Abstract
Human epidermal growth factor receptor 2 (HER‐2) is overexpressed in 20–30% of human breast cancers, associated with poor prognosis and tumour aggression. The aim of this study was the production of trastuzumab‐targeted Ecoflex nanoparticles (NPs) loaded with docetaxel and in vitro evaluation of their cytotoxicity and cellular uptake. The NPs were manufactured by electrospraying and characterised regarding size, zeta potential, drug loading, and release behaviour. Then their cytotoxicity was evaluated by MTT assay against an HER‐2‐positive cell line, BT‐474, and an HER‐2‐negative cell line, MDA‐MB‐468. The cellular uptake was studied by flow cytometry and fluorescent microscope. The particle size of NPs was in an appropriate range, with relatively high drug entrapment and acceptable release efficiency. The results showed no cytotoxicity for the polymer, but the significant increment of cytotoxicity was observed by treatment with docetaxel‐loaded NPs in both HER‐2‐positive and HER‐2‐negative cell lines, in comparison with the free drug. The trastuzumab‐targeted NPs also significantly enhanced cytotoxicity against BT‐474 cells, compared with non‐targeted NPs.
Inspec keywords: cancer, proteins, biomedical materials, nanofabrication, drug delivery systems, cellular biophysics, biological organs, nanomedicine, toxicology, tumours, nanoparticles, biomedical optical imaging, fluorescence, particle size
Other keywords: human breast cancers, tumour aggression, trastuzumab‐targeted Ecoflex nanoparticles, cellular uptake, zeta potential drug loading, HER‐2‐positive cell line, HER‐2‐negative cell line, MDA‐MB‐468, particle size, trastuzumab‐conjugated nanoparticles, electrospraying technique, human epidermal growth factor receptor, cytotoxicity, nontargeted nanoparticles, butylene adipate‐co‐butylene terephthalate, trastuzumab‐targeted NP, docetaxel‐loaded NP
1 Introduction
In spite of the increasing diagnostic and therapeutic investigations on breast cancer, it is still regarded as the most prevalent type of cancer in women all over the world, playing a major role in cancer‐related morbidity and mortality. Currently, there is no absolute cure when the patient is involved with metastatic breast cancer, and the proposed chemotherapeutic treatments exacerbate the patient's quality of life due to their adverse effects [1, 2, 3, 4].
Docetaxel (Taxotere® Sanofi Aventis) is a lipophilic semisynthetic analogue of paclitaxel. It is considered as one of the most notable cytotoxic agents, with proven clinical efficiency in patients suffering from several types of cancers including ovarian, endometrial, colon, and breast cancer. The mechanism of the cytotoxic effect of docetaxel (DTX) is preventing the formation of new cells, as well as inducing apoptosis in the existing cells. In further details, inhibition of the function of microtubules, which are critical for cell survival [5] and inhibition of the anti‐apoptotic gene of Bcl2 that leads to expression of p2t and inhibition of the cell cycle are among the other mechanisms of the cytotoxic effect of this drug [6, 7, 8].
However, these mechanisms are performed on healthy cells along with tumour cells, causing DTX‐related adverse effects which could negatively affect the health status of the patient. This leads to depriving the patient of the potential benefits of treatment due to obligatory cessation or reduction of the administered dose of DTX. Some of these adverse effects are related to the active pharmaceutical ingredient, including neutropenic fever, anaemia, fluid retention, myalgia, peripheral neuropathy, skin and nail toxicity and epiphora [7, 9]. Moreover, there are some adverse effects caused by the formulation components, Tween 80 and ethanol, which are needed to enhance the aqueous solubility of DTX but may also cause hypersensitivity reactions and decreased accumulation in tumour tissues [8].
