Abstract
The aim of this study is to introduce natural‐based polymers, chitosan and starch, to design a remedial nanocomposite, comprising of cerium oxide nanoparticles and silver nanoparticles, to investigate their effects in accelerating wound healing and in wound microbial load. Cerium oxide nanoparticles synthesized in starch solution added to the colloidal dispersion of synthesized silver nanoparticles in chitosan to make a three‐component nanomaterial. Mice were anaesthetized and two parallel full‐thickness round wounds were excised under aseptic conditions with the help of sterile dermal biopsy punch. Furthermore, effects of silver‐chitosan and silver‐cerium‐chitosan nanocomposite had evaluated on rate of wound closure and collagen density and on microbial load of wound in full‐thickness model. Results showed that both silver chitosan and silver‐cerium‐chitosan had significant impact on acceleration of wound closure and collagen content and on reduction of wound microbial load in comparison with control group, which was, received no treatments. However, the silver‐cerium‐chitosan nanocomposite is more potent than silver‐chitosan group and control group in wound closure. The wound healing effects of silver‐cerium‐chitosan nanocomposite are due to unique features of its three components and this nanocomposite promises impressive remedies for clinical application.
Inspec keywords: wounds, nanocomposites, nanomedicine, nanoparticles, proteins, cerium, silver, polymers, colloids, patient treatment
Other keywords: biopolymer‐based nanocomposite wound dressing, wound healing properties, wound microbial load, natural‐based polymers, chitosan, remedial nanocomposite, cerium oxide nanoparticles, nanoceria, silver nanoparticles, starch solution, three‐component nanomaterial, synthesised silver nanoparticles, ketamine intraperitoneal injection, silver‐cerium‐chitosan nanocomposite, wound closure, collagen density, wound healing effects, wound care, aseptic conditions, sterile dermal biopsy punch, Ag‐Ce
1 Introduction
Wound healing is the result of a complex tissue repairing process involving several intracellular and intercellular pathways [1]. There are two main groups of wounds, ‘acute’ including mechanical, surgical, chemical and thermal wounds, and ‘chronic’ such as diabetic ulcers and bedsores [2, 3]. Traditional wound dressings including herbal products like crude extract of plants, or animal products such as fat or skin, have been developed and evolved during many years to form the modern wound dressings [4, 5, 6]. The novel generation of wound dressings not only show the ideal wound dressing properties [7, 8], but also have been precisely engineered to carry active pharmaceutical ingredients to the wound site. The modern dressings consist of natural and synthetic polymers such as hydrocolloids, hydrogels, silicone gels, alginates, chitosan and polyurethane films which are incorporated with active agents such as antimicrobial agents, growth factors and so on in order to accelerate the wound healing process [7].
Nanotechnology enables us to provide a nanoscale platform for manufacturing and fabrication materials with applications in medicine [9]. New compositions of herbal biopolymers and nanoparticles would be considered as smart wound dressings which not only provide the opportunities to mimic the lost natural intrinsic environment, but also significantly improve the wound healing condition by drug delivery system [10, 11, 12]. In this respect, with the use of nanoscale science and also biological materials, we decided to design a novel wound dressing with comprehensive features which has three components including silver (Ag) nanoparticles, cerium oxide (CeO) nanoparticles and chitosan. The wound healing potentials of each compound have been evaluated, however current investigation try to accumulate all the wound healing capacity of these three composition in one dressing to explore their additive effects. The current study exploited the chitosan as a basic polymer to form nanocomposite dressing. Chitosan (β ‐1, 4 link of glucosamine) as a derivative of chitin, is one of the most abundant natural polymers which possess the operative biological properties as forming material for establishment of wound dressings. Chitosan has shown healing potential in four overlapping phases of wound healing process (haemostasis, inflammation, proliferation, remodelling) and also have antimicrobial properties according to its intrinsic cationic polymeric structure [13, 14].
