Abstract
Background
It is unknown whether lung-protective ventilation is applied in burn patients and whether they benefit from it. This study aimed to determine ventilation practices in burn intensive care units (ICUs) and investigate the association between lung-protective ventilation and the number of ventilator-free days and alive at day 28 (VFD-28).
Methods
This is an international prospective observational cohort study including adult burn patients requiring mechanical ventilation. Low tidal volume (VT) was defined as VT ≤ 8 mL/kg predicted body weight (PBW). Levels of positive end-expiratory pressure (PEEP) and maximum airway pressures were collected. The association between VT and VFD-28 was analyzed using a competing risk model. Ventilation settings were presented for all patients, focusing on the first day of ventilation. We also compared ventilation settings between patients with and without inhalation trauma.
Results
A total of 160 patients from 28 ICUs in 16 countries were included. Low VT was used in 74% of patients, median VT size was 7.3 [interquartile range (IQR) 6.2–8.3] mL/kg PBW and did not differ between patients with and without inhalation trauma (p = 0.58). Median VFD-28 was 17 (IQR 0–26), without a difference between ventilation with low or high VT (p = 0.98). All patients were ventilated with PEEP levels ≥5 cmH2O; 80% of patients had maximum airway pressures <30 cmH2O.
Conclusion
In this international cohort study we found that lung-protective ventilation is used in the majority of burn patients, irrespective of the presence of inhalation trauma. Use of low VT was not associated with a reduction in VFD-28.
Trial registration
Clinicaltrials.gov NCT02312869. Date of registration: 9 December 2014.
Keywords: Mechanical ventilation, Inhalation trauma, Lung-protective, Critical care
Highlights.
First international prospective observational study investigating mechanical ventilation practices in specialized adult burn intensive care units.
Lung-protective ventilation is used in the majority of burn patients.
Use of lung-protective ventilation settings is irrespective of the presence of inhalation trauma.
Background
Mechanical ventilation (MV) is considered a lifesaving intervention, but it also causes lung injury [1,2]. Ventilator settings important in ‘ventilator-induced lung injury’ (VILI) include tidal volume (VT) and positive end-expiratory pressure (PEEP). To limit VILI, ‘lung-protective’ MV strategies have become standard care in the general intensive care unit (ICU) [3,4]. VT sizes of ≤8 mL/kg predicted body weight (PBW) are preferred [3,4] as patients with and without acute respiratory distress syndrome (ARDS) benefit from low VT [1,5–8]. Current guidelines suggest the use of higher PEEP (e.g. >10 cmH2O) for patients with moderate to severe ARDS [9,10]. The optimal PEEP for patients without ARDS remains debatable. However, a trend towards the use of moderate PEEP, generally between 5 and 10 cmH2O, has been reported [4,11].
Burn patients often suffer from inhalation trauma. Both thermal and inhalation trauma may result in respiratory dysfunction, necessitating MV [12–14]. Whether lung-protective ventilation is applied in burn patients is yet unknown. There are no evidence-based guidelines for MV in burn patients. Furthermore, it is unknown whether this specific population benefits from lung-protective ventilation as data on the association between ventilation practices and clinical outcomes are scant [15].
To determine ventilation practices in burn ICUs worldwide we performed an international prospective observational cohort study entitled ‘Local Assessment of MaNAgement in BuRn Patients’ (LAMiNAR). We expected extensive variability in ventilation practices. The secondary objective was to determine the association between ventilation settings, focusing on VT size and levels of PEEP, and duration of ventilation in burn patients, with the number of ventilator-free days and alive at day 28 (VFD-28) as the primary outcome measure.
Methods
Design
The LAMiNAR study is a prospective observational international cohort study in specialized burn ICUs. Burn patients were included during a 3-month period per participating center. The study protocol was centrally approved by the Institutional Review Board (IRB) of the Academic Medical Center at the University of Amsterdam, The Netherlands (W14_314#15.0178). Locally, ethical approval was obtained in compliance with the local regulatory requirements. If applicable, written informed consent from individual patients or legal representatives was obtained prior to enrollment. The study was registered at www.clinicaltrials.gov (NCT02312869). National coordinators were appointed in participating countries (see online supplementary appendix, Table 2); they recruited collaborating centers and assisted local coordinators.
Study population
Consecutive adult (≥18 years) burn patients admitted to a participating burn ICU who needed invasive MV, irrespective of severity of burn injury or presence of inhalation trauma, were eligible for inclusion. There were no exclusion criteria.
Data collection
Patient characteristics and baseline data were collected on the day of ICU admission: Simplified Acute Physiology Score (SAPS) II, Lung injury scores (LIS) [16] and Sequential Organ Failure Assessment (SOFA) scores [17]; data on etiology and severity of burn injury (e.g. percentage of total body surface area burned (TBSA %); presence of inhalation trauma: not suspected, clinical diagnosis, or bronchoscopically confirmed; severity of inhalation trauma graded as mild (i.e. minor or patchy areas of erythema, carbonaceous deposits in bronchi), moderate (i.e. moderate degree of erythema, carbonaceous deposits, bronchorrhea, or bronchial obstruction) or severe (i.e. severe inflammation with friability, copious carbonaceous deposits, bronchorrhea, obstruction or evidence of mucosal sloughing, necrosis or obliteration) [18]. Data on the timing of bronchoscopy and applied nebulization protocols (e.g. use of nebulized heparin, mucolytics or bronchodilators) were not collected.
For MV parameters, day 0 was defined as the first day of MV in the ICU. Ventilator data (described below) and clinical outcome parameters were collected daily until day seven, death or discharge from ICU, whichever came first. All ventilatory data were single measurements collected at the same time point. Daily data were collected from the morning round if the patient was stable for at least 1 h; i.e. closest to 08:00 am, otherwise data from 1 h earlier or later was collected. Whether a patient was considered stable was left at the discretion of the attending physician.