Epidermal growth factor receptors, also known as ErbB, constitute a family of receptor tyrosine kinases which play a substantial role in proliferation and differentiation of embryonic and adult cells. The overexpression of these receptors could be associated with various types of cancer. Human epidermal growth factor receptor 2 (HER‐2) is a member of this family which is overexpressed in 20–30% of human breast cancer cases and is associated with poor prognosis and aggression of tumours. Trastuzumab is a monoclonal antibody against HER‐2 and is currently applied in the treatment of HER‐2‐positive breast cancer patients with promising outcomes [10].
Polymeric nanoparticles (NPs), manufactured with biodegradable polymers, have attracted a great deal of interest as drug delivery systems in recent decades. The advantages of using polymeric NPs in cancer drug delivery are the possibility of controlling the release of drug cargo, as well as their ability to target the tumour site either passively via enhanced permeation and retention effect or actively by addition of specific targeting agents on their surface [11].
Considering the various advantages of poly(butylene adipate‐co‐buylene terephthalate), a biodegradable polymer which was introduced BASF Inc. as Ecoflex® [12], the aim of this study was to compare the in vitro efficacy of targeted and non‐targeted DTX‐loaded Ecoflex® NPs with the free drug. Some physicochemical characteristics of this polymer have eliminated the studies regarding fabrication and investigation of Ecoflex® NPs. In this study, NPs of Ecoflex® were constructed using electrospraying technique for the first time in order to provide a biodegradable and biocompatible nanoparticulate system, then they were conjugated to trastuzumab, HER‐2‐specific monoclonal antibody, for targeted delivery of DTX to HER‐2 overexpressing breast cancer cells.
To the best of our knowledge, there are no reports on the production of trastuzumab‐targeted Ecoflex® NPs fabricated by the electrospraying method.
2 Experimental
2.1 Materials
DTX was purchased from Cipla, India, Ecoflex® (Mw = 100,000) from BASF, Germany, Pluronic F‐127 from Sigma, USA, polyethylene glycol (PEG) (Mw = 6000), acetonitrile, dichloromethane, and N, N ‐dimethyl formamide (DMF) from Merck, Germany. 3‐[4, 5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyl tetrazolium bromide (MTT) were supplied by Sigma Company (USA). Roswell Park Memorial Institute (RPMI) medium, fetal bovine serum (FBS), trypsin/EDTA, penicillin/streptomycin were obtained from Biosera Europe, ZI du Bousquet, France. Trastuzumab from Roche, Switzerland, N ‐(3‐ dimethylaminopropyl) ‐N ′‐ ethylcarbodiimide hydrochloride (EDC) from Sigma‐Aldrich, Germany, and N ‐hydroxysuccinimide (NHS) from Merck, Germany.
2.2 Fabrication of Ecoflex® NPs loaded with DTX
The electrospraying technique described by Varshosaz et al. [13] was utilised to prepare the DTX‐loaded Ecoflex® NPs (DTX‐NPs). Briefly, the organic phase was constructed by dissolving DTX, Ecoflex®, and PEG 6000 in a solvent system composed of a 2.7:1 mixture of dichloromethane (DCM): DMF and was placed in a syringe. The aqueous phase, composed of 1% Pluronic‐F127 dissolved in deionised water, was placed as the collector. This organic phase was dispersed in the aqueous phase via electrospraying at the voltage of 19.8 kV, using a syringe pump at the feeding rate of 1 mL/h. The obtained NP suspensions were lyophilised after fabrication. Three different formulations containing 7:1, 4:1 and 3:1 of polymer to drug ratio was used to manufacture the DTX‐NPs and the optimum formulation was selected considering the physicochemical characteristics of the NPs.
2.3 Physical characterisation of the DTX‐NPs
A laser light scattering particle size analyser (Malvern instrument, UK) was used to characterise the DTX‐NPs regarding particle size, particle size distribution and zeta potential. Samples were suspended in deionised water and each measurement was performed in triplicate [14].
