Abstract
The silver nanoparticles (AgNPs) with their unique chemical and physical properties are proving as a new therapeutical agent. In the present study, the AgNPs synthesised from an aqueous extract of a macrofungus, Earliella scabrosa, were characterised by field emission scanning electron microscopy (FESEM), energy dispersive X‐ray analysis (EDX), high‐resolution transmission electron microscopy, X‐ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and further evaluate for its in vitro antibacterial and wound healing efficacy. The mycosynthesised AgNPs exhibited the surface plasmon resonance peak at 410 nm with good stability over a period of a month. The FESEM and EDX analyses revealed the spherical‐shaped AgNPs of an average size of 20 nm and the presence of elemental Ag, respectively. The XRD pattern showed the crystalline nature of AgNPs. The FTIR spectra confirmed the conversion of Ag+ ions to AgNPs due to reduction by biomolecules of macrofungus extract. The mycosynthesised AgNPs showed effective antibacterial activity against two Gram‐positive bacteria, namely Bacillus subtilis and Staphylococcus aureus, and two Gram‐negative bacteria Escherichia coli and Pseudomonas aeruginosa. The pathogens were highly sensitive to AgNPs, whereas less sensitive to AgNO3. The mycosynthesised AgNPs showed significant wound healing potential with 68.58% of wound closure.
Inspec keywords: surface plasmon resonance, wounds, X‐ray diffraction, nanoparticles, molecular biophysics, nanomedicine, antibacterial activity, biomedical materials, reduction (chemical), silver, microorganisms, X‐ray chemical analysis, nanofabrication, transmission electron microscopy, particle size, field emission scanning electron microscopy, Fourier transform infrared spectra
Other keywords: high‐resolution transmission electron microscopy, healing efficacy, mycosynthesised AgNPs, spherical‐shaped AgNPs, wound healing agent, in vitro antibacterial efficacy, Earliella scabrosa, silver nanoparticles, physical properties, chemical properties, therapeutical agent, aqueous extract, macrofungus, field emission scanning electron microscopy, FESEM, energy dispersive X‐ray analysis, EDX, X‐ray diffraction, XRD, Fourier transform infrared spectroscopy, FTIR spectroscopy, surface plasmon resonance peak, crystalline nature, biomolecules, Gram‐positive bacteria, Bacillus subtilis, Staphylococcus aureus, Gram‐negative bacteria, Escherichia coli, Pseudomonas aeruginosa, pathogens, wound closure, Ag
1 Introduction
Nanotechnology is a multidisciplinary field of research and technology development in many areas of science. The capability of synthesising nanoscale materials with specific physiochemical and optoelectronic properties has increasingly important roles in the development of future technology [1]. The use of nanotechnology for the synthesis of nanomaterials is a rapidly developing emerging field. In recent years, there has been an increased interest in the use of biological agents as tools for synthesising nanoparticles (NPs) due to the limitations of physical and chemical methods [2, 3].
Myconanotechnology (myco = fungi and nanotechnology = the creation and exploitation of materials in the size range of 1–100 nm) is a new term defined as the synthesis of NPs by fungi and their subsequent application, particularly in medicine. It is the interface between mycology and nanotechnology and which is a new applied interdisciplinary science that may have considerable potential, partly due to the wide range and diversity of fungi [1]. There is an increasing interest in the use of fungi for these processes, and fungi may have the potential to provide relatively quick and ecologically ‘clean’ biofactories for metallic NPs. Fungi have a number of advantages for NP synthesis compared with other organisms, particularly as they are relatively easy to isolate and culture. They secrete large amounts of extracellular enzymes [4]. The fungal proteins are capable of hydrolysing metal ions quickly and through non‐hazardous processes. As a result, fungi have the potential to produce NPs faster than some chemical synthesis methods. In addition, NPs of high monodispersity and dimensions can be obtained from fungi [5, 6, 7], and the downstream processing and the handling of fungal biomass can be much simpler than the processes needed for chemical synthesis [4].
