Abstract
Cell‐seeded microcarriers (MCs) are currently one of the most promising topics in biotechnology. These systems are supportive structures for cell growth and expansion that allow efficient nutrient and gas transfer between the media and the attached cells. Silk proteins have been increasingly used for this purpose in the past few years due to their biocompatibility, biodegradability and non‐toxicity. To date, several silk fibroin spherical MCs in combination with alginate, gelatin and calcium phosphates have been reported with very interesting outcomes. In addition, other silk‐based three‐dimensional structures such as microparticles with chitosan and collagen, as well as organoids, have been increasingly studied. In this study, the physicochemical and biological properties of these biomaterials, as well as the recent methodologies for their processing and for cell culture, are discussed. The potential biomedical applications are also addressed. In addition, an analysis of the future perspectives is presented, where the potential of innovative silk‐based MCs processing technologies is highlighted.
Inspec keywords: biodegradable materials, proteins, calcium compounds, gelatin, biomedical materials, cellular biophysics, molecular biophysics
Other keywords: supportive structures, cell growth, gas transfer, attached cells, silk proteins, biodegradability, nontoxicity, silk fibroin spherical MCs, gelatin, calcium phosphates, silk‐based three‐dimensional structures, chitosan, collagen, physicochemical properties, biological properties, cell culture, silk‐based microcarriers, cell‐seeded microcarriers, biotechnology, efficient nutrient transfer, biocompatibility, alginate, biomedical applications
1 Introduction
1.1 Role of microcarriers (MCs) in modern biotechnology
The global Biomaterials Market is expected to reach USD 139 billion by 2022, at an annual growth rate of over 11.8% [1]. Nowadays, the application of synthetic or natural materials is essential to respond to the increasing cases of chronic diseases, trauma injuries leading consequently to market growth. Advanced biomaterials for cell adhesion, proliferation and differentiation are used to improve the functionality of damaged tissues and organs [2]. Among these, small and stable and spherical MCs have played an important role in the expansion and differentiation of anchorage‐dependent cells, enabling potential scale‐up of stem cell‐derived products in large bioreactors [3, 4]. A wide range of MCs is commercially available and has different physicochemical parameters, such as degree of porosity, chemical composition, surface topography and diameter (comprised between 100 and 400 µm) [5, 6].
MCs allow the investigation of cell behaviour in vitro in a three‐dimensional (3D) environment similar to their natural surroundings in vivo and may provide an alternative to animal studies [5, 7, 8]. Moreover, the microtissues formed through MCs and cells can be directly delivered to the site of the defect, thus eliminating the digestion of cells before transfer from a monolayer culture [3, 9].
Although current studies on silk‐based MCs focus on microparticles, downsizing some of these systems onto nanocarriers, is an area of interest in biomedical engineering. Both micro and nanocarrier‐based vaccine delivery systems have been explored, to act as an effective antigen carrier, to modulate the immune responses, to protect the antigens from a deleterious environment until delivered to the immune cells and to allow a controlled release of antigen [10].
However, due to their small size and large surface‐area‐to‐volume ratio, nanoparticles can interact with biomolecules, both extracellularly and intracellularly [11]. In this context, the investigation of silk proteins as a drug carrier has been widely expanded over the last few years, since sericin and fibroin can be tailored via genetic engineering to contain specific chemical features [12].
However, when engineered to nanosize, nanocarriers may suffer degradation and exhibit toxic biological effects. Biodegraded nanoparticles may accumulate within cells and lead to intracellular changes such as disruption of organelle integrity or gene alternations. In contrast, the physicochemical and biological properties of MCs are easily controlled [13].
1.2 Cell culturing systems with MCs
Conventional routine cell culture with MCs is performed under static conditions in Petri dishes, T‐flasks or roller bottles [14]. In spite of being easy to use, disposable and low‐cost, these systems require individual handling. Alteration of cell‐specific extracellular matrix secretion, loss of specific morphology and phenotype can occur during passaging [15]. Furthermore, controlled environmental parameters and homogeneous diffusion of nutrients, gas–liquid oxygen and metabolites is generally impossible [7, 8]. Thus, these conditions often have low seeding efficiencies.
To overcome these limitations, dynamic culturing in bioreactors has been used for the seeding and proliferation of cells on MCs [16, 17]. The use of bioreactors, such as stirred tank and rotating chamber reactors, has several advantages. These systems are widely used for microbes [18, 19] and mammalian [20, 21] cells culture under monitored and controlled operational parameters (e.g. pH, temperature, oxygen tension, and nutrient supply) up to an industrial scale [8]. Furthermore, bioreactors allow batch and continuous operation [3, 9]. While batch mode operation only provides limited amounts of material, the continuous mode allows a more efficient use of reagents and enhanced reproducibility [22].
However, the ability of the cells to populate the MCs largely depends on their physicochemical properties, such as their surface properties and particle size. Moreover, a narrow crystal size distribution allows a homogeneous cell growth around the particles. Thus, the physicochemical properties of MCs can determine their behaviour in a dynamic culture [3, 5]. MCs core materials can be divided into synthetic or natural polymers. Even though synthetic polymers exhibit good reproducibility and mechanical properties, their cell adhesion and proliferation properties are low. On the other hand, in addition to being easier and cheaper to obtain, natural polymers show better biological responses [7].
2 Silk MCs
2.1 Silk proteins
Silk proteins represent a unique family of natural fibrous proteins due to their unique properties for the development of MCs [23]. These proteins exhibit non‐toxicity, biodegradability, self‐assembly, mechanical stability and have a controllable structure [24, 25]. There are several species of insects that produce natural silk, such as silkworms, spiders, scorpions, mites, and flies [26]. The silkworm is a native insect from North China and currently the most used in silk production, being Bombyx mori, the most common species of domesticated silkworm [27]. Regarding the type and origin of silk, most of the literature reported studies to use Bombyx mori cocoons as silk source [17, 28, 29]. Silk threads from silkworm cocoons consist primarily of two protein components, fibroin, a semi‐crystalline fibrillar protein, and sericin, the water‐soluble glue‐like protein that operates as a binder to maintain the structural integrity of the cocoon [26] (Fig. 1).
Fig. 1.
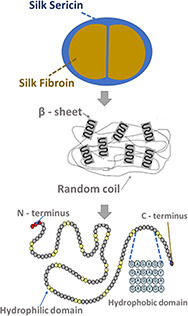
Structure of silkworm Bombyx mori silk sericin and fibroin
Silk fibroin has been used for centuries in the textile industry and for decades in biomaterials, providing stiffness and resistance. Besides providing good mechanical properties and allowing the formation of 3D structures, fibroin is also biocompatible [30]. On the other hand, sericin was traditionally disposed of in silk processing wastewater. More recently, this protein has been reported to improve biocompatibility in vitro and increase cell adhesion and proliferation of several mammalian cell lines [31]. The main advantages and disadvantages of each silk protein are included in Table 1.
Table 1.
Summary of the main characteristics of silkworm proteins: silk fibroin and silk sericin main reported advantages and disadvantages
| Silk proteins | Main physiological function | Advantages | Disadvantages | References |
|---|---|---|---|---|
| fibroin | structural integrity | ‐ biocompatibility; | ‐ stringent protein isolation and purification protocols; | [44, 45, 46, 47, 48, 49, 50, 51, 52] |
| ‐ biodegradability; | ||||
| ‐ good oxygen permeability; | ||||
| ‐ mechanical strength; | ||||
| ‐ elasticity; | ‐ low osteogenic capacity; | |||
| ‐ easily processable; | ||||
| ‐ low cost | ||||
| sericin | protection | ‐ biocompatibility; | ‐ stringent protein isolation and purification protocols; | [53, 54, 55, 56, 57] |
| ‐ biodegradability; | ||||
| ‐ moisturising power; | ||||
| ‐ resistance to oxidation, bacteria, and ultraviolet light; | ‐ weak mechanical strength | |||
| ‐ usually discarded during silk processing |
2.2 Composite silk‐based MCs
Despite their characteristics, silk proteins are usually modified by chemical and physical methods to enhance their properties [32, 33]. A more widespread approach is the combination of silk sericin or fibroin with other materials, forming composites to accommodate a broader spectrum of functional requirements [34].
