Abstract
Although mRNA-based vaccines BNT162b2 and mRNA-1273 exhibit a remarkable efficacy and effectiveness in preventing particularly severe Covid-19 with an overall favorable adverse event profile, their use has been associated with rare cases of acute myocarditis. These occur most commonly after the second dose, with the highest incidence among young male recipients. This complication has not been frequently observed among adenoviral vector vaccine receivers, and its clinical, laboratory and imaging features resemble those of other common causes of acute myocarditis. The pathogenesis of mRNA-vaccine associated myocarditis has not yet been elucidated, although a number of mechanisms have been proposed, typically implicating the administered S-protein mRNA and likely mediated through an autoimmune mechanism. Nonetheless, other mechanisms may be implicated given the fact that myocarditis cases are very rarely observed among recipients of non mRNA vaccines. The recent observation of a similar adverse event in a recipient of the non-mRNA, peptide-based NVX-CoV2373 in the frame of a phase III clinical trial with 7020 participants in the active treatment arm raises the question whether the lipid nanoparticle sheath, which is a common structural component of these platforms could be implicated in the pathogenesis of vaccine-induced myocarditis.
Keywords: Covid-19, Lipid nanoparticles, mRNA vaccine, Myocarditis, SARS-CoV-2
As the coordinated efforts towards development of safe and effective therapies against SARS-CoV-2 infection are in progress [1], vaccination strategies constitute the spearhead of worldwide strategies against Covid-19 [2]. Amidst the current broad-scale nationwide vaccination programs, mRNA-based platforms constitute the bulk of vaccines used in the US as well as in Europe. The two currently available preparations, namely the BNT162b2 marketed by BioNTech/Pfizer and the mRNA-1273 by Moderna were the first agents to be approved for use for immunization against SARS-CoV-2 by the FDA and EMA.
Both available mRNA-based vaccines have shown remarkable efficacy in early clinical trials with rates of prevention of COVID-19 symptomatic infection as high as 95% and 94.1% respectively [3,4]. It should be noted that in the initial phase III clinical trial of mRNA-1273, all 30 observed cases of severe COVID-19 among 30,420 participants were among receivers of placebo [4]. Real-world data from the nation-wide application of BNT162b2 in Israel demonstrated protection rates of 92%, 87% and 94% against symptomatic SARS-CoV-2 infection, hospitalization and severe disease, respectively among fully vaccinated individuals [5]. Accordingly, in an analysis of data from 21 US hospitals, both vaccines were shown to be highly effective as regards the prevention of hospitalizations (88% and 93% for BNT162b2 and mRNA-1273, respectively) [6]. For both preparations, two doses are required for adequate protection, typically administered 3 (BioNTech/Pfizer) or 4 (Moderna) weeks apart, although longer intervals between the two doses have been also applied during the early course of the pandemic. The conferred immunity after both vaccines seems to wane over the course several months following the 2nd dose and probably somewhat more rapidly among BNT162b2 receivers, albeit with durable protective effects as regards a severe COVID-19 course requiring hospitalization, mechanical ventilation, or death [7,8]. However, both vaccines seem to be effective against the so far emerged dominant SARS-VoV-2 variants [7,8], and furthermore, administration of a third booster dose seems to rapidly restore a high-level of protection against the acute infection [7].
As a fundamental feature of their structure, both currently available mRNA platforms implement an in vitro transcribed mRNA sequence, structurally optimized to enhance its half-life and translation [9]. Τhis encodes the amino acid sequence of the viral spike protein antigen, which enables viral cell entry upon binding to its membrane surface receptor, namely the angiotensin converting enzyme–2 (ACE2). After entry into receiver cells, the delivered mRNA is translated into the S-protein, against which the development of a humoral and cellular immune reaction occurs. To counter the inherent instability of free mRNA and facilitate its entry into selected host cells, a lipid nanoparticle sheath is used as a delivery vehicle; the most crucial element of the lipid nanoparticles is the variable ionizable lipid (SM-102 for Moderna and ALC-0315 for Pfizer/BioNTech), which under acidic manufacturing conditions binds to the mRNA molecule. Cholesterol molecules, distearoylphosphatidylcholine and PEGylated lipids are additional components which contribute to stability and surface fluidity of LNPs, while facilitating their uptake and fusion with the endosome membrane of host cells [9].
