Abstract
In the present study, Bipolaris maydis was used to synthesise silver nanoparticles (AgNPs). Several parameters that influence the synthesis of AgNPs such as fungus age, the concentration of Ag nitrate (AgNO3), and incubation time were explored to find the optimum synthesis condition. Furthermore, the antifungal activity of AgNPs against Exserohilum turcicum was determined by measuring inhibition zone diameter, colony formation, and conidia germination. The optimal biosynthesis system included fungus age of 7 days, 8 mM AgNO3, and an incubation time of 120 h. Under these conditions, synthesised NPs were near round, and the average particle size was about 21 nm. At the experiment, the diameter of the inhibition zone reached a maximum of 8 mM AgNO3 and 72 h. In addition, the inhibition rate of colony and conidia reached 83.39 and 100%, respectively, with 200 μg/ml AgNPs. The results offer a novel pathway for phytopathogen control and make it likely to develop new eco‐friendly antimicrobial.
Inspec keywords: silver, nanoparticles, antibacterial activity, microorganisms, particle size, nanomedicine, nanofabrication
Other keywords: biosynthesis, B. maydis, antifungal effect, Exserohilum turcicum, Bipolaris maydis, silver nanoparticles, fungus age, silver nitrate concentration, incubation time, inhibition zone diameter, colony formation, conidia germination, particle size, phytopathogen control, time 72 h, time 120 h, Ag
1 Introduction
The importance of nanotechnology has been shown by extensive applications in the present society. Conventional materials are facilitated into nanoscale, 1–100 nm at least in one dimension [1]. On the nanoscale level, materials have different chemical, physical, magnetic, electrical, and optical properties compared with their bulk counterparts due to their high specific surface area [2].
Silver nanoparticles (AgNPs) have drawn the attention of an increasing number of researchers in the fields of medicine, electronic, optics, and chemistry [3, 4]. Synthesis of NPs can be achieved via different methods such as ultraviolet (UV) irradiation [5], microwave‐assisted [6, 7], electrochemical [8], sonochemical [9], microfluidic route [10], microorganism‐mediated [11, 12, 13, 14, 15, 16], and plant extract‐mediated methods [17, 18, 19, 20, 21, 22]. Physical methods consume energy, while chemical methods contain toxic chemicals; therefore, it is necessary to advocate green synthesis of NPs. Production of NPs based on microorganism and plant extract confirm to the concept of green chemistry. During the past years, various living organisms were applied to synthesise AgNPs; for example, Bacillus koriensis [11], Bacillus sp. [15], Fusarium oxysporum [23], Trichoderma viride [13], Aspergillus fumigatus [16], Septoria apii [14], Pulicaria gnaphalodes [24], Caulerpa racemosa [25], Brassica rapa L. [26], Ginkgo biloba [27], and Osmanthus fragrans [28]. In recent years, animal species such as arthropods and metabolites [29], bio‐resources as agro‐wastes, enzymes, and pigments were also used to synthesise AgNPs [30].
Ag was considered as a bacteriostatic agent in the 19th century. Afterwards, antimicrobial, antioxidant, anticoagulant, and thrombolytic activity of Ag and relevant products were extensively measured [29, 30]. Among these forms, nanoscale particles proved to be the most effective. It has been reported that AgNPs synthesised by date palm pit aqueous extract exhibited prominent antifungal effect at 83% at 25 μg/ml against Descurainia sophia, and minimum bactericide concentration against Klebsiella pneumonia and Acinetobacter baumannii were recorded at 1.56 and 3.12 µg/ml [31]. Khatami et al. reported that AgNPs synthesised by D. sophia showed a maximum inhibitory effect against Rhizoctonia solani at 86% at 25 μg/ml, and the reported minimum bactericidal concentration of AgNPs against Agrobacterium tumefaciens and Agrobacterium rhizogenes were 4 and 8 μg/ml, respectively [32]. Xia et al. [33] evaluated the effect of AgNPs against Trichosporon asahii and the minimum inhibitory concentration was 0.5 μg/ml. Nejad et al. [34] applied green‐synthesised AgNPs to interact with R. solani and the highest inhibition levels against sclerotia formation and mycelia growth were 92 and 85%, respectively, at a concentration of 50 ppm. Swamy and Nargund [35] measured the antifungal effect of AgNPs against Curvularia lunata, and it was found that more than 95% of spores were inhibited at the tested concentration.