Silk fibroin‐based biomaterials with alginate (Section 3.1), gelatin (Section 3.2), calcium phosphates (Section 3.3) and pullulan (Section 3.4) are the most produced silk MCs. Although MCs are traditionally spherical in order to provide a high surface area to volume ratio, other 3D shapes have been reported [7]. Spherical‐like morphology can be challenging to sort or further process. In addition to being directly exposed to surrounding flows and surfaces, the cells attached to spherical MCs are constantly changing locations over time. Moreover, conventional methods to obtain microspheres include emulsification and polymerisation, followed by additional treatments, hindering its continuous production [35, 36]. Thus, other reported works focus on the development of other silk 3D constructs to self‐organise into properly differentiated functional cell types. Among them, silk fibroin microparticles with chitosan and collagen (Section 3.4) and silk organoids (Section 3.5) have been recently developed.
Studies on the efficiency of these silk‐based MCs and microparticles usually involve seeding with mesenchymal stem cells (MSCs). The therapeutic function of these cells is related to their multi‐differentiation potential, which gives these cells the capacity to form bone, cartilage, fat and other multipotent cells [6]. Further, these cells remain at the injury site, secreting trophic and immunomodulatory bioactive factors [37].
Silk proteins are proven to promote cell adhesion, proliferation and differentiation of MSCs [38].
Although there are several studies in which sericin is combined with other inorganic and organic polymers to synthesise different biomaterials such as porous and fibrous scaffolds [39], hydrogels [40], films [41] and microspheres [42], to our knowledge, studies focusing on the development of sericin‐based MCs are still inexistent [24], due to its weak mechanical properties and water solubility [43].
Therefore, the present work focuses on reported studies on the synthesis and physicochemical characterisation of silk fibroin‐based MCs, on the biological response and cell culturing conditions used, as well as on the potential applications of these biomaterials (Fig. 2).
Fig. 2.
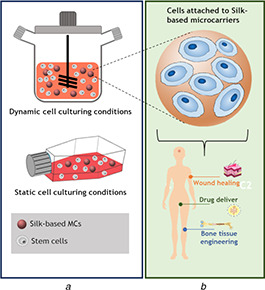
Cell culturing systems and its applications in biomedical engineering
(a) Static and dynamic culturing systems used to produce 3D constructs, (b) Conventional structure of silk‐based MCs reported in the literature and their main applications
3 Silk fibroin‐based MCs
Pure silk fibroin has been used to develop MCs for cell culture under mild processing conditions. In the work of Wang et al. [63], fabrication of these materials was achieved using a high voltage electrostatic field, in order to obtain fibroin spherical droplets. The silk spheres were posteriorly subject to freeze‐drying, resulting in highly porous biomaterials with diameters ranging from 208.4 to 727.3 μm. The smaller particles (200–300 μm) were more suitable for murine fibroblasts cell line L‐929 adhesion and proliferation when compared to larger particles. Fibroblasts, besides being responsible for the production of the extracellular matrix, also play an important role regulating interstitial fluid volume and pressure and wound healing, thus being suitable to study MCs for different biomedical applications [64].
Bessa et al. [65, 66] proposed the use of fibroin microparticles for bone tissue engineering (bone‐TE). In these works, silk MCs were obtained as a delivery carrier for bone morphogenetic protein‐2 (BMP‐2), through a coprecipitation method. Spherical particles with a mean size of ∼580.0 nm and BMP‐2 encapsulation efficiency of 97.7 ± 2.0% were obtained [66]. BMP‐2 released from the MCs was able to induce rapid cell differentiation of immortalised cell line C2C12, originally isolated from mouse muscle [67], into osteoblasts and mineralisation. In vivo assays on a rat ectopic model showed that after four weeks, new bone was formed and an increase in bone density over time resulted from adding BMP‐2 loaded MCs. For non‐loaded MCs, bone formation was not detected, thus meaning that fibroin/BMP‐2 MCs retain growth factors at the site of the injury for a period of time without being immediately degraded [68].
In addition to pure silk fibroin MCs, different biopolymers in combination with silk have been investigated for MC formulation, in order to improve and tailor both mechanical and biological properties [69, 70] (Fig. 3).
Fig. 3.
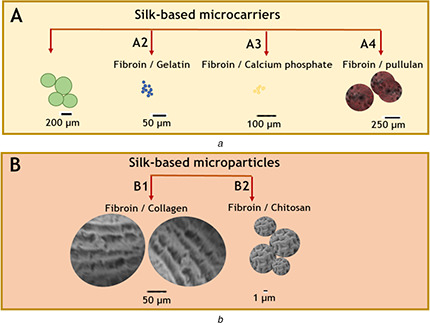
Illustration of the conventional structure of
(a) Silk‐based MCs reported in the literature: (A1) fibroin/alginate MCs based on [58], (A2) fibroin/gelatin MCs based on [59], (A3) fibroin/calcium phosphate adapted based on [29] and (A4) fibroin/pullulan based on [60], (b) Silk‐based microparticles: fibroin/collagen (B1) based on [61], fibroin/chitosan (B2) based on [62]
3.1 Silk/alginate MCs
Alginate is a naturally occurring anionic polymer typically obtained from brown seaweed, which is used as a core, giving stability in MCs due to its biocompatibility, low toxicity, low cost, and fast sol–gel transition in contact with divalent cations. However, this polymer is unable to specifically interact with mammalian cells due to the presence of negative charges and its deficiency of integrin domains [71, 72, 73]. Thus, conventional methods to overcome this disadvantage include blending with natural proteins such as silk proteins.
In the reported works [28, 58, 74], the preparation of alginate cores was achieved by dropping alginate droplets in a calcium chloride water solution. Silk/alginate biomaterials were obtained after immersing the cores in a 1.5% w/v silk fibroin solution. Conventionally, these composites are immersed or washed with 96% (v/v) ethanol to induce the conformational transition of silk fibroin from insoluble β‐sheet conformation to a water‐soluble structure [28, 58, 74]. The biological response of the resulting materials was evaluated after seeding with adipose‐derived stem/stromal cells (ASCs). Adipose tissue is one of the most convenient sources of MSCs due to its availability and accessibility [75]. ASCs adhered rapidly to the spherical MCs with an average diameter of 400 µm. After 7 days, the cells were homogeneously distributed and built connections on the biomaterial surface. After 14 days, numerous 3D constructions were created by the interaction between adherent cells. Evaluation of osteogenic, adipogenic, and chondrogenic potential showed that ASCs adhered to the surface of the MCS can produce mineralised matrix and form lipid droplets, indicating that the cells maintained their viability and multipotency [28, 58].
Perteghella et al. [58] and Duchi et al. [28] followed a conventional static cell seeding process of MSCs in the MCs, whereas, in the work of Perucca Orfei et al. [74], cells were co‐incubated with MCs in a bioreactor for different time intervals and conditions. In order to make silk fibroin/alginate more stable, the MCs were freeze‐dried before optimising the seeding conditions. It was later confirmed that lyophilisation did not affect the MCs structure. Cell density and sample quantity in an oscillating shaker were fixed while the duration of time, stirring speed, dynamic culture and volume of MCs/cell suspension were the variable process parameters. Both adipose‐derived stem cells (hASCs) and human bone marrow stem cells (hBMSCs) showed a good cell adhesion rate on the fibroin coated materials. hASCs were able to adhere to the MCs in <2 h, under suitable conditions. In particular, the dynamic seeding of cells provided the best outcomes in comparison with static cultures, in terms of cell adhesion and viability. The best outcome was achieved for the following seeding conditions: 98 min of incubation time, 12.3 rpm of stirring speed, and 401.5 µl volume of MCs/cell suspension‐cultured with the intermittent dynamic condition. The last parameter was found to deeply influence the final cell adhesion, proliferation, and differentiation.
3.2 Silk/gelatin MCs
Gelatin, a biocompatible and biodegradable protein obtained from the hydrolysis of collagen, can be easily cross‐linked due to its large number of functional groups. Its characteristic RGD sequence (arginine–glycine–aspartic acid) is essential for stable relationships between the cells and the surrounding extracellular matrix, being recognised by integrins and cell surface receptors. Despite its good biological properties, gelatin has poor mechanical properties and is therefore combined with other materials such as silk [76].
