Abstract
The herbal nanoparticles were prepared from shade dried Tridax procumbens plant leaves employing ball milling technique using different process parameters, like ball ratio/size and milling time. The obtained nanoparticles were comprehensively characterised using X‐ray diffraction, Fourier transform infrared spectroscopy, UV–visible spectroscopy, dynamic light scattering, scanning electron microscopy and antimicrobial analysis techniques. The crystallinity of the nanoparticles was retained without altering even though the particle size changes due to milling periods. The antibacterial activities of the prepared herbal nanoparticles against Staphylococcus aureus and Escherichia coli were explored to understand the influence of particle size on antimicrobial activities and their functional properties. The increase in ball ratio and milling time periods leads to a decrease in nanoparticle size from 114 to 45 nm which in turn increases the antimicrobial activities. The above study confirms that antimicrobial activity relies on nanoparticle size. The observed knowledge on influence of particle size on antimicrobial activities will help to optimise the production of potential herbal nanoparticles for different biomedical applications.
Inspec keywords: nanoparticles, antibacterial activity, biomedical materials, nanomedicine, X‐ray diffraction, Fourier transform infrared spectra, scanning electron microscopy, ultraviolet spectra, visible spectra, microorganisms, particle size
Other keywords: antimicrobial properties, Tridax procumbens leaf nanoparticles, herbal nanoparticles, ball milling technique, X‐ray diffraction, Fourier transform infrared spectroscopy, UV‐visible spectroscopy, dynamic light scattering, scanning electron microscopy, antimicrobial analysis techniques, Staphylococcus aureus, Escherichia coli, nanoparticle size
1 Introduction
Recently, the synthetic therapeutic compounds have led pathogenic microorganisms to develop drug resistance against various chemotherapeutic agents due to its improper use. In addition, these compounds are economically expensive and their synergistic activity in physiological metabolism produces adverse effects [1, 2]. It is the time to identify and develop new therapeutic compounds from natural sources and to produce cheap and potential antibiotics that are not susceptible to drug resistant microbes [3]. In ancient times, people knew that plants and their phytochemicals can cure many diseases. Nowadays, new discoveries have helped to develop herbal drugs that have no side effects and have high therapeutic activities [4]. Green chemistry plays an important role in the field of nanoscience and nanotechnology, which are very important in synthesising nanoparticles for different applications. The antimicrobial activity of herbal products has already been investigated in traditional medicines. The most important properties of herbal materials are their non‐hazardous nature and exert less or no side effect when compared with synthetic drugs. In addition, phytochemicals differ in their structure and mode of action [5]. The antibiotic activity of phytochemicals is due to the presence of secondary metabolites such as flavonoids, terpenoids, alkaloids, tannins and saponins. Presently, herbals are needed in the modern medicine to control and cure rapidly growing diseases like viral diseases, cancer and diabetes [6].
Tridax procumbens (T. procumbens) is one of the good and abundant medicinal plants in Asia particularly in India. T. procumbens is a well‐known medicinal plant used to treat diseases like respiratory and intestinal tract diseases. In addition, it is also used to check haemorrhage from cuts, bruises and wounds. The leaf of the plant is used as anticoagulant, anticancer, antifungal and insect repellent applications. The phytochemical constituents existing in the T. procumbens plant leaves are dexamethasone luteolin, glucoluteolin, β‐sitosterol quercetin, β‐sitosterol‐3‐O‐β‐d‐xylopyranoside and procumbenetin [7]. In addition, the plant leaves also has good antibacterial activities against bacteria like Escherichia coli (E. coli), Proteus mirabilis (P. mirabilis) and Staphylococcus aureus (S. aureus) [8].
Therefore, the knowledge and a comprehensive study on the herbal T. procumbens plants powder at nanoscale is the need of the hour for existing and its newly emerging different biomedical applications. Generally, it is known that the structure and properties of nanoparticles depends on their process techniques/methods. Ball milling is one of the top‐down approaches used to produce homogeneous nanoparticles [9]. It is the aim of this study to produce the nanoparticles from herbal T. procumbens leaf powder employing ball milling technique and to explore the influences of antimicrobial activities on particle sizes.
2 Materials and methods
2.1 Plant materials
The 45 days old, healthy and fresh leaves of T. procumbens herbal plant were collected from in and around Tiruchengode, Tamil Nadu, India. The collected leaves were washed twice with tap water followed by in double‐distilled water for multiple times to remove dust particles from the surface of the leaves. The washed leaves were shade dried at room temperature for two weeks.
