Abstract
A unique urban encephalitis epidemic in Romania signaled the emergence of neurological infection due to West Nile (WN) virus as a novel public health threat in Eastern Europe and provided an opportunity to evaluate patterns of immunoglobulin G (IgG) and IgM reactivity in IgM capture and IgG enzyme-linked immunosorbent assays (ELISAs). WN virus infection was diagnosed serologically in 236 of 290 patients from whom acute serum or cerebrospinal fluid (CSF) samples were available. In 37% of serum samples and in 25% of CSF samples collected in the first week of illness, anti-WN virus IgM antibody was detected in the absence of virus-specific IgG. The switch to an IgG antibody response occurred after 4 to 5 days of illness and earlier in CSF than in serum. A specific humoral immune response was detected in the CSF before the serum in some patients for whom paired CSF and serum samples from the same day were available. IgM antibody in convalescent serum samples persisted beyond 2 months after the onset of illness in more than 50% of patients. ELISA optical density values and antibody concentrations were well correlated for both IgM and IgG immunoassays. Anti-WN virus IgM antibody in acute-phase samples did not cross-react significantly with flaviviruses in other antigenic groups.
West Nile (WN) fever is a mosquito-borne flaviviral infection transmitted among vertebrates and various Culex mosquito vectors in Africa, the Middle East, areas of Europe, and Asia; virus isolates have also been recovered from Australia and recently from the United States (1, 3, 12, 16, 17, 20). Humans and horses may develop illness after infection, but they do not contribute to further viral amplification (12, 20). Although the infection is considered to be transmitted mainly in an endemic pattern, especially in Africa, sizeable epidemics, numbering hundreds or thousands of cases, have occurred on that continent and in Israel (12, 15, 16, 20, 32). Smaller outbreaks of human and/or equine cases have been reported in India, Egypt, Algeria, Morocco, central and southern Europe, the Camargue of France, and in the United States (1, 3, 13, 19). Clinically, WN fever is an acute self-limited febrile illness accompanied by headache, polyarthropathy, rash, and lymphadenopathy (15, 18). Rarely, acute hepatitis or pancreatitis has been reported, and cases in the elderly have sometimes been complicated by central nervous system (CNS) infection (5, 6, 10, 11, 20, 21, 32; D. G. Tsereteli, R. A. Tsiklauri, and E. A. Ivanidze, Proc. 8th Int. Congr. Infect. Dis., abstr. 60.006, p. 206, 1998).
Between July and September 1996, a WN fever epidemic centered in the capital city of Bucharest led to over 800 suspected cases in southern Romania (26, 31). A serosurvey in Bucharest disclosed low rates of WN virus antibodies, reflecting an immunologically naive population in which a novel viral infection produced disease in epidemic proportion (31). The severity of illness in the outbreak was unusual. Most patients were hospitalized with signs of CNS infection, and the fatality rate in elderly people was 6%. WN virus was confirmed as the etiology of the outbreak serologically and by the isolation of WN virus from an acute-phase cerebrospinal fluid (CSF) sample in one case (24, 31). The virus was also isolated from a pool of Culex pipiens mosquitoes collected in Bucharest (26). Individual cases were confirmed serologically using previously unevaluated immunoglobulin M (IgM) antibody capture (MAC) and IgG direct enzyme-linked immunosorbent assays (ELISAs). The purpose of the present study was to characterize the peripheral and intrathecal antibody responses to infection and to describe the performance of these immunoenzymatic assays.
MATERIALS AND METHODS
Patients and samples.
Study patients were admitted to two infectious disease hospitals in Bucharest during an outbreak of viral meningoencephalitis from late July through early October 1996. The clinical case definition used in the epidemic investigation was acute aseptic meningitis, encephalitis, or meningoencephalitis of suspected viral etiology, with a CSF pleocytosis. One or more serum and/or CSF samples were received from 290 patients for laboratory diagnosis, including some patients who did not meet all clinical criteria of the case definition but who had other signs of an acute infection during the epidemic period (Table 1). Each sample was aliquoted and stored at −20°C for a maximum of 2 months and thawed just before being tested. Computerized clinical and epidemiological records were available for each patient.
TABLE 1.
Serologic diagnosis of WN virus infection in hospitalized patients by clinical diagnosis
| Clinical diagnosis | No. of patients tested | WN virus infection, confirmed and presumptive cases
|
||
|---|---|---|---|---|
| WN virus-specific IgM antibodies in CSFa | WN virus-specific antibodies in serumb | Total (%) | ||
| Neurological cases meeting case definition (total) | 257 | 75 | 141 | 216 (84) |
| Meningoencephalitis | 115 | 34 | 67 | 101 (89) |
| Encephalitis | 49 | 23 | 18 | 41 (84) |
| Meningitis | 93 | 18 | 56 | 74 (80) |
| Other clinical diagnoses (total) | 33 | 9 | 11 | 20 (61) |
| Acute fever and headache | 10 | 6 | 2 | 8 (80) |
| Respiratory tract infection | 5 | 5 | 5 (100) | |
| Other acute febrile illness | 18 | 3 | 4 | 7 (39) |
With or without serum virus-specific antibodies.