Silk composites with 30% gelatin were obtained by cryodestruction in the studies conducted by Arkhipova et al. [59, 77]. Morphological evaluation of the synthesized MCs evidenced the formation of an irregular‐shaped and porous surface. Increased cell adhesion and proliferation of 3T3 murine fibroblast of silk/gelatin MCs in vitro were confirmed by Arkhipova et al. [59] under static culture conditions. Although complete wound healing was observed in the control group and in the fibroin and fibroin/gelatin MCs, the composites promoted regeneration of damaged areas to a greater extend by regenerating muscle tissue and subcutaneous hypodermis [77]. In vivo, subcutaneous administration in mice of silk/gelatin MCs was performed in another study conducted by Arkhipova et al. [77], demonstrating that these biomaterials are also suitable for the treatment of deep skin wounds. These MCs increase expression of proinflammatory cytokines, which are the key mediators of inflammation [78].
Spherical and porous MCs for osteogenic tissue engineering were first studied by Luetchfird et al. [17]. The MCs were synthesized under dynamic culture conditions using a microfluidic flow system to reproducibly obtain MCs with controlled properties [17]. Gelatin and fibroin solutions were mixed into blends with varying fibroin/gelatin ratios and linked to the inner phase of a T‐junction flow‐focusing device. The outer phase consisted of oleic acid, methanol and Span 80 to induce ‐sheet conformational change in silk fibroin. The inner and outer flow rates were fixed in order to compare the physicochemical characteristics of the different MCs collected (device output) and to generate biomaterials with diameters comparable with commercially available MCs. It was observed that the MCs synthetized, besides having a mean particle diameter in the range of 300–400 μm, also have a narrow size distribution.
Homogeneous MCs were mainly formed in fibroin/gelatin 50:50 and 25:75% w/v, having these blends higher mechanical properties and seeding efficiencies. Contrary to what was expected, pure gelatin materials did not promote rat MSC (rMSC) isolation proliferation. Gelatin swelling resulting from water absorption of the culture medium can occur, reducing the biomaterial mechanical properties and making it unsuitable to stimulate cell adhesion [79]. By adding fibroin, for all conditions studied, cell adhesion and osteogenic proliferation were significantly increased. Contrary to the other reported works on silk/gelatin MCs [59, 77], the system used has the advantage of allowing scale up production of therapeutic cell systems [17].
3.3 Silk/calcium phosphate MCs
Besides natural polymers, inorganic components such as calcium phosphates have also been used to synthetise silk MCs. These compounds are the main components of hard tissues, exhibiting properties of biocompatibility, bioactivity and osteoconductivity [80].
Fibroin MCs modified by calcium phosphate mineralisation have also been studied. In the work of Kotliarova et al. [29] and Goncharenko et al. [81], porous MCs with the size ranging from 100 to 250 μm were obtained by cryodestruction of the matrices to generate fibroin, fibroin/gelatin, fibroin/calcium phosphate and fibroin/gelatin/calcium phosphate materials. Osteosarcoma cell lines (MG‐63) and MSCs cell adhesion occurred for all types of MCs. Cell proliferation was found to be significatively lower for mineralised MCs, probably since substrate mineralisation contributed to the induction of osteogenic differentiation in the absence of inductors, reducing the rate of proliferation [81]. While gelatin can be added to the material in order to promote the cellular response [76], calcium phosphate surface increases the MCs roughness. Although this mechanical factor is not linked to significant changes in cell proliferation, it can promote osteogenic differentiation [82]. Therefore, these fibroin/calcium phosphate MCs can be used to stimulate bone regeneration.
3.4 Silk/pullulan MCs
In addition to the silk bioconjugates covered so far, there is also a work conducted by Aydogdu et al. [60] in which fibroin is used as a coating instead of being used as the core of MCs, as conventionally reported. Instead, pullulan, a natural polymer, was used as the main component of the MCs due to its non‐toxic, non‐immunogenic and biodegradable nature [83].
Pullulan (15%, w/v) was cross‐linked by trisodium trimetaphosphate (7.1% w/v), being this homogeneous solution further mixed with calcium carbonate microparticles with a mean size of 20 μm. The final mixture, in combination with biodegradable surfactants, was added to a stirred tank batch reactor with a stirring motor at 500 rpm. To obtain a porous construct, a porogen leaching phase was implemented after the water‐in‐oil emulsion methodology. The silk coating was obtained via reductive amination, producing particles with an average size of 169.9 ± 45.4 μm. To improve both biological and mechanical properties, pullulan/fibroin MCs were further processed using biomimetic mineralisation by incubation in simulated body fluid (SBF). After 7 days of SBF incubation, sphere‐like MCs with ∼150.1 μm were obtained. As expected, higher mechanical properties and cytocompatibility were obtained by increasing the MCs stability and their bioactivity upon treatment with SBF. The in vitro osteogenic potency of the MCs was investigated using SaoS‐2 cell line, which displays several osteoblastic features, in static and dynamic culture conditions. Similar cell adhesion was obtained for fibroin‐coated MCs and mineralised MCs, in both culture conditions after 1 day of incubation. Additionally, pure pullulan MCs have lower cell adhesion when compared to fibroin/pullulan MC. However, in dynamic conditions, significantly higher cell attachment was observed. Regarding cell proliferation, contrary to what was expected, the overall profiles of cell viability and proliferation were found to be similar for static and dynamic culture conditions. This can be due to the parameters fixed for the dynamic cell culture. According to the review presented by Egger et al. [84], a higher cell number in dynamic cultivation conditions is usually obtained for moderate culture mixing speeds.
3.5 Silk/chitosan and silk/collagen microparticles
There are other works in which silk fibroin‐based microspheres are developed, though the term MCs is not used. They are based on 3D materials at the microscale and with the same morphology to promote cell adhesion and expansion. The combination of silk fibroin with positive charge polymers, such as chitosan and collagen, to obtain microparticles to apply in mucus or similar physiological environments has been the subject of study [62].
On the one hand, chitosan is a biocompatible polymer that promotes cell adhesion and migration, being thus combined with fibroin [85]. On the other hand, collagen is the most abundant protein present in mammalian tissues, tendons, ligaments, bone and skin and is therefore widely used in biomaterials for biomedical engineering. Besides promoting cell attachment, this natural protein has excellent biocompatible properties and can be easily degraded and resorbed by the body [86]. However, poor physical and mechanical properties, and poor thermal stability, justify combination with fibroin in order to obtain a material with suitable structural integrity [87].
Nimisha et al. [61] compared the biological characteristics of fibroin, fibroin/chitosan and fibroin/collagen microparticles. To do so, silk microparticles were obtained by dropping droplets of a fibroin/HFIP (hexafluoroisopropanol) solution in a methanol coagulant bath. The surface of these microparticles was then modified with 1 wt% chitosan or collagen. The physicochemical properties of the three experimental conditions were similar, with pores of the order of 100 mm. However, the coated materials led to an increase in the number of viable MG 63 osteoblast‐like cells. Further, cell attachment and viability was higher in fibroin/collagen composites. However, the osteogenic differentiation was better in fibroin/chitosan microparticles.
Chitosan is also widely used in the controlled release of bioactive proteins or peptide drugs. Nevertheless, chitosan microparticles have limitations such as a high initial burst release rate and a short period of sustained‐release [88]. Several works focus on the synthesis of fibroin/chitosan to increase the release period, using a spray‐drying technique [62, 89] or an emulsification approach [90, 91, 92]. The microspheres obtained usually present a smooth [88, 90] or wrinkled surface [90, 91, 92] with a diameter that can range from ∼7 to 150 μm [90].
The dissolution of the microparticles can be controlled by adjusting the fibroin/chitosan blend ratio. According to the study of Cheerarot and Baimark [91], increasing the chitosan content in the blend led to a further decrease in the dissolution percentage. Adding different components is also a route to obtain different release periods. In the work of Chung et al. [62], an antibiotic (tetracycline‐Hcl) was encapsulated into silk/chitosan and silk/chitosan/tri‐polyphosphate (TPP). The release period of pure fibroin (two days) was prolonged to four and ten days for fibroin/chitosan (10/4.0 wt%) and silk/chitosan/TPP (0.05%) microparticles, respectively [62]. A lower initial burst release and a prolonged cumulative release of up to 21 days were achieved in [90], by using genipin as a cross‐linking agent to produce fibroin/chitosan microparticles (1:1%wt) to encapsulate bovine serum albumin (BSA). It was also found that by using 0.05 g of genipin, particles with the most uniform particle‐size distribution were obtained. In addition to the in vitro release studies conducted in the reviewed papers, Hu et al. [92] confirmed the potential of these composites in vivo. The microparticles were orally administered to rats, and their blood concentration was measured at specific time intervals.
