Abstract
The biosynthesis of silver nanoparticles (AgNPs) has been proved to be a cost effective and environmental friendly approach toward chemical and physical methods. In the present study, biosynthesis of AgNPs was carried out using aqueous extract of Zea mays (Zm) husk. The initial colour change from golden yellow to orange was observed between 410 and 450 nm which confirmed the synthesis of AgNPs. Also, dynamic light scattering‐particle size analysis confirmed the average size to be 113 nm and zeta potential value of −28 kV. The morphology of synthesised Zm AgNPs displayed flower‐shaped structure, X‐ray diffraction pattern revealed the strongest peaks at 2θ = 38.6° and 64° which proved that the nanoparticle has the face centred crystalline structure. The Fourier transform infrared spectroscopy results showed strong absorption bands at 1394.53, 2980.02 and 2980.02 cm−1 due to the presence of alkynes, carboxylic acids, alcoholic and phenolic groups. The maximum zone of inhibition was observed against Salmonella typhi (22 mm) and Candida albicans (18 mm). The synthesised nanoparticles exhibited more free radical scavenging activity than the aqueous plant extract. This is the first report on the synthesis of AgNP from Zm husk, delivers the efficient and stable Zm AgNPs through simple feasible approach toward green biotechnology.
Inspec keywords: silver, nanoparticles, nanofabrication, light scattering, particle size, X‐ray diffraction, crystal structure, Fourier transform infrared spectra, absorption coefficients, free radicals
Other keywords: green synthesis, silver nanoparticles, biosynthesis, environmental friendly approach, aqueous extract, Zea mays husk, colour change, golden yellow, dynamic light scattering‐particle size analysis, average size, zeta potential value, flower‐shaped structure, X‐ray diffraction pattern, face centred crystalline structure, Fourier transform infrared spectroscopy, absorption bands, alkynes, carboxylic acids, alcoholic groups, phenolic groups, Salmonella typhi, Candida albicans, free radical scavenging activity, aqueous plant extraction, green biotechnology, size 113 nm, wavelength 410 nm to 450 nm
1 Introduction
The naturally synthesised products have greater medicinal values and are better alternatives to these chemically synthesised drugs as they have lesser side effects and lead to long‐term cure for the illness [1]. Biosynthesis of nanoparticles has advantages such as slower kinetics, better manipulation and control over crystal growth and excellent stability. In the recent past, various plant sources such as Mimosops elengi. Linn [1], Bixa orellana [2] and Arbutus unedo [3] have been used for the synthesis of silver nanoparticles (AgNPs). Ag is a better substitute compared with the other metals as an antimicrobe as it has very less or no toxic effect [4].
In the present paper, a novel approach for the green synthesis of AgNPs using Zea mays (Zm) husk extract has been used, where husk is easily available and cost effective. The husk extract has been reported to have anti‐inflammatory and analgesic properties based on the in vivo studies conducted on male rats [5]. The corn husk also reported to have arabinoxylan which is one of the main components of soluble and insoluble dietary fibres. The antimicrobial studies using the aqueous extract of Zm husk and the synthesised Zm AgNPs have been carried out against both bacterial and fungal clinical isolates. Antioxidants are micronutrients that have gained importance recently due to their ability to neutralise or reduce the free radical activity [6]. Many plants have been reported to have antioxidant properties. Till to date no extensive work has been carried out to analyse the antioxidant property of Zm husk. Therefore, the present paper intends to biosynthesise AgNPs from Zm and also to explore their biological applications such as antimicrobial and antioxidant activities.
2 Methods
2.1 Materials
Ag nitrate (AgNO3), ethyl alcohol (C2 H6 O), carbinol (CH4 O) and 2,2‐dipheny‐l‐picrylhydrazyl (DPPH) (C18 H12 N5 O6) were of analytical grade and used without further purification. Zm husk were collected from agricultural fields in and around Alandurai, Coimbatore, during the harvest period. Plant species authentication was done at Botanical Survey of India (BSI), Coimbatore, South India (Ref no. BSI/SRC/5/23/2014‐15/194). The clinical isolates such as Staphylococcus aureus, Salmonella typhi, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Candida albicans, Fusarium sp. and Rhizopus oryza were obtained from Microbiology laboratory, Kovai Medical Center and Hospital, Coimbatore, South India.
