Abstract
Green synthesis of silver nanoparticles (AgNPs) using plant extracts has been achieved by eco‐friendly reducing and capping agents. The present study was conducted to evaluate the larvicidal efficacies of AgNPs synthesized using aqueous leaf extracts of Excoecaria agallocha against dengue vector, Aedes aegypti. The 3rd and 4th instar larvae of A. aegypti were exposed to various concentrations of aqueous extracts of E. agallocha, synthesized AgNPs and also crude solvent extracts (methanol and chloroform) for 24 h. The formation of AgNPs using aqueous leaf extracts was observed after 30 min with a characteristic colour change. The results recorded from UV‐Vis spectrum, XRD, FTIR, EDX, SEM and HR‐TEM were used to characterize and confirm the biosynthesis of AgNPs. The highest larvicidal efficacy of synthesized AgNPs was observed against 3rd instar larvae at LC50 4.65 mg/L, LC90 14.17 mg/L and 4th instar larvae with a concentration of LC50 6.10 mg/L, LC90 15.64 mg/L. A significant larvicidal activity was also observed with crude methanolic extracts against 3rd instar larvae at a concentration LC50 41.74 mg/L, LC90 123.61 mg/L and 4th instar larvae at a concentration of LC50 52.06 mg/L, LC90 166.40 mg/L as compared to the chloroform extract.
Inspec keywords: silver, nanoparticles, nanofabrication, microorganisms, cellular biophysics, organic compounds, ultraviolet spectra, visible spectra, X‐ray diffraction, Fourier transform infrared spectra, X‐ray chemical analysis, scanning electron microscopy, transmission electron microscopy
Other keywords: larvicidal activity, green synthesised silver nanoparticles, Excoecaria agallocha L. leaf extract, Aedes aegypti, plant extracts, capping agents, larvicidal efficacies, aqueous leaf extracts, excoecaria agallocha, dengue vector, Aedes aegypti, aegypti, aqueous extraction, E. agallocha, crude solvent extracts, methanol, chloroform, characteristic colour change, ultraviolet‐visible spectrum, X‐ray diffraction, Fourier‐transform infrared spectroscopy, EDX, scanning electron microscopy, high‐resolution transmission electron microscopy, AgNP biosynthesis, larvicidal efficacy, third instar larvae, instar larvae, crude methanolic extracts, chloroform extraction, time 24 h
1 Introduction
Green biosynthesis of nanoparticles has emerged as an important branch of nanobiotechnology. The rich diversity among mangrove plants, phytoconstituents has the potentiality for the synthesis of nanoparticles and could be regarded as prominent biofactories for synthesis of nanoparticles. Vector‐borne disease control is very difficult nowadays due to the increased resistance of mosquitoes against synthetic insecticides and chemotherapeutic drugs [1, 2, 3, 4]. Mosquito borne diseases accounts for the death of more than a million people world‐wide every year. In many diseases of human such as dengue, malaria, chikungunya, filariasis, Japanese encephalitis and leishmaniasis, mosquitoes serves as a vector [5]. Mosquitoes can transmit more diseases than any other organisms and influences the health of millions of people throughout the world [6]. Vector‐borne mosquito diseases are prevalent in many countries across the globe, infecting over billions of people every year world wide and 40,000,000 among the Indian population. Dengue, yellow fever and chickungunya are the most prevalent endemic diseases spread by the vector, Aedes aegypti throughout Southeast Asia, the Pacific island, Africa, America including India. The A. aegypti mosquito is a leading cause of dengue in Southeast Asia [7]. This mosquito is a well‐known vector for arbovirus which causes dengue fever. Dengue fever has become a complicated health problem with the number of cases increasing day by day, mostly with different forms of the diseases, which includes dengue haemorrhagic fever and dengue shock syndrome or with unusual manifestations such as involvement of central nervous system. Pesticides manufactured using plants, provides an alternative to synthetic pesticides because of their huge advantages as they cause generally low environmental pollution and low toxicity to humans [8]. Before the discovery of synthetic organic insecticides many plant extracts have been used as potent botanical insecticides [9]. No effective drug or vaccine is available so far for minimising the wide spread of mosquito borne diseases. The interruption of mosquito borne diseases transmission by killing or preventing mosquitoes from attaining pesticide resistance is an environmental friendly way and important in terms of public health aspects [10, 11]. The killing of mosquitoes at larval stage is an emerging strategy to prevent mosquito borne diseases [12]. Plants are a rich source of phytoconstituents which act against various insect species in a target specific manner; they are rapidly biodegradable and have less chances of development of resistance in insects due to their structural complexity and novelty [13]. The active phytochemicals may act as insecticides, antifeedants, molting hormones, antimolting hormones, as well as attractants [14]. Several plant extracts and bioactive compounds from different plants have been examined for their potential larvicidal activities [15]. About 2000 species of terrestrial plants have been evaluated for their insecticidal activities [16, 17]. The rapid biologically synthesised small spherical shaped and highly crystalline silver‐protein lipid nanoparticles using Sterculia foetida seed extract having strong and promising larvicidal activity was reported against A. aegypti, Anopheles stephensi and Culex quinquefasciatus [18]. Biogenic green synthesis owes huge advantages than chemical and physical methods as it is cost effective, eco‐friendly, easy scale up for large‐scale synthesis and in this method nanoparticles are produced without high pressure, energy, temperature and toxic compounds [19]. The biogenic synthesis of nanoparticles with less ecological damage have been developed and targeted as a promising tool