IgM and/or IgG, with or without seroconversion.
The investigation was conducted in accordance with human experimentation guidelines of the Romanian Ministry of Health and with those of the Centers for Disease Control and Prevention for studies conducted in rapid response to public health emergencies.
WN MAC- and direct IgG ELISAs.
The antigens used in ELISAs were prepared and optimized according to published methods (7, 29). Propagation of viruses was carried out in a biosafety level 3 laboratory. Briefly, antigens were prepared by infecting confluent monolayers of monkey kidney cells (Vero cells; American Type Culture Collection, catalog number CRL1587) with virus at approximately 0.1 PFU per cell. Virus-infected cell cultures were harvested when they exhibited three-plus cytopathic effect (CPE). Cell culture supernatants were clarified by low-speed centrifugation, aliquoted, and frozen at −70°C. Infected cell pellets were resuspended with standard borate saline, pH 9.6, containing 1% Triton X-100, sonicated, and clarified by centrifugation. Cell lysates and culture supernatants were inactivated by cobalt gamma irradiation (3 million rads) and safety tested to ensure inactivation. This was accomplished by inoculating Vero cells with the treated antigens and observing the cell monolayers for CPE. To determine the optimal antigen dilution, we performed checkerboard titrations against homologous reference sera. Cell lysates were used to coat polyvinylchloride microtiter plates (Dynatech, Vienna, Va.) for direct IgG ELISA, and the infected cell culture supernatants were used as the antigen in the IgM capture ELISA (MAC-ELISA). Mock antigens from uninfected cells were prepared in a similar manner and used in control wells.
Viruses used to infect Vero cells included the EG101 strain of WN virus, which was originally isolated in Egypt; the 17d strain (Connaught) of yellow fever (YF) virus, derived by Theiler in 1937; the New Guinea C strain of dengue 2 (DEN2) virus, a human isolate from 1944; and the Hypr strain of Central European encephalitis (tick-borne encephalitis [TBE]) virus, originally isolated in 1953 from a patient in Czechoslovakia.
Serum samples were screened at a 1:100 dilution, and a subset of these sera were serially diluted fourfold from 1:100 to 1:6,400 in the ELISA plate. CSF was screened at 1:10. Homologous hyperimmune mouse ascitic fluid was used as the detector antibody in the MAC-ELISA. This reagent was prepared and optimized according to the methods of Brandt et al. (2). The MAC-ELISA and the indirect assay for IgG antibodies were described previously (7, 27, 29). Optical densities (OD) were determined with an automatic ELISA plate reader (Diagnostics Pasteur) at 405 nm. OD values for control antigen wells were subtracted from the values for corresponding viral-antigen wells to obtain the adjusted OD value of each test sample. Cutoff values were established by determining the mean adjusted OD of four negative serum samples plus 3 standard deviations. WN virus antigen was prepared by infecting confluent Vero cell monolayers with the Eg101 strain at approximately 0.1 PFU per cell. Virus-infected cell cultures were harvested when they exhibited three-plus CPE. Cell culture supernatants were clarified by low-speed centrifugation, aliquoted, and frozen at −70°C. Infected cell pellets were resuspended with borate saline, pH 9.6, with 1% Triton X-100, sonicated for 10 min on ice, and clarified by low-speed centrifugation. Cell lysates and culture supernatants were inactivated by cobalt gamma irradiation (3 million rads) and safety tested prior to use. Cell lysate at an optimized dilution was used to coat plates for the IgG ELISA, and the infected cell culture supernatant was used as the MAC-ELISA antigen. Mock antigens from uninfected cells were prepared in a similar manner and used to coat control wells.
A sample was considered positive if it had an adjusted OD value equal to or greater than the cutoff value. The titer was equal to the reciprocal of the last dilution that was above or equal to the OD cutoff value. Positive control samples were sera from vaccinated or naturally infected persons that exhibited activity in the appropriate flavivirus ELISA. Negative control serum samples were obtained from flavivirus-naive individuals.
Acute WN infection was confirmed if (i) anti-WN virus IgM with or without IgG antibodies was present in the CSF or (ii) a seroconversion or a seroreversion of anti-WN virus IgG or IgM antibodies was demonstrated in sequential serum samples from the same patient. The diagnosis was considered presumptive if virus-specific IgM was detected only in serum or if virus-specific IgG was elevated (a titer of ≥1:400) in one or more serum samples (30). Patients with low levels of virus-specific IgG (a titer of <1:400) without IgM were classified as having had a past WN virus infection.
Statistical analysis.
Statistical analysis was performed with Microsoft Excel software. A two-tailed t test was used to compare paired data. Logarithmic, polynomial, and linear regressions and the Pearson r correlation were used to determine the relationship between paired variables.
