Abstract
The current study was performed to synthesize stable, eco‐friendly and bio‐compatible silver nano‐particles (AgNPs) of Agave americana, Mentha spicata and Mangifera indica leaves and to screen them for biological activities. The ultraviolet‐visible spectroscopic analysis revealed that λ‐max for AgNPs range from 350–500 nm. All AgNPs possessed polycrystalline structure as notified as intense graphical peaks in complete spectrum of 20 values ranging from 10–80° in X‐ray diffraction measurements and supported by scanning electron microscopy data. The size of the nano‐particles was confirmed by transmission electron microscopy (30–150 nm). Mass loss at variable temperatures was evaluated by simultaneous thermogravimetric and differential thermal analysis revealed reduction in mass and activity of compounds was notified by temperature increase from 200 to 800 °C, thus concluding it as thermally sensitive compounds. A. americana AgNPs showed significant (96%) activity against Methicillin resistant Staphylococcus aureus, Escherichia coli (95%) and Fusarium oxysporum (89%). Good antioxidant activity was shown by M. spicata AgNPs at 300 µl (79%). M. indica AgNPs showed significant phytotoxic activity (88%) at highest concentration. No haemagglutination reaction was observed for the test samples. The above results revealed that AgNPs synthesized from selected plant species possesses significant antimicrobial and phytotoxic effect.
Inspec keywords: silver, nanoparticles, nanofabrication, X‐ray diffraction, transmission electron microscopy, scanning electron microscopy, differential thermal analysis, microorganisms, antibacterial activity, nanomedicine, particle size, toxicology
Other keywords: green synthesis, biological evaluation, Agave americana aqueous leave extract, Mentha spicata aqueous leave extract, Mangifera indica aqueous leave extract, stable ecofriendly biocompatible silver nanoparticles, ultraviolet‐visible spectroscopy, polycrystalline structure, X‐ray diffraction, scanning electron microscopy, nanoparticle size, transmission electron microscopy, thermogravimetric analysis, differential thermal analysis, mass loss, thermally sensitive compounds, Methicillin resistant Staphylococcus aureus, Escherichia coli, Fusarium oxysporum, antioxidant activity, phytotoxic activity, plant species, antimicrobial effect, temperature 200 degC to 800 degC, Ag
1 Introduction
Green synthesis of silver nano‐particles (AgNPs) is transpiring domain of nanotechnology as the techniques exploited are reckoned to be environment‐friendly and impregnable than other alternative conventional strategies [1]. Manipulations of AgNPs specifically from plant extracts are entailed by the global commerce due to their substantial pertinence in health and industrial sphere [2]. AgNPs play a prime part in fields of clinical therapeutics, predominantly antimicrobial and anti‐inflammatory prospective are highly reputed. They are also accounted to be utilised in medical devices along bare quandary of nanotoxicity [3].
Agave americana, a monocarpic and succulent plant belonging to family Agavaceae, having 2 m lingering greyish‐green leaves and huge yellow flowers [4]. It is native drought tolerant Mexican plant but is dispersedly cultivated across Pakistan, India and other Mediterranean countries [5]. The long fibres that exist in the leaves are availed to produce rope, mats and sturdy clothing. A. americana saccharine nectar is commercialised as natural sugar comprehending less glycemic index due to exorbitant fructose contents. The sap are cumulated and brewed from flowering stems to produce alcoholic drinks such as tequila and pulque [6].
Mentha spicata habitually entitled as spear mint is herbaceous perennial small plant that belongs to family Lamiaceae [7]. It is native to Europe and South Asia but widely distributed across the planet [8]. It is used to manufacture spearmint oil which possesses carminative and aromatic properties. It is also used in manufacturing of soaps, shampoos, toothpaste and confectioneries due to its strong essence [9]. It possesses tremendous antioxidant, antimicrobial properties and is widely used as seasoning by the Indians [10, 11].
Mangifera indica conventionally known as mango plant belongs to family Anacardiaceae. It is cultivated extensively across temperate regions of the world but wild variety is native to India. It is used in traditional folk medicine as anti‐emetic, anti‐diuretic, anti‐diarrheal, acidity, kidney and heart problems [12].