2.2 Preparation of aqueous leaf extract
The husk of Zm was washed thoroughly with distilled water several times to remove the dust particles. Exactly 10 g of powdered husks were boiled with 200 ml of deionised water for 15 min at 60 °C to obtain the extract. The crude extract was then filtered using Whatman No. 1 filter paper (Maidstone, UK) and stored at room temperature for further experiments.
2.3 Synthesis of AgNPs
Exactly 30 ml of the husk extract was added to 100 ml of 1 mM AgNO3 solution and incubated until colour change. The solution turned to golden yellow to orange colour indicates the formation of Zm AgNPs. The mixture was boiled at 80 °C for 10 min with continuous stirring and filtered through Whatman filter paper No. 1 (Maidstone, UK). The filtrate was lyophilised at Defense Research and Development Organisation, Bharathiar University, Coimbatore for further studies.
2.4 Characterisation of synthesised AgNPs
The synthesised AgNPs were initially confirmed by ultraviolet–visible (UV–vis) analysis at different time intervals and the absorption maxima was scanned between 300 and 700 nm wavelengths using Hitachi U2910 spectrophotometer. The particle size and zeta potential of the synthesised Zm AgNPs were analysed using Malvern particle size analyser using dynamic light scattering technique. Also, the morphological, structural and chemical compositions of Zm AgNPs were analysed by scanning electron microscopy with energy dispersive X‐ray spectroscopy (SEM‐EDX) Jeol model JSM (JEO‐JSM 6390, Japan) and X‐ray diffraction (XRD) (XRD‐6000 X‐ray diffractometer, Shimadzu, Japan). The functional groups present in Zm AgNPs causing the reduction was recorded by Fourier transform infrared spectroscopy (FTIR) (Shimadzu 8400S, Japan).
2.5 Determination of antimicrobial activity of ZmAgNPs
In vitro antimicrobial activity of the synthesised Zm AgNPs was determined by agar well diffusion method [7]. Fresh overnight inoculums (100 µl of 105 –106 CFU/ml) of each clinical isolates were swabbed uniformly on Mueller Hinton agar plates using sterile cotton swab. Then, three wells of 6 mm diameter were made using sterile well borer. About 100 µl of Zm AgNPs solutions with various concentrations (75, 100 and 150 µg/ml) was poured into the corresponding well. The plates were incubated at 37 °C for 24 and 48 h for the bacterial and fungal cultures, respectively. The diameter of inhibition zone was measured after incubation. Deionised water was used as a negative control and the antibiotics ofloxacin (5 µg/disc), tobramycin (10 µg/disc), ciprofloxacin (30 µg/disc) and gentamicin (30 µg/disc) were used as positive controls. Triplicates were maintained and average values were calculated.
2.6 In vitro antioxidant assays
2.6.1 DPPH free radical scavenging assay
The DPPH free radical scavenging assay was conducted based on the method of Chang et al. [8]. About 1 µl of 0.1 mM DPPH (in ethanol) was added to different concentrations (50, 100, 150 and 200 µg/ml) of Zm husk extract and Zm AgNPs. The reaction mixtures were incubated in the dark for 30 min. The absorbance at 517 nm was measured against a blank (ethanol). Ascorbic acid was used as the standard. The lower absorbance of the reaction mixture indicated a higher percentage of scavenging activity. The percentage of inhibition or scavenging of free radicals was determined by the following formula:
3 Results
3.1 UV–vis spectral studies
The colour change of the husk extract was noted from golden yellow to orange after four days of incubation with AgNO3 solution (Fig. 1). Absorption spectrum of the incubated solution at linear wavelengths range from 350 to 600 nm revealed a peak between 410 and 450 nm (Fig. 2). The husk extract without AgNO3 did not show any change in colour. The colour intensity also increased with the duration of incubation.
Fig. 1.
