Abstract
In this study, the authors investigated antimicrobial activity of TiO2 nanoparticles (NPs) synthesised by sol–gel method. As synthesised TiO2 NPs were characterised by X‐ray diffraction, scanning electron microscopy and ultraviolet‐visible absorption spectroscopy. The antimicrobial activity of calcined TiO2 nanoparticle samples was examined in day light on Gram positive bacteria (Staphylococcus aureus, Streptococcus pneumonia and Bacillus subtilis), Gram negative bacteria (Proteus vulgaris, Pseudomonas aeruginosa and Escherichia coli) and fungal test pathogen Candida albicans. The synthesised TiO2 NPs were found to be effective in visible light against Streptococcus pneumonia, Staphylococcus aureus, Proteus vulgaris, Pseudomonas aeruginosa and Candida albicans.
Inspec keywords: titanium compounds, microorganisms, nanomedicine, biomedical materials, nanofabrication, sol‐gel processing, ultraviolet spectra, visible spectra, X‐ray diffraction, scanning electron microscopy, nanoparticles, antibacterial activity
Other keywords: microbicidal activity, titanium dioxide nanoparticle, sol‐gel method, antimicrobial activity, X‐ray diffraction, scanning electron microscopy, ultraviolet‐visible absorption spectroscopy, Gram positive bacteria, Staphylococcus aureus, Streptococcus pneumonia, Bacillus subtilis, TiO2 , Candida albicans, fungal test pathogen, Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, Gram negative bacteria
1 Introduction
The emergence of infectious diseases poses a serious threat to public health worldwide, especially with the emergence of antibiotic‐resistant bacterial strains. Survival of these microbes on environment and medical devices causes nosocomial disease transmission. Over the years, antibiotics have been used to control infections resulting from community and hospital environments [1, 2, 3]. Such infections are frequently connected with resistant organisms, including methicillin‐resistant Staphylococcus aureus (MRSA), penicillin‐resistant Streptococcus pneumoniae, multidrug‐resistant Acinetobacter baumannii and multidrug‐resistant Pseudomonas aeruginosa [4, 5].
Advances in the field of nanobiotechnology lead to the development of new antibacterial agents. Considering the unique properties of nanoparticles (NPs), nano‐sized organic and inorganic particles are being generated for ultimate use in medical practices, such as metal oxides of zinc, copper, and iron in biomedical research and antimicrobial activity [6, 7, 8]. Antimicrobial activity of NPs has largely been studied with human pathogenic bacteria such as Escherichia coli (E. coli) [9] and Staphylococcus aureus [10]. Bactericidal activity of NPs in part depends on size, stability and concentration in the growth medium. While growing in medium amended with NPs, the bacterial population growth can be inhibited by specific nanoparticle interactions [11]. In general, bacterial cell size is in the micrometre range, while its outer cellular membranes have pores in the nanometre range. NPs have a unique ability of crossing the cell membrane because their sizes are smaller than bacterial pores.
Nanocrystalline Titanium dioxide (TiO2) is a well‐known semi‐conductor with photocatalytic activities, having great potential as a self‐cleaning and self‐disinfecting material in many applications. TiO2 has been used broadly for killing different groups of microorganisms including bacteria, fungi and viruses, because it has high photoreactivity, broad‐spectrum antibiosis and chemical stability [12, 13, 14, 15, 16, 17, 18, 19]. TiO2 NPs decompose organic compounds by the formation and constant release of hydroxyl radicals and superoxide ions when exposed to non‐lethal ultraviolet (UV) light, which is highly efficient in inhibiting the growth of MRSA [20]. This potent oxidising power of TiO2 NPs can be used against bacteria and other organic substances [21, 22, 23].
In comparison with published reports on physical, chemical and biological properties, very limited information is available on the broad spectrum of antimicrobial properties of TiO2 NPs in visible light. The present study is designed to evaluate the antimicrobial activity of TiO2 NPs synthesised by sol–gel method. As prepared TiO2 NPs samples calcined at three different temperatures, 400, 600 and 800°C, are used against the microbes such as Gram positive bacteria: Staphylococcus aureus, Streptococcus pneumonia, Bacillus subtilis; Gram negative bacteria: Proteus vulgaris, Pseudomonas aeruginosa, E. coli and the fungal test pathogen: Candida albicans.