The green synthetic AgNPs have a significant action against various health problems; the present research activity was performed by using the aqueous leaves extracts of A. americana, M. spicata and M. indica for the synthesis, characterisation and in vitro biological screening of AgNPs.
2 Materials and methods
2.1 Plant materials
Leaves of A. americana, M. spicata and M. indica were collected from District Peshawar, Khyber Pakhtunkhwa, Pakistan and were identified by Ghulam Jelani, Department of Botany, University of Peshawar, Pakistan.
2.2 Extraction
Leaves of selected plants were washed with water. The cleaned leaves were shade dried and pulverised into fine powder using electrical grinder. Aqueous plant extracts were prepared by boiling 25 g of pulverised leaves in 500 ml of sterile distilled water for 30 min. The aqueous extracts were cooled and filtered at room temperature with Whattman No. 1.
2.3 Synthesis of silver nanoparticles
Aqueous extract (10 ml) was added to 90 ml of AgNO3 (1 mM) solution at room temperature. The concoction was subjected to shaking water bath at 75° for 1 h, changing the color from yellow to dark brownish black indicating reduction of Ag+ ions to Ago nanoparticles. Finally prepared nanoparticles solution was concentrated under vacuum at 50° using rotary evaporator. Concentrated green synthesised AgNPs were dried at room temperature in sterile petri plates.
2.4 Characterisation of silver nanoparticles
2.4.1 Ultraviolet–visible spectroscopy (UV–vis) spectroscopy
The optical properties of AgNPs were measured by UV–vis spectrophotometer (Shimadzu UV‐1601), operated at a resolution of 10 nm between 350–500 nm.
2.4.2 X‐ray diffraction measurements (XRD)
Crystalline metallic properties of AgNPs were examined by XRD (JDX‐3532) equipped with Cu Ê (á) radiation of 1.54187 nm, using Ni as filter at 30 kV/30 mA.
2.4.3 Scanning electron microscopy (SEM)
Using SEM (JEOL‐JSM‐5910), thin films of AgNPs were subjected on carbon coated copper grid. Extra solution was wiped out with blotting paper. The thin film was then allowed to dry by placing it under mercury lamp for 5 min and subjected to electron microscope for evaluation at 150, 500 and 1000× magnification.
2.4.4 Transmission electron microscopy (TEM)
TEM was conducted utilising a TEM (Techni‐G2‐300kV). By dropping very small amount of sample onto the carbon coated copper grid, thin films of sample were prepared. Extra solution was scraped off with blotting paper and then was allowed to dry by putting it under a mercury lamp for 5 min [13].
2.4.5 Energy‐dispersive X‐ray spectroscopy (EDX)
xElemental composition of AgNPs was determined by EDX (INCA‐200) to eliminate the possibility of presence of other elements.
2.4.6 Simultaneous thermogravimetric and differential thermal analysis (TG‐DTA)
Evaluation of physical and chemical properties of AgNPs was determined by simultaneous thermo‐gravimetric and differential thermal analysis (Shimadzu DTG‐60/DTG‐60A). Mass gain and loss of nanoparticles was determined at variable increasing temperatures.
2.5 In vitro biological studies of silver nanoparticles
2.5.1 Antibacterial activity
Antibacterial activity of AgNPs were determined against Pseudomonas aeruginosa, Methicillin resistant Staphylococcus aureus (MRSA), Vancomycin resistant S. aureus (VRSA), Proteus mirabilis, Escherichia coli, Streptomyces griseus and Bacillus subtilis using the agar well diffusion assay [14].
Autoclaved nutrient agar (Sigma‐Aldrich, Germany) was poured into sterilised Petri‐plates. Uniform bacterial lawn was prepared on Petri‐plates using 18–24 h old culture and 6 mm wells were made using a sterile borer. 100 µl from stock solution (3 mg/ml) of AgNPs in dimethyl sulphoxide (DMSO, <1%) was poured into respective wells. Amoxicillin and DMSO were used as positive and negative control, respectively. After 24 h of incubation at 37°, zones of inhibition (mm) were measured and by using the given formula, per cent inhibition was calculated.