In this research, Bipolaris maydis was used to biosynthesise AgNPs, and the optimisation of AgNPs formation was achieved through regulating the incubation time of the isolate, the concentration of Ag nitrate (AgNO3), and the fungus age. The synthesised AgNPs were characterised using UV–visible (UV–vis) spectrophotometry, transmission electron microscopy (TEM), scanning electron microscopy (SEM), and energy dispersive X‐ray (EDX). Furthermore, the antifungal effect of AgNPs was measured through inhibition zone, colony formation, and conidia germination against Exserohilum turcicum.
2 Materials and methods
2.1 Microorganism isolation
The fungus used here for AgNPs production was B. maydis, and the fungus used for antifungal effect was E. turcicum, both of which were conserved in the Plant Protection laboratory of the Anhui Science and Technology University.
2.2 Preparation of hyphae filtrate
The hyphae filtrate was prepared as follows: B. maydis was grown on Potato Dextrose Agar (PDA) plates at 30°C for 3–15 days. Subsequently, it was inoculated into Potato Dextrose (PD) liquid medium, followed by shaking at the speed of 150 r/min for more than 24 h. Then, the liquid cultures were filtered through sterile filter papers, and the filtrate was conserved at 4°C.
2.3 Optimal biosynthesis of AgNPs
AgNPs was synthesised by appending the obtained filtrate to deionised water at a specific rate of 1:9 (v:v); different concentrations of AgNO3 was followed. This confirmed the formation of AgNPs as the solution colour changed. Several parameters were amended to optimise synthesis process including different spawn ages (3, 7, and 15 days), concentrations of AgNO3 (1, 2, 4, and 8 mM), and co‐cultivation time (12, 24, 48, 72, 96, and 120 h). AgNPs synthesised through such conditions were recorded via digital images and UV–vis absorption spectra.
2.4 Characterisation of AgNPs
Co‐cultivation of diluted filtrate and AgNO3 was selected every 24 h to measure the absorption spectra via UV–vis spectroscopy (TU‐1950). The solution obtained via the optimal synthesis process was applied to scan the morphology using TEM (JEM‐2100F) after totally drying on the copper grid. For SEM (S‐4800) analysis, such synthesised AgNPs were deposited on a sample plate, followed by platinum coating. EDX was utilised to analyse the components and their contents in the solution.
2.5 Antifungal effect of AgNPs
2.5.1 Inhibition zone assay
Antifungal activity of AgNPs against E. turcicum was implemented by measuring the diameter of the inhibition zone. About 0.1 ml of spore suspension (106 /ml) was uniformly smeared on solidified PDA plates. Then, 7 μl of various samples such as AgNPs, sterile water, and filtrate was dripped onto the fixed sterile filter paper discs (5 mm×5 mm). After 5 min of standing, the plates were incubated at 30°C for 3 days. Three replicates were conducted per treatment.
2.5.2 Inhibition of colony growth
Oven‐dried AgNPs were dissolved in sterile water as the stock solution (10 mg/ml). A volume of 5 ml of diluted stock solution was added to 45 ml of PDA medium at an approximate temperature of 55–60°C, and final concentrations of AgNPs of 6.25, 12.5, 25, 50, 100, and 200 μg/ml were obtained via dilution with sterile water. The control set contained 5 ml of sterile water without AgNPs. A fungus block (φ = 5 mm) was inoculated in the centre of each Petri dish containing different concentrations of AgNPs, followed by incubation at 28°C for 3–5 days. Each control and experimental treatment was performed in three replicates.
