Abstract
Soft‐rot of ginger (Zingiber officinale) is the most important disease usually caused by Fusarium oxysporum (F. oxysporum) leading to significant yield loss. In this study, chitosan, copper and sulphur nanoparticles synthesised from leaf extract of selected plants were screened against two isolates of F. oxysporum recovered from the infected rhizome of ginger and soil samples. Moreover, among these, sulphur nanoparticles showed maximum inhibition of F. oxysporum isolated from soil samples (ZOI = 12.33 mm) followed by copper (ZOI = >12 mm) and chitosan nanoparticles (ZOI = >9 mm). Similarly, in the case of F. oxysporum isolated from infected ginger, sulphur nanoparticles showed maximum inhibition (ZOI = 13.33) as compared to copper (ZOI = >11 mm) and chitosan nanoparticles (ZOI = >9 mm). Considering the high efficacy, sulphur nanoparticles were further evaluated in combination with commercial fungicides, viz., bavistin, ridomil gold, sunflex and streptocycline. The combination of sulphur nanoparticles with bavistin demonstrated maximum inhibition (ZOI = 45.16 mm, MIC −20 µg/ml), whereas the minimum inhibition was shown by its combination with ridomil gold (ZOI = 10.5 mm, MIC –40 µg/ml). Therefore, it can be concluded that the combination of sulphur nanoparticles with bavistin can be used for effective and eco‐friendly management of F. oxysporum causing soft‐rot of ginger.
Inspec keywords: nanoparticles, biotechnology, microorganisms, toxicology, mass spectroscopic chemical analysis, antibacterial activity, plant diseases
Other keywords: fusarium oxysporum, sulphur nanoparticles, f. oxysporum, soil samples, bavistin demonstrated maximum inhibition, biogenically engineered nanoparticles, ginger soft‐rot, chitosan nanoparticles, ridomil gold
1 Introduction
Ginger (Zingiber officinale) is an important commercial crop in tropical and sub‐tropical regions of different countries including India, China, Japan, Indonesia, Australia, Nigeria and West Indies Islands [1]. The rhizome of ginger is used as a spice in food and as a flavouring agent in food‐products. In India, it is also used as traditional medicine due to its antimicrobial, anti‐inflammatory, antipyretic, antioxidative, hypoglycaemic, hepatoprotective and diuretic properties [2]. India is a leading producer of ginger in the world. Although, the recent data are not available, during the year 2012–2013 the country produced 0.745 million tonnes of ginger from an area of 157,839 hectares [3].
Soft‐rot is a common disease of ginger generally caused by Pythium and Fusarium spp. The deterioration of soft‐tissues of plant by pathogenic fungi is mainly responsible for disease induction [4, 5, 6]. The pathogenic fungi present in soil infect plant roots and enter inside thereby affecting their tissues particularly the vascular system. Due to the overgrowth of fungi in vascular tissues, the plant's water supply is affected; consequently, the stomata of the leaves close, which leads to chlorosis followed by necrosis. Once the disease is induced, it spreads gradually to the adjacent clumps mostly through the soil water by means of zoospores, hyphal fragments and so on both under rain‐fed and irrigated conditions.
Presently, application of chemical fungicides is a major approach used for the management of soft‐rot of ginger. However, reports suggested that fungicides have been causing a hazardous environmental impact for several years. In addition, extensive use of chemical fungicides also resulted in the development of resistance in phytopathogenic fungi to such fungicides [7].
However, various attempts have been made to overcome these problems using biological agents. Several microorganisms, including bacteria and fungi, have been used as biological agents for the management of soft‐rot in ginger. Among the bacteria, Pseudomonas fluorescens, Enterobacter agglomerans, Bacillus sp. and so on, most preferably used for inhibiting the growth of causative agents of soft‐rot [8]. Similarly, fungi such as Trichoderma viride and T. harzianum have been reported to have potential inhibitory effects on Fusarium oxysporum f.sp. zingiberi causing rhizome‐rot of ginger (Khatso and Ao, 2013). Although biological agents help in the eco‐friendly management of pathogens causing soft‐rot, there is a need for the development of novel and more efficient approaches for the management of soft‐rot disease of ginger.
In this context, nanotechnology is considered as a new hope due to its widespread application in the agriculture sector. It offers an eco‐friendly alternative for plant disease management and has many advantages over conventional chemical methods, which are associated with eco‐toxicity. Nanomaterials also play an important role in promoting sustainable agriculture and provide better food globally [9]. Nanoparticles can sustainably intensify the new paradigm in agriculture production and mitigate existing implications [9, 10]. Different nanoparticles have attracted a great deal of attention due to their remarkable antifungal potential. Nanoparticles of silver [11], copper [12], sulphur [13] and so on have been reported to have significant efficacy against a variety of plant pathogenic fungi. In addition, other nanoparticles such as gold, titanium, zinc, silica, aluminium, chitosan and so on also possess potent antifungal potential [14, 15].
An effective management of soft‐rot of ginger by the eco‐friendly and economically viable method is the need of the hour. Hence, the present study is mainly focused on: (i) isolation and identification of isolates of Fusarium from infected rhizomes of ginger and soil samples (ii) evaluation of antifungal activity of biologically synthesised chitosan, copper and sulphur nanoparticles and their combination with commercially available fungicides for management of soft‐rot disease of ginger.
