Abstract
In this study, the ketoconazole‐conjugated zinc oxide (ZnO) nanoparticles were prepared in a single‐step approach using dextrose as an intermediate compound. The physical parameters confirmed the drug conjugation with ZnO and their size was around 70–75 nm. The drug loading and in vivo drug release studies indicated that the –CHO group from the dextrose increase the drug loading up to 65% and their release kinetics were also studied. The anti‐fungal studies indicated that the prepared nanoparticles exhibit strong anti‐fungal activity and the minimum concentration needed is 10 mg/ml. The nanoparticles loaded semi‐solid gel was prepared using carbopol, methylparaben, propyl paraben and propylene glycol. The in vitro penetration of the ketoconazole‐conjugated nanoparticles was studied using the skin. The results indicated that the semi‐solid gel preparations influenced the penetration and also favoured the accumulation into the skin membrane. The veterinary clinical studies indicated that the prepared gel is highly suitable for treatment of Malassezia.
Inspec keywords: II‐VI semiconductors, skin, biomedical materials, antibacterial activity, wide band gap semiconductors, drug delivery systems, nanomedicine, drugs, diseases, gels, nanofabrication, nanoparticles, zinc compounds, biomembranes, veterinary medicine
Other keywords: strong anti‐fungal activity, propyl paraben, propylene glycol, semisolid gel preparations, skin membrane, veterinary clinical studies, semisolid formulation, skin disease, ketoconazole‐conjugated zinc oxide nanoparticles, single‐step approach, physical parameters, drug conjugation, drug loading, release kinetics, dextrose, in vivo drug release studies, carbopol, methylparaben, in vitro penetration, Malassezia, ZnO
1 Introduction
The nanoparticles based therapeutic agents pave the way to replace the traditional anti‐microbial, fungicidal and wound healing agents and it has been used as a major component in a wide variety of consumer, pharmaceutical and industrial products [1, 2, 3]. The nanoparticles offered the design of multifunctional agents in a single unit with various properties. Zinc oxide (ZnO) nanomaterial gained a lot of interest in bio‐medicinal field [4]. The ZnO nanoparticles exhibit large of –OH group on the surface and easily modify with drug or other bio‐molecules and slowly dissolve in acidic and strong basic conditions [5]. Previous studies also indicated that double‐layer polymer shell coating makes the ZnO nanoparticles as water soluble [6]. The ZnO nanoparticles generate reactive oxygen species, which would then result in the formation of hydrogen peroxide [7]. The ZnO nanoparticles highly suitable for dermal applications due to easily imaged through a microscope in the skin and it mostly stayed in stratum corneum without any toxic effect [8]. The synthesis of ZnO nanoparticles has been carried out by several methods such as hydrothermal [9], micro‐emulsion [10], ultrasonic [11], sol–gel method [12], wet chemical synthesis [13], spray pyrolysis [14] and solid‐state reaction [15]. The nanoparticles based drug delivery systems minimising the toxicity associated with systemic absorption or gastrointestinal problems. The topical drug administration generally carried out through ophthalmic, rectal, vaginal and skin routes. The skin‐based topical delivery of nanoparticles could penetrate via intra‐cellular or transappendageal route, resulting in delivery into skin layer or subcutaneous tissue and systemic circulations. The therapeutic effectiveness of the existing drug is improved by formulating them in an advantageous way [16]. The topical treatment of dermatological diseases mainly based on semi‐solid formulations. The effective semi‐solid formulations relay on the active compounds incorporated into the semi‐solid base must reach the site of action. Three different semi‐solid bases are currently manufactured by the pharmaceutical companies and they are: (i) hydrophobic, (ii) hydrophilic and (iii) emulsion (a mixture of hydrophilic and hydrophobic phases) [17, 18]. Ketoconazole is well known oral anti‐fungal agent and it is used for systemic treatment of otitis and dermatitis caused by Malassezia species as well as of infections caused by Candida species and dermatophytosis caused by Microsporum canis. The topical creams and shampoos were made using the ketoconazole. The anti‐fungal activity of the cream is limited due to poor solubility of the drug. Previously, micro‐emulsion [19], noisome [20], liposome [21] and lipid nanoparticle‐based [22] ketoconazole delivery were reported to improve the water solubility and skin permeation of hydrophobic drugs. In the veterinary sector, the canine populations are frequently suffered by the fungus infections. The chronic fungus infection develops severe greasy scale, hyperpigmentation, lichenification and rancid odour. The common sites of the microflora overgrowth are seen on the ventral neck, axillae, inter‐digital spaces, ears and muzzle and perianal skin. Currently, there were two kinds of treatments for fungus infections: systemic treatment and the topical applications. The systemic treatment with the antimycotic agents such as fluconazole, itraconazole etc. showed liver damage and the reduction in the testosterone production. Similarly, the topical application in the form of shampoos requires long‐term application for the treatment and the chances of re‐growth on the skin are possible [23]. Hence, this research focuses on the formulation of ketoconazole‐conjugated ZnO nanoparticle‐based cream and their topical application. For the nanoparticles preparation, the dextrose‐mediated synthesis method was developed for the production of ketoconazole‐conjugated ZnO nanoparticles. The anti‐dermal cream was formulated using a semi‐solid gel base based on the hydrophilic and hydrophobic mixture. To study their clinical applications, dogs with the fungus disease were treated with this cream and the results indicated that the developed cream showed very strong anti‐fungal effects.
2 Material and methods
2.1 Synthesis of ketoconazole‐coated ZnO nanoparticles
For the synthesis, 0.3 g of dextrose, 1.89 g of zinc nitrate (0.1 M) mixed with 100 ml of distilled water and then 10 ml of 0.2 M potassium hydroxide solution was added and stirred for 2 h, and then 2 g of ketoconazole drug was added. After 2 h, the solution was centrifuged at 4000 rpm for 10 min. The obtained pellet was then washed twice with ethanol. The final pellet was kept for calcification at 100°C in the hot air oven. The dried pellet was then powdered in mortar and pistil. The chemical composition of nanoparticles and their crystalline structures were examined by using scanning electron microscope–energy dispersive X‐ray spectroscopy (SEM–EDX) (Quanta 200 FEG), Fourier‐transform infrared spectroscopy (FTIR) (Perkin Elmer Spectrum1 FTIR instrument) and X‐ray diffraction (XRD) (Bruker) at the Sophisticated Analytical Instrumentation Facility, Indian Institute of Technology, Chennai using established methods.
2.2 Ketoconazole loading efficiency
The drug loading efficiency of the ketoconazole was estimated at 225 nm at spectrometer. The calculation formula for ketoconazole loading capacity is listed as follows:
2.3 In vitro drug release studies
The release of drug from the nanoparticles was studied using membrane diffusion technique. The ketoconazole‐coated nanoparticles were dialysed into a beaker containing 100 ml of phosphate buffered saline pH 7.4 containing 10% v/v methanol (to maintain sink condition), which acted as a receptor compartment. The temperature of the receptor medium was maintained at 37 ± 0.5°C and agitated using a magnetic stirrer. Aliquots of 5 ml sample were withdrawn periodically, and after each withdrawal the same volume of the medium was replaced. The collected samples were analysed using an ultraviolet (UV) spectrophotometer at 225 nm. The tests were carried out in triplicate.
2.4 In vitro anti‐fungal activity
Ketoconazole‐coated ZnO nanoparticles were dissolved in sterile dimethyl sulphoxide at a concentration of 10 mg/ml. The free ketoconazole or ketoconazole–ZnO was serially diluted to 1–10 mg/ml. The anti‐fungal studies, the inoculants of Malassezia ( density of 0.5 in McFarland scale) were prepared in the sterile sabouraud liquid medium. Then, the strains were suspended in sabouraud's liquid medium (final density of 1 × 104 CFU/ml) and put into 96 well plates. Solutions of free drug, ketoconazole–ZnO and suspensions were inoculated into 96 well plates. The medium containing fungi and sterile medium was used as growth control and sterility control, respectively. The plates were cultivated under normal atmospheric conditions at 25°C for 48 h and the crystal violet solution was added to study the viability. The absorbance of crystal violet of each plate was measured in a microplate reader to calculate minimum inhibitory concentration values, defined as the lowest concentration required inhibiting the growth of half of the fungi. The experiments were performed in three parallel samples.