2.5.2 Antifungal activity
Antifungal activity of AgNPs was determined against Verticillium dahliae, Aspergillus niger, Aspergillus parasitica, F. oxysporum and Penicillium notatum using agar tube dilution assay [14]. Stock solution (24 mg/ml) of AgNPs was prepared in DMSO. 4 ml Sabouraud dextrose agar (Sigma‐Aldrich Germany) was poured in test tubes and autoclaved at 121° for 15 min at 21 Psi. After autoclavation the tubes were allowed to cool (50°), 66.6 µl from stock solution was added and allowed to solidify in slanting position. With the help of sterilised inoculating loop, fresh culture of test fungi was inoculated to labelled slants. Miconazole and DMSO were used as positive and negative control, respectively. All the test tubes were incubated at 28 ± 1° for 5–7 days. After incubation, the visible linear growth inhibitions of fungal strains were calculated in comparison with controls.
2.5.3 Antioxidant activity
Antioxidant activity of AgNPs were evaluated by their ability to eliminate the free radicals using 1, 1‐diphenyl‐2‐8 picrylhydrazyl (DPPH) as per reported procedure [14]. The test samples were prepared at concentration of 100, 200 and 300 µg/ml. Absorbance at 517 nm was determined after half an hour at room temperature and finally antioxidant activity were calculated as percentage of free radical reduction. Experiment was run in triplicate. DPPH was used as a reference compound.
2.5.4 Phytotoxic activity
AgNPs were evaluated for phytotoxic activity against Lemna minor as per reported procedure [14]. 20 mg/ml stock solutions of nanoparticles were prepared in methanol. E‐medium was prepared for the growth of L. minor. Test samples at concentration of 10, 100 and 1000 µg/ml were poured to sterile flask and methanol was allowed to evaporate. After evaporation, 20 ml of the E‐medium was poured to all flasks. Finally 16 healthy L. minor were picked and transferred to each flask and incubated at 27 ± 1° for 7 days. The results were recorded at the end of incubation period by counting the damaged plants.
2.5.5 Haemagglutination activity
Haemagglutination activity of AgNPs was determined as per procedure [14]. Stock solution (1 mg/ml) was prepared in phosphate buffer (pH 7.0) and different dilutions (1:2, 1:4, 1:8 and 1:16) were prepared. Fresh blood samples from healthy volunteers were collected and centrifuged to isolate red blood cells (RBC). Using phosphate buffer, 2% RBC's suspension was prepared. From each dilution, 1 ml of test sample was transferred to sterilised test tube and 1 ml of the RBC's suspension was then added to it. Reaction mixture was incubated at 37 °C for 30 min. The test tubes were centrifuged to observe positive and negative results indicated by rough and smooth button formation, respectively.
3 Results and discussion
3.1 Characterisation of silver nanoparticles
3.1.1 UV–vis spectroscopy
From UV–vis spectroscopic analysis it was validated that the ƛ‐max for the AgNPs lies in range of 350–500 nm. The ƛ‐max for A. americana and M. indica AgNPs was 430 nm with absorbance of 0.72 and 0.81, respectively, while for M. spicata, it was 410 nm with 0.68 absorbance. Results of spectroscopic analysis are presented in Fig. 1.
Fig. 1.

UV–vis spectroscopic analysis of green synthesised AgNP from selected plants
3.1.2 X‐ray diffraction measurements
From crystallographic evaluation of the biosynthesised AgNPs, it was validated that all of the three AgNPs possessed polycrystalline structure with an intense graphical peaks in complete spectrum of 2θ values, ranging from 10–80° as shown in Figs. 2 a –c.
Fig. 2.
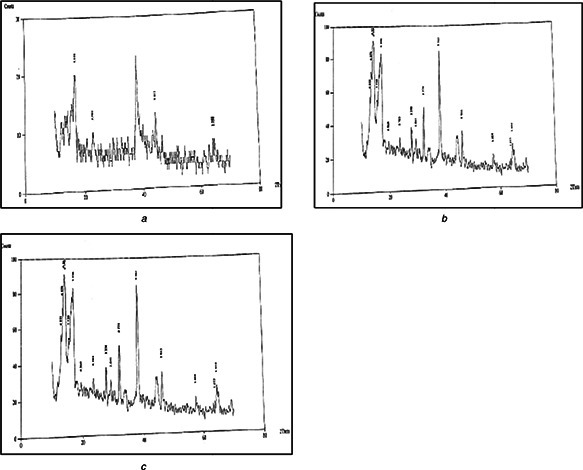
a, b, c: XRD spectra of AgNPs of the selected plants
a Agave americana
b Mentha spicata
c Mangifera indica
3.1.3 Scanning electron microscopy
At 500 and 1000× in SEM, the structure and shape of synthesised AgNPs were revealed as polycrystalline as shown in Figs. 3 a –c.