Clinical outcome parameters included: VFD-28, a VFD was defined as a period of 24 consecutive hours in which the patient was alive and without MV, in hospital and ICU length of stay (LOS) and all-cause mortality. Other outcome parameters included: development of pneumonia (i.e. new or progressive radiographic infiltrate plus at least two of the following: fever >38°C, leukocytosis, leucopenia and/or purulent secretions) [4] or ARDS according to the Berlin definition [19] and development of acute renal failure according to acute kidney injury network criteria [20]. Data on the duration of MV, LOS and mortality (in ICU and hospital) were assessed on days 28 and 90. We also collected data on: PaO2/fraction of inspired oxygen (FiO2) (mmHg), respiratory rate (breaths per minute), pulmonary compliance (mL/cmH2O), minute ventilation and arterial blood gas (PaCO2, pH, bicarbonate).
Ventilatory data included: VT, PEEP, FiO2, ventilator mode (e.g. high-frequency ventilation, spontaneous or controlled modes), peak airway pressure (Ppeak) and plateau pressure (Pplat) for volume-controlled ventilation; maximum airway pressure (Pmax) for pressure-controlled ventilation; and driving pressure [calculated as Pplat (or equivalent) minus PEEP] [21].
Anonymized patient data were entered into a web-based, password-secured, electronic case record form (Openclinica, Boston, MA, USA).
Study objectives and parameters
The objective of this study was to determine current ventilation practices in burn patients. The main ventilatory parameters were VT and PEEP. Secondary ventilatory parameters included FiO2, ventilator mode, Ppeak and Pplat for volume-controlled ventilation; Pmax for pressure-controlled ventilation; and driving pressure. The main outcome parameter was number of VFD-28.
Sample size and statistical analysis
We aimed to include 300 patients to enable a multivariate analysis to determine the association between ventilator settings and number of VFD-28. A sample size of 300 patients was required to have a power of 0.80, with a significance level of 0.05, using an estimated effect size of 0.40 [22], while using four independent variables (i.e. VT, PEEP, FiO2 and ventilator mode) in the model. Inclusion in the 3-month period per participating center was much lower than expected. Therefore, we decided to deviate from our originally planned analysis for our secondary objective, and only analyzed the association between one ventilatory parameter (VT) and the number of VFD-28. We did not analyze the association between levels of PEEP and the number of VFD-28 as only five patients were ventilated with high PEEP levels (i.e. >10 cmH2O).
Continuous not normally distributed variables were expressed by medians and interquartile ranges (IQRs). Categorical variables were expressed as n (%). Groups were compared with the Mann–Whitney U test. Categorical variables were compared with the Chi–square or Fisher’s exact tests.
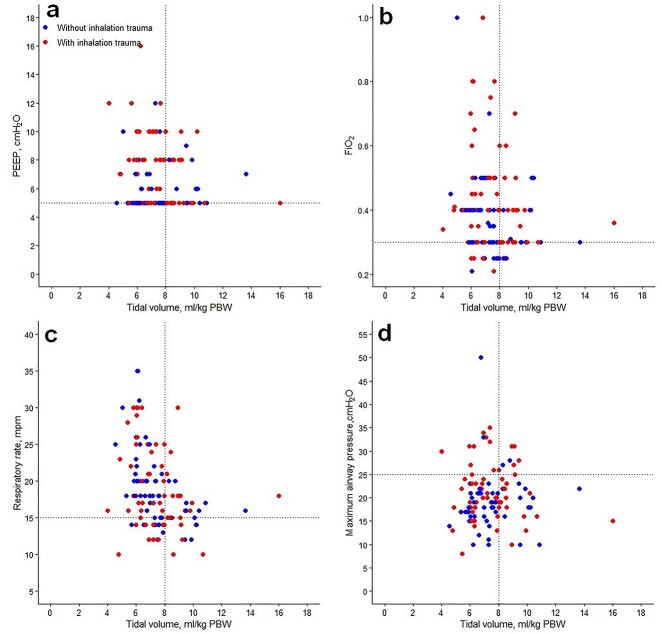
V T was presented as volume normalized for PBW (mL/kg PBW) [5]. Patients in whom PBW could not be calculated were omitted from the analysis. We used scatterplots to present distributions of VT vs PEEP, VT vs respiratory rate, VT vs FiO2 and VT vs Pmax. Widely accepted cut-off values of 8 mL/kg PBW for VT, 14 breaths per min for respiratory rate, 5 cmH2O for PEEP, 0.6 for FiO2 and 30 cmH2O for Pmax, were used to form the matrices [4].
In a post hoc analysis we evaluated differences in ventilation management between patients with and without inhalation trauma, and analyzed the association between the presence of inhalation trauma and number of VFD-28. Patients were stratified into two groups based on the presence [defined as any suspected (clinical diagnosis) or bronchoscopically confirmed inhalation trauma] or absence of inhalation trauma.
We presented ventilation settings for all patients and compared ventilation management between patients with and without inhalation trauma, focusing on the first day of ventilation (day 1).
The median VT on the first day of ventilation was used to determine whether ventilation was ‘lung-protective’ (low VT): ≤8 mL/kg PBW; VT >8 mL/kg PBW was considered as non-protective (high VT).
The association between (1) VT size (VT ≤8 vs VT >8 mL/kg PBW) and (2) inhalation trauma and the number of VFD-28 was analyzed using a competing risk model with death before extubation as competing risk. Data were presented with cumulative incidence curves. The subdistribution hazard ratio for VT and inhalation trauma were calculated using a Cox proportional hazards model, and Schoenfeld residuals were used to test the proportional hazard assumption [23]. Duration of ventilation in survivors was compared using median difference from a quantile regression.
We made no assumptions for missing data and did not adjust for multiplicity across analyses. Patients that were enrolled without subsequent data entry (e.g. no daily data collection and no follow-up data) were excluded from analysis. Statistical significance was considered at p < 0.05. All analyses were performed with R v.2·12·0 (http://www.r-project.org).
Results
Patients
Patients were enrolled between September 2015 and April 2017. In total, 28 specialized burn ICUs in 16 countries participated (see online supplementary appendix Tables 1 and 2). Patient recruitment was lower than expected. Although we expected to include 300 patients within the 3-month periods, only 170 patients were enrolled, of which 160 patients could be included in the analysis (see online supplementary appendix Figure S1).
