), and (200) orientations, respectively, which confirm the wurtzite crystalline phase of ZnO nanoparticles [36].
Table 1 shows the MC of starch/agar (control films) along with starch/agar nanocomposite films (test films). The analysis of MC helps to determine the hydrophobicity of the films, in other words, higher the MC, the higher is the ability of the films to retain water. It is an important property which is in relation to the total water molecules occupied in the complex network of microstructures with nanoparticle embedded films [37, 38].
Table 1.
MC analysis
| Concentration of Ag and ZnO nanoparticles, mM | Control film CS/A | CS/A‐AgNC film | CS/A‐ZnONC film |
|---|---|---|---|
| 0.5 | 34.44 | 18.66 | 25.26 |
| 1.0 | 34.44 | 14.28 | 18.75 |
| 1.5 | 34.44 | 12.75 | 15.38 |
| 2 | 34.44 | 11.64 | 12.19 |
One of the most fundamental properties of packaging films is to maintain optimum moisture levels within the packaged product, because too low or too high levels will damage the product. The MC and total soluble matter of films decide the suitability of bio‐based materials for food packaging. The results shown in Table 1 demonstrate higher water resistance and hydrophobicity of the starch/agar AgNC film and starch/agar ZnONC films compared to the starch/agar control films. This is due to the presence of hydrophobic components embedded in the films, which are the metal nanoparticles. To elaborate upon the results, we can clearly infer that the CS/A AgNC films are more hydrophobic than CS/A ZnONC for all concentrations.
WS provides the necessary information on the film's water affinity [38]. According to the previous table, we can fixate upon the fact that the addition of nanoparticles in the starch/agar tends to establish a more hydrophobic mixture. The results show that there is a significant decrease in the percentage of WS when compared to the control film (CS/A) and the nanoparticle incorporated films (CS/A AgNC and CS/A ZnONC). These films can be used as hydrophobic agents due to the presence of their hydrophobic components such as silver nanoparticles and zinc oxide nanoparticles. Moreover, the silver nanoparticle‐based films have shown the highest level of water resistance when compared to zinc oxide nanoparticle‐based films. On the other hand, there is a significant decrease in hydrophilicity with increasing concentration. The application of WS comes into play when the films are in contact with high moisture food products and food products with high water activity and during storage (Table 2).
Table 2.
WS analysis
| Concentration of Ag and ZnO nanoparticles, mM | Control film CS/A | CS/A‐AgNC film | CS/A‐ZnONC film |
|---|---|---|---|
| 0.5 | 45.25 | 26.96 | 41.66 |
| 1.0 | 45.25 | 23.70 | 35.71 |
| 1.5 | 45.25 | 21.42 | 28.57 |
| 2 | 45.25 | 20.21 | 28.09 |
The WVP of starch/agar control film was (2.02 ± 0.22) × 10−10 g/m s Pa quite higher than that of CS/A‐AgNC films and CS/A ZnONC films. This substantial increase may be attributed to the incorporated Ag and ZnO nanoparticles. Also, the WVP of the Ag and ZnO nanocomposite test films decreased with an increase in the concentration of both the nanoparticles. The presence of hydrophobic components such as silver nanoparticles and zinc oxide nanoparticles in the films have individually decreased the hydrophilicity of blend mixture due to a decrease in the number of hydrophilic groups (–OH) and low amorphous content in the blends [39]. According to the previous research, the lower WVP of nanocomposite films has been related to the tortuous path structure for water vapour diffusion produced by impervious silver and zinc oxide nanoparticles in the film matrix [1] (Table 3).
Table 3.
WVP of the films
| Film composition | WVP (×10−10 g/m s Pa) |
|---|---|
| CS/A (control film) | 2.02 ± 0.22 |
| CS/A‐AgNC film (0.5 mM) | 1.73 ± 0.15 |
| CS/A‐AgNC film (1.0 mM) | 1.53 ± 0.23 |
| CS/A‐ZnONC film (0.5 mM) | 1.88 ± 0.32 |
| CS/A/ZnONC film (1 mM) | 1.79 ± 0.20 |
TGA was done to find the thermal stability in terms of chemical and physical properties. The thermal stability of both the nanocomposite films was almost similar (Figs. 4 a and b).
