Abstract
With the progression of nanotechnology, the use of nanoparticles (NPs) in consumer products has increased dramatically and green synthesis is one of the cheapest and eco‐friendly methods to obtain non‐hazardous NPs. In the current research zinc (Zn) NPs synthesis was carried out by using the fresh and healthy leaves of Mentha arvensis L. followed by characterisation through ultraviolet (UV)–visible spectroscopy, X‐ray diffraction (XRD) and scanning electron microscopy (SEM). UV–visible spectroscopy confirmed the green synthesis of ZnNPs, while XRD confirmed the size of NPs, which was 30–70 nm. SEM shows that the shape of ZnNPs was irregular. The effects of green synthesised NPs on two different varieties of Brassica napus were evaluated. Exposure to ZnNPs (5, 15, and 25 mg/l−1) caused a significant increase in root and shoot length of B. napus. The application of NPs significantly improved plant germination and triggered the production of secondary metabolite and antioxidant enzymes. ZnNPs showed a significant increase in chlorophyll, superoxide dismutase, total flavonoid content (TFC) and antioxidant enzymes while total phenolic content was decreased when TFC increased. Thus, it has been concluded from the current study that ZnNPs may possibly trigger the production of antioxidant enzymes and various biochemical compounds.
Inspec keywords: zinc, nanoparticles, nanofabrication, ultraviolet spectra, visible spectra, X‐ray diffraction, scanning electron microscopy, particle size, enzymes, molecular biophysics, biochemistry, nanobiotechnology, botany
Other keywords: biochemical profiling, Brassica napus, biosynthesised zinc nanoparticles, nanotechnology, Mentha arvensis L, ultraviolet‐visible spectroscopy, X‐ray diffraction, Zn, biochemical compounds, total phenolic content, total flavonoid content, superoxide dismutase, chlorophyll, antioxidant enzymes, secondary metabolite, plant germination, green synthesis, SEM, scanning electron microscopy, XRD
1 Introduction
Nanotechnology is regarded as 21st century sciences, owing to its tremendous applications in biology, physics, chemistry, and various other fields [1]. Nanotechnology has tremendous potential to boost the agriculture with innovative tools as well as perk up the plants potential to uptake essential nutrients. Nowadays, the application of nanoparticles (NPs) in agriculture for the increasing crop quality and for increased growth and disease control in plants is becoming a focal point for scientists. Numerous studies highlighted the biological effects of NPs on higher plants and the research and development in this regard is increasing day‐by‐day.
Rapeseed (Brassica napus L.) belonging to family Brassicaceae is the potential source of vegetable oils. Rapeseed meal encompassing significant available natural resource is regarded as a by‐product of the oil extraction process. Since [2] studied the protein of the rapeseed meal and reported it as a source of novel peptides possessing ACE‐inhibitory activity, it emerges to be a chief source of many other bioactive compounds such as polyphenols, tocopherols and phytosterols all these compounds play a role in the prevention as well as treatment of several diseases [3]. Massive research has been performed to elucidate the important constituents as sources of natural antioxidants from rapeseed meal. To date, many phytochemical studies revealed the extraction of several phenolic compounds and peptides hydrolysed from rapeseed oil and protein [4, 5].
Brassica species are stated to acquire tremendous cancer curing properties [6] and these properties are owing to the presence of glucosinolate as well as their derivative products [7]. The presence of flavonoids and many other phenolics also play a significant role in anticancer properties [8]. While fundamentally temperate, Brassica oleracea varieties are now cultivated in entire regions all over the world [9].
Research evidence reveals that the utilisation of fruits and vegetables is, overall, quite beneficial to health because of the significant protection offered by the antioxidant compounds which are chiefly and naturally found in them [10]. Indeed, the presence of several phytochemicals, in addition to vitamins and pro‐vitamins, has been regarded as an immense nutritional concern in the prevention of many chronic diseases, for instance, cancer, diabetes mellitus, arteriosclerosis, nephritis, ischemic, rheumatism, and cardiovascular diseases. These compounds also possess anti‐aging properties, in which oxidants or free radicals take part [11, 12].