Silver NPs (AgNPs) have been capitalised for their unique properties and their vast applications in biomedicine. AgNPs are recognised as an alternative antimicrobial agent exhibiting low toxicity in human and have diverse in vitro and in vivo applications [8]. The antimicrobial agents are used to prevent wound infection and accelerate the wound healing process. In medicines, AgNPs have an ample application in skin ointments and creams, to prevent infection of burns and open wounds [9]. Wound healing happens as a cellular response to injuries and in turn activates the fibroblasts, endothelial cells and macrophages [10]. Fibroblast proliferation is involved in the restoration of structure and function in the wound site [11].
Mushrooms are well known for their antimicrobial, antioxidant, anti‐inflammatory, antitumour and anticancer activities [12, 13]. It has been reported that the mushroom extracts from Agaricus bisporus, Pleurotus florida, Pleurotus citrinopileatus, Pleurotus cornucopiae var. citrinopileatus have served as bioreductant in AgNPs synthesis [14, 15, 16, 17]. Mushrooms characteristically contain many different bioactive compounds with diverse biological activity [18]. Earliella is a monotypic fungal genus in the family Polyporaceae, comprising of single species Earliella scabrosa. It produces enzymes for dyes biotransformation in solid‐state fermentation [19]. The antifungal activity of E. scabrosa in order to inhibit the growth of wood‐degrading fungi of rubberwood has been reported [20]. Till date, very limited work has been performed in E. scabrosa and there is no earlier report related to synthesis of AgNPs. Since the Ag having strong toxicity against a wide range of microorganisms and the mushrooms (protein rich) acting as reducing, as well as capping agent, this present paper aims the synthesis of AgNPs using macrofungus (E. scabrosa) and to evaluate its antimicrobial and wound healing efficiency.
2 Materials and methods
2.1 Collection of macrofungus
The fruiting bodies of macrofungus, namely E. scabrosa, Flavodon sp., Pleurotus ostreatus and Ganoderma sp. were collected from Yercaud Hills, Yercaud (12.309N and 78.343E) in the province of Tamil Nadu, South India and were brought to the laboratory for further studies.
2.2 Preparation of the fungal extract
The dried macrofungi were washed repeatedly with distilled water to remove any organic impurities. The cleaned mushrooms were cut into small pieces and were powdered into coarse particles. Four grams of each powdered sample was mixed with 100 ml of distilled water and boiled for 15 min at 55°C. The aqueous extracts were filtered through Whatman No. 1 filter paper and the filtrates were stored at 4°C for further use [21].
2.3 Mycosynthesis of AgNPs
For the synthesis of AgNPs, an aqueous solution of Ag nitrate (AgNO3) (1 mM) was prepared. Specifically, 1 ml of mushroom aqueous extracts were added to 9 ml of 1 mM AgNO3 solution. The reactions were performed in dark at room temperature for 24 h and observed for a change in colour. The extracts without AgNO3 solution were maintained as a control [22].
2.4 Characterisation of mycosynthesised AgNPs
2.4.1 Ultraviolet–visible (UV–vis) spectrum and stability
The UV–vis spectral analysis of mycosynthesised AgNPs was performed using PC‐based Systronics Double Beam Spectrophotometer 2202. The stability of AgNPs was also analysed by measuring the absorbance from the range of 300–700 nm at regular time intervals (24 h, 3rd day, 10th day, 20th day, 30th day and 40th day).
2.4.2 Field emission scanning electron microscopy (FESEM) and energy dispersive X‐ray analysis
The morphology and average size of the mycosynthesised AgNPs were observed by FESEM (Hitachi SO‐6600, Japan). Thin films of the sample were prepared on an aluminium‐coated copper grid by just dropping a very small amount of the sample on the grid, the extra solution was removed with the help of a blotting paper and the film on the grid was allowed to dry by placing it under a mercury lamp for 5 min. The energy dispersive X‐ray analysis (EDX) analysis was conducted using the above‐mentioned instrument to confirm the presence of elemental Ag in the sample.