2.5 Gel formulations
For the semi‐solid gel preparations, carbopol 1 g, methylparaben (0.5%) 1.2 ml, propyl paraben (0.2%) 0.1 ml, propylene glycol 5 ml, triethanolamine 1.2 ml and 10 mg of ketoconazole‐coated Ag nanoparticles were mixed and packed in airtight containers.
2.6 Skin deposited and penetration studies
The dermal surface of a skin sample (area 2 cm2) was applied with 1000 mg of cream. After 4 h, the skin sample was cut into small pieces and placed for 12 h in an extraction medium [mobile phase (acetonitrile, hexane)] followed by sonication of the mixture for 15 min to extract the nanoparticles deposited within the membrane. The extraction medium was finally analysed by high‐performance liquid chromatography (HPLC‐UV) to quantify skin deposition of nanoparticles.
2.7 Clinical applications
To study the clinical aspects, the dogs with fungus infection (Madras veterinary college pet animal house dogs) were treated with the ketoconazole–ZnO nanoparticles based gel on their infected area and their severity of the infection. The cream was applied over the infection with the interval of 2 days up to 14 days. After 14 days, the erythematic lesions, scaling, hyperpigmentation, itching, primary lesions and greasy exudates were studied.
3 Results and discussion
The ketoconazole‐coated ZnO nanoparticles were synthesised using dextrose and zinc nitrate and the reaction was catalysed by using potassium hydroxide. The formed ketoconazole‐coated ZnO nanoparticles were confirmed by using SEM, FTIR, XRD and EDX spectra. The SEM‐based results indicated that the drug‐coated ZnO nanoparticles were appeared as a crystal with similar size and shape and it is also arranged in a periodic pattern and the size was around 85–90 nm (Fig. 1 a). The FTIR spectra results indicated that the ketoconazole‐coated nanoparticles showed the ZnO band at 538 cm−1, N–H stretching peak at 3085.89 cm–1, aliphatic C–H stretching at 2927.74 and 2740.66 cm−1 and the carbonyl stretching vibrations (C = O) was absorbed in 1743.53 and 1689.53 cm–1. A peak at 1612 cm–1 indicates the aromatic ring and a peak at 1238 cm–1 is due to C–O argon group of ketoconazole (Fig. 1 b). The XRD pattern results of ketoconazole‐coated ZnO nanoparticles showed the 2θ value around 32, 34, 36, 47, 57, 63, 67 and it corresponds to (60), (60), (101), (38), (50), (40) and (35). The average grain size of the nanoparticles was determined using the Debye–Scherrer formula and the diffraction peaks were more intensive and narrower, implying a good crystallinity (Fig. 1 c). The EDX detector indicated that the prepared ketoconazole‐coated nanoparticles contains C – 48.86%, N – 8.22%, O – 21.88%, Cl – 3.87%, ZnO – 16.36% and K – 0.81%. The EDX results confirmed the ketoconazole coating on the nanoparticles (Fig. 1 d). In this work, we used dextrose as a mediating agent and it offered a high concentration of –CHO functional group and it leads to a high concentration of ketoconazole loading. Without dextrose, we found the ketoconazole drug loading to the nanoparticles is 20.15 ± 1.69. However, in the dextrose‐mediated synthesis, the entrapment efficiency was increased to 65.14 ± 1.40 and the results indicated that the dextrose offered more functional molecules for drug loading. The in vitro drug efficiency studies were conducted at pH 7.4 with 10% methanol. The methanol was added to increase the diffusion of the drug. The particles showed lower drug elution from the nanoparticles and the results indicated maximum 65.85% after 24 h and it may be due to high concentration of –CHO functional group offered by the dextrose and the kinetics were also studied and it was shown in Figs. 2 a –d.
Fig. 1.
