Abstract
Sulphonylureas are extensively used in the treatment of type II diabetes; however, these drugs have complications of hypoglycaemia and weight gain. The current study aims at developing a potent antidiabetic drug that has lesser side effects and better management of its associated conditions. Zinc oxide nanoparticles (ZnO NPs) were synthesised using Syzygium cumini seed extract with an average size of 18.92 nm. In vitro studies on rat insulinoma (RIN‐5F) cells revealed that cells treated with synthesised ZnO NPs showed a dose‐dependent increase in insulin secretion. Streptozotocin‐fructose‐induced type II diabetic rats treated with ZnO NPS exhibited a significant reduction (p < 0.01) in the blood glucose levels, total cholesterol, triglycerides, and low‐density lipoprotein levels and increase (p < 0.01) in serum insulin and liver antioxidant enzyme levels proclaiming its role as a hypoglycaemic and hypolipidaemic drug. Treatment of ZnO NPs in diabetic rats exhibited an increased number of beta cells which was responsible for its increased insulin levels and reduced glucose levels. From the overall observations, biosynthesised ZnO NPs exhibited an efficacious hypoglycaemic effect in diabetic rats, so it can be suggested as a potent antidiabetic drug.
Inspec keywords: biochemistry, II‐VI semiconductors, drug delivery systems, drugs, patient treatment, blood, enzymes, zinc compounds, molecular biophysics, sugar, cellular biophysics, nanofabrication, liver, nanoparticles, diseases, biomedical materials, nanomedicine
Other keywords: protective role, biosynthesised zinc oxide nanoparticles, pancreatic beta cells, vivo approach, type II diabetes, drugs, hypoglycaemia, weight gain, potent antidiabetic drug, lesser side effects, associated conditions, Syzygium cumini seed, rat insulinoma, synthesised ZnO NPs, dose‐dependent increase, insulin secretion, streptozotocin‐fructose‐induced type II diabetic rats, blood glucose levels, low‐density lipoprotein levels, serum insulin, liver antioxidant enzyme levels, hypoglycaemic drug, hypolipidaemic drug, increased insulin levels, reduced glucose levels, biosynthesised ZnO NPs, efficacious hypoglycaemic effect, size 18.92 nm, temperature 5.0 F, ZnO
1 Introduction
Diabetes mellitus is a chronic metabolic disorder that arises when the pancreas fails to produce the required amount of insulin or when the body could not use the insulin effectively, which leads to the hyperglycaemic condition. Diabetes is majorly classified into three types, namely type 1, type 2 and gestational diabetes, whereas type 2 diabetes accounts for the majority of people diagnosed with diabetes. Type 1 diabetes is due to the inability of the body to produce insulin while type 2 diabetes is caused when the body could not utilise the insulin effectively and gestational diabetes is a hyperglycaemic condition that occurs during the pregnancy period [1]. The prevalence of diabetes in India is estimated to be about 10.4% of the adult population which had a mortality of 1 million deaths [2]. Type 2 diabetes is mostly caused due to various factors such as sedentary lifestyles, urbanisation and unhealthy food habits which are mostly prevailing in the developing countries.
Prolonged hyperglycaemic condition stimulates the endoplasmic reticulum to produce more transcriptional proteins to meet the insulin demand which accumulates leading to endoplasmic reticulum stress that sets off the unfolded protein response leading to beta‐cell destruction which in turn reduces the insulin productivity. The hyperglycaemic condition also leads to various microvascular and macrovascular complications such as nephropathy, retinopathy, neuropathy, peripheral vascular disease, and cardiovascular disease. Currently, there are various drugs available commercially to treat diabetes such as sulphonylureas, biguanides, alpha‐glucosidase inhibitors, thiazolidinediones, and meglitinides but have various side effects such as hypoglycaemia, weight gain, nausea, diarrhoea, fluid retention and abdominal bloating [3]. These complications emphasise the need for new antidiabetic drugs that have lesser side effects which led to the current study on the antidiabetic potential of green synthesised zinc oxide nanoparticles (ZnO NPs) on diabetic wistar rats.