2.4.3 High‐resolution transmission electron microscopy (HRTEM) and dynamic light scattering
A drop of mycosynthesised AgNPs was placed on carbon‐coated copper grids and allowed to stand for 2.0 min, and the excess solution was removed using a blotting paper and allowed to dry at room temperature. The samples were then observed under HRTEM FEI‐TECNAI G2 T‐30 S‐TWIN instrument. The size distribution of AgNPs was measured using dynamic light scattering (DLS) (Zetasizer Nano ZS ZEN3600, Malvern, UK), resulting in the mean size of particles inside the sample along with the correlation between the number of particles of a particular size versus the size of the NPs.
2.4.4 X‐ray diffraction
The crystallographic structures of mycosynthesised AgNPs phase properties were revealed by X‐ray diffraction (XRD) measurements. Powder X‐ray diffractometry (SEIFERT JSO‐DEBYEFLEX 2002) was used to study the crystalline nature of AgNPs. The NPs were attained by the process of drying and lyophilisation and the XRD pattern was scanned in the 2θ range from 35° to 70°.
2.4.5 Fourier transform infrared spectroscopy
The biomolecules responsible for the formation of AgNPs were analysed using Fourier transform infrared spectroscopy (FTIR) spectroscopy (Hitachi Ltd., Japan). The analysis was performed with potassium bromide pellets and recorded in the range of 400–4000 cm−1. The various modes of vibrations were identified and assigned to determine the different functional groups present in the samples.
2.5 Antibacterial activity of mycosynthesised AgNPs
The antibacterial activity of mycosynthesised AgNPs was carried out by the agar well diffusion test against the Gram‐positive bacteria such as Bacillus subtilis [American Type Culture Collection (ATCC) 6051], Staphylococcus aureus (ATCC 9144) and Gram‐negative bacteria such as Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853). All strains were obtained from the ATCC. The freshly cultured pathogens were smeared on Muller–Hinton agar plates and wells of 6 mm diameter were made with the help of a sterile cork borer. The wells were loaded with different concentrations (10, 20, 30, 40 µl) of the mycosynthesised AgNPs, 20 µl of 1 mM AgNO3 and 40 µl of 10 µg gentamycin. The plates were incubated at 37°C for 24 h and the zone of inhibition (ZOI; mm) appearing around the wells was recorded.
2.6 In vitro wound scratch assay
This experiment was performed according to the previously reported and standardised protocol [23]. The Vero cells were seeded in 6‐well plates (8 × 105 cells/well) and grown until reached a confluence of 90%, in the optimum culture conditions. In the middle of the cell monolayer, a scratch was made by a P10 pipette tip, to mimic a wound, and cell debris was removed by washing with fresh medium. The wound was exposed to 50 µg/ml mycosynthesised AgNPs and 50 µg/ml of commercial drug (Cipladine; positive control) for 48 h at 37°C in a humidified atmosphere of 5% carbon dioxide. The negative control cells were maintained without any treatment. Scratch wound closure was analysed under the inverted microscope (Magnus INVI, Noida), and two digital images were taken at the 0th day (t 0) and 2nd day (t 2) (static imaging). The closure of the scratch was quantified by measuring the difference between the wound widths at t 0 and t 2, using the ImageJ processing software (http://rsbweb.nih.gov/ij/). Scratch closure rate was calculated as described by Felice et al. [24], using the following formula:
where At 0 is the scratch area at time 0 and At 2 is the scratch area at 2nd day. Results were reported as the mean of three independent experiments ± standard deviation.