Now, it became a centre point for researchers to exploit the effects of nanomaterials on the biosynthesis of economically as well as commercially viable secondary metabolites in medicinal plant species [13]. The application of NPs in soil led to an increase in the shoot/root ratio in different plants. These nanomaterials do not directly affect plant growth, but they show their impacts on the mechanisms that lead to change in the plant growth and development [14].
The chemical composition, as well as surface, shape, and size of NPs, play a crucial role in the toxic effects of metal NPs on plant growth and development [15]. Furthermore, the toxic levels of NPs increase the production of reactive oxygen species (ROS) and free hydroxyl radicals that cause huge damage to the cell membranes leading to change in the permeability of plasma membrane. Therefore, the entrance of NPs into different plant cells become quite easier and stress persuaded by these NPs leads to the production of secondary metabolites [16].
The current study has been designed to investigate the effect of green synthesised zinc (Zn) NP on B. napus L. According to the best of our knowledge, this is the first report from Pakistan that the ZnNPs effect has been investigated on B. napus L. plant.
2 Material methods
2.1 Collection of plant materials
For the synthesis of ZnNPs, fresh leaves of Mentha arvensis L. plants were used as reducing and capping agents. Leaves were air dried under aseptic conditions for 10 days after that the plant materials were ground into fine powder. About 10 g of powdered plant material was subjected to 100 ml of deionised water followed by shaking for 24 h. The resultant solution was filtered twice with Whatman 21 filter paper. The filtrate was kept in a refrigerator at 4°C for further use [17].
2.2 Synthesis of ZnNPs
Synthesis of ZnNPs was carried out by using the M. arvensis L. extract and zinc nitrate hexahydrate (ZnNO3 ·6H2 O) salt. About 1 mM solution of zinc nitrate salt was prepared in deionised water. The solution was boiled on a hotplate and continuously stirred while adding plant extract until the colour changed to light yellow. The solution was centrifuged at 12,000 rpm for 15 min. The supernatant was discarded, and the lower pellet was again suspended in deionised water followed by centrifugation for 5 min. The process was repeated three times to remove impurities any present [18].
2.2.1 Characterisation of ZnNPs
The synthesised ZnNPs were characterised by ultraviolet (UV)–visible spectroscopy, X‐ray diffraction (XRD) and scanning electron microscopy (SEM).
2.3 Plant cultivation
Seeds of B. napus (Faisal canola and Shiralee varieties) were obtained from the National Agriculture Research Center, Islamabad, Pakistan. The seeds were sterilised for 24 h with hypercaloric acid following the methods proposed by Kouhi et al. [19]. After sterilisation, seeds were cultured on Petri plates having different concentrations of green synthesised ZnNPs (5, 15, and 25 mg/l−1).
2.3.1 Germination parameter
Germination percentage: Germination percentage of seedling of B. napus was recorded after 7 days by using the method of Abdul‐Baki and Anderson [20].
2.3.2 Shoot and root length
Root and shoot length of seedling was recorded from the date of germination in cm.
2.3.3 Seedling vigour index (SVI)
SVI was recorded by using the method of Abdul‐Baki and Anderson [20] and expressed in terms of means and standard deviation (SD)
2.4 Biochemical and physiological attributes
2.4.1 Determination of total chlorophyll content (TCC)
The determination of the TCC was done by taking 0.2 g plant tissue that was briefly ground in cold 80% acetone solution and the absorbance was measured at 652 nm using the method proposed by Arnon [21].
2.4.2 Determination of total sugar content (TSC)
For the determination of the TSC, the method proposed by Dubois et al. [22] was followed. About 0.2 g fresh leaves were ground in 80% ethanol solution. The mixture was kept in the water bath for 1 h at 80°C. About 0.5 ml of the mixture, followed by addition of 1 ml of 18% of phenolic solution and mixture was kept for one hour at room temperature. After the addition of 2 ml of sulphuric acid, the mixture was constantly shacked and absorbance was noted at 490 nm.
