Abstract
A growing trend within nanomedicine has been the fabrication of self‐delivering supramolecular nanomedicines containing a high and fixed drug content ensuring eco‐friendly conditions. This study reports on green synthesis of silica nanoparticles (Si‐NPs) using Azadirachta indica leaves extract as an effective chelating agent. X‐ray diffraction analysis and Fourier transform‐infra‐red spectroscopic examination were studied. Scanning electron microscopy analysis revealed that the average size of particles formed via plant extract as reducing agent without any surfactant is in the range of 100–170 nm while addition of cetyltrimethyl ammonium bromide were more uniform with 200 nm in size. Streptomycin as model drug was successfully loaded to green synthesised Si‐NPs, sustain release of the drug from this conjugate unit were examined. Prolong release pattern of the adsorbed drug ensure that Si‐NPs have great potential in nano‐drug delivery keeping the environment preferably biocompatible, future cytotoxic studies in this connection is helpful in achieving safe mode for nano‐drug delivery.
Inspec keywords: silicon compounds, nanofabrication, nanomedicine, drug delivery systems, nanoparticles, X‐ray diffraction, Fourier transform infrared spectra, scanning electron microscopy
Other keywords: nanosilica, streptomycin, nanoscale drug delivery, nanomedicine, silica nanoparticles, Azadirachta indica leaves extract, X‐ray diffraction analysis, Fourier transform‐infrared spectroscopy, scanning electron microscopy, cetyltrimethyl ammonium bromide, SiO2
1 Introduction
Nanomedicine, the use of nanotechnology for medical applications, has undergone swift development in the past several decades [1, 2, 3]. The goal of nanomedicine is for an engineer drug delivery system that can carry sufficient drug loads, efficiently cross‐physiological barriers to reach target sites. Several organic nanomedicines, as well as liposomes, drug–polymer conjugates, dendrimers, polymeric micelles and nanoparticles (NPs), have been widely considered as drug delivery systems [4].
Inorganic drug delivery systems such as gold (Au)‐NPs, quantum dots, silica NPs (Si‐NPs), FeO‐NPs, carbon nanotubes and other inorganic NPs with hollow or porous morphology have emerged as capable alternatives to organic systems for a broad range of biomedical applications. In the middle of these NPs, Si‐NPs have gained significant interest because of their sole properties acquiescent for in vivo applications [5, 6]. Mesoporous silica nanomaterials (MSNs) are widely studied for drug delivery applications [7, 8]. Therapeutic formulations of different compounds such as anti‐inflammatory agents (i.e. naproxen), antibiotics (i.e. vancomycin) and anticancer agents (i.e. carboplatin) have been developed using mobile crystalline material‐41 [8, 9], a type of MSN, was first fabricated by Beck et al. in 1992 [10].
Various physical and chemical methods are used for the synthesis of NPs. The use of toxic and hazardous chemicals in the synthesis and stabilisation of NPs leads to non‐environmentally products. Physical methods, i. e. ultraviolet (UV) irradiation, aerosol and lithography, are also not considered environment‐friendly [11, 12]. The synthesis of non‐toxic and eco‐friendly products can be achieved by replacing hazardous methods with the green chemistry procedure [13]. Plants contain clusters of phytochemicals [14], effectively used as reducing agent for eco‐friendly least toxic synthesis of NPs [15, 16].
Current study reports on green synthesis of Si‐NPs using Azadirachta indica aqueous leaf extract as an effective reducing agent without standard chemicals and secondly hybrid synthesis with plant extract and cetyltrimethyl ammonium bromide (CTAB) as surfactant and size stabilising agent. Streptomycin is used as model drug to test out the sustain release behaviour of green fabricated Si‐NPs.