Fig. 4.
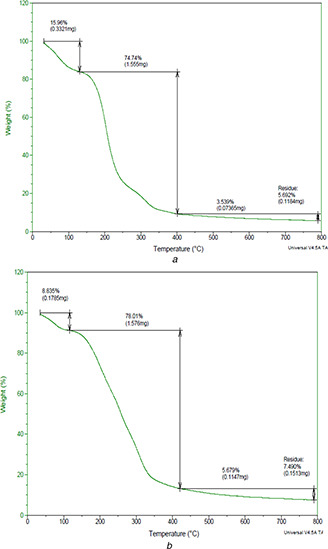
TGA analysis of
(a) CS/A‐AgNC film, (b) CS/A‐ZnONC film
In both cases the thermal decomposition starts at 31.88°C. For CS/A‐AgNC film, 15.96% of degradation was observed till 150°C and rapid degradation of 74.74% was seen till 400°C (Fig. 4 a). Similarly CS/A‐ZnONC film showed 8.835% weight loss till 143°C and a rapid loss of weight (78%) occurred from 150 to 400°C (Fig. 5 b). The evaporation of the retained solvent in the film resulted in the initial thermal decomposition [40]. The later decomposition is attributed by the decomposition of agar which is the base film matrix that would have been completely destroyed at 400°C [9]. Additionally, the TGA curves of both nanocomposite films display small shoulders at 150°C, which is perhaps due to the thermal destruction of the plasticiser glycerol used in casting the film.
Fig. 5.
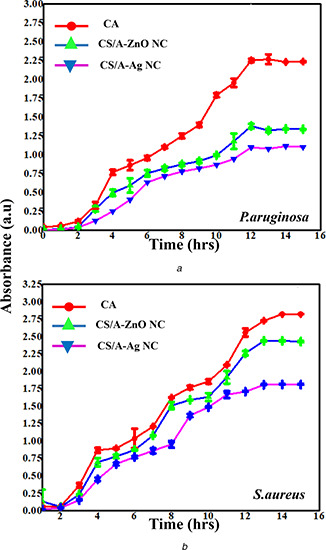
Antimicrobial activity of CS/A, CS/A‐AgNC and CS/A‐ZnONC films against
(a) P. aeruginosa, (b) S. aureus
The SEM micrographs have been shown in Fig. 6. SEM micrographs show uniform blending of Ag nanoparticles with starch/agar matrix with tiny wave‐like appearance on the surface indicating that a homogeneous blending has achieved. The width of the Ag nanocomposite film was found to be 20.5 mm as viewed under 1.01k magnifications (Fig. 6 a). The ZnO film was found to be slightly wrinkled and uniformly blended onto cassava agar film as viewed under SEM. The width of the film was13.0 mm as viewed at a magnification of 3.0k and 20.0 kV (Fig. 6 b). The strong adherence of the nanoparticles to the agar matrix could be observed and this is probably responsible for the modification of the physical and chemical characteristics of the films.
Fig. 6.