2 Materials and methods
2.1 Materials
Leaves of two plants, viz., Hibiscus rosa‐sinensis (H. rosa‐sinensis) and Citrus medica (C. medica) used for the synthesis of nanoparticles were collected from the garden of Department of Biotechnology, Sant Gadge Baba Amravati University, Amravati, Maharashtra, India. Infected rhizomes of ginger were collected from the local vegetable market of Amravati. However, the soil of a ginger grown field was received from a farmer of village in Karnataka, India. Among the chemicals and media, chitosan, glacial acetic acid, RPMI (Roswell Park Memorial Institute), sodium thiosulphate, copper sulphate, ascorbic acid, potato dextrose agar (PDA), corn meal agar (CMA) and other materials like sterile cotton swab and discs were purchased from Hi‐Media Pvt. Ltd. Mumbai, India. Different fungicides viz. ridomil gold (active constituent metalaxyl‐M and Mancozeb), bavistin (Carbendazim), streptomycin (Streptomycin sulphate‐22.4%) and sunflex (sulphur 80%) were purchased from local chemical and fertiliser shop of Amravati, India. Moreover, all the glassware used were purchased from Borosil® Glass Works Limited, India.
2.2 Methods
2.2.1 Collection and surface sterilisation of infected ginger
The rhizomes of infected ginger were aseptically collected from vegetable market of Amravati, Maharashtra, India and brought to the laboratory in paper envelopes. Initially, the ginger samples were washed with sterilised distilled water two to three times. After washing, the infected rhizomes were surface sterilised by dipping in 70% ethanol for 30 s followed by rinsing with sterilised distilled water for three to four times and finally, it was blotted on sterile filter paper.
2.2.2 Isolation and identification of Fusarium sp. from infected ginger and soil samples
The surface sterilised infected rhizomes were used for inoculation on culture medium. Two types of culture media, namely PDA and CMA were used for isolation of Fusarium species. The explants of infected ginger were cut into small pieces (approx. size 2 mm × 2 mm) with the help of sterile scalpel and inoculated onto the solidified agar plates with the help of sterile forceps. After inoculation, the plates were incubated at 28 ± 2°C for 48–72 h and were observed for growth of Fusarium.
Moreover, Fusarium sp. was also isolated from soil of ginger grown field obtained from the field of a farmer from Karnataka, India. The 15 mg soil was crushed with the help of sterilised spatula in Petri plate. Then 1 ml sterilised distilled water was added to it. Thereafter, 10–15 ml sterilised and molten PDA containing Rose Bengal was poured on it. The plates were allowed for incubation at 28 ± 2°C for 24–48 h. The small colonies of the fungus appeared, which were picked up with a sterilised needle and transferred to fresh PDA plates. After one week of incubation, the cultures were identified on the basis of morphological, cultural and microscopic characteristics using ‘The Fusarium laboratory manual’ [16]. Later, the Fusarium isolates were maintained on slants of PDA and CMA at 4°C in a refrigerator.
2.2.3 Synthesis of chitosan, sulphur and copper nanoparticles
Chitosan and sulphur nanoparticles were synthesised using leaf extracts of H. rosa‐sinensis, whereas copper nanoparticles were synthesised from the leaf extract of C. medica. The leaves were extracted by boiling 10 gm of leaves of each plant in 100 ml distilled water. The extract was allowed to cool at room temperature, filtered through filter paper and centrifuged at 4000 rpm for 30 min. The resultant filtrate was used for the synthesis of nanoparticles.
For the synthesis of chitosan nanoparticles method proposed by Nagaonkar et al. [17] was followed. Chitosan was dissolved in aqueous solution of 1% glacial acetic acid at the concentration of 2 mg/ml with continuous stirring at 100 rpm. For the preparation of chitosan nanoparticles, H. rosa‐sinensis leaf extract was added drop wise to chitosan solution under constant stirring at 100 rpm for 30 min. The resultant reaction mixture was centrifugated at 13,000 rpm for 20 min. The pellets of nanoparticle were collected and washed with distilled water.
Sulphur nanoparticles were synthesised using liquid co‐precipitation method demonstrated by Awwad et al. [18]. Here, leaf extract of H. rosa‐sinensis was treated with 50 mM aqueous solution of sodium thiosulfate followed by drop wise addition of conc. HCl for precipitation of sulphur nanoparticles. The resultant nanoparticles solution was centrifugated at 4000 rpm for 30 min to obtain the pallet.
Copper sulphate solution (100 mM) was used as a precursor salt for the synthesis of copper nanoparticles. For that 150 ml, C. medica leaf extract mixed with 150 ml copper sulphate solution was boiled in an aluminium vessel. After 5 min of boiling 10 ml, ascorbic acid was added in drop wise manner. The resultant reaction mixture was allowed to cool at room temperature, the supernatant was discarded and brown colour copper nanoparticles deposited on aluminium vessel were collected by scrapping.