3 Results and discussion
3.1 Mycosynthesis of AgNPs
In the present investigation, four different macrofungi such as E. scabrosa, Flavodon sp., Pleurotus ostreatus, and Ganoderma sp. were used for the synthesis of AgNPs (Fig. 1). Among the four samples, the aqueous extract of E. scabrosa resulted in dark reddish brown colour with good stability, whereas the other tested macrofungi extracts showed poor stability and aggregation during AgNPs synthesis, hence E. scabrosa was chosen for further studies (Fig. 2) [25]. Balashanmugam et al. [21] reported that among the five fungal extracts, Microporus xanthopus extract showed good stability. Philip et al. [26] have reported the biosynthesis of AgNPs using mushroom which strongly supports our result.
Fig. 1.

Fruiting body of macrofungi collected
(a) E. scabrosa, (b) Flavodon sp., (c) Pleurotus ostreatus, (d) Ganoderma sp.
Fig. 2.

Mycosynthesis of AgNPs
(a) E. scabrosa, (b) Flavodon sp., (c) Pleurotus ostreatus, (d) Ganoderma sp.; C – control and T – test
3.2 Characterisation of mycosynthesised AgNPs
3.2.1 UV–vis spectrum and stability of AgNPs
The UV–vis spectra of mycosynthesised AgNPs showed a broad peak at 408 nm (Fig. 3 a) indicating the presence of AgNPs, due to the excitation of surface plasmon resonance (SPR) in the metal NPs [27]. This formation of AgNPs would have been mediated by the active biomolecules present in the mushroom extract. According to Mie's theory, only a single SPR band is observed in the absorption spectra of spherical metal NPs [28].
Fig. 3.
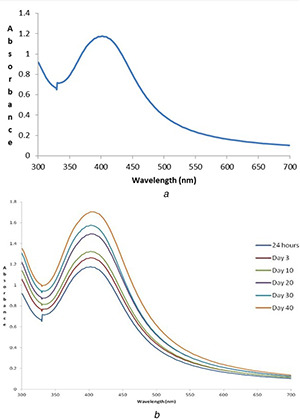
UV–vis spectra of mycosynthesised AgNPs
(a) at 24 h, (b) Stability
The stability of the AgNPs was monitored at different time durations (i.e. 24 h, 3rd day, 10th day, 20th day, 30th day and 40th day) by using UV–vis spectral analysis. It can be summarised that after 24 h of incubation, the SPR showed a peak at 408 nm. After 3 days, the peak shifted to 410 nm and was maintained for more than 1 month. It was observed that with increased contact time, the peak becomes sharper, suggesting the formation of monodisperse NPs [22]. The results revealed that there was no much alteration in the peak even after 1 month of incubation under dark room at 37°C, and thus indicating the higher stability of mycosynthesised AgNPs of E. scabrosa with no signs of aggregation (Fig. 3 b).
3.2.2 FESEM and EDX
The morphology and the particle size of mycosynthesised AgNPs were determined using FESEM micrograph. The particles were spherical in shape with an average size of 20 nm (Fig. 4 a). The particle size distribution analysis of mycosynthesised AgNPs showed diameter size ranging from 10 to 50 nm (Fig. 4 b). The average size of AgNPs synthesised using edible mushrooms such as P. florida and Volvariella volvacea was 20 and 15 nm, respectively [15, 25]. The EDX spectra reveal a strong signal for Ag at 3 keV, correspond to the binding energies of metallic NPs (Fig. 4 c). Throughout the scanning range of binding energies, no peak belonging to impurity was detected. The results indicated that the reaction product was composed of high purity AgNPs [21].
Fig. 4.

Characterisation of mycosynthesised AgNPs
(a) FESEM image of spherical‐shaped AgNPs, (b) Particles size distribution histogram, (c) EDX spectrum
3.2.3 HRTEM and DLS
The HRTEM images of mycosynthesised AgNPs at 100 and 50 nm scales are shown in Figs. 5 a and b, respectively. The AgNPs were distinctly spherical in shape with maximum particles in size range within 9–60 nm with a mean diameter of 16.65 nm. The HRTEM images of AgNPs in the present paper indicated good crystalline nature of the NPs, in turn further evidenced by XRD. The macrofungus P. cornucopiae var. citrinopileatus synthesised NPs reveals the polydispersity and an average size ranging from 20 to 30 nm [17].
