Abstract
A simple and eco‐friendly method for efficient synthesis of stable colloidal silver nanoparticles (AgNPs) using Mentha pulegium extracts is described. A series of reactions was conducted using different types and concentrations of plant extract as well as metal ions to optimize the reaction conditions. AgNPs were characterized by using UV–vis spectroscopy, transmission electron microscopy, atomic force microscopy, dynamic light scattering, zetasizer, energy‐dispersive X‐ray spectroscopy (EDAX) and Fourier transform infrared spectroscopy (FTIR). At the optimized conditions, plate shaped AgNPs with zeta potential value of ‐15.7 and plasmon absorption maximum at 450 nm were obtained using high concentration of aqueous extract. Efficient adsorption of organic compounds on the nanoparticles was confirmed by FTIR and EDAX. The biogenic AgNPs displayed promising antibacterial activity on Escherichia coli, Staphylococcus aureus, and Streptococcus pyogenes. The highest antibacterial activity of 25 µg mL‐1 was obtained for all the strains using aqueous extract synthesized AgNPs. The aqueous extract synthesised AgNPs also showed considerable antifungal activity against fluconazole resistant Candida albicans. The cytotoxicity assay revealed considerable anticancer activity of AgNPs on HeLa and MCF‐7 cancer cells. Overall results indicated high potential of M. pulegium extract to synthesis high quality AgNPs for biomedical applications.
Inspec keywords: silver, nanoparticles, nanofabrication, botany, antibacterial activity, biomedical materials, nanomedicine, ultraviolet spectra, visible spectra, transmission electron microscopy, atomic force microscopy, X‐ray chemical analysis, Fourier transform infrared spectra, electrokinetic effects, microorganisms, cellular biophysics, cancer
Other keywords: antibacterial activity, antifungal activity, anticancer activity, stable colloidal silver nanoparticle, Mentha pulegium, plant extract, UV‐visible spectroscopy, transmission electron microscopy, atomic force microscopy, DLS, zetasizer, energy‐dispersive X‐ray spectroscopy, Fourier transform infrared spectroscopy, methanolic extract, aqueous extract, plate‐shaped silver nanoparticle, zeta potential, plasmon absorption maximum, organic compounds adsorption, biogenic silver nanoparticle, Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes, fluconazole‐resistant Candida albicans, MTT assay, HeLa cancer cell, MCF‐7 cancer cell, Ag
1 Introduction
Noble metal nanoparticles display size‐dependent chemical and physical properties, including mechanical and biological characteristics, catalytic activity, thermal and electrical conductivity and remarkable optical properties. These interesting properties have been considered for widespread applications in catalysis, electronics, optics, environment and biomedicine [1]. Metal nanostructures with highly controlled size, shape and optical properties are in use for biotechnological applications especially for diagnosis and biological imaging [2]. Among them, AgNPs received the highest attention for commercialisation [3] as more than 50% of the total nanomaterial‐based products available in the market are based on the AgNPs [4]. The unique antimicrobial properties of AgNPs have attracted great attention for developing biomedical devices such as wound dressings, surgical instruments and bone substitute materials [5].
Prevalence of multiple antibiotic resistant microorganisms together with subsequent increase in health care costs has stimulated the researchers to develop novel and cost‐effective approaches for effective treatment of microbial infections. Nanosilver‐based antiseptics have been considered as promising alternatives to solve this problem [6]. However, widespread application of the nanomaterials has also increased toxicity issues, raising concerns to human health and environment [7]. In this regard, selection of appropriate and environmentally benign functional agents is essential for increasing their stability in different media, prevention of undesired effects and reduction of their risks for environmental contamination [8]. Development of the improved and eco‐friendly methods is necessary for the design of greener nanomaterials with high reproducibility and purity [9]. To achieve this goal, the application of hazardous materials and production of toxic by‐products should be avoided [10]. Selection of environmentally benign solvents and non‐toxic reducing agents together with appropriate capping agents for stabilisation of the nanoparticles would be considered especially for the green production of metal nanoparticles [11]. Biogenic methods based on the extracted organic compounds from organisms have been considered recently as one of the best green chemistry approaches for production of metal nanoparticles [9]. This approach reduces the common concerns raised from physical and chemical methods and can be used for efficient large scale production of high‐quality nanoparticles. The various metabolites in plant tissues that can act as reducing and capping agents in the synthesis of metal nanoparticles have been considered as promising alternatives to hazardous chemicals for synthesis of these nanoparticles [12]. In this regard, various plant extracts have been successfully used for biogenic synthesis of AgNPs [9].