2.2 Synthesis of herbal nanoparticles
Initially, a mixer grinder was used to grind the shade dried leaves. The ground powders were collected in the form of coarse powders. The obtained coarse powders were milled for 1 h using 20 mm sized ball (Zirconia) using ball mill (PM100; Retsch, Germany), to get fine powders. Then, the obtained fine powders were equally (nearly 5 mg) divided into four parts. The four separated fine powders were again milled (10 mm balls: 300 rpm) in ball mill for different milling periods namely 6, 9, 12 and 15 h (hereafter termed, respectively, as sample TP6, TP9, TP12 and TP15). The synthesis of herbal nanoparticles from T. procumbens plant leaves is shown in flowchart in Fig. 1. All the obtained nanoparticle samples were comprehensively characterised and used for antimicrobial activity studies.
Fig. 1.

Synthesis of herbal nanoparticles – schematic representation
2.3 Characterisation of herbal nanoparticles
The phase purity and structure of T. procumbens nanoparticles were tested out by X‐ray diffraction (XRD) technique. The XRD measurement was carried out using an X‐ray powder diffractometer (X'Pert PRO; PANalytical, The Netherlands) with a long and fine focus of Cu anode operated at 40 kV and 30 mA in Bragg–Brentano geometry. The XRD spectra of herbal nanoparticles were obtained in the 2θ range from 10° to 80° in a step‐scan mode with 2θ step of 0.02°. The Fourier transform infrared (FTIR) spectra of the T. procumbens nanoparticles were obtained using an FTIR spectrometer (Spectrum 100; PerkinElmer, Waltham, Massachusetts) in the frequency range from 400 to 4000 cm−1 using a KBr pellet. The pellet was obtained by mixing 200:1 ratio of KBr nanoparticles. The KBr mixture was ground initially in an agate mortar and then, the pellet was obtained using a hydraulic pellet maker (Perkin Elmer, Mumbai, India). The obtained pellet was used to ascertain the functional groups through measurement of infrared spectra. The absorption spectra of all herbal nanoparticles were obtained using UV–visible (UV–Vis) spectrophotometer (Cary 8454; Agilent, Singapore) operated from the UV to near‐infrared (180–800 nm) spectral regions at a step size of 5 Å. The dispersed nanoparticles (0.1 mg of nanoparticles was dispersed in 2 ml deionised water) was taken in a cuvette. The UV–Vis spectra of the resulting diluents were monitored as a function of reaction time and biomaterial dosage at a resolution rate of 1 nm.
The prepared herbal nanoparticles were subjected to scanning electron microscopy (SEM) coupled with energy‐dispersive X‐ray (SEM‐EDX, JSM 6360 JEOL, Japan) analysis to identify the morphology and microstructure of the prepared nanoparticles. The obtained herbal nanoparticles were excited by bombarding them with high‐energy X‐rays for conducting elemental analysis. The purity and stoichiometry of the prepared green nanoparticles were determined from the elements analysed using an X‐ray fluorescence (XRF) spectrometer (EDX‐720; Shimadzu, Kyoto, Japan). The XRF analysis of T. procumbens nanoparticles is given in Table 1. The particle size distribution was carried out with a submicrometre particle size analyser (Nanophox; Sympatec, Zellerfeld, Germany) using dynamic light‐scattering technique. The particle size distribution was made employing three‐dimensional photon cross‐correlation techniques.
Table 1.
XRF analysis of T. procumbens nanoparticles
| Analyte | Weight, % | |||
|---|---|---|---|---|
| Milling time, h | ||||
| TP6 | TP9 | TP12 | TP15 | |
| K | 49.89 | 49.65 | 49.72 | 49.19 |
| Ca | 42.59 | 41.48 | 41.66 | 41.57 |
| Si | 4.84 | 5.93 | 5.26 | 5.16 |
| Fe | 1.32 | 1.53 | 1.86 | 2.48 |
| Cl | 0.33 | 0.36 | 0.38 | 0.28 |
| Zr | 0.28 | 0.35 | 0.38 | 0.48 |
| S | 0.24 | 0.29 | 0.26 | 0.30 |
| Ti | 0.20 | 0.20 | 0.36 | 0.24 |
| Mn | 0.20 | 0.19 | 0.21 | 0.29 |
2.4 Antimicrobial studies
2.4.1 Collection of microorganisms and culture maintenance
The bacterial cultures namely gram positive (S. aureus, ATCC 6538P) and gram negative (E. coli, ATCC 9677) were obtained from the National Collection of Industrial Microorganisms, National Chemical Laboratory, Pune, Maharashtra, India. The test bacterial cultures were periodically sub‐cultured at room temperature (37°C) for 24 h. The bacterial cultures were maintained on a nutrient agar slant (HiMedia, India) for further experiments at 4°C.