for replacement of synthetic chemical insecticides. Plant‐derived compounds have proved to be potential larvicides with more effectiveness due to the presence of saponins [20], steroids [21, 22], isoflavonoids [23], essential oils [24], alkaloids and tannins [25]. Plant metabolites and their synthetic derivatives may also provide another alternative source in the control of mosquito vectors [26]. Phytochemicals with mosquitocidal activity are now recognised as potent alternative to synthetic insecticides in control of mosquitoes as they possess excellent larvicidal, pupicidal and adulticidal properties. Most of the synthetic insecticides and naturally occurring chemicals have been shown to influence oviposition in mosquitoes [27, 28]. Silver nanoparticles (AgNPs) synthesised using leaf extract of Leucas aspera shows a significant larvicidal activity as compared with the traditional solvent extracts, against A. aegypti [29]. Biolarvicidal and pupicidal activity of AgNPs synthesised with Euphorbia hirta has been extensively reported against A. stephensi [30]. The solvent crude leaf extracts of Acalypha indica, Achyranthes aspera, Leucas aspera, Morinda tinctoria and Ocimum sanctum were reported against the early fourth instar larvae of A. aegypti and C. quinquefasciatus [31]. The plants such as Azadirachta indica [32], Aloe vera [33], Emblica officinalis [34], Cinnamomum camphora [35] and Brassica juncea [36] were used in the extracellular synthesis of silver and gold nanoparticles. Beauveria bassiana mycelial extract mediated AgNPs synthesised showed potential biolarvicidal activity for the control of dengue vector, A. aegypti [37]. The size controlled synthesis of AgNPs using different plant extracts provides advantages in the direction of biogenic process and also represents the pre‐eminence over the chemical synthesis in providing green, environmentally safer method of nanoparticle production. Corangi mangroves, Andhra Pradesh, India are rich sources of biodiversity and the mangrove plants serve as an excellent source of novel compounds. Synthesis and application of green metal nanomaterials is of great interest nowadays because of their wide applications and advantages. The green chemistry approach has many advantages towards the synthesis of AgNPs such as, ease in scaled up, economic, shelf life and viability, and so on. The larvicidal activity of AgNPs synthesised with Rhizophora mucronata leaf extracts showed significant activity against mosquitoes [38]. The present study was envisaged to investigate the potential larvicidal application of mangrove plant, Excoecaria agallocha L. (Euphorbiaceae) leaf extract mediated green AgNPs third and fourth instar larvae of A. aegypti.
2 Materials and methods
2.1 Chemicals and collection of plant material
Silver nitrate (AgNO3, 99.02% purity) was purchased from Hi‐Media Laboratories, Mumbai, India. Other chemicals used in the study were purchased from Merck and SRL, India. All the aqueous solutions were prepared using milliQ water. Fresh leaves of E. agallocha L. (Euphorbiaceae) were collected from Kandikuppa mangrove forest area, which is extended from Corangi mangrove wetland forest up to Konaseema deltaic zone through Godavari estuarine located at 16°35′12.89″ N and 82°16′17.03″ E of East Godavari district, Andhra Pradesh, India. The plant material was identified taxonomically and a voucher preserved.
2.2 Preparation of leaf aqueous extract
Fresh and healthy leaves of E. agallocha collected from the plants in the morning hours and washed with distilled water to ensure that the leaves are dust free and clean. The leaf aqueous extract was prepared by taking 10 g of finely cut and ground leaves. The grounded material was mixed with 200 ml of sterile milliQ water in a 500 ml Erlenmeyer flask and boiled for 5 min. After boiling the content was filtered using whatman No. 1 filter paper and the filtrate was stored in 4 °C for further biological acitivity.
2.3 Synthesis of AgNPs
AgNPs synthesis was carried out by taking 10 ml of fresh aqueous leaf broth and adding 190 ml of 1 × 10−3 M aqueous silver nitrate (AgNO3) solution at room temperature under bright light conditions. The change in colour and reduction of silver ions in the reaction solution was monitored at regular intervals by measuring the absorbance using Systronics, double beam AU‐2701 spectrophotometer from 300 to 800 nm at the resolution of 1 nm [39]. For further characterisation of the nanoparticles, the reaction mixture was centrifuged using REMI cooling centrifuge C‐24BL at 10,000 g for 30 min. The harvested nanoparticles were washed thoroughly twice with sterile distilled water and air dried at room temperature.
2.4 Characterisation of AgNPs
Dried powdered form of AgNPs was subjected to scanning electron microscope (SEM) and EDX analyses using SEM (SEM, S‐3500N, Hitachi Co., Tokyo, Japan) equipped with EDX detector (Thermo Electron Corporation Instrument, USA) to analyse morphology and elemental composition. The Fourier‐transform infra‐red (FTIR) analysis was performed to detect the presence of functional groups and interaction of the synthesised AgNPs with capping agent. Air dried AgNPs was mixed with KBr salt in 1:100 ratio and made into pellet and FTIR analysis was carried out using Thermo Scientific Nicolet iN‐10 spectrophotometer at the resolution of 4 cm−1 in the range of 4000–500 cm−1. The diffracted intensities of the air dried samples of AgNPs (150 mg) was recorded to determine the crystal structure and size of the nanoparticles using X‐ray diffraction spectroscopy (XRD, Rigaku, Ultima IV, X‐ray diffractometer system). The diffracted intensities were recorded at a voltage of 40 kV and current of 30 mA at scan range of 20–80° 2θ under CuKα radiation. The size, morphology and crystal structure of the AgNPs was determined using high resolution transmission electron microscopic (HR‐TEM), JEOL 3010 TEM‐HR instrument equipped with Gatan digital camera, operated at accelerated voltage of 200 kV [39].