Demographic, baseline and etiological characteristics are presented in Table 1. The median percentage of TBSA was 25% (IQR 10–40). Inhalation trauma was clinically suspected in 84 out of 159 patients (52.8%) (1 patient had no data available on the presence of inhalation trauma) and was confirmed by bronchoscopy in 45 of these patients (53.6%). Bronchoscopically confirmed inhalation trauma was graded as mild, moderate or severe in respectively 16, 18 and 11 patients. The number of surgical procedures performed in the first day of mechanical ventilation was similar between patients with and without inhalation trauma and included burn wound excisions, performed in 9 patients, debridement (n = 7) and escharotomy (n = 4).
Table 1.
Patient characteristics and severity of burn injury. Mann–Whitney U or Chi square test. One patient without data on burn etiology and severity. All values given as median (interquartile range) if not stated otherwise. Data from ICU admission day
| All n = 160 | With inhalation trauma n = 84 | Without inhalation trauma n = 75 | P value | |
|---|---|---|---|---|
| Gender, male, n (%) | 119 (75%) | 64 (76%) | 55 (73%) | 0.82 |
| Age (years) | 46 (30–60) | 48 (30–60) | 44 (29–58) | 0.48 |
| Height (cm), n | 175 (167–180), 150 | 175 (166–180), 81 | 174 (167–180), 69 | 0.58 |
| Weight (kg), n | 80 (70–90), 158 | 80 (70–90), 84 | 80 (72–90), 74 | 0.68 |
| SAPS II | 48 (35–60) | 49 (37–62) | 43 (35–57) | 0.08 |
| LIS | 0.75 (0.33–1.33) | 1 (0.33–1.5) | 0.75 (0.27–1.25) | 0.16 |
| SOFA (total) | 9 (8–10) | 9 (8–11) | 8 (7–10) | 0.09 |
| Type of burn injurya, n (%) | ||||
| Flames or explosion | 137 (86.2%) | 75 (89.3%) | 62 (82.7%) | |
| Scalds or steam | 5 (3.1%) | 2 (2.3%) | 3 (4%) | |
| Contact burns | 5 (3.1%) | 0 | 5 (6.7%) | |
| Other | 12 (7.5%) | 7 (8.3%) | 5 (6.7%) | 0.10 |
| TBSA (%) | 25 (10–40) | 24 (10–40) | 25 (13–40) | 0.38 |
| Presence of full thickness burn, n (%) | 97 (60.6%) | 50 (59.5%) | 47 (62.6%) | 0.68 |
aOne patient had no data.LIS lung injury score at admission, n number of patients, SAPS II simplified acute physiology score, SOFA sequential organ failure assessment, TBSA total body surface area of burn
Ventilator settings
Low VT were used in 74% of the patients (Table 2, Figures 1 and 2). The median VT size was 7.3 (IQR 6.2–8.3) mL/kg PBW for all patients and did not differ significantly between patients with and without inhalation trauma (p = 0.58; Table 2). VT sizes were similar between patients with clinical and bronchoscopically confirmed diagnosis of inhalation trauma, irrespective of the severity of inhalation trauma (p = 0.31). Median VT size was significantly higher in patients ventilated with spontaneous [VT 8 mL/kg PWB (IQR 7.3–9.5)] compared to controlled ventilation modes [VT 7 mL/kg PWB (IQR 6.1–8), p = 0.007].
Table 2.
Ventilatory parameters on the first day of ventilation. Values given as median (interquartile range) if not stated otherwise. All ventilation and arterial blood gas parameters were collected at the same time point (e.g. 08.00 am if the patient was stable for at least 1 h, otherwise 1 h earlier or later)
| All | With inhalation trauma | Without inhalation trauma | P value | |
|---|---|---|---|---|
| N = 160 | N = 84 | N = 75 | ||
| V T (mL/kg PBWa), n | 7.3 (6.2–8.3), 130 | 7.3 (6.2–8.5), 71 | 7.3 (6.2–7.8), 59 | 0.58 |
| ≤8, n | 96 | 50 | 46 | |
| >8, n | 34 | 21 | 13 | |
| Absolute VT (mL), n/N | 500 (430–584), 137/160 | 500 (440–600), 73/84 | 480 (420–560), 63/75 | 0.33 |
| Controlled mode, n | 7 (6.1–8), 107 | 7.1 (6.1–8.2), 59 | 7 (6.1–7.8), 48 | 0.58 |
| Spontaneous mode, n | 8 (7.3–9.5), 22 | 8.3 (7.5–9.2), 12 | 7.6 (7–9.5), 10 | 0.82 |
| PEEP (cmH2O), n/N | 6 (5–8), 135 | 8 (5–10), 74 | 5 (5–8), 62 | 0.004 |
| 5, n | 66 | 30 | 37 | |
| 6–10, n | 64 | 40 | 24 | |
| >10, n | 5 | 4 | 1 | |
| FiO2, n | 0.35 (0.3–0.4), 155 | 0.39 (0.3–0.5), 82 | 0.35 (0.3–0.4), 73 | 0.36 |
| Peak pressure (cmH2O), n | 23 (19–31), 67 | 31 (23–35), 29 | 20 (17–25), 38 | <0.001 |
| P plat (cmH2O), n | 18 (16–23), 40 | 21 (18–24), 20 | 17 (15–23), 20 | 0.12 |
| P max (cmH2O), n | 20 (17–24), 59 | 20 (17–25), 37 | 20 (17–22), 22 | 0.46 |
| Maximum airway pressureb, n | 21 (18–30), 121 | 24 (19–31), 64 | 20 (17–24), 58 | 0.007 |
| Driving pressurec (cmH2O), n | 13 (11–17), 118 | 14 (11–18), 62 | 13 (10–16), 56 | 0.55 |
| Other parameters | ||||
| PaO2/FiO2 (mmHg) | 288 (186–402) | 275 (172–358) | 320 (229–415) | 0.05 |
| n (%) | 154 (96) | 73 (90) | 6(85) | |
| Respiratory rate (breaths per minute), n | 18 (15–21), 138 | 18 (15–22), 74 | 18 (15–20), 63 | 0.71 |
| Compliance (mL/cm H2O)d, n | 35.6 (21.2–50), 92 | 34.3 (20.4–48.8), 61 | 36.2 (21.7–51.8), 31 | 0.59 |
| Minute ventilation (L/min), n | 8.8 (7.6–10.8), 130 | 8.8 (7.5–11), 71 | 8.8 (7.6–10), 59 | 0.42 |
| PaCO2 (mmHg), n | 41 (36–45), 142 | 41 (36–47), 73 | 41 (36–44), 68 | 0.46 |
| Arterial blood pH, n | 7.39 (7.32–7.43), 142 | 7.38 (7.31–7.43), 73 | 7.40 (7.34–7.43), 68 | 0.31 |
| HCO2 (mEq/L), n | 23 (21–26), 142 | 23 (12–25), 73 | 24 (22–26), 68 | 0.10 |
N number, VT tidal volume, mL/kg PBW milliliters per kilogram predicted body weight, PEEP positive end-expiratory pressure, Ppeak peak airway pressure, Pplat plateau pressure, Pmax maximum airway pressure, PaO2 partial pressure of oxygen in arterial blood, FiO2 fraction of inspired oxygen
aCalculated for males as follows: 50 + 0.91 (cm of height−152.4) and for females as 45.5 + 0.91 (cm of height−152.4). Patients for which PBW could not be calculated were omitted from the analysis.