2 Experimental section
2.1 Materials
All solvents and chemicals used were of analytical grade. Tetra‐ethyl‐ortho‐silicate (TEOS) Si(OC2 H5)4), CTAB (C16 H33)N(CH3)3 Br, ethanol, ethidium bromide (EtBr) and phosphate‐buffered saline (PBS) were purchased from Sigma–Aldrich. Purified streptomycin was procured from Abbott Healthcare Pvt. Ltd.
2.2 Processing of plant material
Authenticated A. indica leaves were collected from National Agricultural Research Centre Pakistan. The fresh leaves were washed with deionised H2 O then dried under shade at room temperature to avoid photolysis. For extract preparation, 20 g of dried leaves was finely grinded, powder was added in 200 ml of deionised H2 O and placed this mixture undisturbed for 2.5 h at room temperature. The mixture was then placed in shaking incubator for 2 h at 45°C at 50 rpm. Thereafter, obtained extract was filtered using Whatman No. 1 filter paper and the filtrate was collected in 250 ml Erlenmeyer flask and stored at room temperature for further usage.
2.3 Synthesis procedure
2.3.1 Green synthesis of Si‐NPs via A. indica leaf extract
About 30 ml of plant extract and 30 ml TEOS were slowly mixed together. During the process 50–60°C heat and magnetic stirring was given overnight. The following day supernatant was decanted after centrifugation at 8000 rpm and the particles were dried in drying oven at 60°C. The green fabricated Si‐NPs were then washed with distiled water 4–5 times. After water washings a sole ethanol washing was given in the same manner and dried again at 60°C in hot air oven for 2 h. Calcination was performed at 500°C for 5 h. A schematic diagram for Si‐NPs fabrication is displayed in Fig. 1.
Fig. 1.
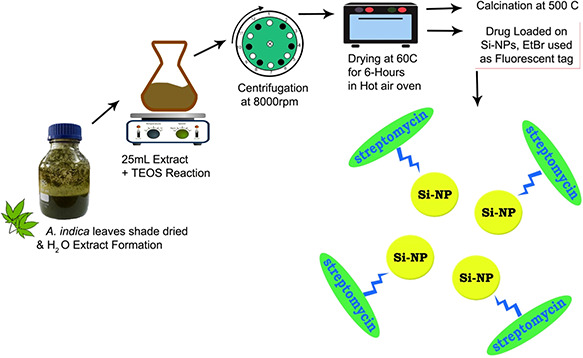
Schematic diagram of Si‐NPs green synthesis using A. indica leaf extract
2.3.2 Hybrid synthesis of Si‐NPs using A. indica leaf extract along with CTAB
For hybrid synthesis of Si‐NPs, 20 ml of plant extract and 20 ml TEOS were slowly mixed together. The ethanol–CTAB (1:0.1 ratio) solution was added to the foregoing mixture. During the process 50–60°C heat and magnetic stirring was given overnight. The next day supernatant was decanted and the particles were dried in drying oven at 60°C. About 4–5 successive washings were given to the Si‐NPs. During the washing process, distiled water was added to the NPs and stirred using magnetic stirrer and then allowed to settle. After 15–20 min the NPS got settled at the bottom and the supernatant was decanted. After water washings a sole ethanol washing was given in the same manner. After that Si‐NPs were dried in hot air oven at 60°C and calcinated at 500°C for 5 h to remove volatile impurities.
2.3.3 Fluorescent tagging with EtBr
About 0.01 gm of EtBr was added in 3 ml of distiled water. About 2.5 µl stock solutions were mixed in 15 ml of distiled water. Si‐NPs were added in the solution of EtBr and constantly stirred for 30 min at room temperature. After stirring the supernatant was decanted and 4–5 successive washings were given to the Si‐NPs. Under UV light the molecule attached with EtBr fluorescence with orange colour.
2.3.4 Si‐NPs loading with streptomycin
For Si‐NPs loading with streptomycin, 0.5 gm of streptomycin was dissolved in 10 ml of acetone. In the drug solution, 0.5 gm of Si‐NPs were added. The above mixture was stirred for 24 h at room temperature. After stirring, it was centrifuged and air dried to obtain the drug loaded Si‐NPs.