2.5.3 Influence on conidia germination
The densities of E. turcicum conidia were adjusted to 106 /ml via counting chamber. Next, various concentrations of AgNPs (6.25, 12.5, 25, 50, 100, and 200 μg/ml) and conidia suspensions were added into sterile centrifuge tubes at a ratio of 1:9 (v:v), the conidia suspension served as control. Then, incubation at 25°C for 1–2 days was performed. Images of conidia in the control and experimental treatments were obtained under a microscope (100×).
3 Results
3.1 Formation of AgNPs
As shown in Fig. 1, the colour of diluted filtrate changed from light green (a) to reddish brown (b) after incubating with 1 mM AgNO3 at 30°C for 96 h, denoting synthesis of AgNPs. The UV–vis spectrum showed a strong and broad surface plasmon resonance peak at 430 nm (c), which is characteristic for AgNPs.
Fig. 1.

Synthesis of AgNPs by B. maydis
(a) Digital image of diluted filtrate without AgNO3, (b) Digital image of filtrate with 1 mM AgNO3, (c) UV–vis absorption spectrum of AgNPs
3.2 Optimal biosynthesis of AgNPs
3.2.1 Effect of fungus age
Taking a certain concentration (2 mM) of AgNO3 as an example, the co‐culture of AgNO3 and B. maydis (3–15 days) appeared reddish brown compared with the filtrate, and there were no obvious differences in Figs. 2 a –c. However, UV–vis spectra of AgNPs based on different fungus age varied. Overall, maximum absorption decreased along with increasing fungus age. Only from the point of maximum absorption peak, the maximum was reached for the isolate of 3 days; however, the corresponding absorption wavelength was 350 nm, which conflicted with the surface plasmon resonance of AgNPs. Therefore, the isolate of 7 days was optimised to synthesise AgNPs.
Fig. 2.

Effect of varied fungus age on AgNPs formation
(a)–(c) Digital photographs of AgNPs with fungus ages of 3, 7, and 15 days, (d) , UV–vis spectra of synthesised AgNPs based on different fungus ages
3.2.2 Influence of various concentrations of AgNO3
The solution colour darkened via gradually increasing the concentration of AgNO3 (Fig. 3 a). Correspondingly, the absorption peak increased continuously, and a maximum was achieved at 8 mM AgNO3 (Fig. 3 b).
Fig. 3.

Influence of various concentrations of AgNO3 on AgNPs synthesis
(a) Digital photographs of AgNPs with 1, 2, 4, and 8 mM AgNO3, (b) UV–vis spectra of synthesised AgNPs based on different concentrations of AgNO3
3.2.3 Effect of different incubation times
Under the condition of 8 mM AgNO3, the solution colour of AgNPs deepened compared with those that contained low concentrations of AgNO3, and it became dark brown when the incubation time reached 120 h (Fig. 4 b). As shown in Fig. 4 c, the maximum absorption of AgNPs decreased as the incubation time prolonged from 24 to 120 h, reaching a minimum at 96 h.
Fig. 4.

Effect of different incubation times on AgNPs formation
(a), (b) Synthesised AgNPs based at 24 and 120 h, (c) UV–vis spectra of synthesised AgNPs based on different incubation times
3.2.4 TEM detection of AgNPs
Fig. 5 showed a representative TEM image of the synthesised AgNPs. The particles were round or near round with favourable dispersibility (Fig. 5 a). To define particle size and size distribution, 200 particles were randomly chosen from several TEM images. Biosynthesised AgNPs were confined to be 8–41 nm with an average particle size of about 21 nm (Fig. 5 b).
Fig. 5.

TEM detection of AgNPs
(a) TEM micrograph, (b) Size distribution of AgNPs
3.2.5 SEM and EDX analyses of AgNPs
Fig. 6 a showed quantities of particles deposited on the substrate. Peaks near 3 kev indicate Ag, and the remaining peaks should be attributed to copper grid and elements of fungus filtrate.
Fig. 6.

