Abstract
In recent years, carbon aerogels have attracted much attention in basic research and as potential applications in many fields. Herein, the authors report a novel approach using bamboo powder as raw material to fabricate cellulose nanofibers (CNFs)/multi‐walled carbon nanotubes (MWCNTs) carbon aerogels by a simple dipping and carbonisation process. The developed material exhibits many exciting properties including low density (0.056 g cm−3), high porosity (95%), efficient capability for separation of oily droplets from water, and high adsorption capacity for a variety of oils and organic solvents by up to 110 times its own weight. Furthermore, the CNF/MWCNT carbon aerogels (CMCA) can be recycled many times by distillation and combustion, satisfying the requirements of practical oil‐water separation. Taken together with its economical, environmentally benign manufacturing process, sustainability of the precursor and versatility of material, the CMCA developed in this study will be a promising candidate for addressing the problems arising from the spills of oily compounds.
Inspec keywords: aerogels, adsorption, nanofibres, filled polymers, nanocomposites, multi‐wall carbon nanotubes, porosity, drops, distillation, combustion, nanofabrication, polymer fibres
Other keywords: CNF‐MWCNT carbon aerogel, adsorbents, bamboo powder, cellulose nanofibers, multiwalled carbon nanotubes, dipping, carbonisation, density, porosity, oily droplets, adsorption capacity, organic solvents, distillation, combustion, practical oil‐water separation, manufacturing process, sustainability, C
1 Introduction
In recent years, frequent occurrence of water pollution from oil spillage and chemical leakage has caused huge economic losses and serious environmental pollution [1, 2]. Traditional methods of addressing these problems include oil containment booms, chemical dispersants, mechanical extraction, bioremediation, in situ burning [3, 4, 5], and the use of oil absorbents. Due to the efficiency and economy of separation of oil from the water surface, physical separation by using oil‐absorbents is regarded as one of the most ideal options for the collection of oil. Currently, there are a large number of absorbent materials such as natural materials [6, 7, 8], inorganic minerals [9, 10] and complex materials [11, 12, 13] widely using in practical applications. However, several shortcomings such as low oil absorption capacity, high cost, environmental incompatibility, and selective absorption have limited their widespread applications. Therefore, there is still a great demand for the development and exploitation of oil absorbents with low cost, high absorption capacity, excellent selectivity, adequate buoyancy, efficiency, and environmental compatibility.
Aerogels are ultra‐light solid materials with three‐dimensional networks and are usually prepared by exchanging the solvent with gas without collapsing the network. Due to the unique moulding methods, aerogels show properties such as high specific surface area, low‐density, low thermal conductivity and low dielectric constant [14, 15]. Cellulose‐based aerogels (CBAs) based on native cellulose nanofibrils have attracted much attention because they not only exhibit the high porosity and large surface area of aerogels but are also bio‐based, non‐toxic, sustainable, abundantly available, and renewable [16, 17]. Therefore, CBAs are ideal materials for use as oil‐absorbents.
Even though CBAs exhibit a combination of favourable absorbent properties, several challenges must be solved before competition with the traditional oil absorbents. The major shortcoming of the aerogels arises from the strong hydrophilic property of cellulosic skeleton, which leads to poor oil/water selective adsorption of CBAs [18, 19]. Several possible approaches have been investigated for CBAs with hydrophobicity, oleophilicity, and high oil/water selectivity could be obtained by atomic layer deposition, chemical vapour deposition, esterification, and fluorination. However, such hydrophobisation processes of CBAs are complicated and heterogeneous. Furthermore, some of the hydrophobising agents are costly, toxic, and not environmentally friendly.