2.5 Preparation of organic solvent extracts of E. agallocha leaf
The fresh leaves of E. agallocha was dried under shade, powdered with a mechanical grinder and stored in an airtight container. The powder obtained (100 g) was subjected to successive soxhlet extraction with the organic solvents (methanol and chloroform) in 1:3 ratio (w/v). The crude extracts were evaporated to dryness using rotary evaporator.
2.6 Gas chromatography–mass spectrometry (GC‐MS) analysis
The GC‐MS analysis of the dried leaf powder solvent extracts of E. agallocha was performed using a Perkin Elmer GC (Model Perkin Elmer Clarus 680) and MS (Model Perkin Elmer Clarus 600) equipped with an Elite‐5 MS silica capillary column (30.0 m × 0.25 mm ID, 250 μm df). The oven temperature was programmed at 60 °C for 2 min then increased to 300 °C for 6 min at the rate of 10 °C/min. Helium was used as carrier gas at flow rate of 1.0 ml/min. The injector temperature was 250 °C, injection size 1.0 μl neat, with split ratio 10:1. Mass detector turbo mass gold‐Perkin Elmer was used as detector. The phytochemicals present in the extract was identified using the NIST‐2008 library database.
2.7 Mosquito rearing
A. aegypti larvae were collected from drainage of local residential area of Guntur town, Andhra Pradesh, India. The larvae were kept in plastic trays containing tap water and were maintained in the laboratory. Larvae were fed with yeast and dog biscuits. All the experiments were carried out at 27 ± 2 °C and 75–85% relative humidity under 14:10 light and dark cycles.
2.8 Larvicidal bioassay
Larvicidal bioassays were performed using early third and fourth instar larvae of A. aegypti according to WHO protocol [6]. Twenty five mosquito larvae were treated with different concentrations of AgNPs ranging from 2 to 14 mg/l. Similarly, another set of third and fourth instar larvae were treated with 1 ml plant extracts (aqueous, methanol and chloroform extract) at different concentrations of 20–160 mg/l. All the experiments were conducted in triplicate and control (silver nitrate and Millipore water) was also set. After exposure for 24 h, the number of dead larvae were counted and percentage mortality (LC50 and LC90) was calculated using probit analysis, to determine the acute toxicity of the extracts and synthesised AgNPS on third and fourth instar larvae of A. aegypti mosquitoes [29].
2.9 Dose‐response bioassay
Crude extracts of leaves of E. agallocha and synthesised AgNPs were evaluated by dose response bioassay for larvicidal activity against A. aegypti. Different concentrations ranging from 20 to 160 mg/l (for solvent crude extract) and 2.0 to 14 mg/l (for synthesised AgNPs) were prepared for larvicidal activity. The number of dead larvae was counted after 24 h of exposure and the percentage of mortality was recorded from the average of triplicate values.
2.10 Data analysis
The average larval mortality data were subjected to probit analysis for calculating LC50 and LC90 at 95% fiducial limits of upper confidence limits (UCL) and lower confidence limits (LCL). The chi‐square values were calculated by using Finney's method [40].
3 Results and discussion
The larvicidal activity of aqueous extracts, synthesised AgNPs and solvent crude extracts of E. agallocha against early third and fourth instar larvae of A. aegypti were investigated and tabulated (Table 1). The highest mortality was found in case of synthesised AgNPs against third (LC50 = 4.65 mg/l) and fourth instar larvae (LC50 = 6.10 mg/l) followed by methanol extract with LC50 value of 41.74 and 52.06 mg/l against third instar larvae and fourth instar larvae, respectively. The differences in the lethal concentrations might have resulted due to the difference in phytoconstituents and capping materials on the AgNPs. Similar observations were reported for AgNPs synthesised using leaf extracts of Avicennia marina having larvicidal activity against the larvae of A. aegypti and A. stephensi and the LC50 values recorded as 4.374 and 7.406 mg/l, respectively [41].
Table 1.