In the first months that followed the release of COVID-19 vaccines into the market which were marked by rapid population-wide immunization, the first cases of vaccine-induced thrombotic thrombocytopenia (VITT) associated with the administration of adenovirus vector platforms ChAdOx1 and Ad26.COV2.S appeared. The possibility of this rare but potentially life-threatening complication being associated with every vaccine platform initially caused turmoil within the medical community [10]. Nonetheless, the two available mRNA vaccines were soon deemed safe with respect to VITT, despite being implicated in sporadic cases of autoimmune thrombocytopenia [11,12]. Besides, they were used as alternative boosters after incomplete vaccination with adenoviral vector-based platforms in many countries after retraction of these agents from the market due to the subsequent VITT-related safety concerns.
Shortly afterwards however, the first reports over a possible association between mRNA platform-based vaccinations and cases of myocarditis appeared. The clinical presentation as well as laboratory and imaging studies of these cases are compatible with that of acute myocarditis from other, commonly viral causes; cases typically present with chest pain with an onset usually within 3–4 days (and, in virtually all cases, within 14 days) following either BNT162b2 or mRNA-1273 vaccination, most frequently the second dose [13], [14], [15], [34]. Although both genders in a broad age spectrum may be affected, the risk appears to be highest among young males; an early series of 136 definite or probable myocarditis cases associated with BNT162b2, the rate ratio within the 30 days following the second dose among fully vaccinated individuals was found to be 2.35 overall and 8.96 in the 16–19 age category compared with unvaccinated individuals. The overall frequency of this complication after the second dose of BNT162b2 was estimated at 1 per 26,000 males and 1 per 218,000 females [14]. Another study reported an overall prevalence of 2.13 per 100,000 persons among those who received at least one BNT162b2 dose, with the highest rates among males in the age group between 16 and 29 years estimated at 10.69 per 100000 [16]. Notably, cases falling out of this typical age- and time-pattern have been reported [17], although according to recent data from the United Kingdom the increased risk of myocarditis seems to solely affect individuals under 40 years of age, and may be substantially greater among those vaccinated with mRNA-1273 compared with BNT162b2 in this age group [34]. It should be noted that in this report and in stark contrast to other available observations a small overall increase in myocarditis risk was observed after the first dose of ChAdOx1 vaccine [34]. Cardiac enzyme elevations are universally observed and may be accompanied by corresponding electrocardiographic changes [14], while the findings in cardiac MRI do not differ from those of myocarditis due to other causes [18]. The clinical course is typically benign and rapidly self-limiting, although cases with a more severe course including a significant left ventricular dysfunction or even death have been reported [14,16].
The scarcity of reported cases within the vast and rapidly expanding population of recipients led the Center for Disease Control and Prevention, the Food and Drug Administration as well as the European Medicines Agency to the statement that the equivocal benefits from the usage of mRNA platforms outweigh the risks for both across all age categories [15,19,20].
With respect to the pathogenesis of mRNA vaccine-related myocarditis cases, a number of potential explanations have been attempted. In order to explain the skewed gender distribution of cases, the influence of sex steroid hormones (estrogen, testosterone) has been suggested. Not surprisingly, in the foreground stand immune or autoimmune mediated processes as possible mechanisms, and the highest frequency of occurrence after the second vaccine dose (after allowing for a presumed sensibilization process to take place after the first dose) seems to strengthen this notion. Besides, mRNA vaccines have been already causally implicated in a number of immune-mediated adverse events such as autoimmune thrombocytopenia and thyroiditis [11,21]. Among others, supported by the relatively frequent occurrence of myocardial damage and myocarditis in the frame of SARS-CoV-2 infection, a mechanism of molecular mimicry between the viral S-protein and various self-antigens (i.e. α-myosin) has been suggested [22]. In this case, relatively similar rates of myocarditis occurrence would be expected among receivers of adenoviral vector-based platforms. the currently available evidence presents a rather solid counterargument against this scenario; while cases of myocarditis/pericarditis in association with administration of the ChAdOx1 vaccine (Vaxzevria, AstraZeneca) have also been reported [34], they do not seem to occur more frequently than expected in the absence of vaccination according to most available evidence [23,24], while there is so far one published only 1 case reported after Janssen Ad26.COV2.S [25]. Furthermore, mRNA strands are immunogenic and may themselves trigger an immune response directed against cardiomyocyte epitopes or adversely influence the myocardium in the frame of an exaggerated systemic reaction [22]. Furthermore, a general mechanism implicating the production of anti-idiotype antibodies against immunogenic regions of antigen-specific antibodies has been recently proposed. Τhis could in theory lead to tissue-specific adverse events through the formation of immune complexes, activation, blockade and/or down-regulation of membrane receptors (e.g. ACE2), as well as complement- or immune cell-mediated cellular damage [26].
