Abstract
For being applied in medicine as therapeutic agents, nanostructures need to be biocompatible and eco‐friendly. Plant‐derived phenolic acids have been utilised for green synthesis of metallic or metallic oxide nanoparticles (NPs). The phenolic acids play role as both reducing agents and stabilisers in the process of NPs synthesis. Many experiments have been dedicated to develop efficient green synthesis techniques for producing metal NPs. Using phenolic acids represents a reproducible, simple, profitable, and cost‐effective strategy to synthesise metal NPs. As a phytochemical for metal NPs synthesis, phenolic acids are antioxidants that represent many health benefits. However, limited studies have been dedicated to the synthesis and characterisation of NPs produced by phenolic acids. Thus, this review focused on phenolic acids mediated nanomaterial synthesis and its biomedical applications. It should be noted the mechanism of metal ion bioreduction, phenolic acids surface adsorption, characterisation, and toxicity of metal NPs made with different phenolic acids have been discussed in this review.
Inspec keywords: bio‐inspired materials, organic compounds, adsorption, nanofabrication, nanoparticles, biomedical materials, nanomedicine
Other keywords: toxicity, biomedical applications, antioxidants, phytochemical synthesis, reducing agents, therapeutic agents, medicine, metallic oxide nanoparticles, plant‐derived phenolic acids, natural phenolic acids, metal nanoparticles synthesis, phenolic acids surface adsorption, metal ion bioreduction, nanomaterial synthesis, efficient green synthesis techniques
1 Introduction
The techniques for nanomaterial fabrication are among rapidly growing fields of nanoscience. Regarding their unique optical, catalytic, and electrical properties, metal NPs have been widely applied in pharmaceutical, medical, nutritional, as well as cosmetic industries [1]. A variety of techniques can be used for synthesising metallic NPs. In practice, two main synthesis approaches have been introduced including bottom‐up and top‐down techniques [2]. The top‐down approaches such as laser ablation, attrition, and pyrolysis are usually inconvenient, expensive, and non‐feasible [3]. Other main disadvantages of the top‐down methods include the imperfect surface structure of the synthesised NPs [4], as well as enormous energy consumption of this strategy [5]. These limitations render the bottom‐up approaches much more applicable than the top‐down methods.
The chemical reduction of the metal ions applying organic or inorganic reducing agents such as sodium citrate [6, 7], lithium or sodium borohydride [8, 9, 10], and amine‐borane complexes [11] comprises a common bottom‐up method for the preparation of metallic NPs. Monodispersed metal NPs can also be prepared by using chemical stabilisers such as polymers and surfactants. The chemicals recruited as either reducing agents, precursors, stabilisers, or even solvents cannot be easily removed from the synthesised NPs which is a concerning issue as some of these agents can be highly toxic [12, 13]. The safety and toxicity considerations are among primary requirements before metal NPs can be applied in biomedical fields such as pharmaceutical, nutritional, and cosmetic industries. Therefore, synthesising metal NPs using safe protocols without toxic materials is a prerequisite for their applications in biomedicine.
Biological synthesis techniques using plants or microorganisms have been introduced as non‐toxic approaches for the synthesis of metal NPs [14, 15, 16]. However, these approaches may lead to unwanted immunogenic responses [17]. Furthermore, these approaches generally result in non‐homogenous NPs [14]. Nanomaterials such as gold and silver NPs have been widely studied for being applied as medical diagnostic and therapeutic compounds [18, 19]. Using phytochemicals as either reducers or stabilisers delivered a safe synthesis approach producing desirable metal NPs with acceptable structural properties.
Medicinal plants have a rich history as old as medicine [1]. Nowadays, medicinal plants are applied as either therapeutic or preventive modalities in cancer [20, 21], heart diseases [22], type‐2 diabetes [23], and microbial infections [24]. Based on their metabolism, plant‐derived compounds are categorised as primary and secondary chemicals. Amino acids, proteins, sugars, nucleic acids, chlorophylls, and other basic metabolites are primary constituents while other phytochemicals comprise the secondary compounds [25]. Plant extracts remain important materials used for the synthesis of metal NPs. Among various plant compounds, we here focused on the applications of natural phenolic acids in the synthesis of metal NPs. These structures are simple, small, non‐toxic, and highly effective for the synthesis of metal nanoparticles (NPs). Herein, this review focused on the synthesis of metal NPs with natural phenolic acids which possess some medical applications.
2 Phenolic acids
Phenolic compounds protect plants against reactive oxygen species (ROS) which are produced during photosynthesis and in exposition to anthropogenic pollutions [26]. Phenolic acids which are phenolic derivatives with at least one functional carboxylic group have been widely used in medicine because of their potent antioxidant activities. Most of the phenolic acids are found as parts of larger polyphenols and other organic and structural compounds [27, 28].
The two main categories of natural phenolic acids include derivatives of benzoic acids and cinnamic acids which are categorised based on the number of hydroxylation sites of aromatic rings (Fig. 1) [29]. The hydroxyl groups in the structure of these compounds can scavenge ROS and free radicals [30]. Chemical structures of both groups are depicted in Fig. 1.
Fig. 1.
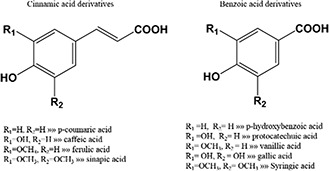
Basic chemical structures of most of phenolic acids that have been used for metal NPs synthesis
3 Benefits of phenolic acids
Plants secondary metabolites which are present in large quantities in vegetable and fruits are able to prevent diseases and elaborate health. Medicinal plants with beneficial health effects have large quantities of phenolic compounds as secondary metabolites. These phytochemicals also exist in a wide range of plant‐based foods and beverages. Although many health benefits have been noted for a diverse variety of these natural compounds, phenolic acids are well known for their antioxidant, anti‐cancer, and anti‐inflammatory properties [31].
The natural antioxidants such as phenolic acids available in foods and beverages have globally been used for their anti‐oxidant activities. Reduction in endogenous antioxidant capacity leads to elevated ROS and free radicals which participate in the pathogenesis of many chronic diseases such as cancer, diabetes, Alzheimer disease, as well as kidney and cardiovascular diseases [32]. Phenolic compounds exert most of their beneficial effects through their antioxidant activities [33]. As well, the radical scavenging activity of these compounds is mediated by their phenolic hydrogens. The antioxidant properties of phenolic acids have been extensively evaluated by researchers. The antioxidant activities of phenolic acids depend on their different functional groups and chemical structures. Likewise, the functional groups in plants secondary metabolites also promote radical scavenging behaviour of these compounds. In addition, the radical scavenging properties of phenolic compounds largely depend on the availability of carboxy, hydroxy, ortho‐dihydroxy, amino‐, catechol, and other functional groups, as well as the presence of conjugated double bonds in their structure [34]. Also, the anti‐cancer function of phenolic acids and other polyphenols has been attributed to their antioxidant activity [35, 36].