Currently, carbon aerogels have attracted much attention due to their outstanding sorption capability, intrinsic hydrophobic and oleophilic nature, excellent chemical and thermal stability. Traditionally, carbon aerogels are fabricated by carbonisation of resorcinol‐formaldehyde organic aerogels or other organic aerogels in an inert atmosphere to form a developed network carbon structure [20]. Likewise, the carbon aerogels can also be pyrolysed by cellulose aerogels as precursors through the same process. The hydrophilic groups such as C–H, C–O, C=O, and O–H in the cellulose molecules are removed during the pyrolysis process, which gives rise to the oleophilic properties [21, 22, 23, 24, 25, 26, 27, 28, 29]. Several efforts have been made to develop carbon aerogels with a larger specific surface area, higher porosity, superior mechanical properties and high oil absorbing capacity. For example, Meng et al. [21] carbonised composite aerogels using freeze‐drying to prepare a mixture of NFC and commercial cross‐linker (Kymene resin) to creating sponge‐like carbon aerogels. It was found that the peak temperature and heating rate at different levels had a considerable impact on the char yield. The optimum process parameters have been found at a heating rate of 8.00°C/min and a peak temperature of 300°C. Han et al. [28] prepared carbon aerogels with low‐density and excellent hydrophobicity with a water contact angle (WCA) of 132° derived from waste newspaper using a freeze‐drying method followed by pyrolysis. The absorption capacity of aerogels could be ∼29–51 times its own weight for organic compounds. Moreover, the harvested organic pollutants can be recycled by three methods (e.g. squeezing, combustion, and distillation). However, there are still some problems such as poor shape‐retention ability and high‐dimensional shrinkage that limits the practical application of carbon aerogels.
cellulose nanofibers (CNFs) were extracted from natural cellulose with many outstanding properties such as high mechanical strength (the elastic modulus of 140 GPa), non‐toxic, recyclable, and low cost. The stable network structure can be formed in water attributed to high surface area and high aspect ratio of CNF [17, 21, 24]. Owing to the mechanical stability and high aspect ratios, multi‐walled carbon nanotube (MWCNT) can be an ideal nanofiller to improve the thermal stability of the composite [30, 31]. In this work, carbon aerogels were first prepared from the CNF/MWCNT composite aerogel. The addition of carbon materials can improve the dimensional stability of aerogels in the carbonisation process. The carbon aerogels could be used as versatile adsorbents due to their low density (0.056 g cm−3), high porosity (95%), high absorption capacity (maximum up to 110 times its own weight), hydrophobic but lipophilic behaviour and repeatability. Furthermore, the excellent shape‐retention ability and low‐dimensional shrinkage will expand the application of carbon aerogels. We believe that such novel carbon aerogels with excellent performance will be prominent candidates for the treatment of oil spills.
2 Experimental
Materials : All chemicals were of reagent grade and used without further purification. Bamboo powders were sieved by 60 mesh filter, then vacuum dried and set aside. MWCNTs were purchased from Aladdin.
Synthesis of CNFs : The chemical purification of bamboo fibres were based on the previous studies [28]; then, the CNF slurry was mechanically lapped by a grinder (MKCA6‐2, Masuko Sangyo Co., Ltd., Japan) at 1500 rpm [16].
Synthesis of CNF/MWCNT aerogels (CMA) : Firstly, 1 wt% CNF slurry was treated by an ultrasonic bath and a high‐speed mixer and poured into the desired moulds. The CNF gels were frozen at −22°C for 12 h and CNF aerogels were obtained by freeze‐drying at −60°C for 48 h. Then, the CNF aerogels were dipped in MWCNT solution for 2 h. The weight ratio between the MWCNTs and CNFs was controlled at 1:40 in the final CNF/MWCNT aerogels. The followed synthesis was by freezing at −22°C for 12 h and CMA was obtained by freeze‐drying −60°C for 48 h.
Synthesis of CNF/MWCNT carbon aerogel (CMCA) : The CMA was heated to 500°C in argon flow with the heating rate of 5°C min−1 and holding at 500°C for 120 min to obtaining the CMCA.
Characterisation: The densities of samples were determined as
| (1) |
where m is the sample weight and v is the sample volume.
The porosity of samples was determined according to
| (2) |
where ρ and ρ s are the densities of aerogel and the solid material, respectively.