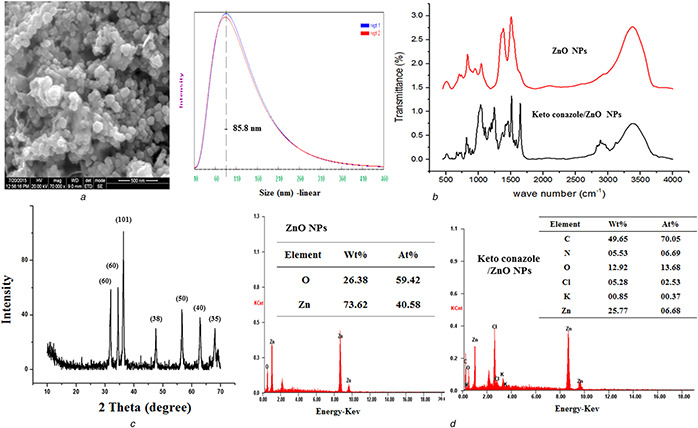
Results of physicochemical characterisations
(a) Results of SEM and the dynamic light scattering‐based particles size analysis, (b) FTIR spectrum of (i) ZnO nanoparticles and ketoconazole‐conjugated ZnO nanoparticles, (c) XRD pattern results of ketoconazole‐conjugated ZnO nanoparticles, (d) EDX spectra of ZnO nanoparticles and ketoconazole‐conjugated ZnO nanoparticles
Fig. 2.
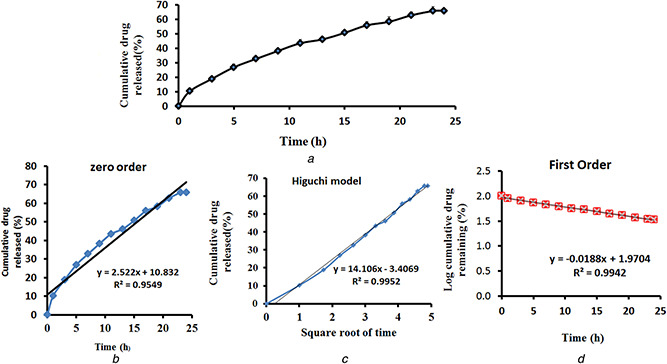
CHO functional group offered by the dextrose and the kinetics
(a) In vitro release curve of ketoconazole‐conjugated ZnO nanoparticles, (b) Results of zero‐order kinetics study, (c) Higuchi model study kinetics, (d) Results of first‐order study kinetics
For the anti‐fungal studies, the Malassezia fungus isolated from a dog is incubated serially with ketoconazole‐coated ZnO nanoparticles and the results were shown in Fig. 3. The results indicated maximum inhibition concentration was around 10 mg/ml. For the skin penetration studies, initially, 10 g of nanoparticles containing ointment was prepared. The nanoparticles permeation and deposition on skin was quantified using HPLC‐UV at a wavelength of 235 nm and the result was shown in Table 1. The cumulative permeation and deposition indicated a maximum 45% of penetration was recorded after 4 h. The ketoconazole‐coated ZnO nanoparticles gel was validated in the animal house of Madras Veterinary College, Chennai, Tamil Nadu for the treatment of Malassezia. The various parameters observed for the validation were erythema, hyperpigmentation, greasy exudates, scaling, primary lesions, itching and number of yeast colonies per frame. The scores were calculated for each of the symptom prevalent in the disease. There was a significant reduction in the score calculated for the validation of the cream. There was a complete reduction in the erythematic lesions in the dogs shown in Figs. 4 a –c. Moreover, we also observed that the hyperpigmentation of the dog was reduced after the application of the gel.
Fig. 3.
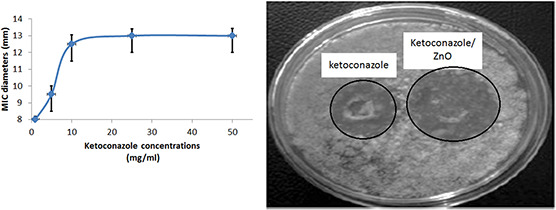
Anti‐fungal activity of ketoconazole‐conjugated ZnO nanoparticles
Table 1.