bMaximum airway pressure: Ppeak pressure, Pmax or Pplat depending on ventilator mode.
cCalculated as Pplat (or equivalent)−PEEP.
dCompliance = VT/(Pplat (or equivalent)−PEEP)
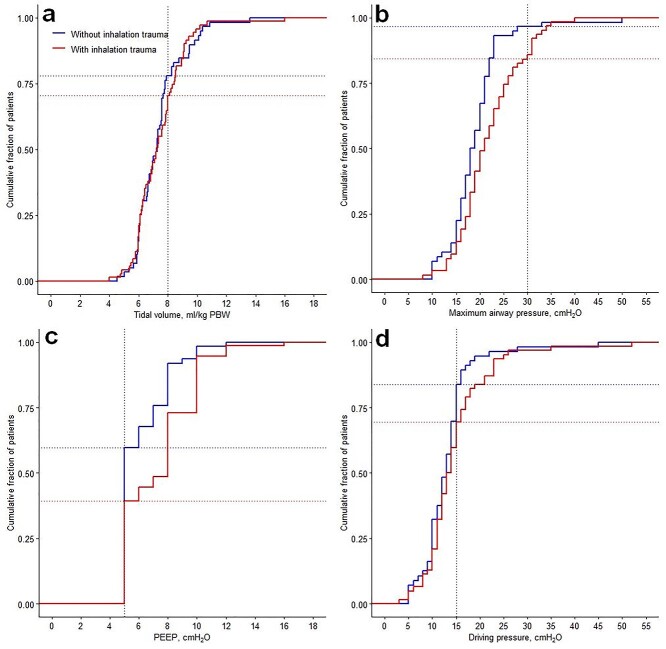
Figure 1.
Ventilator settings on the first day of ventilation of patients with and without inhalation trauma. Cumulative frequency distributions from the following parameters measured on the first day of mechanical ventilation: (a) VT, (b) maximum airway pressure, (c) PEEP, (d) driving pressure. Vertical dotted lines: predefined cut-off values for each variable. Horizontal dotted lines: proportion of patients reaching the cut-offs. Driving pressure: plateau (or peak) pressure minus PEEP. VT tidal volume, PEEP positive end-expiratory pressure, PBW predicted body weight
Figure 2.
Distribution of ventilatory parameters on the first day of mechanical ventilation. Distribution of positive end-expiratory pressure (PEEP), inspired fraction of oxygen (FiO2), respiratory rate and maximum airway pressure vs tidal volume (VT). Dotted lines (horizontal and vertical) represent cut-off values for each variable. (a) PEEP, (b) FiO2, (c) respiratory rate, (d) maximum airway pressure
All patients were ventilated with PEEP of 5 cmH2O or higher, and patients with inhalation trauma received higher PEEP compared to patients without inhalation trauma [median 8 cmH2O (IQR 5–10) vs 5 cmH2O (IQR 5–8); p = 0.004; Table 2]. The median FiO2 did not differ between groups (Table 2). Controlled modes of ventilation were applied more frequently, with no significant differences in type of ventilator mode used for patients with or without inhalation trauma (Table 2). High-frequency ventilation was applied in 2 patients, both with inhalation trauma. Pmax values <30 cmH2O were used in 80% of patients (Figure 1b). Median Ppeak was significantly higher in inhalation trauma patients compared to patients without inhalation trauma [31 cmH2O (IQR 23–35) vs 20 cmH2O (IQR 17–25), p < 0.001]. Driving pressure did not differ between patients with and without inhalation trauma [14 cmH2O (IQR 11–18) vs 13 cmH2O (IQR 10–16), p = 0.55] and was <15 cmH2O in 59% of patients (Figure 1d and Table 2). Ventilatory data over the first 7 days are presented in the online supplementary appendix Figure S2.
Clinical outcomes
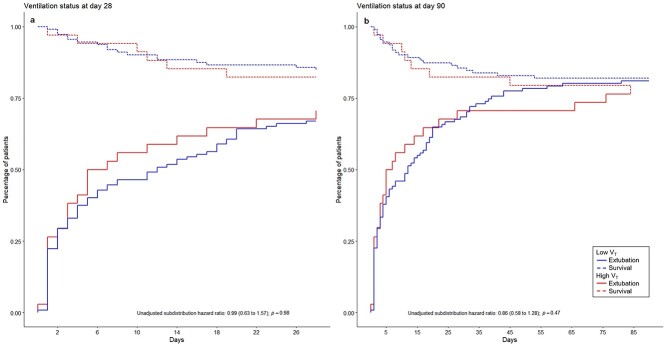
The median number of VFD-28 was 17 (IQR 0–26) and did not differ significantly between patients ventilated with VT ≤8 mL/kg PWB compared to VT >8 mL/kg PWB. The subdistribution hazard ratio for extubation in patients ventilated with VT ≤8 mL/kg PWB while considering death as a competing risk was 0.99 (0.63–1.57), p = 0.98 (Figure 3 and online supplementary appendix Table 3).