SEM and EDX analyses of AgNPs
(a) SEM micrograph, (b) EDX spectroscopy of AgNPs
3.2.6 Inhibition zone assay
Fig. 7 shows strong antifungal effect of AgNPs against E. turcicum. Apparent inhibition zones emerged in the plates with AgNPs based on different incubation times and concentrations of AgNO3. However, no inhibition zone was found when the sample contained sterile water or fungus filtrate.
Fig. 7.

Inhibition zones formed by AgNPs against E. turcicum
(a)–(e) Incubation times of 48, 72, 96, and 120 h, respectively. In each plate, the label of 0 and 1 correspond to sterile water and fungus filtrate; 2, 3, 4, and 5 represent different concentrations of AgNO3 as 1, 2, 4, and 8 mM
To see the result more clearly, the diameter of inhibition zone was measured via crossing method. Taking a certain fungus age (7 days) as an example, this ranged between 5.0 ± 0.84 and 10.7 ± 1.26 mm. The maximum corresponded to AgNPs based on incubation time of 72 h and 8 mM AgNO3 (Table 1).
Table 1.
Diameter of inhibition zone under condition of various AgNPs
| Different incubation times, h | Biosynthesised AgNPs diameter of inhibition zone, mm | |||
|---|---|---|---|---|
| 1 | 2 | 4 | 8 | |
| 24 | 6.0 ± 0.84 | 7.2 ± 2.32 | 8.5 ± 2.48 | 10.2 ± 0.99 |
| 48 | 6.2 ± 0.51 | 6.8 ± 2.23 | 8.5 ± 2.04 | 9.5 ± 0.76 |
| 72 | 7.2 ± 2.85 | 7.7 ± 2.57 | 8.7 ± 2.13 | 10.7 ± 1.26 |
| 96 | 6.0 ± 1.52 | 7.2 ± 2.37 | 7.7 ± 1.69 | 9.7 ± 0.85 |
| 120 | 6.2 ± 1.94 | 6.7 ± 2.13 | 8.3 ± 1.98 | 9.7 ± 0.85 |
3.2.7 Inhibition of colony growth
As shown in Fig. 8, AgNPs exhibited a prominent inhibition effect on colony growth. The diameter of E. turcicum without AgNPs measured via the cross‐method was 6.10 cm (Fig. 8 a), and it decreased gradually with increasing concentration of AgNPs (Figs. 8 b –g). The diameter reached a minimum value of 1.43 cm at 200 μg/ml of AgNPs (Fig. 8 g). The inhibition rate caused by the varied AgNPs concentrations (6.25–200 μg/ml) in the control and experimental treatments of E. turcicum was in the range of 17.86–83.39%.
Fig. 8.

Inhibition of colony growth of E. turcicum by AgNPs
(a) Colony of the control isolate, (b)–(d) E. turcicum with AgNPs concentrations of 12.5, 25, and 200 μg/ml, respectively
3.2.8 Influence of conidia germination
As seen from the data in Fig. 9, AgNPs dramatically affected the germination of conidia of E. turcicum. In the control, the germination rate was 93.94%; however, it decreased notably with the increase in the concentration of AgNPs, and conidia were totally inhibited at 200 μg/ml.
Fig. 9.
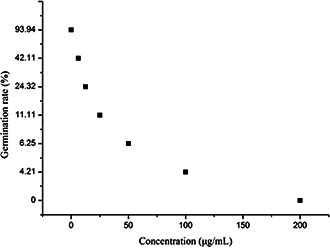
Influence of AgNPs on conidia germination of E. turcicum
4 Discussion
The production of metal NPs, especially of AgNPs, is a popular field, considering the particular properties and broad application values. In this experiment, B. maydis was used to synthesise AgNPs without using extra toxic chemical reagent, and the result is in accordance with ‘green chemistry’. Although several review papers exist about AgNPs synthesised by fungi [11, 29, 30], B. maydis has not been adopted to date, and an optimal synthesis system was determined through adjusting fungus age, the concentration of AgNO3, and incubation time for the first time. In addition, compared with AgNPs synthesised by other fungi reported in previous studies, several differences were found such as solution colour [14, 36], particle shape [36], particle size and size distribution [14, 16, 36], maximum absorption wavelength [36, 37] etc. The reasons for such differences might be fungus species, fungus age, AgNO3 concentration, and incubation time.