2.4.2 Antibacterial activity
Bacterial inoculum was prepared by inoculating a loop of test organisms into a nutrient broth and incubating it at 37°C for 5–8 h until a moderate turbidity was developed. A loop of culture was swabbed on the agar plate. The qualitative assessment of the antibacterial activity of the obtained T. procumbens nanoparticles was carried out by agar well diffusion assay [10]. The cultured agar plates were aseptically punctured using well puncture. The 100 mg/ml concentration of the leaf nanoparticles samples TP6 of T. procumbens was added into the agar well. Then, the plate was incubated at 37°C for 18–24 h. Following the incubation, the zone of inhibition was measured. Similar procedure was employed to prepare culture for nanoparticle samples TP9, TP12 and TP15, and also to determine the zone of inhibition.
3 Results and discussion
The observed results of herbal nanoparticles which are prepared from T. procumbens herbal plant leaves are discussed to understand their antimicrobial activities as a function of particles size. The XRD pattern of TP6, TP9, TP12 and TP15 samples shown in Fig. 2 confirm the absence of any crystalline diffraction peaks at 2θ values in the range of 20–30°. Thus, the XRD pattern of all nanoparticle samples confirms the amorphous nature of the nanoparticles prepared from herbal plant leaf.
Fig. 2.
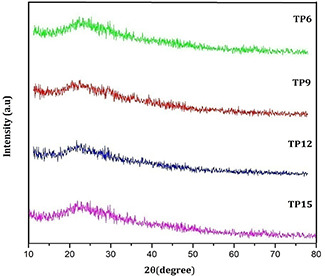
XRD spectra of TP6, TP9, TP12 and TP15 nanoparticles
The FTIR spectra of all nanoparticle samples found to be similar in their functional groups as evident from Fig. 3. The peaks observed at 3396 cm−1 are assigned to primary amide group. The weaker bands observed at 2927 and 2345 cm−1 correspond to asymmetric stretching of C–H groups [11, 12, 13], which are indicative of terpenoid group of compounds in the aqueous solution of T. procumbens. The band observed at 1650 cm−1 corresponds to amide II. The band observed at 1424 cm−1 corresponds to C–H bending mode [14] and that at 1384 cm−1 (germinal methyls) corresponds to primary amine (N–H) bending mode [15, 16]. The prepared sample has appropriate characteristic peaks of flavanones and terpenoids that possess abundant antimicrobial activity in plant extracts [17]. The peak observed at 1384 cm−1 indicates the presence of flavonoids in T. procumbens nanoparticles, suggesting good antimicrobial property. Characteristic absorption bands of isopropyl groups are observed at 1150 cm−1 [18]. The peaks observed at 1104 cm−1 indicate the presence of (–COO–) carboxylate ions [19]. The band observed at 1060 cm−1 suggests the C–N stretching vibrations of aliphatic amines [20]. The peaks at 667 cm−1 are assigned to N–H stretching. The observed peaks at 624 cm−1 mainly correspond to C=C in the herbal nanoparticles.
Fig. 3.