Fig. 5.

Characterisation of mycosynthesised AgNPs
HRTEM image
(a) 100 nm, (b) 50 nm, (c) DLS pattern
The DLS pattern reveals that AgNPs have an average hydrodynamic diameter of 19.59 nm with polydispersity index of 0.396 (Fig. 5 c). As DLS measures the hydrodynamic diameter, DLS measured size is slightly bigger than the particle size measured from TEM micrographs.
3.2.4 X‐ray diffraction
The XRD analysis was performed using dry powders of the AgNPs and the diffracted intensities were recorded from 35° to 70° at 2θ angles. The XRD pattern exhibited the pure crystalline structures of mycosynthesised AgNPs and resulted in three distinct peaks at 38.3°, 43.4° and 64.6° corresponding to the 111, 200 and 220 crystallographic planes (Fig. 6). The peaks were compared with XRD database which strongly supported the presence of AgNPs having face‐centred cubic structure [21].
Fig. 6.
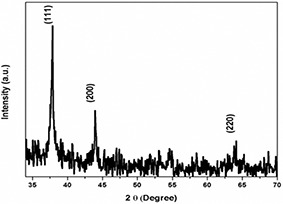
XRD pattern of crystalline AgNPs
3.2.5 Fourier transform infrared spectroscopy
The FTIR spectrum of AgNPs was used to identify the potential biomolecules of E. scabrosa aqueous extract responsible for the reduction of Ag+ ions and capping of mycosynthesised AgNPs for its efficient stabilisation. The FTIR spectrum of the mushroom extract showed a medium peak at 3304 cm−1 corresponding to H‐bonded –OH stretch carboxylic acids and –CH stretch alkene; narrow peak at 1636.4 cm−1 indicating alkenyl C = C stretching [17]. The mycosynthesised AgNPs exhibited two similar peaks as in mushroom extract along with four additional peaks at 1395, 1337, 1184 and 1140 cm−1 corresponding to –OH bending (carboxylic acid), C–C stretching, asymmetric stretching of the SO3– group and ether linkages, respectively, indicating the presence of linkages for ether, sulphur‐oxy compounds as stabilising caps, along with proteins and amino acid residues (Fig. 7). Thus, the FTIR results reveal that the functional groups of alcohols and carboxylic acids present in the mushroom extract may be responsible for the reduction of Ag+ to Ag0 and the proteins and amino acids residues surrounding mycosynthesised AgNPs are responsible for its stability.
Fig. 7.

FTIR spectrum of mycosynthesised AgNPs
3.3 Antibacterial activity of mycosynthesised AgNPs
The mycosynthesised AgNPs of E. scabrosa showed positive antibacterial activity against all the four tested pathogens, i.e. B. subtilis, S. aureus, P. aeruginosa and E. coli. Among the four pathogens, P. aeruginosa was highly sensitive to the mycosynthesised AgNPs, 19 mm ZOI; followed by S. aureus of 14 mm; E. coli of 14 mm and B. subtilis of 11 mm. AgNO3 showed the least activity for S. aureus and E. coli, whereas no zone was observed in P. aeruginosa and B. subtilis. The positive control, gentamycin showed high antibacterial activity, yet, mycosynthesised AgNPs have the comparably similar effect to that of positive (Fig. 8, Table 1). Numerous reports have stated the antibacterial activity of AgNPs against different bacterial strains [21, 29, 30].
Fig. 8.
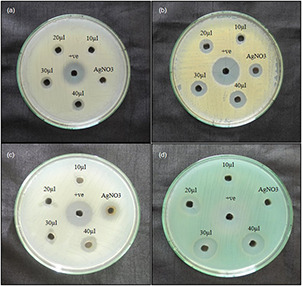
Antibacterial activity of mycosynthesised AgNPs
(a) B. subtilis, (b) S. aureus, (c) E. coli, (d) P. aeruginosa and +ve – gentamycin
Table 1.