2 Materials and methods
Titanium (IV)‐n‐butoxide (Ti(OBu)4) (98%, Alfa Aesar), isopropyl alcohol (99%, Fisher Scientific) and HNO3 (Merck) were used without further purification for the synthesis of nanocrystalline TiO2. Distilled water was used in all synthesis procedures. Synthesis procedures for the preparation of TiO2 NPs used in this work were described elsewhere [24]. The precursor solution was a mixture of 5 cc titanium (IV)‐n‐butoxide (Ti(OBu)4) and 10 cc isopropyl alcohol. This solution was then added drop wise to a pH 2 solution of HNO3. The hydrolysis was performed at room temperature while stirring for 3 h using magnetic stirrer. After complete hydrolysis of Ti(OBu)4, the solution was refluxed at 70°C for 20 h and a sol was formed. Finally, the formed sol was dried at 60°C for 36 h in a hot air oven to obtain TiO2 powders. The obtained powder samples were calcined for 3 h in a muffle furnace at 400, 600 and 800°C in an air atmosphere and are denoted by S1, S2 and S3 respectively.
2.1 Characterisation of TiO2 NPs
X‐ray diffraction (XRD) techniques used in this work were described by our previous study [24]. XRD analysis was carried out in reflection mode with CuKα radiation (λ = 1.5406 Å) using Bruker D8 advance X‐ray diffractometer (step size = 0.020°, step time = 31.2 s and 40 kV, 35 mA) with CuKα radiation in 2θ range from 20 to 70°. The microstructures of the TiO2 samples S1, S2 and S3 were taken using a JOEL Model JSM‐6390 LV scanning electron microscope (SEM). UV–Vis absorption spectra of the samples were recorded in wavelength range 200–800 nm with a Shimadzu UV–Vis spectrophotometer, UV 2600 model. For measurements, NPs were pressed into a thick pellet and placed at entrance port of integrating sphere using a sample holder. Calibration of absorbance scale was done using standard reference materials.
2.2 Source of microorganisms
Microbial strains obtained from the microbial type culture collection (MTCC), Chandigarh. Stock cultures of different Gram‐negative: E. coli (MTCC No: 443), Proteus vulgaris (MTCC No: 1771) and Pseudomonas aeruginosa (MTCC No: 424); Gram‐positive: Bacillus subtilis (MTCC No: 10619), Streptococcus pneumonia (MTCC No: 2672) and Staphylococcus aureus (MTCC No: 96) bacteria and fungus Candida albicans (MTCC No: 227) were sub‐cultured and maintained in nutrient broth/Brain heart Infusion broth and Todd's Hewitt Broth.
2.3 Preparation of TiO2 NPs suspension
150, 200 and 250 microgram (µg) of TiO2 NPs was added to 1 ml of sterile MQ water and shaken vigorously. The suspending solution was treated by ultrasound (100 W, 40 kHz) for 30 min, autoclaved at 121°C for 20 min and then cooled down to room temperature. The sonication was used to break down the agglomerates and aggregation of NPs to make their dispersions.
2.4 Screening of antibiotics
The antibiotic susceptibility pattern was determined by modified Kirby Bauer disc diffusion method [25] against the following antibiotics (drug concentration in µl): Streptomycin (30), ampicillin (10), Erythromycin (30), Tetracycline (30) for bacterial culture and amphotericin B and chloramphenicol (30) for fungal culture. The Mueller–Hinton agar (MHA) plate supplemented with Gram positive (Streptococcus pneumoniae, Staphylococcus aureus, Bacillus subtilis), Gram negative (Proteus vulgaris, Pseudomonas aeruginosa) and fungus (Candida albicans) by a sterile cotton swab and the antibiotics discs were laid on the surface to find antibiotic resistance pattern. It was found that Tetracycline was more sensitive against gram positive and gram negative bacteria. For fungus, chloramphenicol showed more sensitivity than amphotericin. Thus, tetracycline (30 µl/disc) and chloramphenicol (30 µl/disc) were selected as the standards for bacteria and fungus respectively, in antibiotic sensitivity testing.