Despite all the beneficial healing properties of chitosan, this could be used as both reducing agent and capping agent in synthesis of Ag nanoparticles which could offer the enhanced quality of antimicrobial and healing effects [15, 16]. This method is affordable and environmentally friendly [17]. The antimicrobial healing properties of Ag nanoparticles are in the great attention for wound therapy [9, 18].
Existence of reactive oxygen species (ROS) are required in wound healing, particularly for angiogenesis, but extra amounts of generated ROS in oxidative stress has crucial role in the pathogenesis of chronic, non‐healing wounds [19]. However, the use of potent antioxidant agent could have beneficial effects in wound healing process. Cerium is one of the lanthanides or rare‐earth elements and its oxide form nanoparticles comprise of a cerium core inscribed by an oxygen lattice [20]. Studies have demonstrated the regeneration antioxidant features of CeO nanoparticles (nanoceria) under the oxidative stress. Different aspects of wound healing including wound closure, inflammation and angiogenesis could be positively influenced by applying nanoceria [21]. In this study, starch was applied as a stabiliser in formation process of nanoscale particles of CeO. Starch is one of the natural polymers of plant origin, and consists of amylose and amylopectin, which has a history of use as a skin substitute [22, 23].
The aim of this study is to design chitosan‐based nanocomposite comprising of nanoceria and Ag nanoparticles to evaluate the capability of this nanocomposite in accelerating wound healing and also reduction of wound microbial load.
2 Materials and methods
2.1 Synthesis of CeO nanoparticles
To synthesise CeO nanoparticle, 0.2 g of soluble starch powders was dissolved in 20 ml of distilled water and stirred for 10 min at 60°C to achieve a clear starch solution. Under stirring, the required amount of 0.5 M cerium nitrate solution was added to the starch solution, and allowed to further stir for 30 min. Then, ammonia solution (1 M) was added drop‐wise until the solution pH reached 10. The solution was stirred for one more hour. The yellow‐coloured final precipitate was centrifuged and washed several times with acetone and water to make it free from nitrate, ammonia and organic impurities and subsequently dried at 80°C for 12 h. The obtained sample was heat treated at 400°C for 2 h and characterised [24].
2.2 Synthesis of Ag nanoparticles in chitosan
For the preparation of Ag nanoparticles solution, we used the method of Mat Zain and colleagues with some modifications. However, 40 ml of AgNO3 solution (10 mM) was mixed with 40 ml of chitosan solution (3% w/v) and 4 ml of 10% (w/v) ascorbic acid solution. Then mixture was stirred 6 h at 60°C [17].
2.3 Ag–CeO nanocomposite
After the Ag nanoparticles synthesised in chitosan solution, the colloidal dispersion was stirred and meanwhile required amount of CeO nanoparticles gradually added to it, until the concentration of 1 µM of CeO nanoparticles achieved. This Ag–CeO nanocomposite was used for further investigations of this study.
2.4 Animals and wound creation
Adult male albino mice (25–35 g), between 6 and 8 weeks’ age, were obtained from Razi vaccine and serum research institute, Mashhad, Iran (n = 36). They were randomised into three groups (n = 12): (i) animals without any treatment hereafter referred to as control group, (ii) animals which were treated with Ag–citosan colloidal dispersion hereafter referred to as Ag group and (iii) animals which were treated with Ag–CeO–chitosan nanocomposite hereafter referred to as Ag–CeO group.
For wound creation, mice were anaesthetised using an intra‐peritoneal injection of ketamine 90 mg/kg (ketamine 10%, Alfasen, Woerden, Holland) with xylazine 10 mg/kg (xylezine 2%, Alfasen, Woerden, Holland). After depilation of dorsal hair, skin was disinfected with 70% ethanol. Then two parallel full‐thickness round wounds (4 mm in diameter) were excised under aseptic conditions with the help of sterile dermal biopsy punch (Paramount Surgimed LTD., New Delhi, India), on each side of the dorsal midline of each mouse [25]. In groups Ag and Ag–CeO wounds were treated every 24 h after wound creation. In this regard, sterile each swap was impregnated with dispersion of Ag or Ag–CeO, and then applied on each wound 20 times in clockwise manner. All of the experimental protocols were approved by the local institutional committee for animal ethics (approval number: P495/92489.304.83).