The literature is full of scientific evidences confirming the beneficial effects of natural phenols on various diseases such as diabetes [23], cardiovascular diseases [22], and many other chronic conditions [32, 37]. Plant phytochemicals have traditionally been utilised to prevent age‐related brain disorders [38]. The molecular mechanisms underlying the beneficial health impacts of these compounds include signalling pathways preventing oxidative stress [39] and inflammation [40]. Anti‐inflammatory activities of phenolic acids may also be triggered by their antioxidant properties [41]. Due to the potent topical anti‐inflammatory activity of phenolic acids acetate, these have been suggested as potential therapeutics for many cutaneous and systemic inflammatory conditions [42].
Phenolic acids can also act as toxins against different prokaryotic or even some eukaryotic pathogens raising concerns about their cytotoxic effects over excessive usage [43]. Antiviral and antimicrobial activities of phenolic acids and other phytochemicals have been well discussed [24, 44]. Unicellular or multicellular eukaryotic pathogens have also been sensitive to phenolic acids. Teodoro et al. has reviewed the antifungal effects of phenolic acids against Candida agents [45]. The elevated levels of some phenolic acids such as gallic acid and caffeic acid have been shown in tobacco cutworm infested cotton plant [46].
4 Metal NP synthesised by phenolic acids
Many active plant chemicals with medicinal or nutritional properties have been applied for synthesising nanomaterials. Among these, flavonoids and phenolic acids have been extensively under attention for their applications in nanomedicine. Very recently, Sathishkumar et al. [47] has reviewed the roles of flavonoids as mediators in metal NPs synthesis. Many phenolic acids have also been investigated as either reducers or stabilisers for metal NPs synthesis. Here we reviewed the applications of phenolic acids as potential mediators for synthesising metal NPs.
H‐donating capability of phenolic acids has been investigated by many techniques [48, 49]. The antioxidant activity and the reducing capacity of phenolic acids in the process of metal NPs synthesis were reported by Wang et al. who produced cinnamic acid‐capped gold particles in 2006 [50]. In the same year, phenolic acids including propyl gallate, caffeic acid, protocatechuic acid, ferulic acid, and vanillic acid have been investigated for synthesising gold NPs by Scampicchio et al. [51]. All of the investigated phytochemicals were able to reduce gold (III) to gold NPs. However, the potential biomedical applications of the synthesised gold NPs are yet to be investigated. In addition to these compounds, sinapinic and syringic acids have also been studied as potential mediators for synthesising metal NPs by Zougagh and co‐workers [52]. In this report, the gold NPs produced by phenolic acid were able to catalyse the reduction of 4‐nitrophenol to 4‐aminophenol in excess of NaBH4.
Some other phenolic acids have also been applied for synthesising metal NPs in recent years. The biomedical applications of the synthesised NPs by different phenolic acids have been shown in Table 1. Among different phenolic acids, gallic acid has been widely used to synthesise different metal or metal oxide NPs. Gallic acid is a natural antioxidant [69] representing antibacterial [70], antiviral [71], and anticancer [72, 73] properties. Gallic acid has been used as either reducing or stabiliser agent for synthesising gold, silver, and selenium NPs (Table 1). The antibacterial and antifungal activities of silver NPs synthesised by gallic acid (GA@AgNPs) have been evaluated by different research groups [74, 75]. Also, functionalised ZnO NPs synthesised by gallic acid represented antibacterial activity [66]. GA@AgNPs not only inhibited the growth of E. coli, S. aureus, and C. albicans, but also represented selective cytotoxicity against cancerous cells but not normal cells [76]. Similar properties have been reported for silver‐selenium alloy NPs synthesised by gallic acid and quercetin [60], as well as selenium‐ruthenium alloy NPs synthesised by gallic acid [62]. Gallic acid‐coated polymeric NPs have been applied as therapeutic agents for amnesia [77] and anxiety [78]. These effects may also be reproducible for metal NPs coated with gallic acid.
Table 1.
Phenolic acid mediated metal NPs synthesis and application
| No. | Phenolic acids | Materials | Size, nm | Role of phenolic acids in synthesis procedure | Applications | Ref. |
|---|---|---|---|---|---|---|
| 1 | caffeic acid | AgNPs | 6.7 ± 0.3 | reducing agent and stabiliser | anticancer activity | [53] |
| AuNPs | 30.0 ± 7.4 | reducing agent and stabiliser | analytical application | [54] | ||
| FeNPs | 10–150 | the interaction between caffeic acid, with iron results in the formation of meta‐stable colloidal NPs | characterisation of antioxidant properties | [55] | ||
| 2 | chlorogenic acid | AgNPs | 19.3 ± 8.2 | reducing agent and stabiliser | antibacterial activity | [56] |
| AuNPs | 22.2 ± 4.8 | reducing agent and stabiliser | anti‐inflammatory properties | [57] | ||
| 3 | cinnamic acid | AgNPs and AuNPs | 10 ± 5 | synthesis of silver (I) or gold (I) carboxylates precursor for light activated metal NPs synthesis | [58] | |
| AuNPs | <10 | stabiliser | NPs self‐assembly | [50] | ||
| 4 | cumaric acid | FeNPs | 20–50 | interaction between caffeic acid, with iron results in the formation of NPs | characterisation of antioxidant properties | [55] |
| 5 | freulic acid, | AgNPs | 28–33 | reducing agent | detection of ferulic acid through formation of Ag NPs | [59] |
| 6 | vanilic acid, sinapinic acid, propyl gallate | AuNPs | different sizes | reducing agent and stabiliser | analytical application | [51, 52] |
| AgNPs | different sizes | reducing agent and stabiliser | antimicrobial activity | [56] | ||
| protocatechuic acid and syringic acid | ||||||
| 7 | gallic acid | Ag@Se NPs | 30–35 | reducing agent and stabiliser alongside quercetin | anticancer activity | [60] |
| AuNPs | 11.9 ± 1.6 | reducing agent | DNA hybridisation | [61] | ||
| Se@Ru NPs | 70 | reducing agent and stabiliser | anticancer activity | [62] | ||
| SeNPs | 50–75 | reducing agent and stabiliser | anticancer activity, blocking angiogenesis | [62, 63, 64] | ||
| FeNPs | 5 | interaction between gallic acid, with iron results in the formation of NPs | multifunctional theranostic nano‐agents for cancer diagnostic and therapy | [65] | ||
| ZnONPs | 11.5 ± 4.4 | stabiliser and functionalisation | antioxidative and antibacterial activities | [66] | ||
| AuNPs | >120 | reducing agent and stabiliser | analytical application | [51, 52] | ||
| 8 | salicylic acid | AuNRs | ∼50–70 nm | reducing agent | [67] | |
| 9 | salicylic acid | Fe3 O4 NPs | ∼52 | biocompatible shell structure and stabiliser | controlled intravascular accumulation, tissues targeting | [68] |
| Au NPs | different sizes | reducing agent and stabiliser | analytical application | [52] |
Pyrogallol, a decarboxylated derivative of gallic acid, has been applied for synthesising silver NPs. Jiang and Yu reported a simple procedure for synthesising silver NPs using pyrogallol as a reducing and stabiliser agent. The hydrogen bonds of pyrogallol‐coated silver NPs delivers a chain structure which results in a new plasmon resonance peak in near‐infrared wavelength region [79]. Similar behaviour has been reported for cinnamic acid‐coated gold NPs by Wang et al. [50]. Another gallic acid derivate that has been applied for synthesising gold [51] and silver [80] NPs is propyl gallate which is an ester derivative of propanol and gallic acid. Nevertheless, the biomedical applications of such NPs have not been yet studied.