Antifungal activity showed that AgNPs synthesised by B. maydis exhibit excellent inhibition effect against E. turcicum. On the basis of existing results, this showed an apparent discrepancy compared with other relevant reports. It has been reported that the antifungal effect of the same AgNPs against different pathogens differentiated [16, 25, 37]. AgNPs synthesised through different methods, microorganisms or plants showed different inhibition activity against the same pathogen [31, 32, 34]. In addition, the antimicrobial activity of AgNPs was influenced by other parameters such as the pH of the solution [28], the stabiliser [38], and the modified substances [19, 30]. These results may offer a novel approach for the comprehensive control of phytopathogens, and it also avoids pathogen resistance to the utmost extent.
5 Conclusion
On the basis of these results, one type of important phytopathogen called B. maydis that caused severe damage to maize could synthesise AgNPs, and several parameters which influence its synthesis were conducted to ascertain the optimal condition. In addition, as‐synthesised AgNPs showed prominent antifungal activity against E. turcicum including inhibition zone, colony formation, and conidia germination. In the near future, experiments should focus on parameters that affect antifungal activity such as aggregation, charge, UV ray, and high temperature.
6 Acknowledgments
The Key Discipline of Plant Protection in University of Science and Technology of Anhui (AKZDXK2015C04), the Natural Science Fund of Education Department of Anhui province (KJ2017A510), the Talent introduction project in Anhui Science and Technology University (NXYJ201602), and the Innovation project of university students (201710879072).
7 References
- 1. Khademhosseini A. Parak W.J. Weiss P.S.: ‘Nanoscience and nanotechnology around the world’, ACS Nano, 2016, 10, pp. 4883 –4884 [DOI] [PubMed] [Google Scholar]
- 2. Bakshi M. Singh H.B. Abhilash P.C.: ‘The unseen impact of nanoparticles: more or less?’, Curr. Sci.., 2014, 106, pp. 350 –352 [Google Scholar]
- 3. Hajipour M.J. Santoso M.R. Rezaee F. et al.: ‘Advances in Alzheimer's diagnosis and therapy: the implications of nanotechnology’, Trends Biotechnol., 2017, 35, pp. 937 –953 [DOI] [PubMed] [Google Scholar]
- 4. Khatami M. Pourseyedi S. Khatami M. et al.: ‘Synthesis of silver nanoparticles using seed exudates of Sinapis arvensis as a novel bioresource, and evaluation of their antifungal activity’, Bioresources Bioprocessing, 2015, 2, pp. 19 –25 [Google Scholar]
- 5. Zhang X. Yang C.W. Yu H.Q. et al.: ‘Light‐induced reduction of silver ions to silver nanoparticles in aquatic environments by microbial extracellular polymeric substances (EPS)’, Water Res., 2016, 106, pp. 242 –248 [DOI] [PubMed] [Google Scholar]
- 6. Parveen M. Ahmad F. Malla A.M. et al.: ‘Microwave‐assisted green synthesis of silver nanoparticles from Fraxinus excelsior leaf extract and its antioxidant assay’, Appl. Nanosci., 2016, 6, pp. 267 –276 [Google Scholar]
- 7. Aitenneite H. Abboud Y. Tanane O. et al.: ‘Rapid and green microwave‐assisted synthesis of silver nanoparticles using aqueous Phoenix dactylifera L. (date palm) leaf extract and their catalytic activity for 4‐nitrophenol reduction’, J. Mater. Environ. Sci., 2016, 7, pp. 2335 –2339 [Google Scholar]