2.5 Antimicrobial assay
Antimicrobial activities of the synthesised metal oxide (TiO2) NPs were performed against Gram positive bacteria (Streptococcus pneumoniae, Staphylococcus aureus, Bacillus subtilis), Gram negative bacteria (Proteus vulgaris, Pseudomonas aeruginosa, E. coli) and Fungus (Candida albicans). The Kirby–Bauer disc diffusion method was used for the antimicrobial assay [25]. Using a sterile cotton swab lawn, cultures of the test organisms were made on nutrient agar plates under aseptic conditions. Plates were left standing for 10 min to let the culture get absorbed. Then, 8 mm wells were punched into the MHA plates for testing the antimicrobial activity of TiO2 nanoparticle samples S1, S2 and S3. Wells were sealed with one drop of molten agar (0.8% agar) to prevent leakage of nanomaterials from the bottom of the wells. Using a micropipette, 30 μl of each sample at different concentrations (150, 200 and 250 μg/ml) of nanoparticle suspension was poured into each wells on three sets of petriplate taken for S1, S2 and S3. The plates are incubated at 37 ± 1°C and 27 ± 1°C respectively for bacteria and fungus for a period of 12–24 h both in dark and visible light. After incubation the zone diameter was measured.
3 Results and discussion
The XRD patterns of calcined TiO2 samples are shown in Fig. 1. The XRD pattern of the sample calcinated at 400°C (S1) reveals the presence of anatase phase. Rutile peaks started to appear and a mixture of both the anatase and rutile phases of TiO2 existed at 600°C (S2). As calcinations temperature was increased further, a phase transition from anatase to rutile was observed, and the transition to rutile phase is almost completed at 800°C (S3). The average crystallite size of TiO2 NPs were estimated using Scherrer's equation, t = 0.9λ /β cosθ, where λ is the X‐ray wavelength, β the full width at half maximum (FWHM) of the peak and θ the Bragg's angle. The size of the anatase crystallites (S1) increases from 6 to 25.8 nm when the calcination temperature is raised to 600°C (S2), and increases to 33 nm as temperature is further raised to 800°C (S3, rutile). The XRD spectra shows that the diffraction peaks become intense and their FWHM gradually decreases with increasing calcinations temperature, verifying the increase in particle size. At higher calcinations temperatures, the formed crystallites are larger in size, which can be attributed to the thermally promoted crystallite growth [24].
Fig. 1.

XRD spectra of TiO2 samples S1, S2 and S3
All the peaks of anatase TiO2 sample resemble to a tetragonal structure (space group: I 41 /amd) containing twelve atoms per unit cell with lattice parameters a = 3.776 Å and c = 9.486 Å, which is in agreement with the JCPDS file No. 73‐1764 for TiO2. All the XRD peaks of rutile TiO2 sample correspond to a tetragonal structure (space group: P 42 /mnm) with lattice parameters a = 4.582 Å and c = 2.953 Å, which is in agreement with the JCPDS file No. 78‐1510 for TiO2.
Fig. 2 shows SEM images of the samples S1, S2 and S3 respectively. The micrograph shows polycrystalline texture of the material with a large number of interfaces. It shows that all samples have almost spherical shaped grains with some voids are distributed almost uniformly over the entire surface of samples. Moreover, grains of unequal sizes are distributed throughout the samples. Increase of particle size due to calcination is also evident from SEM images of samples.
Fig. 2.

SEM images of TiO2 samples S1, S2 and S3
Fig. 3 shows UV–Vis absorbance spectra of TiO2 nanoparticle samples. It is observed that absorbance spectrum of anatase TiO2 is blue shifted due to quantum size effect. Direct band gap (E g) of samples is determined by fitting absorption data to direct transition equation, αhν = E d (hν – E g)1/2, where α is optical absorption coefficient, hν photon energy, E g direct band gap and E d a constant. Band gap of TiO2 samples have been measured by plotting (αhν)2 as a function of photon energy and extrapolating linear portion of the curve to absorption equal to zero. The band gap values obtained are 2.87, 2.76 and 2.67 eV for samples S1, S2 and S3 respectively. These values are lower than their bulk and literature values [26, 27]. This lowering of band gap may be due to chemical defects or vacancies present in crystal generating new energy levels. The small band gap values of TiO2 NP samples are useful for higher photocatalytic as well as antimicrobial activities because their energy gap values are comparable with energy of visible or UV light photons. It is also found that band gap decreases with increase in calcination due to particle size variation.