2.5 Wound closure assessment
The evaluation of wound closure was studied according to the method we have previously done [26]. Briefly, at certain time intervals (on day 0, 2, 4 and 6) images were taken with digital camera (powershot S2IS Canon; Canon Corp., Tokyo, Japan). Camera lens poses vertically to wounds and distance was 2 cm. These imaging conditions were fixed for all wounds. Then, the selected wound area of images was calculated with image processing software (Scion Image; Scion Corporation) [27]. The number of pixels in each wound area was calculated as area of the wound and used to measure the percentage of wound closure. Method of calculation of wound contraction was as follows:
2.6 Evaluation of collagen density
Collagen fibre density was evaluated by microscopic imaging of six random sections, which were stained with Masson's trichrome [26]. This is a special staining for collagen fibres that makes their colour blue. Then, based on the intensity of blue colour, scaling was numbered as follows; 1: rare, 2: minimal, 3: moderate, 4: marked, 5: high marked.
2.7 Evaluation of wounds microbial load
For testing the effect of treatments on wound microbial load, wound microbiology analysis has been done. Accordingly, on days 2, 4 and 6 post injury, samples were taken from the wound area with sterile swab, then cultured on blood agar medium (Quelab, Montreal, Quebec, Canada) and incubated for 24 h at 37°C. After 24 h numbers of colonies (colony forming units or CFU) were counted [25].
2.8 Statistical analysis
One‐way analysis of variance was used to analyse percentage of wound closure, collagen density and numbers of CFU. The level of significance was set at P < 0.05. Statistical analyses were performed by using the SPSS for Windows software (SPSS Inc., USA).
3 Results
3.1 Characterisation of CeO nanoparticle
Fig. 1 shows the transmission electron microscopy (TEM) image of CeO nanoparticles. The picture indicates that the nanoparticles are small in size and uniform in shape.
Fig. 1.
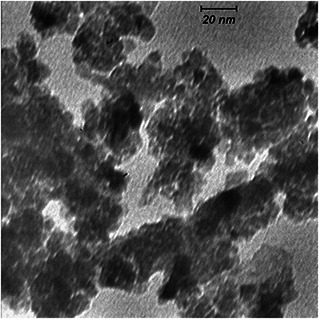
TEM image of CeO nanoparticles[AQ6]
3.2 Characterisation of Ag nanoparticle
Fig. 2 illustrates the TEM micrograph of Ag nanoparticles that synthesised in chitosan solution. The monodispersity of spherical Ag nanoparticles apparently can be distinguished in the TEM image.
Fig. 2.
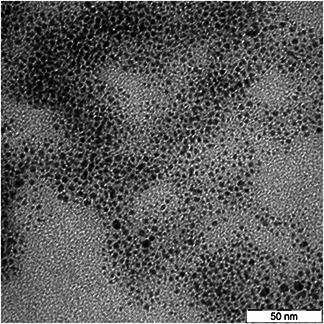
TEM micrograph of Ag nanoparticles that synthesised in chitosan solution
3.3 Wound closure evaluation
Fig. 3 shows the percentage of wound closure, calculated from comparing open wound area with original wound size. On day 2, there was significant difference between Ag–CeO group and control group; thus Ag–CeO has better impact on wound closure. However, on the fourth and sixth days post injury, the differences in wound contraction were increased in both Ag and Ag–CeO groups compared with control (see Fig. 3).
Fig. 3.