2.2.4 Characterisation of chitosan, sulphur and copper nanoparticles
The synthesised nanoparticles were characterised by nanoparticles tracking and analysis (NTA), zeta potential analysis, X‐ray diffraction (XRD) crystallography, Fourier transform infrared (FTIR) spectroscopy and transmission electron microscopy (TEM). Moreover, the same methods were followed for preparation of samples and their analysis using these techniques which are proposed in our previous studies [12, 19].
2.2.5 In vitro screening of nanoparticles to select potential nanoparticles against F. oxysporum isolates
In vitro antifungal activity of all the three nanoparticles was evaluated against isolates of F. oxysporum obtained from both infected ginger and soil sample by disc diffusion and Minimum Inhibitory Concentration (MIC) determination methods as per guidelines of the Clinical Laboratory Standards Institute (CLSI) (http://www.facm.ucl.ac.be/intranet/CLSI/CLSI‐2017‐M100‐S27.pdf).
Disc diffusion method : For evaluation of nanoparticles’ antifungal activity, disc diffusion method was used. Inocula of both isolates of F. oxysporum were prepared by allowing growth of fungal biomass on potato dextrose broth for 8–10 days. The fungal biomass was suspended in Rosewell Park Memorial Institute medium (RPMI 1640 medium). The optical density (OD) was maintained between 0.09 and 0.17 at 530 nm. The fungal spore suspensions were spread on sterile PDA plates with the help of sterile cotton swab. After swabbing the fungal cultures, sterile discs obtained from HiMedia (HiMedia Pvt. Ltd. Mumbai, Maharashtra, India) were placed onto the surface of inoculated PDA plate. Each sterile disc was impregnated with 20 µl of respective (chitosan, sulphur and copper) nanoparticles suspension. The activity of these nanoparticles was evaluated using different concentrations including 1, 2, 3 and 4 mg/ml. The plates were incubated at 28 ± 2°C for 24–48 h till visible growth appears. The zones of inhibition (ZOI) were measured in mm. The experiment was performed in triplicate.
Determination of MIC of nanoparticles : The MIC of nanoparticles (chitosan, sulphur and copper nanoparticles) was determined by micro‐broth dilution method against both isolates of F. oxysporum. For evaluation of MIC, fungi were inoculated in RPMI and incubated for 7–8 days at 28 ± 2°C. The OD of the fungal suspension was adjusted between 0.15 and 0.17 by using RPMI medium. The absorbance was measured by using colorimeter at 530 nm and the fungal spore load was maintained at 0.4 × 104 –0.5 × 104 CFU/ml. The different concentrations of nanoparticles were prepared in the range of 10–100 and 100–1000 µg/ml. The 200 µl of different concentration of each nanoparticle were added in each well of microtiter plate followed by addition of 20 µl of fungal spore inoculum. The negative control was maintained in a well which consists of sterile RPMI broth to check sterility. Similarly, the positive control was also maintained in a well with RPMI inoculated with 20 µl of fungal spore suspension. The microdilution plates were incubated at 28 ± 2°C for 2–3 days. The plates were observed at the interval of 24 h MIC recorded was the lowest concentration of nanoparticles that resulted in visual inhibition of fungal growth. Each assay was performed in triplicate.
2.2.6 In vitro efficiency of potent nanoparticles in combination with fungicides
Among all three tested nanoparticles, nanoparticles showing potent antifungal activity were further evaluated for their in vitro efficacy in combination with commercially available fungicides using both disc diffusion and MIC determination methods. In vitro antifungal efficacy of potent nanoparticles in combination with commercially available fungicide viz. ridomil gold, bavistin, sunflex and streptocycline sulphate was evaluated against both isolates of F. oxysporum recovered from rhizome of infected ginger and soil. For disc diffusion assay, the fungal spore suspensions were prepared and spread on to the surface of sterile PDA plates with the help of sterile glass spreader. After swabbing, three sterile discs each containing 20 µl of potent nanoparticles (4 mg/ml), 20 µl of commercial fungicide (4 mg/ml) and 20 µl of a combination of potent nanoparticles and each fungicide (1:1 proportion) were placed onto the surface of the inoculated PDA plates. The plates were incubated at 28 ± 2°C for 24–48 h till visible growth appears. Further, the ZOI were measured in mm. The experiment was performed in triplicate.
In addition, MIC for a combination of potent nanoparticles and commercially available fungicides was also evaluated against both the isolates of F. oxysporum recovered from infected ginger and soil. For combination, 1:1 ratio of potent nanoparticles and fungicide were used. The MIC was determined using micro‐broth dilution method as described above in determination of MIC of nanoparticles.
The MIC of nanoparticles (chitosan, copper and sulphur nanoparticles) was determined by micro‐broth dilution method for both F. oxysporum isolates recovered from the ginger rhizome and soil in accordance with NCCLS protocol. Fungi were inoculated in RPMI medium and incubated for 7–8 days at 25 ± 2°C. The OD of the fungal suspension was adjusted between 0.15 and 0.17 by using RPMI medium. The absorbance was measured by using colorimeter at 530 nm and the fungal load was maintained at 0.4 × 104 –0.5 × 104 CFU/ml. The different concentrations of nanoparticles were prepared in the range of 10–100 and 100–1000 µg/ml. The 200 µl of different concentration of nanoparticles were added in each well of microtiter plate followed by addition of 20 µl of fungal inoculum. Negative control well consisted of sterile RPMI broth to check sterility while positive control wells were filled with RPMI inoculated with 20 µl of fungal suspension. The micro dilution plates were incubated at 28 ± 2°C for 2–3 days. The plates were observed at the interval of 24 h. Each assay was performed in triplicate. The MIC recorded was the lowest concentration of nanoparticles that resulted in visual inhibition of fungal growth.