- 8. Nasretdinova G. Fazleeva R. Osin Y. et al.: ‘Methylviologen‐mediated electrochemical synthesis of silver nanoparticles via the reduction of AgCl nanospheres stabilized by cetyltrimethylammonium chloride’, Russ. J. Electrochem., 2017, 53, pp. 25 –38 [Google Scholar]
- 9. Vinoth V. Wu J.J. Asiri A.M. et al.: ‘Sonochemical synthesis of silver nanoparticles anchored reduced graphene oxide nanosheets for selective and sensitive detection of glutathione’, Ultrason. Sonochem., 2017, 39, pp. 363 –373 [DOI] [PubMed] [Google Scholar]
- 10. Xu L. Peng J.H. Yan M. et al.: ‘Droplet synthesis of silver nanoparticles by a microfluidic device’, Chem. Eng. Process. Process Intensification, 2016, 102, pp. 186 –193 [Google Scholar]
- 11. Singh P. Kim Y.‐J. Zhang D.B. et al.: ‘Biological synthesis of nanoparticles from plants and microorganisms’, Trends Biotechnol., 2016, 34, pp. 588 –599 [DOI] [PubMed] [Google Scholar]
- 12. Bhakya S. Muthukrishnan S. Sukumaran M. et al.: ‘Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity’, Appl. Nanosci., 2016, 6, pp. 755 –766 [Google Scholar]
- 13. Rajput S. Werezuk R. Lange R.M. et al.: ‘Fungal isolate optimized for biogenesis of silver nanoparticles with enhanced colloidal stability’, Langmuir, 2016, 32, pp. 8688 –8697 [DOI] [PubMed] [Google Scholar]
- 14. Huang W.D. Yan J.J. Wang Y. et al.: ‘Biosynthesis of silver nanoparticles by Septoria apii and Trichoderma koningii ’, Chin. J. Chem., 2013, 31, pp. 529 –533 [Google Scholar]
- 15. Wang C. Kim Y.J. Singh P. et al.: ‘Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity’, Artif. Cells Nanomed. Biotechnol., 2016, 44, pp. 1127 –1132 [DOI] [PubMed] [Google Scholar]
- 16. Sarsar V. Selwal M.K. Selwal K.K.: ‘Biogenic synthesis, optimisation and antibacterial efficacy of extracellular silver nanoparticles using novel fungal isolate Aspergillus fumigatus MA’, IET Nanobiotechnol., 2016, 10, pp. 215 –221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed S. Mudasir S. Swami A.B.L. et al.: ‘Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract’, J. Radiat. Res. Appl. Sci., 2016, 9, pp. 1 –7 [Google Scholar]
- 18. Benelli G.: ‘Plant‐mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review’, Parasitol. Res., 2016, 115, pp. 23 –34 [DOI] [PubMed] [Google Scholar]
- 19. Kumar V. Yadav S.K.: ‘Synthesis of different‐sized silver nanoparticles by simply varying reaction conditions with leaf extracts of Bauhinia variegata L’, IET Nanobiotechnol., 2012, 6, pp. 1 –8 [DOI] [PubMed] [Google Scholar]
- 20. Nesakumar T. Edison J.I. Lee Y.R. et al.: ‘Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow‐12 dye’, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 2016, 161, pp. 122 –129 [DOI] [PubMed] [Google Scholar]
- 21. Velmurugan P. Anbalagan K. Manosathyadevan M. et al.: ‘Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens’, Bioprocess. Biosyst. Eng., 2014, 37, pp. 1935 –1943 [DOI] [PubMed] [Google Scholar]
- 22. Castro L. Blazquez M.L. Gonzalez F. et al.: ‘Biosynthesis of silver and platinum nanoparticles using orange peel extract: characterisation and applications’, IET Nanobiotechnol., 2015, 9, pp. 252 –258 [DOI] [PubMed] [Google Scholar]
- 23. Vijayan S. Divya K. George T.K. et al.: ‘Biogenic synthesis of silver nanoparticles using endophytic fungi Fusarium oxysporum isolated from Withania somnifera (L.), its antibacterial and cytotoxic activity’, J. Bionanosci., 2016, 10, pp. 369 –376 [Google Scholar]