Phenolic acids are not just simple reducing or stabilising agents. These chemicals have been used in complicated NP synthesis procedures. Metal (I) carboxylates precursor has been applied for synthesising light‐activated metal NPs using cinnamic acid as a reducing agent [58]. The application of salicylic acid to reduce Au (III) to Au (I) in the growth solution of gold nanorods prior to the catalytic reduction of Au (I) to Au (0) by ascorbic acid improved the reduction process leading to superior morphology of NPs and monodisperse nanorods [67].
Iron‐chelation properties of phenolic acids make them applicable for the preparation of iron NPs. The interaction between caffeic acid and iron can result in the formation of meta‐stable colloidal NPs [55]. The 64 Cu‐labelled polymeric multifunctional iron‐gallic acid NPs have been used in positron emission tomography imaging‐guided photothermal therapy [65]. Salicylic acid has further been applied as shells in the synthesis of Fe3 O4 NPs. Some advantages of salicylic acid‐coated magnetic NPs have been reported as good bioavailability, biocompatibility, and low vascular embolisation capacity [68].
5 Mechanism of metal NPs synthesis and stabilisation by phenolic acids
5.1 Synthesis mechanisms of metallic NPs by phenolic acids
In‐situ synthesis of phenolic acid‐coated metal NPs can be carried out in aqueous solution (Table 1). Applying phenolic acid as a reducing agent for synthesising metal NPs can be performed using a thermodynamic equilibrium approach. In this synthesis approach, nucleation is initiated by injecting reducing agent (phenolic acids) at the metal ion supersaturation concentration. This is followed by the subsequent growth of metal NPs through gradual ion reduction [81].
The high oxidation tendency of phenolic acids, especially in an alkaline condition, can facilitate the initiation of nucleation process [59]. Kim et al. declared that the oxidation of caffeic acid hydroxyl functional group would provide electron (e‐) required for neutralising gold ions (Au3+) [82]. Scampicchio et al. [51] investigated the reducing capacities of propyl gallate, ferulic acid, caffeic acid, vanillic acid, and protocatechuic acid in the presence of hydrogen tetrachloroaurate by UV–Vis spectra and colourimetry methods. The authors stated that the bioreduction potential of phenolic acids was directly related to the number of their functional hydroxyl groups. The growth process of metal NPs can be mediated by the oxidised phenolic acids attached to the surface of the NPs [57]. The absorbance of the phenolic acid attached to the surface of the metal NPs is resulted from the formation of an adsorbent bond between carboxyl group and the metal atom [50, 58].
The metal ion chelation capability of phenolic acids such as caffeic and coumaric acid also contributes to the iron NPs formation process [55]. Similar NPs have been synthesised through mixing of gallic acid with FeCl3 in the presence of polyvinylpyrrolidone. The chelation capability is further augmented in the presence of catechol or galloyl functional groups [83].
5.2 Phenolic acids adsorption on metal NPs surfaces
Metal NPs synthesised by phenolic compounds represented higher stability than those synthesised by other organic or inorganic reducing agents such as citrate or sodium borohydride [84]. The synthesised metal NPs can be coated on the surface by protonated reducing agents such as citrate through various mechanisms depending on the intermolecular interactions between the absorbed molecules and the metal surface [85]. Natural phenols with functional hydroxyl [47] and carboxyl groups [86] represent protonating and absorbing capabilities. In addition, catechol group of some phenolic acids is a good metal absorbing moiety. This functional group can absorb metal surfaces through three different configurations including bidentate bridging bonding, bidentate chelating bonding, and monodentate ester‐like bonding (Fig. 2) [87, 88]. Zhang et al. investigated the absorption of caffeic acid on the surface of zinc oxide and titanium dioxide NPs by using density functional theory (DFT) calculations. According to their investigation, the absorption of caffeic acid was energy efficient for both metal oxide surfaces with superior results for TiO2 NPs [89]. Caffeic acid has also been used as a reducing agent for synthesising metal gold and silver NPs [53, 54]. Kim and Han investigated the absorption capability of deprotonated caffeic acid for gold NPs applying DFT calculations. The calculated absorption rate of caffeic acid for Au (100), (110), and (111) represented its capacity for being used as a predictor of morphology of the synthesised Au NPs (AuNPs) [90].
Fig. 2.
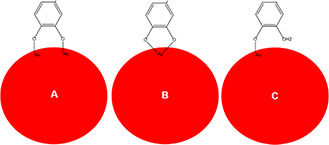
Schematic illustration of three different configurations of catechol group binding on the surface of metallic NPs. Bidentate bridging bonding (A), bidentate chelating bonding (B), and monodentate ester‐like bonding (C)
5.3 Phenolic acid‐coated metal NPs characterisation
Various spectroscopic techniques have been applied for studying the phenolic acid absorption on the surfaces of metal NPs. Although the UV–Vis absorption spectroscopy technique has verified the capability of phenolic acids to reduce metal ions, this method could not verify the attachment of phenolic acids to the surface of the synthesised NPs [91]. On the other hand, it is possible to detect characteristic absorbance peaks for capsaicin, cinnamic acid, gallic acid, salicylic acid, and other phenolic acids attached to the surfaces of metal NPs using Fourier‐transform infrared spectroscopy technique [50, 66, 68, 92]. Some other spectroscopic techniques have also been used for studying phenolic acid absorption on the surface of metal NPs. For example, specific absorption peaks could be attributed to sinapinic acid‐coated gold [93] and Fe [91] nanoclusters using the matrix‐assisted laser desorption/ionisation time of flight mass spectrometry method. Furthermore, the X‐ray photoelectron spectroscopy has been applied for characterising caffeic acid ‐capped gold NPs. In the recent method, a shift in the binding energy for Au0 represented the presence of carboxylic acid functional group in metal NPs surfaces [50]. Finally, it is possible to obtain micrographs of phenolic acids coated on metal and metal oxide NPs by transmission electron microscopy (TEM) (Fig. 3) [54, 60, 79, 94].
Fig. 3.
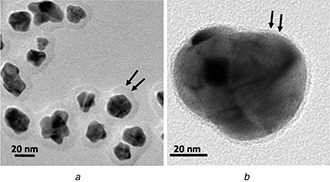
TEM micrographs of
(a) Phenolic acid‐coated gold NPs [54], (b) Silver/selenium alloy NPs [60]
The micrographs are represented with permission of the publishers
6 Phenolic acids‐coated metallic NPs toxicity and safety
Widespread use of common metal and metal oxide NPs is the safety and health concerns of consumers. Also in many cases such as gold NPs, the biodegradability of NPs is not possible and gradual faecal and renal exertion of the NP is the only way of NPs elimination [95, 96]. The lack of biodegradability for some metallic and oxide NPs forced researchers for preparation of more biocompatible nanostructures. Green chemistry through plant biomass or extract is an approach for the production of biocompatible metal NPs.