3 Results and discussion
3.1 Isolation and identification of fungal isolates on the basis of cultural and microscopic characteristics
After inoculation of explants from infected ginger and suspension of soil sample on PDA and CMA media, initial cottony mycelial growth was observed after 48 h of incubation at 28 ± 2° C. These mycelia were further transferred to fresh PDA plates in order to obtain a pure culture of the fungus and maintained at 4°C in refrigerator for further use. Moreover, thus recovered fungi from infected ginger and soil samples were identified on the basis of morphological, cultural and microscopic characteristics using the Fusarium laboratory manual [16]. The fungus recovered from infected ginger ( Fig. 1 A ) showed the growth of white dense aerial mycelia on the front and dark‐violet colour pigmentation on the reverse of agar plate after 7 days of incubation (Figs. 1 B and C). Moreover, microscopic observations include the formation of oval single‐celled microconidia (Fig. 1 D) and two to four septate macroconidia [20] (Fig. 1 E). Moreover, the similar morphological, cultural and microscopic characters were reported for the isolate recovered from soil sample (Figs. 2 A –E) which confirmed the identification of isolates as F. oxysporum. The growth rate of isolate recovered from ginger is comparatively slow as compared to isolate recovered from a soil sample.
Fig. 1.
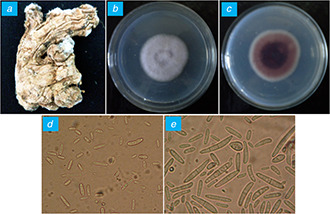
Isolation of Fusarium sp. from infected rhizome of ginger
(a) Infected ginger, (b) Dorsal view, (c) Ventral view, (d) Microconidia, (e) Macroconidia
Fig. 2.
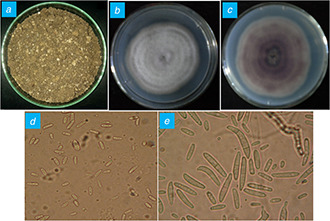
Isolation of Fusarium sp. from an infected soil sample
(a) Soil of ginger grown field, (b) Dorsal view, (c) Ventral view, (d) Microconidia, (e) Macroconidia
3.2 Synthesis of chitosan, sulphur and copper nanoparticles
The change in colour of reaction mixture after the addition of leaf extract of H. rosa‐sinensis from colourless to yellowish indicated the synthesis of chitosan nanoparticles [17].
Similarly, for the synthesis of sulphur nanoparticles formation of light‐yellow precipitation was observed on addition of concentrated HCl to a mixture of leaf extract of H. rosa‐sinensis and 50 mM sodium thiosulphate solution. Formation of off white to pale‐yellow precipitation is the peculiar characteristics for the synthesis of sulphur nanoparticles. It was demonstrated in the case of the synthesis of sulphur nanoparticles using fruit extract of Albizia julibrissin [18].
The treatment of copper sulphate with leaf extract of C. medica and ascorbic acid leads to deposition of shiny reddish‐brown precipitate on the inner wall of aluminium vessel, which indicated the synthesis of copper nanoparticles. This study shows resemblance with the result obtained by Shende et al. [21] who synthesised copper nanoparticles from Citron juice.
3.3 Characterisation of chitosan, copper and sulphur nanoparticles
The synthesised chitosan, sulphur and copper nanoparticles were further characterised by different techniques to study their properties including size, surface charge, crystallinity, shape and morphology.
3.3.1 Nanoparticles tracking and analysis
NTA was performed using Nano Sight LM‐20 (Malvern Instruments, UK). This characterisation is usually performed for the determination of average size and the concentration of synthesised nanoparticles. NTA of chitosan nanoparticles showed that average size of synthesised nanoparticles was 68 ± 38 nm (Fig. 3 A), while the average size of copper nanoparticles was found to be 46 ± 26 nm (Fig. 3 B). Moreover, the average size recorded for sulphur nanoparticles was found to be 80 ± 38 nm (Fig. 3 C).
Fig. 3.
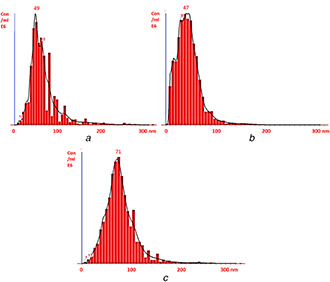
Nanoparticles tracking analysis of
(a) Chitosan nanoparticles, (b) Copper nanoparticles and, (c) Sulphur nanoparticles
The major weaknesses of techniques like dynamic light scattering, or photon‐correlation spectroscopy, which produce an average particle size, have been overcome by NTA because NTA can measure much smaller particle sizes (down to 10 nm) than light obscuration techniques mentioned above, which is an added advantage [22]. In addition, NTA allows a better understanding of the aggregation of nanoparticles very easily, when compared with techniques such as dynamic light scattering and differential centrifugation sedimentation [23].