The compression experiments were performed by Shimadzu CMT4204 (Japan) with a loading rate of 2 mm/min. The diameters of the samples were controlled at a length of 20 mm and a height of 10 mm. The morphologies of the samples were observed by a scanning electron microscope (FE‐SEM, Hitachi S‐4800, Japan). The nitrogen adsorption–desorption isotherm and surface area of CMCA were obtained by an N2 adsorption analyser (TriStar 3000, Quantachrome Instruments, USA) using the (Brunauer‐Emmett‐Teller) BET nitrogen adsorption/desorption technique. The surface wettability of the CMA aerogel and CMCA was evaluated by WCA measurements (OCA20) at ambient temperature. Functional groups of the CNFs, MWCNTs, CMA, and CMCA were identified using a Fourier transform infrared spectrometer (Nicolet iS10, Thermo Scientific Inc, USA). The oil absorption capacity Q t of CMCA was measured using
| (3) |
where Qt is the oil absorption capacity of CMCA at different points of time and m 1 and m 2 are the weight of the aerogel before and after the absorption, respectively. All absorption tests were repeated three times, and the average was obtained, improving the reliability of experimental data.
3 Results and discussion
3.1 Shape of CA and CMA before and after carbonisation, mechanical properties of CMCA, morphology, and surface area of CMCA
As shown in Fig. 1 a, compared with CA, CMA shows better dimensional stability and smaller volume shrinkage. It is indicated that the addition of carbon materials can improve the dimensional stability of aerogels in the carbonisation process. Furthermore, CMCA samples could maintain 80% of their initial heights after 30 times of 100–85% compressing‐release cycles under ∼5000 times of its own weight (Fig. 1 b). To further evaluate mechanical property of the material, compression experiments were carried out using a Shimadzu CMT4204. The result in Fig. 1 c shows that CMCA could tolerate with high compression deformation without damage. The compressive stresses for CMCA was 22.07 kPa at 70% stain. The results suggested that CMCA possessed good flexibility and excellent elasticity, expanding the range of applications of this carbon aerogel.
Fig. 1.
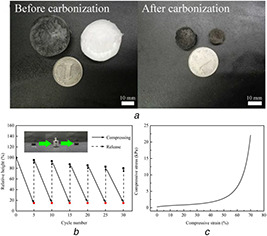
Morphological images and compression test
(a) CA and CMA before and after carbonisation, (b) Height recovery of CMCA as a function of recycle number at 85% compression strain, (c) Compressive stress–strain curve of CMCA
3.2 Morphology, multilayer adsorption of nitrogen and surface area of CMCA
The morphologies of CA, CMA and CMCA were characterised by SEM. Fig. 2 a shows that CA presents a 2D sheet‐like skeletons structure, which is caused by high concentration CNF slurry leading to the insufficient space for the dispersion of the CNFs [32]. Then, the sufficiently large holes of CA will provide channels for convenient entry of MWCNTs inside the aerogels. For the CMA, it can be seen that the morphology of CMA is more homogeneous and shows a compact sheet‐like skeleton structure. After pyrolysis, the CMCA inherited the 2D sheet‐like skeleton structure of CMA (Fig. 2 b). The sheet‐like skeletons of CMCA were contracted and crimped after carbonisation (Fig. 2 c). In addition, the finer holes on the sheet‐like skeletons appeared during the carbonisation process, offering more paths for oil absorption. As shown in Fig. 2 d, the multilayer adsorption of nitrogen has formed in the sample. It was noted that the method for testing surface area was beneficial for the material with micropore (<2 nm) and mesopore (2–50 nm). Therefore, the surface area measured by BET was not reliable (56.782 m2 g−1) due to the whole sample of CMCA with macroporous structure in micron scale (Fig. 2 c).
Fig. 2.
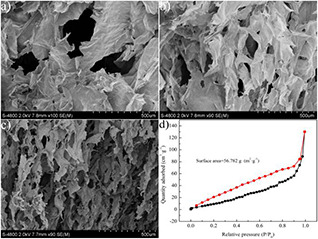
Microstructure and BET measurements
(a)–(c) SEM images of CA, CMA, and CMCA, respectively, (d) BET nitrogen adsorption/desorption isotherm of CMCA
3.3 Surface wettability
The surface wettability of the CA, CMA, and CMCA was estimated by testing the WCA at the surface of samples. In a preliminary experiment, drops (dyed by KMnO4) were deposited on the surfaces of CA, CMA, and CMCA (Fig. 3 a). CA and CMA showed strong hydrophilicity (the WCA near to 0°), as the samples absorbed the drops immediately. In contrast, the CMCA can support a spherical water droplet on its surface with the corresponding WCA of 132°. To further confirm the hydrophobicity of the CMCA, the sample was immersed in water by an external force. It can be seen that the sample was wrapped by a transparent air film, attributed to a continuous air layer between the hydrophobic surface of the sample and surrounding water (Fig. 3 b). After releasing of external force, the sample could float on the water surface immediately as illustrated in the photos. Besides, the CMCA was suspended in an oil layer (dyed by Sudan III) of oil–water mixtures stably, indicating the lipophilicity of CMCA.
