Abstract
Copper oxide nanoparticles (CuO NPs) use has exponentially increased in various applications (such as industrial catalyst, gas sensors, electronic materials, biomedicines, environmental remediation) due to their flexible properties, i.e. large surface area to volume ratio. These broad applications, however, have increased human exposure and thus the potential risk related to their short‐ and long‐term toxicity. Their release in environment has drawn considerable attention which has become an eminent area of research and development. To understand the toxicological impact of CuO NPs, this review summarises the in‐vitro and in‐vivo toxicity of CuO NPs subjected to species (bacterial, algae, fish, rats, human cell lines) used for toxicological hazard assessment. The key factors that influence the toxicity of CuO NPs such as particle shape, size, surface functionalisation, time–dose interaction and animal and cell models are elaborated. The literature evidences that the CuO NPs exposure to the living systems results in reactive oxygen species generation, oxidative stress, inflammation, cytotoxicity, genotoxicity and immunotoxicity. However, the physio‐chemical characteristics of CuO NPs, concentration, mode of exposure, animal model and assessment characteristics are the main perspectives that define toxicology of CuO NPs.
Inspec keywords: catalysts, nanofabrication, reviews, oxidation, toxicology, gas sensors, cellular biophysics, copper compounds, nanoparticles, biochemistry
Other keywords: copper oxide nanoparticles, environmental remediation, short‐ term toxicity, long‐term toxicity, human cell lines, CuO NPs exposure, physiochemical characteristics, mode of exposure, animal model, ssessment characteristics, toxicology, time‐dose interaction, oxidative stress, inflammation, cytotoxicity, genotoxicity, immunotoxicity, toxicological hazard assessment, algae species, bacterial species, fish, rats, CuO
1 Introduction
Nanotechnology is a revolutionary field that deals with the synthesis, characterisation and applications of nanomaterials in the fields of material science, biology, chemistry and physics. The remarkable surface chemistry and chemical reactivity of nanomaterials distinct them from bulk counterparts. The increase in surface‐to‐volume ratio of nanomaterials leads to rise in reactivity or toxicity due to the availability of large number of reactive sites. Toxicology deals with the study of all those noxious elements that have adverse effects on living system and environment. In the last few decades, development in the field of nanotechnology and their subsequent applications has increased the exposure of nanoparticles (NPs) to the environment and human beings. The occurrence of NPs in the environment is of great significance regarding their impact on human health [1], hence nanotechnology coined with toxicology give rise to new term ‘nanotoxicology’ [2]. The mechanisms involved behind the interaction of NPs with the living systems that result in nanotoxicity are not well known yet.
This review focuses on the toxicity of CuO NPs because of their widely usage in various commercial applications such as catalysts, photovoltaic cells, gas sensors, heat transfer nanofluids and antimicrobial agent [3, 4]. CuO NPs have been reported effective against broad range of microorganisms [5, 6]. Additionally, these are also used as an anti‐fungal agent when incorporated in plastics, textiles, coatings and so on [7, 8]. The electrochemical properties of CuO NPs make them appropriate to be used in graphite surface coating to improve its capacitance properties [9]. Besides these, copper (Cu) plays significant role in normal functioning of human body by maintaining its homeostasis while high Cu intake leads to jaundice, haemolysis and ultimately death. It also results in toxicity of respiratory system, gastro intestinal tract (GIT) and skin diseases when exceeds certain limits. Due to high impact usage of CuO NPs, there is an urgent need to critically assess the toxicity associated with CuO NPs. However at present, there is limited knowledge into the potential detrimental outcomes of human exposure to CuO NPs.
Throughout this review, emphasis has been done on the information relating to synthesis methodologies of copper oxide nanoparticles (CuO NPs), characterisation, advantages and disadvantages, experimental designs related to concentration of NPs, models used, duration of the CuO NPs exposure and assessment of endpoints. The information is used to understand the influence of experiments on toxicological examinations and their relevancy. In the light of above discussions, we tried to cover toxicological consequences of CuO NPs. In order to attain this objective, we targeted in‐vitro and in‐vivo toxicity of CuO NPs which include oxidative stress, immunotoxicity, genotoxicity, serum chemistry, haematology, urine chemistry, histology and mechanism involved behind toxicity. The present level of understanding the toxicity of CuO NPs has been achieved, and the gaps are covered, in order to highlight the research areas that should be addressed in the future.
2 Toxicity of metal oxide NPs
The genotoxicity, cytotoxicity and immunotoxicity of many metal oxide NPs have been reported. Due to several contributing factors, the mechanism of metal oxide NPs is not yet fully understood despite of extensive work. Irrefutably, the surface properties greatly affect the toxicity and interaction with the living systems. Before evaluating the toxicity (genotoxicity/cytotoxicity/immunotoxicity) of nanomaterial parameters like morphology, size, crystallographic appearance, chemical composition, surface properties (chemistry, charge) and aggregative behaviour should be characterised [10]. The release of metal ions from NPs is one of the major factors in the toxic potential. In inducing toxicity, the dissolution of metal ions from metal oxide NPs and the environment in which the NPs expose, plays a vital role [11]. Among different NPs, we hereby discuss toxicity of CuO NPs in detail due to its wide spread use in almost all fields.
3 Toxicity of CuO NPs
The complications that result in misunderstanding the toxicity rely on the fact of CuO NPs binding, their interaction to the living cells and subsequent change in surface chemistry. To elucidate the toxicity of CuO NPs, there is a need of understanding the characterisation and surface modification of CuO NPs, routes of exposure and mechanism or pathways that are involved in toxicity. Table 1 demonstrates the correlation between the synthetic methods of CuO NPs, characterisation and their toxic effects. The complications that result in misunderstanding the toxicity rely on the fact of NPs binding, their interaction to the living cells and subsequent change in surface chemistry. To elucidate the toxicity of nanomaterials there is a need of understanding the characterisation and surface modification of NPs, routes of exposure and mechanism or pathways that are involved in toxicity [24, 25, 26].
Table 1.
Synthesis techniques, characterisation and applications of CuO NPs
| S.no | Method | Technique | Chemicals | Characterisation | Results | Advantages | Disadvantages | Applications | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | physical | laser ablation | 99.99% copper target, deionised water, d/f laser focusing conditions | ellipsoidal shaped NPs, monoclinic CuO and cubic Cu2 O 25–200 nm | laser ablation, pressure, temperature ultimately induced structural modifications in NPs | no capping, stabilising agents required | less concentration of NPs produced, high energy is required | nanolubricant, heterogeneous catalyst, sensors and so on | [12] |
| S.no | Method | Technique | Chemicals | Characterisation | Results | Advantages | Disadvantages | Applications | References |
|---|---|---|---|---|---|---|---|---|---|
| 2 | chemical | reduction method | copper (II) succinate as precursor | crystalline 45 nm average diameter | controlled particle size | simple, economical, efficient, surfactants prevents agglomeration of NPs, controlled size and shape of NPs | instability of NPs in solution | antimicrobial | [13] |
| sol–gel method | sodium dodecyl sulphate as a surfactant | monoclinic, crystalline NPs | particle size increase with calcination b/c of agglomeration | controlled size and shape NPs | expensive and less eco‐friendly, costly | applications in gas sensing | [14] | ||
| chemical vapour deposition | copper nitrate tri‐hydrate 0.02M, distilled water, 0.5 g sodium hydroxide at 5C | rod like structures, monoclinic 33 nm | widely dispersed | synthesis of controlled size and shaped NPs | needs high temperature | antimicrobial | [15] | ||
| precipitation method | copper (II) acetate and sodium hydroxide | crystalline NPs 23 nm | reproducable procedure | simple and effective | inappropriate for the synthesis of pure, price stoichiometric phase | biocidal | [16] | ||
| electro‐chemical deposition | supporting electrolytes, NaOH, NaCO3, sodium nitrate in water, water:acetonitrile (12:1), water:methanol (12:1), copper plate | well dispersed, granular spherical, round shaped, 20–25 nm, peaks at 2θ values | small sized particles | cost‐effective, less laborious, pure and controlled size and shaped NPs are synthesised | lack of reproducibility, needs high energy, pressure and temperature | photocatalytic and antibacterial | [14] | ||
| hydrothermal | copper sulphate and NaOH | crystalline monoclinic, 27 nm average size | large optical band energy observed | variable NPs produced | high pressure and temperature is required | — | [17] | ||
| ultrasonication synthesis of CuO/Cu2 O/Cu‐NPs | 2.0 g copper acetate monohydrate, ammonia solution (25% w/w) | monoclinic crystalline,bandgap 1.42 eV, major peaks at 2θ values | simple but specific instrument required | effective, simple, cheap, non‐toxic | scale up problems | could be used as industrial catalysts for photocatalytic degradation of dyes | [18] |
| S.no | Method | Technique | Chemicals | Characterisation | Results | Advantages | Disadvantages | Applications | References |
|---|---|---|---|---|---|---|---|---|---|
| 3 | biological | E. coli mediated | citrate minimal medium, CuSO4, E. coli mass, SDS‐PAGE | micrometre dimensions and variable morphologies | use of SDS‐PAGE resulted in reduction of Cu II to CuO NPs and their stabilisation | high yield, low cost, fast. Clean, reproducibility | tedious purification steps, difficult to understand the mechanism and to achieve control size and shape of NPs | antimicrobial applications | [19] |
| fungi mediated (Trichoderma asperellum) | 5 mM copper nitrate, TA‐CFE (mycelial free water extract) | crystalline and cubic‐faced structure, 110 nm average size | ROS production and cellular observation in A549 cancer cells and the activity increased with TA‐CuO NPs | high yields secretory proteins related to increase NPs, scalability, easy downstream processing | risk of pathogenicity | TA‐CuO NPs could be used as therapeutics in modern medicines | [20, 21] | ||
| plant mediated Hibiscus rosa‐sinensis (flower extracts) | 1 M aqueous copper acetate solution, deionised water | crystalline, spherical, 45–80 nm | antimicrobial | cost‐ effective, eco‐friendly, easy availability, high quality NPs, controlled size and shape | difficulty and time consuming | antimicrobial applications | [22] | ||
| algae mediated (Bifurcaria bifurcate) | 1 mM copper(II) sulphate, 2 ml algal extract | crystalline NPs, 5–45 nm average size | antimicrobial | eco‐friendly, convenient, no chemicals involved | laborious and slow process | pharmaceutical | [23] |
4 Key factors
4.1 Characterisation
Characterisation of CuO NPs involves the size, shape and charge of the particles. There is a correlation between size of the NPs and surface‐to‐volume ratio, smaller the size greater will be the surface‐to‐volume ratio and vice versa. The penetration and reactivity of particles is entirely dependent upon its size. However, it has been postulated that NPs having size >100 nm can penetrate the cells by crossing the cell membrane whilst size <40 nm can enter into blood to nuclei of cells. The mechanisms that are influenced by NPs size are cellular uptake, interaction mechanism and intercellular stability. By making it abridged in comparison to large size NPs, the small NPs show more toxicity and are more suspected to cellular internalisation [24, 25]. The translocation process of NPs through the cell membrane is affected by shape and charge up to 60 orders of magnitude [25, 26]. Due to small size of Cu NPs, they can easily translocate between cells, cross cell membrane and causes cell disruptions (Lee et al., 2016). Few studies have been conducted to demonstrate the shape and particle form of NPs affecting their toxicity and bioaccumulation. The reports elucidate that at the nanoscale, CuO NPs spherical in shape showed increased reactivity (antibacterial activity) than bulk CuO. Nano‐cupric oxide (n‐CuO) CuO nanosheets showed highest surface reactivity, electrochemical properties and antimicrobial activity than CuO.
There are three synthetic methods which influence the characterisation and properties of CuO NPs viz., physical, chemical and biological (Table 1). Laser ablation method has been used for synthesis of CuO NPs that produces 25–200 nm sized NPs with ellipsoidal shape and no capping or stabilising agents are required [12]. The chemical methods involved for CuO NPs synthesis are chemical reduction, sol–gel, chemical vapour deposition, precipitation electrochemical, hydrothermal and ultrasonication. One of the easiest and simple methods for CuO NPs synthesis is the chemical reduction method [13]. The CuO NPs produced are crystalline in nature and having average size of 45 nm which is enough to penetrate the bacterial cell membranes and cause cell disruptions at different levels. The CuO NPs synthesised by chemical vapour deposition and precipitation method produces monoclinic rod like structures, 33 nm in size.