3.3.2 Zeta potential analysis
Zeta potential was performed to measure the surface charge of nanoparticles. If a particle possesses higher negative or positive zeta potential, they will tend to repel each other and do not form aggregate. The zeta potential of synthesised nanoparticles was determined by zeta sizer (Malvern's Zetasizer ZS 90, UK). The magnitude of zeta potential gives an indication of the potential stability of the colloidal system. The general dividing line between stable and unstable suspension is usually taken at either +30 or −30 mV. Particles with zeta potential more positive than +30 mV or more than −30 mV are normally considered stable and zeta potential more positive than −15 mV indicates a threshold of agglomeration.
Zeta potential reported for chitosan nanoparticles was found to be 30.3 mV. The positive zeta potential above 30 mV indicated highly stable nature of chitosan nanoparticles [17] (Fig. 4 A). Similarly, the zeta potential of copper and sulphur nanoparticles was found to be –15.3 and 7.97 mV, respectively (Figs. 4 B and C). The results showed a resemblance with the findings reported by Rajak et al. [24] who reported the negative zeta potential for stable copper nanoparticles. Suryavanshi et al. [25] also demonstrated the stability of sulphur nanoparticles due to their positive zeta potential.
Fig. 4.
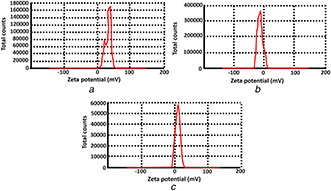
Zeta potential analysis
(a) Chitosan nanoparticles, (b) Copper nanoparticles and, (c) Sulphur nanoparticles
3.3.3 XRD analysis
XRD technique was performed to determine the crystalline nature of nanoparticles. The synthesised copper nanoparticles showed its characteristic diffraction peak at 38°, 50°, 79°, which depicts the presence of (111), (200), (220) facets of an FCC structure (Fig. 5 A). This value showed best matches with JCPDS (Joint Committee on Powder Diffraction, Standard) file no. 04‐0836. The broad bottom area of the peaks indirectly represents the smaller size of the nanoparticles. The similar diffraction pattern of copper nanoparticles was reported by Shende et al. [21]. Moreover, thus synthesised sulphur nanoparticles demonstrated its characteristic diffraction peaks at 15°, 23°, 26°, 28°, 32°, 44°, which depicts the presence of facets of an FCC structure (113), (222), (040), (313), (422), (319) (Fig. 5 B). These values are in agreement with JCPDS (Joint Committee on Powder Diffraction, Standard) file no. N34‐094. The broad bottom area of the peaks indirectly represents the smaller size of the nanoparticles. The diffraction pattern of sulphur nanoparticles shows correspondence with the results obtained by Suleiman et al. [26].
Fig. 5.
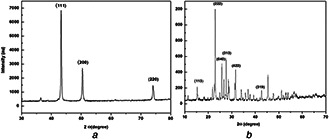
XRD analysis of
(a) Copper nanoparticles, (b) Sulphur nanoparticles
3.3.4 TEM analysis
The TEM analysis was performed to determine the shape and size of chitosan, copper and sulphur nanoparticles. The TEM micrograph confirmed the polydispersive nature of thus synthesised nanoparticles. The size of chitosan nanoparticles was found to be in the range of 20–45 nm with spherical in shape (Fig. 6 A). Moreover, the other two nanoparticles, i.e. copper and sulphur were polydispersed having a size in the range of 50–70 and 60–85 nm, respectively (Figs. 6 B and C).
Fig. 6.

TEM analysis of
(a) Chitosan nanoparticles, (b) Copper nanoparticles and, (c) Sulphur nanoparticles
3.4 In vitro screening of nanoparticles for selection of promising nanoparticles against F. oxysporum isolates
In vitro antifungal activity of all the synthesised nanoparticles namely chitosan, copper and sulphur nanoparticles were evaluated at various concentrations (1, 2, 3 and 4 mg/ml) against both isolates of F. oxysporum recovered from infected ginger and soil sample by Kierby–Baur disc diffusion method [27]. It was observed that biosynthesised sulphur nanoparticles showed a remarkable inhibitory effect on both isolates of F. oxysporum followed by copper and chitosan nanoparticles. In the case of F. oxysporum isolated from soil, it was observed that sulphur nanoparticles at a concentration of 4 mg/ml showed maximum activity (ZOI = 14.33 mm), followed by copper (ZOI = >12 mm) and chitosan nanoparticles (ZOI = >9 mm). Moreover, a similar pattern was observed in the case of F. oxysporum recovered from the infected ginger. In this case also, sulphur nanoparticles at 4 mg/ml showed higher activity (ZOI = 13.33 mm) followed by copper (ZOI = >11 mm) and chitosan nanoparticles (ZOI = >9 mm) at same 4 mg/ml concentration (Table 1). These findings are in accordance with observations reported by Rao and Paria [13]. The authors reported the fungicidal effect of sulphur nanoparticles against two phytopathogens, i.e. Fusarium solani, responsible for early blight of tomato; and Venturia inaequalis causing apple scab disease. Similarly, it was demonstrated that sulphur nanoparticles also inhibit the growth of Aspergillus niger and F. oxysporum, which are common plant pathogenic fungi [28]. Unfortunately, chitosan and copper nanoparticles failed to show significant antifungal activity against both isolates of F. oxysporum.