Mentha pulegium L., commonly known as pennyroyal, is a flowering herb belonging to the family Lamiaceae and native to Europe, Africa, Asia Minor and the Near East. The genus Mentha comprises more than 25 species containing significant amount of essential oils that are commonly used in perfume and food industries [13]. The flowering aerial parts of M. pulegium have been traditionally used for treating colds, sinusitis, cholera, food poisoning, bronchitis and tuberculosis due to their carminative, antitussive and antiseptic effects [14]. The antimicrobial and antioxidant activity of essential oils and extracts of Mentha species have been well documented by several authors [14, 15]. Hence, the use of organic compounds of Mentha for synthesis of AgNPs would be expected to render the novel biological properties to the nanoparticles.
At the first time, the ability of organic compounds of M. pulegium as reducing and capping agents for synthesis of AgNPs was comprehensively investigated in the present work. Following the optimisation of synthesis conditions and characterisation of produced AgNPs, the antibacterial, antifungal and anticancer activities of the obtained colloidal nanoparticles were comprehensively investigated.
2 Materials and methods
2.1 Materials and reagents
All the materials and media were purchased from Sigma (Germany) unless otherwise stated. Organic solvents for the plant extraction were obtained from Merck (Darmstadt, Germany). All of the aqueous solutions were prepared by sterilised double distilled water.
2.2 Preparation of the plant extracts and synthesis of AgNPs
Different solvents including double distilled water, ethanol solution (70% v/v) and absolute methanol were used separately to fractionate the organic compounds of M. pulegium. Briefly, 2 g of dried and powdered leaves of M. pulegium were suspended in 50 ml of the solvent following by sonication for 1 h. The suspension was further shacked overnight and filtered through Whatman no.1 filter paper.
AgNPs were synthesised by reduction of silver nitrate solutions in the presence of M. pulegium extract. Different parameters including type and concentration of the plant extract and concentration of silver nitrate (Table 1) were investigated to optimise the reaction conditions for synthesis of stable and monodisperse colloidal nanoparticles. All the reactions were prepared in the final volume of 20 ml by mixing suitable volumes of double distilled water with appropriate volumes of plant extracts according to Table 1. The appropriate amounts of 10 mM silver nitrate solution were subsequently added drop‐wise to the reactions under sonication. The reactions were incubated at ambient conditions and the synthesis progress was monitored using UV–vis spectroscopy (Biochrom WPA, model Biowave II, UK) in the wavelength range from 300 to 800 nm. The corresponding concentrations of plant extracts in NaNO3 solution were used as blank.
Table 1.
Reaction conditions for the synthesis of stable colloidal solutions of AgNPs by using M. pulegium extracts
| Reaction number | AgNO3, mM | Extraction solvent | Extract volume, ml |
|---|---|---|---|
| 1 | 0.5 | ddH2 O, ethanol (70%), methanol | 1 |
| 2 | 1.0 | ddH2 O, ethanol (70%), methanol | 1 |
| 3 | 0.5 | ddH2 O, ethanol (70%), methanol | 2 |
| 4 | 1.0 | ddH2 O, ethanol (70%), methanol | 2 |
| 5 | 0.5 | ddH2 O, ethanol (70%), methanol | 3 |
| 6 | 1.0 | ddH2 O, ethanol (70%), methanol | 3 |
| 7 | 0.5 | ddH2 O, ethanol (70%), methanol | 4 |
| 8 | 1.0 | ddH2 O, ethanol (70%), methanol | 4 |
2.3 Material characterisations
The optical properties of AgNPs were preliminary characterised using UV–vis spectroscopy. The size and morphology of nanoparticles were determined by transmission electron microscopy (TEM, CM30, Philips, Eindhoven, Netherlands) operating at an accelerating voltage of 100–300 kV. The colloidal nanoparticles were drop coated on a hydrophilic carbon coated copper grid and allowed to air dry before TEM analysis. The morphology and surface topology of nanoparticles were further analysed using atomic force microscopy (AFM, DME, Denmark) in the contact mode on a DualScopeTM scanning probe‐optical microscope equipped with a C‐26 controller. The particle size distribution and zeta potential of the colloidal nanoparticles were determined by ZEN 3600 zetasizer (Malvern, Worcestershire, UK). The Fourier transform infrared spectroscopy (FTIR) spectra were recorded using FT/IR‐6300 Spectrometer (Jasco, Japan) with wavelength range between 4000 and 400 cm−1. For this purpose, the colloidal nanoparticles synthesised using different extracts of M. pulegium were precipitated by centrifugation at 15,000 rpm for 15 min and completely dried using a freeze dryer (VaCo 5, Zirbus, Germany). The pellets were used for FTIR analysis after grinding with KBr. The elemental composition analysis of the biogenic nanoparticles was carried out by energy‐dispersive X‐ray spectroscopy (EDAX) using a Bruker Quantax 200 detector (Bruker AXS Inc., Madison, WI, USA).