2.4 Drug analysis
The DTX concentration in samples was analysed using an high performance liquid chromatography (HPLC) system (Waters, USA) equipped with a UV detector and a C‐18 Column (Waters Spherisorb®, 5 μm ODS2 4.6 × 250 mm). The mobile phase was a 65:35 mixture of acetonitrile (ACN): water. Analyses were performed at 230 nm wavelength and the flow rate was set to 1 mL/min [15]. A standard curve was plotted in the concentration range of 0.25–40 ng/mL. Its linearity was investigated with regard to the inter‐day and intra‐day SDs, and its line equation was used to analyse the peak area of DTX in the chromatograms.
2.5 Calculation of the yield of the procedure and drug entrapment efficiency in NPs
In order to evaluate the efficiency of the procedure, the yield % was calculated; hence, the amount of obtained NPs at the collector site was measured and used in the following equation:
| (1) |
Drug entrapment efficiency was calculated by dispersing the lyophilised DTX‐NPs in an aqueous medium that contained Tween 80. In order to separate the free drug from the NPs, this suspension was placed in the Amicon ultracentrifuge filter with 10,000 Da cut‐off (Millipore, Ireland) and centrifuged (Eppendorf centrifuge 5430, Germany) at 14,000 rpm for 15 min. The concentration of free drug was analysed by HPLC and the following equation was used to calculate the entrapment efficiency:
| (2) |
2.6 In vitro drug‐release behaviour of the NPs
Drug‐release behaviour of the NPs was studied in a phosphate buffered saline medium (pH 7.4 ± 0.2) which contained 0.5% Tween 80. The dialysis bags (cutoff 12,000 Da, Memberacel®, Viskase, USA) filled with an aqueous suspension of NPs were placed in beakers containing release medium. The volume of the release medium was calculated according to the saturation solubility of DTX (9.8 ± 0.3 μg/mL) and the predetermined entrapment efficiency of the NPs, so that the sink condition was provided, <10–15% of the saturation solubility. Drug release was investigated at 37 ± 0.5°C for 30 h and 100 μL samples were taken and replaced with a fresh medium during this time period. Each sample was placed in Amicon Ultra centrifugal filters (cutoff 10,000 Da) and centrifuged at 14,000 rpm for 15 min, proceeded by HPLC analysis regrading DTX concentration.
2.7 Production of trastuzumab‐targeted NPs
The trastuzumab‐targeted DTX‐NPs (trastuzumab‐DTX‐NPs) were obtained by formation of an amide bond between the amine functional group on trastuzumab molecule and carboxylic acid group on Ecoflex® NPs loaded with DTX. For this purpose, the aforementioned −COOH groups were activated by incubation of an aqueous suspension of DTX‐NPs with EDC (400 mM) and NHS (100 mM) with gentle shaking at room temperaturefor 15 min. Afterward, the activated NPs were reacted with trastuzumab molecules. The obtained trastuzumab‐conjugated NPs were washed to remove unbound reagents [16, 17].
2.8 Surface hydrophobicity of NPs
In order to determine the surface hydrophobicity, the binding constant of Rose Bengal to NPs was investigated according to the method reported by Varshosaz et al. [18]. Nanosuspensions containing 250 μg/mL of trastuzumab‐DTX‐NPs, non‐targeted DTX‐NPs, or blank NPs were incubated with Rose Bengal solution with various concentrations of 1–45 μg/mL for 3 h at room temperature. Afterward, the samples were centrifuged for 1 h at 2000 g and the absorbance of each supernatant was measured via spectrophotometry at 542 nm. The related concentrations were calculated using a calibration curve and the resulted data were used in the Scatchard transformation (3) to calculate the binding constant, K b, of Rose Bengal:
| (3) |
In which, r represents the amount of adsorbed Rose Bengal, a is the equilibrium concentration of free Rose Bengal, and N is the maximum amount of bound Rose Bengal. Surface hydrophobicity was determined by calculation of K b, using the slope of the linear plot of r/a versus r experiments were performed in triplicate.