Antibacterial activity of the AgNPs
| Name of the organism | ZOI (mm in diameter) | |||||
|---|---|---|---|---|---|---|
| AgNPs (µl) | AgNO3 | Positive control | ||||
| 10 | 20 | 30 | 40 | |||
| B. subtilis | 06 | 10 | 11 | 11 | — | 20 |
| S. aureus | 07 | 11 | 14 | 14 | 10 | 22 |
| E. coli | 04 | 06 | 09 | 14 | 12 | 20 |
| P. aeruginosa | 11 | 15 | 17 | 19 | — | 20 |
Abbreviations: AgNPs, silver NPs and AgNO3, Ag nitrate.
3.4 In vitro wound scratch assay
The wound healing efficacy of the mycosynthesised AgNPs and the commercial drug was observed under an inverted microscope (Fig. 9) and the percentage of wound healed was calculated. It was observed that on the 2nd day, the wounds were healed to an efficacy of 73.58% with mycosynthesised AgNPs and 85.72% with the commercial drug (positive control) compared with the negative control (17.64%). The morphology of healed cells was looking similar to normal cells. Thus, it was inferred that AgNPs have significantly good healing potential. The earlier reports state that antimicrobial and antioxidant activities seem to have positive effects on wound healing [31, 32] also the cell migration and proliferation play a crucial role in the healing process by initiating the proliferative phase of repair [33, 34].
Fig. 9.

In vitro wound scratch assay
(a) Normal cells, (b) Wounded cells (untreated), (c) AgNPs treated cells, (d) Commercial drug treated cells
4 Conclusion
The macrofungus (mushroom)‐mediated synthesis of AgNPs was successfully developed using E. scabrosa aqueous extract. This is the first report where AgNPs are synthesised using E. scabrosa and was found to be stable over a period of 2 months. Also, the average size of NPs synthesised in the present work is smaller compared with previous studies. The biomolecules of macrofungus have played a major role as reducing and capping agent for the synthesis of stable and functionalised AgNPs. The mycosynthesised AgNPs exhibited a potential bactericidal activity against human pathogens and enhanced wound healing properties. These promising medicinal properties justify the use of AgNPs for the treatment of microbial infections and wounds. Furthermore, an in‐depth mechanism for the wound healing and antibacterial inhibitory action of mycosynthesised AgNPs needs to be further clarified.
5 Acknowledgments
We wish to thank The Director, Avanz Bio Pvt. Ltd. for providing the laboratory facilities. They also thank CSIR‐CLRI, Chennai, SRM University, Chennai and VIT for providing instrumentation facility. Our special thanks to Dr. M. Kumar, Assistant Professor, Madras Christian College for collection and identification of macrofungus samples.