Syzygium cumini (SC) is a tropical evergreen tree that has been used traditionally for their medicinal properties; the seeds are widely used to treat diabetes, diarrhoea, dyspepsia, and blood pressure [4]. The bioactive compounds such as anthocyanins, flavonoids, and phenolic acids in SC have potent antioxidant properties that help to prevent various metabolic disorders [5]. Zinc is an essential metal present in the body, which acts as an enzyme activator and also plays a vital role in glucose metabolism, it improves glucose utilisation by hepatic glycogenesis that acts through the insulin pathway and insulin signalling via enhanced PI3K activity, glycogen synthesis kinase‐3 inhibition, and increased phosphorylation of insulin receptor [6]. It plays an important role in insulin secretion, storage, and biosynthesis [7]. Zinc is proven to have an insulin‐mimetic property and also required for the maintenance of glucose transporter 4 (GLUT 4) which corresponds to its ability to lower the blood glucose levels [8, 9]. Zinc is also essential in the management of diabetes because of its role in the insulin biosynthesis pathway, which led to carry out the current study on ZnO NPs.
2 Materials and methods
2.1 Green synthesised ZnO NPs
ZnO NPs were synthesised using SC seed extract as a reducing agent that produced crystalline NPs. The absorption maximum of the NPs was at 369 nm while the size of the NPs was 16.7–22.9 nm which was revealed from the transmission electron microscopy analysis. X‐ray diffraction analysis revealed the crystalline nature of the synthesised NPs and the size of the NPs as 18.92 nm. Fourier transform‐infrared spectroscopy revealed the presence of phenolic content of the seed extract in the synthesised ZnO NPs. The synthesis and characterisation of ZnO NPs were already reported in our previous study [10]. The synthesised ZnO NPs were used in the following experimental study.
2.2 In vitro insulin secretion analysis
Rat insulinoma (RIN‐5F) cells were used to determine the insulin secretion potential of ZnO NPs. The cell line was procured from National Centre for Cell Science, Pune, India. The cells were maintained in RPMI‐1640 medium supplemented with 10% FBS and 4500 mg/l glucose under 5% CO2 atmosphere. The cytotoxicity of the synthesised ZnO NPs on RIN‐5F was previously studied and the IC50 value was estimated to be 62.06 μg/ml while the commercially available ZnO NPs were cytotoxic above 10 μg/ml concentration [10, 11]. 2 × 105 cells/well were seeded in a 24‐well plate and incubated for 72 h for cell growth after which the medium is changed and allowed to grow for another 48 h. Wells were designated separately for low glucose and high glucose containing 11 and 25 mM of glucose. ZnO NPs were added at a concentration of 10, 20 and 40 μg/ml to the wells and incubated for 4 h. Cell supernatant was then estimated for the insulin quantity by ELISA and it was compared with control well without ZnO NPs treatment.
2.3 Induction of type II diabetes and experimental design
Adult female Wistar rats were used for the current study. The experimental protocol was authenticated and approved by the Institutional Animal Ethical Committee (IAEC) of VIT University, Vellore (VIT/IAEC/11th/October 10th/No.39) and the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA); Ministry of Environment, Forests and Climate Change, Governments of India. Animals were housed in standard laboratory conditions of 27°C and 12‐h day/night cycle. Water was made freely available and the rats were fed with commercially available pelleted feed. To induce type II diabetes in Wistar rats, the animals were given 10% of fructose dissolved in drinking water for 2 weeks, followed by intraperitoneal injection of streptozotocin (STZ) at a dosage of 40 mg/kg body weight (b.wt) dissolved in 0.1 M citrate buffer (pH 4.5) [12]. The animals were then treated with 5% glucose water ad libitum for 6 h to counter sudden hypoglycaemic conditions due to STZ injection. After 4 days of STZ injection, animals were screened for fasting blood glucose levels to confirm the induction of diabetes. Animals with a blood glucose level above 250 mg/dl were considered diabetic, and those animals were used for the study.
For the study, the animals were divided into five groups; each group consisted of six female Wistar rats. Group I : non‐diabetic rats (control); Group II : diabetic rats (diabetic control); Group III : diabetic rats treated with standard drug glibenclamide 0.1 mg/kg b.wt (positive control); Groups IV and V : diabetic rats treated with 5 and 10 mg/kg b.wt of synthesised ZnO NPs, respectively. ZnO NPs and glibenclamide were administered orally for 28 days using a 22‐gauge feeding needle. The body weight of all group animals was measured every week. Blood glucose levels were examined in all the groups at 1, 7, 14, 21 and 28 days by glucose oxidase–peroxidase method [13]. At the end of the experimental period, animals were fasted for 12 h, anaesthetised and sacrificed. Blood samples were collected; the serum was separated by centrifugation (2000g for 20 min) and stored at −20°C for biochemical assays. The liver and pancreas tissues were dissected from the animals, washed in ice‐cold saline and stored at −80°C for further assays.