RESULTS
Specific IgM and IgG antibody response by clinical diagnosis.
Confirmed or presumptive WN virus infections were diagnosed in 236 (81%) of 290 patients hospitalized with various clinical disorders (Table 1). The relatively low percentage of seroreactivity among patients meeting the epidemiological case definition reflects the absence of convalescent serum specimens in 24 cases. Patients meeting the case definition but who lacked appropriate serologic confirmation differed significantly from patients with confirmed cases of infection in age, residence, and clinical diagnosis, suggesting that they represented other summertime infections upon which the epidemic was superimposed (24). WN virus infection was laboratory proven in eight patients not meeting the case definition but who were hospitalized with fever and headache. Remarkably, acute WN virus infection was also diagnosed serologically in five patients with respiratory tract infections and in seven patients hospitalized with nonspecific febrile illnesses.
Kinetics of IgM and IgG antibody responses in serum and CSF.
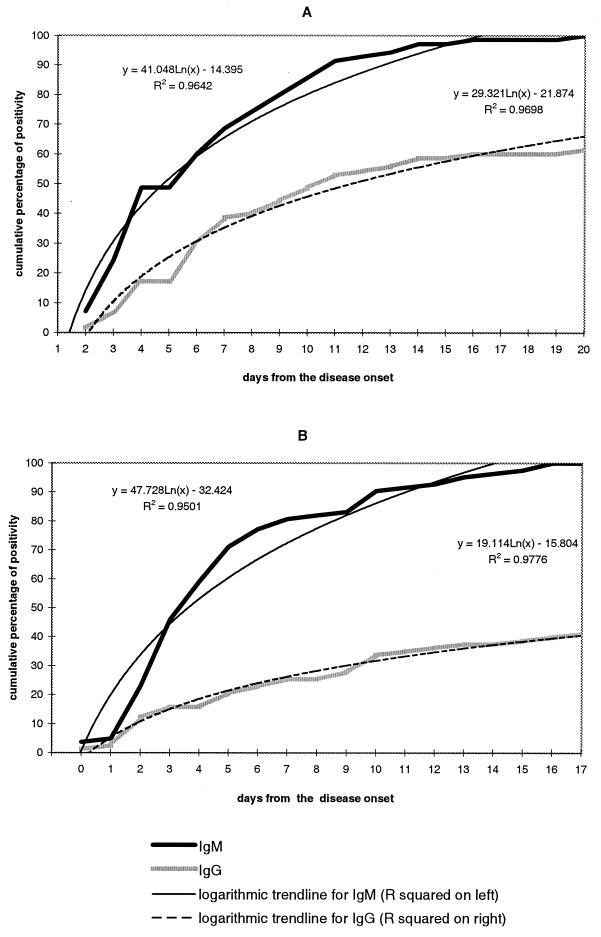
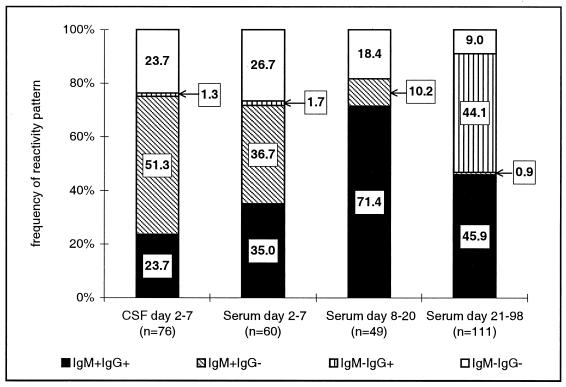
WN virus antibodies were detectable as early as the first hospitalization day in CSF and from the second day after the onset of illness in serum samples (Fig. 1). During the first week of illness, the cumulative percentage of patients who became seropositive rose by approximately 10% each day. Patterns of IgG and IgM reactivity in serum and CSF were examined in detail for patients meeting the clinical case definition (Fig. 2). Among 60 patients who had acute-phase serum samples (mean collection day, 4.1 ± 1.6; range, 2 to 7), 37% had virus-specific IgM only without specific IgG. The mean collection day for these samples was 3.5 ± 0.9, significantly lower than the mean collection day for samples that had both IgM and IgG, which was 4.9 ± 1.6 (P < 0.01). Thus, the switch to an IgG antibody response occurred after 4 to 5 days of illness. CSF samples collected from various days in the first week of illness were available from 76 patients meeting the clinical case definition (mean collection day, 3.7 ± 1.4; range, 2 to 7). The switch to an IgG antibody response appeared to occur slightly earlier in CSF than in serum. The mean collection day for CSF samples with specific IgM only was 3.7 ± 1.2, and for those having both IgM and IgG, the mean day was 3.8 ± 1.2 (P = 0.839) (Fig. 1B). Among the 60 patients who fulfilled the clinical case definition and who had acute-phase serum samples, 72% were seropositive by the seventh day of illness (Fig. 2, column 1). An additional 10% became seropositive by the end of the third week of illness, and another 9% became seropositive only after 4 weeks of illness, giving a total seroreactivity of 91%.