2.9 In vitro cytotoxicity
The in vitro cytotoxicity of the targeted and non‐targeted DTX‐NPs was compared with the free drug using the MTT assay. Briefly, RPMI medium supplemented with 10% FBS and 1% of 1:1 mixture of penicillin (100 IU/mL) streptomycin (100 μg/mL) was used as the culture medium for HER‐2‐positive (BT474) and HER‐2‐negative (MDA‐MB‐468) breast cancer cells [19]. Cell suspension 180 μL containing 6 × 104 cell/mL was seeded in each well of a 96‐well culture plate and placed in an incubator (Memmert, Germany) for 24 h at 37°C, under 98% relative humidity and 5% CO2. After this incubation period which would let the cells attach to the plate, samples including trastuzumab‐DTX‐NPs, DTX‐NPs, and free DTX, containing 10–100 ng/mL DTX equivalent concentration were added to the cells. Also, samples including blank NPs, which did not contain the drug and DMSO, at the maximum concentration used to dissolve the free drug were studied as control groups. The treated cells were incubated for another 24‐h period before addition of 20 μL of MTT solution (5 mg/mL) to each well. Further 3‐h incubation time was needed for the formazan crystals to be formed; afterward, these crystals were dissolved in DMSO to construct solutions with different concentrations according to the number of formazan crystals produced by live cells. The absorbance was analysed at 570 nm using an ELISA plate reader (BioTek Instruments, USA). The cell survivals were calculated using the following equation [20]:
| (4) |
The cell survivals obtained from this assay, which was performed in triplicate, were used to calculate the IC50 of each sample and were statistically analysed by analysis of variance.
2.10 Investigation of cellular uptake
To investigate the uptake of the NPs by cancer cells, fluorescent microscopy was applied using pyrene as a hydrophobic fluorescent agent, which was loaded in the DTX‐free NPs. In the first step, the release profile of pyrene from NPs was studied to make sure that the release was negligible during the time period required for the cellular uptake assay, thus the fluorescence visualised in the fluorescent microscope was not due to the penetration of the released fluorochrome into the cells [21].
In the second step, sterilised 18‐mm coverslips, pre‐treated with poly‐l ‐lysine 0.1% solution, were placed inside each well of a 12‐well cell culture plate. Afterwards, 450 μL of a 105 cell/mL suspension of BT‐474 cells were seeded in each well, followed by an initial incubation period which let the cells attach to the coverslip, 50 μL of samples including pure pyrene, blank NPs, pyrene‐loaded NPs, and trastuzumab‐targeted pyrene‐loaded NPs were added to the wells. After a 2‐hour incubation period, coverslips were removed and placed on separate slides following a gentle wash with PBS. The resulted slides were evaluated by fluorescent and light microscopes (Eclipse Ti‐U; NIKON, Japan).
3 Results and discussions
3.1 Characterisation of Ecoflex® NPs
The physicochemical characteristics of DTX‐NPs are demonstrated in Table 1. According to the obtained results, Ecoflex® NPs loaded with DTX (3:1 polymer:drug), using the aqueous phase with 1% w/v Pluronic‐F127, and the ratio of DCM to DMF of 2.7 with particle size of 202.4 ± 8.3 nm, PDI value of 0.29 ± 0.01 demonstrating almost uniform particle size distribution, zeta potential value of −17.6 ± 0.4 mV, high entrapment efficiency of drug cargo as much as 82.0 ± 8.5%, and drug release efficiency of 45.4 ± 2.7% >30 h, had notable characteristics among the studied formulations.
Table 1.
Physicochemical characteristics of DTX‐NPs
| P:D ratio | Pluronic‐F127% | DCM:DMF ratio | Particle size, nm | PDI | Zeta potential, mV | Yield % | RE30 % | EE % |
|---|---|---|---|---|---|---|---|---|
| 7:1 | 1 | 2.7 | 230.9 ± 1.8 | 0.35 ± 0.04 | 23.53 ± 1.50 | 54 ± 2 | 37.6 ± 2.3 | 85.1 ± 2.7 |
| 4:1 | 1 | 2.7 | 220.1 ± 6.1 | 0.31 ± 0.02 | 17.5 ± 0.85 | 48 ± 2 | 43.5 ± 2.1 | 82.8 ± 3.9 |
| 3:1 | 1 | 2.7 | 202.4 ± 8.3 | 0.29 ± 0.01 | 17.60 ± 0.36 | 37 ± 1 | 47.1 ± 3.3 | 82.0 ± 8.5 |
P:D ratio is polymer to drug ratio.