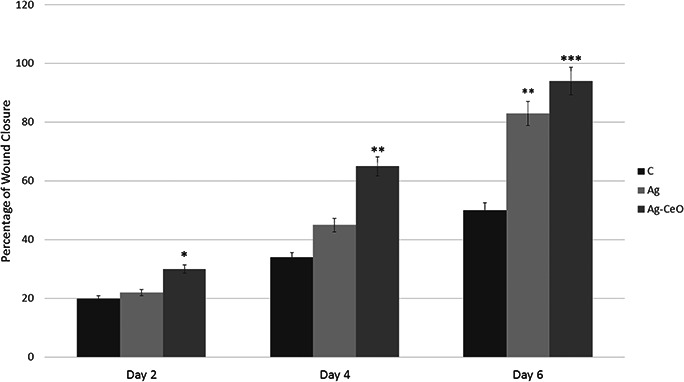
Percentage of wound closure, calculated from comparing open wound area with original wound size C: Control group, Ag: Ag group, Ag–CeO: Ag–CeO2 group. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with control group
3.4 Evaluation of collagen density
Masson's trichrome staining results in collagen fibre colour turn into blue. However, the scaling of the blue colour intensity was used to evaluate the acceleration of wound healing. Figs. 4 and 5 show the collagen content of wound sites. As seen in Fig. 4, on days 2, 4 and 6 amount of collagen significantly increased in Ag–CeO and Ag groups compared with the control group.
Fig. 4.
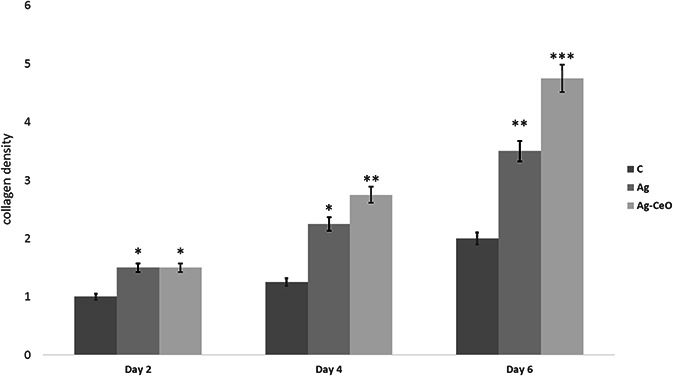
Treatment with Ag–CeO2 and Ag groups can significantly increase collagen content in comparison with control group C: Control group, Ag: Ag group, Ag–CeO: Ag–CeO2 group. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with control group.
Fig. 5.
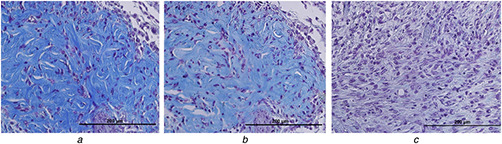
Collagen content of wound sites (A), (B) and (C) show the wounds sections of the Ag–CeO2, Ag and Control groups, respectively
3.5 Evaluation of wound microbial load
Examination of wounds microbiology showed significant effects of both Ag and Ag–CeO2 in decreasing the bacterial load; on days 2, 4 and 6 post injury, both Ag and Ag–CeO2 groups decreased wound bacterial load, and numbers of colonies in control group were noticeably high (see Figs. 6 and 7).
Fig. 6.
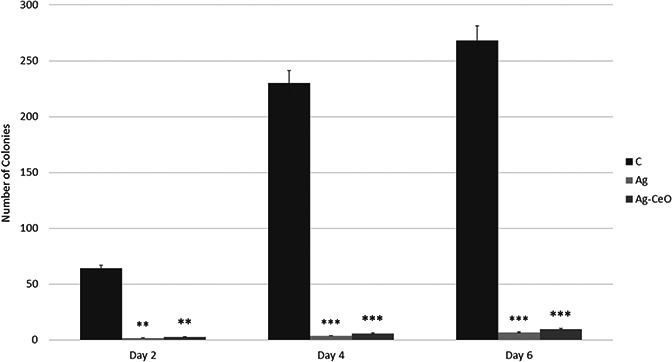
Examination of wounds microbiology showed significant effects of both Ag and Ag–CeO2 in decreasing the bacterial load. C: Control group, Ag: Ag group, Ag–CeO: Ag–CeO2 group. Ag and Control groups, respectively. ** p < 0.01, *** p < 0.001 compared with control group
Fig. 7.