Table 1.
ZOI formed by chitosan, copper and sulphur nanoparticles against the F. oxysporum isolates at different concentrations
| Test organism | Nanoparticles | ZOI formed at a different concentration of nanoparticles, mm | |||
|---|---|---|---|---|---|
| 1 mg/ml | 2 mg/ml | 3 mg/ml | 4 mg/ml | ||
| F. oxysporum (ginger isolate) | chitosan NPs | >7 | >8 | >8 | >8 |
| copper NPs | >9 | >10 | >10 | >11 | |
| sulphur NPs | 10 | 11.33 | 10.66 | 13.33 | |
| F. oxysporum (soil isolate) | chitosan NPs | >8 | >9 | >9 | >9 |
| copper NPs | >11 | >11 | >12 | >12 | |
| sulphur NPs | 12.33 | 11.33 | 14 | 14.33 | |
NPs = nanoparticles.
Moreover, a similar pattern was reported in case of MIC determination of all the three nanoparticles. Among these, sulphur nanoparticles showed significantly lower MIC for both F. oxysporum recovered from infected ginger (MIC = 100 μg/ml) and soil (MIC = 200 μg/ml). However, MIC values for chitosan and copper nanoparticles were comparatively higher for both the isolates (Table 2).
Table 2.
MIC of different nanoparticles for isolates of F. oxysporum recovered from infected ginger and soil samples
| Test organism | MIC, μg/ml | ||
|---|---|---|---|
| Sulphur NPs | Copper NPs | Chitosan NPs | |
| F. oxysporum (ginger isolate) | 100 | 800 | 500 |
| F. oxysporum (soil isolate) | 200 | 500 | 500 |
NPs – nanoparticles, µg – microgram.
3.5 In vitro efficacy of sulphur nanoparticles in combination with fungicides
The screening of chitosan, sulphur and copper nanoparticles for their antifungal efficacy confirmed that sulphur nanoparticles showed remarkable activity as compared to chitosan and copper nanoparticles. Hence, sulphur nanoparticles were selected for further evaluation of their efficacy in combination with commercial fungicides using both disc diffusion and MIC methods. Different fungicides such as bavistin, ridomil gold, sunflex and streptocycline are commonly used for the management of plant pathogenic fungi and hence these antifungal agents were selected for the study. The antifungal assays were performed as discussed above. Combinations of sulphur nanoparticles with bavistin and sunflex fungicides showed significant synergistic activity against both F. oxysporum isolates. ZOI of sulphur nanoparticles in combination with bavistin, sunflex, ridomil gold and streptocycline were found to be 45.16 ± 1.25, 23.33 ± 2.08, 10.5 ± 0.86 and 12.16 ± 1.04 mm, respectively against F. oxysporum isolated from infected ginger (Fig. 7). While, in the case of F. oxysporum isolated from soil sample it was observed that the combination of sulphur nanoparticles with bavistin was more effective (ZOI = 43.16 ± 2.02 mm) followed by its combinations with sunflex (ZOI = 25.16 ± 1.04 mm), ridomil gold (ZOI = 13.5 ± 1.5 mm) and streptocycline (ZOI = 13.6 ± 0.75 mm) (Fig. 8).
Fig. 7.
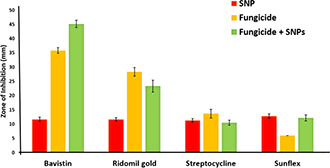
Antifungal activity of sulphur nanoparticles in combination with commercially available fungicides against F. oxysporum isolated from the infected ginger
Fig. 8.
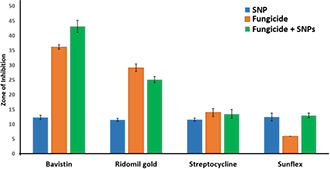
Antifungal activity of sulphur nanoparticles in combination with commercially available fungicides against F. oxysporum isolated from the soil samples
The combination of streptocycline and ridomil gold did not show synergistic activity against F. oxysporum isolated from the infected ginger. The reasons behind the antagonistic effect of the above combinations are not known and at the moment we do not have direct data to give any inference, but it may be possible due to the inhibitory effect of one of the agents involved in combination. Combinations of fungicide with nanoparticles enable the use of smaller quantities of fungicide for effective management of F. oxysporum.
In addition, the efficacy of these combinations was also evaluated by determining the MIC for each combination. From the analysis, it was observed that in case of F. oxysporum (ginger isolate) the combination of sulphur nanoparticles with bavistin and ridomil gold showed similar and significant efficacy followed by the combinations with streptocycline and sunflex (Table 3). Moreover, in the case of F. oxysporum recovered from a soil sample, the combination of sulphur nanoparticles with ridomil gold showed maximum activity followed by the combinations with bavistin, streptocycline and sunflex (Table 3).