The CuO NPs synthesised by sol–gel method, electrochemical and ultra‐sonication showed high gas sensing and strong photocatalytic properties against safronine O and methylene blue dyes [14, 18, 27]. The advantages of these techniques are cost‐effectiveness, non‐toxic, controlled size and shape and efficient one. The disadvantages are lack of reproducibility, scalability, difficulty in understanding the mechanisms.
The biological methods have also been used, i.e. bacteria, fungi, algae and plant for CuO NPs [19, 20, 21, 22]. The NPs synthesised are of various sizes and shapes and shows strong antimicrobial, photocatalytic activities. The NPs synthesised show strong abilities to be used in therapeutics, biotechnological, environmental, pharmaceutical and industrial applications. The advantages of biological methods are cost‐effectiveness, eco‐friendliness, easy handling and availability. The drawbacks of using green techniques are tedious down streaming procedures, laborious, difficult in case of using bacteria, risk of pathanogenictiy in fungi mediated, time consuming and slow in case of algae and plant mediated synthesis of CuO NPs.
4.2 Surface modification
The potentiality of CuO NPs in wide range of applications is because of its high surface reactivity, chemical stability and thermoelectric properties [28]. Greater the surface reactivity, greater will be the reactive oxygen species (ROS) production and vice versa. The relationship between surface chemistry (capping agents) of Cu NPs and generation of ROS has been well documented [29]. Three surface modifiers named 8‐mercaptooctanoic acid (MOA), 12‐mercaptododecanoic acid (MDA) and 16‐mercaptohexadecanoic acid were used and followed by dispersion in water, demonstrating the strong affinity of Cu NPs to thiolated functional groups as compared to carboxylic acids. The 8‐mercaptooctanoic treated Cu‐NPs showed a significant level of ROS activity. The findings showed that ROS generation abilities profoundly decreased with increasing long chains.
4.3 Dissolution
The dissolution of metal ions is another factor affecting the toxicity of NPs. The solubility parameter is one of the meaningful parameter which exploits the reasons of NPs toxicity to many organisms. The NPs exhibit faster dissolution because nanosized particles have larger surface area as compared to bulk ones to interact with solvent molecules. Thus, the nanosized particles exhibit higher toxicity. From literature it has been evidenced that dissolution plays significant role in oxidation of Cu NPs. The Cu‐based NPs (CuO NPs) release sufficed amount of Cu ions in relevant media to affect biological systems [11]. The toxicity of CuO NPs increases by 40‐fold to bulk CuO and evident 40–50 fold increase in toxicity [30]. The toxicity of Cu ions from CuO NPs is significant in the growth medium but lower than the effects of particles themselves [31]. Other possibility is the release of Cu ions after penetration and accumulation in lysosomes, where the solubility parameter increases due to acidic pH leading to highly cytotoxic Cu2 + ions into the cytoplasm [32].
4.4 NP dose
In toxicology, NP dose is one of the key factors contributing towards toxicity. The NP dose applies to the initial concentration to the cells but also to the real number of NPs taken up by a one single cell. For public health risk assessment, it is important to elucidate real and relevant dose regimes for in‐vitro and in‐vivo experimental models. By all means to achieve a biological response, nanotechnologist should examine NPs toxicity based on real‐world doses instead of unrealistic one. In nanomedicines, NP dose is considered critical phenomenon [2]. The human pulmonary epithelial cells (A549) when exposed to CuO NPs concentration in dose‐dependent manner (10, 25, 50 µg/ml) indicated by depletion of glutathione and induction of lipid peroxidation, catalase and superoxide dismutase. The MIT assay indicated that the CuO NPs decreased the cell viability to 75, 66 and 48%, respectively [16].
4.5 Environment
The chemical transformation of nanomaterials in environment or living body significantly affects their toxicity risks. In the environment, NPs interact with air, soil and water. As discussed above, the interaction of NPs with substrates changes the surface properties of NPs or charge and results in aggregation [33]. The aggregation of NPs affects the cellular uptake and ultimately toxicity [34]. The release of Cu NPs in environment via Cu‐based materials used in agriculture (pesticides) or effluent of wastewater treatment undergoes chemical transformations such as sedimentation, aggregation, dissolution, natural organic matter interactions and redox reactions [35].
4.6 Exposure route of Cu NPs
The primary exposure of NPs is by improper handling of raw materials and equipments by the workers during lab‐scale production. The commercial synthesis of nanomaterials is another primary exposure route of NPs. The other forms of exposure route of NPs might be the errors that occur during the categorisation, packing and transportation of nano materials. On contrary, the applications and consumption of nanomaterials based products also lead to lethal effects. The main entry routes for NPs into the body are inhalation, ingestion or through skin and blood stream [36, 37, 38]. After entrance into the body, the NPs are transported to other regions of body through blood circulatory system [39, 40] and cause toxic effects at the different sites.
The Cu NPs exposure route is the main indicator which affects their toxicological outcomes. Due to small size of Cu NPs, they can easily translocate via tissue interstitially (between cells), pass through cell membranes and eventually enter into blood circulatory system. A very significant role is played by circulatory and lymphatic systems in the translocation of NPs from exposure site to downstream effects, ending in accumulation in the body organs.
4.7 Inhalation
Inhalation is the chief route of entrance of NPs into the body. During inhalation NPs penetrate into lungs and interact with the epithelium that leads to inflammation. The olfactory bulb is considered one of the hazardous routes for the inhaled NPs to get access to other organs of the body like CNS [41, 42]. The mechanism that lies behind the translocation of NPs seems to be through endocytosis of alveolar epithelial cells [43]. Amount of NPs, disposition in lungs, dimensional properties of NPs, persistent of NPs, and defence mechanism are the factors that affect the living system on inhalation of NPs. As the size of NPs decrease, the disposition rate of NPs significantly increases in lungs. The occupational workers are the cohort population exposed to Cu NPs and CuO NPs. Industrial emission of Cu NPs takes place in the production of asphalt and rubber [35]. The capability of Cu NPs to remain in lung tissues for indefinite time is accredited to its small size, ends in ROS production followed by oxidative stress and inflammatory responses because of sensitisation and irritation.
4.8 Ingestion
Gastrointestinal tract is also one of the potential exposure routes for NPs. In different food items and drugs, NPs are added directly or indirectly that are taken orally and absorbed through mechanism of GIT from where they enter in lymphatic cells [44]. The factors that influence the absorbance of NPs by GIT are surface chemistry, geometry, charge, shape, size, substrate and attachment potential to substrates [45]. The unstable NPs are excreted by body whereas the NPs that become agglomerated or form aggregates block the GIT and leads to death. It has been observed that accumulation varies in key organs like brain, spleen, heart and liver. The extent ability of body to excrete NPs in urine is not yet studied well or their bioaccumulation in distinguished organs (liver, spleen, heart and brain) and their possibility to block the excretory pathways. When the NPs enter hepatic circulation, they become hepatotoxic and cause gradual fibrosis. However, when NPs enter the GIT, they result in ulcers by altering the lining permeability, cause dysplasia/metaplasia by weakening the epithelium, malabsorption of the nutrients and in severe case may results in chronic bleeding. In viewing such aspects of toxicity of nanomaterials, greater attention ought to be paid in evaluating harmful effects before endorsing any parenteral administration [46, 47]. The Cu NPs present in the food/water entered through direct ingestion or inhalation, exposed to GIT. According to the reports of Chen et al., toxicity of Cu NPs after oral exposure in mouse was evaluated. The fate of the Cu NPs in the GIT followed by reaction with the stomach acid, diffusion into the microvilli, or entered into the intestines ending in systematic transport of Cu NPs by intestinal epithelium to organs [48].
4.9 Skin
Human skin acts as a first barrier towards the NPs and other harmful chemical substances. However, the presence of hair follicles and sweat glands makes this barrier susceptible by allowing the entrance of NPs [44]. There are more chances of penetration of NPs when the protective layer of skin is removed or wounded [49]. The penetration of NPs into the skin decides their fate and shows their diverse toxic forms. They may cause irritation, allergy or can damage the cellular or subcellular parts of body, which may initiate chemical reaction results in production of ROS [50].
Cohen et al. (2012) described the toxicity of CuO micro and NPs in human skin organ cultures using electron microscopy and biochemical markers. The particles adhere to the skin surface, react with the acidic environment leading towards generation of soluble Cu ions that make their way to inner sites. Particles suspension were made and topically applied onto intact/stripped epidermis for 24 and 72 h treatment. The reports evidenced that NPs showed more toxicity as compared to micro‐sized particles and the effects were much stronger when the particles supplied in growth medium. In epidermis, the CuO NPs induced inflammatory responses followed by cytokine secretions and necrosis.
5 In‐vitro toxicity of CuONPs
5.1 ROS and oxidative stress
ROS constitute oxygen containing molecules like O2–, H2 O2 and HO• that exhibit greater reactivity than molecular oxygen. Among antioxidants, GSH is the most copious compound comprising sulfhydryl groups and performs a substantial role in various life processes. It also establishes first line of defence against oxidative damages, and acts as a main redox buffer intracellularly [51] and co‐substrate in the GSH peroxidase catalysed reaction of H2 O2 or lipid peroxidation leading to its depletion. Superoxides, hydroxyl, hydrogen peroxide and other oxygen radicals among ROS are quite important as they are directly involved in oxidation of biological molecules like DNA, proteins and lipids [52]. Under normal physiological conditions, damages induced by ROS are regulated via antioxidative enzymes and non‐enzymatic molecules like catalases, superoxide dimutases, glutathione peroxidase, tocopherol, glutathione and ascorbic acid present in the cell [53]. However, when generation of oxygen containing radicals increase, they generate toxic intermediates that are usually responsible for oxidative stress due to imbalance between the level of ROS and antioxidants that detoxify toxic intermediates and restore damages [54]. Body's defence systems collapse to counterbalance oxidative stress and lead to the malfunctioning of biomolecules [55, 56], change in the level of antioxidant enzymes, depletion of glutathione and perturbation of mitochondria. Damages also induce in DNA and consequently cell death occurs [57]. The report addressed by Mortimer et al. exhibits the effect of CuO NPs (80 mg/l) on T. thermophila cell membrane [31]. T. thermophile regulates its membrane's lipid composition to more rigid configuration by lowering its unsaturated fatty acids and increasing saturated fatty acids. Studies also evidence the lipid peroxidation in rainbow and C. reinhardtii when exposed to CuO NPs [58, 59]. The studies also report cytotoxicity of CuO NPs in primary liver cells of cat fish and HepG2 due to generation of ROS [60, 61].
The cytotoxicity of CuO NPs is also observed in human cell lines like human lung epithelial A549, human cardiac microvascular endothelial, kidney and neuronal cells [16, 62, 63, 64, 65, 66]. CuO NPs cytotoxicity and oxidative stress have been examined in A549 cells that leads to high level of lipid peroxidation and ROS production while lower level of antioxidant (GSH) in HepG2 (human hepatocellular carcinoma) cells. They observed that MDA, a lipid peroxidation marker and antioxidant enzymes like SOD and CAT significantly increased followed by reduction in GSH level. These outcomes suggest that oxidative strain might be the key mechanism behind the CuO NPs [16, 67]. The reports also show that oxidative stress is the main reason for cytotoxicity of CuO NPs [63, 68].
Lanone et al. stated that engineered NPs including CuO NPs alter level of antioxidant enzymes like SOD and CAT along with glutathione [69]. Lower level of GSH and inhibition of SOD and CAT activities by CuO NPs contributing to oxidative damages was also observed in embryo and while change in zebrafish physiology like failure in hatching, small body length and reduction in reproductive potential were also due to CuO NPs affect [70]. CuO NPs produce ROS along with blockage of antioxidant defence system. Rat administered with CuO NPs displayed lower CAT, TAC and GSH activities while higher level of nitric oxide (NO) was observed [71].
Fahmy and Cormier [63] observed no difference in case of SOD activity while 25 and 29% inhibition in catalase and GR activities was observed. They found 100 and 150% increase in 8‐isoprostanes and GPx activity upon exposure of HEp‐2 cells to 80 lg/cm2 of CuO NPs. Increase (150%) in oxidised to total glutathione ratio shows that oxidised GSH directs the failure of epithelial cells to inhibit ROS produced by CuO NPs. This leads to the generation of oxidative stress that was responsible for oxidative damage and cell death. Besides this, CuO NPs was more effective at blocking the antioxidant defence of the cell [63, 72].