FIG. 1.
Cumulative percent positivity of anti-WN virus IgM and IgG ELISA antibodies in sera (A) and CSF (B) of patients with confirmed and presumptive recent WN virus infection by day after onset of illness.
FIG. 2.
Patterns of WN virus-specific IgM and IgG antibody reactivity in CSF and serum by interval after onset of illness.
Cross-reactivity of acute-phase anti-WN antibodies with flaviviruses in other antigenic groups.
Antibody responses of infected subjects in areas where intense flavivirus activity occurs in successive years can give rise to difficulties in serologic diagnosis. As a rule, primary responders exhibit mainly monotypic antibody responses, but with successive infections, the antibody response broadens to include heterotypic reactivity to other flaviviruses in the same or different antigenic groups. The occurrence of this outbreak in a flavivirus-naive population allowed us to evaluate the specificities of the ELISAs in primary WN virus infection. We selected at random 14 acute-phase samples, which were tested for IgM and IgG antibodies against four flavivirus antigens (Table 2): WN virus, DEN virus, YF virus, and TBE virus. The IgM immune responses were monotypic, while the IgG antibodies exhibited a broader range of specificity in accordance with the magnitude of the primary immune response.
TABLE 2.
IgM and IgG reactivity to flaviviral antigens in selected patients with WN encephalitis
| Specimen no. | ELISA titer
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IgM
|
IgG
|
|||||||
| TBE | YF | DEN2 | WN | TBE | YF | DEN | WN | |
| 1 | 50 | 50 | 50 | 1,600 | 50 | 50 | 50 | 100 |
| 2 | 50 | 50 | 100 | 1,600 | 50 | 50 | 50 | 100 |
| 3 | 50 | 400 | 1,600 | 12,800 | 50 | 50 | 50 | 400 |
| 4 | 50 | 50 | 400 | 12,800 | 100 | 50 | 50 | 100 |
| 5 | 50 | 50 | 100 | 12,800 | 50 | 50 | 50 | 50 |
| 6 | 50 | 50 | 400 | 1,600 | 50 | 50 | 50 | 400 |
| 7 | 50 | 50 | 100 | 1,600 | 100 | 50 | 50 | 400 |
| 8 | 50 | 50 | 100 | 400 | 50 | 50 | 50 | 400 |
| 9 | 50 | 50 | 100 | 1,600 | 50 | 50 | 50 | 50 |
| 10 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| 11 | 50 | 50 | 100 | 1,600 | 50 | 50 | 50 | 50 |
| 12 | 50 | 50 | 400 | 6,400 | 50 | 50 | 50 | 50 |
| 13 | 50 | 50 | 20 | 1,600 | 50 | 50 | 50 | 400 |
| 14 | 50 | 100 | 400 | 50 | 50 | 50 | 400 | |
Correlation between OD values and serum antibody titer.
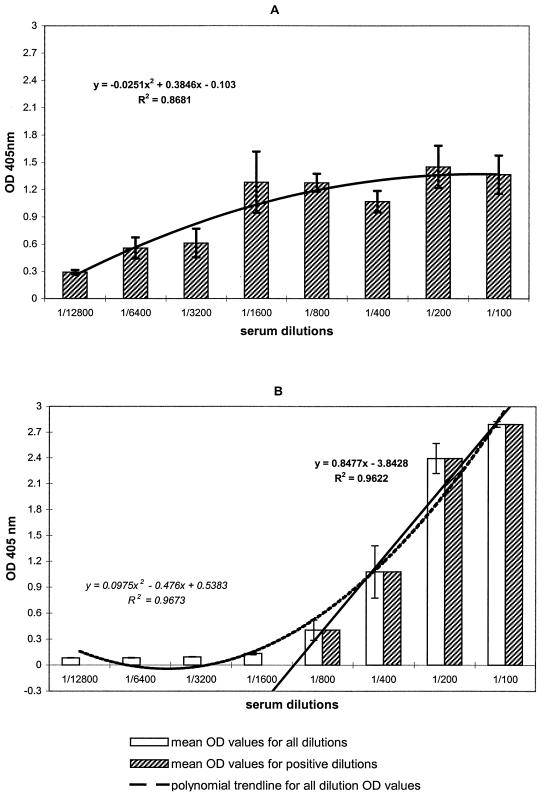
In Fig. 3, the mean OD values for a twofold-dilution series of serum samples (beginning at 1:100) are displayed for IgM and IgG. As expected from the multistep protocol used, OD values in the MAC-ELISA show great variability and practically no linearity. The best-fit regression for IgM OD values was a polynomial relationship (R = 0.93) in which low OD values corresponded to low antibody concentration and high OD values corresponded to high antibody concentration. Reactivity in the MAC-ELISA usually persisted in dilutions of >1:10,000 and occasionally >1:50,000 (data not shown), reflecting the presence of very high levels of WN virus-specific IgM antibodies in the samples and/or a high sensitivity of the assay system. With respect to IgG OD values for the same dilutions, Fig. 3B shows a much lower variability among replicates and a better polynomial correlation between OD values and dilutions (R = 0.98). The linearity of the relationship in the region with serum diluted 1:100 to 1:800 (Fig. 3B) allowed an accurate estimation of IgG serum concentration from the sample OD value. After serial dilution, reactivity was extinguished more rapidly for IgG than for IgM, with no reactivity beyond a dilution of 1:6,400.