Fig. 3.

a, b and c: SEM of AgNPs of the selected plants
a Agave americana
b Mentha spicata
c Mangifera indica
3.1.4 Transmission electron microscopy
The TEM analysis revealed that major particle size falls in the range of 30–150 nm. However, few particles of 15–20 nm were also spotted. Conformations of fabricated AgNPs were localised as spherical, semi‐spherical, oblong, rods and scarily triangular. The obtained results were supported by the reported work of Jingqi et al. [15] Results are displayed in Figs. 4 a –c.
Fig. 4.

a, b and c: Transmission Electron Microscopic analysis of AgNPs of selected plants
a Agave americana
b Mentha spicata
c Mangifera indica
3.1.5 Energy‐dispersive X‐ray spectroscopy
From EDX analysis (Figs. 5 a –c), it was revealed that composition of AgNPs is precise and consists of 34.91% (by weight) of silver for A. americana, 9.96% for M. spicata and 9.93% for M. indica, along with other elements; organic carbon, oxygen, magnesium, silicon, sulphur, chlorine, potassium and calcium.
Fig. 5.

a, b and c: EDX of AgNPs of selected plants
a Agave americana
b Mentha spicata
c Mangifera indica
3.1.6 Simultaneous thermogravimetric and differential thermal analysis
The reduction in mass and activity of nanoparticles occurred as temperature increased from 200–800 °C as depicted in Figs. 6 a –c of TG‐DTA spectra. The analysis revealed that the stated nanoparticles are temperature sensitive.
Fig. 6.

a, b and c: TG‐DTA of AgNPs of selected plants
a Agave americana
b Mentha spicata
c Mangifera indica
3.2 Biological activities of silver nanoparticles
3.2.1 Antibacterial activity
AgNPs of A. americana possess significant antibacterial potential against MRSA (96%) and E. coli (95%). Good activity was observed against VRSA (76%), P. mirabilis (72%) and B. subtilus (64%). Moderate activity was observed against P. aeruginosa (48%) and S. griseus (45%). AgNPs of M. spicata showed significant inhibitory potential against E. coli (83%), moderate activity was observed against MRSA (57%), P. merabilis (56%), P. aeruginosa (55%), VRSA (46%) and S. griseus (45%) and low activity against B. subtilis (20%). M. indica AgNPs exhibited good antibacterial activity against; B. subtilis (76%), P. aeruginosa (74%), S. griseus (70%), MRSA (69%) and P. mirabilis (60%). Moderate activity was observed against E. coli (52%) and VRSA (42%). Results are presented in Fig. 7.
Fig. 7.

Antibacterial activity of AgNPs of selected plants
From antibacterial studies of biosynthesised AgNPs, it has been manifested that AgNPs possessed pre‐eminent antibacterial prospective to disrupt microbial membranes, prevent biofilm formation, obstruct replication by intercalating between bases or proliferate reactive species formations which act as bacteriostatic and bactericidal agents, which can assist in industrial, agricultural and medical sphere to cease and terminate pandemics of both Gram positive and negative bacterial infections particularly caused by Klebsiella, E. coli, Proteus, Pseudomonas and Staphylococcus species [16, 17, 18].
3.2.2 Antifungal activity
The AgNPs of A. americana possessed significant antifungal activity against F. oxysporum (89%) and V. dahliae (82%), good activity against A. niger (70%) and moderate activity was observed against P. notatum (55%). M. indica AgNPs possessed significant inhibitory effect against A. niger (80%), moderate inhibitory effect was observed against P. notatum (51%) and F. oxysporum (41%). The test sample was found inactive against V. dahliae and A. parasiticus. M. spicata AgNPs showed low activity against P. notatum (39%) and V. dahliae (12%) while it was inactive against the other test fungi. Results are given in Fig. 8.