2.4 Antimicrobial activity of AgNPs
Reference strains of Esherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923) and Streptococcus pyogenes (ATCC 1447) were used to investigate the potential antibacterial activity of biogenic AgNPs by disk diffusion assay and minimum inhibitory concentration (MIC) assay.
For disk diffusion assay, sterilised paper disks were impregnated with 20 µl of the freshly prepared AgNPs at a final content of 10 µg disk−1 and dried before use. A single colony of each bacterial strain was inoculated overnight in tryptic soy broth medium under shaking (150 rpm) at 37°C. The overnight cultures were diluted with 0.9% NaCl to the 0.5 McFarland standard and used to inoculate the Muller‐Hinton agar plates. The AgNPs impregnated discs and the standard ampicillin disk (10 µg disk−1) were then applied on the plates and the zones of inhibition were measured after incubation at 37°C for 24 h.
To determine the MIC of AgNPs synthesised by different extracts of M. pulegium, the original stock solutions of AgNPs (100 µg ml−1) were serially diluted two‐fold to obtain the concentration range of 0.78–100 µg ml−1. About 100 μl of each dilution together with 100 μl of cultured E. coli cells at concentration of 105 CFU ml−1 were added to individual microtitre wells. Two controls including nutrient media containing corresponding concentrations of AgNPs (positive control) and inoculated nutrient media without AgNPs (negative control) were used to compare the results. The bacterial growth after 24 h incubation at 37°C was measured using microplate reader at 600 nm. The absorbance value of each treated well was corrected by deducting the corresponding absorbance value of the positive control. MIC was indicated as concentration of AgNPs at which no increase of the OD600 of the pathogen was observed. This procedure was also performed using S. aureus and S. pyogenes strains.
The antifungal activity of AgNPs against fluconazole resistant strain of Candida albicans was investigated using MIC method. The colloidal AgNPs were serially diluted two folds in yeast extract peptone dextrose to obtain the final concentrations ranging from 100 to 0.78 µg ml−1 and added into microtitre plates. The overnight culture of the tested yeast was then inoculated onto microtitre plates so that the final inoculum density of 106 CFU ml−1 was obtained in each well. The 50% inhibitory concentration (IC50) was defined as the lowest concentration that inhibited 50% of the growth as determined by a comparison with the cell growth in the control wells. The cell growth was determined by monitoring absorption at 630 nm using microplate reader (Bio‐Tek Instruments, USA).
2.5 Anticancer activity of AgNPs
The anticancer effects of AgNPs on MCF‐7 (breast) and HeLa (cerevical) cancer cell lines were investigated by MTT assay according to the previously reported method [9]. Briefly, the cells were first seeded on 96‐well plates at a density of 104 cells ml−1 and incubated overnight at 37°C in the presence of 5% CO2. The cells were then exposed with different concentrations of AgNPs (5, 10, 20, 50 and 100 µg ml−1) at the same conditions for 48 h. After discarding the medium and addition of 100 μl MTT (0.5 mg ml−1 in media) into each well, the cells were incubated again at 37°C for 4 h. The resulting crystals of formazan were dissolved in 150 µl dimethyl sulfoxide (DMSO) and the absorbance was measured at 570 nm. The cell viability was determined as the ratio of absorbance value of each treatment to the control. The morphological changes of MCF‐7 and HeLa cells after incubation with AgNPs were also studied. For this purpose, the cells were first seeded at a density of 1 × 106 cells ml−1 in 24‐well plate and incubated overnight in a humidified incubator at 37°C with 5% CO2. The cells were washed with phosphate‐buffered saline (PBS) following the exposure to AgNPs for 48 h and imaged by using optical microscope.