Specific plant or algae may be rich of some phenolic acids compound however still, there is plenty of others material which makes it difficult to study the role of phenolic acids compound in the observed toxicity results for synthesised metal NPs. In general, the reported toxicity effect of metal NPs synthesised through plant extract is controversial in pieces of literature. While the non‐toxic effect has been observed for metal NPs that are synthesised from garlic [97] or tea [98] extract. However, cytotoxicity and genotoxicity were reported for synthesised silver NPs from pandanus odorifer [99], piper longum extracts [100, 101]. Some claimed that green synthesised metal NPs are more biocompatible in comparison to chemically prepared metal NPs [102]. However, the higher biocidal activity of metal NPs that have been synthesised through plant extract in comparison to chemically prepared NPs was reported by other [103].
The diverse library of various chemicals in a crude extract of a plant and natural differences between plants is the primary reason for obtained controversial results. However, the clean separation of metal NPs from a plant extract or biomass is also very important. In some cases, the synthesised metal NPs through plant biomass were extracted with the help of dangerous detergent such as cetyltrimethylammonium bromide [104]. These detergents are one of the main cytotoxic chemicals that render metal NP application [12]. In another approach, plants mediated synthesised metal NPs were washed through centrifugation and decantation [105]. Plants, microstructures, and macromolecule that have been existed in the extract are also precipitated in the palate after centrifugation and disrupt washing performance. The observed toxic effects for extract mediated synthesised metal NPs may be due to the presence of these residual materials with NPs.
Cytotoxicity of nanomaterial is depended on many factors such as particle size, shape, and surface chemistry [12, 106, 107]. Nearly all of synthesised phenolic acid‐coated metal NPs are spherical with various sizes (Table 1). So, the surface chemistry of synthesised metal NPs could be a comparative factor for biocompatibility. Surface chemistry of NPs that have been synthesised through natural phenols is considered more biocompatible in comparison to citrate‐capped NPs [84]. Low or no cytotoxic effect was reported by many researchers for phenolic acids‐coated metal NPs [57, 108, 109]. However, few studies compare the cytotoxicity of phenolic acids‐coated NPs with NPs that have been coated by common chemicals.
Li et al. investigated the cytotoxic effect of gallic acid‐coated silver NPs in hepatocyte cells (HL‐7702) and Hela cells. Their reports represent no cytotoxic effect for gallic acid‐coated silver NPs in HL‐7702 up to 30 mg/ml. However for Hela cells, small cytotoxicity was observed at concentrations of 24 and 30 mg/ml [76]. Zhou et al.’s study showed more significant apoptotic cell death in Hela cells in comparison to normal epithelial kidney cells (HK‐2) with gallic acid‐coated Se/Ru NPs. Gallic acid‐coated Se/Ru NPs shown to be active in suppressing matrix metalloproteinase (MMP‐2 and MMP‐9) [62]. Similar results were acquired in Chen et al. report. Gallic acid‐coated gold NPs shown to be active in inhibiting MMP‐9 expressions. MMPs play an important role in the metastasis of cancers through EGF/EGFR malignant progression [109].
7 Future perspective
Applying bioactive natural compound instead of whole or part of plant extract for green synthesis of metal NPs is a new approach for green synthesis of metal NPs [110]. Recently, few researches have been conducted on the synthesis and biomedical application of phytochemicals based metal nanostructures. The diversity of natural phenolic acids, alongside their metal ions bioreduction capability, represent these phytochemicals as an ideal choice for preparation of biocompatible metal NPs with numerous biomedical applications. Metal NPs that have been synthesised through phenolic acids are only applied for few biomedical application. Both metal NPs [111] and phenolic acids [44] have many biomedical applications. Due to the lack of compelling results for biomedical properties and safety consideration, most of the biomedical applications of phenolic compound‐coated metal NPs were not developed in comparison with common chemically‐coated metal NPs [47]. A deep lab analysis which includes various parameters such as size, surface chemistry, and the type of the phenolic acids and metal NPs must be performed through proper animal models and well‐designed molecular studies. However, the ROS scavenging capability of phenolic‐coated metal NPs has been shown [84], and more reliable results are needed. Last, not the least, the capability of many natural phenolic acids and their derivate is needed to be investigated for various metal NPs syntheses and our current knowledge in this area is still very inadequate.
8 Conclusion
Metal NPs have attracted a lot of attention in the biomedical industries. Natural phenolic compounds are potential substitutes for the common toxic chemicals and used for the synthesis and stabilisation of metal NPs. Many phenolic acids have been applied for the synthesis and stabilisation of metal NPs and many others are yet to be investigated for this purpose. Metal NPs have many applications in biomedical fields such as tissue engineering, antimicrobial coating, applying as biosensors, carrying drugs and genes, and treating various cancers. However, the potential biomedical applications of phenolic acid‐coated metal NPs have only been investigated in few in‐vitro studies. There is a need for proper in‐vivo studies to scrutinise the toxicity and safety of these NPs in pre‐clinical settings.
9 Acknowledgment
This work was supported under grant number 96‐04‐223‐32620 from Iran University of Medical Sciences (IUMS).