2.4.3 Determination of total phenolic content (TPC)
The determination of TPC was carried out following the protocol proposed by Veilaogu et al. [23]; the folin–ciocalteu reagent was used for quantification. About 0.2 g powdered plant material was crushed with 80% ethanol and then 100 μl plant extract was mixed with 0.75 ml of folin–ciocalteu reagent followed by incubation at 21°C for 10 min. The mixture was equipped with 0.75 ml of sodium bicarbonate solution and kept for 90 min at 21°C followed by absorbance at 725 nm by using a Cecil UV–visible spectrophotometer.
2.4.4 Determination of total flavonoid content (TFC)
Quantification of TFC was carried out by using the protocol proposed by Chang et al. [24]; about 10 mg of quercetin was dissolved in 80% ethanol followed by further dilution. Dilution involved mixing with 1.5 ml of ethanol, 1 M CH3 CO2 K, 2.5 ml of water and 0.2 ml Al2 Cl3. The reaction mixture was kept at room temperature for 60 min. Using a Cecil UV–visible spectrophotometer, the absorbance of the mixture was recorded by setting wavelength at 415 nm.
2.4.5 Determination of soluble protein content (SPC)
For the determination of the SPC, about 0.5 g of fresh leaves were ground in phosphate buffer with the help of pestle mortar. About 0.5 ml of the above extract was mixed with 0.5 ml of distilled water and 3 ml of bio red dye. The homogenised mixture was shaken well and then the absorbance of the sample was observed at 650 nm by using the Cecil 2021 spectrophotometer made in Japan [25].
2.4.6 Superoxide dismutase (SOD) activity
For determination of SOD, 10 ml sodium phosphate buffer was used to grind 0.5 g leaf material. After settling down of the solution, a separate set of test tubes containing 0.1 ml of the extract was supplemented with 0.1 ml riboflavin and 3 ml of phosphate buffer. This reaction mixture was placed under the fluorescent lamp for 8 min to start the reaction. The same reaction mixture was prepared for dark reaction in another set of test tubes. The absorbance of both sets was recorded at 560 nm wavelength [26]
2.5 Statistical analysis
The whole experiment was performed in triplicate. Results are presented as mean ± SD. A significant difference (at P ≤ 0.05) and means comparison were done using one‐way analysis of variance. Statistical analysis was carried out by using SPSS 20 software.
3 Result and discussion
UV–visible spectroscopy of green synthesised ZnNPs confirmed the synthesis of ZnNPs and absorption spectra were 220–450 nm already published in [27] (Fig. 1). The plant extract may contain possible reducing compounds which triggered the synthesis of NPs [28]. After confirmation by UV–visible spectroscopy, the NPs were calcined and dried in the electric furnace at 500°C for 24 h to remove oxygen and pure ZnNPs were obtained in the form of fine powder. ZnNPs were then subjected to XRD at the Department of Chemistry, Quaid‐e‐Azam University, Islamabad, Pakistan. The XRD pattern showed that ZnNPs are irregularly shaped and crystalline in nature [29]. SEM of ZnNPs was carried out at the Institute of Space Technology Islamabad, Pakistan. Technology by using SIGMA model (MIRA3; TESCAN Brno). The SEM picture confirmed the size in the range of 30–70 nm and morphology of the synthesised ZnNPs was non‐spherical, irregular in shape previously reported by [30].
Fig. 1.

Characterisation of ZnNPs
(a) UV–visible scheme, (b) SEM analysis of green synthesised ZnNPs, (c) XRD of green synthesised ZnNPs
3.1 Germination percentage of B. napus seedlings
In the current study, germination percentage was recorded after 7 days of exposure to different concentrations of 5, 15 and 25 mg/l−1 ZnNPs. Both the varieties showed significantly higher germination percentage than control variety as shown in Fig. 1. It can be attributed that the maximum germination was recorded at 15 mg/l−1 of ZnNPs in both V1 and V2 followed by 25 mg/l−1 of ZnNPs as compared with control in a dose‐dependent manner. Other researchers have already reported that ZnNPs enhanced germination percentage in wheat crop [31] (Fig. 2).
Fig. 2.