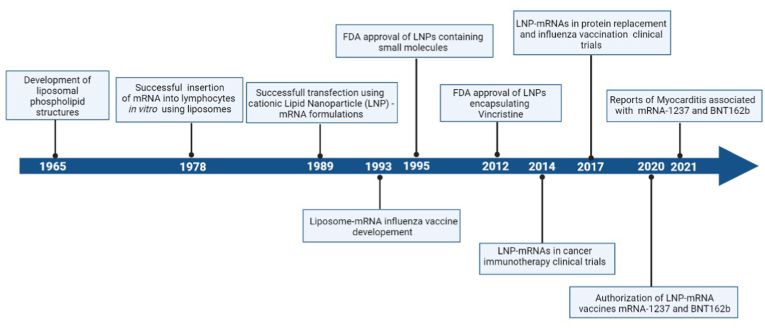
A previously overlooked component of the mRNA vaccines relates to the lipid nanoparticle sheath which encapsulates the mRNA strand encoding the S-protein sequence. The Moderna and Pfizer/BioNTech vaccines are not the first applications of this technology, since liposomal and nanoparticle formulations have already been used as drug delivery vehicles, particularly chemotherapeutics, for over two decades now (Fig. 1) [27]. Since myocarditis cases after Covid-19 vaccination are virtually exclusively confined among mRNA-based recipients, the question arises if specific lipid molecules or their combination in the structure of the lipid nanoparticle component of these vaccines could play a role in the pathogenesis of myocardial damage associated with their use. Within this context, a hypothesis could be made in the direction of either a direct deleterious effect on myocardial cells of LPN itself or of that of an immune reaction against it or even its aggregate with the mRNA strand within the vaccine preparations. One could argue that there have been up until now essentially no reports of a similar clinical picture among receivers of other non-vaccine, LPN-containing treatments (Fig. 1). This could be a mere result of the rarity of this adverse event combined with the massive vaccination programs, which could have allowed for the clustering and recognition of such cases. It should be also noted that the population most susceptible to vaccine-induced myocarditis, namely the younger age groups are typically underrepresented among those who receive treatment with lipid nanoparticle – formulated chemotherapeutics. Besides, the polyethylene glycol (PEG) component and several other ingredients of the LNP sheath have been also implicated in other hypersensitivity reactions, most notably in extremely rare but potentially life-threatening immediate cases of anaphylaxis following mRNA vaccine administration [28,29].
Fig. 1.
Timeline of important events in the evolution of lipid nanoparticle technology. Created with BioRender.com.
To further fuel this argument, in the recently published results of the recombinant nanoparticle NVX-CoV2373 (Novavax) vaccine safety and efficacy trial, a case of myocarditis among 7020 participants in the treatment arm occurred was reported, which also occurred 3 days after receiving the 2nd vaccination [30]. The NVX-CoV2373 contains no mRNA strands, but rather the viral S-protein itself, synthesized in moth cells after infection with an engineered Baculovirus which carries the S-Protein DNA sequence. The produced S-Proteins are incorporated on a lipid nanoparticle delivery vehicle consisting of phospholipids, cholesterol and saponin [31,32]. The case of myocarditis within the NVX-CoV2373 clinical trial was reviewed by an independent safety monitoring which determined that it was likely of viral origin and not related to the vaccination itself. Although this single case may have emerged as a result of sheer chance, it should be taken into account that the scarcity of these events among mRNA vaccine receivers did not allow for the recognition of this complication during the premarketing stage. Furthermore, the only homology between NVX-CoV2373 and mRNA platforms lies in the implementation of lipid nanoparticles, although they structurally differ between these two modalities.
With the need for a third mRNA-vaccine booster dose to sustain an adequate level of immunity against SARS-CoV-2 becoming increasingly apparent, it is likely that our knowledge with respect to the epidemiological and clinical features of vaccine-induced myocarditis will unfortunately continue to expand. This urgently calls for a deeper understanding of the exact pathogenetic mechanisms responsible for this rare entity, in order to reliably identify individuals at the highest risk for this rare complication and to accordingly adjust the corresponding vaccination strategies. In this context, the shift of investigation to include the lipid nanoparticle component of these platforms may constitute a field of particular relevance.
Financial support
None.
Declaration of competing interest
No conflict of interest to disclose.
Contributor Information
Dimitrios Tsilingiris, Email: tsilingirisd@gmail.com.
Maria Dalamaga, Email: madalamaga@med.uoa.gr.