Fibroin/chitosan composites have also been explored in the work of Yang et al. [93] as possible cardiac patches. Microspheres with particle sizes ranging from 70 to 147 μm, obtained through spray‐drying, led to a better relative growth rate of rMSCs than pure chitosan. In addition, the cardiomyogenic differentiations of rMSCs on the silk‐based patches in vitro were further enhanced by adding hyaluronic acid.
3.6 Silk organoids
Other innovative silk 3D constructs that have been developed to promote cell adhesion and proliferation are organoids. These structures form organ‐like tissues that mimic natural organs, structurally and functionally in vitro. Lyophilised silk scaffolds were formed and seeded with kidney progenitors [94]. The main limitation highlighted in these structures is the lack of in vivo‐like functionality, such as vascularisation. For tissue to grow beyond 100–200 µm, expanding into a microtissue, new blood‐vessel formation is required to supply the individual cells with nutrients and oxygen. Otherwise, nutrient deficiencies, hypoxia, on‐uniform cell differentiation and integration can occur in the tissue [95].
Potential strategies for enabling organoid vascularisation include the use of silk fibroin in laser photo‐ablation methodologies [96]. Gupta et al. [97] evaluated the capacity of silk to serve as an organoid to form kidney tissue from primary cells and human‐induced pluripotent stem cells (iPSCs). This trending silk technology was found to be well suited to support iPSCs growth and differentiation into kidney tissue. However, the proliferation of stromal cells within the graft and tissue organisation stills presents a challenge to overcome [97].
According to the final application, different types of silk composites and cell types can be used, as summarised in Table 2.
Table 2.
Silk‐based 3D structures reviewed, cell types used and potential applications
| Biomaterial | Synthesis methods | Cell type used | Potential applications | References |
|---|---|---|---|---|
| silk fibroin MCs | droplet production | murine fibroblasts L‐929 | wound healing | [63] |
| co‐precipitation | C2C12 cell line | drug delivery system for TE applications | [65, 66] | |
| silk fibroin/alginate MCs | droplet production followed by fibroin coating | hASCs | cell delivery for advanced therapy and regenerative medicine of bone and cartilage diseases or defects | [28, 58, 74] |
| hBMSCs | ||||
| silk fibroin/gelatin MCs | freezing thawing | 3T3 murine fibroblast | wound healing | [59, 77] |
| mixing | rMSC | bone TE | [17] | |
| silk fibroin/calcium phosphate MCs | freezing thawing | MG‐63 | bone‐TE | [29, 81] |
| MSCs | ||||
| silk fibroin/pullulan MCs | water‐in‐oil emulsion | SaoS‐2 cell line | bone‐TE | [60] |
| silk fibroin/chitosan microparticles | spray‐drying | rMSCs | cardiac patches | [93] |
| — | drug delivery systems | [62, 89] | ||
| emulsification | — | [90, 91, 92] | ||
| silk fibroin/Collagen microparticles | droplet production followed by coating | MG 63 osteoblast‐like cell line | bone‐TE (load‐bearing osteo‐regenerative applications). | [61] |
| silk organoids | freeze‐drying | iPSCs | regenerative medicine – kidney tissue | [97] |
4 Future perspectives
MCs offer an environment that resembles biological systems, where cells can proliferate, maintain their cell phenotype and expand on a larger scale. Due to these properties, MC systems have the potential to produce 3D microtissues, which can be used to regenerate and restore damaged tissue. Thus, MCs have a huge potential in regenerative medicine [7].
MCs are a recent technology, being most of the reported work from the last 5 years. Despite the growing interest in this topic, there are still several aspects to be studied and explored.
4.1 Exploring silk‐based MCs for biomedical applications
Although silk‐sericin MCs have not yet been reported, this type of biomaterial has a great interest in biomedical engineering. Sericin can be easily incorporated in the reviewed MCs through the coating, in order to improve the biological activity of MCs [24]. Even though most silk‐based MCs use fibroin as the core material, the works carried out with pullulan and alginate (Sections 3.1 and 3.4) demonstrates that other materials can be used to give integrity to the material and that silk protein can be incorporated through different methodologies. An increasing number of studies have shown that sericin improves cell adhesion and proliferation when used as an organic matrix or a medium for cell growth [98, 99, 100]. Moreover, sericin can be used as serum‐free media, substituting foetal bovine serum, thus being recovered for biomedical applications [101, 102]. These properties can be useful to maintain the integrity, structure, and intrinsic function of microtissues developed from MCs, allowing the creation of stocks for later use or even opening new avenues for the transportation of synthetic tissue substitutes to remote locations. Nevertheless, recently proposed technologies for enhancing the stability of sericin, such as enzymatic cross‐linking (WO/2018/011732), might open room for the development of viable MCs.
Additionally, specific growth factors or extracellular matrix proteins, besides BMP, can be included in silk‐based MCs to further aid cell adhesion and expansion. Hence, these 3D materials can serve a dual role as both delivery systems of bioactive factors and scaffolds for cell proliferation and differentiation. Moreover, other components that can be used in the development of new silk‐based MCs for different biomedical applications are dextran, cellulose, plastic or glass [106].
4.2 Dynamic culture systems to generate silk‐based MCs
Although the use of bioreactors to perform cell seeding on MCs is a promising way to mimic and simulate the biological environment in order to obtain 3D constructs, studies of fibroin‐based MCs with dynamic cell culture are still scarce [60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74].
In addition, conventional spinner‐flask and stirred tank bioreactors to have limitations related to mass transfer phenomena that can affect both the producibility and the product characteristics [7]. Since the characteristics of MCs (e.g. chemical composition, surface topography, charge density, particle size etc.) are intrinsically related to their ability to promote cell adhesion and proliferation, it is important to find solutions to these limitations [7, 8, 107]. Further, for clinical applications, it is also important to obtain a fully functional microtissue, overcoming the main limitation of the silk organoids reported: lack of vascularisation [97, 108]. Employing these systems is also related with other critical issues such as the standardisation of bioprocesses and limited upscaling [14].
In that regard, oscillatory flow reactors (OFRs) are an example of a ‘technology ready to deliver’ [109, 110]. In particular, a recently developed meso‐OFR (meso‐OFR) [110] has been shown as a promising tool in multiphase systems, namely by promoting a significant increase in the intensification of mixing leading to the formation of materials with controlled properties [111, 112]. The device consists of a glass tube at the mesoscale provided with smooth periodic constrictions (SPCs) operating under oscillatory flow mixing. The intensity of the mixture is controlled by the frequency and amplitude of the fluid oscillation [110, 113, 114]. This reactor has been successfully used in the precipitation of non‐toxic, pure hydroxyapatite and hydroxyapatite/silk sericin materials with improved cell viability when compared to a commercially available hydroxyapatite powder [43]. Moreover, meso‐OFR has been successfully implemented in the growth of fungi cells [113]. Thereby, the OFR has the potential for both the synthesis of MCs and for posterior tissue maturation. Further, the scale of these devices can be easily extended and the devices can operate within a batch and continuous flow regimes (Fig. 4 a) [115]. Large‐scale culture technology is crucial in MCs and organoid technology to obtain the number of stem cells required for clinical application [6, 97].
Fig. 4.
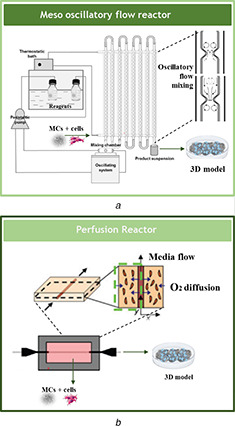
Illustration of the implementation of recently developed bioreactors in the production of an MC‐based 3D construct
(a) Meso‐OFR (image adapted from [103]), (b) Perfusion bioreactor (image adapted from [104, 105])
Post‐processing of the 3D structure can also be achieved using a perfusion bioreactor. These reactors improve mass transfer not only on the external surfaces of tissue scaffolds, but also around internal areas, stimulating tissue growth and maturation in a homogeneous manner [116, 117] In the work of Zhang et al. [118] and Grasman et al. [104] a perfection reactor was used for the integration of vascular cells into silk scaffolds (Fig. 4 b).