DCM:DMF, dichloromethane to N, N ‐dimethylformamide ratio; EE%, a per cent of entrapment efficiency; PDI, polydispersity index; RE30%, release efficiency in 30 h.
As indicated by the results, the increment of polymer to drug ration resulted in increased particle size of NPs; however, it also caused a favourable increment in yield of procedure. Besides, a significant increment of absolute zeta potential value was observed with the increment of polymer to drug ratio from 4:1 to 7:1. The release efficiency percent was inversely related to the polymer to drug ratio which could be due to the reduction of release ratio by an increment of the amount of the polymer in the formulation.
As illustrated in Fig. 1, in all studied formulations drug release reached the plateau state within 30 h.
Fig. 1.
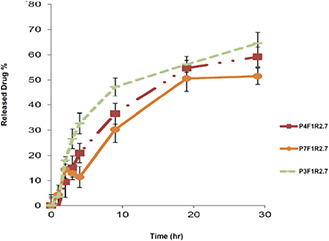
Diagrams of the drug release profile from different formulations of DTX‐NP. The aqueous release medium was composed of phosphate buffered saline (pH 7.4 ± 0.2) and Tween 80 (0.5% w/v)
Ecoflex® NPs loaded with DTX were fabricated by electrospraying technique, which provided a suitable technique regarding the physicochemical characteristics of Ecoflex®. When the organic phase is pumped into the high‐voltage electrical field, the liquid is atomised due to the Coulomb repulsion forces. This results in the formation of small charged droplets, which would be dried into solid NPs upon reaching the collector electrode; this would reduce the poly‐dispersity index (PDI) of NPs in electrospraying technique.
In some other investigations, such as one performed by Yang et al., [22] conjugation to trastuzumab was done by pre‐treating the antibody molecule using 2‐iminothiolane (known as Traut's reagent). In their method, Traut's reagent was used to react with primary amines on trastuzumab to produce sulfhydryl groups. Afterward, the thiolated trastuzumab could easily attach to maleimide‐derivatised liposomes via thiol‐ether bonds formed between thiol and maleimide functional groups. Mandler et al. [23] confirmed that pre‐treatment with Traut's reagent and conjugation does not negatively affect the antigen recognition and cell binding of trastuzumab. For this purpose, a modified cell binding assay was performed on an HER‐2‐transfected cell line, using native and conjugated trastuzumab that was labelled with 125I. Comparison of K d values revealed no significant difference after conjugation. Therefore, it could be deduced that conjugation of the primary amine of trastuzumab to the carboxylic groups on Ecoflex® NPs that was performed in this study could not compromise the immune‐reactivity of this antibody.
The Rose Bengal binding constants obtained in the surface hydrophobicity study are demonstrated in Table 2. According to the results, the attachment of trastuzumab molecules to the NPs reduced the obtained Kb value, which represents the reduction of surface hydrophobicity of the NPs [18].
Table 2.
Binding constant (K b) of Rose Bengal to targeted, non‐targeted and blank NPs
| Blank NPs | DTX‐NPs | Tratuzumab‐DTX‐NPs | |||
|---|---|---|---|---|---|
| K b | R 2 | K b | R 2 | K b | R 2 |
| 0.151 ± 0.010 | 0.9944 | 0.417 ± 0.021 | 0.9773 | 0.113 ± 0.011 | 0.9924 |
R 2 shows the correlation coefficient of the data.