Larvicidal activity of AgNPs synthesised E. agallocha crude leaf extract and solvent extracts against third and fourth instar larvae of A. aegypti
| Extracts name | Number | LC50, mg L−1 | LC90, mg L−1 | LCL | UCL | X 2 | |
|---|---|---|---|---|---|---|---|
| Third instar larvae | |||||||
| aqueous extract + AgNO3 | AgNPs | 25 | 4.65 | 14.17 | 3.67 | 18.38 | 3.86 |
| solvent extracts of E. agallocha |
methanol chloroform |
25 25 |
41.74 58.79 |
123.61 129.92 |
32.70 45.24 |
153.26 167.72 |
7.24 4.40 |
| aqueous extract | extract | 25 | 69.33 | 180.51 | 52.37 | 254.78 | 2.78 |
| Fourth instar larvae | |||||||
| aqueous extract + AgNO3 | AgNPs | 25 | 6.10 | 15.64 | 5.11 | 19.82 | 5.11 |
| solvent extracts of E. agallocha |
methanol chloroform |
25 25 |
52.06 73.38 |
166.40 199.43 |
41.53 55.15 |
214.57 190.42 |
5.02 3.47 |
| aqueous extract | extract | 25 | 81.49 | 272.13 | 67.07 | 384.90 | 13.00 |
AgNPs were synthesised using 10 ml of fresh aqueous leaf broth solution to the 190 ml of 1 × 10−3 M aqueous AgNO3 solution at room temperature under bright light conditions. After immediate addition, the appearance of brown colour was a characteristic feature for the formation of AgNPs in the solution. The colour intensity of the fresh leaf extracts of E. agallocha before and after 30 min of reaction is shown in Fig. 1. Reduction of silver ions in the aqueous solution of silver complex during the reaction with phytochemical constituents present in the leaf extract of E. agallocha was confirmed by the ultraviolet–visible (UV–Vis) spectroscopy. The spectral scan recorded after 30 min of reaction showed an intense peak at 440 nm (Fig. 2), which was the characteristic feature of AgNPs and the peak arise due to excitation of surface plasmon resonance in AgNPs. On further characterisation using SEM, the nanoparticles were found to be polydispersed, spherical in shape with a size ranging from 18 to 50 nm (Fig. 3) and a characteristic peak at 3 kev in EDX spectrum was obtained, where it indicates the presence of Ag (Fig. 4).
Fig. 1.

Photographs showing aqueous leaf extract(A) and AgNPs(B) synthesised from E. agallocha
Fig. 2.
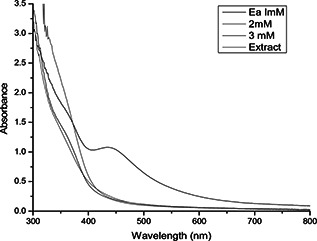
UV–Vis spectra of AgNPs with E. agallocha leaf extract at different concentrations of silver nitrate solution and aqueous leaf extract
Fig. 3.

SEM micrographic image of AgNPs synthesised using E. agallocha leaf extract
Fig. 4.
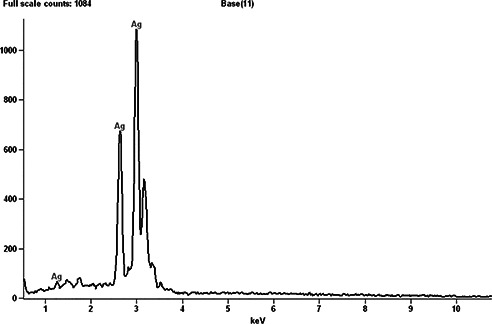
EDX spectrum of AgNPs synthesised from E. agallocha, taken in point and shoot mode showing only silver peaks
FTIR analysis was carried out to identify the prominent phytochemical constituents responsible for the reduction of Ag+ ions and capping of the nanoparticles along with the phytochemicals present in the leaf extracts of E. agallocha. FTIR analysis of the purified nanoparticles showed the presence of prominent peaks at 2357.0, 1564.6, 1303.1, 1056.8, 1026.7, 786.2, 723.0, 674.4, 590.2 and 502.7 cm−1 (Fig. 5). The sharp absorption peak at 2357.0 cm−1 corresponding to NH3 stretching of any ammonium ions, the sharp band at 1564.6 cm−1 corresponding to C=C stretching and the weaker band at 1303.1 cm−1 corresponding to N–O stretching of nitro compounds. The peaks at 1056.8 and 1026.7 cm−1 correspond to C–O stretching vibration of aliphatic amines. The peaks at 788.2 and 674.4 cm−1 also represent the presence of alkyl halides, corresponding to either C–Cl stretch or C–Br stretch. The XRD pattern of AgNPs produced by leaf extracts of E. agallocha is shown in Fig. 6. The XRD pattern of AgNPs were reflected in 2θ on 38.11°, 44.28°, 64.5° and 77.12° corresponding to 111, 200, 220 and 311 planes of silver, respectively, which respects the JCPDS 652871 and confirmed the crystalline nature of the AgNPs. The size and crystal structure of the AgNPs were further determined using HR‐TEM (Fig. 7). The HR‐TEM images depicted polydispersed, spherical AgNPs ranging in size from 18 to 50 nm which was in in good agreement with that of the results obtained by SEM and XRD analysis.
Fig. 5.
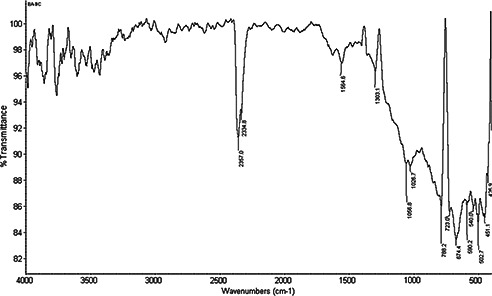
FTIR spectrum of AgNPs synthesised using E. agallocha leaf extract
Fig. 6.