2.6 Statistical analysis
The results were expressed as the mean value ± standard deviation of three independent experiments. The data were analysed using Student's t‐test (Microsoft Excel, Microscoft Corporation, USA) and P values of <0.05 were considered as statistically significant.
3 Results and discussion
3.1 Synthesis and characterisation of AgNPs
The reaction conditions were first optimised using different types and concentrations of the plant extract as well as the concentrations of silver nitrate in order to achieve stable and monodisperse nanoparticles. The reducing and stabilising agents have been considered as significant parameters on the physicochemical properties of AgNPs [16, 17]. Therefore, the optimisation of reaction is an essential step toward the production of stable colloidal AgNPs with controlled size and shape. UV–vis spectroscopy showed different optical properties of AgNPs based on the type of extract. A single and symmetric peak with maximum absorption around 450 nm was obtained from the nanoparticles synthesised using aqueous or methanolic extracts. However, application of ethanolic extract led to the synthesis of AgNPs with an asymmetric and more extended peak containing two maximum absorptions around 400 and 550 nm (Fig. 1). This feature of absorption spectra could be attributed to the variable shapes and sizes of nanoparticles. Monitoring the reaction progress by UV–vis spectroscopy as well as the colour of colloidal solutions revealed the obvious differences between the reactions containing different extracts. The rate of nanoparticle synthesis in the presence of ethanolic extract was considerably slower than two others [the colour of ethanolic colloidal solutions gradually changed from white to light grey within a week while the colour of methanolic and aqueous solutions changed from white to grey during up to two days (Fig. 1)]. This observation is in accordance with the recent report [9] regarding to the rapid reduction of silver nitrate by aqueous extract of Taxus baccata due to the high quantity of carbohydrates in the extract. The results could be attributed to the capability of the solvents for extraction of various organic compounds with different reduction potentials. Plant extracts contain different organic compounds including carbohydrates, proteins, phenolics, alkaloids and terpenoids with different chemical properties which may act as reducing or stabilising agents in the synthesis of metal nanoparticles [12, 18, 19]. Due to the diversity of plant biomolecules, the composition and concentration of plant extracts can significantly affect the yield and quality of products [12]. Therefore, selection of a suitable extract with appropriate concentration seems to be a crucial step for optimisation of the reaction conditions. Moreover, the fractionation of plant compounds using different solvents may facilitate control of the reaction and interpretation of the results.
Fig. 1.
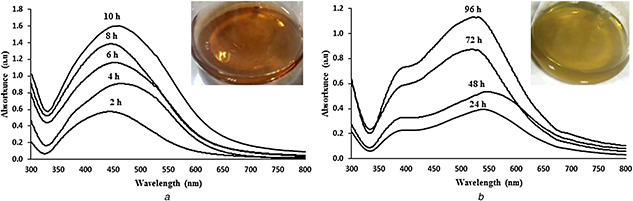
Time‐dependent UV–vis spectroscopy of AgNPs synthesised by using high concentration of aqueous (left) and methanolic (right) extracts of M. pulegium
Increasing the concentration of extract or silver nitrate led to the improved reaction rate. However, the colloidal stability of AgNPs significantly decreased in high concentration of silver nitrate as significant precipitation of nanoparticles was observed after a few days due to the agglomeration. While the ethanolic extract synthesised nanoparticles were considerably unstable and precipitated after a few days, nanoparticles synthesised using aqueous and methanolic extracts remained stable after 6 months without significant agglomeration. The rapid synthesis and subsequent agglomeration of AgNPs in the presence of ethanolic extract could be attributed to the major role of ethanolic extracted compounds in the reduction of silver nitrate than stabilising the nanoparticles. In the best conditions, the nanoparticles synthesised using high amount of aqueous extract were found stable with zeta potential value of −15.7 over 6 months.
TEM analysis of the nanoparticles synthesised using aqueous and methanolic extracts confirmed their monodispersity and narrow size distribution (Fig. 2). The majority of nanoparticles were anisotropic in shape and between 5 and 50 nm in size. The aqueous extract synthesised AgNPs displayed significantly smaller size than those synthesised by methanolic extract indicating the significant role of extract on the size and colloidal stability of nanoparticles. AFM analysis of surface topography also showed a thin and planar morphology of aqueous AgNPs with a thickness ranging from 2.46 to 3.15 nm while completely different morphology was observed for methanolic AgNPs (Fig. 2).
Fig. 2.