Germination percentage of B. napus varieties after exposure to green synthesised ZnNPs
3.2 Root length, shoot length and SVI
The effect of green synthesised ZnNPs on root and shoot of B. napus (Faisal canola and Shiralee) was recorded after an interval of 7 days. The highly varied trend was observed on different concentrations of ZnNPs (5, 15 and 25 mg/l−1). The maximum root and shoot length was observed at 15 mg/l−1 of ZnNPs in V1 (2.99 cm) and (8.3 cm), respectively. It has been observed that ZnNPs can stimulate the growth of root and shoot significantly on augmentation. Our results are in conformity with [32], which previously reported the effect of NP on root and shoot length of the Artemisia plant. Maximum seedling vigour index was observed in both varieties of B. napus V1 (1106.42 ± 3.6) and V2 791.04 ± 7.1 as compared to control shown in Table 1. Shah and Belozerova also reported an increase in root and shoot growth of Lactuca seeds under ZnNP applications [33].
Table 1.
Effect of green synthesised ZnNPs on germination parameters of B. napus
| Varieties | Treatment of Zn NPs | Germination, % | Germination index | Seedling vigour index | Shoot length | Root length |
|---|---|---|---|---|---|---|
| Faisal canola (V1) | Control | 60 ± 1.5 | 19.78 ± 0.9 | 440.0 ± 5.9 | 5.8 ± 0.6 | 1.54 ± 0.8 |
| 5 mg/l−1 | 62 ± 0.9 | 18.0 ± 0.2 | 612.56 ± 6.8 | 7.9 ± 0.8 | 1.98 ± 0.5 | |
| 15 mg/l−1 | 98 ± 1.4 | 20.56 ± 1.2 | 1106.42 ± 3.6 | 8.3 ± 0.5 | 2.99 ± 1.8 | |
| 25 mg/l−1 | 88 ± 0.4 | 17.98 ± 0.2 | 766.48 ± 2.9 | 6.7 ± 0.5 | 2.01 ± 1.3 | |
| Shiralee (V2) | Control | 65 ± 0.9 | 18.12 ± 1.5 | 403.00 ± 3.2 | 4.3 ± 0.09 | 1.90 ± 0.5 |
| 5 mg/l−1 | 60 ± 0.8 | 17.45 ± 1.2 | 438.00 ± 5.1 | 5.7 ± 0.7 | 1.6 ± 0.1 | |
| 15 mg/l−1 | 96 ± 0.7 | 19.67 ± 0.9 | 791.04 ± 7.1 | 6.2 ± 0.2 | 2.04 ± 0.4 | |
| 25 mg/l−1 | 80 ± 0.1 | 17.67 ± 0.4 | 583.2 ± 4.9 | 5.3 ± 0.1 | 1.99 ± 1.3 |
The data are presented in the form of means and SD, while the P value was kept >0.05.
Nanoscale Zn showed large root growth of seedling compared to bulk ZnSO4 and control. Such a promotory effect of nanoscale SiO2 and TiO2 on germination was also reported in soya bean [34]. Germination means breaking of seed testa and emergence of seedling up to 1–8 mm [35]. A significant increase was observed in the root and shoot of the wheat plant by applying the different concentration of ZnNPs [31].
3.3 Chlorophyll and sugar content
The chlorophyll content is more important to plant species and play a vital role in photosynthesis. A significant increase was observed in both the tested varieties. In the current study, the high content of total chlorophyll was recorded at 15 mg/l of ZnNPs in V1 (Faizal canola) as compared to V2 (Shiralee) variety, respectively. Overall, in all concentrations of ZnNPs both the varieties showed a significant increase in the chlorophyll content as compared to control. Our results are in agreement with [36], which reported the increase in the TPC in the leaves of Soybean variety. Raliya et al. [37] also reported that ZnNPs increase 63% of the chlorophyll content in the leaves of tomato as compared to control plant. It was observed that the sugar content of both varieties in response to the different concentrations of ZnNPs showed reliable results as compared to control after 7 and 14 days of seedling while after 21 days of seedling no significant increase was observed on any concentration of ZnNPs. The highest sugar content was observed at 25 mg/l−1 of ZnNPs in V1 (Faisal Canola) as compared with V2 (Shiralee) which was (3.9 µg/ml fresh weight (FW)) and (2.99 µg/ml FW) respectively. The role of green synthesised ZnNPs is widely reported in triggering plant biochemical responses [38] (Fig. 3).