Higher level of 8‐isoprostane in the supernatant of HEp‐2 cells upon treatment of CuO NPs demonstrate oxidative damages to the cells [73]. CuO NPs also significantly increase SOD, and TBARS while reduce CAT activity [74]. Jing et al. conducted an experiment on HBEC and A549 cells exposed to CuO NPs. They observed that upon 4 h exposure, significant higher level of ROS was found in HBEC followed by A549 cells [75].
Furthermore, scientists have found that cytotoxicity links with CuO NPs due to the elevated ROS production in several cell lines like HL60 cells, human laryngeal and alveolar type‐I epithelial cells [63, 76, 77, 78, 79]. Results show downregulation of catalase around 3.18 and CA3 to 4.05 fold in the liver while 2.19 fold of glutathione peroxidase 3 in the kidney via using 2D‐DIGE technology in rats intoxicated with CuO NPs (200 mg/kg). Decline in the levels of NPSH (non‐protein thiol groups) and PSH (protein thiol groups) were also observed in the liver and kidney. These outcomes demonstrate that CuO NPs weaken antioxidant system of the liver and kidney. Besides this, increment in the level of MDA suggests that lipid peroxidation also establishes in liver and kidney when exposed to CuO NPs [80]. Studies support that CuO NPs cause cytotoxicity through primarily generating oxidative stress followed by genotoxicity.
6 Genotoxicity and nanomaterials
Genotoxicity is chromosomal aberrations, oxidative DNA damage and DNA strand breaks caused by different mutagens that lead to mutations. There is paradigmatic relationship between the syntheses of ROS and DNA damage [81]. The proposed mechanism of toxicity studied by Thit et al. demonstrates the uptake of NPs by cell via endocytosis that results in synthesis of ROS [82]. ROS causes DNA damage that triggers a signalling pathway which ends in apoptosis or cell death. Genotoxicity of nanomaterials is of particular concern since an alteration of the genetic material may favour cancer development or fertility impairment.
6.1 How nanomaterials induce genotoxicity?
Nanomaterials induce DNA alterations by two pathways: via direct association with DNA strands or through indirect route by induction of oxidative stress upon exposure to NPs (Fig. 1). In direct genotoxicity, NPs penetrate either through nuclear pores or during mitosis directly interact with DNA molecules or disrupt their replication and transcription. During indirect genotoxicity, NPs induce toxicity by interaction with the nuclear proteins, mitotic spindles, checkpoints and inhibition of antioxidant defence mechanism. The secondary genotoxicity of NPs is mediated by ROS production in inflammatory cells (phagocytes, macrophages). The oxidative burst caused by activated phagocytes might be a potential explanation for the genotoxicity of NPs [83].
Fig. 1.
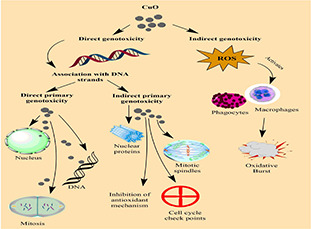
Pathways of genotoxicity of NPs
There are many factors that are involved in genotoxicity induced by nanomaterials. Magdolenova et al. and Sun et al. elucidated the factors that influence the genotoxic potential of NPs [83, 84]. These include size of NPs, shape, surface properties, composition, solubility, aggregation/agglomeration, particle uptake, presence of mutagens and transition metals affiliated with the particles also influence the mechanisms of genotoxicity. CuO NPs carry out higher proportion of DNA damages and cell deaths as compared to other metallic NPs [16, 85]. The genotoxic potential of CuO NPs has been observed by Wang et al. in the nucleus by hypothetically disrupting the nuclear membrane, permitting the NPs to pass through the cell nucleus and directly interact with DNA [11].
6.2 CuO NPs and genotoxicity
Plenty of reports are there that verify the genotoxic potential of CuO NPs among different organisms. Table 2 presents data on the test organisms that have used for the determination of genotoxicity exhibited by CuO NPs. The genotoxicity is dependent upon size, shape, duration of exposure and concentration of NPs. Generation of free oxygen radical and oxidative stress evoke a cascade of cellular events including DNA damage and apoptosis.
Table 2.
Genotoxic potential of CuO NPs in different organism
| Organism | Part used | Shape | Sizes | NPs mode of concentration | Effects | References |
|---|---|---|---|---|---|---|
| frog | kidney epithelial cells | polydispersed | 6 nm, <100 nm, <6nm | Size‐dependent NPs, <100 nm NPs more toxic than 6 nm | <100 nm NPs more toxic than 6 nm DNA damage, decreased cell viability, reduced levels of GSH, cell death | [82] |
| Danio rerio (fish) | not specified | polydispersed | <50 nm | dose‐dependent manner | small size, cross‐cell membrane, release Cu2+, ROS production, DNA damage, cell death | [86] |
| mice | lung tissues | nearly spherical | <30–>80 nm | dose‐dependent manner | cell apoptosis | [87] |
| mouse | neuroblastoma cell line | spherical | 30–40 nm | dose‐dependent manner | DNA fragmentation, DNA methylation, chromosomal damage, lipid peroxidation, micronucleus formation | [65] |
| rat | lung tissues | spherical | 15–20 nm | time‐ and dose‐dependent manner | cell proliferation, inflammation, upregulation of proinflammatory cytokines | [88] |
| human | A549 cells | spherical | <100 nm | time‐ and dose‐dependent manner | DNA damage, DNA lesions, neurotoxicity, cytotoxicity | [32] |
| human | pulmonary epithelial cells | spherical | 50 nm | dose‐dependent manner | DNA damage mediated through lipid peroxidation and oxidative stress, apoptosis | [16] |
| human | cell line A549 | spherical | 50 nm, 3 µm | size‐dependent manner | Cu NPs more toxic than micro‐NPs, DNA damage | [89] |
| human | HepG2 cells | spherical | 22 nm | dose‐dependent manner | induce cytotoxicity, ROS production, p53 and apoptotic gene caspase‐3 upregulation, apoptosis in HepG2 cells via mitochondrial pathway | [77] |
| human | bronchial epithelial cells BEAS‐2B | approx. spherical | 20–200 nm | size‐dependent manner | CuO NPs more toxic than CuO MPs, induction of oxidative stress, cell cycle arrest, apoptosis | [90] |
| human | skin keratinocytes cells | spherical | 50 nm | time‐ and dose‐dependent manner | apoptosis, necrosis, DNA damage via oxidative stress | [91] |
| human | airway epithelial cells | spherical | — | dose‐dependent manner | increased inflammation, collagen deposition in lungs | [92] |
| human | cancer cell lines HT‐29 and SW620 | polydispersed | 20 nm | dose‐dependent manner | apoptosis, inhibition of proteins expression | [93] |
The p53 protein is known as master guardian of the cell cycle check points, DNA repair and apoptosis. In case of genetic toxicity, the p53 protein is triggered to arrest the cell cycle to provide time for the DNA repair mechanism or ultimate apoptosis [94] The production of ROS induces the expression of p53 and p38 proteins that damage the DNA. The ROS production also leads to the synthesis of inflammatory cytokines. These findings support that the CuONPs causes cytotoxicity through mediation of oxidative stress followed by genotoxicity.
7 In‐vivo toxicity of CuONPs
7.1 CuO NPs toxicity and behavioural analysis
The toxic effects of nano‐sized CuO towards neurons results in hippocampal dysfunction (impairing the voltage gated sodium channels) which attributes to learning and memory. The nano‐CuO caused spatial impairment and electrophysical alteration in rats [95]. In behavioural experiment, Morris water maze (MWM) showed significant decrease in memory and learning in rats treated with nano‐CuO for 14‐days. The accumulation of Cu in the hippocampus region remarkably evidenced the notions that NPs have ability to cross blood brain barrier and disrupt central nervous system physiological functions. The occurrence of oxidation–antioxidation imbalance, disruption of homeostasis, damaging of neurons in the hippocampus, oxidative damages induced by nano‐CuO resulted in neurotoxicity. Apart from cognitive malfunctions and reduction in spontaneous activity, there were no evidences of anxiety/stress observed in the rats treated with nano‐CuO.
To evaluate learning and memory behaviours, the MWM test was administrated with CuO NPs to Male Wister rats at 0.5 mg/kg/day for 2 weeks [96]. The test showed the toxic behaviours of CuO NPs on the rat's cognitive functions. After the MWM test, the long‐term potentiation test was conducted to demonstrate the field excitatory postsynaptic potential. The adverse effects of CuO NPs on the cognitive behaviour of rats is because of the suppression of postsynaptic receptors and release of presynaptic glutamate ended in diminishing long‐term potentiation and correspondingly other induced cognitive deficits.
Sun et al. examined dose‐dependent toxic effects of CuO NPs on the behaviour (locomotion) of zebra fish larvae [97]. The locomotion behaviour was estimated by total movement distance, angular velocity, velocity, fast movement time, medium movement time and slow movement time. Three groups were used in the trial, control, exposed to 12.5 and 50 mg/l CuO NPs. The behavioural tests were performed three times after exposing the larvae groups to CuO NPs for six days. The larvae groups exposed to CuO NPs showed reduction in movement distance, velocity and angular velocity and no significant difference was examined in fast movement time. Moreover, the exposed groups medium movement time and slow movement time were longer than the control group. The study also showed that the CuO NPs exposure to zebra fish larvae resulted in reduced locomotion capacity. The delayed development of the central nervous system or the effective neuromuscular system ended in the changed larval behaviours.
A study conducted by Mashock et al. quantified the Caenorhabditis elegans (nematode) behaviour (feeding and average body length) and reproduction in response to CuO NPs and copper sulphate exposure [98]. Three strains of wild nematode and wild type strain were examined to explore the influence of Cu on their physiological responses. As compared to CuSO4, CuO NPs showed significantly effects on the average body length of the wild strains at 7.9 mg/l concentration. Whilst at 15.9 mg/l copper sulphate demonstrated no impact on the average body length. In terms of feeding behaviour, CuO NPs showed greater toxicity trend than CuSO4 in Caenorhabditis elegans strains. Reproduction was considerably reduced only at the utmost Cu dose, though still more noticeable with CuO NPs than copper sulphate treatment.
8 Biochemical alterations induced by CuO NPs
Many reports verify substantial toxicity of CuO NPs, induced damages in nephrotic and hepatic tissues of rats that affect physiology and biochemical functioning of kidney and liver [11]. Mohammadyari et al. [99] found rise in ALT and ALP upon treatment of CuO NPs demonstrating disturbance in liver's membrane that lead to cellular release of these enzymes. Lee et al. [100] observed dose‐dependent increase in AST, ALP, ALT, TBIL, CRE, BUN and LDH while the level of TP (total protein) and TG (triglyceride) decreased significantly. Moreover, rats displayed dysregulation in the electrolytes balance. Manna et al. [101] also observed elevation in AST, ALT, TB, BUN and CRE. Arafa et al. [71] conducted an experiment on rats treated with 3 and 50 mg/kg of CuO NPs. They found significant increase in liver enzymes (ALT, AST and ALP) in dose‐dependent manner as compared to the control. Lei et al. reported significant biochemical alterations in rats administered with 200 mg/kg as compared to the control group [102]. They noticed five‐fold increase in AST, TB, CRE and BUN while about two‐fold increase were observed in ALT, TG and total bile. Furthermore, level of ALP and TCHO (total cholesterol) reduced while TCHO and TG were slightly increased in mid‐ and low‐dose treated groups. Comparative intoxication of male and female rats with CuO NPs was done by Wang et al. [103]. Rats treated with 1250 mg/kg CuO NPs showed reduction in TG, Na and Cl and elevation in ALT, AST, BUN, LDH and TCHO in male while increased level of CPK and LDH were found in female rats. Additionally, significant increase in the level of ALT, AST, TCHO, CPK, LDH and total proteins and decrease in TG, Cl and K were reported in female rats exposed with 2500 mg/kg CuO NPs. Kim et al. also observed strong inflammation in mice upon exposure of CuO NPs that led to higher neutrophils and total cell recruitment in lungs while LDH activity was enhanced in the bronchus [104]. Doudi and Setorki studied the effect of 10, 100 and 300 mg/kg of CuO NPs on hepatic enzymes, i.e. SGOT (serum glutamate oxaloacetate transaminase) and SGPT (serum glutamate pyruvate transaminase). Their results showed that after two weeks, noteworthy difference was observed in SGOT in the rats treated with 10 and 300 mg/kg of CuO NPs while the level of SGPT elevated significantly in rats intoxicated with low does (10 mg/kg) [105]. Meng et al. [106] compared effect of micro, nano and ionic Cu in mice and found that nano‐Cu induced higher toxicity as compared to others due to higher SC level and lower elimination rate. On the other hand, Chen et al. found that nano‐Cu was more toxic as compared to micro and ionic Cu due to higher level of TBA, ALP, Cr and BUN [48].