FIG. 3.
Correlation between OD values and IgM (A) and IgG (B) antibody concentrations in serum. Sera with high IgM and IgG WN virus-specific antibody titers were tested in twofold dilutions; the bars represent the mean OD value for each given serum dilution, and error bars were set at ±1 standard deviation of the mean. The trend line equation and R2 values are shown.
Correlation between OD values and illness day.
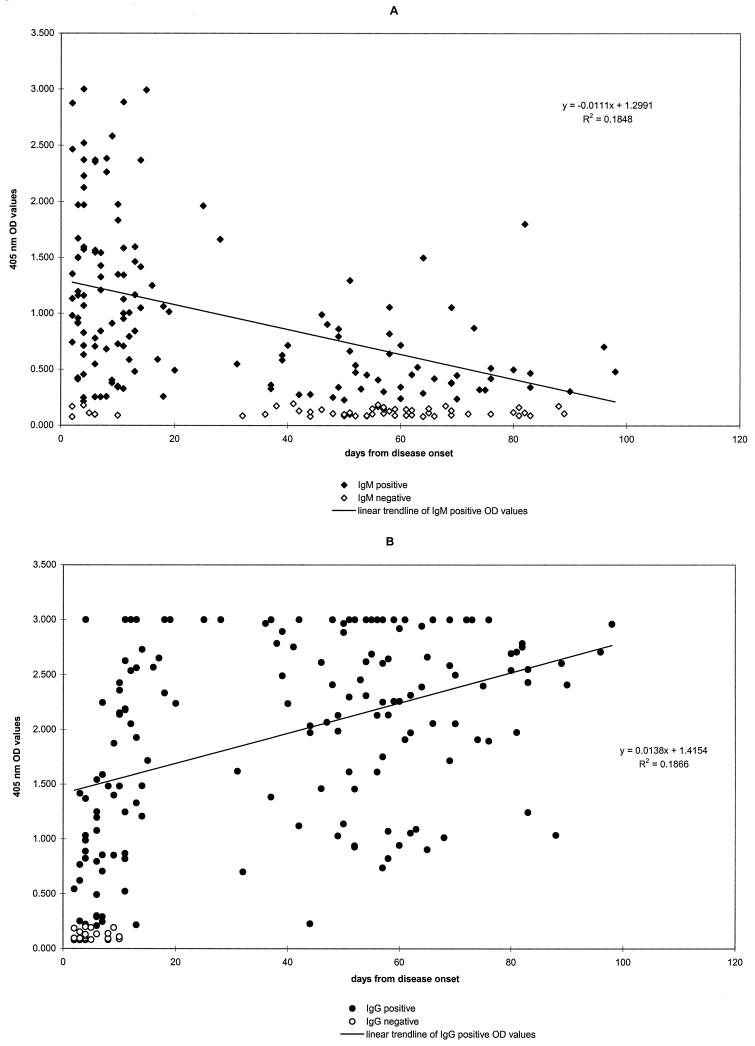
OD values for serum IgM showed a general decline through the first 3 months after the onset of illness (Fig. 4A). The mean OD value of samples in the first 20 days of illness, 1.230 ± 0.733, was significantly higher (P < 0.0001) than the mean OD of later samples, 0.618 ± 0.401. Generally, high OD values in the IgM assay corresponded to a recent infection while low OD values corresponded to a more distantly acquired infection, 1 to 3 months before serum collection (slope, −16.7; R = −0.43; P < 0.01). More precisely, an adjusted IgM OD value of more than 1.000 had a probability of 0.62 of indicating a recent infection (within 1 month), and an IgM OD value of less than 1.000 had a probability of 0.95 of indicating an infection older than 1 month. More than 50% of cases had IgM persisting beyond 2 months after the onset of illness. OD values for IgG were more scattered (Fig. 4B), although a tendency toward an incremental increase is evident (slope, 13.5; R = 0.432; P < 0.01). The mean IgG OD value during the first 20 days of illness (1.519 ± 0.880) was significantly lower than the mean OD value found in later samples (2.249 ± 0.732; P < 0.01).
FIG. 4.
Correlation plots between OD values and day of disease for IgM (A) an IgG (B).