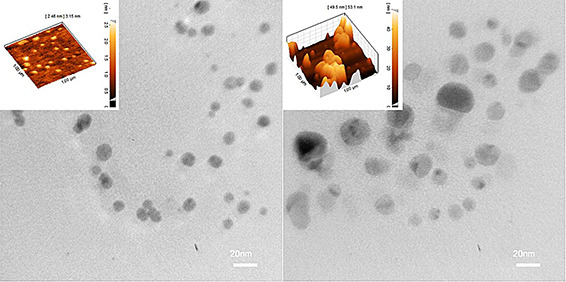
TEM images of AgNPs synthesised using aqueous (left) and methanolic (right) extract of M. pulegium. Corresponding AFM images are shown as inset
FTIR was used to investigate the attachment of biological compounds to the surface of nanoparticles. As shown in Fig. 3, FTIR spectra of AgNPs contain several distinct peaks that could be mainly attributed to the surface adsorbed organic compounds such as aliphatic, aromatic or phenolic molecules as well as proteins and polysaccharides. The results showed the presence of different functional groups such as OH, CH, C = O and NH on the nanoparticles surfaces indicating the prominent role of the organic compounds as capping agents for stabilising the nanoparticles. However, considerably less intensity was observed for the FTIR spectrum of ethanolic AgNPs indicating inefficient adsorption of the ethanolic extracted compounds on the nanoparticles.
Fig. 3.
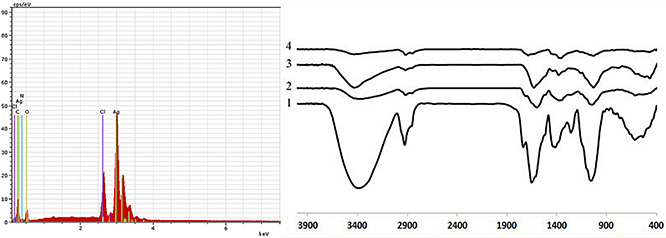
EDAX spectra of AgNPs synthesised using M. pulegium extract (left) and FTIR spectra (right) of dried powder of M. pulegium leaves (curve 1); AgNPs synthesised using methanolic (curve 2), aqueous (curve 3) and ethanolic extract of M. pulegium (curve 4)
EDAX analysis of the elemental composition of AgNPs confirmed the presence of a considerable silver peak. Other elemental signals were also observed in the spectrum that attribute to the adsorbed plant compounds on the nanoparticles (Fig. 3). The overall results indicated the high potential of aqueous and methanolic extracts of M. pulegium for synthesis and stabilisation of the high‐quality colloidal AgNPs that are suitable for the biomedical applications.
3.2 Antibacterial activity of AgNPs
The antimicrobial properties of silver against a broad spectrum of microorganisms have been well known for decades. The nanosilver‐based biomaterials and devices have found widespread applications in biomedicine, water and air purification, food production and preservation, cosmetics, clothing industry and numerous household products [20, 21]. Several mechanisms have been reported for antibacterial activity of AgNPs including direct damage to the bacterial cell membrane, the release of silver ions and subsequent generation of reactive oxygen species which finally lead to the increased membrane permeability and DNA damage [20, 22].
It has been reported that the size, shape and surface chemistry of AgNPs significantly affect their potential for cell penetration as well as their cytotoxicity and antibacterial activity. Therefore, selection of the suitable stabilising agents for efficient penetration of nanoparticles into the bacterial cells can improve their antibacterial activity which finally leads to the decreased therapeutic dose [21]. Moreover, the use of organic compounds, with well‐known antimicrobial activity, for functionalisation of AgNPs may increase their therapeutic effects. The capping agents can also improve the colloidal stability of nanoparticles by avoiding their aggregation and significantly affect their interactions with in vivo components [23]. To investigate the mentioned hypothesis, antibacterial activity of the biogenic AgNPs was assessed on different bacterial strains including E. coli (gram negative), S. aureus and S. pyogenes (gram positive).
Recently, the extracts and essential oils of M. pulegium have attracted some attention as a potential alternative to chemical additives for the food industry due to their promising antimicrobial activity against several pathogenic strains [14, 24]. The use of M. pulegium extracts for the green synthesis of AgNPs may decrease the concerns raised from chemical synthesis and result in the development of nanoparticles with improved antimicrobial activities.