2.4 Serum biochemical assays
The insulin levels in the blood serum were measured using rat insulin ELISA (Enzyme‐Linked Immunosorbent Assay) kit from RayBio®, USA. The content of total cholesterol (TC), triglycerides (TG), high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), liver marker enzymes such as aspartate transaminase (AST) and alanine transaminase (ALT) in the serum were analysed as per commercial kit protocol (ARKRAY®, India).
2.5 Antioxidant and lipid peroxidation (LP) assays
Liver tissues were homogenised in medium containing 1 mM EDTA, 0.25 M sucrose, 10 mM HEPES‐NaOH and centrifuged at 4°C for 20 min at 9000 rpm [14]. The resulting supernatant was used to determine liver Catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) and LP levels. The liver CAT activity was estimated using the method described by Sinha and expressed in μmole of H2 O2 consumed per minute per mg protein [15]. SOD level was estimated using a modified Kakkar et al. [16] method and expressed in nmol of enzyme/g tissue. The level of GPx in the liver tissue supernatant was analysed using the Hafeman method and expressed in U/mg protein [17]. LP assay was accomplished according to the standard procedure illustrated by Ohkawa et al. [18], and expressed in nmol MDA formed/mg tissue.
2.6 Histological studies
A 10% neutral buffered formalin was used to fix the liver and pancreas from each group of animals. Following the fixation, tissues were sliced using an automated tissue processor and embedded in wax. Tissue sections were cut into a thickness of 5 μm using a Leica microtome and stained by haematoxylin and eosin staining. The slides thus obtained were subjected to histological examination.
2.7 Statistical analysis
All data were expressed as mean±standard error mean (SEM). One‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests was carried out to determine the significance between the experimental groups. All data were analysed using GraphPad Prism® version 7.
3 Results and discussion
3.1 Insulin secretion activity by ZnO NPs on RIN‐5F cells
Treatment of RIN‐5F cells with ZnO NPs exhibited a dose‐dependent increase in insulin levels (Fig. 1). Insulin levels of the high glucose control cells exhibited increased insulin secretion when compared to the low glucose control cells. Insulin level in the cells treated with 40 μg/ml of ZnO NPs did not show much increase when compared to the cells treated with 20 μg/ml of ZnO NPs. The overall observations indicated that the treatment of ZnO NPs at 10 and 20 μg/ml concentration increased insulin secretion by RIN‐5F cells. The increase in insulin secretion could be attributed by the role of zinc in secretion, storage and biosynthesis of insulin [7], the same was observed from the results obtained.
Fig. 1.
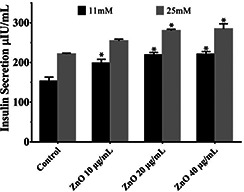
Effect of ZnO NPs on insulin secretion activity by RIN‐5F cells. Values are mean±SEM, n = 6, * = p < 0.05. Statistical analysis – ANOVA followed by Tukey's comparison. * = comparison with control
3.2 Hypoglycaemic activity of the synthesised ZnO nanoparticles
In the current study, the antidiabetic activity of the biosynthesised ZnO NPs was evaluated and compared with standard hypoglycaemic drug glibenclamide. The effect of ZnO NPs on the bodyweight of the experimental rats is shown in Fig. 2. There was a significant reduction in the bodyweight of group II diabetic control rats when compared to the initial body weight. Similarly, ZnO NP‐treated rats showed a slight decrease in the body weight; however, they managed to normalise at the later period of treatment. The reduction of body weight was prominent in the untreated group which should have been due to osmotic diuresis which leads to excessive loss of fluids by polyuria [19].
Fig. 2.