Fig. 8.

Antifungal activity of AgNPs of the selected plants
From the antifungal studies of AgNPs conducted earlier, it has been manifested that green synthesised AgNPs have the capacity to hinder the growth of fungal strains particularly Alternaria brassicicola, Trichoderma harizanum and F. oxysporum by shattering their membranes and increased endocytosis leads to ROS formation which terminate growth of fungal species by blocking its genomic machinery and thus can be used in treatment of fabrics mainly silk and cotton [18].
3.2.3 Antioxidant activity
All the tested AgNPs presented good antioxidant potential at the dilutions of 200 and 300 µl with values of 73 and 68%, 79 and 73% and 64 and 62% for A. americana, M. spicata and M. indica, respectively, as shown in Fig. 9. Earlier reported studies have displayed that biosynthesised AgNPs of various plants exhibited good antioxidant activity in comparison with the plant extract and thus they are helpful to treat many oxidative stress related diseases [19, 20].
Fig. 9.

Antioxidant activity of AgNPs of the selected plants
3.2.4 Phytotoxic activity
The synthesised AgNPs were screened for phytotoxic activity against L. minor at various concentrations. The results revealed that AgNPs of M. indica showed significant phytotoxic potential (88%) at concentration of 1000 µl, moderate (50%) at 100 µl and low activity (25%) was observed at 10 µl. A. americana AgNPs showed good (63%) phytotoxic activity at higher concentration (1000 µl) while at lower concentrations (100 and 10 µl) the test sample showed low activity. The phytotoxic activity of AgNPs of M. spicata was 50, 31 and 13% at 1000, 100 and 10 µl, respectively, as shown in Fig. 10. Results depict strong herbicidal potentials by blocking its vegetative pathways particularly vascular bundles.
Fig. 10.

Phytotoxic activity of AgNPs of the selected plants
3.2.5 Haemagglutination activity
The result of haemagglutination activity of AgNPs at all dilutions; 1:2, 1:4, 1:8 and 1:16 was negative as shown by smooth button formation. The results revealed that the nano‐particles of the selected plants species lack phytolectins.
4 Conclusion
From the above results it may be concluded that the selected plant species can be used for the synthesis of AgNPs. AgNPs (30–150 nm) of A. americana, M. spicata and M. indica leaves extract possessed remarkable antimicrobial activity and thus can be exploited in pharmaceutical industry to formulate topical creams and ointments. It can also be used in food and dairy industry to prevent food borne infections and spoilage. At various dilutions, good and moderate antioxidant activity was observed by the biosynthesised AgNPs. Significant phytotoxic activity of the AgNPs may be a green sign for its utilisation by industrial and agricultural sectors to manufacture non‐toxic herbicides. Finally no haemagglutination activity was observed due to absence of phytolectins.
5 Acknowledgment
The authors are thankful to Directorate of Science and Technology (DOST) Government of Khyber Pakhtunkhwa, Pakistan, for financial support.