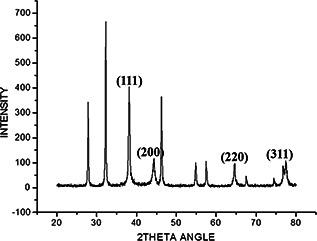
XRD pattern of AgNPs synthesised using E. agallocha leaf extracts
Fig. 7.
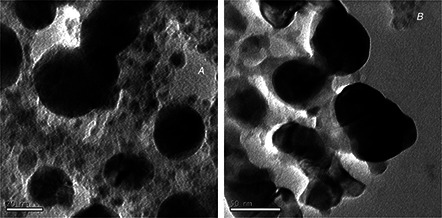
HR‐TEM micrographs of AgNPs taken at different magnifications ranging from (A) 20nm to (B) 50 nm. From the above images it is concluded that the AgNPs are polydispersed, spherical in shape and ranges in size 18–50 nm
GCMS analysis of methanol and chloroform extracts of leaves of E. agallocha showed the presence of different phytochemical constituents indicated by the characteristic peaks (Fig. 8). The mass spectra of the phytoconstituents were compared with NIST library search. The major phytochemical compounds identified in methanol extracts of E. agallocha were (Table 2) n‐hexadecanolic acid; myo‐inositol, 4‐c‐methyl; phytol; methyl 8,11,14‐heptadecatrienoate; squalene; 3,3′ dimenthol; gamma tocopherol; alpha tocopherol; gamma sitosterol; hop‐22(29)‐en‐3.beta.‐ol; betulin; β‐amyrin; 2R‐ace0toxymethyl‐1,3,3‐trimethyl‐4t‐(3‐methyl‐2‐buten‐1‐yl)‐1t‐cyclohexanol; lupeol; 4,4,6A,6B,8A,11,11,14B‐octamethyl,1,4,4A,5,6A,6B,7,8,8A,9,10,11,12,12A,14,14A, 14B‐octadecahydro‐2; 9,19‐cyclolanost‐25‐EN‐3‐OL, 24‐methyl‐, (3. Beta,24S). On the basis of the above investigations, the leaf extract of E. agallocha is regarded as potential larvicidal agents based on the variety of phytochemicals such as diterpenes, triterpenes, steroids and palmitic acid. These phytoconstituents might contribute to the reduction of AgNO3 to form functionalised AgNPs. In 2014, Manjari et al. [42] reported that the phytoconstituents present in leaf extracts of Clausena dentata possess an excellent larvicidal activity against A. aegypti. Various parts of E. agallocha extracts have been proved as a potential source of a mosquito larvicidal agent [43]. It has been reported that the synergistic activity of leaf extracts of A. marina and Avicennia officinalis showed significant larvicidal activity with a concentration of 34.622, 87.681 and 206.047 μg/ml (LC50) against A. aegypti, C. quinquefasciatus and A. stephensi, respectively [44]. The present study may lead to the development of natural larvicidal agent as an alternative to the synthetic insecticides.
Fig. 8.
Phytochemical compounds identified in methanol extracts of E. agallocha, Inst() acquistion parameters‐oven: initial temperature 60 °C for 2 min, ramp 10 °C/min–300 °C, holsd 6 min, InjAauto = 250 °C, volume = 0 μL, split = 10:1, carrier gas = He, solvent delay = 2.00 min, transfer temperature = 240 °C, source temperature = 240 °C, scan: 50–600 Da, column 30.0 m × 250 μm
Table 2.
Chemical composition of methanol leaf extracts of E. agallocha
| S.No | RT | Area | Area, % | Compound name | Activity |
|---|---|---|---|---|---|
| 1 | 15.549 | 58,725,120.0 | 21.400 | myo‐Inositol,4‐c‐methyl‐ | no known activity |
| 2 | 17.815 | 7,829,957.0 | 2.853 | N‐hexadecanoic acid | insecticidal property |
| 3 | 19.150 | 3,293,158.5 | 1.200 | Phytol | antimicrobial activity |
| 4 | 19.465 | 10,038,550.0 | 3.658 | methyl 8,11,14‐heptadecatrienoate | no known activity |
| 5 | 24.677 | 8,243,940.0 | 3.004 | 2,6,10,14,18,22‐tetracosahexane,2,6,10,15,19,23‐hexa,ethyl‐, (all‐e)‐ | pesticide, antioxidant activity |
| 6 | 25.053 | 3,742,039.2 | 1.364 | 3,3′‐dimenthol | antibacterial activity |
| 7 | 26.308 | 4,112,334.2 | 1.499 | gamma‐tocopherol | antioxidant, antimicrobial |
| 8 | 26.888 | 47,870,352.0 | 17.444 | alpha‐tocopherol | antioxidant, antimicrobial |
| 9 | 28.629 | 14,447,939.0 | 5.265 | gamma‐sitosterol | antidiabetic, antimicrobial |
| 10 | 28.749 | 18,494,970.0 | 6.740 | betulin | antimalarial, anticancer, antibacterial activity |
| 11 | 28.909 | 12,068,890.0 | 4.398 | betulin | antimalarial, anticancer, antibacterial activity |
| 12 | 29.104 | 12,519,889.0 | 4.562 | 4,4,6B,8A,11,11,14B‐octamethyl‐1,4,4A,5,6,6A,6B,7,8,8A,9,10,11,12, 12A,14,14A,14B‐Octadecahhydro‐2 | antimalarial, antibacterial activity |
| 13 | 29.284 | 3,208,045.2 | 1.169 | 2R‐acetoxymethyl‐1,3,3‐trimethyl‐4t‐(3‐methyl‐2‐buten‐1‐yl)‐1t‐cyclohexanol | anti‐inflammatory activity |