Fig. 3.

Total chlorophyll and sugar content in B. napus varieties in response to green synthesised ZnNPs
3.4 TPC and TFC
After the introduction of green synthesised ZnNP into seedling of B. napus varieties, the data was recorded at different days interval, a significant increase was seen in the TPC of B. napus varieties. The maximum TPC content calculated was (12.97 µg/mg dry weight (DW)) after an interval of 14 days and great variation has been seen in both varieties at different days interval. The result of my study is in comparison with [18], which already reported TPC in Citrus reticulata plant. It has been seen in the current study that the TPC and TFC in relation to FW were significantly high in seedlings treated with ZnNPs after 7 days. Maximum production of TFC was found after 7 days interval, which was (0.7 µg/mg DW) in B. napus (V1 Faisal canola) followed by V2 (Shiralee) variety as compared with 14 and 21 days of interval. Comparable results of broccoli floral leaf were recorded [39]. Furthermore, the production of TPC with green synthesised NPs showed a positive correlation with TFC. It can be explained in terms of role green synthesised ZnNPs in the biosynthesis of TPC and TFC. Abiotic stresses cause increased production of phenolic compounds in plant [40] (Fig. 4).
Fig. 4.
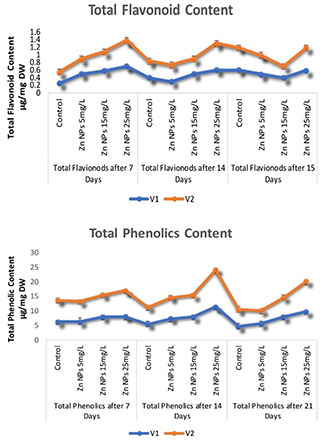
TPC and TFC in B. napus varieties in response to green synthesised ZnNPs at different days interval
3.5 Protein and SOD
Protein and lipids exchange play a key role in plant metabolism when an exposed stress of any nature. Plants react to different stress stimuli by triggering their immune system. Exposure to such ZnNPs may initiate a series of stress signalling pathways helping in the production of different classes of defensive compounds called secondary metabolites [41]. In this study, the total protein and SOD were investigated at different interval of days on different concentrations of green synthesised ZnNPs. After seven days of harvesting the highest protein content was observed at 15 mg/l−1 of green synthesised ZnNPs (195 µg bovine serum albumin equivalent/mg FW) in V1(Faisal Canola) as compared with V2 (Shiralee), respectively. Both varieties showed a significant increase in its protein content as compared to control. This increase could be due to the shape and medium‐size of green synthesised ZnNPs, which triggered the production of protein. The nutritional profile of the canola protein would play a pivotal role in determining its suitability as a food ingredient [41]. As canola proteins are almost exclusively used for animal feed, knowledge of their nutritional value to humans is quite limited [42]. In the current research, B. napus varieties treated with ZnNPs of 5, 15 and 25 mg/l−1 showed increased SOD activity 0.49 µg/ml in V1 and V2 as compared to control, respectively. This may be due to the chemical and metallic stress of green synthesised ZnNPs. Plant defensive secondary metabolites such as SOD, peroxidase, catalase etc. are commercially and medicinally important as health‐promoting compounds [43]. SOD is the first line defence against any ROS. They can convert superoxide anion O2 to low harmful ROS [44]. However, the production of protein is in correlation to SOD concentration. The application of ZnNPs in different forms enhanced the SOD, POD and catalase production in B. napus L. Differences in the pattern is due to the chemical nature, NPs surface and size. The enzymatic role of SOD and POD and the catalase activities of different medicinal plants are extensively reported [45, 46] (Fig. 5).
Fig. 5.