DISCUSSION
The epidemic in Romania was the first significant WN fever outbreak reported in Europe. The uniformly low seroprevalence rates in all age groups recorded in a postepidemic serosurvey suggests that the virus was newly introduced into an immunologically naive population (31). This may explain the unprecedented number of neurological cases and deaths, especially among elderly persons. In the 1996 epidemic, 835 clinically suspected cases were reported, but in 326 that met the case definition, appropriate clinical specimens to confirm a laboratory diagnosis were unavailable. This gap underscores a critical need for clinical laboratory procedures that can be applied to specimens obtained early in the illness.
The accepted diagnostic categories for defining a case of arboviral encephalitis specify a compatible clinical syndrome and (i) virus-specific IgM in CSF or a fourfold rise in serum antibody titer by ELISA, indirect immunofluorescent antibody assay (IFA), complement fixation (CF), hemagglutination inhibition (HI), or neutralization (confirmed case); (ii) elevated antibody titer in a single serum sample (e.g., >320 by HI, >256 by IFA, >128 by CF, >160 by 90% plaque reduction neutralization, or virus-specific IgM in the serum) (probable case); or (iii) occurrence during a period when arbovirus transmission is likely (suspected case) (30).
During the Romanian epidemic, 84% of patients meeting the clinical case criteria were laboratory confirmed or were considered probable, based on the presence of anti-WN virus IgM antibodies in CSF or serum samples collected in the first week of illness. In serum samples collected in the same interval from patients hospitalized with other clinical conditions, only 61% had virus-specific IgM antibodies. In 51% of patients with samples available during the first week after hospital admission, IgM antibodies were detected in the CSF only, while IgM was detected in only 37% of early serum samples. In some cases, when both serum and CSF samples taken the same day were available, the onset of IgM antibody synthesis was reported first in the CSF. This observation underscores the importance of collecting CSF specimens for serologic testing at the earliest stages of illness. The MAC-ELISA has demonstrated a high degree of sensitivity and specificity in the serologic diagnosis of other neurotropic flaviviral infections when both serum and CSF samples were tested, paralleling the results reported here (4, 14).
The appearance of IgM in the CSF before it appears in the serum indicates that antibody production began locally in the CNS and that its presence did not merely reflect transudation from the systemic circulation (4, 5, 14). IgM antibodies were still present 2 months after the onset of illness in more than 50% of convalescent serum samples. The overall decline in antibody titers with time does not exclude the possibility that high titers in some samples could reflect viral persistence. Chronic neurological infections have been produced in monkeys experimentally infected with WN virus, and naturally acquired Japanese encephalitis infections have been followed by delays in virus clearance, by clinical relapses, and by the persistence of intrathecal viral antigen for several weeks and intrathecal IgM antibodies for several months (22, 25, 28). The persistence of serum IgM antibodies for up to 3 decades in recipients of live attenuated YF vaccine has been reported, presumably reflecting viral persistence (23). Efforts are being made to follow recovered WN encephalitis patients in this outbreak to study the disappearance or continued persistence of IgM antibodies.
Serum IgG antibodies were present in 90% of convalescent sera collected between 2 and 3 months after the onset of illness. The absence of IgG antibodies in the seroprevalence study conducted during the epidemic investigation is evidence that WN virus was newly introduced to Bucharest in 1996. The only other flaviviral infection recognized in Romania is TBE virus infection, which is transmitted in northern Romania, where it is the etiology of 20 to 30 encephalitis cases annually (8, 9). In primary infections, antibodies to TBE and WN viruses are likely to show cross-reactivity by either HI or ELISA only in samples with high antibody titers. Our evaluation showed that anti-WN virus IgM antibodies detected by MAC-ELISA were highly specific but that IgG ELISA antibodies exhibited some cross-reactivity.
In the wake of the 1996 epidemic, surveillance of acute encephalitis cases was established in districts affected by the outbreak, including Bucharest, and 13 sporadic cases were detected in 1997 and 1998 (6). The gradual diminution of viral transmission in the years after an outbreak, also observed after St. Louis encephalitis epidemics in the United States, may reflect the continued depletion of susceptible amplifying and end hosts, extension of intermediate-term meteorological patterns favoring viral transmission, or the changing sensitivity of case detection. Previous field studies in the Danube Delta in southeastern Romania had suggested that the virus was transmitted in a local sylvatic cycle (8, 9). Details of the sylvatic transmission cycle there or elsewhere in Europe have not been well defined, and the novel circumstances that led to epidemic urban transmission in 1996 are even more obscure. By analogy from the epidemiology of St. Louis encephalitis in the western United States, WN virus may be transmitted perennially in rural areas of southern Europe, producing an endemic pattern of infection in the local population. Under unusual circumstances of amplified transmission, the virus may spill over to urban locations, where susceptible human populations and the proximity and abundance of vector mosquitoes and of intermediate avian amplifying hosts provide conditions for epidemic transmission (18, 31).