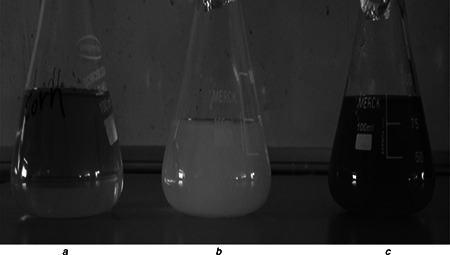
UV–vis spectral studies
a Aqueous husk extract
b AgNO3 solution
c Synthesised ZmAgNPs
Fig. 2.
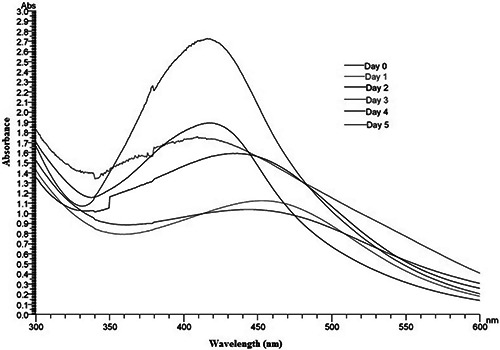
UV–vis spectrum of AgNO3 (1 mM) and the aqueous husk extract of Zm reaction mixture at different time intervals
3.2 Particle size and zeta potential measurements
Particle size determination of the synthesised AgNPs was shown under by intensity. Laser diffraction revealed that particles obtained are polydisperse mixture with the size ranging from 50 to 70 nm (Fig. 3). The average diameter of the particles was found to be 113.5 nm. Meanwhile, zeta potential measurement of synthesised AgNPs showed the surface charges of the particles and exhibited a value of −28.7 mV (Fig. 4), suggesting higher stability of AgNPs. The large negative potential value could be due to the capping of polyphenolic constituents present in the husk extract of Zm.
Fig. 3.
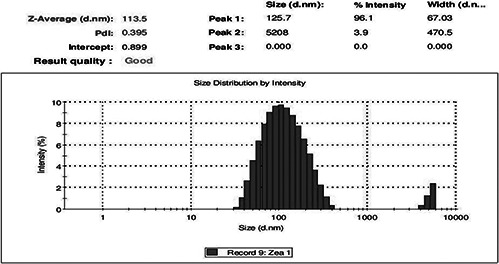
Particle size distribution curve for synthesised AgNPs
Fig. 4.
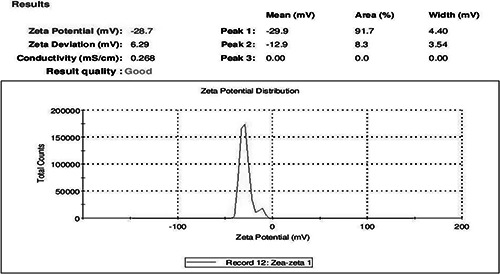
Zeta potential measurement of synthesised AgNPs
3.3 Characterisation studies
The XRD pattern of the biosynthesised Ag nanostructure produced by the husk extract was further demonstrated and confirmed by the characteristic peaks observed in the XRD image (Fig. 5). The XRD pattern showed two intense correspondence peaks at 38.6° and 64° in the whole spectrum of 2θ value ranging from 10 to 90. SEM analysis was carried out to understand the topology and the size of the synthesised AgNPs, which showed the synthesis of higher density polydispersed spherical AgNPs of various sizes that ranged from 106 to 240 nm (Fig. 6). The EDX spectrum of synthesised AgNPs (Fig. 7) clearly exhibited the absence of elemental nitrogen and oxygen peaks and the presence of elemental Ag metal. The sharp peak of Ag strongly confirmed the reduction of AgNO3 to AgNPs. FTIR measurements of both the aqueous husk extract and ZmAgNPs were carried out to identify the possible biomolecules responsible for the reduction of the Ag+ ions and capping of the bio‐reduced AgNPs synthesised by the husk extract. The major peaks in the FTIR spectrum of AgNPs (Fig. 8) were observed at ∼1042.42, ∼1394.53, ∼1643.35 and ∼2980 cm−1. Similarly, in the FTIR spectrum of the Zm aqueous husk extract, the major peaks were observed at ∼1045.42, ∼1637.56, ∼2360.87 and ∼2981.95 cm−1. The peak ∼2360.87 observed in Zm aqueous husk extract was completely reduced in FTIR spectrum of AgNPs which evidently proved that the functional group nitriles are responsible for the reduction of AgNO3 to AgNPs.