6 References
- 1. Awwad A.M. Salem N. Abdeen A.: ‘Biosynthesis of silver nanoparticles using Olea europaea leaves extract and its antibacterial activity’, J. Nanosci. Nanotechnol., 2012, 2, (6), pp. 164 –170 (doi: 10.5923/j.nn.20120206.03) [DOI] [Google Scholar]
- 2. Kholoud M. El‐Nour A. Eftaiha A. et al.: ‘Synthesis and applications of silver nanoparticles’, Arab. J. Chem., 2010, 3, (3), pp. 135 –140 (doi: 10.1016/j.arabjc.2010.04.008) [DOI] [Google Scholar]
- 3. Prabhu S. Poulose E.K.: ‘Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effect’, Int. Nano Lett., 2012, 2, p. 32 (doi: 10.1186/2228-5326-2-32) [DOI] [Google Scholar]
- 4. Royal Horticulture Society : ‘A – Z encyclopedia of garden plants’ (Dorling Kindersley, United Kingdom, 2008), p. 1136 [Google Scholar]
- 5. Heywood V.H. Valentine D.H. Tutin T.G. et al.: ‘Flora Europaea’, Cambridge University Press, Cambridge, 1993, p. 464 [Google Scholar]
- 6. Oudhia P.: ‘ Agave americana L. medicinal plants/plantes médicinales 1. Wageningen, Netherlands’, Prota, 2007, 11, (1), pp. 1 –3 [Google Scholar]
- 7. Blamey M. Wilson G.C.: ‘Flora of Britain and Northern Europe’. 1989, available at http://en.wikipedia.org/wiki/Sorbus_chamaemespilus. Accessed 15 January 2015
- 8. Mentha spicata, Natural resources conservation Service plants database. USDA. Available at http://plants.usda.gov/core/profile?symbol=Mesp3. Accessed 15 January 2015
- 9. Hussain I.A. Anwar F Nigam S.P. et al.: ‘Seasonal variation in content, chemical composition, antimicrobial and cytotoxic activities of essential oils from four Mentha species’, Sci. Food Agric., 2010, 90, (11), pp. 1827 –1836 [DOI] [PubMed] [Google Scholar]
- 10. Konstantia A. Afroditi S. Stella K. et al.: ‘Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi’, J. Agric. Food Chem., 1998, 46, (5), pp. 1739 –1745 (doi: 10.1021/jf9708296) [DOI] [Google Scholar]
- 11. Kanatt S.R. Ramesh C. Arun S.: ‘Antioxidant potential of mint (Mentha spicata) in radiation‐processed lamb meat’, Food Chemistry., 2007, 100, (2), pp. 451 –458 (doi: 10.1016/j.foodchem.2005.09.066) [DOI] [Google Scholar]
- 12. Barreto J.C. Trevisan T.S. Hull W.E. et al.: ‘Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.)’, J. Agric. Food Chem., 2008, 56, (14), pp. 5599 –5610 (doi: 10.1021/jf800738r) [DOI] [PubMed] [Google Scholar]
- 13. Sally D.S. Mozghan B. Aravindan V.J. et al.: ‘Synthesis and study of silver nanoparticles’, J. Chem. Educ., 2007, 84, (2), p. 322 (doi: 10.1021/ed084p322) [DOI] [Google Scholar]
- 14. Bashir A. Sadiq A. Shumaila B. et al.: ‘Antiinflammatory and enzyme inhibitory activities of crude extract and a new pterocarpan isolated from the aerial parts of Vitex agnus castus ’, Biotechnol. J., 2010, 5, (11), pp. 1207 –1215 (doi: 10.1002/biot.201000020) [DOI] [PubMed] [Google Scholar]
- 15. Jingqi T. Sen L. Yingwei Z. et al.: ‘Environmentally friendly, one‐pot synthesis of Ag nanoparticle‐decorated reduced graphene oxide composites and their application to photocurrent generation’, Inorg. Chem., 2012, 51, (8), pp. 4742 –4746 (doi: 10.1021/ic300332x) [DOI] [PubMed] [Google Scholar]
- 16. Kaushik R. Supratim B. Pataki C.B.: ‘Green synthesis of silver nanoparticles by using Grape (Vitis vinifera) fruit extract, characterization of the particles and study of antibacterial activity’, 2013, 4, (1), pp. 1271 –1278 [Google Scholar]
- 17. Lavanya M. Veenavardhini S. Gim G.H. et al.: ‘Synthesis, Characterization and evaluation of antimicrobial efficacy of silver nanoparticles using Paederia foetida L. leaf extract’, Int. Res. J. Biol. Sci., 2013, 2, (3), pp. 28 –34 [Google Scholar]
- 18. Padma S.V Shukla D.: ‘Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric’, Appl. Nanosci., 2012, 2, pp. 163 –168 (doi: 10.1007/s13204-011-0051-y) [DOI] [Google Scholar]
- 19. Abdel‐Aziz M. Shaheen M. El‐Nekeety A. et al.: ‘Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract’, J. Saudi Chem. Soc., 2013, 9, p. 11 [Google Scholar]
- 20. Bungheza R. Patrascuc B. Badead N. et al.: ‘Antioxidant silver nanoparticles green synthesized using ornamental plants’, J. Optoelectron. Adv. Mater., 2013, 14, (11–12), pp. 1016 –1022 [Google Scholar]