Disk diffusion assay revealed the promising antibacterial effects of the nanoparticles on all the tested bacterial strains (Table 2). Among the strains, E. coli showed the most sensitivity to the M. pulegium synthesised AgNPs. To determine the potential synergistic effect of the plant extracts on antibacterial activity of AgNPs, the nanoparticles synthesised using low and high concentrations of the extracts were used in the experiments. The results showed different antibacterial activities of AgNPs depending on the type and concentration of M. pulegium extract. In general, AgNPs synthesised in the presence of low concentration of the extracts showed higher antibacterial activity than AgNPs synthesised using high concentration of the extracts. Efficient encapsulation of nanoparticles by using high concentration of organic compounds may inhibit the significant release of silver ions and decrease their antibacterial effects. Interestingly, the considerable antibacterial activity was obtained from the nanoparticles synthesised using low concentration of aqueous extract while no significant antibacterial activity was observed for the nanoparticles synthesised using high concentration of aqueous extract.
Table 2.
Mean zone of inhibition of AgNPs synthesised using 1 or 4 ml of aqueous (A), ethanolic (E) or methanolic (M) extract of M. pulegium
| E. coli | S. aureus | S. pyogenes | |
|---|---|---|---|
| A1 | 10.20 ± 1.20 | 9.65 ± 0.64 | 10.63 ± 0.67 |
| A4 | 0.00 | 0.00 | 0.00 |
| E1 | 9.60 ± 0.70 | 9.35 ± 1.20 | 8.65 ± 0.78 |
| E4 | 7.60 ± 0.70 | 8.60 ± 0.70 | 7.50 ± 0.42 |
| M1 | 11.00 ± 0.14 | 11.40 ± 0.14 | 11.90 ± 0.57 |
| M4 | 8.65 ± 0.77 | 9.40 ± 0.57 | 9.20 ± 0.14 |
Based on the results of MIC assay (Table 3), the highest antibacterial activity was obtained from AgNPs synthesised in the presence of the low concentration of aqueous extract. Apart from the type of extract, the results could be attributed to the different sizes and shapes of the aqueous extract synthesised nanoparticles in comparison to the other AgNPs. In accordance with the previous report by Fayaz et al. [6], the results showed less inhibitory effects of AgNPs on the growth of gram‐positive bacteria (S. aureus and S. pyogenes) than the gram‐negative strain (E. coli). AgNPs synthesised using M. pulegium extracts displayed higher antibacterial activity than the AgNPs synthesised using other plant extracts [6, 16, 25].
Table 3.
MIC of AgNPs synthesised in the presence of 1 ml of aqueous (A), ethanolic (E) or methanolic (M) extract of M. pulegium on different bacterial strains
| E. coli, µg ml−1 | S. aureus, µg ml−1 | S. pyogenes, µg ml−1 | |
|---|---|---|---|
| A | 25 | 25 | 25 |
| E | 25 | 50 | 25 |
| M | 25 | 50 | 50 |
The antifungal activity of the AgNPs was studied by using the standard microdilution method. The results showed the inhibited cell growth of C. albicans based on the type and concentration of AgNPs. The lowest MIC value of 100 µg ml−1 was obtained in the presence of aqueous extract synthesised AgNPs that inhibited up to 50% of cell growth. Under the same conditions, up to 34 and 43% cell growth inhibitions were obtained using the ethanolic and methanolic extract synthesised AgNPs, respectively.
3.3 Anticancer activity of AgNPs
The aqueous extract synthesised AgNPs were selected for investigation of the potential anticancer activity, due to their appropriate physicochemical and antimicrobial properties. The results indicated the promising anticancer effects of the AgNPs on MCF‐7 and HeLa cancer cells. In the best conditions, more than 47 and 58% mortalities were obtained after 48 h exposure of HeLa and MCF‐7 cells, respectively, to 100 µg ml−1 AgNPs (Table 4). The nanoparticles showed dose and cell line dependent anticancer activity. The significantly higher mortality was obtained for MCF‐7 cells than Hela cells and the anticancer activity increased depending on the concentration of AgNPs.
Table 4.