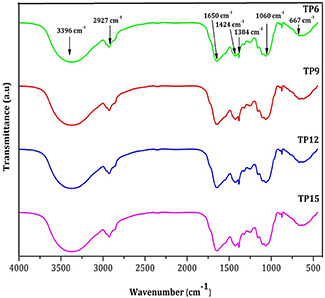
FTIR spectra of TP6, TP9, TP12 and TP15 nanoparticles
All the nanoparticles almost show similar elements as evident from XRF analysis as shown in Table 1. The elemental compositions of the prepared nanoparticles show that the K (49.88%) and Ca (42.58%) are the major elements present in the herbal nanoparticles. It is inferred from the above observation that, the variation in milling time period does not reveal any influence on the functional groups and elemental compositions, rather it reduces the particle size. The UV absorption spectra (Fig. 4) of all herbal nanoparticles show UV absorption at 279, 437 and 678 nm, which show the existence of silver nanoparticles as reported elsewhere [21, 22, 23]. The herbal nanoparticles show the absorption band at 279 nm band (II) near by the silver nanoparticles which confirms the presence of related flavonoids compound such as anthocyanidins and anthocyanins. The peak at 437 nm associated with strong negative band corresponds to carotenoid region and the positive band at 678 nm corresponds to chlorophyll region was absorbed in hebal nanoparticles. Similar absorption peaks at 272, 460 and 572 nm are reported on nanosilver particles synthesised from T. procumbens plant leaf extract. The SEM images of herbal nanoparticles (Fig. 5) exhibit the topographical nature of obtained nanoparticles for different milling time periods namely 6, 9, 12 and 15 h. The samples TP6 and TP9 (Figs. 5 a and b) illustrate the aggregated irregular surface morphology with larger particles. In Figs. 5 c and d, higher ball milling time, respectively, (TP12 and TP15) illustrates the discrete particles with flake like structure comparatively smaller than TP6 and TP9. The particle size analysis of all the samples shows that the average particle size of TP6, TP9, TP12 and TP15 samples is, respectively, 114, 88, 65 and 45 nm (Fig. 6). The size of nanoparticles decreases from 114 to 45 nm as the time periods of ball milling of the herbal particles increases from 6 to 15 h. The above results indicate that the milling time period of the herbal plant leaves play a dominant role in reducing size of the produced nanoparticles.
Fig. 4.
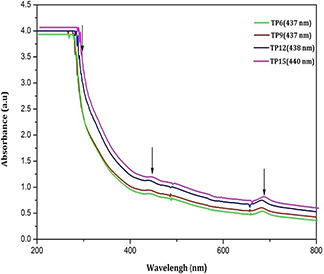
UV–Vis spectra of TP6, TP9, TP12 and TP15 nanoparticles
Fig. 5.
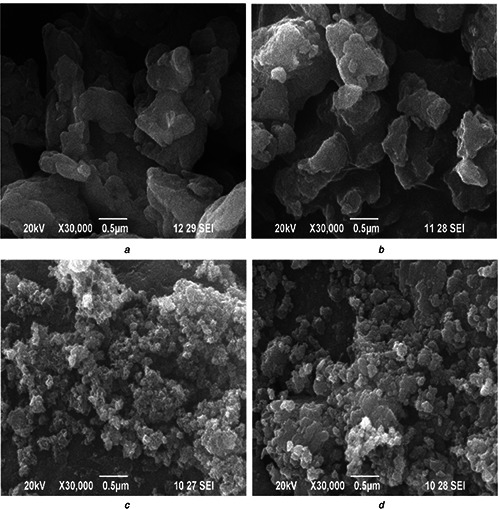
SEM image of
(a) TP6, (b) TP9, (c) TP12, (d) TP15 nanoparticles
Fig. 6.
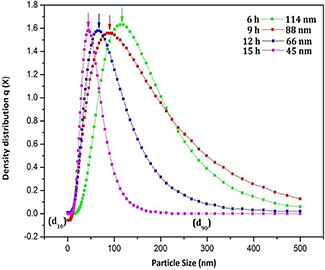
Particle size distribution of TP6, TP9, TP12 and TP15 nanoparticles processed at different time periods
3.1 Antimicrobial assessment of T. procumbens nanopowders
The qualitative assessments of the antibacterial activity of the herbal nanoparticles exhibit interesting observation as evident from Fig. 7. The zone of inhibition varies for samples obtained under different ball milling times namely TP6, TP9, TP12 and TP15 (Fig. 7 and Table 2). The maximum formation of zone of inhibition for E. coli and S. aureus is observed as 45 nm for the sample TP15, i.e. the higher time period of milling (15 h) of the plant leaves powders. At this higher milling time, the size of the nanoparticles observed is only 45 nm which is very low when compared with any other milling time. The smaller particles are found to exhibit the formation of maximum zone of inhibition when compared with larger particles. The surface area of the smaller particles is larger than the higher particles size. The larger surface area of smaller nanoparticles helps for the easy penetration and denature of the bacterial cell walls. This in turn restricts the DNA replications [24, 25, 26] and hence, leads to formation of higher zone of inhibition than the small surface area of the larger nanoparticles. The present studies confirm that the surface area of nanoparticles influences on the formation of zone of inhibition.
Fig. 7.
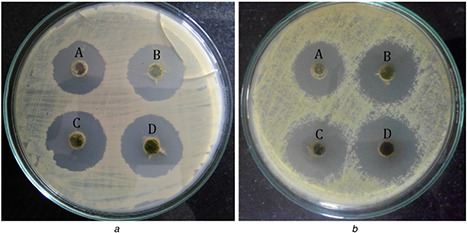
Antimicrobial activity of TP6, TP9, TP12 and TP15 nanoparticles against
(a) E. coli, (b) S. aureus bacteria
Table 2.