Little is known of the incidence or geographic distribution of WN encephalitis in Europe because the disease is not routinely considered in the differential diagnosis of neurological infection and because laboratory diagnosis is not readily available. The 1996 epidemic in Romania, associated suspect cases in Bulgaria, an equine epizootic in Italy, and a recent study from western Georgia in which WN virus antibodies were reported in 31% of patients with acute CNS infection suggest that WN virus infections may have public health significance in a larger area of eastern and southern Europe than has been appreciated previously (6, 13; Tsereteli et al., Proc. 8th Int. Congr. Infect. Dis.). In addition, the recent emergence of a WN virus-like outbreak in the United States signals the potential for an even wider, global distribution (1, 3). The dissemination of sensitive and specific laboratory test systems, such as MAC-ELISA, to confirm the diagnosis and of IgG ELISAs to aid in serosurveys will greatly facilitate comprehension of the public health burden of this previously neglected infection.
ACKNOWLEDGMENTS
We are grateful to the staff epidemiologists of the Bucharest Preventive Medicine Department for tracing cases and to the staff clinicians of the Dr. Victor Babes and N. Gh. Lupu Hospitals of infectious diseases in Bucharest for providing clinical samples and data.
The investigation was supported under an interagency agreement between the United States Agency for International Development and the Department of Health and Human Services.
REFERENCES
- 1.Anonymous. Outbreak of West Nile-like viral encephalitis–New York, 1999. Morb Mortal Wkly Rep. 1999;48:845–849. [PubMed] [Google Scholar]
- 2.Brandt W, Buescher E L, Hetrick F M. Production and characterization of arbovirus antibody in mouse ascitic fluid. Am J Trop Med Hyg. 1967;16:339–347. doi: 10.4269/ajtmh.1967.16.339. [DOI] [PubMed] [Google Scholar]
- 3.Briese T, Jia X Y, Huang C, Grady L J, Lipkin W I. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. Lancet. 1999;354:1261–1262. doi: 10.1016/s0140-6736(99)04576-6. [DOI] [PubMed] [Google Scholar]
- 4.Burke D S, Nisalak A, Ussery M A, Laorakongse T, Chantavibul S. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J Infect Dis. 1985;151:1093–1099. doi: 10.1093/infdis/151.6.1093. [DOI] [PubMed] [Google Scholar]
- 5.Cernescu C, Ruta S M, Tardei G, Grancea C, Moldovan L, Spulbar E, Tsai T F. A high number of severe neurologic clinical forms during an epidemic of West Nile virus infection. Rom J Virol. 1997;48:13–25. [PubMed] [Google Scholar]
- 6.Cernescu C, Nedelcu N I, Tardei G, Ruta S, Tsai T F. Continued transmission of West Nile virus to humans in southeastern Romania, 1997–1998. J Infect Dis. 2000;181:710–712. doi: 10.1086/315225. [DOI] [PubMed] [Google Scholar]
- 7.Chu Y K, Rossi C, LeDu J, Lee H, Schmaljohn C, Dalrymple J. Serological relationships among viruses in the Hantavirus genus, family Bunyaviridae. Virology. 1994;198:196–204. doi: 10.1006/viro.1994.1022. [DOI] [PubMed] [Google Scholar]
- 8.Draganescu N, Garjabu E, Iftimovici R, Klebleev E. Études sérologiques sur la présence de quelques arbovirus dans la région de sylvo-steppe de Tulcea. Rev Roum Virol. 1991;42:47–51. [PubMed] [Google Scholar]
- 9.Draganescu N, Girjabu E, Iftimovici R. Investigations sérologiques chez l'homme et chez differentes espèces d'animaux domestiques de la région de sylvo-steppe de Roumanie, pour le dépistage de quelques Togaviridae et Bunyaviridae. Rev Roum Virol. 1993;44:207–210. [PubMed] [Google Scholar]
- 10.George N J, Lesbordes J L, Georges-Courbet M C, Meunier DMY, Gonzalez J P. Fatal hepatitis from West Nile virus. Ann Inst Pasteur. 1987;138:237–244. [Google Scholar]
- 11.George S, Prasad S R, Rao J A, Yergolkar P N, Sreenivasaiah Setty C V. Isolation of Japanese encephalitis and West Nile viruses from fatal cases of encephalitis in Kolar district of Karnataka. Indian J Med Res. 1987;86:131–134. [PubMed] [Google Scholar]
- 12.Hayes C G. West Nile fever. In: Monath T F, editor. The arboviruses: epidemiology and ecology. V. Boca Raton, Fla: CRC Press; 1989. pp. 59–88. [Google Scholar]