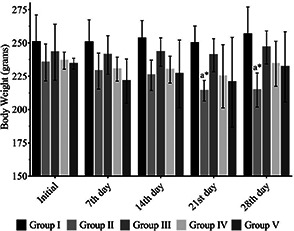
Effect of ZnO NPs on the bodyweight of the experimental animals. Values are mean±SEM, n = 6, * = p < 0.05. Statistical analysis – ANOVA followed by Tukey's comparison test. a = Comparison of bodyweight with initial weight
The group of rats treated with STZ exhibited a significant increase (p < 0.01) in fasting blood glucose levels initially when compared to the control group I rats. From the initial day to 28th day a significant increase (p < 0.01) in blood glucose levels was observed in group II diabetic control rats when compared to the control group I rats. The oral administration of glibenclamide in group III rats and ZnO NPs at the concentration of 5 mg/kg b.wt and 10 mg/kg b.wt in group IV and V rats, respectively, exhibited a significant reduction (p < 0.01) in blood glucose level on the 7th, 14th, 21st, and 28th days when compared to group II diabetic control rats (Table 1). In previous findings, SC has been reported to have anti‐hyperglycaemic and anti‐hyperlipaemia effects on STZ and alloxan‐induced rats [20, 21]. It is also proven that zinc has an insulin‐mimetic property and also required for the maintenance of GLUT 4 which corresponds to its ability to lower the blood glucose levels [8, 9]. The biochemical evaluations of the rats in the study have enlightened that the treatment with ZnO NPs has significantly reduced the glucose levels in group IV and V rats which were possible through the action of zinc and SC.
Table 1.
Glucose level of the experimental animals treated with ZnO NPs
| Group I (control) | Group II (diabetic control) | Group III (positive control – glibenclamide) | Group IV (ZnO NPs 5 mg/kg b.wt) | Group V (ZnO NPs 10 mg/kg b.wt) | |
|---|---|---|---|---|---|
| initial day (mg/dl) | 90.50 ± 8.78 | 380.33 ± 38.15 a** | 349.83 ± 30.89 a** | 329.33 ± 34.27 a** | 337.67 ± 19.37 a** |
| 7th day (mg/dl) | 95.00 ± 10.05 | 422.83 ± 23.31 a** | 152.67 ± 18.19 b** | 197.83 ± 8.03 a**b** | 237.67 ± 13.22 a**b** |
| 14th day (mg/dl) | 80.17 ± 8.27 | 409.50 ± 43.32 a** | 121.33 ± 9.97 b** | 219.33 ± 13.70 a** b** | 205.33 ± 20.94 a** b** |
| 21st day (mg/dl) | 80.83 ± 11.09 | 423.50 ± 23.91 a** | 119.50 ± 9.91 b** | 194.16 ± 7.87 a** b** | 189.167 ± 6.88 a** b** |
| 28th day (mg/dl) | 100.83 ± 6.18 | 383.00 ± 27.57 a** | 116.16 ± 5.12 b** | 193.83 ± 5.74 a** b** | 179.00 ± 7.48 a* b** |
Values are mean±SEM, n = 6, * p < 0.05, ** p < 0.01. Statistical analysis – ANOVA followed by Tukey's comparison test. a: comparison of group I with other groups and b: comparison of group II with other groups.
3.3 Effect of ZnO NPs on serum biochemical parameters
The serum insulin levels estimated by radioimmunoassay (Fig. 3) in group II diabetic control rats showed a significant reduction (p < 0.01) in insulin levels in comparison with the group I rats; reduction in insulin level indicates the extent of beta‐cell damage. A marked increase (p < 0.01) in the level of insulin was observed in glibenclamide‐treated group III rats in comparison with group II diabetic control rats. A similar result was observed (p < 0.01) in ZnO NP‐treated (10 mg/kg b.wt) group V rats. The serum insulin levels were significantly increased when compared with group II diabetic control rats and showed no difference when compared to group I rats. This is in accordance with the previous reports that suggest ZnO NPs could increase insulin secretion and also its sensitivity [22]. The result also acknowledges the result obtained from the in vitro data which showed increased insulin secretion by the treatment of the synthesised ZnO NPs.
Fig. 3.