Effects of treatments on wound microbial load. (A), (B) and (C) show the Number of colonies of the Ag–CeO2, Ag and Control groups on day 6, respectively
4 Discussion
Combination of nanotechnology into medicine has exploited great potentials of nanoparticles in many aspects of medicine including, wound healing process. Among these nanoparticles Ag nanoparticle, CeO2 nanoparticles have shown wound healing and antimicrobial properties. Having the potential as blood coagulants and blocking nerve endings which reduce pain, chitosan has shown great potential treatment of wounds [28]. Here the wound healing properties of Ag–CeO2 nanocomposite dispersion in chitosan, as well as its potential in reduction of wound microbial load was evaluated.
Three properties of CeO2 nanoparticle make it good potential candidate in wound healing. These features included antioxidant and anti‐inflammatory activities, as well as its pro‐angiogenic properties. Many studies have indicated the antioxidant activity of CeO2 nanoparticles and reported its potency to scavenge superoxide radical [29, 30], hydrogen peroxide [31], hydroxyl radical [32] and nitric oxide radical [33]. Suzanne et al. (2009) have shown the potential of CeO2 nanoparticles to reduce ROS production in inflammatory conditions and suggesting its application in chronic inflammation therapy [34]. Furthermore, in vitro and in vivo studies have shown the ability of CeO2 nanoparticles to induce angiogenesis [35]. However, Chigurupati et al. (2013) have demonstrated the wound healing properties of CeO2 nanoparticles, in which the proliferation and migration of keratinocytes and fibroblasts, and induction of angiogenesis by endothelial cells were increased [20]. However, this study showed the concentration of 1 µM or more have the ability to direct these properties; hence, we choose concentration of 1 µM here. Our findings show the healing effects of Ag–CeO2 in acceleration of wound closure or re‐epithelialisation which is proportional to keratinocytes migration and proliferation [36].
The process of wound repair depends on collagen which produced by fibroblast cells. Collagen fibres provide the integrity of wound site by forming cross‐linkages between fibres. Many studies have shown the key role of collagen content on acceleration of wound tensile strength and cell migration [25]. Our results showed that treatment with Ag–CeO2 and Ag groups can significantly increase collagen content in comparison with control group (see Fig. 4).
Wound bacterial infection can significantly influence wound healing process, so prevention of bacterial growth on wound site is an essential prerequisite [37]. Ag nanoparticles, on one hand, have antibacterial effects against wide spectrum of bacteria that considerably reduce the chances of developing resistance [9]. This is the favourable property in management of wound healing process, in which wound environment makes bacteria to grow and proliferate, and particularly develop wound infection [38]. On the other hand, Ag nanoparticles decrease matrix metalloprotease activity, cytokine‐related inflammation and negatively regulate TGF‐β, hence directly incorporates in wound healing process [39]. Furthermore, Ag nanoparticles involve in migration of keratinocyte and differentiation of fibroblast into myofibroblast [40]. Our results were consistent with these studies, since Ag group shows the potency in wound closure (see Fig. 3), not as much as Ag–CeO2 group, but the capacity of Ag group in reduction of wound microbial load was little more than Ag–CeO2 group (see Figs. 6 and 7). The Ag antibacterial mechanism of action is due to release of Ag ions which interact with thiol groups of critical enzymes and inactive them. This action eventually leads to production of ROS which have known deleterious effects on cell viability [41]. It could be suggested that the difference between Ag group and Ag–CeO2 groups is due to the above mentioned ability of CeO2 in scavenging ROS, thus intervening in Ag mechanism of action. However, CeO2 nanoparticles has antibacterial properties per se [42]. Nevertheless, further analysis should perform about intervention of Ag and CeO2 nanoparticles in the context of antimicrobial properties.
Here, we chose chitosan due to its ability to stabilise colloidal Ag nanoparticles [43] and also to promote wound healing activity [13]. Archana et al. (2013) [44] evaluated the wound healing and antimicrobial properties of chitosan nanodressing both in vitro and in vivo; the result of the study indicated the antimicrobial and wound healing potentials of chitosan which has positive effects on promoting wound closure.