Antimicrobial assessment of the T. procumbens milled in different time durations
| Zone of inhibition, mm | ||||
|---|---|---|---|---|
| Test organisms | TP6 | TP9 | TP12 | TP15 |
| E. coli | 18.61 ± 0.61 | 21.78 ± 0.55 | 23.27 ± 0.39 | 24.02 ± 0.34 |
| S. aureus | 20.87 ± 0.66 | 21.91 ± 0.52 | 25.54 ± 0.51 | 26.11 ± 0.28 |
4 Conclusion
The herbal nanoparticles were prepared from the shade dried leaves of T. procumbens plant employing ball milling technique. The nanoparticles obtained from four different time periods of milling confirms the amorphous nature without exhibiting any crystalline peaks in the XRD spectra. The observed functional groups and elemental compositions of the herbal nanoparticles were found to be uniform in all samples. The higher absorption peaks in the region of 279, 437 and 678 nm shows the absorption of T. procumbens nanoparticles in the UV–Vis region, which is higher in TP15 sample. The milling time periods play an important role in the formation of homogeneous nanoparticles, which enhances the antimicrobial action of T. procumbens plant leaves nanoparticles. The maximum zone of inhibition was found at 45 nm nanoparticles in TP15 samples against gram‐positive and gram‐negative bacteria. This study confirms that smaller nanoparticles with high surface area show the formation of maximum zone of inhibition than higher particle size with smaller surface area. In addition, Size dependent antimicrobial analysis of nanoparticles gives the promising results in this study and hence, it will be more helpful in obtaining homogeneous herbal nanoparticles with improved biological activity.
5 Acknowledgment
The authors acknowledge the financial support provided by the Board of Research and Nuclear Science (BRNS), Mumbai (sanction letter no.: 2013/34/30/BRNS/1127 dt.19.09.2013).
6 References
- 1. Karaman L. Sahin F. Gulluce M. et al.: ‘Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L’, J. Ethnopharmacol., 2003, 85, pp. 231 –235 [DOI] [PubMed] [Google Scholar]
- 2. Schinor E.C. Salvador M.J. Ito I.Y. et al.: ‘Evaluation of the antimicrobial activity of crude extracts and isolated constituents from Chrestascapigera’, Braz. J. Microbiol., 2007, 38, pp. 145 –149 [Google Scholar]
- 3. Sharma B. Kumar P.: ‘Extraction and pharmacological evaluation of some extracts of Tridax procumbens and Capparis decidua ‘, Int. J. Appl. Res. Nat. Prod., 2009, 1, pp. 5 –12 [Google Scholar]
- 4. Jones R.N.: ‘Can antimicrobial activity be sustained? An appraisal of orally administered drugs used for respiratory tract infections’, Diagn. Microbiol. Infect. Dis., 1997, 27, pp. 21 –28 [DOI] [PubMed] [Google Scholar]
- 5. Fabricant D.S. Fansworth N.R.: ‘The value of plants used in traditional medicine for drug discovery’, Environ. Health Perspect., 2001, 109, pp. 69 –75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowan M.M.: ‘Plant products as antimicrobial agents’, Clin. Microbiol. Rev., 1999, 12, (4), pp. 564 –582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sunil C. Kulathivel T.M. Agastian P.: ‘Phytochemical and antibacterial studies of leaves of Tridax procumbens L’, Asian Pac. J. Trop. Biomed., 2012, pp. S159 –S161 [Google Scholar]
- 8. Salahdeen H.M. Yemitan O.K. Alada A.R.A.: ‘Effect of aqueous leaf extract of Tridax procumbens on blood pressure and heart rate in rats’, Afr. J. Biomed. Res., 2004, 7, pp. 27 –29 [Google Scholar]
- 9. Vinoth M. Suriyaprapha R. Arunmetha S. et al.: ‘Synthesis of Nothapodytes nimmoniana leaf nanoparticles for antireflective and self‐cleaning applications’. Synth. React. Iorg. Met.‐Org. Nano‐Met. Chem., 2015, 46, pp. 1445 –1449 [Google Scholar]