Figure 3.
Cumulative incidence curves for ventilation status of patients ventilated with low vs high tidal volume size at day 28 and day 90. Sub-distribution hazard ratio: the magnitude is affected by both time to extubation and probability of death; calculated using the Cox proportional hazard model. (a) Ventilation status at day 28, (b) ventilation status at day 90. VT tidal volume
Patients with inhalation trauma had fewer VFD-28 compared to patients without inhalation trauma [16 (IQR 0–26) vs 21 (IQR 0–26)], with a subdistribution hazard ratio for extubation in inhalation trauma patients of 0.61 (0.42–0.82; p = 0.01), considering death before extubation as a competing risk (see online supplementary appendix Table S3 and Figure S3). Hence, patients with inhalation trauma had a 39% lower probability of being successfully liberated from MV by day 28 when compared to patients without inhalation trauma. This difference is primarily driven by the longer duration of MV (p = 0.02) rather than a higher mortality (p = 0.70, see online supplementary appendix Table S3).
Pneumonia was diagnosed significantly more often in patients with inhalation trauma (see online supplementary appendix Table S4). Most pneumonias occurred in the first week of ICU admission (53 out of 59 patients), and were diagnosed while the patient was mechanically ventilated (56 out of 59 patients). Pneumonia was microbiologically confirmed in 20 out of 38 patients with inhalation trauma vs 11 out of 21 patients without inhalation trauma. ARDS was reported in 28 out of 160 patients; the incidence of ARDS did not differ significantly between patients with and without inhalation trauma. The LOS in the ICU, or in hospital, and 90-day mortality did not differ between groups (see supplementary appendix Table 4).
Discussion
This international prospective observational study investigated ventilation practices in specialized adult burn ICUs. It is one of the largest prospective cohort studies in this specific patient population over a short timeframe.
Ventilation practices in critically ill burn patients were less variable than expected as about three-quarters of the patients were ventilated with low VT, irrespective of the presence of inhalation trauma. Use of PEEP between 5 and 10 cmH2O and Pmax values <30 cmH2O have also been largely adopted. This suggests that lung-protective ventilation strategies are implemented in burn patients. We found no difference in VFD-28 between patients ventilated with low compared to high VT.
The VT in our study was marginally lower than reported VT in nonburn patients without or at risk for ARDS [4] or ARDS patients [3]. The implementation of low VT in the majority of patients in our study contrasts with a survey on mechanical ventilation practices amongst North American burn centers conducted in 2014. The American survey showed that ventilation practices tended to deviate from lung-protective strategies as only 26% of the respondents adhered to the ARDSNet protocol in burn patients with severe ARDS [24]. A systematic review on MV in burn patients showed a high variability in MV practices with a trend towards implementation of lung-protective settings in recent years [15]. Indeed, VT declined from 14 mL/kg in studies performed before 2006 to ~8 mL/kg in more recent studies [15]. In our study, applied VT did not differ between patients with or without inhalation trauma. However, patients with inhalation trauma were ventilated with significantly higher PEEP and Pmax. This was also seen in the systematic review on applied MV strategies in burn patients [15], where the majority of studies reported PEEP levels of up to 10 cmH2O. Higher PEEP levels were frequently used in studies including patients with inhalation trauma [15]. Also, studies conducted in the last decade reported the use of lower Pmax values when compared to earlier studies [15]. In the present study, Pplat values were only reported for a few patients. We used Pmax as a surrogate parameter for Pplat to calculate driving pressures [21]. Consequently, the calculated driving pressure could be an overestimation of the actual driving pressure. Still, driving pressures did not differ significantly between groups and were within suggested safety limits [3,25,26]. Finally, in our study, applied FiO2 did not differ significantly between patients with and without inhalation trauma and medians were comparable to FiO2 applied to critically ill patients without ARDS [4]. Currently, there are no clear recommendations on what FiO2 to use or what oxygen pressures in blood to aim for in burn patients. The optimal target to guide oxygen supplementation in nonburn critically ill patients also remains a subject of debate as large trials comparing liberal with conservative oxygen strategies in ventilated patients showed conflicting results [27–30]. Ventilatory data was collected at 08.00 am, provided that the patient was stable. As this was an observational study, no protocol to limit airway interventions before data collection was used. If such interventions were performed, they could have influenced the ventilatory data. For instance, VT size could have been affected by the performance of suctioning, which may result in alveolar derecruitment [31], or by recruitment of collapsed alveoli though recruitment maneuvers [32]. Extrapolation of lung-protective ventilation strategies to the burn population is controversial as burn patients were generally excluded from benchmark ventilation studies [5,33–35]. Specific characteristics of burn patients may hamper applicability of lung-protective ventilation settings. Higher driving and plateau pressures may be required in patients with decreased pulmonary and chest wall compliance caused by circumferential abdominal and thoracic burns. We did not collect data on the percentage of total body surface area with third degree burns nor on the presence of circumferential thoracic burns requiring thoracic escharotomy. Notably, eschar formation or pulmonary edema from the injury itself or from aggressive fluid resuscitation, can increase pulmonary problems [24,36].
Also, low VT ventilation can lead to ‘permissive hypercapnia’ [37]. Such hypercapnia may not be acceptable in burn patients who frequently require a high minute ventilation due to the markedly increased carbon dioxide production caused by the hypermetabolic response after a severe burn injury [36]. Higher VT may be required to improve oxygenation and ventilation in burn patients [24,38,39]. The only randomized controlled trial comparing a low VT strategy with high-frequency ventilation in adult burn patients was stopped prematurely after inclusion of 62 out of the 170 planned patients [38]. There were safety concerns as approximately one-third of the patients ventilated with low VT failed to meet oxygenation and ventilation goals [38]. Although low VT ventilation was applied in the majority of patients in our study, it remains uncertain whether burn patients benefit from this strategy. The lack of a relationship between VT and patient outcome was also reported in recent cohort studies in nonburn patients [21,40]. A randomized controlled trial comparing low with intermediate VT (i.e. 7 vs 9 mL/kg PBW) in nonburn ICU patients without ARDS also showed no significant difference in the number of VFD-28 or other clinical outcomes [41]. Future studies on ventilation strategies in burn patients could also target a driving pressure <15 cmH2O to investigate whether VT adjusted for respiratory compliance provides better outcomes.