Fig. 3.
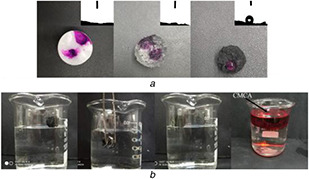
Surface wettability measurements
(a) Surface wettability and WCA of the CA, CMA, and CMCA; (b) Surface wettability of CMCA to different probe liquids
3.4 FT‐IR analysis
To study the change of the functional groups of the CMA and CMCA, we examined the functional groups of the CMCA, CMA, MWCNTs, and CNFs, respectively. As shown in Fig. 4 a–d, CMA had inherited several peaks representing oxygen‐containing groups such as –OH (3320 cm−1), –CH (2900 cm−1), –C=O (1639 cm−1), O–H (1367 cm−1) and C–O–C (1023 cm−1) from CNFs and MWCNTs. After the carbonisation process, the final aerogel only preserved the characteristic diffraction peaks of MWCNTs due to the good heat resistance of MWCNTs. Meanwhile, the disappearance and weakening of the major oxygen‐containing groups indicated the removal of the hydrophilic moieties during the carbonisation process (Fig. 4 d). The mechanism behind the changes of WCA before and after the carbonisation can be interpreted as the weakening and disappearance of the hydrophilic moieties from the carbon structure after carbonisation.
Fig. 4.
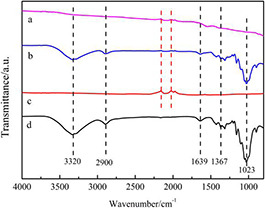
FTIR spectra of samples
(a) CA, (b) CMA, (c) MWCNTs, and (d) CMCA
3.5 Absorption capacity of CMCA
Based on its 3D porous structure, low‐density, high porosity, excellent hydrophobicity and good mechanical properties, CMCA will be an ideal candidate material for absorption of organic pollutants/oils. As displayed in Fig. 5 a, the oil contaminants (dyed by Sudan III) were rapidly and completely absorbed by the CMCA sample from the water surface even after slight contact between the sample and the target oil. The sample could float on the water surface after absorption due to its low density and excellent hydrophobicity. In Fig. 5 b, when a piece of CMCA was immersed in water and contacted the motor oil (dyed by Sudan III), the motor oil was also absorbed completely in several seconds. All of these characteristics indicated that CMCA is a promising absorption material for the facile removal of oil spillage and chemical leakage.
Fig. 5.
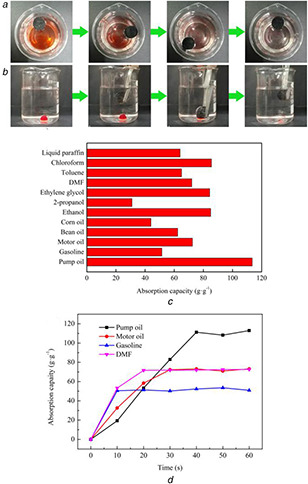
Adsorption of organic liquids by CMCA
(a) Photographs showing the adsorption process of CMCA towards gasoline. (b) Photographs showing the adsorption process of chloroform by CMCA. (c) Adsorption efficiency of CMCA towards various organic liquids. (d) Oil absorption capacity of CMCA as a function of time
Various types of organic liquids such as commercial oils (pump oil, gasoline, motor oil, bean oil and corn oil), water‐miscible solvents (ethanol, 2‐propanol, ethylene glycol and DMF), and water‐immiscible solvents (toluene, liquid paraffin and chloroform) were tested to further quantitatively evaluate the absorption efficiency of CMCA (Fig. 5 c). Briefly, it was found that CMCA could absorb organic liquids at 35–110 times its own weight. The different absorption capacities for oils were attributed not only to the surface properties of CMCA but also to the oil characteristics such as hydrophobicity, density, and surface tension [33]. The high organic liquids absorption capacity may be caused by the high porosity, which could provide enough dedicated space for organic liquids. Additionally, the capillary effect was considered to favour driving organic liquids into the aerogel. The capillary effect of CMCA was contributed by the crossed carbon nanofibers between the carbon skeleton, which could offer small passages for liquid flows [25, 34, 35]. It is noteworthy that CMCA needs about 30–40 s to achieve absorption equilibrium for motor oil and pump oil, while gasoline and DMF need less time in other organic solvents (Fig. 5 d). The movement velocity of the liquid molecules was obviously affected by viscosity of liquid. Therefore, the samples with lower viscosity (viscosity: pump oil > motor oil > DMF > gasoline) can reach absorption equilibrium in less time.