2.4 Characterisation
2.4.1 Field emission scanning electron microscopy
Si‐NPs and CTAB Si‐NPs scanning electron microscopy (SEM) analysis were performed using MIRA3 TESCAN [field emission (FE)‐SEM] operating at 20 kV. Si‐NPs and CTAB Si‐NPs were suspended in deionised water at a concentration of 1 mg/ml and then sonicated using a sonicator bath until the sample forms a homogeneous suspension. For size measurement, the sonicated stock solution of Si‐NPs and CTAB Si‐NPs (1 mg/ml) was diluted 20 times. After this one drop of the sonicated aqueous solution was taken on a glass plate and allowed to dry. Further the sample was Au coated and images were taken. Energy‐dispersive X‐ray (EDX) spectrum attached along with the FE‐SEM was also plotted and analysed.
2.4.2 X‐ray diffraction (XRD) spectroscopy
To examine crystallographic structure of green synthesised Si‐NPs and green synthesised CTAB Si‐NPs, XRD analysis was carried out using PANalytical X'Pert3 powder with nickel monochromator in the range of 2θ from 10° to 80° using Cu Kα radiation of wavelength 1.5406 Å. Operating voltage of 40 kV with 30 mA current provided at room temperature.
2.4.3 Fourier transform infra‐red spectroscopy
For furthermore structural evaluation of EtBr‐tagged Si‐NPs, EtBr‐tagged CTAB Si‐NPs, streptomycin loaded EtBr‐tagged Si‐NPs, streptomycin loaded EtBr‐tagged CTAB Si‐NPs, A. indica green extract and a residue, Fourier transform‐infra‐red (FT‐IR) spectroscopy were performed using potassium bromide pellet methodology by SHIMADZU FT‐IR wave number ranged 400‐4000 cm−1. FT‐IR spectra of all samples were recorded separately and analysed comparatively.
2.5 Drug (streptomycin) sustain release pattern
To observe sustain release pattern of streptomycin from Si‐NPs, 0.5 mg drug loaded Si‐NPs were mixed in 1 ml PBS (pH = 7.4) buffer. After that mixture was kept in the 37°C shaking water bath for 30 min. After 30 min the mixture was centrifuged for 2 min at 8000 rpm. The PBS buffer was decanted and its optical density was checked at 220 nm under UV–visible spectrophotometer.
3 Results and discussion
3.1 Si‐NPs synthesis analysis
In the green synthesis of Si‐NPs, A. indica leaves extract was used as reducing and capping agent. Si‐NPs were prepared using two different experimental procedures. In both procedures, TEOS was used as a silica precursor and A. indica leaves extract as reducing agent, whereas in the second procedure CTAB was used as a surfactant. White powdered Si‐NPs were obtained from both procedures as shown in Fig. 2. The physical difference observed in the particles was their weight, particles in which CTAB was used as surfactant were light in weight as compared with the particles without it. Less dense hollow particles were formed by the addition of CTAB as it acts as structure directing agent.
Fig. 2.

White powdered Si‐NPs
(a) Prepared from plant extract, (b) TEOS and CTAB, prepared from plant extract and TEOS
3.2 FE‐SEM and EDX analyses
Figs. 3 and 4 show the FE‐SEM images of the green synthesised Si‐NPs. The figures show that the Si‐NPs are spherical in shape. The average size of particles formed using plant extract as reducing agent without any surfactant is in the range of 100–170 nm. The other types of particles synthesised with the addition of CTAB were uniform with 200 nm in size. The SEM images also revealed that the spherical Si‐NPs formed are unevenly distributed and agglomerated. Chitra and Annadurai [17] also synthesised spherical unevenly distributed agglomerated Si‐NPs in the size range of 151–165 nm by using chemical methods.
Fig. 3.

FE‐SEM images of Si‐NPs synthesised using plant extract
Fig. 4.