Innovative technologies covered in this review, which promote cell adhesion and expansion, such as bioprinting and conventional chemical processing methodologies, can be further complemented with the highlighted bioreactors.
Despite being a well‐established method to assemble cells and specific extracellular matrix within a 3D construct, the development of bioprinted skin‐like constructs still faces several challenges, including the promotion of critical cell–cell interactions and signal communications. The selection of suitable bioinks and of in vitro culture systems that allow for cell expansion are among the methods used to improve tissue authenticity over conventional tissue equivalents [106]. In this context, silk‐based MCs are a developing technology used to promote a high‐yield culture of anchorage‐dependent cells providing appropriate microenvironments for cell interaction in vitro and allowing the standardisation of bioprocesses. Fibroin MCs with tailored properties, can be obtained using an OFR and loaded in the bioink to obtain a specific 3D microtissue. The perfusion reactor can be used as a final step for the development and maturation of vascular and neuronal tissues.
5 Conclusions
MCs systems have a porous microstructure, in order to allow cells to attach and grow. These 3D constructs conventionally adopt a spherical morphology; however, other morphologies have been explored. Besides providing a high surface area to volume ratio for maximising cell number during cell expansion, MCs allow efficient gas–liquid oxygen exchanges, being cost‐effective and space‐saving. In this context, the scientific interest in silk‐based composites has considerably increased in recent years. Silk fibroin combines biological properties, such as biodegradability and non‐toxicity, with mechanical strength and stability. Silk fibroin is the most widely studied silk protein.
In short, the herein reviewed literature highlights the diversity of potential biomedical applications of MCs and other recent 3D silk‐based structures. Despite the advances made in the last decade, there are still several studies that need to be conducted. The use of silk sericin in MC systems, as well as the development of further studies on the behaviour of MCs in conventional and new biological reactors, is among the future perspectives discussed in this paper.
6 Acknowledgments
This work was financially supported by Base Funding – UIDB/00511/2020 of the Laboratory for Process Engineering, Environment, Biotechnology and Energy – LEPABE – funded by national funds through the FCT/MCTES (PIDDAC). The authors also acknowledge Portuguese National Funds from FCT – Fundação para a Ciência e a Tecnologia through project UID/Multi/04044/2019. This work was supported by project Biotherapies‐Bioengineered Therapies for Infectious Diseases and Tissue Regeneration and Interreg V‐A POCTEP Programme through FEDER funds from the European Union [0245_IBEROS_1_E] C. A.
7 References
- 1. Allied Market Research : ‘Biomaterials market by type (metallic, polymeric, ceramic, and natural) and application (cardiovascular, dental, orthopedic, wound healing, plastic, surgery, ophthalmology, tissue engineering) ‐ global opportunity analysis and industry forecast, 2014–20’, https://www.premiummarketinsights.com/reports‐amr/biomaterials‐market, accessed February 2020
- 2. Hossain A., Roy S., Guin P.S.: ‘The importance of advance biomaterials in modern technology: a review’, Asian J. Res. Chem., 2017, 10, (4), p. 441 [Google Scholar]
- 3. Martin Y., Eldardiri M., Lawrence‐Watt D.J. et al.: ‘Microcarriers and their potential in tissue regeneration’, Tissue Eng. B Rev., 2011, 17, (1), pp. 71–80 [DOI] [PubMed] [Google Scholar]
- 4. Sart S., Agathos S.N., Li Y.: ‘Engineering stem cell fate with biochemical and biomechanical properties of microcarriers’, Biotechnol. Prog., 2013, 29, (6), pp. 1354–1366 [DOI] [PubMed] [Google Scholar]
- 5. Malda J., Frondoza C.G.: ‘Microcarriers in the engineering of cartilage and bone’, Trends Biotechnol., 2006, 24, (7), pp. 299–304 [DOI] [PubMed] [Google Scholar]
- 6. Tavassoli H., Alhosseini S.N., Tay A. et al.: ‘Large‐scale production of stem cells utilizing microcarriers: a biomaterials engineering perspective from academic research to commercialized products’, Biomaterials, 2018, 181, pp. 333–346 [DOI] [PubMed] [Google Scholar]
- 7. Li B., Wang X., Wang Y. et al.: ‘Past, present, and future of microcarrier‐based tissue engineering’, J. Orthop. Trans., 2015, 3, (2), pp. 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun L.‐Y., Lin S.‐Z., Li Y.‐S. et al.: ‘Functional cells cultured on microcarriers for use in regenerative medicine research’, Cell Transplant., 2011, 20, (1), pp. 49–62 [DOI] [PubMed] [Google Scholar]
- 9. Pörtner R., Nagel‐Heyer S., Goepfert C. et al.: ‘Bioreactor design for tissue engineering’, J. Biosci. Bioeng., 2005, 100, (3), pp. 235–245 [DOI] [PubMed] [Google Scholar]
- 10. Saleem I., Petkar K., Somavarapu S.: ‘Rationale for pulmonary vaccine delivery: formulation and device considerations’, in Skwarczynski M., Toth I. (Eds.): ‘Micro and nanotechnology in vaccine development’ (Elsevier, Amsterdam, 2017), pp. 357–371 [Google Scholar]
- 11. Mottaghitalab F., Farokhi M., Shokrgozar M.A. et al.: ‘Silk fibroin nanoparticle as a novel drug delivery system’, J. Controlled Release, 2015, 206, pp. 161–176 [DOI] [PubMed] [Google Scholar]
- 12. Numata K., Kaplan D.L.: ‘Silk‐based delivery systems of bioactive molecules’, Adv. Drug Deliv. Rev., 2010, 62, (15), pp. 1497–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gwinn M.R., Vallyathan V.: ‘Nanoparticles: health effects – pros and cons’, Environ. Health Perspect., 2006, 114, (12), pp. 1818–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massai D., Isu G., Madeddu D. et al.: ‘A versatile bioreactor for dynamic suspension cell culture. application to the culture of cancer cell spheroids’, PLOS One, 2016, 11, (5), pp. 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goepfert C., Lutz V., Lünse S. et al.: ‘Evaluation of cartilage specific matrix synthesis of human articular chondrocytes after extended propagation on microcarriers by image analysis’, Int. J. Artif. Organs, 2010, 33, (4), pp. 204–218 [PubMed] [Google Scholar]
- 16. Perez R.A., Riccardi K., Altankov G. et al.: ‘Dynamic cell culture on calcium phosphate microcarriers for bone tissue engineering applications’, J. Tissue Eng., 2014, 5, pp. 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luetchford K.A., Chaudhuri J.B., De Bank P.A.: ‘Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering’, Mater. Sci. Eng. C, 2020, 106, (September 2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Price J.R., Shieh W.K., Sales C.M.: ‘A novel bioreactor for high density cultivation of diverse microbial communities’, J. Visualized Exp., 2015, 2015, (106), pp. 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svobodová K., Novotný Č.: ‘Bioreactors based on immobilized fungi: bioremediation under non‐sterile conditions’, Appl. Microbiol. Biotechnol., 2018, 102, (1), pp. 39–46 [DOI] [PubMed] [Google Scholar]