Fig. 5.
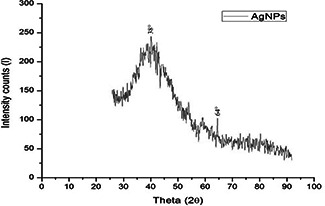
XRD pattern of ZmAgNPs
Fig. 6.

SEM image of synthesised ZmAgNPs
Fig. 7.
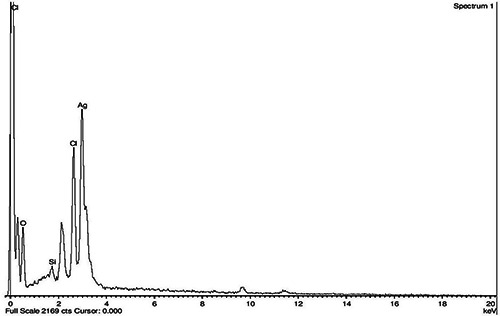
EDX image of ZmAgNPs
Fig. 8.
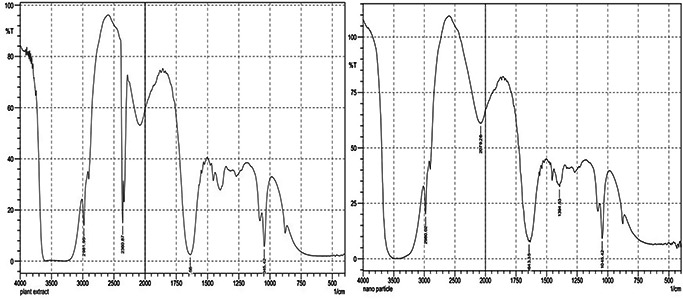
FTIR spectrum of the husk extract and ZmAgNPs
3.4 Antimicrobial studies
The antibacterial activity of the synthesised ZmAgNPs in different concentrations (100–200 μl) was quantitatively assessed on the basis of zone of inhibition (Table 1). According to the Clinical and Laboratory Standards Institute guidelines, the bacterial pathogens were resistant to all five antibiotics (positive control) tested in the study. Among the Gram negative bacteria, S. typhi was strongly inhibited by ZmAgNPs with zone of inhibition of 22.3 ± 2.08 at 200 μl. Similarly, the growth of E. coli was inhibited at 150 μl (14.6 ± 0.57 mm). In case of gram positive, growth of S. aureus was inhibited at 200 μl with the zone of inhibition of 10.6 ± 1.15 mm. Moreover, the antifungal activity of ZmAgNPs exhibited higher zone of inhibition of 14.6 ± 0.57 mm (200 μl) against C. albicans. The present study clearly indicates that ZmAgNPs exhibited strong antibacterial and antifungal activities against all the clinical isolates even at lower concentration (100 μl). The zone of inhibition was accurately measured and compared with the standard antibiotics given in Table 2.
Table 1.
Antimicrobial activity of Zm AgNPs against human pathogens
| Microorganisms | ZmAgNPs, µg/ml/zone of inhibition, mm | ||
|---|---|---|---|
| 100 µl | 150 µl | 200 µl | |
| E. coli | 11.3 ± 1.15 | 13 ± 1.0 | 14.6 ± 0.57 |
| S. aureus | 9 ± 1.0 | 9.6 ± 1.15 | 10.6 ± 1.15 |
| K. pneumoniae | 7.6 ± 1.15 | 8.5 ± 0.57 | 9.1 ± 0.52 |
| P. aeruginosa | 8.3 ± 1.15 | 7.6 ± 1.15 | 8.6 ± 0.57 |
| S. typhi | 19.6 ± 1.5 | 20.3 ± 1.15 | 22.3 ± 2.08 |
| Fusarium sp. | 7.6 ± 1.15 | 10.3 ± 1.15 | 12.6 ± 1.52 |
| Rhizopus oryzae | 8.6 ± 0.57 | 9.1 ± 0.52 | 12 ± 1.0 |
| C. albicans | 10 ± 1.0 | 11.6 ± 0.57 | 14.6 ± 0.57 |
Table 2.