- 24. Chitsazi M.R. Korbekandi H. Asghari G. et al.: ‘Synthesis of silver nanoparticles using methanol and dichloromethane extracts of Pulicaria gnaphalodes (vent.) boiss. Aerial parts’, Artif. Cells Nanomed. Biotechnol., 2016, 44, pp. 328 –333 [DOI] [PubMed] [Google Scholar]
- 25. Kathiraven T. Sundaramanickam A. Shanmugam N. et al.: ‘Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens’, Appl. Nanosci., 2014, 5, pp. 499 –504 [Google Scholar]
- 26. Narayanan K.B. Park H.H.: ‘Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens’, Eur. J. Plant Pathol., 2014, 140, pp. 185 –192 [Google Scholar]
- 27. Huang W.D. Bao Y. Duan H.M. et al.: ‘Antifungal effect of green synthesized silver nanoparticles against Setosphaeria turcica ’, IET Nanobiotechnol., 2017, 11, pp. 803 –808 [Google Scholar]
- 28. Huang W.D. Chen X.Y. Duan H.M. et al.: ‘Optimized biosynthesis and antifungal activity of Osmanthus fragrans leaf extract‐mediated silver nanoparticles’, Int. J. Agric. Biol., 2017, 19, pp. 668 –672 [Google Scholar]
- 29. Lateef A. Ojo S.A. Elegbede J.A.: ‘The emerging roles of arthropods and their metabolites in the green synthesis of metallic nanoparticles’, Nanotechnol. Rev., 2016, 5, pp. 601 –622 [Google Scholar]
- 30. Adelere I.A. Lateef A.: ‘Novel approach to the green synthesis of metallic nanoparticles: the use of agro‐wastes, enzymes and pigments’, Nanotechnol. Rev., 2016, 5, pp. 567 –587 [Google Scholar]
- 31. Khatami M. Pourseyedi S.: ‘ Phoenix dactylifera (date palm) pit aqueous extract mediated novel route for synthesis high stable silver nanoparticles with high antifungal and antibacterial activity’, IET Nanobiotechnol., 2015, 9, pp. 184 –190 [DOI] [PubMed] [Google Scholar]
- 32. Khatami M. Mehnipor R. Poor M.H.S. et al.: ‘Facile biosynthesis of silver nanoparticles using Descurainia sophia and evaluation of their antibacterial and antifungal properties’, J. Cluster Sci., 2016, 27, pp. 1601 –1612 [Google Scholar]
- 33. Xia Z.‐K. Ma Q.‐H. Li S.‐Y. et al.: ‘The antifungal effect of silver nanoparticles on Trichosporon asahii ’, J. Microbiol. Immunol. Infect., 2016, 49, pp. 182 –188 [DOI] [PubMed] [Google Scholar]
- 34. Nejad M.S. Bonjar G.H.S. Khatami M. et al.: ‘In vitro and in vivo antifungal properties of silver nanoparticles against Rhizoctonia solani, a common agent of rice sheath blight disease’, IET Nanobiotechnol., 2017, 11, pp. 236 –240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swamy C. Nargund V.B.: ‘Sunlight induced mediated silver nanoparticles from seeds of Thevetia peruviana L., characterization and their antifungal efficacy against Curvularia lunata (Wakker) Boedijn’, Int. J. Curr. Microbiol. Appl. Sci., 2017, 6, pp. 1008 –1013 [Google Scholar]
- 36. Amerasan D. Nataraj T. Murugan K. et al.: ‘Myco‐synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: culicidae)’, J. Pest Sci., 2016, 89, pp. 249 –256 [Google Scholar]
- 37. Waghmare S.R. Mulla M.N. Marathe S.R. et al.: ‘Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms’, Biotechnology, 2015, 5, pp. 33 –38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhatia D. Mittal A. Malik D.K.: ‘Antimicrobial activity of PVP coated silver nanoparticles synthesized by Lysinibacillus varians ’, Biotechnology, 2016, 6, pp. 196 –203 [DOI] [PMC free article] [PubMed] [Google Scholar]