Total protein content and SOD activity in B. napus varieties in response to green synthesised ZnNPs at different days interval
4 Conclusion
From the current experiment, it has been concluded that green synthesised ZnNPs have the potential to improve the germination and biochemical aspects of the B. napus L. plant. ZnNPs have also been reported to play a vital role in the production of auxin and many other hormones which are responsible for plant growth and yield. The size and surface structure of NPs is of prime importance and play a key role in the production of antioxidant enzymes. Once NPs enter the plant they play a vital role in triggering different defence mechanisms of the plant. Green synthesis of NPs is the cheapest source of obtaining NPs but it is still a mystery and much work is needed to explore which compounds of plant play a key role in the reducing and capping agent.
5 References
- 1. Zaka M. Abbasi B.H. Rahman L. et al.: ‘Synthesis and characterization of metal nanoparticles and their effects on seed germination and seedling growth in commercially important Eruca sativa ’, IET Nanobiotechnol., 2016, 10, (3), pp. 1 –7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marczak E.D. Usui H. Fujita H. et al.: ‘New antihypertensive peptides isolated from rapeseed’, Peptides, 2003, 24, (6), pp. 791 –798 [DOI] [PubMed] [Google Scholar]
- 3. Szydlowska‐Czerniak A. Bartkowiak‐Broda I. Karlovic I. et al.: ‘Antioxidant capacity, total phenolics, glucosinolate and color parameters of rapeseed cultivars’, Food Chem., 2011, 127, (2), pp. 556 –563 [DOI] [PubMed] [Google Scholar]
- 4. Harbaum‐Piayda B. Oehlke K. Sonnichsen F.D. et al.: ‘New polyphenolic compounds in commercial deodistillate and rapeseed oils’, Food Chem., 2010, 123, (3), pp. 607 –615 [Google Scholar]
- 5. Kuwahara H. Kanazawa A. Wakamatu D. et al.: ‘Antioxidative and antimutagenic activities of 4‐vinyl‐2,6‐dimethoxyphenol (canolol) isolated from canola oil’, J. Agric. Food Chem., 2004, 52, (14), pp. 4380 –4387 [DOI] [PubMed] [Google Scholar]
- 6. Beecher C. W.: ‘Cancer preventive properties of varieties of Brassica oleracea: a review’, Am. J. Clin. Nutr., 1994, 59, (5), pp. 1165S –11170 [DOI] [PubMed] [Google Scholar]
- 7. Stoewsand G.S.: ‘Bioactive organosulfur phytochemicals in Brassica oleracea vegetables, a review’, Food Chem. Toxicol., 1995, 33, (6), pp. 537 –543 [DOI] [PubMed] [Google Scholar]
- 8. Wang C. C. Chang S. C. Inbaraj B. S. et al.: ‘Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity’, Food Chem., 2010, 120, (1), pp. 184 –192 [Google Scholar]
- 9. Vaughan J.G. Geissler C.A.: ‘The new Oxford book of food plants’ (Oxford University Press, New York, USA, 1997), pp. 166 –167 [Google Scholar]
- 10. Kahkonen M.P. Hopia A.I. Vuorela H.J. et al.: ‘Antioxidant activity of plant extracts containing phenolic compounds’, J. Agric. Food Chem., 1999, 47, (10), pp. 3952 –3962 [DOI] [PubMed] [Google Scholar]
- 11. Pulido R. Bravo L. Saura‐Calixto F.: ‘Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay’, J. Agric. Food Chem., 2000, 48, (8), pp. 3396 –3402 [DOI] [PubMed] [Google Scholar]
- 12. Behl C. Moosmann B.: ‘Antioxidant neuroprotection in Alzheimer's disease as preventive and therapeutic approach’, Free Radical Biol. Med., 2002, 33, (2), pp. 182 –191 [DOI] [PubMed] [Google Scholar]