3.6 Recyclability and repeatability
The recyclability of CMCA and the recoverability of organic pollutants have vital economic significance for oil/chemical cleanup applications. To date, many methods have been used to recycle sorbents and recover pollutants. In this study, two major methods, namely distillation and combustion, were used to test the recycling performance of CMCA based on the characteristics of different organic pollutants. Distillation is regarded as an effective approach for the removal of the valuable and low boiling point organic pollutants. CMCA was recovered by heating the sample at 80 ℃ (boiling point of ethanol). Fig. 6 a shows that the absorption capacity displays negligible changes after six absorption–distillation cycles.
Fig. 6.
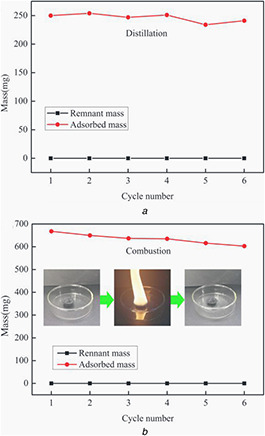
Recyclability of CMCA using different methods and corresponding morphologies after cyclic operation six times
(a) Ethanol‐adsorbed CMCA recycled by distillation; (b) Gasoline‐adsorbed CMCA recycled by combustion
To further demonstrate the recycling performance of CMCA, adsorption–combustion experiments were carried out by using gasoline as the absorbent to estimate the recyclability of CMCA. As shown in Fig. 6 b, CMCA burned stably and quietly and still preserved a cylindrical shape during the combustion process, showing the excellent thermal stability and fire resistance of CMCA. The adsorption capacity of CMCA also drops by only 8.2% after six cycles, demonstrating the stable adsorption and recyclability of CMCA.
4 Conclusions
In conclusion, we have successfully used a simple method for the fabrication of low density (0.056 g cm−3), high porosity (95%), compressible and multifunctional carbon aerogels based on bamboo powder. Unlike the cellulose that is frequently used as a unique carbon source, a small amount of introduced MWCNTs decreased the volume shrinkage of cellulose aerogels. Furthermore, the low cost, abundantly available raw material sources, and environmentally friendly procedure are also characteristic of this method. Therefore, we have good reason to believe that this hydrophobic, stable, recyclable, and low production cost material is an ideal adsorbent for collection of oily compounds on the surface of the water.
5 Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 31770607), the National Key Research and Development Program of China (grant no. 2017YFD0600204), the Natural Science Foundation of Jiangsu Province of China (grant no. BK20171450), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors acknowledge the Advanced Analysis & Testing Center of Nanjing Forestry University.