10 References
- 1. Liao H. Nehl C.L. Hafner J.H.: ‘Biomedical applications of plasmon resonant metal nanoparticles’, Nanomedicine, 2006, 1, (2), 201 –208. doi:10.2217/17435889.1.2.201 [DOI] [PubMed] [Google Scholar]
- 2. Cao G. Wang Y.: ‘Nanostructures and nanomaterials: synthesis, properties, and applications’ (World Scientific, London, UK, 2004) [Google Scholar]
- 3. Cunningham A. Bürgi T.: ‘Bottom‐up organisation of metallic nanoparticles’, Amorphous Nanophotonics (Springer, Berlin, Germany, 2013) [Google Scholar]
- 4. Li Y. Duan X. Qian Y. et al.: ‘Nanocrystalline silver particles: synthesis, agglomeration, and sputtering induced by electron beam’, J. Colloid Interface Sci., 1999, 209, (2), pp. 347 –349 [DOI] [PubMed] [Google Scholar]
- 5. Magnusson M.H. Deppert K. Malm J.‐O. et al.: ‘Gold nanoparticles: production, reshaping, and thermal charging’, J. Nanopart. Res., 1999, 1, (2), pp. 243 –251 [Google Scholar]
- 6. Esmaeili‐bandboni A. Amini S.M. Faridi‐majidi R. et al.: ‘Cross‐linking gold nanoparticles aggregation method based on localised surface plasmon resonance for quantitative detection of MIR‐155’, IET Nanobiotechnol., 2018, 12, (4), pp. 453 –458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fatemi F. Amini S.M. Kharrazi S. et al.: ‘Construction of genetically engineered M13K07 helper phage for simultaneous phage display of gold binding peptide 1 and nuclear matrix protein 22 SCFV antibody’, Colloids Surf. B, Biointerfaces, 2017, 159, pp. 770 –780 [DOI] [PubMed] [Google Scholar]
- 8. Agarwal S.V. Reddy S.S. Dhayal M.: ‘Ultra‐small gold nanoparticles synthesized in aqueous solution and their application in fluorometric collagen estimation using bi‐ligand functionalization’, RSC Adv., 2014, 4, (35), pp. 18250 –18256 [Google Scholar]
- 9. Amini S.M. Kharrazi S. Jaafari M.R.: ‘Radio frequency hyperthermia of cancerous cells with gold nanoclusters: an in vitro investigation’, Gold Bull., 2017, 50, (1), pp. 43 –50 [Google Scholar]
- 10. Zarchi A.A.K. Amini S.M. Salimi A. et al.: ‘Synthesis and characterisation of liposomal doxorubicin with loaded gold nanoparticles’, IET nanobiotechnol., 2018, 12, (6), pp. 846 –849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng N. Fan J. Stucky G.D.: ‘One‐step one‐phase synthesis of monodisperse noble‐metallic nanoparticles and their colloidal crystals’, J. Am. Chem. Soc., 2006, 128, (20), pp. 6550 –6551 [DOI] [PubMed] [Google Scholar]
- 12. Amini S.M. Kharrazi S. Rezayat S.M. et al.: ‘Radiofrequency electric field hyperthermia with gold nanostructures: role of particle shape and surface chemistry’, Artif. Cells Nanomed. Biotechnol., 2017, 46, pp. 1 –11 [DOI] [PubMed] [Google Scholar]
- 13. Khademi S. Sarkar S. Kharrazi S. et al.: ‘Evaluation of size, morphology, concentration, and surface effect of gold nanoparticles on X‐ray attenuation in computed tomography’, Phys. Medica, 2018, 45, pp. 127 –133 [DOI] [PubMed] [Google Scholar]
- 14. Iravani S. Korbekandi H. Mirmohammadi S.V. et al.: ‘Synthesis of silver nanoparticles: chemical, physical and biological methods’, Res. Pharm. Sci., 2014, 9, (6), pp. 385 –406 [PMC free article] [PubMed] [Google Scholar]
- 15. Thakkar K.N. Mhatre S.S. Parikh R.Y.: ‘Biological synthesis of metallic nanoparticles’, Nanomed. Nanotechnol. Biol. Med., 2010, 6, (2), pp. 257 –262 [DOI] [PubMed] [Google Scholar]
- 16. Mohanpuria P. Rana N.K. Yadav S.K.: ‘Biosynthesis of nanoparticles: technological concepts and future applications’, J. Nanopart. Res., 2008, 10, (3), pp. 507 –517 [Google Scholar]
- 17. Thomas C.E. Ehrhardt A. Kay M.A.: ‘Progress and problems with the use of viral vectors for gene therapy’, Nat. Rev. Genetics, 2003, 4, (5), p. 346 [DOI] [PubMed] [Google Scholar]
- 18. Giljohann D.A. Seferos D.S. Daniel W.L. et al.: ‘Gold nanoparticles for biology and medicine’, Angew. Chem. Int. Ed., 2010, 49, (19), pp. 3280 –3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prabhu S. Poulose E.K.: ‘Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects’, Int. Nano Lett., 2012, 2, (1), p. 32 [Google Scholar]
- 20. Liu R.H.: ‘Potential synergy of phytochemicals in cancer prevention: mechanism of action’, J. Nutr., 2004, 134, (12), pp. 3479S –3485S [DOI] [PubMed] [Google Scholar]
- 21. Surh Y.‐J.: ‘Cancer chemoprevention with dietary phytochemicals’, Nat. Rev. Cancer, 2003, 3, (10), p. 768 [DOI] [PubMed] [Google Scholar]
- 22. Visioli F. Borsani L. Galli C.: ‘Diet and prevention of coronary heart disease: the potential role of phytochemicals’, Cardiovasc. Res., 2000, 47, (3), pp. 419 –425 [DOI] [PubMed] [Google Scholar]
- 23. Dembinska‐Kiec A. Mykkänen O. Kiec‐Wilk B. et al.: ‘Antioxidant phytochemicals against type 2 diabetes’, Br. J. Nutr., 2008, 99, (E‐S1), pp. ES109 –ES117 [DOI] [PubMed] [Google Scholar]
- 24. Cowan M.M.: ‘Plant products as antimicrobial agents’, Clin. Microbiol. Rev., 1999, 12, (4), pp. 564 –582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hahn N.I.: ‘Are phytoestrogens nature's cure for what ails us? A look at the research’, J. Am. Diet. Assoc., 1998, 98, (9), pp. 974 –976 [DOI] [PubMed] [Google Scholar]
- 26. Dumay O. Costa J. Desjobert J.‐M. et al.: ‘Variations in the concentration of phenolic compounds in the seagrass posidonia oceanica under conditions of competition’, Phytochemistry, 2004, 65, (24), pp. 3211 –3220 [DOI] [PubMed] [Google Scholar]
- 27. Lam T. Kadoya K. Iiyama K.: ‘Bonding of hydroxycinnamic acids to lignin: ferulic and p‐coumaric acids are predominantly linked at the benzyl position of lignin, not the Β‐position, in grass cell walls’, Phytochemistry, 2001, 57, (6), pp. 987 –992 [DOI] [PubMed] [Google Scholar]
- 28. Klick S. Herrmann K.: ‘Glucosides and glucose esters of hydroxybenzoic acids in plants’, Phytochemistry, 1988, 27, (7), pp. 2177 –2180 [Google Scholar]
- 29. Balasundram N. Sundram K. Samman S.: ‘Phenolic compounds in plants and agri‐industrial by‐products: antioxidant activity, occurrence, and potential uses’, Food Chem., 2006, 99, (1), pp. 191 –203 [Google Scholar]