- 13. Gomes S.I.L. Novais S.C. Gravato C. et al.: ‘Effect of Cu‐nanoparticles versus one Cu‐salt: analysis of stress biomarkers response in Enchytraeus albidus (Oligochaeta)’, Nanotoxicology, 2012, 6, (2), pp. 134 –143 [DOI] [PubMed] [Google Scholar]
- 14. Shah V. Belozerova I.: ‘Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds’, Water Air Soil Pollut., 2009, 197, (1–4), pp. 143 –148 [Google Scholar]
- 15. Masarovicova E. Kralova K.: ‘Metal nanoparticles and plants’, Ecol. Chem. Eng. Sci., 2013, 20, (1), pp. 9 –22 [Google Scholar]
- 16. Kim J.S. Kuk E. Yu K.N. et al.: ‘Antimicrobial effects of silver nanoparticles’, Nanomedicine, 2007, 3, (1), pp. 95 –101 [DOI] [PubMed] [Google Scholar]
- 17. Sohail I. Rahman U. Iqbal Z. et al.: ‘In vitro antimicrobial study of Aspergillus flavus mycelial extract against different bacterial and fungal pathogenic strains’, Int. J. Biosci., 2014, 4, (6), pp. 223 –228 [Google Scholar]
- 18. Hussain M. Raja N.I. Mashwani Z.R. et al.: ‘In vitro germination and biochemical profiling of Citrus reticulata in response to green synthesized zinc and copper nanoparticles’, IET Nanobiotechnol., 2017, 22, (7), pp. 790 –796 [Google Scholar]
- 19. Kouhi S. M. M. Lahouti M. Ganjeali A. et al.: ‘Long‐term exposure of rapeseed (Brassica napus L.) to ZnO nanoparticles: anatomical and ultrastructural responses’, Environ. Sci. Pollut. Res., 2015, 22, (14), pp. 10733 –10743 [DOI] [PubMed] [Google Scholar]
- 20. Abdul‐Baki A.A. Anderson J.D.: ‘Vigor determination in soybean and seed multiple criteria’, Crop Sci., 1973, 13, (6), pp. 630 –633 [Google Scholar]
- 21. Arnon D. I.: ‘Copper enzymes in isolated chloroplasts. Polyphenol oxidase in beta vulgaris’, Plant Physiol., 1949, 24, (1), pp. 1 –15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubois M. Gilles K. A. Hamilton J. K. et al.: ‘Colorimetric method for determination of sugars and related substances’, Anal. Chem., 1956, 28, (3), pp. 350 –356 [Google Scholar]
- 23. Velioglu Y.S. Mazza G. Gao L. et al.: ‘Antioxidant activity and total phenolics in selected fruits, vegetables and grains products’, J. Agric. Food Chem., 1998, 46, (10), pp. 4113 –4117 [Google Scholar]
- 24. Chang C. Yang M. Wen H. et al.: ‘Estimation of total flavonoid content in propolis by two complimentary colorimetric method’, J. Food Drug Anal., 2002, 10, (3), pp. 178 –182 [Google Scholar]
- 25. Lowry O. H. Rosebrough N. J. Farr A. L. et al.: ‘Protein measurement with the folin phenol reagent’, J. Biol. Chem., 1951, 193, (1), pp. 265 –275 [PubMed] [Google Scholar]
- 26. Ullah N. Haq I.U. Safdar N.: ‘Physiological and biochemical mechanisms of allelopathy mediated by the allelochemical extracts of Phytolacca latbenia (Moq.) H. Walter’, Toxicol. Ind. Health, 2015, 31, (10), pp. 931 –937 [DOI] [PubMed] [Google Scholar]
- 27. Santhoshkumar J. Kumar S.V. Rajeshkumar S.: ‘Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen’, Resour. Efficient Technol., 2017, 3, (4), pp. 459 –465 [Google Scholar]
- 28. Rauwel P. Kuunal S. Ferdov S. et al.: ‘A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM’, Adv. Mater Sci. Eng., 2015, 2015, pp. 1 –9 [Google Scholar]
- 29. Jamdagni P. Khatri P. Rana J. S.: ‘Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor‐tristis and their antifungal activity’, J. King Saud Univ. Sci., 2016, 30, (2), pp. 168 –175 [Google Scholar]
- 30. Sangeetha G. Thambavani S.: ‘One pot synthesis of zinc oxide nanoparticles via chemical and green method’, J. Mater. Sci. Res., 2013, 1, (7), pp. 1 –8 [Google Scholar]