In our study, ARDS occurred in ~18% of patients, with no significant difference between patients with and without inhalation trauma. This is considerably higher when compared to nonburn patients [3,4]. In burn patients, the reported incidence of ARDS ranges widely [15,33,42]. Pneumonia occurred significantly more often in patients with inhalation trauma. Similar to prior studies, most cases were diagnosed within the first week post-burn [18,43]. Indeed, pneumonia is a common complication following inhalation injury and is considered an important risk factor for mortality in those patients [44].
The LAMiNAR results come with limitations. Given the lower than needed number of patients included, we simplified our analysis and focused on the impact of VT or presence of inhalation trauma on the number of VFD-28. Still, the results of this analysis should be regarded with caution as the study was underpowered. VFD-28 was used as the main clinical outcome parameter. This composite outcome parameter does not discriminate between mortality and ventilation duration of more than 28 days [45]. To account for this limitation, we performed a competing risk analysis and reported both components of our composite outcome [45].
We did not account for potential confounders such as the severity of burn injury, severity of inhalation trauma, applied nebulizer protocols, applied fluid strategy and use of sedatives and analgesics. Timely burn wound excision and grafting could impact mechanical ventilation as it improves chest wall compliance, limits the hypermetabolic response and potentially reduces the volumes required for fluid resuscitation [46–49]. As limited data on surgical aspects of burn care were collected, we did not account for surgical procedures as possible confounding factors. Although the large number of participating specialized burn centers increases generalizability, it also means that there were only a few patients per center. We analyzed pooled data to describe current ventilation practices and did not adjust for between-center differences [50]. Also, participation bias may have occurred, as burn centers with particular interest in ventilation practices could have been more prone to participate in LAMiNAR.
Conclusions
In this international cohort study we found that lung-protective ventilation is used in the majority of burn patients, irrespective of the presence of inhalation trauma. Use of low VT was not associated with a reduction in VFD-28. LAMiNAR provides relevant insights into current ventilation practices in burn patients, which could serve as a baseline in future randomized trials investigating MV strategies in burn patients.
Abbreviations
ARDS: acute respiratory distress syndrome; FiO2: fraction of inspired oxygen; ICU: intensive care unit; IQR: interquartile range; IRB: institutional review board; LAMiNAR: Local Assessment of MaNAgement in BuRn Patients; LIS: Lung injury scores; LOS: length of stay; MV: mechanical ventilation; PBW: predicted body weight; PEEP: positive end-expiratory pressure; Pmax; maximum airway pressure: Ppeak: peak airway pressure; Pplat: plateau pressure; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; VFD-28: ventilator-free days and alive at day 28; VILI: ventilator-induced lung injury; VT: tidal volume
Funding
Funded by ‘Nederlandse Brandwonden Stichting’ (the Dutch Burn Association, Beverwijk, The Netherlands).
Availability of data and material
De-identified individual participant data will be available on request from researchers, after approval of proposal by the steering committee. The data will be available by contacting the principal investigator after publication.
Authors’ contributions
Steering committee: concept and design of the study and writing of the study protocol. National coordinators and steering committee: recruitment of participating centers. National and local coordinators: ensured the study conducted conforms to good clinical practice. Local coordinators and collaborators: patient enrollment and data collection. ASN, JMB, GJG: statistical analysis. Writing committee: data interpretation and writing of the manuscript. Steering committee and national coordinators: critical revision of the manuscript for important intellectual content. MJS: principal investigator.
Ethics approval
The study protocol was centrally approved by the Institutional Review Board (IRB) of the Academic Medical Center at the University of Amsterdam, The Netherlands (W14_314#15.0178). All participating sites submitted the study protocol to their (local) IRB or regulatory authority and obtained ethical approval prior to initiation of the study, in compliance with the applicable local regulatory requirements.
Consent for publication
All authors approved the manuscript and this submission.
Competing interests
None declared.
Supplementary Material
Acknowledgments
We sincerely thank all participating investigators and patients.
Contributor Information
Gerie J Glas, Academic Medical Center, University of Amsterdam, Amsterdam, AZ 1105, The Netherlands.
Janneke Horn, Academic Medical Center, University of Amsterdam, Amsterdam, AZ 1105, The Netherlands.
Markus W Hollmann, Academic Medical Center, University of Amsterdam, Amsterdam, AZ 1105, The Netherlands.
Benedikt Preckel, Academic Medical Center, University of Amsterdam, Amsterdam, AZ 1105, The Netherlands.
Kirsten Colpaert, Department of Anaesthesia and Intensive Therapy Medical University of Lublin Aleje Racklawickie 1 – 20-059 Lublin – Poland.
Manu Malbrain, AZ JAN PALFIJN GENT Watersportlaan 5 – 9000 Gent – Belgium; Department of Anaesthesia and Intensive Therapy Medical University of Lublin Aleje Racklawickie 1 – 20-059 Lublin – Poland.
Ary Serpa Neto, ABC Medical School, São Paulo, Bangú, SP 5001, Brazil; Australian and New Zealand Intensive Care Research Centre. Monash University, Melbourne, VIC 3004, Australia; GH St-Louis- Lariboisière, APHP, Paris 75010, France.
Karim Asehnoune, Service d'Anesthésie Réanimation Chirurgicale, Nantes 44093, France.
Marcello Gamma de Abreu, University Hospital Carl Gustav Carus, Dresden 01307, Germany.
Ignacio Martin-Loeches, St James University Hospital, Dublin D08 NHY1, Ireland.
Paolo Pelosi, University of Genoa, Genoa, GE 16128, Italy.
Folke Sjöberg, Linköping University Hospital, Linköping 581 85, Sweden.
Jan M Binnekade, Academic Medical Center, University of Amsterdam, Amsterdam, AZ 1105, The Netherlands.
Berry Cleffken, Maasstad Hospital, Rotterdam, DZ 3079, The Netherlands.