In order to attach the NPs to trastuzumab molecules, Sun et al. [24] simply decorated DTX‐loaded NPs with trastuzumab molecules by overnight incubation at room temperature. Although this method is easier to perform, the possibility of detachment of ligand from the NPs is faced as a major disadvantage, due to the lack of a chemical conjugation step.
Taking advantage of the presence of −COOH functional group on Ecoflex®, the DTX‐NPs could be attached to trastuzumab molecules chemically via an amide bond between the −COOH and the primary amine functional groups on trastuzumab.
3.2 Cytotoxicity assay
The survival rate of BT‐474 and MDA‐MB‐468 cells which were treated with DTX‐NP suspension, trastuzumab‐targeted DTX‐NP, free drug, and control samples are presented in Figs. 2 a and b.
Fig. 2.
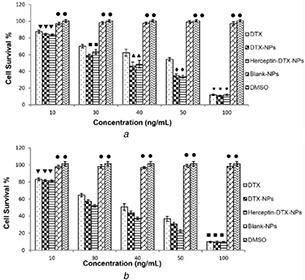
Survival per cent of
(a) MDA‐MB‐468 cells and, (b) BT‐474 cells after 24‐h incubation with free DTX, non‐targeted DTX‐NPs, and trastuzumab‐conjugated DTX‐NPs, containing 10–100 ng/mL DTX equivalent. DTX‐free NPS and DMSO with the maximum concentration used in the test groups were used as control samples. (Diagrams marked with similar symbols did not have a significant difference with each other)
The results of the cytotoxicity study indicated that even the highest concentration of drug‐free NPs, blank NPs in Fig. 2, used as control did not show any significant cytotoxic effect on the HER‐2‐positive and HER‐2‐negative cell lines; hence, the safety of implemented delivery system could be confirmed (Fig. 2). Furthermore, at 30–50 ng/mL DTX equivalent concentrations, the cell survivals of both cell lines treated with DTX‐NPs were significantly lower than those treated with the free DTX (Fig. 2). Besides, incubation of the HER‐2 overexpressing cells with trastuzumab‐targeted DTX‐NPs resulted in a significantly lower cell survival than the non‐targeted NPs, whereas the difference between these two treatments was not significant in an MDA‐MB‐468 cell line (Fig. 2).
Overall, the results of MTT assay demonstrated that the Ecoflex® NPs could improve the in vitro cytotoxicity of their drug cargo, for instance, DTX in this study. Moreover, the addition of trastuzumab as a targeting ligand amplifies this effect on receptor‐positive cells. This could be due to the effect of ligand–receptor attachment, which could lead to enhanced cellular uptake of the NPs that are specifically targeted for HER‐2 overexpressing breast cancer cells.
3.3 Cellular uptake of the NPs
Ecoflex® NPs were loaded with pyrene, as a lipophilic fluorophore cargo instead of DTX, thus the cellular uptake of targeted and non‐targeted Ecoflex® NPs could be evaluated. After being exposed to pyrene‐NPs the cells were washed and investigated by fluorescence microscopy. The micrographs representing BT‐474 cells treated with pyrene‐loaded NPs and free pyrene are demonstrated in Fig. 3.
Fig. 3.
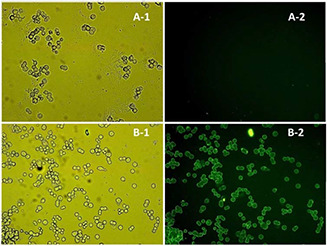
Micrographs of BT‐474 cells treated with
(a) Free pyrene, (b) Pyrene‐loaded nanoparticles obtained by (1) visible or (2) fluorescent light (×40 magnification)
Detection of fluorescence in cells confirmed that the NPs were uptaken by the cells as the cells were washed after being exposed to the NPs. As free pyrene did not enter the cells, no fluorescence was observed in fluorescence micrographs while the cells could be observed in visible light microscopy. In contrast, the pyrene‐loaded NPs were shown to be up‐taken by the cancer cells that could verify the ability of this nanoparticulate delivery system to enter the cancer cells.