Antimicrobial activity of Standard antibiotics
| Microorganisms | Antibiotics/zone of inhibition, mm | |||
|---|---|---|---|---|
| Ofloxacin (5 mcg/disc) | Tobramycin (10 mcg/disc) | Gentamicin (30 mcg/disc) | Ciprofloxacin (30 mcg/disc) | |
| E.coli | 11 | 15 | 7 | 14 |
| S. aureus | 8 | 10 | 8 | 12 |
| K. pneumoniae | 12 | 13 | 9 | 15 |
| P. aeruginosa | 7 | 9 | 6 | 9 |
| S. typhi | 8 | 11 | 9 | 8 |
3.5 In vitro antioxidant activity
Positive DPPH tests demonstrated that Zm and ZmAgNPs are free radical scavengers. The DPPH scavenging assay exhibited less effective inhibition activity of both Zm and ZmAgNPs when compared with the standard, ascorbic acid (Fig. 9). The DPPH activity of the nanoparticles was found to increase in a dose‐dependent manner. However, the ZmAgNPs exhibited more inhibition with more than 80% scavenging activity of DPPH than Zm. Zm exhibited potent scavenging effects against DPPH with an Inhibitory concentration (IC50) value of 147.53 ± 0.42 μg/ml and ZmAgNPs shown that IC50 value of 101.50 ± 1.95 μg/ ml. The IC50 value of standard ascorbic acid for this assay was 60.78 ± 1.79 µg/ml.
Fig. 9.
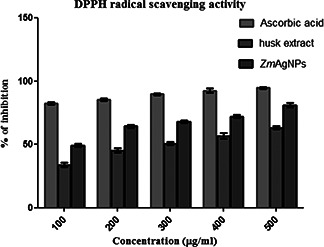
Reducing power assay of ZmAgNPs, Zm and ascorbic acid
4 Discussion
In the present paper, the green synthesis of benign AgNPs was carried out successfully under optimised conditions. The husk extract of corn was used as the primary source which exhibited various medicinal properties [5]. The bio‐reduction of AgNO3 is due to the presence of phytochemicals such as flavonoids, saponins, tannins and polyphenols present in the corn husk [5]. The AgNO3 concentration was slightly increased and the aqueous husk extract yield was also increased to obtain better results.
The peak value was observed within the range of 410–450 nm at various time intervals by using UV–vis spectrophotometer. The similar U–V readings within the range of 440–450 nm have been reported by Prakash et al. [1] for the green synthesis of AgNPs from the plant Mimusops elengi. Linn. Similarly, Mehrdad and Khalil [9] synthesised AgNPs from soaproot – Acantha phylum bracteatum and obtained peak value in 425 nm. Moreover, AgNPs synthesised by three different plant extracts are at 447, 445 and 482 nm [10]. Likewise, Stefano et al. [11] reported 440 nm maximum absorption for AgNPs capped with L‐cysteine with 1 : 5 ratio. This exhibited that present study results were highly correlated with previously published reports. The particle size of synthesised nanoparticles was found to be in the range 113 nm. Since the husk is the outer covering which is dry in nature, more plant metabolites could not be extracted under laboratory conditions. By subjecting the husk powder to sonication, more yield could be obtained and the nanometre size will be reduced subsequently. The reason for large size distribution was due to time fluctuation in the intensity of light scattering by autocorrelator, which determines the signal autocorrelation function of nanoparticles [12]. The particle size of Malus domestica AgNPs was reported in the range of 50–300 nm with average of 150 nm [13], whereas in the current study, it was found to be 113 nm, which was well supported. Moreover, Zm AgNPs were found to be moderately stable with a stability of −28 mv, whereas Achillea biebersteinii AgNPs showed between −20 and −40 mV [14] and Lantana camara leaf extract found to be −25 and −50 mV [15] which is highly significant with present study. The reason may be due to high electric charge on the surface of nanoparticles that creates desired zeta potential resulting in capping of bio‐organic compounds present in the husk extract [16].