- 20. Obom K.M., Cummings P.J., Ciafardoni J.A. et al.: ‘Cultivation of mammalian cells using a single‐use pneumatic bioreactor system’, J. Visualized Exp., 2014, e52008, (92), pp. 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frahm B., Brod H., Langer U.: ‘Improving bioreactor cultivation conditions for sensitive cell lines by dynamic membrane aeration’, Cytotechnology, 2009, 59, (1), pp. 17–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawton S., Steele G., Shering P. et al.: ‘Continuous crystallization of pharmaceuticals using a continuous oscillatory baffled crystallizer’, Org. Process Res. Dev., 2009, 13, (6), pp. 1357–1363 [Google Scholar]
- 23. Verma D., Gulati N., Kaul S. et al.: ‘Protein based nanostructures for drug delivery’, J. Pharm., 2018, 2018, pp. 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veiga A., Castro F., Rocha F. et al.: ‘Recent advances in silk sericin/calcium phosphate biomaterials’, Front. Mater., 2020, 7, (February), pp. 1–14 [Google Scholar]
- 25. Patra J.K., Baek K.‐H.: ‘Biosynthesis of silver nanoparticles using aqueous extract of silky hairs of corn and investigation of its antibacterial and anticandidal synergistic activity and antioxidant potential’, IET Nanobiotechnol., 2016, 10, (5), pp. 326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanson‐manful P., Patrick W.M.: ‘Protein nanotechnology’ (Humana Press, USA., 2013) [Google Scholar]
- 27. Mondal M., Trivedy K., Kumar S.N.: ‘The silk proteins, sericin and fibroin in silkworm, Bombyx mori linn., ‐ a review’, Casp. J. Environ. Sci., 2006, 25, (4), pp. 77–84 [Google Scholar]
- 28. Duchi S., Piccinini F., Pierini M. et al.: ‘A new holistic 3D non‐invasive analysis of cellular distribution and motility on fibroin‐alginate microcarriers using light sheet fluorescent microscopy’, PLOS One, 2017, 12, (8), p. e0183336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kotliarova M.S., Zhuikov V.A., Chudinova Y. V. et al.: ‘Induction of osteogenic differentiation of osteoblast‐like cells MG‐63 during cultivation on fibroin microcarriers’, Moscow Univ. Biol. Sci. Bull., 2016, 71, (4), pp. 212–217 [Google Scholar]
- 30. Nguyen T.P., Nguyen Q.V., Nguyen V. et al.: ‘Silk fibroin‐based biomaterials for biomedical applications: a review’, Polymers (Basel)., 2019, 11, (12), p. 1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Terada S., Nishimura T., Sasaki M. et al.: ‘Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma’, Cytotechnology, 2002, 40, (1–3), pp. 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teramoto H., Nakajima K., Takabayashi C.: ‘Chemical modification of silk sericin in lithium chloride/dimethyl sulfoxide solvent with 4‐cyanophenyl isocyanate’, Biomacromolecules, 2004, 5, (4), pp. 1392–1398 [DOI] [PubMed] [Google Scholar]
- 33. Sofia S., McCarthy M.B., Gronowicz G. et al.: ‘Functionalized silk‐based biomaterials for bone formation’, J. Biomed. Mater. Res., 2001, 54, (1), pp. 139–148 [DOI] [PubMed] [Google Scholar]
- 34. Roohani‐Esfahani S.I., Lu Z.F., Li J.J. et al.: ‘Effect of self‐assembled nanofibrous silk/polycaprolactone layer on the osteoconductivity and mechanical properties of biphasic calcium phosphate scaffolds’, Acta Biomater., 2012, 8, (1), pp. 302–312 [DOI] [PubMed] [Google Scholar]
- 35. Nilsson K.: ‘Microcarrier cell culture’, Biotechnol. Genet. Eng. Rev., 1988, 6, (1), pp. 404–439 [PubMed] [Google Scholar]
- 36. Park J.‐H., Lee E.‐J., Knowles J.C. et al.: ‘Preparation of in situ hardening composite microcarriers: calcium phosphate cement combined with alginate for bone regeneration’, J. Biomater. Appl., 2014, 28, (7), pp. 1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caplan A.I.: ‘Mesenchymal stem cells: time to change the name!’, Stem Cells Transl. Med., 2017, 6, (6), pp. 1445–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Costa F., Silva R., Boccaccini A.R.: ‘Fibrous protein‐based biomaterials (silk, keratin, elastin, and resilin proteins) for tissue regeneration and repair’, in Barbosa M., Martins C. (Eds.): ‘Peptides and proteins as biomaterials for tissue regeneration and repair’ (Elsevier, Woodhead Publishing, UK., 2017), pp. 175–204 [Google Scholar]
- 39. Lungu A., Albu M.G., Stancu I.C. et al.: ‘Superporous collagen‐sericin scaffolds’, J. Appl. Polym. Sci., 2013, 127, (3), pp. 2269–2279 [Google Scholar]
- 40. Wang Z., Zhang Y., Zhang J. et al.: ‘Exploring natural silk protein sericin for regenerative medicine: an injectable, photoluminescent, cell‐adhesive 3D hydrogel’, Sci. Rep., 2015, 4, (1), p. 7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kwak H.W., Lee H., Lee M.E. et al.: ‘Facile and green fabrication of silk sericin films reinforced with bamboo‐derived cellulose nanofibrils’, J. Clean. Prod., 2018, 200, pp. 1034–1042 [Google Scholar]
- 42. Khandai M., Chakraborty S., Sharma A. et al.: ‘Preparation and evaluation of algino‐sericin mucoadhesive microspheres: an approach for sustained drug delivery’, J. Adv. Pharm. Res., 2010, 1, (1), pp. 48–60 [Google Scholar]
- 43. Veiga A., Castro F., Reis C.C. et al.: ‘Hydroxyapatite/sericin composites: a simple synthesis route under near‐physiological conditions of temperature and pH and preliminary study of the effect of sericin on the biomineralization process’, Mater. Sci. Eng. C, 2020, 108, p. 110400 [DOI] [PubMed] [Google Scholar]
- 44. Wang L., Nemoto R., Senna M.: ‘Changes in microstructure and physico‐chemical properties of hydroxyapatite‐silk fibroin nanocomposite with varying silk fibroin content’, J. Eur. Ceram. Soc., 2004, 24, (9), pp. 2707–2715 [Google Scholar]
- 45. Zou R., Niu L., Liu Q. et al.: ‘A novel nanocomposite particle of hydroxyapatite and silk fibroin: biomimetic synthesis and its biocompatibility’, J. Nanomater., 2010, 2010, pp. 1–6 [Google Scholar]
- 46. Kong X.D., Cui F.Z., Wang X.M. et al.: ‘Silk fibroin regulated mineralization of hydroxyapatite nanocrystals’, J. Cryst. Growth, 2004, 270, (1‐2), pp. 197–202 [Google Scholar]
- 47. Zakharov N.A., Demina L.I., Aliev A.D. et al.: ‘Synthesis and properties of calcium hydroxyapatite/silk fibroin organomineral composites’, Inorg. Mater., 2017, 53, (3), pp. 333–342 [Google Scholar]
- 48. Miroiu F.M., Socol G., Visan A. et al.: ‘Composite biocompatible hydroxyapatite‐silk fibroin coatings for medical implants obtained by matrix assisted pulsed Laser evaporation’, Mater. Sci. Eng. B Solid‐State Mater. Adv. Technol., 2010, 169, (1–3), pp. 151–158 [Google Scholar]
- 49. Chen L., Hu J., Ran J. et al.: ‘Preparation and evaluation of collagen‐silk fibroin/hydroxyapatite nanocomposites for bone tissue engineering’, Int. J. Biol. Macromol., 2014, 65, (4), pp. 1–7 [DOI] [PubMed] [Google Scholar]
- 50. Rockwood D.D.N., Preda R.R.C., Yücel T. et al.: ‘Materials fabrication from Bombyx mori silk fibroin’, Nat. Protoc., 2011, 6, (10), pp. 1–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bhardwaj N., Kundu S.C.: ‘Silk fibroin protein and chitosan polyelectrolyte complex porous scaffolds for tissue engineering applications’, Carbohydr. Polym., 2011, 85, (2), pp. 325–333 [Google Scholar]
- 52. Farokhi M., Mottaghitalab F., Samani S. et al.: ‘Silk fibroin/hydroxyapatite composites for bone tissue engineering’, Biotechnol. Adv., 2018, 36, (1), pp. 68–91 [DOI] [PubMed] [Google Scholar]