FE‐SEM images of Si‐NPs synthesised using plant extract and CTAB
EDX spectrum of green synthesised Si‐NPs is shown in Figs. 5 a and b. The EDX results confirm the presence of element silica. The green fabricated Si‐NPs show optical absorption band peak at 1.8 keV also reported in previous studies [17]. There is no difference in results for particles synthesised with CTAB and without CTAB. The EDX spectrum also displays the presence of oxygen also complies with FT‐IR studies.
Fig. 5.
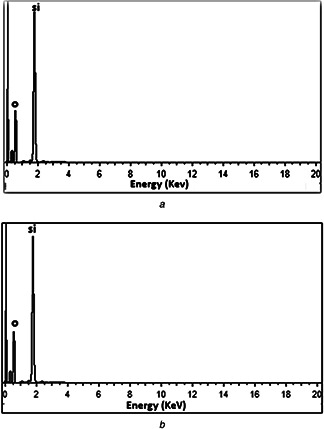
EDX spectrum of Si‐NPs
(a) Without CTAB, (b) With CTAB
3.3 XRD analysis
Fig. 6 shows the XRD spectrum of green synthesised Si‐NPs. The XRD spectrum shows that the Si‐NPs are amorphous in nature. The XRD results show a characteristic broad halo peaks at 23.5° and 22.6° on both types of particles. The 2θ value of the present XRD spectrum result was compared and confirmed by using standard JCPDS file and the obtained XRD result is matched with JCPDS file (79‐1711). The characteristic peak in the XRD pattern states that the Si‐NPs are amorphous [18]. The particles synthesised with and without surfactant CTAB show the same results. The consequences also indicates that plant extract can also synthesise amorphous Si‐NPs.
Fig. 6.
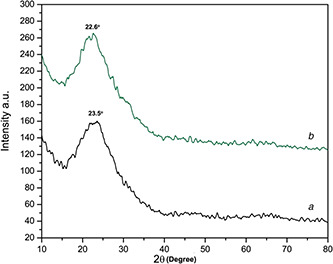
XRD spectrum
(a) Green synthesised Si‐NPs, (b) Green synthesised CTAB Si‐NPs
3.4 FT‐IR spectroscopy analysis
Fig. 7 reflects FT‐IR spectrum of Si‐NPs and streptomycin loaded Si‐NPs, respectively. Figs. 7 a and b show stretching frequencies at ∼800 cm−1 is due to Si–O bond [18]. The strongest IR band range at ∼1650 cm−1 shows the presence of Si–O–Si bonding, whereas absorption bands at ∼1035 to ∼1065 cm−1 also show Si–O–Si bonding. The intensity of bond is more in the sample without surfactant. The absorption band ∼1800 cm−1 due to the presence of –OH group [17, 19]. The band ∼1200 cm−1 in Fig. 7 b depicts the presence of CTAB. In Figs. 7 c and d, both samples show similar absorption spectrum with stretching frequency ∼400, 1000–1250 and 1600–1700 cm−1. The frequency range of 1000–1250 and 1600–1700 cm−1 present the presence of streptomycin [20]. Figs. 7 e and f represent the formulation of nano‐silica through A. indica plant extract, comparative analysis shows that nitro‐compounds from plant extract were acting as active capping agents in tuning nano‐silica. Peak shift in residue (Fig. 7 f) further validates the synthesis procedure.
Fig. 7.