4 Conclusion
Ecoflex® is a biodegradable and biocompatible polymer, implemented in this study to fabricate NPs via electrospraying technique. In this technique, organic phase in the syringe is broken into fine droplets inside a high‐voltage electrical field which accelerates the evaporation of organic solvents before the NPs reached the opposite electrode, placed in a gently stirring aqueous phase containing a surfactant to prevent the coalescence of the NPs.
In contrast, the possibility of attachment of trastuzumab molecules to the DTX‐NPs provides the opportunity of fabricating a non‐toxic targeted delivery system for delivery of hydrophobic anticancer agents which could notably reduce the adverse effects proposed by non‐targeted biodistribution of the cytotoxic agent as well as the ingredients of the formulation needed to enhance its solubility to make an injectable dosage form.
However, further studies are underway to evaluate the antitumour effects, biodistribution, and pharmacokinetic properties of the designed NPs.
5 References
- 1. Bidgoli S.A. Ahmadi R. Zavarhei M.D.: ‘Role of hormonal and environmental factors on early incidence of breast cancer in Iran’, Sci. Total Environ., 2010, 408, (19), pp. 4056 –4061 [DOI] [PubMed] [Google Scholar]
- 2. Bray F. McCarron P. Parkin D.M.: ‘The changing global patterns of female breast cancer incidence and mortality’, Breast Cancer Res., 2004, 6, pp. 229 –239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slamon D.J. Leyland‐Jones B. Shak S. et al.: ‘Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2’, N. Engl. J. Med., 2001, 344, pp. 783 –792 [DOI] [PubMed] [Google Scholar]
- 4. Ghislain I. Zikos E. Coens C. et al.: ‘Health‐related quality of life in locally advanced and metastatic breast cancer: methodological and clinical issues in randomised controlled trials’, Lancet Oncol., 2016, 17, pp. 294 –304 [DOI] [PubMed] [Google Scholar]
- 5. Shelley M. Harrison C. Coles B. et al.: ‘Chemotherapy for hormone‐refractory prostate cancer’,, Cochrane Database Syst. Rev., 2006, 4, pp. 1 –70 [DOI] [PubMed] [Google Scholar]
- 6. Poppel H.V.: ‘Recent docetaxel studies establish a new standard of care in hormone refractory prostate cancer’, Can. J. Urol., 2005, 12, pp. 81 –85 [PubMed] [Google Scholar]
- 7. Baker J. Ajani J. Scotte F. et al.: ‘Docetaxel‐related side effects and their management’, Eur. J. Oncol. Nurs., 2008, 12, (3), pp. 49 –59 [DOI] [PubMed] [Google Scholar]
- 8. Sumera S. Anwar A. Ovais M. et al.: ‘Docetaxel‐loaded solid lipid nanoparticles: a novel drug delivery system’, IET Nanobiotechnol., 2017, 11, (6), pp. 621 –629 [Google Scholar]
- 9. Esmaeli B. Valero V. Ahmadi M.A. et al.: ‘Canalicular stenosis secondary to docetaxel (taxotere) a newly recognized side effect’, Ophthalmology, 2001, 108, (5), pp. 994 –995 [DOI] [PubMed] [Google Scholar]
- 10. Cho H.S. Mason K. Ramyar K.X. et al.: ‘Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab’, Nature, 2003, 421, (6924), pp. 756 –760 [DOI] [PubMed] [Google Scholar]
- 11. Liua Q. Li R. Zhub Z. et al.: ‘Enhanced antitumor efficacy, biodistribution and penetration of docetaxel‐loaded biodegradable nanoparticles’, Int. J. Pharm., 2012, 430, (1–2), pp. 350 –358 [DOI] [PubMed] [Google Scholar]