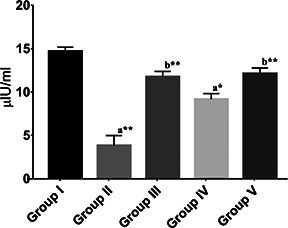
Serum Insulin levels of different groups expressed in μIU/ml. Values are mean±SEM, n = 6, * = p < 0.05, ** = p < 0.01. Statistical analysis – ANOVA followed by Tukey's comparison test. a = comparison of group I with other groups and b = comparison of group II with other groups
Table 2 depicts the biochemically estimated lipid profile and liver function test of rats on the 28th day after various treatments. The concentrations of TC, TG, HDL and LDL in the control group I rats was 188.28 ± 17.60, 267.52 ± 2.61, 87.07 ± 9.16 and 47.71 ± 15.25 mg/dl, respectively. In group II diabetic control rats, there was a significant (p < 0.01) increase in TC, TG and HDL levels when compared to control group I rats. Oral administration of glibenclamide in group III rats reduced (p < 0.01) the TC, TG, LDL and HDL level in comparison with group II diabetic control rats. Similarly, group IV and group V rats treated with ZnO NPs significantly reduced (p < 0.01) the levels of TC and LDL when compared to group II diabetic control rats. A marked decrease in HDL (p < 0.05) level was also observed in group IV and V rats. The primary pathogenesis of diabetes in lipid metabolism is the increase in free fatty acids in the blood by mobilising the fatty acid from adipose tissues which are esterified to form TG [23]. The increased TG in the diabetic group II rats emphasises the effect of diabetes on the lipid profile which was comparatively less in ZnO NP‐treated groups, the ratio of HDL level to LDL were high in treated groups when compared to group II rats which reveal the hypolipidemic effect of the synthesised ZnO NPs.
Table 2.
Effect of ZnO NPs on serum lipid profile and liver markers
| Group I (control) | Group II (diabetic control) | Group III (positive control – glibenclamide) | Group IV (ZnO NPs 5 mg/kg b.wt) | Group V (ZnO NPs 10 mg/kg b.wt) | |
|---|---|---|---|---|---|
| TC (mg/dl) | 188.28 ± 17.60 | 434.64 ± 29.41 a** | 153.65 ± 19.86 b** | 177.86 ± 26.64 b** | 145.83 ± 10.83 b** |
| triglycerides (mg/dl) | 267.52 ± 2.61 | 293.66 ± 4.35 a** | 255.16 ± 5.35 b** | 274.58 ± 3.78 b* | 254.77 ± 1.64 b** |
| LDL (mg/dl) | 47.71 ± 15.25 | 258.17 ± 30.60 a** | 36.24 ± 15.86 b** | 43.80 ± 19.65 b** | 21.81 ± 3.61 b** |
| HDL (mg/dl) | 87.07 ± 9.16 | 117.73 ± 2.84 | 66.37 ± 6.70 b** | 79.15 ± 11.20 b* | 73.07 ± 11.39 b* |
| ALT (IU/l) | 40.96 ± 5.09 | 77.50 ± 3.53 a* | 58.05 ± 8.92 | 47.44 ± 3.61 b** | 48.47 ± 4.49 b* |
| AST (IU/l) | 58.79 ± 6.38 | 91.49 ± 5.39 a* | 54.96 ± 13.23 | 52.89 ± 6.72 | 69.54 ± 5.27 |
Values are mean±SEM, n = 6, *p < 0.05, **p < 0.01. Statistical analysis – ANOVA followed by Tukey's comparison test. a: comparison of group I with other groups and b: comparison of group II with other groups.
Levels of ALT and AST significantly increased (p < 0.05) in group II diabetic control rats when compared to group I control rats. A non‐significant reduction of ALT and AST levels were observed in group III positive control rats when compared with group II. Group IV (p < 0.01) and group V (p < 0.05) rats treated with ZnO NPs exhibited a significant reduction in ALT level and a non‐significant reduction in the AST level in comparison with group II diabetic rats. The levels of ALT and AST were normalised in groups that were administered with ZnO NPs.