8.1 Immunotoxicity induced by NPs
The function of immune system is to preserve the integrity of body by preventing it from environmental agents like chemicals, microbes or any entity that could disturb the homeostasis [107]. The interaction of NPs with the immune system has been an active research area in the field of biotechnology and nanotechnology. Tremendous efforts have been made in providing novel tactics to the existing therapies and diagnostics in health sector. The applications of nanotechnology in medical are trying to proffer many therapeutic solutions in the form of desirable physico chemical characteristic of NPs to enhance bioavailabilty, biodistribution and to reduce toxicity [108].
There are four possible mechanisms of NPs interactions with immune cells that are phagocytosis, endocytosis, passive uptake and receptor interaction based uptake [108]. The phagocytosis mechanism is well known for removing biological agents like microbes. The capture and internalisation of NPs take place in the phagosomes following by lysosomal degradation. The pH level of phagolysosomes results in the release of metallic ions that ends in disruption of mitochondrial processes leading to ROS production via Fenton type reactions. Similarly, in clathrin‐independent and dependent endocytosis, lysosomal fusion with the endosome takes place [109, 110].
The passive uptake involves the internalisation of CuO NPs which passively enter the cell or escape the endocytic/phagocytic vesicles, directly interacts with normal autophagic process and modulate the NLRP3 inflammasome [111, 112, 113]. The interaction of CuO NPs with cell surface receptors results in intracellular cascades activation such as the scavenger receptor pathway, TLR4 cascade, MAPK pathway and the lectin pathway. The extracellular consequences include exocytosis, cytokine secretion and ended in complement activation [108].
8.2 Immunotoxicity induced by CuO NPs
Immunotoxic effects of dose‐dependent CuO NPs on macrophages and signalling pathways of peritonitis model (mouse) showed that after 1 h of exposure, the injection site had noticeably macrophages recruitment (Arancibia et al., 2015). The reports showed that the CuO NPs recruited the first cells of the innate immune system. In comparison, no significant changes were observed in the control group, T and B cells, natural killer cells and mast cells. The inflammatory mediators and free radicals (NO) are required by macrophages for the removal of pathogens and other harmful substances. For evaluation, peritoneal macrophages were modified with lipopolysaccharide (LPS) and treated with different NPs exposure to demonstrate their effect on NO production. The in‐vitro results elucidated that the LPS‐mediated NO production was not affected by SiNP, TiNP and AINP while stimulation of primary macrophages treated with LPS, co‐treatment with CuO NPs prevented the production of NO. The production of intracellular free radicals by CuO NPs leads to activation of genes and different signalling pathways and ends in upregulation of arginase activity that indirectly enhances protein expressions. The arginase activity regulation results in modulation of CuO NPs induced proinflammatory cytokines secretion (TNFα, MIP‐1β) and induction of COX‐2 (cyclooxygenases derived prostaglandin) and prostaglandin E2. These results clearly evidence that CuO NPs suppresses macrophaged innate immune response by upregulating the arginase activity.
The induction of ROS generation by CuO NPs exposure caused death in human lymphocytes through the generation of oxidative damage [114]. From healthy male individuals, lymphocytes were isolated via Ficoll polysaccharide followed by gradient centrifugation. The lymphocytes were exposed to varying concentrations of CuO NPs and incubated for 6 h. CuO NPs resulted in decreased cell viability, ROS formation, lipid peroxidation, alteration in glutathione levels followed by mitochondria and lysosomal damage. Another findings by Yaqub et al. elucidated the effect of sub‐lethal concentrations of CuO NPs on the haematological parameters (white blood cells, red blood cells, haemoglobin and platelets count) of Mus musculus (Home mouse) [115]. The intravenous injection of sub‐lethal concentration of CuO NPs into the Mus resulted in elevated level of WBCs, evidential decrease in RBCs, Hgb and platelets. The findings also evidenced the toxic effects of CuO NPs on haematological and metabolic enzymes of caspian trout (Juvenile) [116]. The experiment was run in triplicate in which the Caspian trout was exposed to lethal concentration of CuO NPs (LC50) for 28 days. Blood samples were taken to examine the short‐ and long‐term effects of CuO NPs. The biochemical tests revealed an elevation in WBCs, neutrophils, monocytes, RBCs, Hgb and Hct after acute exposure of CuO NPs as compared to control group (p < 0.05). Moreover, after CuO NPs induction, there was a significant elevation in the alkaline phosphatase, lactate dehydrogenase and aspartate aminotransferase levels.
The functional analysis of RAW264.7 cell line macrophages revealed a decrease in glutathione levels, phagocytosis inhibition, LPS‐mediated NO production [117]. The findings evidenced that the Cu ‐based NPs profoundly affect the macrophages functions. The proteomic analysis also showed alterations in oxidative stress responses (superoxidases and peroxidases), mitochondrial proteins, glutathione biosynthesis, actomyosin.
The immunotoxic effect of CuO NPs and CuSO4 on Metaphore Posthuma (Indian earthworm) immune associated parameters [phagocytic response, total count, cytotoxic molecules (superoxide anion, NO)], enzymes activities (alkaline phosphatase, catalase, superoxide dismutase, phenoloxidase, acid phosphatase) and coelomocytes total protein has shown that the exposure of 1000 mg/kg of CuO NPs and CuSO4 for 14 days resulted in meaningful decrease in total coelomocyte followed by NO production, lower phenoloxidase and cytotoxic activity in 7–14 days, respectively [118]. Immune compromisation and decrease in earthworm's population density is observed among the species habituating in unrestricted contaminated soil by CuO and CuSO4 NPs.
The impact of Cu‐based NPs (Cu and CuO) on adult mussel's immune system showed that the soluble Cu accumulated in gills and haemolymph whilst CuO NPs noticeably caused damages to gills and cumulated in the haemocytes. The increased ROS production, lower multi‐drug resistance transporter activity and lysosome abundance in the haemocytes were results of the Cu and CuO NPs cellular toxicity. The exposure of Cu and CuO NPs to adult mussels resulted in lowering their haemocytes phagocytic activity and elaborating the risks in pathogenic bacterial proliferations.
8.3 Haematological alterations induced by CuO NPs
Significant changes have been observed in the haematological profile of rats upon administration of CuO NPs. Lee et al. observed that CuO NPs induced haemolysis of RBCs (red blood cells), decrease in level of RBCs, HB (haemoglobin), iron, HCT (haematocrit), MCV (mean corpuscular volume) while increased RET (reticulocytes) was observed [100]. They also found reduction in LYM (lymphocytes) percentage was due to the differential WBCs count that in turn effect defence system of the organisms. Meanwhile, increased percentages of MON and NEU (monocytes and neutrophils) indicate inflammation in the exposed organs. Other scientists also observed similar finding where they found destruction of RBCs in rodents that cause anaemia and reducing RBCs count, HCT, MCH, HB, MCV and WBCs (leukocytes) [119, 120]. Moreover, higher level of Cu prevents absorption and utilisation of iron hence level of iron reduces and eventually diminishes in the serum [121, 122]. These findings demonstrate that CuO NPs disturb RBCs count and immune system of the treated organism.
8.4 Urine chemistry alterations induced by CuO NPs
Administration of CuO NPs also effect urine parameters as reported by many researchers. Lei et al. observed higher level of glucose, citrate, amino acid, acetate, lactate, succinate and TAMO while creatinine level dropped in urine spectra of rats treated with Cu NPs [102]. Lee et al. [100] reported significant increase in protein, white blood cells, ketone bodies, specific gravity, nitrite and occult blood in urine. Meng et al. [106] compared biochemical analysis (serum Cu, SC; serum ceruloplasmin, CP; and urine Cu, UC) of mice after 24 and 72 h intoxication with micro, nano and ionic Cu. They found higher level of SC after 24 h and that remained higher after 72 h in case of nano‐Cu as compared to micro‐Cu while ion Cu reduced after 72 h. This was due to accumulation and transformation of nano‐Cu into ions while macro‐Cu cannot. The UC level increased significantly in mice treated with both ion‐ and nano‐Cu as compared to control while no increase was found in micro‐Cu. Moreover, higher CP level was found in ionic Cu than nano‐Cu might be due to binding of serum Cu with CP, i.e. acute phase protein type reactant.
A study conducted by Lischkova et al. [123] erected the presence of NPs in the biological samples of exposed occupational individuals. During the study two groups were subjected to engineered NPs (Fe, Mn and C compounds) and CuO NPs. Biological samples of the post‐shift were collected and analysis was performed using transmission electron microscopy (TEM) and energy‐dispersive spectroscopy. The biogenic ones (K, C, Cl and O) were the most identified chemical elements whilst the metals containing NPs were found in urine, blood sample and exhaled breath condensate.
8.5 Histological alterations induced by CuO NPs
Administration of CuO NPs causes deleterious effects in the structure of various organs that can be traced via histopathological studies. Many reports verify toxic effects of CuO NPs in different organs (Table 3).
Table 3.