Fig. 3.

Absorption spectra for TiO2 NP samples
The TiO2 NPs synthesised at three different temperatures were used for checking the antibiotic sensitivity at three different concentrations (150, 200 and 250 µg/ml). No significant antimicrobial activity was detected for samples kept in the dark for 12–24 h. Table 1 shows inhibition zone of TiO2 NPs against pathogens under visible light. Figs. 6, 5, 4 show well diffusion assay for TiO2 NPs against Gram positive Staphylococcus aureus, Streptococcus pneumoniae and Bacillus subtilis bacteria; Gram negative Proteus vulgaris, Pseudomonas aeruginosa and E. coli bacteria and Candida albicans fungus respectively in visible light. The sample S1 (anatase) shows sensitivity to Streptococcus pneumonia (2.3 cm), Staphylococcus aureus (2.0 cm), Proteus vulgaris (1.9 cm) and Pseudomonas aeruginosa (2.2 cm) bacteria, and Candida albicans (2.5 cm) fungus (Table 1 and Fig. 3, 6, 5, 4). The sample S2 (TiO2 ‐600) shows sensitivity to Streptococcus pneumonia (2.3 cm), Staphylococcus aureus (2.2 cm), Proteus vulgaris (2 cm), Pseudomonas aeruginosa (2.1 cm) and E. coli (2.0 cm) bacteria, and Candida albicans (2.6 cm) fungus. However, Sample S3 (rutile) shows sensitivity to Streptococcus pneumonia (2.1 cm), Staphylococcus aureus (2.1 cm) and Pseudomonas aeruginosa (2.4 cm) bacteria and Candida albicans (2.1 cm) fungus only. It can be seen from Table 1 and Figs. 6, 5, 4 that the sample S1, (anatase TiO2), shows significant antimicrobial activity compared with samples S2 and S3 (rutile TiO2). Similar results are reported in literature [28]. The calcination of TiO2 NPs causes increase of particle size, which in turn results in the reduction of antimicrobial potency.
Table 1.
Zone of inhibition of TiO2 NPs against pathogens
| Microorganism | S1 | S2 | S3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone of inhibition, cm | ||||||||||||
| Control | Concentrations, µg/ml | Control | Concentrations, µg/ml | Control | Concentrations, µg/ml | |||||||
| 150 | 200 | 250 | 150 | 200 | 250 | 150 | 200 | 250 | ||||
| Streptococcus Pneumonia | 3.5 | — | 2.1 | 2.3 | 3.4 | — | 1.9 | 2.3 | 3.4 | — | 1.8 | 2.1 |
| Staphylococcus Aureus | 3.1 | 0.6 | 1.9 | 2.1 | 3.2 | 0.6 | 1.8 | 2.2 | 2.7 | — | 1.6 | 2.1 |
| Bacillus subtilis | 3.3 | — | 1.4 | 1.6 | 3.2 | — | 1.6 | 1.8 | 3.2 | — | 1.4 | 1.8 |
| Proteus Vulgaris | 2.1 | — | 1.5 | 1.9 | 1.6 | — | 1.7 | 2.0 | 1.7 | — | 1.6 | 1.7 |
| Pseudomonas aeruginosa | 2.1 | 1.1 | 2.0 | 2.2 | 2.4 | — | 1.8 | 2.1 | 2.1 | — | 2.2 | 2.4 |
| E. coli | 2.3 | — | 1.6 | 1.8 | 2.3 | — | 1.4 | 2.0 | 2.2 | — | 1.3 | 1.6 |
| Candida Albicans | 3.4 | — | 2.2 | 2.5 | 4 | 0.6 | 2.4 | 2.6 | 3.2 | — | 2.0 | 2.1 |
a Sensitive – 1.9 cm; Intermediate – 1.5–1.8 cm; Resistance – <1.4 cm.
Fig. 6.

Well diffusion assay for TiO2 NPs against Candida albicans
Fig. 5.