References
- 1.Vallianou N.G., Tsilingiris D., Christodoulatos G.S., Karampela I., Dalamaga M. Anti-viral treatment for SARS-CoV-2 infection: a race against time amidst the ongoing pandemic. Metabolism open. 2021;10:100096. doi: 10.1016/j.metop.2021.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavilani A., Abbasi E., Kian Ara F., Darini A., Asefy Z. COVID-19 vaccines: current evidence and considerations. Metabolism open. 2021;12:100124. doi: 10.1016/j.metop.2021.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Self W.H., Tenforde M.W., Rhoads J.P., Gaglani M., Ginde A.A., Douin D.J., et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions - United States, March-August 2021. MMWR Morbidity and mortality weekly report. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pegu A., O'Connell S.E., Schmidt S.D. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. 2021;373:1372–1377. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhary N., Weissman D., Whitehead K.A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. 2021;20:817–838. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsilingiris D., Vallianou N.G., Karampela I., Dalamaga M. Vaccine induced thrombotic thrombocytopenia: the shady chapter of a success story. Metabolism open. 2021;11:100101. doi: 10.1016/j.metop.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fragkou P.C., Dimopoulou D. Serious complications of COVID-19 vaccines: a mini-review. Metabolism open. 2021;12:100145. doi: 10.1016/j.metop.2021.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez Y., Levy E.R., Joshi A.Y., Virk A., Rodriguez-Porcel M., Johnson M., et al. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2021. Myocarditis following COVID-19 mRNA vaccine: a case series and incidence rate determination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mevorach D., Anis E., Cedar N., Bromberg M. 2021. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargano J.W.W.M., Hadler S.C., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization Practices — United States. June 2021. MMWR Morbidity and mortality weekly report. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., et al. Myocarditis after covid-19 vaccination in a large health care organization. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautam N., Saluja P., Fudim M., Jambhekar K., Pandey T., Al'Aref S. A late presentation of COVID-19 vaccine-induced myocarditis. Cureus. 2021;13 doi: 10.7759/cureus.17890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chelala L., Jeudy J., Hossain R., Rosenthal G., Pietris N., White C. Cardiac MRI findings of myocarditis after COVID-19 mRNA vaccination in adolescents. AJR American journal of roentgenology. 2021 doi: 10.2214/ajr.21.26853. [DOI] [PubMed] [Google Scholar]
- 19.Document FDA Briefing. Vaccines and Related Biological Products Advisory Committee Meeting; 2021. EUA amendment request for Pfizer-BioNTech COVID-19 Vaccine for use in children 5 through 11 years of age. [Google Scholar]
- 20.Agency E.M. 2021. Comirnaty and Spikevax: possible link to very rare cases of myocarditis and pericarditis. [Google Scholar]
- 21.Siolos A., Gartzonika K., Tigas S. Thyroiditis following vaccination against COVID-19: report of two cases and review of the literature. Metabolism open. 2021;12:100136. doi: 10.1016/j.metop.2021.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/circulationaha.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberta Office of the chief medical officer of health. Myocarditis and/or Pericarditis following COVID-19 Vaccines. 2021 https://www.alberta.ca/assets/documents/health-myocarditis-and-pericarditis-following-covid.pdf [Google Scholar]
- 24.Australian Government. Department of Health . 2021. COVID-19 vaccination – guidance on myocarditis and pericarditis after mRNA COVID-19 vaccines. [Google Scholar]
- 25.Sulemankhil I., Abdelrahman M., Negi S.I. Temporal association between the COVID-19 Ad26.COV2.S vaccine and acute myocarditis: a case report and literature review. Cardiovasc Revascularization Med : Mol Interv. 2021 doi: 10.1016/j.carrev.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy W.J., Longo D.L. A possible role for anti-idiotype Antibodies in SARS-CoV-2 infection and vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMcibr2113694. [DOI] [PubMed] [Google Scholar]
- 27.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021:1–17. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatziantoniou S., Maltezou H.C., Tsakris A., Poland G.A., Anastassopoulou C. Anaphylactic reactions to mRNA COVID-19 vaccines: a call for further study. Vaccine. 2021;39:2605–2607. doi: 10.1016/j.vaccine.2021.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moghimi S.M. Allergic reactions and anaphylaxis to LNP-based COVID-19 vaccines. Molecular Therapy: the journal of the American Society of Gene Therapy. 2021;29:898–900. doi: 10.1016/j.ymthe.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 covid-19 vaccine. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadman M. The long shot. Science (New York, N.Y.) 2020;370:649–653. doi: 10.1126/science.370.6517.649. [DOI] [PubMed] [Google Scholar]
- 32.Milane L., Amiji M. Clinical approval of nanotechnology-based SARS-CoV-2 mRNA vaccines: impact on translational nanomedicine. Drug delivery and translational research. 2021;11:1309–1315. doi: 10.1007/s13346-021-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patone Martina. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]