6 References
- 1. Duan B. Gao H. He M. et al.: ‘Hydrophobic modification on surface of chitin sponges for highly effective separation of oil’, ACS Appl. Mater. Interfaces, 2014, 6, (22), pp. 19933 –19942 [DOI] [PubMed] [Google Scholar]
- 2. Sai H. Fu R. Xing L. et al.: ‘Surface modification of bacterial cellulose aerogels’ web‐like skeleton for oil/water separation’, ACS Appl. Mater. Interfaces, 2015, 7, (13), pp. 7373 –7381 [DOI] [PubMed] [Google Scholar]
- 3. Zhang Z. Sèbe G. Rentsch D. et al.: ‘Ultralightweight and flexible silylated nanocellulose sponges for the selective removal of oil from water’, Chem. Mater., 2014, 26, (8), pp. 2659 –2668 [Google Scholar]
- 4. Korhonen J.T. Kettunen M. Ras R.H.A. et al.: ‘Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents’, ACS Appl. Mater. Interfaces, 2011, 3, (6), pp. 1813 –1816 [DOI] [PubMed] [Google Scholar]
- 5. Wu Z.Y. Li C. Liang H.W. et al.: ‘Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions’, Sci. Rep., 2014, 4, pp. 4079, DOI :10.1038/srep04079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi H.M. Cloud R.M.: ‘Natural sorbents in oil spill cleanup’, Environ. Sci. Technol., 1992, 26, (4), pp. 772 –776 [Google Scholar]
- 7. Moriwaki H. Kitajima S. Kurashima M. et al.: ‘Utilization of silkworm cocoon waste as a sorbent for the removal of oil from water’, J. Hazard. Mater., 2009, 165, (1), pp. 266 –270 [DOI] [PubMed] [Google Scholar]
- 8. Radetic M. Ilic V. Radojevic D. et al.: ‘Efficiency of recycled wool‐based nonwoven material for the removal of oils from water’, Chemosphere, 2008, 70, (3), pp. 525 –530 [DOI] [PubMed] [Google Scholar]
- 9. Rajaković‐Ognjanović V. Aleksić G. Rajaković L.: ‘Governing factors for motor oil removal from water with different sorption materials’, J. Hazard. Mater., 2008, 154, (1), pp. 558 –563 [DOI] [PubMed] [Google Scholar]
- 10. Teas C. Kalligeros S. Zanikos F. et al.: ‘Investigation of the effectiveness of absorbent materials in oil spills clean up’, Desalination, 2001, 140, (3), pp. 259 –264 [Google Scholar]
- 11. Lin C. Hong Y.J. Hu A.H.: ‘Using a composite material containing waste tire powder and polypropylene fiber cut end to recover spilled oil’, Waste Manage., 2010, 30, (2), pp. 263 –267 [DOI] [PubMed] [Google Scholar]
- 12. Oh Y.S. Maeng J. Kim S.J.: ‘Use of microorganism‐immobilized polyurethane foams to absorb and degrade oil on water surface’, Appl. Microbiol. Biotechnol., 2000, 54, (3), pp. 418 –423 [DOI] [PubMed] [Google Scholar]
- 13. Lin J. Shang Y. Ding B. et al.: ‘Nanoporous polystyrene fibers for oil spill cleanup’, Mar. Pollut. Bull., 2012, 64, (2), pp. 347 –352 [DOI] [PubMed] [Google Scholar]
- 14. Yang X. Cranston E.D.: ‘Chemically cross‐linked cellulose nanocrystal aerogels with shape recovery and superabsorbent properties’, Chem. Mater., 2014, 26, (20), pp. 6016 –6025 [Google Scholar]
- 15. Zhang W. Zhang Y. Lu C. et al.: ‘Aerogels from crosslinked cellulose nano/micro‐fibrils and their fast shape recovery property in water’, J. Mater. Chem., 2012, 22, (23), pp. 11642 –11650 [Google Scholar]
- 16. Chen W. Li Q. Wang Y. et al.: ‘Comparative study of aerogels obtained from differently prepared nanocellulose fibers’, Chem. Sus. Chem., 2014, 7, (1), pp. 154 –161 [DOI] [PubMed] [Google Scholar]
- 17. Pääkkö M. Vapaavuori J. Silvennoinen R. et al.: ‘Long and entangled native cellulose I nanofibers allow flexible aerogels and hierarchically porous templates for functionalities’, Soft Mat., 2008, 4, (12), pp. 2492 –2499 [Google Scholar]
- 18. Jiang F. Hsieh Y.L.: ‘Amphiphilic superabsorbent cellulose nanofibril aerogels’, J. Mater. Chem. A, 2014, 2, (18), pp. 6337 –6342 [Google Scholar]