The two strong XRD peaks revealed that the nanoparticle possessed face centred cubic lattice shape, and hence it confirmed the crystalline nature of the Zm AgNPs. The average particle shape of the nanoparticles was derived from the full width at half maximum of the corresponding peak (111) and (011) planes with cubic (2θ = 38.6°), face‐centred cubic (2θ = 64°) structures of the Zm AgNPs [17]. The findings of the current study clearly specified that the AgNPs formed by the reduction of Ag+ ions by the Zm husk leaf extract are crystalline in nature. Anal and Prasad [18] synthesised AgNPs using Cycas leaf extract and found out the nanoparticles to be face centred cubic unit cell. They also reported that the broadening of the peaks observed was due to the small particle size of the synthesised nanoparticle. The broadening of the peaks seen arises due to the presence of various crystalline biomolecules present in the aqueous husk extract [19]. Various distributed irregularly shaped particles were obtained in the nanometre range during SEM analysis which showed the increased nanometre range of 106–240 nm of Zm AgNPs. Rajkiran et al. [20] reported that AgNPs synthesised from Carcia papaya showed 50–300 nm range under SEM analysis which indicated that the present results were significant with previous reports. In Energy dispersive X‐ray analysis (EDAX), the Ag metal was found to be in high concentrations and this shows that AgNPs were not oxidised during the incubation period. The optical absorption band of EDX peaks were in the range of 3–4 keV which is typical for absorption of metallic AgNPs [21]. The weight percentage of Ag obtained for Zm AgNPs was found to be 18.45% which is in line with the previous studies.
The FTIR results showed biomolecules responsible for the reduction of AgNO3 to AgNPs. The strong peak at 2360.87 cm−1 was due to formation of alkynes. The alcoholic phenolic carboxylic and the alkynes were seen at various band stretches and were responsible for the bio‐reduction of AgNO3 to AgNPs. The AgNPs synthesised using Nerium oleander showed strong bands at 3698.28 cm−1, 1622.25 cm, 1500.39 cm, 1529.52 cm−1 and 1216.78 cm−1 [22]. The FTIR peaks of Zm and ZmAgNPs were compared and observed that withdrawal of peaks at major spectrum occurred with decrease in their intensity. This is due to the Ag ions reduction by polyols and phenols which were oxidised to unsaturated CH4 O groups [6].
The antimicrobial activity against the clinically isolated pathogens had appreciable results. The synthesised Zm AgNPs have excellent zone of inhibition for S. typhi (22 mm) and C. albicans (18 mm). Similarly, Abraham et al. [23] synthesised nanoparticles from the commercially available plant powders such as Solanum tricobatum and analysed its antibacterial activity against human pathogenic bacteria: namely, E. coli, S. aureus, P. aeruginosa using agar well diffusion method and showed excellent zone of inhibition. The current study results indicated that the antimicrobial activity of AgNPs were moderate for bacteria when compared with fungal pathogens. This is due to the Ag cations released from the Zm AgNPs pertaining to the changes in the membrane structure of the microbes which leads to increased membrane permeability of the microbe leading to cell death [1].
The antioxidant activity understood from the radical scavenging activity indicated that the synthesised Zm AgNPs will have excellent medicinal values in future against various cardiovascular diseases, cancer and so on. Similarly, Bunghez et al. [24] studied the antioxidant activity of green synthesised AgNPs using ornamental plants Hyacinthus orientalis and Dianthus caryophyllus and reported that strong antioxidant properties ranging between 86.46 and 95.16% were noted for plant extracts and 88.30 and 97.38%, for herbal AgNPs. Phenols and polyphenolic compounds such as flavonoids are widely found in food products derived from plant sources, and they have been shown to possess significant antioxidant activities [25].