Histological alteration induced by CuO NPs in animal models
| Organism | Concentration, mg/kg | Organs | Histological observations | References |
|---|---|---|---|---|
| rats | 10, 100 and 300 | liver | vasculature in central veins and portal triad vessels, loss of hexagonal lobules | [105] |
| lungs | thickening of air sacs, increase in fibrous tissues | |||
| rats | 100 and 200 | liver | necrosis of tissues | [102] |
| kidney | necrosis in proximal renal tubule, cellular fragments around tubule lumen, swelling of proximal tubule | |||
| rats | 1250 and 2500 | liver | slight inflammatory cell's infiltration, dilation in sinusoids vacuolation | [103] |
| spleen | multinucleated giant cell, decline in cell's white pulp | |||
| kidney | inflammatory cells infiltration, tubular dilation, renal glomerulus atrophy | |||
| rats | CuO NPs and conjugated with quercetin | liver | lobular hepatic architecture, hepatocyte with ballooning, binucleated hepatocytes, sinusoidal dilatation, lymphocytes aggregates | [71] |
| rat | nano‐ and micro‐Cu | spleen | appearance of atrophic white pulp, increased yellow pigmentation reduction of cellularity, functional and structural damage | [100] |
| thymus, | disrupted demarcation of medulla/cortex, decreased cellularity of medulla/cortex, cytoplasmic vacuolation | |||
| liver | mononuclear cell infiltration, dilated sinusoid, degenerated hepatocytes, binucleated hepatocytes | |||
| kidney | dilated tubules, degenerated tubular cells, inflammatory cell infiltration | |||
| rats | 50, 100 and 200 | kidney | necrosis in proximal tubule, cell debris present in tubule's lumen, proximal tubule swelling | [81] |
| liver | no changes were observed in liver | |||
| rat | kidney | swelling of glomerulus, glomerulonephritis, deterioration in the lumen of Bowman's capsule | [106] | |
| mice | nano and micro Cu | kidney | proximal tubule injuries, swollen glomerulus, dwindling in Bowman's capsule lumen, deterioration of epithelial cells of proximal convoluted tubules | [48] |
| mice | CuO NPs and conjugated with thiamine | ovary | degeneration of granulosa cells, disintegration of corpus luteum | [75] |
Doudi and Setorki [105] treated rats with different doses (10, 100 and 300 mg/kg) of CuO NPs and studied their effect on liver and lungs. They found that all doses of CuO NPs showed changes in the liver, i.e. appearance of central vein vasculature, portal triad vessels and loss of hexagonal lobules while thickening of air sacs and increase in fibrous tissues in the lungs. Lei et al. intoxicated rats with 100 and 200 mg/kg of Cu NPs and observed their effects on liver and kidney [80]. The Cu NPs caused necrosis in rat's liver intoxicated with 200 mg/kg while 100 mg/kg Cu NPs did not cause any alteration. Noteworthy changes were also observed in nephrotic tissues including extensive necrosis in proximal renal tubule along with presence of cellular fragments tubule lumen upon treatment with 200 mg/kg of Cu NPs. Rats treated with 100 mg/kg of Cu NPs showed swelling of proximal tubule. Wang et al. [103] studied gender‐based effect of different doses of Cu NPs on rats. They noticed histological alterations in liver, spleen and kidney in male (1250 mg/kg), and female (2500 mg/kg) rats. Liver of male and female rats with above‐mentioned doses also displayed slight inflammatory cell's infiltration, dilation in sinusoids and vacuolation. Same was observed when male and female rats treated with Cu ions (625 mg/kg). Inflammatory cell's infiltration, cellular fragments deposition in the tubule, hyaline cast, tubular dilation, atrophy of glomerulus were found in kidney treated with Cu NPs while mild cast and dilation was observed in the kidney's tubule when exposed to Cu ions. Multinucleated spleen along with decline in cell's white pulp was observed in both sexes administered with Cu NPs while no such alterations were found in Cu ion treated rats. Arafa et al. [71] conducted an experiment on the female rats treated with CuO NPs and CuO NPs conjugated with quercetin. They observed effects in liver. Rats administered with CuO NPs induced severe damages to liver like lobular liver structure, liver cells with ballooning and bi‐nucleated cell infiltration, microsteatotic, dilated sinusoids and congested central vein as compared to control and CuO NPs conjugated with quercetin. Lee et al. [100] studied the comparative effect of nano‐ and micro‐Cu on spleen, thymus, hepatic and nephrotic tissues of rat. Their results determined that nano‐Cu is more toxic and causes major alterations in these organs while micro‐Cu did not exhibit any change. Major alterations found in these organs when exposed to nano‐Cu were appearance of atrophic white pulp, yellow coloration and decreased cellularity and follicular number observed in spleen, interrupted demarcation of cortex and medulla. Reduced cells and vacuolation in cytoplasm were exhibited by thymus, hepatic tissues represented sinusoid dilation, mononuclear cell infiltration, deteriorated or binucleated liver cells, tubule dilation, cell fragments with purple or pink pigmented tubular casts, while nephrotic cells displayed disintegrated tubule cells along with inflammatory cell infiltration. Lei et al. used 50, 100 and 200 mg/kg of nano‐Cu and examined their pathological effects on kidney and liver [102]. They found that 200 mg/kg of nano‐Cu caused toxic effects including prevalent necrosis in proximal tubule, cell debris present in tubule's lumen where deposition of orange crystalline material was seen in the renal tissues while scattered dot necrosis in liver cells were also observed at same dose. Moreover, other doses (50 and 100 mg/kg) caused proximal tubule swelling in renal tissues and did not exhibited any toxic sign in liver. Meng et al. [106] reported diverse range of irregularities in kidney including swelling of glomerulus, deterioration in the lumen of Bowman's capsule revealing glomerulonephritis and purplish deposits were also found. Chen et al. [124] observed the pathological effects of different doses of nano‐ and micro‐Cu on various organs of both sexes of mice. Mice exposed to micro‐Cu did not display any overt effect in any organ of any sex expect at higher dose (5000 mg/kg) in which both male and female mice died and ileus were shown in their intestine. Contrary to these observations, mice exposed to nano‐Cu at all concentrations had gravely effected organs. Effect of nano‐Cu on tissue damage was dose dependent, i.e. higher dose showed severe damage and vice versa. Exposure to lower dose (108, 158, 232 mg/kg) nano‐Cu to mice's kidney caused proximal tubule injuries with swollen glomerulus and dwindling in Bowman's capsule lumen being contributing factors towards glomerulonephritis. Moreover, deterioration of epithelial cells of proximal convoluted tubules occurs at medium dose (341 mg/kg) while extensive necrobiosis was presented at higher dose (1080 mg/kg) mice. Protein fluid filled in renal tubules were seen in which purplish deposits were found in mice kidney treated with medium and higher dose but not observed in lower dose mice. Additionally, nucleus of epithelial cells of renal tubules were seen in lower dose, reduces in medium and ultimately diminished at higher dose. In hepatic tissues, mice exposed to medium and higher dose exhibited steatosis around liver's central vein while nothing was observed in lower dose mice. Spleen of treated mice showed various abnormalities like atrophy, splenic interstitium fibrosis, reduction in splenic units and lymphocytes in mice exposed to various doses (232, 341, 501, 736 and 1080 mg/kg). Fatahian‐Dehkordi et al. compared the effects of CuO NPs, CuO; and conjugated CuO NPs and CuO with thiamine on the architecture of mice ovary [74]. They observed that both CuO NPs and CuO caused degeneration of granulosa cells, inflammatory cell infiltration especially in lymphocytes raised around blood vessels determining disintegration of corpus luteum while no such abnormalities were observed in mice treated with CuO NPs‐thiamine and CuO‐thiamine.
8.6 Ultrastructral alterations of cell induced by CuO NPs
The reports of Chen et al. [125] focused CuO NPs (using papaya lead extracts) antibacterial activity on soil born pathogenic bacteria (Ralstonia solanacearum). The soil born pathogenic bacteria exposed to 250 μg ml−1 concentrations of CuO NPs and the findings showed significant killing of Ralstonia solanacearum. The CuO NPs prevented biofilm formation, lowered swarming motility, and disrupted the ATP production by interacting with bacterial cells. After adsorption of CuO NPs, the TEM revealed significant ultrastructural variations in the cytomembrane. The molecular observations elucidated the down‐regulations of genes responsible for the mechanisms of pathogenesis and motility.
The cytotoxic effects of surface modified CuO NPs in mouse macrophage cell lines demonstrated that the different surface modifications did not disturb dissolution of the NPs [126]. The CuO NPs showed no ultrastructural changes observed in the exposed cells of macrophages. The internalisation of NPs resulted in agglomeration of NPs in membrane enclosed vesicles. In other subcellular compartments no CuO NPs agglomerates were found. The results of Minigalieva et al. [127] demonstrated the ultrastructural alterations in the brain revealed by TEM when the rats exposed to CuO NPs and observed nervous fibre demyelination. CuO NPs also showed minimum ultrastructural damage to the mitochondrial membranes and cristae [128].
The time‐dependent changes in the ultrastructure of Daphnia magna's midgut epithelium upon exposure to CuO NPs than bulk CuO showed that after 10 min of exposure the dispersion of CuO NPs occurred in the lumen of midgut as compared to bulk CuO where clumps were formed [129]. Upon CuO NPs exposure at the 48 h, subsequent ultrastructural changes observed in the midgut which were protrusion of epithelial cells in the lumen of the midgut, occurrence of circular CuO NPs similar to membrane vesicles from holocrine secretion in the midgut lumen. Surprisingly, bacterial colonisation observed in the midgut upon CuO NPs. The effect of CuO NPs on the ultrastructural alterations in the midgut of D. magna NPs but not to bulk CuO refer to its nanosize‐related adverse effects. Similar results have shown 50‐fold higher acute toxicity of CuO NPs compared to bulk CuO to Daphnia magna [130].
8.7 Morphometric analysis and CuO NPs toxicity
CuO NPs synthesised through green method (Anabaena specie) exposed to fishes exhibited no visual abnormality [131]. The fish (Channa Punctatus) showed normal swimming behaviour when exposed to fresh water (control) or CuO NPs. However, after 72 h of CuO NPs (40 µg/ml) exposure resulted 11% mortality. To demonstrate the CuO NPs effects on cell viability, mammalian cell lines (Human gastric epithelium) were treated for 24–48 h at different concentrations. The MTT analyses elucidated that at 2.5% concentration, the cells showed 86% survival whilst at 3% the survival significantly dropped to 55%. The findings of Bhattacharya et al. inferred that up to 2.5% CuO NPs did not show much distortion in mammalian cell shapes and morphology and considered as safe for human [131].
The toxicity of CuO NPs in rat's intestine epithelial cells (IEC‐6) and human intestinal cells has shown that cell viability was significantly lowered at 4 µg/ml CuO NPs, with a greater response following 24 h exposure (75% cell viability at 4 h and 57% at 24 h). The highest dose, 80 µg/ml, had the greatest effect on viability with ∼25 and 12% of the cells viable at 4 and 24 h, respectively. At 80 µg/ml CuO NPs, the alteration in cellular morphology was noticeable and some cells released cellular contents. To assess the viability under dose and time dependent, the human intestinal cells were exposed to CuO NPs (80 µg/ml) for 4 h. The two‐way analysis of variance exhibited noticeable time, dose interaction effects (p < 0.0001) on the cells viability. The cells viability significantly lowered at 24 h.
Furthermore, prenatal administration of CuO NPs in mice had significant effect on body weight and mortality during basic development and gestation period. They found that weight gain during gestation period was slower in mothers and pups after delivery. Moreover, mortality rate was also higher in pups as compared to the control [132].
Dose‐dependent cytotoxic effects of CuO NPs synthesised through F. religiosa on human lung cancer cells (A549) have been observed by Sankar et al. (2014). At 50 μg/ml exposure to CuO NPs, A549 cells showed 70% viability whilst the viability noticeably lowered to 6% at 500 μg/ml. The results demonstrated morphological alterations (shape alterations, cell clumping, cell communication inhibition and chromatin condensation) in the cancer cells after 36 h. The cells treated with CuO NPs showed loss in membrane integrity, alterations in mitochondrial membrane potential due to ROS production that leads to apoptosis cascade.
The study conducted by Han et al. on Drosophila melanogaster showed that Cu NPs effect development, life span and sperm quality. They observed that Cu NPs decreased adult longevity characterised by slow development and reduced sperm competition [133].
9 Mechanism behind toxicity of CuO NPs
The major toxicological mechanisms of CuO NPs are ROS generation and oxidative stress induction [30]. CuO NPs cause direct toxicity via activation of ROS production and indirect via stimulating redox system of cell which leads to ROS production (Fig. 2). Upon entry through any exposure route (inhalation, ingestion, skin), CuO NPs interact with the acidic environment of lysosomes or with mitochondria (oxidative organelles) leading towards ROS induction that become a persuasive mechanism behind toxicity associated with CuO NPs [16, 63]. The CuO NPs initiated ROS generation that leads to a wide range of biological responses that entirely depends on the abundancy of biochemical factor (ROS), kind of cellular pathways and the antioxidant response elements that are involved in oxidative stress [134]. The CuO NPs act as a pro‐oxidant, i.e. they encourage oxidative stress via generating ROS or impeding antioxidants. The ROS collectively constitute hydrogen peroxide, anionic superoxide and hydroxyl free radical. Among the free radicals hydroxyl (OH) toxic effect is considered as one of the most lethal one among the other ROS species. Hence, through direct method the CuO NPs cause their effect through production of ROS inside the cell comprising mitochondrial respiration and triggering NADPH‐dependent enzyme systems [124, 135, 136]. Production of ROS also causes inflammatory responses and damages cell membrane, DNA and proteins [137]. Wang et al. verified the potential of CuO NPs to induce damages in DNA and mitochondria resulting in cell death when taken up by the cells (A549) via endocytosis as evidently situate inside the cell nucleus and mitochondria [11]. Moreover in indirect method, upon entry of CuO NPS they can also stimulate redox system of the cell most commonly in lungs where alveolar macrophages and neutrophils of immune system direct as ROS inducers through NADPH oxidase enzyme system [124, 138]. These radicals subsequently oxidise biological molecules that lead to substantial oxidative stress and cell death [139]. Both of direct and indirect means of ROS production ultimately leads to damage cellular structures (mitochondria, DNA, protein) which results in cell death. While in case of mitochondria another mechanism participates in cell death via depolarisation of mitochondrial membrane that stimulate NADPH‐dependent enzymes and cause cell death. Other leading oxidative stress response is the release of Ca2+, which ends to mitochondrial disruption and cell death. To oxidative stress many lethal diseases are linked such as cardiovascular, autoimmune, lung diseases and ageing [134, 140].
Fig. 2.