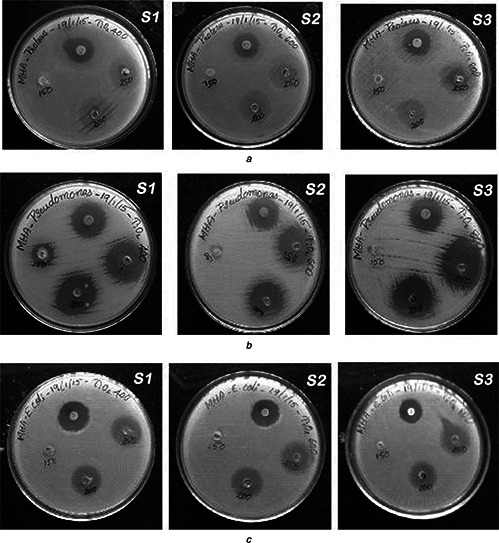
Well diffusion assay for TiO2 NPs against
a Proteus vulgaris
b Pseudomonas aeruginosa
c Escherichia coli
Fig. 4.

Well diffusion assay for TiO2 NPs against
a Staphylococcus aureus
b Streptococcus pneumonia
c Bacillus subtilis
The antimicrobial activity of sample S3 is found higher inhibition zone than the standard antibiotics Tetracycline for Pseudomonas aeruginosa. All the samples show higher sensitivity to pathogens at 250 µg concentration. No samples found inhibited or sensitive against Bacillus subtilis bacteria. TiO2 NPs samples calcined at various temperatures (400, 600 and 800°C) possesses different sensitivity against microbes due to size variation caused by calcination process. The present studies found that presence of light, concentration of TiO2 and size of NPs are the factors for efficient antimicrobial activity against pathogens. Moreover, anatase TiO2 NPs samples showed considerably good activity against Streptococcus pneumonia, Staphylococcus aureus, Proteus vulgaris and Pseudomonas aeruginosa bacteria and Candida albicans.
Antibacterial activity of TiO2 NPs can be attributed to the decomposition of bacterial outer membranes by reactive oxygen species (ROS), primarily hydroxyl radicals (OH), which leads to phospholipid peroxidation and finally cell death [20, 21, 22, 23, 29]. Photocatalytic oxidation reactions are initiated when a photon of energy level higher or equal to the band gap energy is absorbed by a TiO2 catalyst. This reaction promotes an electron (e−) from the valence band to the conduction band with simultaneous generation of a positive hole (h+) in the valence band. The positive hole of TiO2 breaks water molecule to form hydrogen gas (H2) and hydroxyl radicals (OH . ). The negative‐electron reacts with atmospheric oxygen molecule to form super oxide ions. These hydroxyl radicals contact with each other to produce hydroxyl peroxide (H2 O2). These ROS can decompose organic compounds and extinguish cellular activity. TiO2 photocatalytic action induces significant damages in cell membranes by ROS, followed by loss of essential functions [25, 26, 28].
The present investigation found that TiO2 NPs of suitable size may act as promising antibacterial and antifungal agents in future. Further, the present experimental work is done at day light hence, it is important in terms of potential applications of these NPs for antimicrobial modification of various surfaces. In hospital environments, where resistant strains could be transmitted easily and cause infection in patients with surgical wounds and burnt, TiO2 NPs could be used as the suitable disinfectant. Moreover, it could be possible to manufacture the suture or wound bands by cotton fabrics with antibacterial material TiO2 to decrease the rate of infection in patients.
4 Conclusions
TiO2 NPs samples were synthesised by sol–gel method and were characterised by XRD, SEM and UV–Vis spectroscopy. TiO2 NPs calcined at 400, 600 and 800°C were used for antimicrobial study of Gram positive bacteria (Staphylococcus aureus, Streptococcus pneumonia, Bacillus subtilis), Gram negative bacteria (Proteus vulgaris, Pseudomonas aerogenosa, E. coli) and fungal test pathogen Candida albicans. The synthesised TiO2 NPs were found to have antimicrobial potency in day light against five bacterial strains Streptococcus pneumonia, Staphylococcus aureus, Pseudomonas aerogenosa, Proteus vulgaris and E. coli and a fungal strain Candida albicans. The calcination of TiO2 NPs results changes in particle size and optical band gap, which in turn changing antimicrobial potency.
5 Acknowledgments
Authors thank Nirmala College, Muvattupuzha and Mangalore University, Mangalagangotri, Karnataka for providing opportunity to undertake this study. Authors are grateful to KSCSTE, Thiruvananthapuram for providing financial support to carry out this investigation.