5 Conclusion
The green synthesis of benign AgNPs using the aqueous husk extract of Zm proved to be a rapid, environment friendly and cost‐effective method. The synthesised AgNPs had a thin layer of alcohol, phenol, alkynes and carboxylic acids were responsible for the bio‐reduction of AgNO3 to Ag. Appreciable zone of inhibition was observed against the clinical pathogens proved that they may have immense applications in medical, textiles and other related fields. The free radical scavenging activity of the synthesised nanoparticles was better than crude husk extract and will act as a tool against cancer in future. From a technology point of view, the obtained Zm AgNPs has potential applications in the bio‐medical field.
6 Acknowledgments
The author(s) acknowledge the facilities provided by the Department of Biotechnology, Karunya University and also grateful to Chancellor (Dr. Paul Dhinakaran), Vice Chancellor (Dr. Sundar Manoharan) and Registrar (Dr. Joseph Kennady), Karunya University, Coimbatore, India for their kind support to carry out this publication.
7 References
- 1. Prakash P. Gnanaprakasam P. Emmanuel R. et al.: ‘Green synthesis of silver nanoparticles from leaf extract of Mimusops eleni, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates’, Colloids Surf. B, 2013, 108, pp. 255 –259 (doi: 10.1016/j.colsurfb.2013.03.017) [DOI] [PubMed] [Google Scholar]
- 2. Thilagam M. Tamilselvi A. Chandrasekeran B. et al.: ‘Photosynthesis of silver nanoparticles using medicinal and dye yielding plant of Bixa Orellama L. leaf extract’, J. Pharm. Sci. Innov., 2013, 2, pp. 9 –13 (doi: 10.7897/2277-4572.02441) [DOI] [Google Scholar]
- 3. Pantelis K. Andreas D. Vassilis Z. et al.: ‘Green synthesis of silver nanoparticles produced using Arbutus Unedo leaf extract’, Mater. Lett., 2010, 76, pp. 18 –20 [Google Scholar]
- 4. Sukumaran P. Eldho K.P.: ‘Silver nanoparticles mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects’, Int. Nano Lett., 2012, 2, p. 32 (doi: 10.1186/2228-5326-2-32) [DOI] [Google Scholar]
- 5. Joseph O. Bamidele V. Owoyele M.N. et al.: ‘Analgesic and anti‐inflammatory effects of aqueous extract of Zea mays husk in male Wistar rats’, J. Med. Food, 2010, 13, pp. 343 –347 (doi: 10.1089/jmf.2008.0311) [DOI] [PubMed] [Google Scholar]
- 6. Dipankar C. Murugan S.: ‘The green synthesis characterization and evaluation of the biological activities of silver nanoparticles using Irestene Herbstii aqueous leaf extracts’, Colloids surf. B, Biointerfaces, 2012, 98, pp. 112 –119 (doi: 10.1016/j.colsurfb.2012.04.006) [DOI] [PubMed] [Google Scholar]
- 7. Perez C. Paul M. Bazerque P.: ‘An antibiotic assay by the agar well diffusion method’, Acta Biol. Med. Exp., 1990, 15, pp. 113 –115 [Google Scholar]
- 8. Chang C.W. Sei K.C. Soon H.S. et al.: ‘Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay‐guided comparison’, Plant Sci., 2002, 163, pp. 1161 –1168 (doi: 10.1016/S0168-9452(02)00063-8) [DOI] [Google Scholar]
- 9. Mehrdad F. Khalil F.: ‘Biological and green synthesis of silver nanoparticles’, Turk. J. Eng. Environ. Sci., 2010, 34, pp. 281 –287 [Google Scholar]
- 10. Ya‐yuan M. Yan‐kui T. Sheng‐ye W. et al.: ‘Green synthesis of silver nanoparticles using eucalyptus leaf extract’, Mater. Lett., 2015, 144, pp. 165 –167 (doi: 10.1016/j.matlet.2015.01.004) [DOI] [Google Scholar]