Table 3.
MIC of the combination of fungicide and sulphur nanoparticles for F. oxysporum isolated from infected ginger and soil samples
| Test organism | MIC, μg/ml | |||
|---|---|---|---|---|
| Bavistin + SNPs | Ridomil gold + SNPs | Sunflex + SNPs | Streptocycline + SNPs | |
| F. oxysporum (ginger isolate) | 20 | 20 | 40 | 30 |
| F. oxysporum (soil isolate) | 30 | 20 | 50 | 30 |
SNPs – sulphur nanoparticles, µg – microgram.
Sulphur is one of the most important elements used in various biomedical fields. Being a part of various amino acids, it plays a key role in many metabolic activities and body functions of human beings [29]. In addition to its biomedical applications, recently sulphur and its nanoparticles are being used for the management of plant pathogenic fungi due to their fascinating antifungal properties [13, 30].
Considering the significant antifungal efficacy of sulphur nanoparticles singly and in combination with commercial fungicides such as bavistin and ridomil gold, it is believed that sulphur nanoparticles can be effectively used against F. oxysporum causing soft‐rot of ginger.
4 Conclusions
The soft‐rot of ginger is one of the most important diseases leading to significant yield and economic loss. Among the causative agents, F. oxysporum is most frequently associated with disease. The morphological, cultural and microscopic characteristics confirmed the identity of both the isolates as F. oxysporum. Further, the biological approaches used in the synthesis of sulphur, copper and chitosan nanoparticles are found to be rapid, novel and eco‐friendly. Among all nanoparticles tested, sulphur nanoparticles showed significant activity against both isolates of F. oxysporum recovered from infected ginger and ginger grown soil. Moreover, a combination of sulphur nanoparticles with commercially available fungicide also showed remarkable growth inhibition of soft‐rot causing F. oxysporum. Considering the phenomenal antifungal potential of sulphur nanoparticles singly and in a combination of fungicides, it can be recommended to use as novel and next generation nanofungicide for effective management of F. oxysporum causing soft‐rot of ginger. However, there is a need to evaluate the toxicity of nanoparticles in human beings and environment to generate more information for clear understanding about the toxic effects, if any.
Finally, to the best of our knowledge, so far there is no report available on the evaluation of antifungal activity of sulphur nanoparticles in combination with commercial fungicides. Therefore, this study is the first report and the significant observations reported here will be used as a reference for future studies.
5 References
- 1. Gupta M. Kaushal M.: ‘Diseases infecting ginger (Zingiber officinale Roscoe): a review’, Agric. Rev., 2017, 38, (1), pp. 15 –28 [Google Scholar]
- 2. Karuppiah P. Rajaram S.: ‘Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple‐drug resistant clinical pathogens’, Asian Pac. J. Trop. Biomed., 2012, 2, (8), pp. 597 –601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jayashree E. Kandiannan K. Prasath D. et al.: ‘Ginger’, Ind. Agric. Res., 2015, 682, pp. 1 –12 [Google Scholar]
- 4. Pegg K. Moffett M. Colbran R.: ‘Diseases of ginger in Queensland’, Qld. Agric. J., 1974, 100, pp. 611 –618 [Google Scholar]
- 5. Rajan P.P. Gupta R.S. Sarma R.Y. et al.: ‘Disease of ginger and their control with Tricoderma harzianum ’, Ind. Phytopathol., 2002, 55, (2), pp. 173 –177 [Google Scholar]
- 6. Dohroo N.P.: ‘Diseases of ginger’, in Ravindran P.N. Nirmal Babu K. (Eds.): ‘Ginger, the genus Zingiber’ (CRC Press, Boca Raton, 2005), pp. 305 –340 [Google Scholar]
- 7. Shanmugam V. Gupta S. Dohroo N.: ‘Selection of a compatible biocontrol strain mixture based on co‐cultivation to control rhizome rot of ginger’, Crop Prot., 2013, 43, pp. 119 –127 [Google Scholar]
- 8. Bhai R.S. Kishore V.K. Kumar A. et al.: ‘Screening of rhizobacterial isolates against soft rot disease of ginger (Zingiber officinale Rosc.)’, J. Spices Aromat. Crops, 2005, 14, (2), pp. 130 –136 [Google Scholar]
- 9. Fraceta L.F. Grillo R. de Medeiros G.A. et al.: ‘Nanotechnology in agriculture: which innovation potential does it have?’, Front Environ. Sci., 2016, 4, p. 20 [Google Scholar]
- 10. Gogos A. Knauer K. Bucheli T.D.: ‘Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities’, J. Agric. Food Chem., 2012, 60, pp. 9781 –9792 [DOI] [PubMed] [Google Scholar]