3.4 Effect of ZnO NPs on liver antioxidant enzymes and LP
The liver is an important organ responsible for various functions including storage of glycogen, detoxification and conversion of waste to aid excretion [24]. Estimation of the antioxidant enzymes in liver tissues determined the nature of administered nanoparticles in vivo and its toxicity condition. The levels of antioxidant enzymes such as CAT, SOD and GPx and LP of the experimental animals were shown in Table 3. Group II diabetic control exhibited a significant increase (p < 0.05) in CAT in comparison to the group I control rats. However, after 4 weeks of treatment of glibenclamide and ZnO NPs (10 mg/kg b.wt) in group III and V rats exhibited a remarkable increase (p < 0.01) in CAT levels. The rats treated with 5 mg/kg b.wt ZnO NPs showed a non‐significant increase in the CAT level. In comparison with the control group I animals a significant reduction of SOD levels in group II (p < 0.01) and group III rats (p < 0.05) were observed. The reduction in SOD levels may be an indication of the utilisation of antioxidants to overcome the stress caused by diabetes. However, the SOD level was higher in group IV (p < 0.01) and group V (p < 0.05) animals when compared to group II diabetic animals suggests the restoration role of ZnO NPs. No significant difference was observed in the GPx level in group IV and group V rats. Group II diabetic control rats clearly showed elevated levels (p < 0.01) of LP, whereas the levels got reduced (p < 0.01) in groups III, IV and IV due to the treatment with glibenclamide and ZnO nanoparticles when compared to group II diabetic control. Oxidative stress is an important factor in diabetes that leads to the development of many complications. LPx levels were elevated due to blood glucose which leads to the formation of reactive oxygen species [25]. The pro‐immune property of zinc and the antioxidant activity of phenolics present in the ZnO NPs could have attributed to the increased levels of antioxidant enzymes CAT and SOD in ZnO NP‐treated rats [26]. Phenolic content present in SC seeds has been previously reported to have antihyperglycaemic and antihyperlipidaemic effects on diabetes‐induced rats [27, 28] and also prevents insulin resistance, lipid and glucose dysregulation [29, 30].
Table 3.
Effect of ZnO NPs on liver antioxidant enzymes and LP
| Group I (control) | Group II (diabetic control) | Group III (positive control – glibenclamide) | Group IV (ZnO NPs 5 mg/kg b.wt) | Group V (ZnO NPs 10 mg/kg b.wt) | |
|---|---|---|---|---|---|
| CAT (μmole of H2 O2 consumed/min mg protein) | 471.45 ± 38.35 | 276.81 ± 18.09a* | 741.93 ± 60.38a*b** | 448.84 ± 47.84 | 691.17 ± 56.79 b** |
| SOD (nmol of enzyme/g tissue) | 189.86 ± 4.11 | 146.27 ± 4.93 a** | 150.00 ± 7.23 a* | 193.38 ± 7.12 b** | 173.20 ± 6.34 b* |
| GPx (U/mg protein) | 19.37 ± 1.61 | 13.59 ± 0.70 a* | 23.22 ± 2.63 b* | 15.84 ± 0.83 | 18.28 ± 1.70 |
| LPx (nmol/mg protein) | 0.09 ± 0.00 | 0.18 ± 0.01 a** | 0.07 ± 0.02 b** | 0.11 ± 0.02 b** | 0.09 ± 0.01 b** |
Values are mean±SEM, n = 6, *p < 0.05, **p < 0.01. Statistical analysis – ANOVA followed by Tukey's comparison test. a: comparison of group I with other groups and b: comparison of group II with other groups.
3.5 Histological analysis
Histological analysis of the pancreas showed normal cell architecture in the control group I animals (Fig. 4 a). It was observed that there was a decreased number of beta cells in the islet of Langerhans in group II diabetic control rats, which explain the adverse effect of STZ on beta cells (Fig. 4 b). Pancreatic exocrine glands of all the groups showed normal morphology. Group III positive control group rats (Fig. 4 c) showed a reduced density of islet cells, but the number of cells was more in the ZnO NP‐treated group V (Fig. 4 e) animals when compared to group II diabetic rats. Zinc was previously reported to help in the regeneration of beta cells in diabetic rats which may also have helped in the antidiabetic activity of the synthesised nanoparticles [31]. It is observed that ZnO NPs administration is effectively increasing the beta‐cell mass in experimental rats, improving the insulin secretion.
Fig. 4.
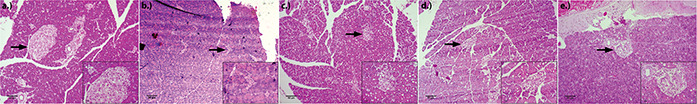
Histopathological observations of pancreas at 40× magnification and inlay at 100× magnification
(a) Group I, (b) Group II, (c) Group III, (d) Group IV (ZnO NPs 5 mg/kg b.wt), (e) Group IV ZnO NPs (10 mg/kg b.wt)
Microscopic observations of the liver sections of the experimental animals were represented in Fig. 5.
Fig. 5.