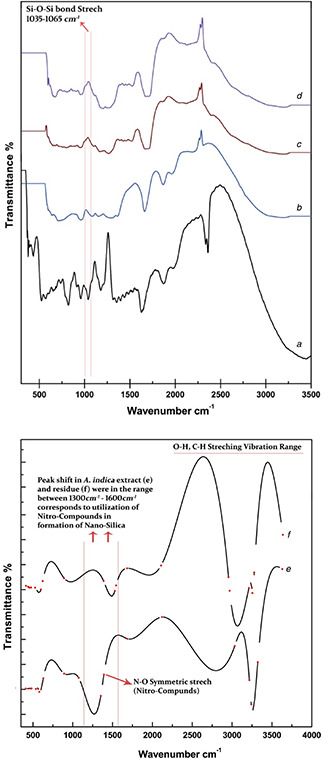
FT‐IR spectrum of
(a) EtBr‐tagged Si‐NPs, (b) EtBr‐tagged CTAB Si‐NPs, (c) Streptomycin loaded EtBr‐tagged Si‐NPs, (d) Streptomycin loaded EtBr‐tagged CTAB Si‐NPs, (e) A. indica leaf extract, (f) Residue drained out after reaction
3.5 Drug (streptomycin) sustain release pattern studies
Fig. 8 shows the drug streptomycin releasing pattern from Si‐NPs. The release was checked in PBS buffer with pH 7.4 at 37°C. The pH value of 7.4 mimics the physiological pH of blood [21]. The drug release pattern shows a continuous release till 570 min with a maximum of 15% adsorbed drug released from silica nano‐carriers prepared with surfactant and 15.5% maximum release for Si‐NPs prepared without surfactant. The Si‐NPs prepared without surfactant showed 15% maximum release at 330 min as compared with Si‐NPs prepared with surfactant which showed it at 450 min. The continuous release pattern shows that the green synthesised Si‐NPs potentially act as a vehicle for drug delivery with the advantage of biocompatibility and non‐toxic protective surface.
Fig. 8.
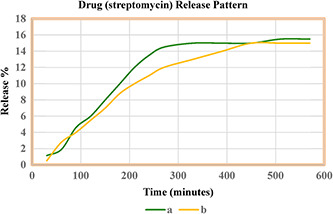
Drug streptomycin releasing pattern from Si‐NPs
(a) Si‐NPs synthesised using plant extract without surfactant, (b) Si‐NPs synthesised using plant extract with surfactant
4 Conclusion
From the above investigation, it can be deduced that Si‐NPs can be synthesised utilising green chemistry approach without addition of any synthetic chemical as reducing agent. The use of plant extract is not only economical but also environment‐friendly. Present study reflects that plant extract had efficiently worked as reducing agent as like any other chemical reducing agent commonly applied in the synthesis of metallic NPs. The green synthesised biocompatible Si‐NPs will further be investigated in the future to observe their cytotoxic behaviour for healthy cells, work in this regard helps us to use these nano‐engineered structures in safe and targeted drug delivery.
5 Acknowledgment
Author acknowledges Muhammed Fahad Shams of Preston Institute of Nano Science and Technology (PINSAT) for providing technical assistance regarding SEM operations.
6 References
- 1. Jain R.K. Stylianopoulos T.: ‘Delivering nanomedicine to solid tumors’, Nat. Rev. Clin. Oncol., 2010, 7, (11), pp. 653 –664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peer D. Karp J.M. Hong S. et al.: ‘Nanocarriers as an emerging platform for cancer therapy’, Nat. Nanotechnol., 2007, 2, (12), pp. 751 –760 [DOI] [PubMed] [Google Scholar]
- 3. Kim B.Y. Rutka J.T. Chan W.C.: ‘Nanomedicine’, N. Engl. J. Med., 2010, 363, (25), pp. 2434 –2443 [DOI] [PubMed] [Google Scholar]
- 4. O'brien M.E.R. Wigler N. Inbar M.C.B.C.S.G. et al.: ‘Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first‐line treatment of metastatic breast cancer’, Ann. Oncol., 2004, 15, (3), pp. 440 –449 [DOI] [PubMed] [Google Scholar]
- 5. Slowing I.I. Vivero‐Escoto J.L. Wu C.W. et al.: ‘Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers’, Adv. Drug Deliv. Rev., 2008, 60, (11), pp. 1278 –1288 [DOI] [PubMed] [Google Scholar]