Finally, in the both contexts of wound closure and wound microbial load that evaluated here, our findings imply the acceleration of wound healing process. However, the wound healing effects of this nanocomposite is due to unique features of its three components, namely CeO2 nanoparticles, Ag nanoparticles and chitosan. Due to the healing and antimicrobial properties of Ag–CeO2 nanocomposite that introduced here, more studies are needed in the field of clinical use.
5 Acknowledgment
This work was supported by Islamic Azad University, Mashhad Branch, Mashhad, Iran, as a research project and therefore, is appreciated by the authors.
6 References
- 1. Gurtner G.C. Werner S. Barrandon Y. et al.: ‘Wound repair and regeneration’, Nature, 2008, 453, (7193), pp. 314 –321 [DOI] [PubMed] [Google Scholar]
- 2. Ferreira M.C. Tuma P. Júnior Carvalho V.F. et al.: ‘Complex wounds’, Clinics (Sao Paulo, Brazil), 2006, 61, pp. 571 –578 [DOI] [PubMed] [Google Scholar]
- 3. Percival N.J.: ‘Classification of wounds and their management’, Surgery – Oxford Int Edit., 20, (5), pp. 114 –117 [Google Scholar]
- 4. Kudi A.C. Umoh J.U. Eduvie L.O. et al.: ‘Screening of some Nigerian medicinal plants for antibacterial activity’, J. Ethnopharmacol., 1999, 67, (2), pp. 225 –228 [DOI] [PubMed] [Google Scholar]
- 5. Inngjerdingen K. Nergard C.S. Diallo D. et al.: ‘An ethnopharmacological survey of plants used for wound healing in Dogonland, Mali, West Africa’, J. Ethnopharmacol., 2004, 92, (2‐3), pp. 233 –244 [DOI] [PubMed] [Google Scholar]
- 6. Mensah A.Y. Houghton P.J. Dickson R.A. et al.: ‘In vitro evaluation of effects of two Ghanaian plants relevant to wound healing’, Phytother. Res, PTR, 2006, 20, (11), pp. 941 –944 [DOI] [PubMed] [Google Scholar]
- 7. Boateng J.S. Matthews K.H. Stevens H.N. et al.: ‘Wound healing dressings and drug delivery systems: a review’, J. Pharm. Sci., 2008, 97, (8), pp. 2892 –2923 [DOI] [PubMed] [Google Scholar]
- 8. Gaikwad V.V. Patil A.B. Gaikwad M.V.: ‘Scaffolds for drug delivery in tissue engineering’, Int. J. Pharm. Sci. Nanotechnol., 2008, 1, pp. 113 –122 [Google Scholar]
- 9. Tocco I. Zavan B. Bassetto F. et al.: ‘Nanotechnology‐based therapies for skin wound regeneration’, J. Nanomater., 2012, 2012, p. 4, 11 [Google Scholar]
- 10. Mayet N. Choonara Y.E. Kumar P. et al.: ‘A comprehensive review of advanced biopolymeric wound healing systems’, J. Pharm. Sci., 2014, 103, (8), pp. 2211 –2230 [DOI] [PubMed] [Google Scholar]
- 11. Ding F. Deng H. Du Y. et al.: ‘Emerging chitin and chitosan nanofibrous materials for biomedical applications’, Nanoscale, 2014, 6, (16), pp. 9477 –9493 [DOI] [PubMed] [Google Scholar]
- 12. Virlan M. Miricescu D. Radulescu R. et al.: ‘Organic nanomaterials and their applications in the treatment of oral diseases’, Molecules, 2016, 21, (2), p. 207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai T. Tanaka M. Huang Y.Y. et al.: ‘Chitosan preparations for wounds and burns: antimicrobial and wound‐healing effects’, Expert Rev. Anti‐Infect. Ther., 2011, 9, (7), pp. 857 –879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomas V. Yallapu M.M. Sreedhar B. et al.: ‘Fabrication, characterization of chitosan/nanosilver film and its potential antibacterial application’, J. Biomater. Sci. Polym. Ed., 2009, 20, (14), pp. 2129 –2144 [DOI] [PubMed] [Google Scholar]