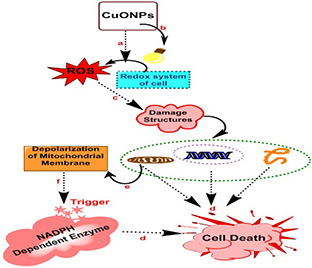
Mechanism of action involved in the cytotoxicity of CuO NPs comprises of multiple steps
(a) Production of ROS directly via exposure of CuO NPs, (b) Stimulation of redox system of cell that leads to production of ROS, (c) Generation of ROS ultimately leads to damage structures like mitochondria, DNA, proteins and so on, (d) Damaged structures promotes cell death, (e) Damages to mitochondria involve depolarisation of mitochondrial membrane, (f) Depolarised mitochondrial membrane trigger NADPH‐dependent enzymes that cause cell death
10 Conclusions
CuO NPs have emerged as an important class of nanomaterials for a wide range of applications (medicinal, environmental, industrial) that have potential risks to organisms and environment. In this review, an overview about CuO NPs synthetic methods, their advantages and disadvantages, applications, exposure routes, toxicological evaluations, relationship between ROS production and oxidative stress, immunotoxicity, cytotoxicity and genotoxicity, their testing in in‐vitro and in‐vivo in different organisms, and factors affecting the toxicity of CuO NPs are discussed. The factors that influence the toxicity of CuO NPs are shape, size, surface modification, morphology and concentration. Conclusively, to ensure that the CuO NPs are safe to environment and organisms, the toxicity of CuO NPs must be reduced to the non‐significant level. The objective is to focus on the toxicity factors that will weaken toxicity mechanisms. Innovated work on surface modification, size, dissolution factor, selection of adequate exposure route will minimise the toxicity of metal oxide NPs [30]. As can be seen, these preliminary findings warrant more comprehensive studies to clearly elucidate the mechanisms of CuO NPs induced toxicity. Henceforth, the CuO NPs environmental fate must be determined carefully, and criteria for sustainable applications in different fields must be defined. In future, we recommend that much work should be done in the area of genotoxicity exhibited by CuO NPs as this area is less explored. Research must be carried out in the context of present risk assessments linked with CuO NPs, their applications, distribution and release into the environment.
11 References
- 1. Galdiero S. Falanga A. Vitiello M. et al.: ‘Silver nanoparticles as potential antiviral agents’, Molecules, 2011, 16, (10), pp. 8894 –8918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elsaesser A. Howard C.V.: ‘Toxicology of nanoparticles’, Adv. Drug Deliv. Rev., 2012, 64, (2), pp. 129 –137 [DOI] [PubMed] [Google Scholar]
- 3. Singh G. Beddow J. Mee C. et al.: ‘Cytotoxicity study of Textile fabrics impregnated with CuO nanoparticles in mammalian cells’, Int. J. Toxicol., 2017, 36, (6), pp. 478 –484 [DOI] [PubMed] [Google Scholar]
- 4. Katsumiti A. Thorley A.J. Arostegui I. et al.: ‘Cytotoxicity and cellular mechanisms of toxicity of CuO NPs in mussel cells in vitro and comparative sensitivity with human cells’, Toxicol. in Vitro, 2018, 48, pp. 146 –158 [DOI] [PubMed] [Google Scholar]
- 5. Ren G. Hu D. Cheng E.W. et al.: ‘Characterisation of copper oxide nanoparticles for antimicrobial applications’, Int. J. Antimicrob. Agents, 2009, 33, (6), pp. 587 –590 [DOI] [PubMed] [Google Scholar]
- 6. Applerot G. Lellouche J. Lipovsky A. et al.: ‘Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress’, Small, 2012, 8, (21), pp. 3326 –3337 [DOI] [PubMed] [Google Scholar]
- 7. Naika H.R. Lingaraju K. Manjunath K. et al.: ‘Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity’, J. Taibah Univ.for Sci., 2015, 9, (1), pp. 7 –12 [Google Scholar]
- 8. Borkow G. Gabbay J.: ‘Copper as a biocidal tool’, Curr. Med. Chem., 2005, 12, (18), pp. 2163 –2175 [DOI] [PubMed] [Google Scholar]
- 9. Pendashteh A. Mousavi M.F. Rahmanifar M.S.: ‘Fabrication of anchored copper oxide nanoparticles on graphene oxide nanosheets via an electrostatic coprecipitation and its application as supercapacitor’, Electrochim. Acta., 2013, 88, pp. 347 –357 [Google Scholar]
- 10. Djurišić A.B. Leung Y.H. Ng A.M. et al.: ‘Toxicity of metal oxide nanoparticles: mechanisms, characterization, and avoiding experimental artefacts’, Small, 2015, 11, (1), pp. 26 –44 [DOI] [PubMed] [Google Scholar]
- 11. Wang Z. Li N. Zhao J. et al.: ‘CuO nanoparticle interaction with human epithelial cells: cellular uptake, location, export, and genotoxicity’, Chem. Res. Toxicol., 2012, 25, (7), pp. 1512 –1521 [DOI] [PubMed] [Google Scholar]
- 12. Kawasaki M.: ‘Laser‐induced fragmentative decomposition of fine CuO powder in acetone as highly productive pathway to Cu and Cu2 O nanoparticles’, J. Phys. Chem. C, 2011, 115, (12), pp. 5165 –5173 [Google Scholar]
- 13. Karthik L. Gaurav K. Rao K.B.: ‘Environmental and human impact on marine microorganisms synthesized nanoparticles’, in Kim S.K. (Ed.): ‘Marine biomaterials: characterization, isolation and applications’ (CRC Press, Boca Raton, 2013), pp. 253 –272 [Google Scholar]
- 14. Nithya K. Yuvasree P. Neelakandeswari N. et al.: ‘Preparation and characterization of copper oxide nanoparticles’, Int. J. ChemTech Res., 2014, 6, pp. 2220 –2222 [Google Scholar]
- 15. Jillani S. Jelani M. Hassan N.U. et al.: ‘Synthesis, characterization and biological studies of copper oxide nanostructures’, Mater. Res. Express, 2018, 5, (4), p. 045006 [Google Scholar]
- 16. Ahamed M. Siddiqui M.A. Akhtar M.J. et al.: ‘Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells’, Biochem. Biophys. Res. Commun., 2010, 396, (2), pp. 578 –583 [DOI] [PubMed] [Google Scholar]
- 17. Arun L. Karthikeyan C. Philip D. et al.: ‘Effect of Ni 2 + doping on chemocatalytic and supercapacitor performance of biosynthesized nanostructured CuO’, J. Mater. Sci., Mater. Electron., 2018, 29, (24), pp. 21180 –21193 [Google Scholar]
- 18. Mosleh S. Rahimi M.R. Ghaedi M. et al.: ‘Sonochemical‐assisted synthesis of CuO/Cu2 O/Cu nanoparticles as efficient photocatalyst for simultaneous degradation of pollutant dyes in rotating packed bed reactor: LED illumination and central composite design optimization’, Ultrason. Sonochem., 2018, 40, pp. 601 –610 [DOI] [PubMed] [Google Scholar]
- 19. Singh A.V. Patil R. Anand A. et al.: ‘Biological synthesis of copper oxide nano particles using Escherichia coli’, Curr. Nanosci., 2010, 6, (4), pp. 365 –369 [Google Scholar]
- 20. Kumar K. Priya A. Arun A. et al.: ‘Antibacterial and natural room‐light driven photocatalytic activities of CuO nanorods’, Mater. Chem. Phys., 2019, 226, pp. 106 –112 [Google Scholar]
- 21. Pantidos N. Horsfall L.E.: ‘Biological synthesis of metallic nanoparticles by bacteria, fungi and plants’, J. Nanomed. Nanotechnol., 2014, 05, (5), p. 1 [Google Scholar]
- 22. Rajendran V. Deepa B. Mekala R.: ‘Studies on structural, morphological, optical and antibacterial activity of pure and Cu‐doped MgO nanoparticles synthesized by co‐precipitation method’, Mater. Today: Proc., 2018, 5, (2), pp. 8796 –8803 [Google Scholar]
- 23. El‐Kassas H.Y. Okbah M.A.E.A.: ‘Phytotoxic effects of seaweed mediated copper nanoparticles against the harmful alga: Lyngbya majuscula’, J. Genet. Eng. Biotechnol., 2017, 15, (1), pp. 41 –48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jefferson D.A.: ‘The surface activity of ultrafine particles’, Philos. Trans. R. Soc. Lond. A, Math. Phys. Eng. Sci., 2000, 358, (1775), pp. 2683 –2692 [Google Scholar]
- 25. Sajid M. Ilyas M. Basheer C. et al.: ‘Impact of nanoparticles on human and environment: review of toxicity factors, exposures, control strategies, and future prospects’, Environ. Sci. Pollut. Res., 2015, 22, (6), pp. 4122 –4143 [DOI] [PubMed] [Google Scholar]
- 26. Nangia S. Sureshkumar R.: ‘Effects of nanoparticle charge and shape anisotropy on translocation through cell membranes’, Langmuir, 2012, 28, (51), pp. 17666 –17671 [DOI] [PubMed] [Google Scholar]
- 27. Katwal R. Kaur H. Sharma G. et al.: ‘Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity’, J. Ind. Eng. Chem., 2015, 31, pp. 173 –184 [Google Scholar]
- 28. Henson T.E. Navratilova J. Tennant A.H. et al.: ‘In vitro intestinal toxicity of copper oxide nanoparticles in rat and human cell models’, Nanotoxicology, 2019, 13, pp. 795 –811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi M. Kwon H.S. Peng Z. et al.: ‘Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles’, ACS Nano, 2012, 6, (3), pp. 2157 –2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang Y.N. Zhang M. Xia L. et al.: ‘The toxic effects and mechanisms of CuO and ZnO nanoparticles’, Materials. (Basel), 2012, 5, (12), pp. 2850 –2871 [Google Scholar]
- 31. Mortimer M. Kasemets K. Vodovnik M. et al.: ‘Exposure to CuO nanoparticles changes the fatty acid composition of protozoa Tetrahymena thermophila’, Environ. Sci. Technol., 2011, 45, (15), pp. 6617 –6624 [DOI] [PubMed] [Google Scholar]
- 32. Costa P.M. Gosens I. Williams A. et al.: ‘Transcriptional profiling reveals gene expression changes associated with inflammation and cell proliferation following short‐term inhalation exposure to copper oxide nanoparticles’, J. Appl. Toxicol., 2018, 38, (3), pp. 385 –397 [DOI] [PubMed] [Google Scholar]
- 33. Handy R.D. Von der Kammer F. Lead J.R. et al.: ‘The ecotoxicology and chemistry of manufactured nanoparticles’, Ecotoxicology, 2008, 17, (4), pp. 287 –314 [DOI] [PubMed] [Google Scholar]
- 34. Albanese A. Chan W.C.: ‘Effect of gold nanoparticle aggregation on cell uptake and toxicity’, ACS Nano., 2011, 5, (7), pp. 5478 –5489 [DOI] [PubMed] [Google Scholar]
- 35. Ameh T. Sayes C.M.: ‘The potential exposure and hazards of copper nanoparticles: a review’, Environ. Toxicol. Pharmacol., 2019, 71, p. 103220 [DOI] [PubMed] [Google Scholar]
- 36. Docter D. Westmeier D. Markiewicz M. et al.: ‘The nanoparticle biomolecule corona: lessons learned–challenge accepted?’, Chem. Soc. Rev., 2015, 44, (17), pp. 6094 –6121 [DOI] [PubMed] [Google Scholar]
- 37. Stern S.T. McNeil S.E.: ‘Nanotechnology safety concerns revisited’, Toxicological Sci., 2007, 101, (1), pp. 4 –21 [DOI] [PubMed] [Google Scholar]
- 38. Caracciolo G. Farokhzad O.C. Mahmoudi M.: ‘Biological identity of nanoparticles in vivo: clinical implications of the protein corona’, Trends Biotechnol., 2017, 35, (3), pp. 257 –264 [DOI] [PubMed] [Google Scholar]
- 39. Brigger I. Morizet J. Aubert G. et al.: ‘Poly (ethylene glycol)‐coated hexadecylcyanoacrylate nanospheres display a combined effect for brain tumor targeting’, J. Pharmacology Exp. Therapeutics, 2002, 303, (3), pp. 928 –936 [DOI] [PubMed] [Google Scholar]
- 40. Pattan G. Kaul G.: ‘Health hazards associated with nanomaterials’, Toxicol. Ind. Health, 2014, 30, (6), pp. 499 –519 [DOI] [PubMed] [Google Scholar]
- 41. Liu Y. Gao Y. Zhang L. et al.: ‘Potential health impact on mice after nasal instillation of nano‐sized copper particles and their translocation in mice’, J. Nanosci. Nanotechnol., 2009, 9, (11), pp. 6335 –6343 [DOI] [PubMed] [Google Scholar]