6 References
- 1. Rai M. Yadav A. Bridge P. et al.: ‘Myconanotechnology: a new and emerging science’, in Rai M. Bridge P. (Eds.): ‘Applied mycology’ (CAB International, New York, 2009, 14th edn.), pp. 258 –267 [Google Scholar]
- 2. Kohler J.M. Hubner U. Romanus H. et al.: ‘Formation of star‐like and core–shell Au–Ag nanoparticles during two and three step preparation in batch and microfluidic systems’, J. Nanomater., 2007, 1155, pp. 98134 –98141 [Google Scholar]
- 3. Kowshik M. Deshmukh N. Kulkarni S.K. et al.: ‘Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in fabrication of an ideal diode’, Biotechnol. Bioeng., 2002, 78, (5), pp. 583 –588 [DOI] [PubMed] [Google Scholar]
- 4. Mandal D. Bolander M.E. Mukhopadhyay D. et al.: ‘The use of microorganisms for the formation of metal nanoparticles and their application’, Appl. Microbiol. Biotechnol., 2006, 69, pp. 485 –492 [DOI] [PubMed] [Google Scholar]
- 5. Bhattacharya D. Gupta R.K.: ‘Nanotechnology and potential of microorganisms’, Crit. Rev. Biotechnol., 2005, 24, (4), pp. 199 –204 [DOI] [PubMed] [Google Scholar]
- 6. Mohanpuria P. Rana N.K. Yadav S.K.: ‘Biosynthesis of nanoparticles: technological concepts and future applications’, J. Nanopart. Res., 2007, 7, pp. 9275 –9280 [Google Scholar]
- 7. Mukherjee P. Roy M. Mandal B.P. et al.: ‘Green synthesis of highly stabilized nanocrystalline silver particles by a non‐pathogenic and agriculturally important fungus T. asperellum ’, Nanotechnology, 2008, 19, pp. 103 –110 [DOI] [PubMed] [Google Scholar]
- 8. Farooqui M.A. Chauhan P.S. Krishnamoorthy P. et al.: ‘Extraction of silver nano‐particles from the leaf extracts of Clerodendrum inerme ’, Dig. J. Nanomater. Biostruct., 2010, 5, pp. 43 –49 [Google Scholar]
- 9. Duran N. Marcato P.D. Alves O.L. et al.: ‘Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains’, J. Nanobiotechnol., 2005, 3, pp. 8 –14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark R.A.F.: ‘Fibrin and wound healing’, Ann. New York Acad. Sci., 2001, 936, (1), pp. 355 –367 [DOI] [PubMed] [Google Scholar]
- 11. Mensah A.Y. Sampson J. Houghton P.J. et al.: ‘Effects of Buddleja globosa leaf and its constituents relevant to wound healing’, J. Ethnopharmacol., 2001, 77, pp. 219 –226 [DOI] [PubMed] [Google Scholar]
- 12. Ajith T.A. Janardhanan K.K.: ‘Indian medicinal mushrooms as a source of antioxidant and antitumor agents’, J. Clin. Biochem. Nutr., 2007, 40, p. 157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turkoglu A. Duru M.E. Mercan N. et al.: ‘Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill’, Food Chem., 2007, 101, pp. 267 –273 [Google Scholar]
- 14. Al‐Hamadani A.H. Kareem A.A.: ‘Combination effect of edible mushroom – sliver nanoparticles and antibiotics against selected multidrug biofilm pathogens’, Iraq Med. J., 2017, 1, (3), pp. 68 –74 [Google Scholar]
- 15. Bhat R. Deshpande R. Ganachari S.V. et al.: ‘Photoirradiated biosynthesis of silver nanoparticles using edible mushroom Pleurotus florida and their antibacterial activity studies’, Bioinorg. Chem. Appl., 2011, 2011, Article ID: 650979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maurya S. Bhardwaj A.K. Gupta K.K. et al.: ‘Green synthesis of silver nanoparticles using Pleurotus and its bactericidal activity’, Cell. Mol. Biol., 2016, 62, p. 131 [Google Scholar]
- 17. Owaid M.N. Raman J. Lakshmanan H. et al.: ‘Mycosynthesis of silver nanoparticles by Pleurotus cornucopiae var. Citrinopileatus and its inhibitory effects against Candida sp.’, Mater. Lett., 2015, 153, pp. 186 –190 [Google Scholar]