6 References
- 1. Lowy F.: ‘Staphylococcus aureus infections’, N. Engl. J. Med., 1998, 339, pp. 520 –532 (doi: 10.1056/NEJM199808203390806) [DOI] [PubMed] [Google Scholar]
- 2. Komolafe O.O.: ‘Antibiotic resistance in bacteria – an emerging public health problem’, Malawi Med. J., 2003, 15, pp. 63 –67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hawkey P.M.: ‘The growing burden of antimicrobial resistance’, J. Antimicrob Chemother., 2008, 62, pp. i1 –i9 (doi: 10.1093/jac/dkn241) [DOI] [PubMed] [Google Scholar]
- 4. Kramer A. Schwebke I. Kampf G.: ‘How long do nosocomial pathogens persist on inanimate surfaces? A systematic review’, BMC Infect. Dis., 2006, 6:130, pp. 1 –8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber D.J. Rutala W.A.: ‘Use of germicides in the home and the healthcare setting: Is there a relationship between germicide use and antibiotic resistance?’, Infect. Control Hosp. Epidemiol., 2006, 27, pp. 1107 –1119 (doi: 10.1086/507964) [DOI] [PubMed] [Google Scholar]
- 6. Mahapatra O. Bhagat M. Gopalakrishnan C. et al.: ‘Ultrafine dispersed CuO nanoparticles and their antibacterial activity’, J. Exp. Nanosci., 2008, 3, pp. 185 –193 (doi: 10.1080/17458080802395460) [DOI] [Google Scholar]
- 7. Tran N. Mir A. Mallik D. et al.: ‘Bactericidal effects of iron oxide nanoparticles on Staphylococcus aureus ‘, Int. J. Nanomedicine, 2010, 5, pp. 277 –283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones N. Ray B. Ranjit K.T. et al.: ‘Antibacterial activity of ZnO nanoparticles suspensions on a broad spectrum of microorganisms’, FEMS Microbiol. Lett., 2008, 279, pp. 71 –76 (doi: 10.1111/j.1574-6968.2007.01012.x) [DOI] [PubMed] [Google Scholar]
- 9. Yoon K.Y. Byeon J.H. Park J.H. et al.: ‘Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles’, Sci. Total Environ., 2007, 373, pp. 572 –575 (doi: 10.1016/j.scitotenv.2006.11.007) [DOI] [PubMed] [Google Scholar]
- 10. Ruparelia J.P. Chatterjee A.K. Duttagupta S.P. et al.: ‘Strain specificity in antimicrobial activity of silver and copper nanoparticles’, Acta Biomater., 2008, 4, pp. 707 –716 (doi: 10.1016/j.actbio.2007.11.006) [DOI] [PubMed] [Google Scholar]
- 11. Raghupati K.R. Koodali R.T. Manna A.C.: ‘Size‐dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles’, Langmuir, 2011, 27, pp. 4020 –4028 (doi: 10.1021/la104825u) [DOI] [PubMed] [Google Scholar]
- 12. Nadtochenko V. Denisov N. Sarkisov O. et al.: ‘Laser kinetic spectroscopy of the interfacial charge transfer between membrane cell walls of E. coli and TiO2 ’, J. Photochem. Photobiol. A: Chem., 2006, 181, pp. 401 –407 (doi: 10.1016/j.jphotochem.2005.12.028) [DOI] [Google Scholar]
- 13. Rincon A.G. Pulgarin C.: ‘Effect of pH, inorganic ions, organic matter and H2 O2 on E. coli K12 photocatalytic inactivation by TiO2: Implications in solar water disinfection’, Appl. Catal. B: Environ., 2004, 51, pp. 283 –302 (doi: 10.1016/j.apcatb.2004.03.007) [DOI] [Google Scholar]
- 14. Yu J.C. Ho W. Lin J. et al.: ‘Photocatalytic activity, antibacterial effect, and photoinduced hydrophilicity of TiO2 films coated on a stainless steel substrate’, Environ. Sci. Technol., 2003, 37, pp. 2296 –2301 (doi: 10.1021/es0259483) [DOI] [PubMed] [Google Scholar]