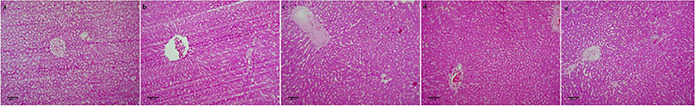
Histopathological observations of liver at 40× magnification
(a) Group I, (b) Group II, (c) Group III, (d) Group IV (ZnO NPs 5 mg/kg b.wt), (e) Group IV ZnO NPs (10 mg/kg b.wt)
Group 1 control showed normal liver architecture (Fig. 5 a). Group II diabetic control showed damaged histology with a more dilated central vein (Fig. 5 b). In group III positive control congestion of central and portal veins and Kupffer cell hyperplasia were observed (Fig. 5 c). Group IV (Fig. 5 d) and group V (Fig. 5 e) animals treated with ZnO NPs exhibited mild dilation of central and portal veins and minimal Kupffer cell hyperplasia.
4 Conclusion
ZnO NPs subjected to treat STZ‐induced diabetic rats exhibited hypoglycaemic activity with a significant reduction in blood glucose (p < 0.01) and an increase in serum insulin levels in diabetic rats. It also presented an antioxidant role by increasing SOD and catalase levels in liver tissues. The insulin levels were significantly increased in both cell lines and experimental rats. The histopathological study of the pancreas in ZnO NP‐treated rats showed improved beta‐cell mass and structure. Hence, the biosynthesised ZnO NPs can be used as an efficient antidiabetic agent, and further studies have to be carried out to explore the mechanism involved. From the observations made it is hypothesised that the ZnO NPs and phenolics present in it play an essential role in the antidiabetic property majorly by reducing the blood glucose level and in the restoration of beta islets density.
5 Acknowledgments
The authors thank the Vellore Institute of Technology (VIT), Vellore for the facilities and funding through the SEED grant to carry out the research work.
6 References
- 1. WHO : ‘Global report on diabetes’ World Health Organization, Geneva, 2016. [Google Scholar]
- 2. International Diabetes Federation : ‘IDF Diabetes Atlas, 8th edition.’, 2017.
- 3. Modi P.: ‘Diabetes beyond insulin: review of new drugs for treatment of diabetes mellitus’, Curr. Drug Discov. Technol., 2007, 4, (1), pp. 39–47 [DOI] [PubMed] [Google Scholar]
- 4. Morton J.F.: ‘Fruits of warm climates’ (Julia F. Morton, Miami, FL, USA, 1987) [Google Scholar]
- 5. Ayya N., Nalwade V., Khan T.N.: ‘Effect of jamun (Syzygium cumini L.) seed powder supplementation on blood glucose level of type‐II diabetic subject’, Food Sci. Res. J., 2015, 6, (2), pp. 353–356 [Google Scholar]
- 6. Jansen J., Karges W., Rink L.: ‘Zinc and diabetes – clinical links and molecular mechanisms’, J. Nutr. Biochem., 2009, 20, (6), pp. 399–417 [DOI] [PubMed] [Google Scholar]
- 7. Sun Q., Van Dam R.M., Willett W.C. et al.: ‘A prospective study of zinc intake and risk of type 2 diabetes in women’, Diabetes Care, 2009, 32, pp. 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sakurai H., Adachi Y.: ‘The pharmacology of the insulinomimetic effect of zinc complexes’, Biometals, 2005, 18, (4), pp. 319–323 [DOI] [PubMed] [Google Scholar]
- 9. Keller S.R.: ‘Role of the insulin‐regulated aminopeptidase IRAP in insulin action and diabetes’, Biol. Pharm. Bull., 2004, 27, (6), pp. 761–764 [DOI] [PubMed] [Google Scholar]
- 10. Daniel Ja., Devi Sa.: ‘Inhibition of key digestive enzymes involved in glucose metabolism by biosynthesized zinc oxide nanoparticles from Syzygium cumini (L.): an in vitro and in silico approach’, Pharmacogn. Mag., 2019, 15, (66), p. 502 [Google Scholar]
- 11. Umrani R.D., Paknikar K.M.: ‘Zinc oxide nanoparticles show antidiabetic activity in streptozotocin‐induced type 1 and 2 diabetic rats’, Nanomedicine, 2014, 9, (1), pp. 89–104 [DOI] [PubMed] [Google Scholar]
- 12. Wilson R.D., Islam M.S.: ‘Fructose‐fed streptozotocin‐injected rat: an alternative model for type 2 diabetes’, Pharmacol. Rep., 2012, 64, (1), pp. 129–139 [DOI] [PubMed] [Google Scholar]