- 19. Wang Y. Yadav S. Heinlein T. et al.: ‘Ultra‐light nanocomposite aerogels of bacterial cellulose and reduced graphene oxide for specific absorption and separation of organic liquids’, RSC Adv., 2014, 4, (41), pp. 21553 –21558 [Google Scholar]
- 20. Wu D. Fu R.: ‘Requirements of organic gels for a successful ambient pressure drying preparation of carbon aerogels’, J. Porous Mater., 2008, 15, (1), pp. 29 –34 [Google Scholar]
- 21. Meng Y Young T.M. Liu P. et al.: ‘Ultralight carbon aerogel from nanocellulose as a highly selective oil absorption material’, Cellulose, 2014, 10, (17), pp. 3544 –3550 [Google Scholar]
- 22. Bi H. Huang X. Wu X. et al.: ‘Carbon microbelt aerogel prepared by waste paper: an efficient and recyclable sorbent for oils and organic solvents’, Small, 2014, 10, (17), pp. 3544 –3550 [DOI] [PubMed] [Google Scholar]
- 23. Xu X. Zhou J. Nagaraju D.H. et al.: ‘Flexible, highly graphitized carbon aerogels based on bacterial cellulose/lignin: catalyst‐free synthesis and its application in energy storage devices’, Adv. Funct. Mater., 2015, 25, (21), pp. 3193 –3202 [Google Scholar]
- 24. Wang H. Gong Y. Wang Y.: ‘Cellulose‐based hydrophobic carbon aerogels as versatile and superior adsorbents for sewage treatment’, RSC Adv., 2014, 4, (86), pp. 45753 –45759 [Google Scholar]
- 25. Lai F. Miao Y.E. Zuo L. et al.: ‘Carbon aerogels derived from bacterial cellulose/polyimide composites as versatile adsorbents and supercapacitor electrodes’, Chem. Nano. Mat., 2016, 2, (3), pp. 212 –219 [Google Scholar]
- 26. Li Y.Q. Samad Y.A. Polychronopoulou K. et al.: ‘Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption’, ACS Sust. Chem. Eng., 2014, 2, (6), pp. 1492 –1497 [Google Scholar]
- 27. Yang S. Chen L. Mu L. et al.: ‘Low cost carbon fiber aerogel derived from bamboo for the adsorption of oils and organic solvents with excellent performances’, RSC Adv., 2015, 5, (48), pp. 38470 –38478 [Google Scholar]
- 28. Han S. Sun Q. Zheng H. et al.: ‘Green and facile fabrication of carbon aerogels from cellulose‐based waste newspaper for solving organic pollution’, Carbohydrate Polym., 2016, 136, pp. 95 –100 [DOI] [PubMed] [Google Scholar]
- 29. Wan C. Lu Y. Jiao Y. et al.: ‘Fabrication of hydrophobic, electrically conductive and flame‐resistant carbon aerogels by pyrolysis of regenerated cellulose aerogels’, Carbohydrate Polym., 2015, (118), pp. 115 –118 [DOI] [PubMed] [Google Scholar]
- 30. Du F. Fischer J.E. Winey K.I.: ‘Coagulation method for preparing single‐walled carbon nanotube/poly (methyl methacrylate) composites and their modulus, electrical conductivity, and thermal stability’, J. Polym. Sci. B Polym. Phys., 2003, 41, (24), pp. 3333 –3338 [Google Scholar]
- 31. Kashiwagi T. Grulke E. Hilding J. et al.: ‘Thermal degradation and flammability properties of poly (propylene)/carbon nanotube composites’, Macromol. Rapid Commun., 2002, 23, (13), pp. 761 –765 [Google Scholar]
- 32. Abe K. Yano H.: ‘Comparison of the characteristics of cellulose microfibril aggregates of wood, rice straw and potato tuber’, Cellulose, 2009, 16, (6), pp. 10 –17 [Google Scholar]
- 33. Kim H. Lee C. Kim M.H. et al.: ‘Drop impact characteristics and structure effects of hydrophobic surfaces with micro‐and/or nanoscaled structures’, Langmuir, 2012, 28, (30), pp. 11250 –11257 [DOI] [PubMed] [Google Scholar]
- 34. Yao X. Yu W. Xu X. et al.: ‘Amphiphilic, ultralight, and multifunctional graphene/nanofibrillated cellulose aerogel achieved by cation‐induced gelation and chemical reduction’, Nanoscale, 2015, 7, (9), pp. 3959 –3964 [DOI] [PubMed] [Google Scholar]
- 35. Kabiri S. Tran D.N.H. Altalhi T. et al.: ‘Outstanding adsorption performance of graphene‐carbon nanotube aerogels for continuous oil removal’, Carbon, 2014, (80), pp. 523 –533 [Google Scholar]