- 12. Varshosaz J. Riahi S. Ghassami E. et al.: ‘Transferrin‐targeted poly (butylene adipate)/terephthalate nanoparticles for targeted delivery of 5‐fluorouracil in HT29 colorectal cancer cell line’, J. Bioac. Compat. Polym., 2017, 32, (5), pp. 503 –527 [Google Scholar]
- 13. Varshosaz J. Ghassami E. Noorbakhsh A. et al.: ‘Poly(butylene adipate‐co‐butylene terephthalate) nanoparticles prepared by electrospraying technique for docetaxel delivery in ovarian cancer induced mice’, Drug Dev. Ind. Pharm., 2018, 44, (6), pp. 1012 –1022 [DOI] [PubMed] [Google Scholar]
- 14. Zakeri‐Milania P. Loveymib B.D. Jelvehgarid M. et al.: ‘The characteristics and improved intestinal permeability of vancomycin PLGA‐nanoparticles as colloidal drug delivery system’, Colloids Surf. B, Biointerfaces, 2013, 103, pp. 174 –181 [DOI] [PubMed] [Google Scholar]
- 15. Taymouri S. Varshosaz J. Hassanzadeh F. et al.: ‘Optimisation of processing variables effective on self‐assembly of folate targeted synpronic‐based micelles for docetaxel delivery in melanoma cells’, IET Nanobiotechnol., 2015, 9, (5), pp. 306 –313 [DOI] [PubMed] [Google Scholar]
- 16. Nam K. Kimura T. Kishida A.: ‘Controlling coupling reaction of EDC and NHS for preparation of collagen gels using ethanol/water co‐solvents’, Macromol. Biosci., 2008, 8, pp. 32 –37 [DOI] [PubMed] [Google Scholar]
- 17. Aravind A. Jeyamohan P. Nair R. et al.: ‘AS1411 aptamer tagged PLGA‐lecithin‐PEGNanoparticles for tumor cell targeting and drug delivery’, Biotechnol. Bioeng., 2012, 109, (11), pp. 2920 –2931 [DOI] [PubMed] [Google Scholar]
- 18. Varshosaz J. Sadeghi H. Shafipour F.: ‘Biological safety evaluation of stealth solid lipid nanoparticles of risperidone: effect of surface characteristics on their hydrophobicity, haemolysis and macrophage phagocytosis’, Farmacia, 2012, 60, (1), pp. 64 –79 [Google Scholar]
- 19. Anido J. Matar P. Albanell J. et al.: ‘ZD1839, a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulin signaling in HER2‐overexpressing breast cancer cells’, Clin. Cancer Res., 2003, 9, (4), pp. 1274 –1283 [PubMed] [Google Scholar]
- 20. Ghassami E. Varshosaz J. Jahanian‐Najafabadi A. et al.: ‘Pharmacokinetics and in vitro/in vivo antitumor efficacy of aptamer‐targeted Ecoflex® nanoparticles for docetaxel delivery in ovarian cancer’, Int. J. Nanomed., 2018, 13, pp. 493 –504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayashida O. Eguchi C. Kimura K. et al.: ‘Guest binding, cellular uptake, and molecular delivery of water‐soluble cyclophanes having a pyrene moiety’, Chem. Lett., 2010, 39, pp. 1321 –1322 [Google Scholar]
- 22. Yang T. Choi M.K. Cui F.D. et al.: ‘Preparation and evaluation of paclitaxel‐loaded PEGylated immunoliposome’, J. Control Release, 2007, 120, (3), pp. 169 –177 [DOI] [PubMed] [Google Scholar]
- 23. Mandler R. Kobayashi H. Davis M.Y. et al.: ‘Modifications in synthesis strategy improve the yield and efficacy of geldanamycin‐herceptin immunoconjugates’, Bioconjug. Chem., 2002, 13, (4), pp. 786 –791 [DOI] [PubMed] [Google Scholar]
- 24. Sun B. Ranganathana B. Feng S.: ‘Multifunctional poly(D,L‐lactide‐co‐glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by trastuzumab for targeted chemotherapy of breast cancer’, Biomaterials, 2008, 29, (4), pp. 475 –486 [DOI] [PubMed] [Google Scholar]