SEM micrographs of
(a) CS/A‐AgNC film, (b) CS/A‐ZnONC film
3.3 Antimicrobial activity
The growth kinetics of P. aeruginosa and S. aureus was studied for a period of 16 h in the presence of nanocomposite films (CS/A‐AGNC and CS/A‐ZnONC film) and control films (Figs. 5 a and 5 b). Both CS/A‐AgNC and CS/A‐ZnONC films highly retarded the growth of Gram‐negative bacteria P. aeruginosa compared to the Gram‐positive bacteria S. aureus. It is deceptive that the control films did not significantly inhibit the growth of pathogenic bacteria whereas the composite films with Ag and ZnO nanoparticles demonstrated strong antibacterial activity against Gram‐negative bacteria P. aeruginosa. However, the degree of antimicrobial activity of the films varied with the nanoparticle. The nanocomposite film with Ag nanoparticle exhibited slightly higher activity against both the test organisms. Furthermore, both P. aeruginosa and S. aureus were highly inhibited in the presence of nanocomposite films with increasing contact time. Generally, the antibacterial activity of silver is influenced by the disposal of ionic silver for bacterial contact [41]. The mechanism of action of silver nanoparticles has been proposed by several researchers and generally stated that positively charged silver ions can interact with negatively charged biomacromolecular components, causing structural changes and distortion of bacterial cell walls and membranes leading to disruption of metabolic processes followed by cell death [42, 43].
4 Conclusion
According to the results of the experiments that we conducted, starch/agar nanocomposite films Ag and ZnO have a considerable antimicrobial activity. With Ag nanoparticle incorporated films having the most antimicrobial activity followed by ZnO nanoparticle. The hydrophobicity and other physical attributes also suggest the suitability of these films for food packaging applications.
. The biodegradability of the biopolymer blend nanocomposite films and antibacterial activity of the nanocomposite packages shows promising use of these nancomposite films in the near future, in food packaging to increase the shelf life of edible food items.
5 References
- 1. Rhim J., Ng P.K.: ‘Natural biopolymer‐based nanocomposite films for packaging applications’, Crit. Rev. Food Sci. Nutr., 2007, 47, pp. 411–433 [DOI] [PubMed] [Google Scholar]
- 2. Tang X.Z., Kumar P., Alavi S. et al.: ‘Recent advances in biopolymers and biopolymer‐based nanocomposites for food packaging materials’, Crit. Rev. Food Sci. Nutr., 2012, 52, pp. 426–442 [DOI] [PubMed] [Google Scholar]
- 3. Tharanathan R.N.: ‘Biodegradable films and composite coatings: past, present and future, trends’, Food Sci. Technol., 2003, 14, pp. 71–78 [Google Scholar]
- 4. Kanmani P., Rhim J.: ‘Antimicrobial and physical‐mechanical properties of agar‐based films incorporated with grapefruit seed extract’, Carbohydr. Polym., 2014, 102, pp. 708–716 [DOI] [PubMed] [Google Scholar]
- 5. Mokhothu T.H., John M.J.: ‘Review on hygroscopic aging of cellulose fibres and their biocomposites’, Carbohydr. Polym., 2015, 131, pp. 337–354 [DOI] [PubMed] [Google Scholar]
- 6. Dutta J., Tripathi S., Dutta P.K.: ‘Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: a systematic study needs for food applications’, Food Sci Technol. Int., 2012, 18, pp. 3–34 [DOI] [PubMed] [Google Scholar]
- 7. Orsuwan A., Shankar S., Wang L.F. et al.: ‘Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles’, Food Hydrocoll., 2016, 60, pp. 476–485 [Google Scholar]