- 15. Hur J.S. Koh Y.: ‘Bactericidal activity and water purification of immobilized TiO2 photocatalyst in bean sprout cultivation’, Biotechnol. Lett., 2002, 24, pp. 23 –25 (doi: 10.1023/A:1013849014715) [DOI] [Google Scholar]
- 16. Maness P.C. Smolinski S. Blake D.M. et al.: ‘Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism’, Appl. Environ. Microbiol., 1999, 65, pp. 4094 –4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen X. Mao S.S.: ‘Titanium dioxide nanomaterials: synthesis, properties, modifications and applications’, Chem. Rev., 2007, 107, pp. 2891 –2959 (doi: 10.1021/cr0500535) [DOI] [PubMed] [Google Scholar]
- 18. Kiran G. Singh R.P. Ashutosh P. et al.: ‘Photocatalytic antibacterial performance of TiO2 and Ag‐doped TiO2 against S. aureus. P. aeruginosa and E. coli’, Beilstein J. Nanotechnol., 2013, 4, pp. 345 –351 (doi: 10.3762/bjnano.4.40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saraschandraa N. Pavithrab M. Sivakumar A.: ‘Antimicrobial applications of TiO2 coated modified polyethylene (HDPE) films’, Arch. Appl. Sci. Res., 2013, 5, pp. 189 –194 [Google Scholar]
- 20. Shah M.S.A. Nag M. Kalagara T. et al.: ‘Silver on PEG‐PU‐TiO2 polymer nanocomposite films; an excellent system for antibacterial applications’, Chem. Materials, 2008, 20, pp. 2455 –2460 (doi: 10.1021/cm7033867) [DOI] [Google Scholar]
- 21. Cho K. Park J. Osaka T. et al.: ‘The study of antimicrobial activity and preservative effects of nanosilver ingredient’, Electrochim. Acta, 2005, 51, pp. 956 –960 (doi: 10.1016/j.electacta.2005.04.071) [DOI] [Google Scholar]
- 22. Fujishima A. Rao T.N. Tryk D.A.: ‘Titanium dioxide photocatalysis’, J. Photochem. Photobiol. C: Photochem. Rev., 2000, 1, pp. 1 –21 (doi: 10.1016/S1389-5567(00)00002-2) [DOI] [Google Scholar]
- 23. Shiraishi Y. Hirai T.: ‘Selective organic transformations on titanium oxide‐based photocatalysts’, J. Photochem. Photobiol. C: Photochem. Rev., 2008, 9, pp. 157 –170 (doi: 10.1016/j.jphotochemrev.2008.05.001) [DOI] [Google Scholar]
- 24. Priyanka K.P. Sheena P.A. Sabu N.A. et al.: ‘Sol–Gel Synthesis and Characterization of TiO2 Nanoparticles’, Adv. Sci. Eng. Med., 2014, 6, pp. 253 –256 (doi: 10.1166/asem.2014.1495) [DOI] [Google Scholar]
- 25. Ameer A. Ahmed A.S. Mohammad O. et al.: ‘Antimicrobial activity of metal oxide nanoparticles against Gram‐positive and Gram‐negative bacteria: a comparative study’, Int. J. Nanomedicine, 2012, 7, pp. 6003 –6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ba‐Abbad M.M. Kadhum A.A.H. Mohamad A.B. et al.: ‘Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation’, Int. J. Electrochem. Sci., 2012, 7, pp. 4871 –4888 [Google Scholar]
- 27. Arun Kumar D. Alex Xavier J. Merline J. et al.: ‘Synthesis and structural, optical and electrical properties of TiO2 /SiO2 nanocomposites’, J. Mater. Science, 2013, 48, pp. 3700 –3707 (doi: 10.1007/s10853-013-7167-2) [DOI] [Google Scholar]
- 28. Velhal S.G. Kulakrni S.D. Jaybhaye R.G.: ‘Titanium dioxide nanoparticles for control of microorganisms’, Int. J. Res. Chem. Environ., 2014, 4, pp. 192 –198 [Google Scholar]
- 29. Ishibashi K.I. Fujishima A. Watanabe T. et al.: ‘Generation and deactivation processes of superoxide formed on TiO2 film illuminated by very weak UV light in air or water’, J. Phys. Chem. B, 2000, 104, pp. 4934 –4938 (doi: 10.1021/jp9942670) [DOI] [Google Scholar]