- 13. Trinder P.: ‘Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor’, Ann. Clin. Biochem., 1969, 6, (1), pp. 24–27 [Google Scholar]
- 14. Graham J.: ‘Homogenization of mammalian tissues’, Sci. World J., 2002, 2, pp. 1626–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sinha A.K.: ‘Colorimetric assay of catalase’, Anal. Biochem., 1972, 47, (2), pp. 389–394 [DOI] [PubMed] [Google Scholar]
- 16. Kakkar P., Das B., Viswanathan P.N.: ‘A modified spectrophotometric assay of superoxide dismutase’, 1984. [PubMed]
- 17. Hafeman D.G., Sunde R.A., Hoekstra W.G.: ‘Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat’, J. Nutr., 1974, 104, (5), pp. 580–587 [DOI] [PubMed] [Google Scholar]
- 18. Ohkawa H., Ohishi N., Yagi K.: ‘Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction’, Anal. Biochem., 1979, 95, (2), pp. 351–358 [DOI] [PubMed] [Google Scholar]
- 19. Chiasson J.L., Aris‐Jilwan N., Bélanger R. et al.: ‘Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state’, 2003. [PMC free article] [PubMed]
- 20. Bhuyan Z.A., Rokeya B., Masum N. et al.: ‘Antidiabetic effect of Syzygium cumini l. Seed on type 2 diabetic rats’, Dhaka Univ. J. Biol. Sci., 2010, 19, (2), pp. 157–164 [Google Scholar]
- 21. Siddiqui S., Sharma B., Ram G.: ‘Anti‐hyperglycemic and anti‐hyperlipemia effects of Syzygium cumini seed in alloxan induced diabetes mellitus in Swiss albino mice (Mus musculus)’, Med. Aromat. Plants, 2014, 3, (4), p. 166 [Google Scholar]
- 22. Hussein J., El‐Banna M., Razik T.A. et al.: ‘Biocompatible zinc oxide nanocrystals stabilized via hydroxyethyl cellulose for mitigation of diabetic complications’, Int. J. Biol. Macromol., 2018, 107, pp. 748–754 [DOI] [PubMed] [Google Scholar]
- 23. Singh B.M., Palma M.A., Nattrass M.: ‘Multiple aspects of insulin resistance: comparison of glucose and intermediary metabolite response to incremental insulin infusion in IDDM subjects of short and long duration’, Diabetes, 1987, 36, (6), pp. 740–748 [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y.‐N., Poon W., Tavares A.J. et al.: ‘Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination’, J. Controlled Release, 2016, 240, pp. 332–348 [DOI] [PubMed] [Google Scholar]
- 25. Matough F.A., Budin S.B., Hamid Z.A. et al.: ‘The role of oxidative stress and antioxidants in diabetic complications’, Sultan Qaboos Univ. Med. J., 2012, 12, (1), p. pp. 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rink L., Kirchner H.: ‘Zinc‐altered immune function and cytokine production’, J. Nutr., 2000, 130, (5), pp. 1407S–1411S [DOI] [PubMed] [Google Scholar]
- 27. Ikewuchi J.C., Onyeike E.N., Uwakwe A.A. et al.: ‘Effect of aqueous extract of the leaves of Acalypha wilkesiana ‘Godseffiana’ Muell Arg (Euphorbiaceae) on the hematology, plasma biochemistry and ocular indices of oxidative stress in alloxan induced diabetic rats’, J. Ethnopharmacol., 2011, 137, (3), pp. 1415–1424 [DOI] [PubMed] [Google Scholar]
- 28. Akiyama S., Katsumata S., Suzuki K. et al.: ‘Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin‐clathrated hesperetin in Goto‐Kakizaki rats with type 2 diabetes’, Biosci. Biotechnol. Biochem., 2009, 73, (12), pp. 2779–2782 [DOI] [PubMed] [Google Scholar]
- 29. Ren B., Qin W., Wu F. et al.: ‘Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats’, Eur. J. Pharmacol., 2016, 773, pp. 13–23 [DOI] [PubMed] [Google Scholar]
- 30. Revathy J., Abdullah S.S.: ‘Influence of hesperetin on glycoprotein components in diabetic rats’, Int. J. Sci. Eng. Res., 2016, 7, pp. 214–220 [Google Scholar]
- 31. Horton T.M., Allegretti P.A., Lee S. et al.: ‘Zinc‐chelating small molecules preferentially accumulate and function within pancreatic β cells’, Cell Chem. Biol., 2019, 26, pp. 213–222.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]