- 10. Chitra P. Ujjwala K. Manjusha B. et al.: ‘Antibacterial activity of Tridax procumbens with special reference to Nosocomial pathogens’, Br. J. Pharm. Res., 2011, 1, (4), pp. 164 –173 [Google Scholar]
- 11. Ismail E.H. Khalil M.M.H. Al Seif F.A. et al.: ‘Biosynthesis of gold nanoparticles using extract of grape (Vitisvinifera) leaves and seeds’, Prog. Nanotechnol. Nanomater., 2014, 3, (1), pp. 1 –12 [Google Scholar]
- 12. Namazka S. Ahmad W.A.: ‘Spray‐dried prodigiosin from Serratia marcescens as a colorant’, Biosci. Biotechnol. Res. Asia, 2013, 10, (1), pp. 69 –76 [Google Scholar]
- 13. Heera P. Shanmugam S. Ramachandran J.: ‘Green synthesis of copper nanoparticle using Gymnema sylvestre by different solvent extract’, Int. J. Curr. Res. Acad. Rev., 2015, 3, (10), pp. 268 –275 [Google Scholar]
- 14. Akbari Z. Amanlou M. Karimi‐Sabet J. et al.: ‘Characterization of carbamazepine‐loaded solid lipid nanoparticles prepared by rapid expansion of supercritical solution’, Trop. J. Pharm. Res., 2014, 13, (12), pp. 1955 –1961 [Google Scholar]
- 15. Shankar S.S. Rai A. Ahmad A. et al.: ‘Rapid synthesis of Au, Ag and bimetallic Au core Ag shell nanoparticles using neem (Azadirachta indica) leaf broth’, J. Colloid Interface Sci., 2004, 275, pp. 496 –502 [DOI] [PubMed] [Google Scholar]
- 16. Priya B. Mantosh S. Aniruddha M. et al.: ‘Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis’, Bioresour. Bioprocess., 2014, 1, (3), pp. 1 –10 [Google Scholar]
- 17. Sathya Bama S. Jayasurya Kingsley S. Sankaranarayanan S. et al.: ‘Antibacterial activity of different phytochemical extracts from the leaves of T. procumbens Linn: Identification and mode of action of the terpenoid compound as antibacterial’, Int. J. Pharm. Pharm. Sci., 2012, 4, pp. 557 –564 [Google Scholar]
- 18. Sifontes A.B. Gutierrez B. Mónaco A. et al.: ‘Preparation of functionalized porous nano‐γ‐Al2O3 powders employing colophony extract’, Biotechnol. Rep., 2014, 4, pp. 21 –29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Senthil M. Ramesh C.: ‘Biogenic synthesis of Fe3 O4 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa ‘, Digest J. Nanomater. Biostruct., 2012, 7, (3), pp. 1655 –1660 [Google Scholar]
- 20. Rajan K. Bharalia K. Bhattacharyya G.: ‘Kinetic and thermodynamic studies on fluoride biosorption by deydaru (Polyalthia longifolia) leaf powder’, Octa J. Environ. Res., 2014, 2, (1), pp. 22 –31 [Google Scholar]
- 21. Anitha P. Sakthivel P.: ‘Microwave assisted synthesis and characterization of silver nanoparticles using Tridax procumbens and its anti‐inflammatory activity against human blood cells’, J. Nanomater. Mol. Nanotechnol., 2015, 4, pp. 1 –6 [Google Scholar]
- 22. Dhanalakshmi T. Rajendran S.: ‘Synthesis of silver nanoparticles using Tridax procumbens and its antimicrobial activity’, Arch. Appl. Sci. Res., 2012, 4, (3), pp. 1289 –1293 [Google Scholar]
- 23. Ilioaia C. Johnson M.P. Horton P. et al.: ‘Induction of efficient energy dissipation in the isolated light‐harvesting complex of photosystem II in the absence of protein aggregation’, J. Biol. Chem., 2008, 283, (43), pp. 29505 –29512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agnihotri S. Mukherji S. Mukherji S.: ‘Size‐controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy’, RSC Adv., 2014, 4, pp. 3974 –3983 [Google Scholar]
- 25. Moideen S. Lakshmi Prabha A.: ‘Green synthesis of silver nanoparticles using Luffa Acutangula Roxb. Var. Amara. Lin, and it's antibacterial activity’, Int. J. Pharm. Bio. Sci., 2014, 5(4), (B), pp. 1051 –1061 [Google Scholar]
- 26. Raghupathi K.R. Koodali R.T. Manna A.C.: ‘Size dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles’, Langmuir, 2011, 27, pp. 4020 –4028 [DOI] [PubMed] [Google Scholar]