- 11. Stefano P. Veera H. Polina P.: ‘Biogenic synthesis of antimicrobial silver nanoparticles capped with l‐cysteine’, Colloids Surf. A, Physicochem. Eng. Aspects, 2014, 460, pp. 219 –224 (doi: 10.1016/j.colsurfa.2013.09.034) [DOI] [Google Scholar]
- 12. Kaszuba M. McKnight D. Connah M.T. et al.: ‘Measuring sub nanometre sizes using dynamic light scattering’, J. Nanoparticle Res., 2008, 10, pp. 823 –829 (doi: 10.1007/s11051-007-9317-4) [DOI] [Google Scholar]
- 13. Umoren S.A. Obot I.B. Gasem Z.M.: ‘Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature’, J. Mater. Environ. Sci., 2008, 5, pp. 907 –914 [Google Scholar]
- 14. Javad B. Farideh N. Tayebe R. et al.: ‘Green synthesis of silver nanoparticles using Achillea biebersteinii flower extract and its anti‐angiogenic properties in the rat aortic ring model’, Molecules, 2014, 19, pp. 4624 –4634 (doi: 10.3390/molecules19044624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ajithaa B. Ashok K.R.Y. Sreedhara R.P.: ‘Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract’, Mater. Sci. Eng. C., 2015, 49, pp. 373 –381 (doi: 10.1016/j.msec.2015.01.035) [DOI] [PubMed] [Google Scholar]
- 16. Parameshwaran R. Kalaiselvam S. Jayavel R.: ‘Green synthesis of silver nanoparticles using Beta vulgaris: role of process conditions on size distribution and surface structure’, Mater. Chem. Phys., 2013, 140, pp. 135 –147 (doi: 10.1016/j.matchemphys.2013.03.012) [DOI] [Google Scholar]
- 17. Roopan S.M. Rohit M.G. Rahuman A.A. et al.: ‘Low‐cost and eco‐friendly phyto‐synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity’, Ind. Crops Prod., 2013, 43, pp. 631 –635 (doi: 10.1016/j.indcrop.2012.08.013) [DOI] [Google Scholar]
- 18. Anal K.J. Prasad K.: ‘Green synthesis of silver nanoparticles using Cycas leaf’, Int. J. Green Nanotechnol. Phys. Chem., 2010, 1, pp. 110 –117 (doi: 10.1080/19430871003684572) [DOI] [Google Scholar]
- 19. Akilandeshwari S. Sathiya C.K.: ‘Fabrication and characterization of silver nanoparticles using Delonix leaf broth’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2013, 128, pp. 337 –341 [DOI] [PubMed] [Google Scholar]
- 20. Rajkiran R.B. Veera B.N. Pratap R.K.: ‘Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X‐ray diffraction, electron microscopy and evaluation of bactericidal properties’, Saudi J. Biol. Sci., 2015, 22, pp. 637 –644 (doi: 10.1016/j.sjbs.2015.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magudapathy P. Gangopadhyay P. Panigrahi B.K. et al.: ‘Electrical transport studies of Ag nanoclusters embedded in glass matrix’, Physica B., 2001, 299, pp. 142 –146 (doi: 10.1016/S0921-4526(00)00580-9) [DOI] [Google Scholar]
- 22. Subbaiya R. Shiyamala M. Revathi K. et al.: ‘Biological synthesis of silver nanoparticles from Nerium oleander and its antibacterial and antioxidant property’, Int. J. Current Microbiol. Appl. Sci., 2014, 3, pp. 83 –87 [Google Scholar]
- 23. Abraham J. Logeswari P. Silambarasan S.: ‘Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties’, Sci. Iranica, 2012, 20, pp. 1049 –1054 [Google Scholar]
- 24. Bunghez I.R. Barbinta P.M.E. Badead N. et al.: ‘Antioxidant silver nanoparticles green synthesized using ornamental plants’, J. Optoelectron. Adv. Mater., 2012, 14, pp. 1016 –1022 [Google Scholar]
- 25. Nabavi S.M. Ebrahimzadeh M.A. Nabavi S.F. et al.: ‘In vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrus boissieriana growing in Iran’, Pharmacognosy Mag., 2009, 4, pp. 122 –126 [Google Scholar]