| 14 | 29.389 | 25,794,414.0 | 9.400 | lupeol | antiprotozoal activity |
| 15 | 29.489 | 5,251,148.5 | 1.914 | 2R‐acetoxymethyl‐1,3,3‐trimethyl‐4t‐(3‐methyl‐2‐buten‐1‐yl)‐1t‐cyclohexanol | anti‐inflammatory activity |
| 16 | 29.614 | 25,281,340.0 | 9.213 | 2R‐acetoxymethyl‐1,3,3‐trimethyl‐4t‐(3‐methyl‐2‐buten‐1‐yl)‐1t‐cyclohexanol | anti‐inflammatory activity |
| 17 | 30.040 | 3,493,336.5 | 1.273 | 4,4,6A,6B,8A,11,11,14B‐octamethyl‐1,4,4A,5,6,6A,6B,7,8,8A,9,10,11,12, 12A,14,14A,14B‐Octadecahhydro‐2 | antimalarial, antibacterial activity |
| 18 | 30.290 | 10,006,224.0 | 3.646 | 9,19‐cyclolanost‐25‐en‐3‐ol,24‐methyl‐,(3.beta.,24s)‐ | no known activity |
4 Conclusion
In conclusion, AgNPs synthesised using leaf extracts of E. agallocha is an eco‐friendly and non‐toxic approach in developing larvicidal agents against A. aegypti. GC‐MS analysis of crude extracts showed the presence of terpenes and steroids as major constituents which will serve as a potential environmentally benign larvicides for mosquito control.
5 Acknowledgments
We thank the Centre for Nanoscience and Technology and the Central Instrumentation Facility (CIF), the Pondicherry University for providing facilities of UV‐VIS spectroscopy, FTIR, XRD, EDX and SEM. We thank the Sophisticated analytical instrument facility (SAIF), the IIT Madras for providing facilities to carry out HR‐TEM analysis and also thank SIF,VIT, Vellore, for providing the facility to carry out GC‐MS analysis work.
6 References
- 1. Hargreaves K. Koekemoer L.L. Brooke B.D. et al.: ‘ Anopheles funestus resistant to pyrethroid insecticides in South Africa’, Med. Vet. Entomol., 2000, 14, pp. 181 –189 (doi: 10.1046/j.1365-2915.2000.00234.x) [DOI] [PubMed] [Google Scholar]
- 2. Ranson H. Rossiter L. Ortelli F. et al.: ‘Identification of a novel class of insect glutathione S‐transferases involved in resistance to DDT in the malaria vector, Anopheles gambiae ’, Biochem. J., 2001, 359, pp. 295 –304 (doi: 10.1042/bj3590295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gericke A. Govere J.M. Durrheim D.N.: ‘Insecticide susceptibility in the South African malaria mosquito Anopheles arabiensis (Diptera: Culicidae)’, S. Afr. J. Sci., 2002, 98, pp. 205 –208 [Google Scholar]
- 4. Shelton A.M. Wang P. Zhao J.Z. et al.: ‘Resistance to insect pathogens and strategies to manage resistance: an update’, in Laceyand L.A. Kaya H.K. (Eds.): ‘Field manual of techniques in invertebrate pathology’ (Springer, New York, 2007) [Google Scholar]
- 5. World Health Organization : ‘A global brief on vector‐borne diseases’ WHO/DCO/WHD/2014.1 [Google Scholar]
- 6. WHO‐World Health Organization : ‘Report of the WHO informal consultation on the evaluation on the testing of insecticides’. CTD/ WHO PES/IC/ 96.1, (WHO, Geneva, 1996), p. 69 [Google Scholar]
- 7. Gubler D.J.: ‘Dengue and dengue hemorrhagic fever’, Clin. Microbiol. Rev., 1998, 11, pp. 480 –496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu S.Q. Shi J.J. Cao H. et al.: ‘Survey of pesticidal component in plant’. Entomology in China in 21st Century, Proc. of Conf. of Chinese Entomological Society ed. (Science & Technique Press, 2000), pp. 1098 –1104
- 9. Indian Council of Medical Research (ICMR) Bulletin : ‘Prospects of using herbal products in the control of mosquito vectors’, ICMR Bull., 2003, 33, (1), pp. 1 –10 [Google Scholar]
- 10. Mathew N. Anitha M.G. Bala T.S. et al.: ‘Larvicidal activity of Saraca indica, Nyctanthes arbortristis, and Clitoria ternatea extracts against three mosquito vector species’, Parasitol. Res., 2009, 104, pp. 1017 –1025 (doi: 10.1007/s00436-008-1284-x) [DOI] [PubMed] [Google Scholar]
- 11. Su T. Mulla M.S.: ‘Ovicidal activity of neem products (Azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae)’, J. Am. Mosq. Control Assoc., 1998, 14, pp. 204 –209 [PubMed] [Google Scholar]