- 6. Barbe C. Bartlett J. Kong L. et al.: ‘Silica particles: a novel drug‐delivery system’, Adv. Mater., 2004, 16, (21), pp. 1959 –1966 [Google Scholar]
- 7. Di Pasqua A.J. Yuan H. Chung Y. et al.: ‘Neutron‐activatable holmium‐containing mesoporous silica nanoparticles as a potential radionuclide therapeutic agent for ovarian cancer’, J. Nucl. Med., 2013, 54, (1), pp. 111 –116 [DOI] [PubMed] [Google Scholar]
- 8. Di Pasqua A.J. Wallner S. Kerwood D.J. et al.: ‘Adsorption of the PtII anticancer drug carboplatin by mesoporous silica’, Chem. Biodivers., 2009, 6, (9), pp. 1343 –1349 [DOI] [PubMed] [Google Scholar]
- 9. Halamová D. Zeleňák V.: ‘NSAID naproxen in mesoporous matrix MCM‐41: drug uptake and release properties’, J. Incl. Phenom. Macrocyclic Chem., 2012, 72, (1‐2), pp. 15 –23 [Google Scholar]
- 10. Beck J.S. Vartuli J.C. Roth W.J. et al.: ‘A new family of mesoporous molecular sieves prepared with liquid crystal templates’, J. Am. Chem. Soc., 1992, 114, (27), pp. 10834 –10843 [Google Scholar]
- 11. Li X. Xu H. Chen Z.S. et al.: ‘Biosynthesis of nanoparticles by microorganisms and their applications’, J. Nanomater., 2011, p. 8 [Google Scholar]
- 12. Nath D. Banerjee P.: ‘Green nanotechnology – a new hope for medical biology’, Environ. Toxicol. Pharmacol., 2013, 36, (3), pp. 997 –1014 [DOI] [PubMed] [Google Scholar]
- 13. Park Y.S. Hong Y.N. Weyers A. et al.: ‘Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles’, IET Nanobiotechnol., 2011, 5, (3), pp. 69 –78 [DOI] [PubMed] [Google Scholar]
- 14. Saxena M. Saxena J. Nema R. et al.: ‘Phytochemistry of medicinal plants’, J. Pharmacognosy Phytochemistry, 2013, 1, (6) [Google Scholar]
- 15. Thovhogi N. Diallo A. Gurib‐Fakim A. et al.: ‘Nanoparticles green synthesis by Hibiscus sabdariffa flower extract: main physical properties’, J. Alloys Compd., 2015, 647, pp. 392 –396 [Google Scholar]
- 16. Arumugam A. Karthikeyan C. Hameed A.S.H. et al.: ‘Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties’, Mater. Sci. Eng. C, 2015, 49, pp. 408 –415 [DOI] [PubMed] [Google Scholar]
- 17. Chitra K. Annadurai G.: ‘Fluorescent silica nanoparticles in the detection and control of the growth of pathogen’, J. Nanotechnol., 2013. [Google Scholar]
- 18. Stanley R. Nesaraj A.S.: ‘Effect of surfactants on the wet chemical synthesis of silica nanoparticles’, Int. J. Appl. Sci. Eng., 2014, 12, (1), pp. 9 –21 [Google Scholar]
- 19. Thuc C.N.H. Thuc H.H.: ‘Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method’, Nanoscale Res. Lett., 2013, 8, (1), pp. 1 –10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meanwell R.J. Shama G.: ‘Direct FTIR assay of streptomycin in agar’, Biotechnol. Lett., 2005, 27, (20), pp. 1629 –1631 [DOI] [PubMed] [Google Scholar]
- 21. Suhre K. Shin S.Y. Petersen A.K. et al.: ‘Human metabolic individuality in biomedical and pharmaceutical research’, Nature, 2011, 477, (7362), pp. 54 –60 [DOI] [PMC free article] [PubMed] [Google Scholar]