- 30. Natella F. Nardini M. Di Felice M. et al.: ‘Benzoic and cinnamic acid derivatives as antioxidants: structure–activity relation’, J. Agric. Food Chem., 1999, 47, (4), pp. 1453 –1459 [DOI] [PubMed] [Google Scholar]
- 31. Kassim M. Achoui M. Mustafa M.R. et al.: ‘Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti‐inflammatory activity’, Nutr. Res., 2010, 30, (9), pp. 650 –659 [DOI] [PubMed] [Google Scholar]
- 32. Willcox J.K. Ash S.L. Catignani G.L.: ‘Antioxidants and prevention of chronic disease’, Crit. Rev. Food Sci. Nutr., 2004, 44, (4), pp. 275 –295 [DOI] [PubMed] [Google Scholar]
- 33. Luximon‐Ramma A. Bahorun T. Soobrattee M.A. et al.: ‘Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of cassia fistula’, J. Agric. Food Chem., 2002, 50, (18), pp. 5042 –5047 [DOI] [PubMed] [Google Scholar]
- 34. Edreva A. Velikova V. Tsonev T. et al.: ‘Stress‐protective role of secondary metabolites: diversity of functions and mechanisms’, Gen. Appl. Plant Physiol., 2008, 34, (1‐2), pp. 67 –78 [Google Scholar]
- 35. Mates J M Segura J A Alonso F J et al.: ‘Anticancer antioxidant regulatory functions of phytochemicals’, Curr. Med. Chem., 2011, 18, (15), pp. 2315 –2338 [DOI] [PubMed] [Google Scholar]
- 36. Kampa M. Alexaki V.‐I. Notas G. et al.: ‘Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action’, Breast Cancer Res., 2004, 6, (2), p. R63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dillard C.J. German J.B.: ‘Phytochemicals: nutraceuticals and human health’, J. Sci. Food Agric., 2000, 80, (12), pp. 1744 –1756 [Google Scholar]
- 38. Adams M. Gmünder F. Hamburger M.: ‘Plants traditionally used in age related brain disorders – a survey of ethnobotanical literature’, J. Ethnopharmacol., 2007, 113, (3), pp. 363 –381 [DOI] [PubMed] [Google Scholar]
- 39. Cole G.M. Lim G.P. Yang F. et al.: ‘Prevention of alzheimer's disease: omega‐3 fatty acid and phenolic anti‐oxidant interventions’, Neurobiol. Aging, 2005, 26, (1), pp. 133 –136 [DOI] [PubMed] [Google Scholar]
- 40. Omoigui S.: ‘The interleukin‐6 inflammation pathway from cholesterol to aging–role of statins, bisphosphonates and plant polyphenols in aging and age‐related diseases’, Immun. Ageing., 2007, 4, (1), p. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kroes B.v. Van Den Berg A. Van Ufford H.Q. et al.: ‘Anti‐inflammatory activity of gallic acid’, Planta Med., 1992, 58, (6), pp. 499 –504 [DOI] [PubMed] [Google Scholar]
- 42. Fernandez M. Saenz M. Garcia M.: ‘Natural products: anti‐inflammatory activity in rats and mice of phenolic acids isolated from scrophularia frutescens’, J. Pharm. Pharmacol., 1998, 50, (10), pp. 1183 –1186 [DOI] [PubMed] [Google Scholar]
- 43. Galati G. Lin A. Sultan A.M. et al.: ‘Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins’, Free Radicals Biol. Med., 2006, 40, (4), pp. 570 –580 [DOI] [PubMed] [Google Scholar]
- 44. Özçelik B. Kartal M. Orhan I.: ‘Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids’, Pharm. Biol., 2011, 49, (4), pp. 396 –402 [DOI] [PubMed] [Google Scholar]
- 45. Teodoro G.R. Ellepola K. Seneviratne C.J. et al.: ‘Potential use of phenolic acids as anti‐candida agents: a review’, Front. Microbiol., 2015, 6, (1420), pp. 1 –11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Usha Rani P. Pratyusha S.: ‘Defensive role of Gossypium Hirsutum L. Anti‐oxidative enzymes and phenolic acids in response to Spodoptera Litura F. Feeding’, J. Asia Pac. Entomol., 2013, 16, (2), pp. 131 –136 [Google Scholar]
- 47. Sathishkumar P. Gu F.L. Zhan Q. et al.: ‘Flavonoids mediated ‘green’ nanomaterials: a novel nanomedicine system to treat various diseases – current trends and future perspective’, Mater. Lett., 2018, 210, pp. 26 –30 [Google Scholar]
- 48. Kadoma Y. Fujisawa S.: ‘A comparative study of the radical‐scavenging activity of the phenolcarboxylic acids caffeic acid, P‐coumaric acid, chlorogenic acid and ferulic acid, with or without 2‐mercaptoethanol, a thiol, using the induction period method’, Molecules, 2008, 13, (10), pp. 2488 –2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang J. Stanley R.A. Melton L.D. et al.: ‘Inhibition of lipid oxidation by phenolic antioxidants in relation to their physicochemical properties’, Pharmacol. Online, 2007, 1, pp. 180 –189 [Google Scholar]
- 50. Wang L. Wei G. Sun L. et al.: ‘Self‐assembly of cinnamic acid‐capped gold nanoparticles’, Nanotechnology, 2006, 17, (12), p. 2907 [Google Scholar]
- 51. Scampicchio M. Wang J. Blasco A.J. et al.: ‘Nanoparticle‐based assays of antioxidant activity’, Anal. Chem., 2006, 78, (6), pp. 2060 –2063 [DOI] [PubMed] [Google Scholar]
- 52. Lerma‐García M. Ávila M. Simó‐Alfonso E.F. et al.: ‘Synthesis of gold nanoparticles using phenolic acids and its application in catalysis’, J. Mater. Environ. Sci., 2014, 5, pp. 1919 –1926 [Google Scholar]
- 53. Guo D. Dou D. Ge L. et al.: ‘A caffeic acid mediated facile synthesis of silver nanoparticles with powerful anti‐cancer activity’, Colloids Surf. B, Biointerfaces, 2015, 134, pp. 229 –234 [DOI] [PubMed] [Google Scholar]
- 54. Seo Y.S. Ahn E.‐Y. Park J. et al.: ‘Catalytic reduction of 4‐nitrophenol with gold nanoparticles synthesized by caffeic acid’, Nanoscale Res. Lett., 2017, 12, (1), p. 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nilsson L. Lof D. Bergenståhl B.r.: ‘Phenolic acid nanoparticle formation in iron‐containing aqueous solutions’, J. Agric. Food Chem., 2008, 56, (23), pp. 11453 –11457 [DOI] [PubMed] [Google Scholar]
- 56. Noh H.J. Kim H.‐S. Jun S.H. et al.: ‘Biogenic silver nanoparticles with chlorogenic acid as a bioreducing agent’, J. Nanosci. Nanotechnol., 2013, 13, (8), pp. 5787 –5793 [DOI] [PubMed] [Google Scholar]
- 57. Hwang S.J. Jun S.H. Park Y. et al.: ‘Green synthesis of gold nanoparticles using chlorogenic acid and their enhanced performance for inflammation’, Nanomed. Nanotechnol. Biol. Med., 2015, 11, (7), pp. 1677 –1688 [DOI] [PubMed] [Google Scholar]