- 15. Gopal A. Kant V. Gopalakrishnan A. et al.: ‘Chitosan‐based copper nanocomposite accelerates healing in excision wound model in rats’, Eur. J. Pharmacol., 2014, 731, pp. 8 –19 [DOI] [PubMed] [Google Scholar]
- 16. Anisha B. Biswas R. Chennazhi K. et al.: ‘Chitosan–hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds’, Int. J. Biol. Macromol., 2013, 62, pp. 310 –320 [DOI] [PubMed] [Google Scholar]
- 17. Zain N.M. Stapley A.G.F. Shama G.: ‘Green synthesis of silver and copper nanoparticles using ascorbic acid and chitosan for antimicrobial applications’, Carbohydr. Polym., 2014, 112, pp. 195 –202 [DOI] [PubMed] [Google Scholar]
- 18. Kaler A. Mittal A.K. Katariya M. et al.: ‘An investigation of in vivo wound healing activity of biologically synthesized silver nanoparticles’, J. Nanopart. Res., 2014, 16, (9), pp. 1 –10 [Google Scholar]
- 19. Schäfer M. Werner S.: ‘Oxidative stress in normal and impaired wound repair’, Pharmacol. Res., 2008, 58, (2), pp. 165 –171 [DOI] [PubMed] [Google Scholar]
- 20. Celardo I. Pedersen J.Z. Traversa E. et al.: ‘Pharmacological potential of cerium oxide nanoparticles’, Nanoscale, 2011, 3, (4), pp. 1411 –1420 [DOI] [PubMed] [Google Scholar]
- 21. Chigurupati S. Mughal M.R. Okun E. et al.: ‘Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing’, Biomaterials, 2013, 34, (9), pp. 2194 –2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weinstein‐Oppenheimer C.R. Aceituno A.R. Brown D.I. et al.: ‘The effect of an autologous cellular gel‐matrix integrated implant system on wound healing’, J. Transl. Med., 2010, 8, p. 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shaik S. Kummara M.R. Poluru S. et al.: ‘A green approach to synthesize silver nanoparticles in starch‐co‐poly(acrylamide) hydrogels by tridax procumbens leaf extract and their antibacterial activity’, Int. J. Carbohydr. Chem., 2013, 2013, p. 10 [Google Scholar]
- 24. Darroudi M. Sarani M. Kazemi Oskuee R. et al.: ‘Green synthesis and evaluation of metabolic activity of starch mediated nanoceria’, Ceram. Int., 2014, 40, (1, Part B), pp. 2041 –2045 [Google Scholar]
- 25. Rezazade Bazaz M. Mashreghi M. Mahdavi Shahri N. et al.: ‘Pharmaceutical application of frog skin on full‐thickness skin wound healing in mice’, Pharm. Biol., 2013, 51, (12), pp. 1600 –1606 [DOI] [PubMed] [Google Scholar]
- 26. Mashreghi M. Rezazade Bazaz M. Mahdavi Shahri N. et al.: ‘Topical effects of frog ‘Rana ridibunda’ skin secretions on wound healing and reduction of wound microbial load’, J. Ethnopharmacol., 2013, 145, (3), pp. 793 –797 [DOI] [PubMed] [Google Scholar]
- 27. Shimizu N. Ishida D. Yamamoto A. et al.: ‘Development of a functional wound dressing composed of hyaluronic acid spongy sheet containing bioactive components: evaluation of wound healing potential in animal tests’, J. Biomater. Sci. Polym. Ed., 2014, 25, (12), pp. 1278 –1291 [DOI] [PubMed] [Google Scholar]