- 8. Tian H., Xu G., Yang B. et al.: ‘Microstructure and mechanical properties of soy protein/agar blend films: effect of composition and processing methods’, J. Food Eng.., 2011, 107, pp. 21–26 [Google Scholar]
- 9. El‐Hefian E.A., Nasef M.M., Yahava A.H.: ‘Preparation and characterization of chitosan/agar blended films: part 2. Thermal, mechanical, and surface properties’, Eur. J. Chem., 2012, 9, pp. 510–516 [Google Scholar]
- 10. Arfat Y.A., Ahmed J., Jacob H.: ‘Preparation and characterization of agar‐based nanocomposite films reinforced with bimetallic (Ag‐Cu) alloy nanoparticles’, Carbohydr. Polym., 2017, 155, pp. 382–390 [DOI] [PubMed] [Google Scholar]
- 11. De Bruijn G.H., Fresco L.O.: ‘The importance of cassava in world food production’, Neth J. Agr. Sci., 1989, 37, pp. 21–34 [Google Scholar]
- 12. Mali S., Sakanaka L., Yamashita F. et al.: ‘Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect’, Carbohydr. Polym., 2005, 60, pp. 283–289 [Google Scholar]
- 13. Zhu F.: ‘Composition, structure, physicochemical properties, and modifications of cassava starch’, Carbohydr. Polym., 2015, 122, pp. 456–480 [DOI] [PubMed] [Google Scholar]
- 14. Myllarinen P., Partanen R., Seppala J. et al.: ‘Effect of glycerol on behaviour of amylose and amylopectin films’, Carbohydr. Polym., 2002, 50, pp. 355–361 [Google Scholar]
- 15. Slawson R., Lohmeier‐Vogel E., Lee H. et al.: ‘Silver resistance in Pseudomonas stutzeri’, Biometals., 1994, 7, pp. 30–40 [DOI] [PubMed] [Google Scholar]
- 16. Marslin G., Selvakesavan R.K., Franklin G. et al.: ‘Antimicrobial activity of cream incorporated with silver nanoparticles biosynthesized from Withania somnifera’, Int. J. Nanomedicine, 2015, 10, pp. 5955–5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Danilczuc M., Lund A., Sadlo J. et al.: ‘Conduction electrospin resonance of small silver particles’, Spectrochim. Acta Mol. Biomol. Spectrosc., 2006, 63, pp. 189–191 [DOI] [PubMed] [Google Scholar]
- 18. Jung W.K., Koo H.C., Kim K.W. et al.: ‘Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli’, Appl. Environ. Microbiol., 2008, 74, pp. 2171–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hatchett D.W., White H.S.: ‘Electrochemistry of sulfur adlayers on the low‐index faces of silver’, J. Phys. Chem., 1996, 100, pp. 9854–9859 [Google Scholar]
- 20. Othman S.H., Salam N.R., Zainal N. et al.: ‘Antimicrobial activity of TiO2 nanoparticle‐coated film for potential food packaging applications’, Int. J. Photoenergy, 2014, Article ID: 945930 pp. 1–6, Available at: 10.1155/2014/945930 [DOI] [Google Scholar]
- 21. Bonetta S., Bonetta S., Motta F. et al.: ‘Photocatalytic bacterial inactivation by TiO2‐coated surfaces’, AMB Express, 2013, 3, p. 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao N., Yeung K.L.: ‘Investigation of the performance of TiO2 photocatalytic coatings’, Chem. Eng. J., 2011, 167, pp. 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suo B., Li H., Wang Y. et al.: ‘Effects of ZnO nanoparticle‐coated packaging film on pork meat quality during cold storage’, J. Sci. Food Agr., 2016, 97, pp. 2023–2029 [DOI] [PubMed] [Google Scholar]
- 24. Waliszewski K.N., Aparicio M.A., Bello L.A. et al.: ‘Changes of banana starch by chemical and physical modification’, Carbohydr. Polym., 2003, 52, pp. 237–242 [Google Scholar]
- 25. Rungsinee S., Natcharee P.: ‘Oxygen permeability and mechanical properties of banana films’, Food Res. Int., 2007, 40, pp. 365–370 [Google Scholar]
- 26. Ferdos P.M., Masoumeh K., Parya V.: ‘Synthesis of nano‐Ag particles using sodium borohydride’, Orient. J. Chem., 2015, 31, pp. 1831–1833 [Google Scholar]