- 42. Elder A. Gelein R. Silva V. et al.: ‘Translocation of inhaled ultrafine manganese oxide particles to the central nervous system’, Environ. Health Perspect., 2006, 114, (8), pp. 1172 –1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yacobi N.R. Malmstadt N. Fazlollahi F. et al.: ‘Mechanisms of alveolar epithelial translocation of a defined population of nanoparticles’, Am. J. Respir. Cell Mol. Biol., 2010, 42, (5), pp. 604 –614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teow Y. Asharani P.V. Hande M.P. et al.: ‘Health impact and safety of engineered nanomaterials’, Chem. Commun., 2011, 47, (25), pp. 7025 –7038 [DOI] [PubMed] [Google Scholar]
- 45. Hillyer J.F. Albrecht R.M.: ‘Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles’, J. Pharm. Sci., 2001, 90, (12), pp. 1927 –1936 [DOI] [PubMed] [Google Scholar]
- 46. Oberdörster G. Sharp Z. Atudorei V. et al.: ‘Extrapulmonary translocation of ultrafine carbon particles following whole‐body inhalation exposure of rats’, J. Toxicol. Environ. Health A., 2002, 65, (20), pp. 1531 –1543 [DOI] [PubMed] [Google Scholar]
- 47. Borm P.J. Robbins D. Haubold S. et al.: ‘The potential risks of nanomaterials: a review carried out for ECETOC’, Part Fibre Toxicol., 2006, 3, (1), p. 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Z. Meng H. Xing G. et al.: ‘Acute toxicological effects of copper nanoparticles in vivo’, Toxicol. Lett., 2006, 163, (2), pp. 109 –120 [DOI] [PubMed] [Google Scholar]
- 49. Mavon A. Miquel C. Lejeune O. et al.: ‘In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen’, Skin Pharmacol. Physiol., 2007, 20, (1), pp. 10 –20 [DOI] [PubMed] [Google Scholar]
- 50. Shvedova A. Castranova V. Kisin E. et al.: ‘Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells’, J. Toxicol. Environ Health A., 2003, 66, (20), pp. 1909 –1926 [DOI] [PubMed] [Google Scholar]
- 51. Meister A.: ‘On the biochemistry of glutathione’, Glutathione Centennial: Mol. Perspect. Clin. Implications, 1989, pp. 3 –21 [Google Scholar]
- 52. Chen Y. McMillan‐Ward E. Kong J. et al.: ‘Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells’, Cell Death Differentiation, 2008, 15, (1), pp. 171 –182 [DOI] [PubMed] [Google Scholar]
- 53. Blokhina O. Virolainen E. Fagerstedt K.V.: ‘Antioxidants, oxidative damage and oxygen deprivation stress: a review’, Ann. Botany, 2003, 91, (2), pp. 179 –194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sayre L.M. Moreira P.I. Smith M.A. et al.: ‘Metal ions and oxidative protein modification in neurological disease’, Ann. Ist. Super. Sanita., 2005, 41, (2), pp. 143 –164 [PubMed] [Google Scholar]
- 55. Gutteridge J.M.: ‘Lipid peroxidation and antioxidants as biomarkers of tissue damage’, Clin. Chem., 1995, 41, (12), pp. 1819 –1828 [PubMed] [Google Scholar]
- 56. Ramírez‐Prieto M.T. García‐Río F. Villamor J.: ‘Role of oxidative stress in respiratory diseases and its monitoring’, Med. Clin., 2006, 127, (10), pp. 386 –396 [DOI] [PubMed] [Google Scholar]
- 57. Li N. Hao M. Phalen R.F. et al.: ‘Particulate air pollutants and asthma: a paradigm for the role of oxidative stress in PM‐induced adverse health effects’, Clin. Immunol., 2003, 109, (3), pp. 250 –265 [DOI] [PubMed] [Google Scholar]
- 58. Shaw B.J. Al‐Bairuty G. Handy R.D.: ‘Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchus mykiss): physiology and accumulation’, Aquatic Toxicol., 2012, 116, pp. 90 –101 [DOI] [PubMed] [Google Scholar]
- 59. Cheloni G. Slaveykova V.I.: ‘Optimization of the c11‐BODIPY581/591 dye for the determination of lipid oxidation in Chlamydomonas reinhardtii by flow cytometry’, Cytometry A., 2013, 83, (10), pp. 952 –961 [DOI] [PubMed] [Google Scholar]
- 60. Wang Y. Aker W.G. Hwang H.M. et al.: ‘A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human hepG2 cells’, Sci. Total Environ., 2011, 409, (22), pp. 4753 –4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Piret J.P. Jacques D. Audinot J.N. et al.: ‘Copper (II) oxide nanoparticles penetrate into HepG2 cells, exert cytotoxicity via oxidative stress and induce pro‐inflammatory response’, Nanoscale, 2012, 4, (22), pp. 7168 –7184 [DOI] [PubMed] [Google Scholar]
- 62. Maynard A.D. Kuempel E.D.: ‘Airborne nanostructured particles and occupational health’, J. Nanoparticle Res., 2005, 7, (6), pp. 587 –614 [Google Scholar]
- 63. Fahmy B. Cormier S.A.: ‘Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells’, Toxicol. In Vitro., 2009, 23, (7), pp. 1365 –1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun J. Wang S. Zhao D. et al.: ‘Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells’, Cell Biol. Toxicol., 2011, 27, (5), pp. 333 –342 [DOI] [PubMed] [Google Scholar]
- 65. Perreault F. Melegari S.P. da Costa C.H. et al.: ‘Genotoxic effects of copper oxide nanoparticles in neuro 2A cell cultures’, Sci. Total Environ., 2012, 441, pp. 117 –124 [DOI] [PubMed] [Google Scholar]
- 66. Xu J. Li Z. Xu P. et al.: ‘Nanosized copper oxide induces apoptosis through oxidative stress in podocytes’, Arch. Toxicol., 2013, 87, (6), pp. 1067 –1073 [DOI] [PubMed] [Google Scholar]
- 67. Akhtar M.J. Kumar S. Alhadlaq H.A. et al.: ‘Dose‐dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells’, Toxicol. Ind. Health, 2016, 32, (5), pp. 809 –821 [DOI] [PubMed] [Google Scholar]
- 68. Berntsen P. Park C.Y. Rothen‐Rutishauser B. et al.: ‘Biomechanical effects of environmental and engineered particles on human airway smooth muscle cells’, J. R. Soc. Interface., 2010, 7, p. S27, rsif20100068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lanone S. Rogerieux F. Geys J. et al.: ‘Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines’, Part. Fibre Toxicol., 2009, 6, (1), p. 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu J. Fan D. Wang L. et al.: ‘Effect of ZnO, CuO, Ag and TiO2 nanoparticles on Daphnia magna and early life stages of Zebrafish Danio rerio’, Environ. Protect Eng., 2014, 40, pp. 139 –149 [Google Scholar]
- 71. Arafa A.F. Ghanem H.Z. Soliman M.S. et al.: ‘Modulation effects of quercetin against copper oxide nanoparticles‐induced liver toxicity in rats’, Egypt. Pharm. J., 2017, 16, (2), p. 78 [Google Scholar]
- 72. Sandhu N.S. Chopra D. Kaur S.: ‘Amelioration of paracetamol induced hepatotoxicity by a protein isolated from the leaves of the herb Cajanus acutifolius Linn’, Int. J. Pharm. Pharm. Sci., 2010, 2, (3), pp. 75 –80 [Google Scholar]
- 73. Naz S. Islam M. Tabassum S. et al.: ‘Green synthesis of hematite (α‐Fe2 O3) nanoparticles using Rhus punjabensis extract and their biomedical prospect in pathogenic diseases and cancer’, J. Mol. Struct., 2019, 1185, pp. 1 –7 [Google Scholar]
- 74. Park S. Lee Y.K. Jung M. et al.: ‘Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells’, Inhalation Toxicol., 2007, 19, (sup 1), pp. 59 –65 [DOI] [PubMed] [Google Scholar]
- 75. Fatahian‐Dehkordi R. Reaisi M. Heidarnejad M.S. et al.: ‘Serum biochemical status and morphological changes in mice ovary associated with copper oxide nanoparticles after thiamine therapy’, J. Herbmed Pharmacol., 2017, 6, pp. 21 –26 [Google Scholar]
- 76. Jing X. Park J.H. Peters T.M. et al.: ‘Toxicity of copper oxide nanoparticles in lung epithelial cells exposed at the air–liquid interface compared with in vivo assessment’, Toxicol. Vitro, 2015, 29, (3), pp. 502 –511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Prabhu B.M. Ali S.F. Murdock R.C. et al.: ‘Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat’, Nanotoxicology, 2010, 4, (2), pp. 150 –160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Siddiqui M.A. Alhadlaq H.A. Ahmad J. et al.: ‘Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells’, PloS One, 2013, 8, (8), p. e69534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Misra S.K. Nuseibeh S. Dybowska A. et al.: ‘Comparative study using spheres, rods and spindle‐shaped nanoplatelets on dispersion stability, dissolution and toxicity of CuO nanomaterials’, Nanotoxicology, 2014, 8, (4), pp. 422 –432 [DOI] [PubMed] [Google Scholar]
- 80. Rodhe Y. Skoglund S. Wallinder I.O. et al.: ‘Copper‐based nanoparticles induce high toxicity in leukemic HL60 cells’, Toxicol. in Vitro, 2015, 29, (7), pp. 1711 –1719 [DOI] [PubMed] [Google Scholar]
- 81. Lei R. Yang B. Wu C. et al.: ‘Mitochondrial dysfunction and oxidative damage in the liver and kidney of rats following exposure to copper nanoparticles for five consecutive days’, Toxicol. Res., 2015, 4, (2), pp. 351 –364 [Google Scholar]
- 82. Watson C. Ge J. Cohen J. et al.: ‘High‐throughput screening platform for engineered nanoparticle‐mediated genotoxicity using Comet Chip technology’, ACS Nano, 2014, 8, (3), pp. 2118 –2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Thit A. Selck H. Bjerregaard H.F.: ‘Toxic mechanisms of copper oxide nanoparticles in epithelial kidney cells’, Toxicol. Vitro, 2015, 29, (5), pp. 1053 –1059 [DOI] [PubMed] [Google Scholar]
- 84. Magdolenova Z. Collins A. Kumar A. et al.: ‘Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles’, Nanotoxicology, 2014, 8, (3), pp. 233 –278 [DOI] [PubMed] [Google Scholar]
- 85. Sun T. Yan Y. Zhao Y. et al.: ‘Copper oxide nanoparticles induce autophagic cell death in A549 cells’, PLOS One, 2012, 7, (8), p. e43442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Choi S.J. Oh J.M. Choy J.H.: ‘Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells’, J. Inorg. Biochem., 2009, 103, (3), pp. 463 –471 [DOI] [PubMed] [Google Scholar]
- 87. Hou J. Liu H. Wang L. et al.: ‘Molecular toxicity of metal oxide nanoparticles in Danio rerio’, Environ. Sci. Technol., 2018, 52, (14), pp. 7996 –8004 [DOI] [PubMed] [Google Scholar]
- 88. Lai X. Zhao H. Zhang Y. et al.: ‘Intranasal delivery of copper oxide nanoparticles induces pulmonary toxicity and fibrosis in C57BL/6 mice’, Sci. Rep., 2018, 8, (1), p. 567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Magaye R. Zhao J. Bowman L. et al.: ‘Genotoxicity and carcinogenicity of cobalt‐, nickel‐and copper‐based nanoparticles’, Exp. Therapeutic Med., 2012, 4, (4), pp. 551 –561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Karlsson H.L. Gustafsson J. Cronholm P. et al.: ‘Size‐dependent toxicity of metal oxide particles: a comparison between nano‐and micrometer size’, Toxicol. Lett., 2009, 188, (2), pp. 112 –118 [DOI] [PubMed] [Google Scholar]
- 91. Strauch B.M. Niemand R.K. Winkelbeiner N.L. et al.: ‘Comparison between micro‐and nanosized copper oxide and water soluble copper chloride: interrelationship between intracellular copper concentrations, oxidative stress and DNA damage response in human lung cells’, Part. Fibre Toxicol., 2017, 14, (1), p. 2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Alarifi S. Ali D. Verma A. et al.: ‘Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells’, Int. J. Toxicol., 2013, 32, (4), pp. 296 –307 [DOI] [PubMed] [Google Scholar]