- 28. Jayakumar R. Prabaharan M. Kumar P.S. et al.: ‘Biomaterials based on chitin and chitosan in wound dressing applications’, Biotechnol. Adv., 2011, 29, (3), pp. 322 –337 [DOI] [PubMed] [Google Scholar]
- 29. Karakoti A. Singh S. Dowding J.M. et al.: ‘Self WT. redox‐active radical scavenging nanomaterials’, Chem. Soc. Rev., 2010, 39, (11), pp. 4422 –4432 [DOI] [PubMed] [Google Scholar]
- 30. Korsvik C. Patil S. Seal S. et al.: ‘Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles’, Chem. Commun., 2007, (10), pp. 1056 –1058 [DOI] [PubMed] [Google Scholar]
- 31. Pirmohamed T. Dowding J.M. Singh S. et al.: ‘Nanoceria exhibit redox state‐dependent catalase mimetic activity’, Chem. Commun., 2010, 46, (16), pp. 2736 –2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xue Y. Luan Q. Yang D. et al.: ‘Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles’, J. Phys. Chem. C, 2011, 115, (11), pp. 4433 –4438 [Google Scholar]
- 33. Dowding J.M. Dosani T. Kumar A. et al.: ‘Cerium oxide nanoparticles scavenge nitric oxide radical (NO)’, Chem. Commun., 2012, 48, (40), pp. 4896 –4898 [DOI] [PubMed] [Google Scholar]
- 34. Hirst S.M. Karakoti A.S. Tyler R.D. et al.: ‘Anti‐inflammatory properties of cerium oxide nanoparticles’, Small, 2009, 5, (24), pp. 2848 –2856 [DOI] [PubMed] [Google Scholar]
- 35. Das S. Singh S. Dowding J.M. et al.: ‘The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments’, Biomaterials, 2012, 33, (31), pp. 7746 –7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raja S.K. Garcia M.S. Isseroff RR.: ‘Wound re‐epithelialization: modulating keratinocyte migration in wound healing’, Front. Biosci, 2007, 12, pp. 2849 –2868 [DOI] [PubMed] [Google Scholar]
- 37. Woo C.H. Choi Y.C. Choi J.S. et al.: ‘A bilayer composite composed of TiO2 ‐incorporated electrospun chitosan membrane and human extracellular matrix sheet as a wound dressing’, J. Biomater. Sci. Polym. Ed., 2015, 26, (13), pp. 841 –854 [DOI] [PubMed] [Google Scholar]
- 38. Stojadinovic A. Carlson J.W. Schultz G.S. et al.: ‘Topical advances in wound care’, Gynecologic Oncol., 2008, 111, (2, Supplement), pp. S70 –S80 [DOI] [PubMed] [Google Scholar]
- 39. Widgerow A.D.: ‘Nanocrystalline silver, gelatinases and the clinical implications’, Burns, 2010, 36, (7), pp. 965 –974 [DOI] [PubMed] [Google Scholar]
- 40. Liu X. Lee P.Y. Ho C.M. et al.: ‘Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing’, Chem. Med. Chem., 2010, 5, (3), pp. 468 –475 [DOI] [PubMed] [Google Scholar]
- 41. Prabhu S. Poulose E.K.: ‘Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects’, Int. Nano Lett., 2012, 2, (1), pp. 1 –10 [Google Scholar]
- 42. Kuang Y. He X. Zhang Z. et al.: ‘Comparison study on the antibacterial activity of nano‐ or bulk‐cerium oxide’, J. Nanosci. Nanotechnol., 2011, 11, (5), pp. 4103 –4108 [DOI] [PubMed] [Google Scholar]
- 43. de Lima C.A. da Silva P.S. Spinelli A.: ‘Chitosan‐stabilized silver nanoparticles for voltammetric detection of nitrocompounds’, Sens. Actuators B, Chem., 2014, 196, pp. 39 –45 [Google Scholar]
- 44. Archana D. Dutta J. Dutta P.K.: ‘Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies’, Int. J. Biol. Macromol., 2013, 57, pp. 193 –203 [DOI] [PubMed] [Google Scholar]