Viability percentage of the MCF‐7 and HeLa cancer cells after incubation for 48 h with different concentrations of the aqueous extract synthesised AgNPs
| 5 µg ml−1 | 10 µg ml−1 | 20 µg ml−1 | 50 µg ml−1 | 100 µg ml−1 | |
|---|---|---|---|---|---|
| MCF‐7 | 67.24 ± 3.12 | 65.02 ± 2.41 | 57.33 ± 2.69 | 49.87 ± 2.44 | 41.55 ± 2.39 |
| HeLa | 75.86 ± 3.87 | 69.71 ± 3.04 | 65.3 ± 2.73 | 60.84 ± 3.68 | 52.64 ± 4.02 |
In agreement with the results of MTT assay, the optical microscopic monitoring of cancer cells after exposure to aqueous extract synthesised AgNPs revealed the significant changes of cell morphology including the cellular shrinkage, rounding and clumping. The cell proliferation was also considerably suppressed following the exposure to AgNPs in comparison with the untreated cells (Fig. 4).
Fig. 4.
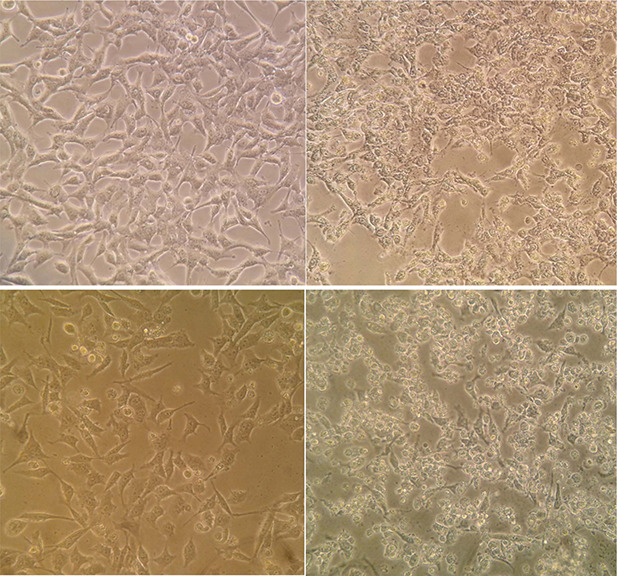
Morphology changes of MCF‐7 (top) and HeLa (bottom) cancer cells after treatment with AgNPs synthesised using aqueous extract of M. pulegium. The control and treated cells have been shown in the left and right, respectively
4 Conclusion
In this study, different extracts of M. pulegium were used for the first time, as reducing and capping agents, for synthesis of AgNPs. The results elucidated the significant effects of the type and concentration of plant extract on the physicochemical characteristics of nanoparticles as well as their biological activity. The colloidal nanoparticles with different shapes, sizes, stabilities and optical properties were synthesised using different types and concentrations of M. pulegium extract. Moreover, the biogenic AgNPs displayed promising antibacterial, antifungal and anticancer activities, indicating the suitability of the present method for synthesis of high‐quality colloidal AgNPs for different biomedical applications. In conclusion, the aqueous extract of M. pulegium is suggested as the best candidate for green synthesis of high‐quality AgNPs with promising antibacterial, antifungal and anticancer effects.
5 Acknowledgment
The financial support of research council of Isfahan University is gratefully acknowledged.
6 References
- 1. Cheng F. Betts J.W. Kelly S.M. et al.: ‘Synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using aminocellulose as a combined reducing and capping reagent’, Green Chem., 2013, 15, p. 989 [Google Scholar]
- 2. El‐Sayed I.H. Huang X. El‐Sayed M.: ‘Surface plasmon resonance scattering and absorption of anti‐EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer’, Nano Lett., 2005, 5, pp. 829 –834 [DOI] [PubMed] [Google Scholar]
- 3. Xiu Z. Zhang Q. Puppala H.L. et al.: ‘Negligible particle‐specific antibacterial activity of silver nanoparticles’, Nano Lett., 2012, 12, pp. 4271 –4275 [DOI] [PubMed] [Google Scholar]
- 4. Asghari S. Johari S.A. Lee J.H. et al.: ‘Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna’, J. Nanobiotechnol., 2012, 10, p. 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suresh A.K. Pelletier D. Wang W. et al.: ‘Cytotoxicity induced by engineered silver nanocrystallites is dependent on surface coatings and cell types’, Langmuir, 2012, 28, pp. 2727 –2735 [DOI] [PubMed] [Google Scholar]
- 6. Fayaz A.M. Balaji K. Girilal M. et al.: ‘Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram‐positive and gram‐negative bacteria’, Nanomedicine, 2010, 6, pp. 103 –109 [DOI] [PubMed] [Google Scholar]
- 7. Park M.V.D.Z. Neigh A.M. Vermeulen J.P. et al.: ‘The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles’, Biomaterials, 2011, 32, pp. 9810 –9817 [DOI] [PubMed] [Google Scholar]