- 12. Liu H. Xu Q. Zhang L. et al.: ‘Chlorpyrifos resistance in mosquito Culex quinquefasciatus ’, J. Med. Entomol., 2005, 42, (5), pp. 815 –820 (doi: https://doi.org/10.1603/0022‐2585(2005)042[0815:CRIMCQ]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 13. Sukumar K. Perich M.J. Boobar L.R.: ‘Botanical derivatives in mosquito control: a review’, J. Am. Mosq. Control Assoc., 1991, 72, pp. 210 –237 [PubMed] [Google Scholar]
- 14. Ignacimuthu S.: ‘The root of botanicals in combating mosquitoes’. Proc. Symp. on Recent Trends in Combating Mosquitoes, Loyola College, Chennai, India, 2000, vol. 19, p. 19 [Google Scholar]
- 15. Markouk M. Bekkouche K. Larhsini M. et al.: ‘Evaluation of some Moroccan medicinal plant extracts for larvicidal activity’, J. Ethnopharmacol., 2000, 73, pp. 93 –297 (doi: 10.1016/S0378-8741(00)00257-9) [DOI] [PubMed] [Google Scholar]
- 16. Feinstein L.: ‘ j‘Insecticides from plants’ In: Insects’ (The Year Book of Agriculture, USA, Washington, 1952), pp. 222 –229 [Google Scholar]
- 17. Veerakumar K. Govindarajan M. Rajeswary M. et al.: ‘Low‐cost and eco‐friendly green synthesis of silver nanoparticles using Feronia elephantum (Rutaceae) against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae)’, Parasitol. Res., 2014, 113, pp. 1775 –1785 (doi: 10.1007/s00436-014-3823-y) [DOI] [PubMed] [Google Scholar]
- 18. Rajasekharreddy P. Usha Rani P.: ‘Biofabrication of silver nanoparticles using Sterculia foetida L. seed extract and their toxic potential against mosquito vectors and HeLa cancer cells’, Mater. Sci. Eng. C, 2014, 39, pp. 203 –212 (doi: 10.1016/j.msec.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 19. Lok C. Ho C. Chen R. et al.: ‘Silver nanoparticles: partial oxidation and antibacterial activities’, J. Biol. Inorg. Chem., 2007, 12, pp. 527 –534 (doi: 10.1007/s00775-007-0208-z) [DOI] [PubMed] [Google Scholar]
- 20. Wiseman Z. Chapagain B.P.: ‘Larvicidal effects of aqueous extracts of Balanites aegyptiaca (desert date) against the larvae of Culex pipiens mosquitoes’, Afr. J. Biotechnol., 2005, 4, (11), pp. 1351 –1354 [Google Scholar]
- 21. Chowdhury N. Ghosh A. Chandra G.: ‘Mosquito larvicidal activities of Solanum villosum berry extract against the dengue vector Stegomyia aegypti ’, BMC Complement Altern. Med., 2008, 8, p. 10 (doi: 10.1186/1472-6882-8-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghosh A. Chowdhury N. Chandra G.: ‘Laboratory evaluation of a phytosteroid compound of mature leaves of day jasmine (Solanaceae: Solanales) against larvae of Culex quinquefasciatus (Diptera: Culicidae) and non‐target organisms’, Parasitol. Res., 2008, 103, pp. 221 –277 (doi: 10.1007/s00436-008-0963-y) [DOI] [PubMed] [Google Scholar]
- 23. Joseph C.C. Ndoile M.M. Malima R.C. et al.: ‘Larvicidal and mosquitocidal extracts, a coumarin, isoflavonoids and pterocarpans from Neorautanenia mitis ’, Trans. R. Soc. Trop. Med. Hyg., 2004, 98, (8), pp. 451 –455 (doi: 10.1016/j.trstmh.2003.10.008) [DOI] [PubMed] [Google Scholar]
- 24. Cavalcanti E.S.B. Morais S.M. Lima M.A.A. et al.: ‘Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L’, Mem. Inst. Oswaldo Cruz, 2004, 99, pp. 541 –544 (doi: 10.1590/S0074-02762004000500015) [DOI] [PubMed] [Google Scholar]
- 25. Khanna V.G. Kannabiran K.: ‘Larvicidal effect of Hemidesmus indicus, Gymnema sylvestre, and Eclipta prostrata against Culex quinquefasciatus mosquito larvae’, Afr. J. Biotechnol., 2007, 3, pp. 307 –311 [Google Scholar]
- 26. Yang Y.C. Le E.H. Lee H.S. et al.: ‘Repellency of aromatic medicinal plant extracts to Aedes aegypti ’, J. Am. Mosq. Control Assoc., 2004, 20, (2), pp. 146 –149 [PubMed] [Google Scholar]
- 27. Olagbemiro T.O. Birkett M.A. Mordue A.J. et al.: ‘Production of (5R, 6S)‐6‐acetoxy‐5‐hexadecanolide, the mosquito oviposition pheromone, from the seed oil of the summer cypress plant, Kochia scoparia (Chenopodiaceae)’, J. Agric. Food Chem., 1999, 47, pp. 3411 –3415 (doi: 10.1021/jf990294b) [DOI] [PubMed] [Google Scholar]
- 28. Veerakumar K. Govindarajan M.: ‘Green synthesis of silver nanoparticles using Sida acuta (Malvaceae) leaf extract against Culex quinquefasciatus, Anopheles stephensi and Aedes aegypti (Diptera: Culicidae)’, Parasitol. Res., 2013, 112, pp. 4073 –4085 (doi: 10.1007/s00436-013-3598-6) [DOI] [PubMed] [Google Scholar]