Nicole P Juffermans, Academic Medical Center, University of Amsterdam, Amsterdam, AZ 1105, The Netherlands.
Paul Knape, Red Cross Hospital, Beverwijk, LE 1942, The Netherlands.
Bert G Loef, Martini Hospital, Groningen, NT 9728, The Netherlands.
David P Mackie, Red Cross Hospital, Beverwijk, LE 1942, The Netherlands.
Perenlei Enkhbaatar, University of Texas Medical Branch, Galveston, TX 77555, USA.
Nadia Depetris, Turin CTO Burn Center, Turin, TO 10126, Italy.
Anders Perner, Rigshospitalet, Copenhagen 2100, Denmark.
Eva Herrero, La Paz University Hospital, Madrid 28046, Spain.
Lucia Cachafeiro, La Paz University Hospital, Madrid 28046, Spain.
Marc Jeschke, Ross Tilley Burn Centre, Sunnybrook Health Sciences Centre, Toronto M4N 3M5, Canada.
Jeffrey Lipman, Royal Brisbane and Women’s Hospital, Queensland University, Herston, QLD 4029, Australia.
Matthieu Legrand, GH St-Louis- Lariboisière, APHP, Paris 75010, France; Hopital Roger Salengro, CHRU Lille, Lille 59037, France.
Johannes Horter, BG Klinik Ludwigshafen, Ludwigshafen 67071, Germany.
Athina Lavrentieva, Papanikoalou Hospital, Thessaloniki 546 21, Greece.
Alex Kazemi, Middlemore Hospital, Otahuhu, Auckland 2025, New Zealand.
Anne Berit Guttormsen, Haukeland University Hospital, Bergen 5021, Norway.
Frederik Huss, Uppsala University Hospital, Uppsala 751 85, Sweden.
Mark Kol, Concord Repatriation General Hospital NSW, University of Sydney, Concord 2139, Australia.
Helen Wong, Concord Repatriation General Hospital NSW, University of Sydney, Concord 2139, Australia.
Therese Starr, Royal Brisbane and Women’s Hospital, Queensland University, Herston, QLD 4029, Australia.
Luc De Crop, Department of Anaesthesia and Intensive Therapy Medical University of Lublin Aleje Racklawickie 1 – 20-059 Lublin – Poland.
Wilson de Oliveira Filho, Ziekenhuis Netwerk Antwerpen–Stuivenberg, Antwerpen 2060, Belgium.
João Manoel Silva Junior, Hospital e Pronto Socorro 28 de Agosto, Manaus 69057-000, Brazil.
Cintia M C Grion, Universidade de Sao Paulo, Sao Paulo 01246-903, Brazil.
Marjorie Burnett, Universidade Estadual de Londrina, Londrina 86057-970, Brazil.
Frederik Mondrup, Rigshospitalet, Copenhagen 2100, Denmark.
Francois Ravat, Sunnybrook Health Sciences Centre, Toronto M4N 3M5, Canada.
Mathieu Fontaine, Sunnybrook Health Sciences Centre, Toronto M4N 3M5, Canada.
Renan Le Floch, CHU Lyon St Luc, Lyon 69007, France.
Mathieu Jeanne, CHU Nantes Service dánesthesie reanimation chirugicale, Nantes 44093, France.
Morgane Bacus, CHU Nantes Service dánesthesie reanimation chirugicale, Nantes 44093, France.
Maïté Chaussard, Hopital Roger Salengro, CHRU Lille, Lille 59037, France.
Marcus Lehnhardt, Saint-Louis Hospital, Paris 75010, France.
Bassem Daniel Mikhail, Saint-Louis Hospital, Paris 75010, France.
Jochen Gille, BG University Hospital Bergmannsheil, Bochum 44789, Germany.
Aidan Sharkey, St James University Hospital, Dublin D08 NHY1, Ireland.
Nicole Trommel, Maasstad Hospital, Rotterdam, DZ 3079, The Netherlands.
Auke C Reidinga, Martini Hospital, Groningen, NT 9728, The Netherlands.
Nadine Vieleers, Red Cross Hospital, Beverwijk, LE 1942, The Netherlands.
Anna Tilsley, Middlemore Hospital, Otahuhu, Auckland 2025, New Zealand.
Henning Onarheim, Haukeland University Hospital, Bergen 5021, Norway.
Maria Teresa Bouza, St George Leipzig, Leipzig 04129, Germany.
Alexander Agrifoglio, La Paz University Hospital, Madrid 28046, Spain.
Filip Fredén, Uppsala University Hospital, Uppsala 751 85, Sweden.
Tina Palmieri, St. James Hospital, Dublin D08 NHY1, Ireland.
Lynda E Painting, St. James Hospital, Dublin D08 NHY1, Ireland.
Marcus J Schultz, Academic Medical Center, University of Amsterdam, Amsterdam, AZ 1105, The Netherlands.