- 53. Yang M., Shuai Y., Zhang C. et al.: ‘Biomimetic nucleation of hydroxyapatite crystals mediated by antheraea pernyi silk sericin promotes osteogenic differentiation of human bone marrow derived mesenchymal stem cells’, Biomacromolecules, 2014, 15, (4), pp. 1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lindenbergh R., Roopani Kumar D., Merchant N. et al.: ‘Extraction and characterization of sericin and its immobilization on hydroxyapatite nanoparticles for tissue engineering applications’, Int. J. ChemTech Res., 2010, 7, (5), pp. 2117–2124 [Google Scholar]
- 55. Li W., Cai Y., Zhong Q. et al.: ‘Silk sericin microcapsules with hydroxyapatite shells: protection and modification of organic microcapsules by biomimetic mineralization’, J. Mater. Chem. B, 2016, 4, (2), pp. 340–347 [DOI] [PubMed] [Google Scholar]
- 56. Yang M., Shuai Y., Zhou G. et al.: ‘Tuning molecular weights of Bombyx mori (B. mori) silk sericin to modify its assembly structures and materials formation’, ACS Appl. Mater. Interfaces, 2014, 6, (16), pp. 13782–13789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barajas‐Gamboa J.A., Serpa‐Guerra A.M., Restrepo‐Osorio A. et al.: ‘Sericin applications: a globular silk protein’, Ing. Y Comput., 2016, 18, (2), pp. 193–206 [Google Scholar]
- 58. Perteghella S., Martella E., de Girolamo L. et al.: ‘Fabrication of innovative silk/alginate microcarriers for mesenchymal stem cell delivery and tissue regeneration’, Int. J. Mol. Sci., 2017, 18, (9), p. 1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arkhipova A.Y., Kotlyarova M.C., Novichkova S.G. et al.: ‘New silk fibroin‐based bioresorbable microcarriers’, Bull. Exp. Biol. Med., 2016, 160, (4), pp. 491–494 [DOI] [PubMed] [Google Scholar]
- 60. Aydogdu H., Keskin D., Baran E.T. et al.: ‘Pullulan microcarriers for bone tissue regeneration’, Mater. Sci. Eng. C, 2016, 63, pp. 439–449 [DOI] [PubMed] [Google Scholar]
- 61. Parekh N., Hushye C., Warunkar S. et al.: ‘In vitro study of novel microparticle based silk fibroin scaffold with osteoblast‐like cells for load‐bearing osteo‐regenerative applications’, RSC Adv., 2017, 7, (43), pp. 26551–26558 [Google Scholar]
- 62. Chung T.W., Chang C.H., Ho C.W.: ‘Incorporating chitosan (CS) and TPP into silk fibroin (SF) in fabricating spray‐dried microparticles prolongs the release of a hydrophilic drug’, J. Taiwan Inst. Chem. Eng., 2011, 42, (4), pp. 592–597 [Google Scholar]
- 63. Wang L., Xia Z.R., Lv L.L. et al.: ‘Preparation of silk fibroin microspheres and its cytocompatibility’, Adv. Mater. Res., 2011, 236–238, (January), pp. 1902–1905 [Google Scholar]
- 64. Gurtner G.C., Werner S., Barrandon Y. et al.: ‘Wound repair and regeneration’, Nature, 2008, 453, (7193), pp. 314–321 [DOI] [PubMed] [Google Scholar]
- 65. Bessa P.C., Balmayor E.R., Hartinger J. et al.: ‘Silk fibroin microparticles as carriers for delivery of human recombinant bone morphogenetic protein‐2: in Vitro and In vivo bioactivity’, Tissue Eng. Part C., 2010, 16, (5), pp. 937–945 [DOI] [PubMed] [Google Scholar]
- 66. Bessa P.C., Balmayor E.R., Azevedo H.S. et al.: ‘Silk fibroin microparticles as carriers for delivery of human recombinant BMPs. Physical characterization and drug release’, J. Tissue Eng. Regen. Med., 2010, 4, (5), pp. 349–355 [DOI] [PubMed] [Google Scholar]
- 67. Montano M.: ‘Model systems’, in Montano M. (Ed.): ‘Translational biology in medicine’ (Elsevier, Woodhead Publishing, UK., 2014), pp. 9–33 [Google Scholar]
- 68. Mumcuoglu D., Siverino C., Tabisz B. et al.: ‘How to use BMP‐2 for clinical applications? a review on pros and cons of existing delivery strategies’, J. Transl. Sci., 2017, 3, (5), pp. 1–11 [Google Scholar]
- 69. Kundu B., Rajkhowa R., Kundu S.C. et al.: ‘Silk fibroin biomaterials for tissue regenerations’, Adv. Drug Deliv. Rev., 2013, 65, (4), pp. 457–470 [DOI] [PubMed] [Google Scholar]
- 70. Hu X., Cebe P., Weiss A.S. et al.: ‘Protein‐based composite materials’, Mater. Today, 2012, 15, (5), pp. 208–215 [Google Scholar]
- 71. Lee K.Y., Mooney D.J.: ‘Alginate: properties and biomedical applications’, Prog. Polym. Sci., 2012, 37, (1), pp. 106–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rowley J.A., Madlambayan G., Mooney D.J.: ‘Alginate hydrogels as synthetic extracellular matrix materials’, Biomaterials, 1999, 20, (1), pp. 45–53 [DOI] [PubMed] [Google Scholar]
- 73. Wu J., Yang R., Zheng J. et al.: ‘Fabrication and improvement of PCL/alginate/PAAm scaffold via selective laser sintering for tissue engineering’, Micro Nano Lett., 2019, 14, (8), pp. 852–855 [Google Scholar]
- 74. Perucca Orfei C., Talò G., Viganò M. et al.: ‘Silk/fibroin microcarriers for mesenchymal stem cell delivery: optimization of cell seeding by the design of experiment’, Pharmaceutics., 2018, 10, (4), p. 200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yan L.‐P., Oliveira J.M., Oliveira A.L. et al.: ‘In vitro evaluation of the biological performance of macro/micro‐porous silk fibroin and silk‐nano calcium phosphate scaffolds’, J. Biomed. Mater. Res. B. Appl. Biomater., 2015, 103, (4), pp. 888–898 [DOI] [PubMed] [Google Scholar]
- 76. Jaipan P., Nguyen A., Narayan R.J.: ‘Gelatin‐based hydrogels for biomedical applications’, MRS Commun., 2017, 7, (3), pp. 416–426 [Google Scholar]
- 77. Arkhipova A.Y., Nosenko M.A., Malyuchenko N.V. et al.: ‘Effects of fibroin microcarriers on inflammation and regeneration of deep skin wounds in mice’, Biochemistry, 2016, 81, (11), pp. 1251–1260 [DOI] [PubMed] [Google Scholar]
- 78. Eming S.A., Krieg T., Davidson J.M.: ‘Inflammation in wound repair: molecular and cellular mechanisms’, J. Invest. Dermatol., 2007, 127, (3), pp. 514–525 [DOI] [PubMed] [Google Scholar]
- 79. Nichol J.W., Koshy S.T., Bae H. et al.: ‘Cell‐laden microengineered gelatin methacrylate hydrogels’, Biomaterials, 2010, 31, (21), pp. 5536–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Baskaran P., Udduttula A., Uthirapathy V.: ‘Development and characterisation of novel Ce‐doped hydroxyapatite–Fe3O4 nanocomposites and their in vitro biological evaluations for biomedical applications’, IET Nanobiotechnol., 2018, 12, (2), pp. 138–146 [Google Scholar]
- 81. Goncharenko A.V., Kotlyarova M.S., Moisenovich A.M. et al.: ‘Osteogenic differentiation of mouse bone marrow stromal cells on fibroin microcarriers’, Dokl. Biochem. Biophys., 2017, 477, (1), pp. 345–348 [DOI] [PubMed] [Google Scholar]
- 82. Li X., Huang Y., Zheng L. et al.: ‘Effect of substrate stiffness on the functions of rat bone marrow and adipose tissue derived mesenchymal stem cells in vitro’, J. Biomed. Mater. Res. – A, 2014, 102, (4), pp. 1092–1101 [DOI] [PubMed] [Google Scholar]
- 83. Singh R.S., Kaur N., Rana V. et al.: ‘Pullulan: a novel molecule for biomedical applications’, Carbohydr. Polym., 2017, 171, pp. 102–121 [DOI] [PubMed] [Google Scholar]
- 84. Egger D., Tripisciano C., Weber V. et al.: ‘Dynamic cultivation of mesenchymal stem cell aggregates’, Bioengineering, 2018, 5, (2), pp. 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Croisier F., Jérôme C.: ‘Chitosan‐based biomaterials for tissue engineering’, Eur. Polym. J., 2013, 49, (4), pp. 780–792 [Google Scholar]