- 18. Chang R.: ‘Functional properties of edible mushrooms’, Nutr. Rev., 1996, 54, pp. S91 –S93 [DOI] [PubMed] [Google Scholar]
- 19. Guerra G. Domínguez O. Leal M.R. et al.: ‘Production of laccase and manganese peroxidase by white‐rot fungi from sugarcane bagasse in solid bed: use for dyes decolourisation’, Sugar Tech, 2008, 10, (3), pp. 260 –264 [Google Scholar]
- 20. Peng T.Y. Don M.M.: ‘Antifungal activity of in‐vitro grown Earliella scabrosa, a Malaysian fungus on selected wood ‐degrading fungi of rubberwood’, J. Phys. Sci., 2013, 24, (2), pp. 21 –33 [Google Scholar]
- 21. Balashanmugam P. Santhosh S. Giyaullah H et al.: ‘Mycosynthesis, characterization and antibacterial activity of silver nanoparticles from Microporus xanthopus: a macro Mushroom’, Int. J. Innov. Res. Sci. Eng. Technol., 2013, 2, (11), pp. 6262 –6270 [Google Scholar]
- 22. Balashanmugam P. Kalaichelvan P.T.: ‘Biosynthesis characterization of silver nanoparticles using Cassia roxburghii DC. Aqueous extract, and coated on cotton cloth for effective antibacterial activity’, Int. J. Nanomed., 2015, 10, pp. 87 –97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang C.C. Park A.Y. Guan J.L.: ‘In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro’, Nat. Protocol, 2007, 2, (2), pp. 329 –333 [DOI] [PubMed] [Google Scholar]
- 24. Felice F. Zambito Y. Belardinelli E. et al.: ‘Effect of different chitosan derivatives on in vitro scratch wound assay: a comparative study’, Int. J. Biol. Macromol., 2015, 76, pp. 236 –241 [DOI] [PubMed] [Google Scholar]
- 25. Philip D.: ‘Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract’, Spectrochim. Acta Mol. Biomol. Spectrosc., 2009, 73, pp. 374 –381 [DOI] [PubMed] [Google Scholar]
- 26. Philip D. Unni C. Aromal S.A. et al.: ‘ Murraya koenigii leaf‐assisted rapid green synthesis of silver and gold nanoparticles’, Spectrochim. Acta Mol. Biomol. Spectrosc., 2011, 78, pp. 899 –904 [DOI] [PubMed] [Google Scholar]
- 27. Mulvaney P.: ‘Surface plasmon spectroscopy of nanosized metal particles’, Langmuir, 1996, 12, pp. 788 –800 [Google Scholar]
- 28. Mie G.: ‘A contribution to the optics of turbid media, especially colloidal metallic suspensions’, Ann. Phys., 1908, 25, pp. 377 –445 [Google Scholar]
- 29. Bankura K.P. Maity D. Mollick M.M.R. et al.: ‘Synthesis, characterization and antimicrobial activity of dextran stabilized silver nanoparticles in aqueous medium’, Carbohydr. Polym., 2012, 89, pp. 1159 –1165 [DOI] [PubMed] [Google Scholar]
- 30. Morones J.R. Elechiguerra J.L. Camacho A. et al.: ‘The bactericidal effect of silver nanoparticles’, J. Nanotechnol., 2005, 16, pp. 2346 –2353 [DOI] [PubMed] [Google Scholar]
- 31. Adetutu A. Morgan W.A. Corcoran O.: ‘Antibacterial, antioxidant and fibroblast growth stimulation activity of crude extracts of Bridelia ferruginea leaf, a wound‐healing plant of Nigeria’, J. Ethnopharmacol., 2011, 133, pp. 116 –119 [DOI] [PubMed] [Google Scholar]
- 32. Annan K. Houghton P.J.: ‘Antibacterial, antioxidant and fibroblast growth stimulation of aqueous extracts of Ficus asperifolia Miq. and Gossypium arboreum L., wound‐healing plants of Ghana’, J. Ethnopharmacol., 2008, 119, pp. 141 –144 [DOI] [PubMed] [Google Scholar]
- 33. Ebeling S. Naumann K. Pollok S. et al.: ‘From a traditional medicinal plant to a rational drug: understanding the clinically proven wound healing efficacy of birch bark extract’, PLoS One, 2014, 9, p. e86147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fronza M. Heinzmann B. Hamburger M. et al.: ‘Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts’, J. Ethnopharmacol., 2009, 126, pp. 463 –467 [DOI] [PubMed] [Google Scholar]