References
- 1. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. [DOI] [PubMed] [Google Scholar]
- 2. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2014;370:980. [DOI] [PubMed] [Google Scholar]
- 3. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. [DOI] [PubMed] [Google Scholar]
- 4. Neto AS, Barbas CS, Simonis FD, Artigas-Raventos A, Canet J, Determann RM, et al. Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study. Lancet Respir Med. 2016;4:882–93. [DOI] [PubMed] [Google Scholar]
- 5. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–8. [DOI] [PubMed] [Google Scholar]
- 6. Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151:566–76. [DOI] [PubMed] [Google Scholar]
- 7. Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Espósito DC, Pasqualucci MO, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–9. [DOI] [PubMed] [Google Scholar]
- 9. Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–63. [DOI] [PubMed] [Google Scholar]
- 10. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- 11. Esteban A, Ferguson ND, Meade MO, Frutos-Vivar F, Apezteguia C, Brochard L, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med. 2008;177:170–7. [DOI] [PubMed] [Google Scholar]
- 12. Steinvall I, Bak Z, Sjoberg F. Acute respiratory distress syndrome is as important as inhalation injury for the development of respiratory dysfunction in major burns. Burns. 2008;34:441–51. [DOI] [PubMed] [Google Scholar]
- 13. Mosier MJ, Pham TN. American burn association practice guidelines for prevention, diagnosis, and treatment of ventilator-associated pneumonia (VAP) in burn patients. Journal of burn care & research: official publication of the American Burn Association. 2009;30:910–28. [DOI] [PubMed] [Google Scholar]
- 14. Badulak JH, Schurr M, Sauaia A, Ivashchenko A, Peltz E. Defining the criteria for intubation of the patient with thermal burns. Burns. 2018;44:531–8. [DOI] [PubMed] [Google Scholar]
- 15. Glas GJ, Horn J, van der Hoeven SM, Hollmann MW, Cleffken B, Colpaert K, et al. Changes in ventilator settings and ventilation-induced lung injury in burn patients-a systematic review. Burns. 2020;46(4):762–70. [DOI] [PubMed] [Google Scholar]
- 16. Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–3. [DOI] [PubMed] [Google Scholar]
- 17. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- 18. Albright JM, Davis CS, Bird MD, Ramirez L, Kim H, Burnham EL, et al. The acute pulmonary inflammatory response to the graded severity of smoke inhalation injury. Crit Care Med. 2012;40:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- 20. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simonis FD, Barbas CSV, Artigas-Raventos A, Canet J, Determann RM, Anstey J, et al. Potentially modifiable respiratory variables contributing to outcome in ICU patients without ARDS: a secondary analysis of PRoVENT. Ann Intensive Care. 2018;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen J. Statistical Power Analysis for the Behavioral Sciences, Vol. 1988, 2nd edn. Hillsdale,NJ: Lawrence Erlbaum, 1988, 2nd edition. [Google Scholar]
- 23. Scheike TH, Zhang MJ. Flexible competing risks regression modeling and goodness-of-fit. Lifetime Data Anal. 2008;14:464–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung KK, Rhie RY, Lundy JB, Cartotto R, Henderson E, Pressman MA, et al. A survey of mechanical ventilator practices across burn Centers in North America. Journal of burn care & research: official publication of the American Burn Association. 2016;37:e131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mekontso Dessap A, Boissier F, Charron C, Begot E, Repesse X, Legras A, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862–70. [DOI] [PubMed] [Google Scholar]
- 26. Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55. [DOI] [PubMed] [Google Scholar]
- 27. Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583–9. [DOI] [PubMed] [Google Scholar]
- 28. Chu DK, Kim LH, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet (London, England). 2018;391:1693–705. [DOI] [PubMed] [Google Scholar]
- 29. Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999–1008. [DOI] [PubMed] [Google Scholar]
- 30. Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382:989–98. [DOI] [PubMed] [Google Scholar]
- 31. Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, et al. Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med. 2003;167:1215–24. [DOI] [PubMed] [Google Scholar]
- 32. Hartland BL, Newell TJ, Damico N. Alveolar recruitment maneuvers under general anesthesia: a systematic review of the literature. Respir Care. 2015;60:609–20. [DOI] [PubMed] [Google Scholar]
- 33. Lundy JB, Chung KK, Pamplin JC, Ainsworth CR, Jeng JC, Friedman BC. Update on severe burn Management for the Intensivist. J Intensive Care Med. 2016;31:499–510. [DOI] [PubMed] [Google Scholar]
- 34. Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–55. [DOI] [PubMed] [Google Scholar]
- 35. Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. [DOI] [PubMed] [Google Scholar]
- 36. Bittner EA, Shank E, Woodson L, Martyn JA. Acute and perioperative care of the burn-injured patient. Anesthesiology. 2015;122:448–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nin N, Muriel A, Peñuelas O, Brochard L, Lorente JA, Ferguson ND, et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017;43:200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung KK, Wolf SE, Renz EM, Allan PF, Aden JK, Merrill GA, et al. High-frequency percussive ventilation and low tidal volume ventilation in burns: a randomized controlled trial. Crit Care Med. 2010;38:1970–7. [DOI] [PubMed] [Google Scholar]
- 39. Sousse LE, Herndon DN, Andersen CR, Ali A, Benjamin NC, Granchi T, et al. High tidal volume decreases adult respiratory distress syndrome, atelectasis, and ventilator days compared with low tidal volume in pediatric burned patients with inhalation injury. J Am Coll Surg. 2015;220:570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42:1865–76. [DOI] [PubMed] [Google Scholar]
- 41. Simonis FD, Serpa Neto A, Binnekade JM, Braber A, Bruin KCM, Determann RM, et al. Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: a randomized clinical trial. JAMA. 2018;320:1872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cartotto R, Li Z, Hanna S, Spano S, Wood D, Chung K, et al. The acute respiratory distress syndrome (ARDS) in mechanically ventilated burn patients: an analysis of risk factors, clinical features, and outcomes using the berlin ARDS definition. Burns. 2016;42:1423–32. [DOI] [PubMed] [Google Scholar]
- 43. Chen MC, Chen MH, Wen BS, Lee MH, Ma H. The impact of inhalation injury in patients with small and moderate burns. Burns. 2014;40:1481–6. [DOI] [PubMed] [Google Scholar]
- 44. Walker PF, Buehner MF, Wood LA, Boyer NL, Driscoll IR, Lundy JB, et al. Diagnosis and management of inhalation injury: an updated review. Crit Care. 2015;19:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Re-appraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch Surg. 2003;138:127–32. [DOI] [PubMed] [Google Scholar]
- 47. Moussa A, Lo CH, Cleland H. Burn wound excision within 24 h: a 9-year review. Burns. 2021;47(6):1300–1307. [DOI] [PubMed] [Google Scholar]
- 48. Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg. 2009;36:583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greenwood JE. Advantages of immediate excision of burn eschar. Anaesth Intensive Care. 2020;48:89–92. [DOI] [PubMed] [Google Scholar]
- 50. Basagaña X, Pedersen M, Barrera-Gómez J, Gehring U, Giorgis-Allemand L, Hoek G, et al. Analysis of multicentre epidemiological studies: contrasting fixed or random effects modelling and meta-analysis. Int J Epidemiol. 2018;47:1343–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data will be available on request from researchers, after approval of proposal by the steering committee. The data will be available by contacting the principal investigator after publication.