- 58. Schliebe C. Jiang K. Schulze S. et al.: ‘A convenient light initiated synthesis of silver and gold nanoparticles using a single source precursor’, Chem. Commun., 2013, 49, (38), pp. 3991 –3993 [DOI] [PubMed] [Google Scholar]
- 59. Wang H.Y. Li Y.F. Huang C.Z.: ‘Detection of ferulic acid based on the plasmon resonance light scattering of silver nanoparticles’, Talanta, 2007, 72, (5), pp. 1698 –1703 [DOI] [PubMed] [Google Scholar]
- 60. Mittal A.K. Kumar S. Banerjee U.C.: ‘Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential’, J. Colloid Interface Sci., 2014, 431, pp. 194 –199 [DOI] [PubMed] [Google Scholar]
- 61. Wang W. Chen Q. Jiang C. et al.: ‘One‐step synthesis of biocompatible gold nanoparticles using gallic acid in the presence of poly‐(N‐vinyl‐2‐pyrrolidone)’, Colloids Surf. A, Physicochem. Eng. Aspects, 2007, 301, (1‐3), pp. 73 –79 [Google Scholar]
- 62. Zhou Y. Xu M. Liu Y. et al.: ‘Green synthesis of Se/Ru alloy nanoparticles using gallic acid and evaluation of theiranti‐invasive effects in Hela cells’, Colloids Surf. B, Biointerfaces, 2016, 144, pp. 118 –124 [DOI] [PubMed] [Google Scholar]
- 63. Bannerjee I.: ‘The spontaneous formation of selenium nanoparticles on gallic acid assemblies and their antioxidant properties’, Fordham Undergraduate Res. J., 2013, 1, (1), p. 3 [Google Scholar]
- 64. Sun D. Liu Y. Yu Q. et al.: ‘The effects of luminescent ruthenium (II) polypyridyl functionalized selenium nanoparticles on BFGF‐induced angiogenesis and AKT/ERK signaling’, Biomaterials, 2013, 34, (1), pp. 171 –180 [DOI] [PubMed] [Google Scholar]
- 65. Jin Q. Zhu W. Jiang D. et al.: ‘Ultra‐small iron‐gallic acid coordination polymer nanoparticles for chelator‐free labeling of 64 Cu and multimodal imaging‐guided photothermal therapy’, Nanoscale, 2017, 9, (34), pp. 12609 –12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee J. Choi K.‐H. Min J. et al.: ‘Functionalized Zno nanoparticles with gallic acid for antioxidant and antibacterial activity against methicillin‐resistant S. Aureus’, Nanomaterials, 2017, 7, (11), p. 365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scarabelli L. Grzelczak M. Liz‐Marzán L.M.: ‘Tuning gold nanorod synthesis through prereduction with salicylic acid’, Chem. Mater., 2013, 25, (21), pp. 4232 –4238 [Google Scholar]
- 68. Mihaiescu D.E. Buteică A.S. Neamţu J. et al.: ‘Fe3 O4 /salicylic acid nanoparticles behavior on chick cam vasculature’, J. Nanopart. Res., 2013, 15, (8), p. 1857 [Google Scholar]
- 69. Sohi K.K. Mittal N. Hundal M.K. et al.: ‘Gallic acid, an antioxidant, exhibits antiapoptotic potential in normal human lymphocytes: a BCL‐2 independent mechanism’, J. Nutr. Sci. Vitaminol., 2003, 49, (4), pp. 221 –227 [DOI] [PubMed] [Google Scholar]
- 70. Kang M.‐S. Oh J.‐S. Kang I.‐C. et al.: ‘Inhibitory effect of methyl gallate and gallic acid on oral bacteria’, J. Microbiol., 2008, 46, (6), pp. 744 –750 [DOI] [PubMed] [Google Scholar]
- 71. Kratz J.M. Andrighetti‐Fröhner C.R. Leal P.C. et al.: ‘Evaluation of anti‐Hsv‐2 activity of gallic acid and pentyl gallate’, Biol. Pharm. Bull., 2008, 31, (5), pp. 903 –907 [DOI] [PubMed] [Google Scholar]
- 72. Hsu C.‐L. Lo W.‐H. Yen G.‐C.: ‘Gallic acid induces apoptosis in 3T3‐L1 pre‐adipocytes via a fas‐and mitochondrial‐mediated pathway’, J. Agric. Food Chem., 2007, 55, (18), pp. 7359 –7365 [DOI] [PubMed] [Google Scholar]
- 73. Chuang C.‐Y. Liu H.‐C. Wu L.‐C. et al.: ‘Gallic acid induces apoptosis of lung fibroblasts via a reactive oxygen species‐dependent ataxia telangiectasia mutated‐P53 activation pathway’, J. Agric. Food Chem., 2010, 58, (5), pp. 2943 –2951 [DOI] [PubMed] [Google Scholar]
- 74. Martinez‐Gutierrez F. Olive P.L. Banuelos A. et al.: ‘Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles’, Nanomed. Nanotechnol. Biol. Med., 2010, 6, (5), pp. 681 –688 [DOI] [PubMed] [Google Scholar]
- 75. Martinez‐Castanon G. Nino‐Martinez N. Martinez‐Gutierrez F. et al.: ‘Synthesis and antibacterial activity of silver nanoparticles with different sizes’, J. Nanopart. Res., 2008, 10, (8), pp. 1343 –1348 [Google Scholar]
- 76. Li D. Liu Z. Yuan Y. et al.: ‘Green synthesis of gallic acid‐coated silver nanoparticles with high antimicrobial activity and low cytotoxicity to normal cells’, Process Biochem., 2015, 50, (3), pp. 357 –366 [Google Scholar]
- 77. Nagpal K. Singh S. Mishra D.: ‘Nanoparticle mediated brain targeted delivery of gallic acid: in vivo behavioral and biochemical studies for protection against scopolamine‐induced amnesia’, Drug Deliv., 2013, 20, (3‐4), pp. 112 –119 [DOI] [PubMed] [Google Scholar]
- 78. Nagpal K. Singh S. Mishra D.: ‘Optimization of brain targeted gallic acid nanoparticles for improved antianxiety‐like activity’, Int. J. Biol. Macromol., 2013, 57, pp. 83 –91 [DOI] [PubMed] [Google Scholar]
- 79. Jiang X. Yu A.: ‘One‐step approach for the synthesis and self‐assembly of silver nanoparticles’, J. Nanosci. Nanotechnol., 2010, 10, (11), pp. 7643 –7647 [DOI] [PubMed] [Google Scholar]
- 80. Ping W. Yong‐Nian N.: ‘Silver nanoparticles preparation using antioxidant propyl gallate and its analytical application’, Chem. J. Chin. Univ.‐Chin., 2013, 34, (4), pp. 837 –840 [Google Scholar]
- 81. Guozhong C.: Nanostructures and nanomaterials: synthesis, properties and applications’ (World Scientific, London, UK, 2004) [Google Scholar]
- 82. Kim H.‐s. Seo Y.S. Kim K. et al.: ‘Concentration effect of reducing agents on green synthesis of gold nanoparticles: size, morphology, and growth mechanism’, Nanoscale Res. Lett., 2016, 11, (1), p. 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Andjelković M. Van Camp J. De Meulenaer B. et al.: ‘Iron‐chelation properties of phenolic acids bearing catechol and galloyl groups’, Food Chem., 2006, 98, (1), pp. 23 –31 [Google Scholar]
- 84. Shaabani E. Amini S.M. Kharrazi S. et al.: ‘Curcumin coated gold nanoparticles: synthesis, characterization, cytotoxicity, antioxidant activity and its comparison with citrate coated gold nanoparticles’, Nanomed. J., 2017, 4, (2), pp. 115 –125 [Google Scholar]