- 27. Suh J.S., DiLella D.P., Moskovits M.: ‘Surface‐enhanced Raman spectroscopy of colloidal metal systems: a two‐dimensional phase equilibrium in p‐aminobenzoic acid adsorbed on silver’, J. Phys. Chem., 1983, 87, pp. 1540–1544 [Google Scholar]
- 28. Kandarp M., Mihir S.: ‘Synthesis of silver nanoparticles by using sodium borohydride as a reducing agent’, Int. J. Eng., 2013, 2, pp. 1–5 [Google Scholar]
- 29. Hasnidawani J.N., Azlina H.N., Norita H. et al.: ‘Synthesis of ZnO nanostructures using sol‐gel method’, Procedia Chem., 2016, 19, pp. 211–216 [Google Scholar]
- 30. Souza A.C., Benze R., Ferrao E.S. et al.: ‘Cassava starch biodegradable films: influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature’, Food Sci. Technol‐Leb., 2012, 46, pp. 110–117 [Google Scholar]
- 31. Rhim J.W., Wang L.F.: ‘Mechanical and water barrier properties of agar/κ‐carrageenan/konjac glucomannan ternary blend biohydrogel films’, Carbohydr. Polym., 2013, 96, pp. 71–81 [DOI] [PubMed] [Google Scholar]
- 32. Gennadios A., Weller C.L., Gooding C.H.: ‘Measurement errors‐ in‐water‐vapor‐permeability of highly permeable, hydrophilic edible films’, J. Food Eng., 1994, 21, (4), pp. 395–409 [Google Scholar]
- 33. Othman S.H.: ‘Bio‐nanocomposite materials for food packaging applications: types of biopolymer and nano‐sized filler’, Agric. Agric. Sci. Procedia., 2014, 2, pp. 296–303 [Google Scholar]
- 34. Reddy J.P., Rhim J.W.: ‘Characterization of bionanocomposite films prepared with agar and paper‐mulberry pulp nanocellulose’, Carbohydr. Polym., 2014, 110, pp. 480–488 [DOI] [PubMed] [Google Scholar]
- 35. Rhim J.W., Wang L.F., Hong S.I.: ‘Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity’, Food Hydrocoll., 2013, 33, pp. 327–335 [Google Scholar]
- 36. Santosh Kumar S., Boro J.C., Ray D. et al.: ‘Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape’, Heliyon, 2019, 5, p. e01867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arham R., Mulyati M.T., Metusalach M. et al.: ‘Physical and mechanical properties of agar based edible film with glycerol plasticizer’, Int. Food. Res. J., 2016, 23, pp. 1669–1675 [Google Scholar]
- 38. Cerqueiraa M.A., Bourbona A.I., Pinheiroa A.C. et al.: ‘Galactomannans use in the development of edible films/coatings for food applications’, Trends Food Sci. Technol., 2011, 22, pp. 662–671 [Google Scholar]
- 39. Shankar S., Teng X., Rhim J.W.: ‘Properties and characterization of agar/CuNP bio nanocomposite films prepared with different copper salts and reducing agents’, Carbohydr. Polym., 2014, 114, pp. 484–492 [DOI] [PubMed] [Google Scholar]
- 40. Rhim J.W., Mohanty A.K., Singh S.P. et al.: ‘Effect of the processing methods on the performance of polylactide films: thermocompression versus solvent casting’, J. Appl. Polym. Sci., 2006, 101, pp. 3736–3742 [Google Scholar]
- 41. Busolo M. A., Fernandez P., Ocio M. J. et al.: ‘Novel silver‐based nanoclay as an antimicrobial in polylactic acid food packaging coatings. Food Additives Contaminants, A Chem,, Anal., Control, Exposure Risk Assess., 2010, 27, pp. 1617–1626 [DOI] [PubMed] [Google Scholar]
- 42. Butkus M.A., Edling L., Labare M.P.: ‘The efficacy of silver as a bactericidal agent: advantages, limitations and considerations for future use’, J. Water Supply Res. T., 2003, 52, pp. 407–416 [Google Scholar]
- 43. Feng Q.L., Wu J., Chen G.Q. et al.: ‘A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus’, J. Biomed. Mater. Res., 2000, 52, pp. 662–668 [DOI] [PubMed] [Google Scholar]