- 86. Wahl D.A., Czernuszka J.T.: ‘Collagen‐hydroxyapatite composites for hard tissue repair’, Eur. Cells Mater., 2006, 11, (3), pp. 43–56 [DOI] [PubMed] [Google Scholar]
- 87. Jiang J.P., Liu X.Y., Zhao F. et al.: ‘Three‐dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury’, Neural Regen. Res., 2020, 15, (5), pp. 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sinha V.., Singla A.., Wadhawan S. et al.: ‘Chitosan microspheres as a potential carrier for drugs’, Int. J. Pharm., 2004, 274, (1–2), pp. 1–33 [DOI] [PubMed] [Google Scholar]
- 89. Lv B.H., Tan W., Zhu C.C. et al.: ‘Properties of a stable and sustained‐release formulation of recombinant human parathyroid hormone (rhPTH) with chitosan and silk fibroin microparticles’, Med. Sci. Monit., 2018, 24, pp. 7532–7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ye M., Zeng S., Gao W. et al.: ‘Preparation and characterization of genipin‐crosslinked silk fibroin/chitosan controlled‐release microspheres’, Nan Fang Yi Ke Da Xue Xue Bao, 2014, 34, (6), pp. 875–879 [PubMed] [Google Scholar]
- 91. Cheerarot O., Baimark Y.: ‘Biodegradable silk fibroin/chitosan blend microparticles prepared by emulsification‐diffusion method’, E‐Polymers, 2015, 15, (2), pp. 67–74 [Google Scholar]
- 92. Hu Y., Liu C., Liu Y. et al.: ‘An alternative sustained release microsphere of silk fibroin‐chitosan: preparation, pharmaceutical characteristics and pharmacokinetic profiles’, Anesth. Analg., 2010, 111, (6), p. 1561 [Google Scholar]
- 93. Yang M.C., Wang S.S., Chou N.K. et al.: ‘The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin‐polysaccharide cardiac patches in vitro’, Biomaterials, 2009, 30, (22), pp. 3757–3765 [DOI] [PubMed] [Google Scholar]
- 94. Morizane R., Bonventre J. V.: ‘Organoids for modeling kidney disease’, in Lawrence M., Davies J.A. (Eds.): ‘Organs and organoids’ (Elsevier, Academic Press, USA., 2018), pp. 227–245 [Google Scholar]
- 95. Rouwkema J., Rivron N.C., van Blitterswijk C.A.: ‘Vascularization in tissue engineering’, Trends Biotechnol., 2008, 26, (8), pp. 434–441 [DOI] [PubMed] [Google Scholar]
- 96. Grebenyuk S., Ranga A.: ‘Engineering organoid vascularization’, Front. Bioeng. Biotechnol., 2019, 7, (MAR), pp. 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gupta A.K., Coburn J.M., Davis‐Knowlton J. et al.: ‘Scaffolding kidney organoids on silk’, J. Tissue Eng. Regen. Med., 2019, 13, (5), pp. 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jiayao Z., Guanshan Z., Jinchi Z. et al.: ‘Antheraea pernyi silk sericin mediating biomimetic nucleation and growth of hydroxylapatite crystals promoting bone matrix formation’, Microsc. Res. Tech., 2017, 80, (3), pp. 305–311 [DOI] [PubMed] [Google Scholar]
- 99. Sunarintyas S., Siswomihardjo W.: ‘The effect of sericin application over hydroxyapatite surface on osteoblast cells proliferation’ Int. Conf. on Instrumentation, Communication, Information Technology and Biomedical Engineering, Bandung, Indonesia, 2011, pp. 145–149 [Google Scholar]
- 100. Cai Y., Jin J., Mei D. et al.: ‘Effect of silk sericin on assembly of hydroxyapatite nanocrystals into enamel prism‐like structure’, J. Mater. Chem., 2009, 19, (32), p. 5751 [Google Scholar]
- 101. Lamboni L., Gauthier M., Yang G. et al.: ‘Silk sericin: a versatile material for tissue engineering and drug delivery’, Biotechnol. Adv., 2015, 33, (8), pp. 1855–1867 [DOI] [PubMed] [Google Scholar]
- 102. Morikawa M., Kimura T., Murakami M. et al.: ‘Rat islet culture in serum‐free medium containing silk protein sericin’, J. Hepatobiliary. Pancreat. Surg., 2009, 16, (2), pp. 223–228 [DOI] [PubMed] [Google Scholar]
- 103. Castro F., Ferreira A., Rocha F. et al.: ‘Precipitation of hydroxyapatite at 37°C in a meso oscillatory flow reactor operated in batch at constant power density’, AIChE J., 2013, 59, (12), pp. 4483–4493 [Google Scholar]
- 104. Grasman J.M., Williams M.D., Razis C.G. et al.: ‘Tissue models for neurogenesis and repair in 3D’, ACS Biomater. Sci. Eng., 2019, 5, (10), pp. 5327–5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lovett M., Rockwood D., Baryshyan A. et al.: ‘Simple modular bioreactors for tissue engineering: a system for characterization of oxygen gradients, human mesenchymal stem cell differentiation, and prevascularization’, Tissue Eng. C Methods, 2010, 16, (6), pp. 1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gupta S.K., Dangi A.K., Smita M. et al.: ‘Effectual bioprocess development for protein production’, in Shukla P. (Ed.): ‘Applied microbiology and bioengineering’ (Elsevier, Academic Press, USA., 2019), pp. 203–227 [Google Scholar]
- 107. Park J.‐H., Pérez R.A., Jin G.‐Z. et al.: ‘Microcarriers designed for cell culture and tissue engineering of bone’, Tissue Eng. B Rev., 2013, 19, (2), pp. 172–190 [DOI] [PubMed] [Google Scholar]
- 108. Yan W.‐C., Davoodi P., Vijayavenkataraman S. et al.: ‘3d bioprinting of skin tissue: from pre‐processing to final product evaluation’, Adv. Drug Delivery Rev., 2018, 132, pp. 270–295 [DOI] [PubMed] [Google Scholar]
- 109. McGlone T., Briggs N.E.B., Clark C.A. et al.: ‘Oscillatory flow reactors (OFRs) for continuous manufacturing and crystallization’, Org. Process Res. Dev., 2015, 19, (9), pp. 1186–1202 [Google Scholar]
- 110. Ferreira A., Rocha F., Teixeira J. et al.: ‘Apparatus for mixing improvement based on oscillatory flow reactors provided with smooth periodic constrictions’, Int. Patent WO 2015/056156 A1.’ PCT/IB2014/065273, 2014.
- 111. Fitch A.W., Jian H., Ni X.: ‘An investigation of the effect of viscosity on mixing in an oscillatory baffled column using digital particle image velocimetry and computational fluid dynamics simulation’, Chem. Eng. J., 2005, 112, (1–3), pp. 197–210 [Google Scholar]
- 112. Mackley M.., Smith K.., Wise N..: ‘The mixing and separation of particle suspensions using oscillatory flow in baffled tubes’, Chem. Eng. Res. Des., 1993, 71, (Part A), pp. 649–655 [Google Scholar]
- 113. Reis N.M.: ‘Novel Oscillatory Flow Reactors for Biotechnological Applications’. PhD thesis at the School of Engineering of the University of Minho, 2006. [Google Scholar]
- 114. Abbott M.S.R., Harvey A.P., Perez G. V. et al.: ‘Biological processing in oscillatory baffled reactors: operation, advantages and potential’, Interface. Focus., 2012, 3, (1), pp. 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Castro F., Ferreira A., Reis R. et al.: ‘Continuous‐flow precipitation as a route to prepare highly controlled nanohydroxyapatite: in vitro mineralization and biological evaluation’, Mater. Res. Express, 2016, 3, (7), pp. 1–13 [Google Scholar]
- 116. Abbott R.D., Raja W.K., Wang R.Y. et al.: ‘Long term perfusion system supporting adipogenesis’, Methods, 2015, 84, pp. 84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Detsch R., Boccaccini A.R.: ‘The role of osteoclasts in bone tissue engineering’, J. Tissue Eng. Regen. Med., 2015, 9, (10), pp. 1133–1149 [DOI] [PubMed] [Google Scholar]
- 118. Zhang X., Wang X., Keshav V. et al.: ‘Dynamic culture conditions to generate silk‐based tissue‐engineered vascular grafts’, Biomaterials, 2009, 30, (19), pp. 3213–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]