- 29. Suganya G. Karthi S. Shivakumar M.S.: ‘Larvicidal potential of silver nanoparticles synthesized from Leucas aspera leaf extracts against dengue vector Aedes aegypti ’, Parasitol. Res., 2014, 113, pp. 875 –880 (doi: 10.1007/s00436-013-3718-3) [DOI] [PubMed] [Google Scholar]
- 30. Priyadarshini K. Murugan K. Panneerselvam C. et al.: ‘Biolarvicidal and pupicidal potential of silver nanoparticles synthesized using Euphorbia hirta against Anopheles stephensi Liston (Diptera: Culicidae)’, Parasitol. Res., 2012, 111, pp. 997 –1006 (doi: 10.1007/s00436-012-2924-8) [DOI] [PubMed] [Google Scholar]
- 31. Bagavan A. Rahuman A. Kamaraj C. et al.: ‘Larvicidal activity of saponin from Achyranthes aspera against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae)’, Parasitol. Res., 2008, 103, (1), pp. 223 –229 (doi: 10.1007/s00436-008-0962-z) [DOI] [PubMed] [Google Scholar]
- 32. Shankar S.S. Rai A. Ahmad A. et al.: ‘Rapid synthesis of Au, Ag and bimetallic Au core–Ag shell nanoparticles using neem (Azadirachta indica) leaf broth’, J. Colloid Interf. Sci., 2004, 275, pp. 496 –502 (doi: 10.1016/j.jcis.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 33. Chandran S.P. Chaudhary M. Pasricha R. et al.: ‘Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract’, Biotechnol. Prog., 2006, 22, pp. 577 –583 (doi: 10.1021/bp0501423) [DOI] [PubMed] [Google Scholar]
- 34. Ankamwar B. Damle C. Ahmad A. et al.: ‘Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution’, J. Nanosci. Nanotechnol., 2005, 5, (10), pp. 1665 –1671 (doi: 10.1166/jnn.2005.184) [DOI] [PubMed] [Google Scholar]
- 35. Huang J. Li Q. Sun D. et al.: ‘Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf’, Nanotechnology, 2007, 18, pp. 105104 –105114 (doi: 10.1088/0957-4484/18/10/105104) [DOI] [Google Scholar]
- 36. Haverkamp R.G. Marshall A.T. Agterveld D.V.: ‘Pick your carats: nanoparticles of gold–silver–copper alloy produced in vivo’, J. Nanoparticle Res., 2007, 9, pp. 697 –700 (doi: 10.1007/s11051-006-9198-y) [DOI] [Google Scholar]
- 37. Najitha B.A. Balasubramanian C.: ‘Myco‐synthesis of silver nanoparticles using Beauveria bassiana against dengue vector, Aedes aegypti (Diptera: Culicidae)’, Parasitol. Res., 2014, 113, pp. 2869 –2877 (doi: 10.1007/s00436-014-3948-z) [DOI] [PubMed] [Google Scholar]
- 38. Gnanadesigan M. Anand M. Ravikumar S. et al.: ‘Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property’, Asian Pac. J. Trop. Med., 2011, 4, pp. 799 –803 (doi: 10.1016/S1995-7645(11)60197-1) [DOI] [PubMed] [Google Scholar]
- 39. Siddhardha B. Jobina R. Bibhuti R. et al.: ‘Green rapid biogenic synthesis of bioactive silver nanoparticles (AgNPs) using Pseudomonas aeruginosa ’, IET Nanobiotechnol., 2014, 8, (4), pp. 267 –274 (doi: 10.1049/iet-nbt.2013.0059) [DOI] [PubMed] [Google Scholar]
- 40. Finney D.J.: ‘Probit analysis’ (Cambridge University Press, Cambridge, 1971, 3rd edn.) [Google Scholar]
- 41. Balakrishnan S. Srinivasan M. Mohanraj J.: ‘Biosynthesis of silver nanoparticles from mangrove plant (Avicennia marina) extract and their potential mosquito larvicidal property’, J. Parasit. Dis., 2014, DOI: 10.1007/s12639‐014‐0621‐5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manjari M.S. Karthi S. Ramkumar G. et al.: ‘Chemical composition and larvicidal activity of plant extracts from Clausena dentata (Willd) (Rutaceae) against dengue, malaria, and filariasis vectors’, Parasitol. Res., 2014, 113, (7), pp. 2475 –2481 (doi: 10.1007/s00436-014-3896-7) [DOI] [PubMed] [Google Scholar]
- 43. Thangam T.S. Kathiresan K.: ‘Marine plants for mosquito control’, in Wildey K.B. (Ed.): ‘Proceedings of the Second International Conference on Urban Pests’, 1996. [Google Scholar]
- 44. Renugadevi G. Ramanathan T. Shanmuga P.R. et al.: ‘Studies on combined effect of mangrove plants against three dangerous mosquitoes’, Int. J. Pharm. Biol. Arch., 2012, 3, (2), pp. 357 –362 [Google Scholar]