- 11. Shaik M.R. Khan M. Kuniyil M. et al.: ‘Plant‐extract‐assisted green synthesis of silver nanoparticles using Origanum vulgare L. Extract and their microbicidal activities’, Sustainability, 2018, 10, (913), pp. 1 –14 [Google Scholar]
- 12. Ingle A.P. Rai M.: ‘Copper nanoflowers as effective antifungal agents for plant pathogenic fungi. IET Nanobiotechnol., 2017, 1, (5), pp. 546 –551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao K.J. Paria S.: ‘Use of sulfur nanoparticles as a green pesticide on Fusarium solani and Venturia inaequalis phytopathogens’, RSC Adv., 2013, 3, pp. 10471 –10478 [Google Scholar]
- 14. Sabir S. Arshad M. Chaudhari S.K.: ‘Zinc oxide nanoparticles for revolutionizing agriculture, synthesis and applications’, Sci. World J., 2014, 2014, pp. 1 –8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banker S. Volova T. Prudnikova S.V. et al.: ‘Nanoagroparticles emerging trends and future prospect in modern agriculture system’, Environ. Toxicol. Pharmacol., 2017, 53, pp. 10 –17 [DOI] [PubMed] [Google Scholar]
- 16. Leslie J.F. Summerell B.A.: ‘The Fusarium laboratory manual’ (Blackwell publishing, Ames, IA, USA, 2006, 3rd edn.) [Google Scholar]
- 17. Nagaonkar D. Gaikwad S. Rai M.: ‘ Catharanthus roseus leaf extract‐synthesized chitosan nanoparticles for controlled in vitro release of chloramphenicol and ketoconazole’, Colloid Poly. Sci., 2015, 293, (5), pp. 1465 –1473 [Google Scholar]
- 18. Awwad A.M. Salem N.M. Abdeen A.O.: ‘Novel approach for synthesis sulfur (S‐NPs) nanoparticles using Albizia julibrissin fruits extract’, Adv. Mat. Lett., 2015, 6, (5), pp. 432 –435 [Google Scholar]
- 19. Paralikar P. Rai M.: ‘Bio‐inspired synthesis of sulphur nanoparticles using leaf extract of four medicinal plants with special reference to their antibacterial activity’, IET Nanobiotechnol., 2017, 12, (1), pp. 25 –31 [Google Scholar]
- 20. Ingle A.P. Rai M.K.: ‘Foodborne toxigenic Fusaria: isolation, taxonomy and mycotoxins’ (LAP Lambert Academic Publisher, Germany, 2012) [Google Scholar]
- 21. Shende S. Ingle A.P. Gade A.: ‘Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity’, World J. Microbiol. Biotechnol., 2015, 31, p. 865 [DOI] [PubMed] [Google Scholar]
- 22. Rai M. Ingle A.P. Gade A.K. et al.: ‘Three Phoma spp. Synthesised novel silver nanoparticles that possess excellent antimicrobial efficacy’, IET Nanobiotechnol., 2015, 9, (5), pp. 280 –287 [DOI] [PubMed] [Google Scholar]
- 23. Tiwari N. Pandit R. Gaikwad S. et al.: ‘Biosynthesis of zinc oxide nanoparticles by petals extract of Rosa indica L., its formulation as nail paint and evaluation of antifungal activity against fungi causing onychomycosis’, IET Nanobiotechnol., 2017, 11, (2), pp. 205 –211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rajak J. Bawaskar M. Rathod D. et al.: ‘Interaction of copper nanoparticles and an endophytic growth promoter Piriformospora indica with Cajanus cajan ’, J. Sci. Food Agric., 2017, 97, (13), pp. 4562 –4570 [DOI] [PubMed] [Google Scholar]
- 25. Suryavanshi P. Pandit R. Gade A. et al.: ‘ Colletotrichum sp. Mediated synthesis of sulphur and aluminium oxide nanoparticles and its in vitro activity against selected food‐borne pathogens’, Food Sci. Technol., 2017, 81, pp. 188 –194 [Google Scholar]
- 26. Suleiman M. Al Ali A. Hussein A. et al.: ‘Sulfur nanoparticles: synthesis, characterizations and their applications’, J. Mater. Environ. Sci., 2013, 4, (6), pp. 1029 –1033 [Google Scholar]
- 27. Bauer A.W. Kirby W.M. Sherris J.C. et al.: ‘Antibiotic susceptibility testing by a standardized single disk method’, Am. J. Clin. Pathol., 1966, 45, pp. 493 –496 [PubMed] [Google Scholar]
- 28. Choudhary R.S. Ghosh M. Mandal A. et al.: ‘Surface modified sulphur nanoparticles: an effective antifungal agent against Aspergillus Niger and Fusarium oxysporum ’, Appl. Microbiol. Biotechnol., 2011, 90, pp. 733 –743 [DOI] [PubMed] [Google Scholar]
- 29. Rai M. Ingle A.P. Paralikar P.: ‘Sulfur and sulfur nanoparticles as potential antimicrobials: from traditional medicine to nanomedicine’, Expert Rev. Anti‐infect. Ther., 2016, 14, (10), pp. 969 –978 [DOI] [PubMed] [Google Scholar]
- 30. Yela A.V. Jimenez V.J. Rodriguez D.V. et al.: ‘Evaluation of the antifungal activity of sulfur and chitosan nanocomposites with active ingredients of Ruta graveolens, Thymus vulgaris and Eucalyptus melliodora on the growth of Botrytis fabae and Fusarium oxysporum ‘, Biol. Med. (Aligarh), 2016, 8, (3), pp. 1 –4 [Google Scholar]