- 8. Virkutyte J. Varma R.S.: ‘Green synthesis of metal nanoparticles: biodegradable polymers and enzymes in stabilization and surface functionalization’, Chem. Sci., 2011, 2, p. 837 [Google Scholar]
- 9. Abbasi Kajani A. Bordbar A.K. Zarkesh Esfahani S.H. et al.: ‘Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract’, RSC Adv., 2014, 4, pp. 61394 –61403 [Google Scholar]
- 10. Hutchison J.E.: ‘Greener nanoscience: a proactive approach to advancing applications and reducing implications of nanotechnology’, ACS Nano, 2008, 2, pp. 395 –402 [DOI] [PubMed] [Google Scholar]
- 11. Raveendran P. Fu J. Wallen S.L.: ‘Completely ‘green’ synthesis and stabilization of metal nanoparticles’, J. Am. Chem. Soc., 2003, 125, pp. 13940 –13941 [DOI] [PubMed] [Google Scholar]
- 12. Iravani S.: ‘Green synthesis of metal nanoparticles using plants’, Green Chem., 2011, 13, p. 2638 [Google Scholar]
- 13. Aghel N.: ‘Supercritical carbon dioxide extraction of Mentha pulegium L. essential oil’, Talanta, 2004, 62, pp. 407 –411 [DOI] [PubMed] [Google Scholar]
- 14. Ghazghazi H. Chedia A. Weslati M. et al.: ‘Chemical composition and in vitro antimicrobial activities of Mentha pulegium leaves extracts against foodborne pathogens’, J. Food Saf., 2013, 33, pp. 239 –246 [Google Scholar]
- 15. Enache T.E.Ş.E. Iacomi B.M. Oprea M. et al.: ‘Antifungal activity of some plant extracts against Botrytis cinerea Pers in the black currant crop (Ribes nigrum L.)’, Acta Sci. Pol. Hortorum Cultus., 2015, 14, pp. 29 –43 [Google Scholar]
- 16. Agnihotri S. Mukherji S. Mukherji S.: ‘Size‐controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy’, RSC Adv., 2014, 4, p. 3974 [Google Scholar]
- 17. Tripathy A. Raichur A.M. Chandrasekaran N. et al.: ‘Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves’, J. Nanoparticle Res., 2010, 12, pp. 237 –246 [Google Scholar]
- 18. Mittal A.K. Chisti Y. Banerjee U.C.: ‘Synthesis of metallic nanoparticles using plant extracts’, Biotechnol. Adv., 2013, 31, pp. 346 –356 [DOI] [PubMed] [Google Scholar]
- 19. Durán N. Marcato P.D. Durán M. et al.: ‘Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants’, Appl. Microbiol. Biotechnol., 2011, 90, pp. 1609 –1624 [DOI] [PubMed] [Google Scholar]
- 20. Kumar V. Yadav S.K.: ‘Plant‐mediated synthesis of silver and gold nanoparticles and their applications’, J. Chem. Technol. Biotechnol., 2009, 84, pp. 151 –157 [Google Scholar]
- 21. Marambio‐Jones C. Hoek E.M.V.: ‘A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment’, J. Nanoparticle Res., 2010, 12, pp. 1531 –1551 [Google Scholar]
- 22. Azócar M.I. Tamayo L. Vejar N. et al.: ‘A systematic study of antibacterial silver nanoparticles: efficiency, enhanced permeability, and cytotoxic effects’, J. Nanoparticle Res., 2014, 16, p. 2465 [Google Scholar]
- 23. Agnihotri S. Mukherji S. Mukherji S.: ‘Immobilized silver nanoparticles enhance contact killing and show highest efficacy: elucidation of the mechanism of bactericidal action of silver’, Nanoscale, 2013, 5, pp. 7328 –7340 [DOI] [PubMed] [Google Scholar]
- 24. Gnanadhas D.P. Ben Thomas M. Thomas R. et al.: ‘Interaction of silver nanoparticles with serum proteins affects their antimicrobial activity in vivo’, Antimicrob. Agents Chemother., 2013, 57, pp. 4945 –4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shahmohamadi R. Sariri R. Rasa M. et al.: ‘Chemical composition and antimicrobial activity of flowering aerial parts Mentha pulegium from gilan’, Pharmacol. Online, 2011, 3, pp. 651 –659 [Google Scholar]