- 93. Ko J.W. Shin N.R. Park J.W. et al.: ‘Copper oxide nanoparticles induce collagen deposition via TGF‐β1/Smad3 signaling in human airway epithelial cells’, Nanotoxicology, 2018, 12, (3), pp. 239 –250 [DOI] [PubMed] [Google Scholar]
- 94. Midander K. Cronholm P. Karlsson H.L. et al.: ‘Surface characteristics, copper release, and toxicity of nano‐and micrometer‐sized copper and copper (II) oxide particles: a cross‐disciplinary study’, Small, 2009, 5, (3), pp. 389 –399 [DOI] [PubMed] [Google Scholar]
- 95. An K. Somorjai G.A.: ‘Size and shape control of metal nanoparticles for reaction selectivity in catalysis’, ChemCatChem, 2012, 4, (10), pp. 1512 –1524 [Google Scholar]
- 96. Li X. Sun W. An L.: ‘Nano‐CuO impairs spatial cognition associated with inhibiting hippocampal long‐term potentiation via affecting glutamatergic neurotransmission in rats’, Toxicol. Ind. Health, 2018, 34, (6), pp. 409 –421 [DOI] [PubMed] [Google Scholar]
- 97. Sun Y. Zhang G. He Z. et al.: ‘Effects of copper oxide nanoparticles on developing zebrafish embryos and larvae’, Int. J. Nanomed., 2016, 11, p. 905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mashock M.J. Zanon T. Kappell A.D. et al.: ‘Copper oxide nanoparticles impact several toxicological endpoints and cause neurodegeneration in Caenorhabditis elegans’, PloS One, 2016, 11, (12), p. e0167613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mohammadyari A. Razavipour S.T. Mohammadbeigi M. et al.: ‘Explore in‐vivo toxicity assessment of copper oxide nanoparticle in Wistar rats’, J. Biol. Today's World, 2014, 3, pp. 124 –128 [Google Scholar]
- 100. Lee I.C. Ko J.W. Park S.H. et al.: ‘Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles’, Part. Fibre Toxicol., 2016, 13, (1), p. e64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Manna P. Ghosh M. Ghosh J. et al.: ‘Contribution of nano‐copper particles to in vivo liver dysfunction and cellular damage: role of IκBα/NF‐κB, MAPKs and mitochondrial signal’, Nanotoxicology, 2012, 6, (1), pp. 1 –21 [DOI] [PubMed] [Google Scholar]
- 102. Lei R. Wu C. Yang B. et al.: ‘Integrated metabolomic analysis of the nano‐sized copper particle‐induced hepatotoxicity and nephrotoxicity in rats: a rapid in vivo screening method for nanotoxicity’, Toxicol. Appl. Pharmacol., 2008, 232, (2), pp. 292 –301 [DOI] [PubMed] [Google Scholar]
- 103. Wang D. Lin Z. Wang T. et al.: ‘Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both?’, J. Hazardous Mater., 2016, 308, pp. 328 –334 [DOI] [PubMed] [Google Scholar]
- 104. Kim J.S. Adamcakova‐Dodd A. O'Shaughnessy P.T. et al.: ‘Effects of copper nanoparticle exposure on host defense in a murine pulmonary infection model’, Part. Fibre Toxicol., 2011, 8, (1), p. 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Doudi M. Setorki M.: ‘Acute effect of nano‐copper on liver tissue and function in rat’, Nanomed. J., 2014, 1, (5), pp. 331 –338 [Google Scholar]
- 106. Meng H. Chen Z. Xing G. et al.: ‘Ultrahigh reactivity provokes nanotoxicity: explanation of oral toxicity of nano‐copper particles’, Toxicol. Lett., 2007, 175, (1–3), pp. 102 –110 [DOI] [PubMed] [Google Scholar]
- 107. Dobrovolskaia M.A. Shurin M. Shvedova A.A.: ‘Current understanding of interactions between nanoparticles and the immune system’, Toxicol. Appl. Pharmacol., 2016, 299, pp. 78 –89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. David C.A. Owen A. Liptrott N.J.: ‘Determining the relationship between nanoparticle characteristics and immunotoxicity: key challenges and approaches’, Nanomedicine, 2016, 11, (11), pp. 1447 –1464 [DOI] [PubMed] [Google Scholar]
- 109. Kirkham M. Parton R.G.: ‘Clathrin‐independent endocytosis: new insights into caveolae and non‐caveolar lipid raft carriers’, Biochim. Biophys. Acta Mol. Cell Res., 2005, 1745, (3), pp. 273 –286 [DOI] [PubMed] [Google Scholar]
- 110. McMahon H.T. Boucrot E.: ‘Molecular mechanism and physiological functions of clathrin‐mediated endocytosis’, Nat. Rev. Mol. Cell Biol., 2011, 12, (8), p. pp. 517 –533 [DOI] [PubMed] [Google Scholar]
- 111. Krpetić Ž. Anguissola S. Garry D. et al.: ‘Nanomaterials: impact on cells and cell organelles’, Nanomaterial, 2014, 811, pp. 135 –156 [DOI] [PubMed] [Google Scholar]
- 112. Huang D. Zhou H. Gao J.: ‘Nanoparticles modulate autophagic effect in a dispersity‐dependent manner’, Sci. Rep., 2015, 5, p. 1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhong Z. Umemura A. Sanchez‐Lopez E. et al.: ‘NF‐κB restricts inflammasome activation via elimination of damaged mitochondria’, Cell, 2016, 164, (5), pp. 896 –910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Assadian E. Zarei M.H. Gilani A.G. et al.: ‘Toxicity of copper oxide (Cuo) nanoparticles on human blood lymphocytes’, Biol. Trace Elem. Res., 2018, 184, (2), pp. 350 –357 [DOI] [PubMed] [Google Scholar]
- 115. Yaqub A. Anjum K.M. Munir A. et al.: ‘Evaluation of acute toxicity and effects of sub‐acute concentrations of copper oxide nanoparticles (CuO‐NPs) on hematology, selected enzymes and histopathology of liver and kidney in Mus musculus’, Indian J. Anim. Sci., 2017, 52, (1), pp. 92 –98 [Google Scholar]
- 116. Kaviani E.F. Naeemi A.S. Salehzadeh A.: ‘Influence of copper oxide nanoparticle on hematology and plasma biochemistry of Caspian Trout (Salmo trutta caspius), following acute and chronic exposure’, Pollution, 2019, 5, (1), pp. 225 –234 [Google Scholar]
- 117. Chevallet M. Aude‐Garcia C. Lelong C. et al.: ‘Effects of nanoparticles on murine macrophages’, J. Phys., Conf. Ser., 2011, 304, (1), p. 012034 [Google Scholar]
- 118. Gautam A. Ray A. Mukherjee S. et al.: ‘Immunotoxicity of copper nanoparticle and copper sulfate in a common Indian earthworm’, Ecotoxicol. Environ. Saf., 2018, 148, pp. 620 –631 [DOI] [PubMed] [Google Scholar]
- 119. Winge D.R. Mehra R.K.: ‘Host defenses against copper toxicity’, Int. Rev. Exp. Pathol., 1990, 31, pp. 47 –83 [DOI] [PubMed] [Google Scholar]
- 120. Al‐Taae E.H. Alsoufi L.A. Al‐Tayar N.H. et al.: ‘Hematological and biochemical evaluation after different orally doses of copper sulfate in rats’, Iraqi J. Veterinary Med., 2014, 38, (1), pp. 83 –91 [Google Scholar]
- 121. Hébert C.D. Elwell M.R. Travlos G.S. et al.: ‘Subchronic toxicity of cupric sulfate administered in drinking water and feed to rats and mice’, Fundam. Appl. Toxicol., 1993, 21, (4), pp. 461 –475 [DOI] [PubMed] [Google Scholar]
- 122. Arredondo M. Núñez M.T.: ‘Iron and copper metabolism’, Mol. Aspects Med., 2005, 26, (4–5), pp. 313 –327 [DOI] [PubMed] [Google Scholar]
- 123. Lischkova L. Pelclova D. Hlusicka J. et al.: ‘Detection and identification of engineered nanoparticles in exhaled breath condensate, blood serum, and urine of occupationally exposed subjects’, Monatsh. Chem., 2019, 150, pp. 511 –523 [Google Scholar]
- 124. Chen H. Yoshioka H. Kim G.S. et al.: ‘Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection’, Antioxidants Redox Signaling, 2011, 14, (8), pp. 1505 –1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chen M. He Y. Ye Q. et al.: ‘Solar thermal conversion and thermal energy storage of CuO/paraffin phase change composites’, Int. J. Heat Mass Transf., 2019, 130, pp. 1133 –1140 [Google Scholar]
- 126. Líbalová H. Costa P.M. Olsson M. et al.: ‘Toxicity of surface‐modified copper oxide nanoparticles in a mouse macrophage cell line: interplay of particles, surface coating and particle dissolution’, Chemosphere, 2018, 196, pp. 482 –493 [DOI] [PubMed] [Google Scholar]
- 127. Minigalieva I.A. Katsnelson B.A. Panov V.G. et al.: ‘In vivo toxicity of copper oxide, lead oxide and zinc oxide nanoparticles acting in different combinations and its attenuation with a complex of innocuous bio‐protectors’, Toxicology, 2017, 380, pp. 72 –93 [DOI] [PubMed] [Google Scholar]
- 128. Katsnelson B.A. Privalova L.I. Sutunkova M.P. et al.: ‘Some inferences from in vivo experiments with metal and metal oxide nanoparticles: the pulmonary phagocytosis response, subchronic systemic toxicity and genotoxicity, regulatory proposals, searching for bioprotectors (a self‐overview)’, Int. J. Nanomed., 2015, 10, pp. 3013 –3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Heinlaan M. Kahru A. Kasemets K. et al.: ‘Changes in the Daphnia magna midgut upon ingestion of copper oxide nanoparticles: a transmission electron microscopy study’, Water Res., 2011, 45, (1), pp. 179 –190 [DOI] [PubMed] [Google Scholar]
- 130. Heinlaan M. Ivask A. Blinova I. et al.: ‘Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus’, Chemosphere, 2008, 71, (7), pp. 1308 –1316 [DOI] [PubMed] [Google Scholar]
- 131. Bhattacharya P. Swarnakar S. Ghosh S. et al.: ‘Disinfection of drinking water via algae mediated green synthesized copper oxide nanoparticles and its toxicity evaluation’, J. Environ. Chem. Eng., 2019, 7, (1), p. 102867 [Google Scholar]
- 132. Adamcakova‐Dodd A. Monick M.M. Powers L.S. et al.: ‘Effects of prenatal inhalation exposure to copper nanoparticles on murine dams and offspring’, Part. Fibre Toxicol., 2015, 12, (1), p. 405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Han J.W. Gurunathan S. Jeong J.K. et al.: ‘Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line’, Nanoscale Res. Lett., 2014, 9, (1), pp. 459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Xia T. Kovochich M. Liong M. et al.: ‘Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties’, ACS Nano, 2008, 2, (10), pp. 2121 –2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jomova K. Baros S. Valko M.: ‘Redox active metal‐induced oxidative stress in biological systems’, Transit. Metal Chem., 2012, 37, (2), pp. 127 –134 [Google Scholar]
- 136. Regoli F. Giuliani M.E.: ‘Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms’, Mar. Environ. Res., 2014, 93, pp. 106 –117, doi: 10.1016/j.marenvres.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 137. Privalova L. Katsnelson B. Loginova N. et al.: ‘Subchronic toxicity of copper oxide nanoparticles and its attenuation with the help of a combination of bioprotectors’, Int. J. Mol. Sci., 2014, 15, (7), pp. 12379 –12406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Soenen S.J. Rivera‐Gil P. Montenegro J.M. et al.: ‘Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation’, Nano. Today, 2011, 6, (5), pp. 446 –465 [Google Scholar]
- 139. Akhtar S. Chandrasekhar B. Attur S. et al.: ‘On the nanotoxicity of PAMAM dendrimers: superfect® stimulates the EGFR–ERK1/2 signal transduction pathway via an oxidative stress‐dependent mechanism in HEK 293 cells’, Int. J. Pharm., 2013, 448, (1), pp. 239 –246 [DOI] [PubMed] [Google Scholar]
- 140. Yamakoshi Y. Umezawa N. Ryu A. et al.: ‘Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2‐• versus 1O2’, J. Am. Chem. Soc., 2003, 125, (42), pp. 12803 –12809 [DOI] [PubMed] [Google Scholar]