Ketoconazole–ZnO nanoparticles semi‐solid gel penetration and the deposition percentage results
| Cream formulations (amount of ketoconazole–ZnO nanoparticles/area, mg cm−2 | Cumulative penetrations, mg cm−2 | Amount deposited, mg cm−2 | Delivery percentage, % | Percentage of deposition |
|---|---|---|---|---|
| 1000 | 450 ± 5.7 | 540 ± 8.75 | 45 | 55 |
Fig. 4.
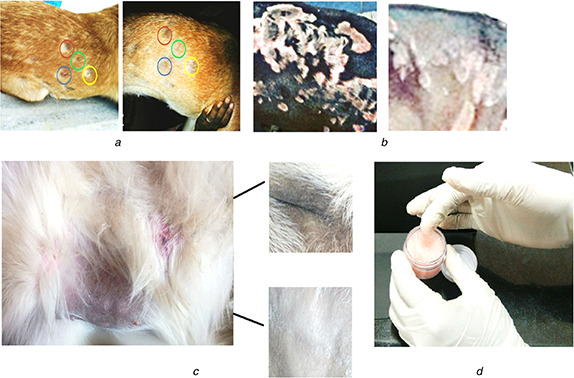
Photograph of the field animal studies
(a) Dog treated with semi‐solid gel contained ketoconazole‐conjugated ZnO nanoparticles: it cured erythematic lesions due to Malassezia, (b) Gel‐based treatment for hyperpigmentation due to Malassezia : after applied the gel, the infection got reduced, (c) Images of the developed semi‐solid gel
4 Conclusion
To conclude, we developed the ketoconazole‐coated ZnO nanoparticle‐based semi‐solid gel for the veterinary application. The nanoparticles exhibit anti‐fungal effects and the semi‐solid gel increases the dispersion of the nanoparticle and also increases the drug retention time over the skin. The semi‐ solid gel‐based delivery offers many advantages such as it can be directly applied over the infected area; small volume is required to bring about the therapeutic effect; eliminate the possibility of the increased drug in blood plasma; increased bioavailability; and increased retention time over the skin.
5 Acknowledgment
This study was funded by the Department of Biotechnology (DBT), New Delhi under the Translational Research Platform for Veterinary Biologicals, partnership programme (DBT Sanction no. 102/IFD/DBT/SAN 2680/2011‐2012) with TANUVAS, Chennai.
6 References
- 1. Petros R.A. DeSimone J.M.: ‘Strategies in the design of nanoparticles for therapeutic applications’, Nat. Rev. Drug Discov., 2010, 9, (8), pp. 615 –627 [DOI] [PubMed] [Google Scholar]
- 2. Cheow W.S. Xu R. Hadinoto K.: ‘Towards sustainability: new approaches to nano‐drug preparation’, Curr. Pharm. Des., 2013, 19, (35), pp. 6229 –6245 [DOI] [PubMed] [Google Scholar]
- 3. Wei L. Lu J. Xu H. et al.: ‘Silver nanoparticles: synthesis, properties, and therapeutic applications’, Drug Discov. Today, 2015, 20, (5), pp. 595 –601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y. Nayak T.R. Hong H. et al.: ‘Biomedical applications of zinc oxide nanomaterials’, Curr. Mol. Med., 2013, 13, (10), pp. 1633 –1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mirzaei H. Darroudi M.: ‘Zinc oxide nanoparticles: biological synthesis and biomedical applications’, Ceram. Int., 2017, 43, (1), pp. 907 –914 [Google Scholar]
- 6. Liu P.: ‘Facile preparation of monodispersed core/shell zinc oxide@polystyrene (ZnO@PS) nanoparticles via soapless seeded microemulsion polymerization’, Colloids Surf. A, Physicochem. Eng. Asp., 2006, 291, (1–3), pp. 155 –161 [Google Scholar]
- 7. Choudhury S.R. Ordaz J. Lo C.L. et al.: ‘Zinc oxide nanoparticles‐induced reactive oxygen species promotes multimodal cyto‐ and epigenetic toxicity’, Toxicol. Sci., 2017, 156, (1), pp. 261 –274 [DOI] [PubMed] [Google Scholar]