- 31. Mazzaglia A. Fortunati E. Kenny J. M. et al.: ‘Nanomaterials in plant protection’, Nanotechnol. Agric. Food Sci., 2017, 4, (1), pp. 189 –95 [Google Scholar]
- 32. Zaka M. Abbasi B.H. Rahman L. et al.: ‘Synthesis and characterisation of metal nanoparticles and their effects on seed germination and seedling growth in commercially important Eruca sativa’, IET Nanobiotechnol.., 2016, 10, (3), pp. 1 –7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah V. Belozerova I.: ‘Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds’, Water Air Soil Pollut., 2009, 97, (1–4), pp. 143 –148 [Google Scholar]
- 34. Lu C. M. Zhang C. Y. Wen J. Q. et al.: ‘Research of the effect of nanometer materials on germination and growth enhancement of glycine max and its mechanism’, Soya Bean Sci., 2002, 21, pp. 168 –172 [Google Scholar]
- 35. Murata M.R. Hammes P.S. Zharare G.E.: ‘Effect of solution pH and calcium concentration on germination and early growth of groundnut’, J. Plant Nutr., 2003, 26, (6), pp. 1247 –1262 [Google Scholar]
- 36. Ghafariyan M. H. Malakouti M. J. Dadpour M. R. et al.: ‘Effects of magnetite nanoparticles on soybean chlorophyll’, Environ. Sci. Technol., 2013, 47, (18), pp. 10645 –10652 [DOI] [PubMed] [Google Scholar]
- 37. Raliya R. Nair R. Chavalmane S. et al.: ‘Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant’, Metallomics, 2015, 7, (12), pp. 1584 –1594 [DOI] [PubMed] [Google Scholar]
- 38. Zafar H. Ali A. Ali J. et al.: ‘Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response’, Front. Plant Sci., 2016, 7, p. 535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heimler D. Vignolini P. Dini M.G. et al.: ‘Antiradical activity and polyphenols composition of local Brassicaceae edible varieties’, Food Chem., 2006, 99, (3), pp. 464 –469 [Google Scholar]
- 40. Young J.W. Mau J.L. Ko P.T. et al.: ‘Antioxidant properties of fermented soybean broth’, Food Chem., 2000, 71, (2), pp. 249 –254 [Google Scholar]
- 41. Tan J. Bednarek P. Liu J. et al.: ‘Universally occurring phenylpropanoid and species‐specific indolic metabolites in infected and uninfected Arabidopsis thaliana roots and leaves’, Phytochemistry, 2004, 65, (6), pp. 691 –699 [DOI] [PubMed] [Google Scholar]
- 42. Bos C. Arinei G. Mariotti F. et al.: ‘The poor digestibility of rapeseed protein is balanced by its very high metabolic utilization in humans’, J. Nutr., 2007, 137, (3), pp. 594 –600 [DOI] [PubMed] [Google Scholar]
- 43. Vardhan P. V. Lata I.S.: ‘Gamma irradiation of medicinally important plants and the enhancement of secondary metabolite production’, Int. J. Radiat. Biol., 2017, 93, (9), pp. 967 –979 [DOI] [PubMed] [Google Scholar]
- 44. Gangwar S. Singh V. P. Tripathi D. K. et al.: ‘Plant responses to metal stress: the emerging role of plant growth hormones in toxicity alleviation’, Int. Emerg. Technol. Manag. Crop Stress Toler., 2014, 2, pp. 215 –248 [Google Scholar]
- 45. Meratan A.A. Gaffari S.M. Nikram V.: ‘In vitro organogenesis and antioxidant enzymes activity in Acanthophyllum sordidum ’, Biol. Plantarum, 2009, 53, (1), pp. 5 –10 [Google Scholar]
- 46. Priyadarshini S. Deepesh B. Zaidi M.G.H. et al.: ‘Silver nanoparticle mediated enhancement in growth and antioxidant status of Brassica juncea ’, Appl. Biochem. Biotechnol., 2012, 167, (8), pp. 2225 –2233 [DOI] [PubMed] [Google Scholar]