- 85. Park J.‐W. Shumaker‐Parry J.S.: ‘Structural study of citrate layers on gold nanoparticles: role of intermolecular interactions in stabilizing nanoparticles’, J. Am. Chem. Soc., 2014, 136, (5), pp. 1907 –1921 [DOI] [PubMed] [Google Scholar]
- 86. Anand B.G. Dubey K. Shekhawat D.S. et al.: ‘Capsaicin‐coated silver nanoparticles inhibit amyloid fibril formation of serum albumin’, Biochemistry, 2016, 55, (24), pp. 3345 –3348 [DOI] [PubMed] [Google Scholar]
- 87. Ye Q. Zhou F. Liu W.: ‘Bioinspired catecholic chemistry for surface modification’, Chem. Soc. Rev., 2011, 40, (7), pp. 4244 –4258 [DOI] [PubMed] [Google Scholar]
- 88. Ata M. Liu Y. Zhitomirsky I.: ‘A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles’, RSC Adv., 2014, 4, (43), pp. 22716 –22732 [Google Scholar]
- 89. Zhang T. Wojtal P. Rubel O. et al.: ‘Density functional theory and experimental studies of caffeic acid adsorption on zinc oxide and titanium dioxide nanoparticles’, RSC Adv., 2015, 5, (129), pp. 106877 –106885 [Google Scholar]
- 90. Kim K. Han J.W.: ‘Effect of caffeic acid adsorption in controlling the morphology of gold nanoparticles: role of surface coverage and functional groups’, Phys. Chem. Chem. Phys., 2016, 18, (40), pp. 27775 –27783 [DOI] [PubMed] [Google Scholar]
- 91. Komori H. Hashizaki R. Osaka I. et al.: ‘Nanoparticle‐assisted laser desorption/ionization using sinapic acid‐modified iron oxide nanoparticles for mass spectrometry analysis’, Analyst, 2015, 140, (24), pp. 8134 –8137 [DOI] [PubMed] [Google Scholar]
- 92. Anand B.G. Shekhawat D.S. Dubey K. et al.: ‘Uniform, polycrystalline, and thermostable piperine‐coated gold nanoparticles to target insulin fibril assembly’, ACS Biomater. Sci. Eng., 2017, 3, (6), pp. 1136 –1145 [DOI] [PubMed] [Google Scholar]
- 93. Chen T.‐H. Yu C.‐J. Tseng W.‐L.: ‘Sinapinic acid‐directed synthesis of gold nanoclusters and their application to quantitative matrix‐assisted laser desorption/ionization mass spectrometry’, Nanoscale, 2014, 6, (3), pp. 1347 –1353 [DOI] [PubMed] [Google Scholar]
- 94. Mîndrilă I. Buteică S. Mihaiescu D. et al.: ‘Fe3 O4 /salicylic acid nanoparticles versatility in magnetic mediated vascular nanoblockage’, J. Nanopart. Res., 2016, 18, (1), p. 10 [Google Scholar]
- 95. Rengan A.K. Bukhari A.B. Pradhan A. et al.: ‘In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer’, Nano Lett., 2015, 15, (2), pp. 842 –848 [DOI] [PubMed] [Google Scholar]
- 96. Mukherjee S. Patra C.R.: ‘Therapeutic application of anti‐angiogenic nanomaterials in cancers’, Nanoscale, 2016, 8, (25), pp. 12444 –12470 [DOI] [PubMed] [Google Scholar]
- 97. Ahamed M. Khan M.M. Siddiqui M. et al.: ‘Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles’, Physica E, Low‐Dimens. Syst. Nanostruct., 2011, 43, (6), pp. 1266 –1271 [Google Scholar]
- 98. Moulton M.C. Braydich‐Stolle L.K. Nadagouda M.N. et al.: ‘Synthesis, characterization and biocompatibility of ‘green’ synthesized silver nanoparticles using tea polyphenols’, Nanoscale, 2010, 2, (5), pp. 763 –770 [DOI] [PubMed] [Google Scholar]
- 99. Panda K.K. Achary V.M.M. Krishnaveni R. et al.: ‘In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants’, Toxicol. in Vitro, 2011, 25, (5), pp. 1097 –1105 [DOI] [PubMed] [Google Scholar]
- 100. Jacob S.J.P. Finub J. Narayanan A.: ‘Synthesis of silver nanoparticles using piper longum leaf extracts and its cytotoxic activity against Hep‐2 cell line’, Colloids Surf. B, Biointerfaces, 2012, 91, pp. 212 –214 [DOI] [PubMed] [Google Scholar]
- 101. Reddy N.J. Nagoor Vali D. Rani M. et al.: ‘Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by piper longum fruit’, Mater. Sci. Eng., C, 2014, 34, pp. 115 –122 [DOI] [PubMed] [Google Scholar]
- 102. Akter M. Sikder M.T. Rahman M.M. et al.: ‘A systematic review on silver nanoparticles‐induced cytotoxicity: physicochemical properties and perspectives’, J. Adv. Res., 2018, 9, pp. 1 –16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sudhasree S. Shakila Banu A. Brindha P. et al.: ‘Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity’, Toxicol. Environ. Chem., 2014, 96, (5), pp. 743 –754 [Google Scholar]
- 104. Armendariz V. Herrera I. Jose‐yacaman M. et al.: ‘Size controlled gold nanoparticle formation by avena sativa biomass: use of plants in nanobiotechnology’, J. Nanopart. Res., 2004, 6, (4), pp. 377 –382 [Google Scholar]
- 105. Singh P. Kim Y.‐J. Zhang D. et al.: ‘Biological synthesis of nanoparticles from plants and microorganisms’, Trends Biotechnol., 2016, 34, (7), pp. 588 –599 [DOI] [PubMed] [Google Scholar]
- 106. Amiri S. Yousefi‐Ahmadipour A. Hosseini M.‐J. et al.: ‘Maternal exposure to silver nanoparticles are associated with behavioral abnormalities in adulthood: role of mitochondria and innate immunity in developmental toxicity’, NeuroToxicology, 2018, 66, pp. 66 –77 [DOI] [PubMed] [Google Scholar]
- 107. Schrand A.M. Rahman M.F. Hussain S.M. et al.: ‘Metal‐based nanoparticles and their toxicity assessment’, Wiley Interdiscip. Rev., Nanomed. Nanobiotechnol., 2010, 2, (5), pp. 544 –568 [DOI] [PubMed] [Google Scholar]
- 108. Lee J. Kim K.S. Na K.: ‘Caffeic acid‐coated multifunctional magnetic nanoparticles for the treatment and bimodal imaging of tumours’, J. Photochem. Photobiol. B, Biol., 2016, 160, pp. 210 –216 [DOI] [PubMed] [Google Scholar]
- 109. Chen Y.‐J. Lee Y.‐C. Huang C.‐H. et al.: ‘Gallic acid‐capped gold nanoparticles inhibit EGF‐induced MMP‐9 expression through suppression of P300 stabilization and NFΚB/C‐Jun activation in breast cancer MDA‐MB‐231 cells’, Toxicol. Appl. Pharmacol., 2016, 310, pp. 98 –107 [DOI] [PubMed] [Google Scholar]
- 110. Amini S.M.: ‘Preparation of antimicrobial metallic nanoparticles with bioactive compounds’, Mater. Sci. Eng., C, 2019, 103, p. 109809 [DOI] [PubMed] [Google Scholar]
- 111. Amini S.M.: ‘Gold nanostructures absorption capacities of various energy forms for thermal therapy applications’, J. Therm. Biol., 2019, 79, pp. 81 –84 [DOI] [PubMed] [Google Scholar]