- 8. Schneider M. Stracke F. Hansen S. et al.: ‘Nanoparticles and their interactions with the dermal barrier’, Dermatoendocrinology, 2009, 1, (4), pp. 197 –206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bharti D.B. Bharti A.V.: ‘Synthesis of ZnO nanoparticles using a hydrothermal method and a study its optical activity’, Luminescence, 2017, 32, (3), pp. 317 –320 [DOI] [PubMed] [Google Scholar]
- 10. Bumajdad A. Madkour M.: ‘In situ growth of ZnO nanoparticles in precursor‐insensitive water‐in‐oil microemulsion as soft nanoreactors’, Nanoscale Res. Lett., 2015, 10, (19), pp. 1 –5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinjari D.V. Pandit A.B. Mhaske S.T.: ‘Ultrasound assisted green synthesis of zinc oxide nanorods at room temperature’, Indian J. Chem. Technol., 2016, 23, (3), pp. 221 –226 [Google Scholar]
- 12. Vafaee M. Sasani Ghamsari M.: ‘Preparation and characterization of ZnO nanoparticles by a novel sol–gel route’, Mater. Lett., 2007, 61, (14–15), pp. 3265 –3268 [Google Scholar]
- 13. Woo Lee B. Koo J.H. Lee T.S. et al.: ‘Synthesis of ZnO nanoparticles via simple wet‐chemical routes’, Adv. Mater. Res., 2013, 699, pp. 133 –137 [Google Scholar]
- 14. Lee S.D. Nam S.H. Kim M.H. et al.: ‘Synthesis and photocatalytic property of ZnO nanoparticles prepared by spray‐pyrolysis method’, Phys. Procedia, 2012, 32, pp. 320 –326 [Google Scholar]
- 15. Zhu Y. Zhou Y.: ‘Preparation of pure ZnO nanoparticles by a simple solid‐state reaction method’, Appl. Phys. A, 2008, 92, pp. 275 –278 [Google Scholar]
- 16. Lee V.H.L. Li V.H.K.: ‘Prodrugs for improved ocular drug delivery’, Adv. Drug Deliv. Rev., 1989, 3, (1), pp. 1 –38 [Google Scholar]
- 17. Cole E.T. Cade D. Benameur H.: ‘Challenges and opportunities in the encapsulation of liquid and semi‐solid formulations into capsules for oral administration’, Adv. Drug Deliv. Rev., 2008, 60, (6), pp. 747 –756 [DOI] [PubMed] [Google Scholar]
- 18. Jankowski A. Dyja R. Sarecka‐hujar B.: ‘Dermal and transdermal delivery of active substances from semisolid bases’, Indian J. Pharm. Sci., 2017, 79, (4), pp. 488 –500 [Google Scholar]
- 19. Patel M.R. Patel R.B. Parikh J.R. et al.: ‘Investigating effect of microemulsion components: in vitro permeation of ketoconazole’, Pharm. Dev. Technol., 2011, 16, (3), pp. 250 –258 [DOI] [PubMed] [Google Scholar]
- 20. Shirsand S.B. Para M.S. Nagendrakumar D. et al.: ‘Formulation and evaluation of ketoconazole niosomal gel drug delivery system’, Int. J. Pharm. Invest., 2012, 2, (4), pp. 201 –207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashe S. Nayak D. Tiwari G. et al.: ‘Development of liposome‐encapsulated ketoconazole: formulation, characterisation and evaluation of pharmacological therapeutic efficacy’, Micro Nano Lett., 2015, 10, (2), pp. 126 –129 [Google Scholar]
- 22. Ramasamy T. Khandasami U.S. Ruttala H. et al.: ‘Development of solid lipid nanoparticles enriched hydrogels for topical delivery of anti‐fungal agent’, Macromol. Res., 2012, 20, (7), pp. 682 –692 [Google Scholar]
- 23. Lehmann P.F.: ‘Immunology of fungal infections in animals’, Vet. Immunol. Immunopathol., 1985, 10, (1), pp. 33 –69 [DOI] [PubMed] [Google Scholar]