- 13.Hubalek Z, Haouzka J. West Nile fever, a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Innis B L, Nisalak S, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke C H. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1991;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 15.Marberg K, Goldblum N, Stork V, Jasinska-Klingberg W, Klingberg M. The natural history of West Nile fever. I. Clinical observations during an epidemic in Israel. Am J Hyg. 1956;69:259–269. doi: 10.1093/oxfordjournals.aje.a119838. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh B M, Jupp P G, Dos Santos I, Meeneham G M. Epidemics of West Nile and Sindbis viruses in South Africa with Culex (Culex) univittatus Theobald as a vector. S Afr J Sci. 1976;72:295–300. [Google Scholar]
- 17.Mitchell C J. Geographic spread of Aedes albopictus and potential for involvement in arbovirus cycles in the Mediterranean Basin. J Vector Ecol. 1995;20:44–58. [Google Scholar]
- 18.Monath T P, Tsai T F. Flaviviruses. In: Richman D D, Whitley R J, Hayden F G, editors. Clinical virology. New York, N.Y: Churchill-Livingstone; 1997. pp. 1133–1186. [Google Scholar]
- 19.Panthier R, Hannoun C, Beytout D, Mouchet J. Epidemiologie du virus West Nile. Etude d'un foyer en Camargue. 3. Les maladies humaines. Ann Inst Pasteur. 1968;115:435–445. [PubMed] [Google Scholar]
- 20.Peiris J S M, Amerasinghe F P. West Nile fever. In: Beran G W, editor. Handbook of zoonoses, section B. Viral. 2nd ed. Boca Raton, Fla: CRC Press; 1994. pp. 139–148. [Google Scholar]
- 21.Perelman A, Stern J. Acute pancreatitis in West Nile fever. Am J Trop Med Hyg. 1974;23:1150–1152. doi: 10.4269/ajtmh.1974.23.1150. [DOI] [PubMed] [Google Scholar]
- 22.Pogodina V V, Frolova M P, Malenko G V, Fokina G I, Koreshkova G V, Kiseleva L L, Bochkova N G, Ralph N M. Study on West Nile virus persistence in monkeys. Arch Virol. 1983;75:71–86. doi: 10.1007/BF01314128. [DOI] [PubMed] [Google Scholar]
- 23.Poland J D, Calisher C H, Monath T P, Downs W G, Murphy K. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull W H O. 1981;59:895–900. [PMC free article] [PubMed] [Google Scholar]
- 24.Porter K R, Summers P L, Dubois D, Puri B, Nelson W, Henchal E, Oprandy J J, Hayes C G. Detection of West Nile virus by the polymerase chain reaction and analysis of nucleotide sequence variation. Am J Trop Med Hyg. 1993;48:440–446. doi: 10.4269/ajtmh.1993.48.440. [DOI] [PubMed] [Google Scholar]
- 25.Ravi V, Desai A, Shenoy PK, Satishchandra P, Chandramuki A, Gourie-Devi M. Persistence of Japanese encephalitis virus in the human nervous system. J Med Virol. 1993;40:326–329. doi: 10.1002/jmv.1890400412. [DOI] [PubMed] [Google Scholar]
- 26.Savage H M, Ceianu C, Nicolescu G, Karabatsos N, Lanciotti R, Vladimirescu A, Laiv L, Ungureanu A, Romanca C, Tsai T F. Entomological and avian investigations of an epidemic of West Nile fever in Romania, 1996, with serological and molecular characterization of a virus isolate from mosquitoes. Am J Trop Med Hyg. 1999;61:600–611. doi: 10.4269/ajtmh.1999.61.600. [DOI] [PubMed] [Google Scholar]
- 27.Schmaljohn C, Vanderzanden L, Bray M, Custer D, Meyer B, Li D, Rossi C, Fuller D, Fuller J, Haynes J, Huggins J. Naked DNA vaccines expressing the prM and E genes of Russian spring summer encephalitis virus and Central European encephalitis virus protect mice from homologous and heterologous challenge. J Virol. 1997;71:9563–9569. doi: 10.1128/jvi.71.12.9563-9569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S, Mathur A, Prakash R, Kulshreshtha R, Kumar R, Chaturvedi U C. Japanese encephalitis virus latency in peripheral blood lymphocytes and recurrence of infection in children. Clin Exp Immunol. 1991;85:85–89. doi: 10.1111/j.1365-2249.1991.tb05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharp T R, Wallace M R, Hayes C G, Sanchez J L, Defraites R F, Arthur R R, Thornton S A, Batchelor R A, Rosmajzl P J, Hanson R K, Wu S J, Iriye C, Burans J P. Dengue fever in US troops during Operation Restore Hope, Somalia, 1992–1993. Am J Trop Med Hyg. 1996;53:89–94. [PubMed] [Google Scholar]
- 30.Tsai T F. Arboviruses. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 1107–1124. [Google Scholar]
- 31.Tsai T F, Popovici F, Cernescu C, Campbell G C, Nedelcu N I the Investigative Team. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767–771. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 32.Varsano M, Basat M B, Rannon L. West Nile virus infection in recent years in Israel. Isr J Med Sci. 1982;19:11. [Google Scholar]