Abstract
STUDY QUESTION
Can abnormalities in retinal microvasculature representing adverse microcirculatory perfusion and inflammation shed light on the pathophysiology of female fecundability?
SUMMARY ANSWER
In our prospective study, abnormalities in retinal vascular geometric morphology (i.e. sparser arteriolar fractal and larger venular bifurcation) during pre-conception phase are temporarily associated with a prolonged time-to-pregnancy (TTP).
WHAT IS KNOWN ALREADY
Suboptimal retinal microcirculatory morphology has been associated with obesity, psychological stress and hypertension, all of which are known risk factors for reduced female fecundability.
STUDY DESIGN, SIZE, DURATION
A total of 652 women of Chinese, Malay or Indian ethnicity 18–45 years of age and planning to conceive spontaneously within the next 12 months were recruited during the pre-conception period into the Singapore PREconception Study of long-Term maternal and child Outcomes (S-PRESTO), from February 2015 to October 2017.
PARTICIPANTS/MATERIALS, SETTING, METHODS
During recruitment, we collected information on socio-demographic factors, menstrual characteristics and lifestyle behaviors and made anthropometric measurements. We assessed the following retinal microvascular features: caliber, branching angle and fractal dimension. We conducted follow-up telephone surveys to track each participant’s pregnancy status at 6, 9 and 12 months after enrolment. We ascertained clinical pregnancies via ultrasonography, with TTP measured by the number of menstrual cycles required to achieve a clinical pregnancy over a 1-year follow-up. Then, we performed discrete-time proportional hazards models to estimate the fecundability odds ratio (FOR) and 95% CI for each retinal microvascular feature in association with TTP, after adjusting for major confounders, including body mass index and fasting glycemic level at study entry.
MAIN RESULTS AND THE ROLE OF THE CHANCE
Among 652 recruited women, 276 (42.3%) successfully conceived within 1 year of follow-up. The mean (and SD) was 1.24 (0.05) Df for retinal arteriolar dimension fraction and 78.45 (9.79) degrees for retinal venular branching angle, respectively. Non-linear relationship testing was performed before multiple adjustment in all associations and a non-monotonic association was detected between retinal venular branching angle and TTP. Compared with women in the highest tertile of retinal arteriolar fractal dimension, women in the second tertile had a prolonged TTP (FOR: 0.68; 95% CI: 0.51–0.92), as did women in the lowest tertile (FOR: 0.73; 95% CI: 0.55–0.98). Compared with women in the middle tertile of retinal venular branching angle, women in the highest tertile had a borderline prolonged TTP (FOR: 0.75; 95% CI: 0.56–1.02). No other retinal vascular features were significantly associated with TTP.
LIMITATIONS, REASONS FOR CAUTION
We were unable to adjust for other potential confounding factors such as female sexual function (e.g. frequency of sexual intercourse), which might introduce a residual bias. Moreover, even though this is a prospective cohort design, our findings can identify the temporal relationship but not necessarily infer a causal relationship between maternal microvasculature and TTP. Lastly, our study involving mainly Chinese, Malay and Indian ethnicities might not be generalizable to other races or ethnicities.
WIDER IMPLICATIONS OF THE FINDINGS
Suboptimal microcirculation may lead to reduced female fecundability. In the future, in addition to conventional ultrasonographic evaluation of ovarian and uterine physiological function, assessing the retinal microvasculature might be useful for assessment of ovarian age, fertility prediction and endometrial evaluation before assisted reproductive techniques for fertility treatments.
STUDY FUNDING/COMPETING INTEREST(S)
This research is supported by the Singapore National Research Foundation (NRF) under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC) (Singapore-NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014) and Singapore National Medical Research Council Transition Award (NMRC TA/0027/2014). The authors have no competing interests to declare.
TRIAL REGISTRATION NUMBER
ClinicalTrials.gov, NCT03531658.
Keywords: retinal microcirculation / retinal microvasculature / time to pregnancy / fecundability / pregnancy
Introduction
The global fertility has fallen sharply in the past half century, with a drop in total fertility rate (TFR) of 49.4% from 4.7 live births per woman in 1950 to 2.4 live births per woman in 2017 (Murray et al., 2018). In Singapore, the TFR fell to 1.10 in 2020, well below the 2.05 required to maintain a zero population growth (Singapore, 2020). Such a low TFR would lead to negative population growth in the absence of reduced mortality or increased immigration, and could further diminish the working population and adversely affect social and economic development over the long term (Murray et al., 2018). As such, it is pivotal to identify risk factors of TFR that may contribute to the prediction of TFR and prevention of low TFR.
One of the major causes of low TFR has been regarded as prolonged time-to-pregnancy (TTP) (Murray et al., 2018). Identified female risk factors of prolonged TTP include advanced age at childbearing, obesity and systemic disease (hypertension, type 2 diabetes [T2D]), as well as abnormal menstrual characteristics (Sharma et al., 2013; McKinnon et al., 2016; Wesselink et al., 2016; Loy et al., 2018; Hong et al., 2019; Loy et al., 2021). Even though the pathophysiological mechanisms underlying these risk factors are not always clear, chronic inflammation, oxidative stress and endothelial dysfunction, as well as impairment of perfusion in uterine and ovarian circulation, in particular, have been speculated to disrupt ovulation and implantation (Abulafia and Sherer, 2000; Smith, 2001; Ou et al., 2012). However, current studies using Doppler ultrasound to study the uterine and ovarian blood flow are greatly hindered by the high inter-examiner variability, while there are very few feasible and reliable examinations assessing inflammation and oxidant stress levels in vivo among female subjects (Dickey, 1997).
Retinal microvasculature examination is a routine practice to detect retinopathy in patients with ocular disease (e.g. age-related macular denegation) and/or systemic disease (e.g. hypertension, diabetes) (Fong et al., 2004; Williams et al., 2004; Kanagasingam et al., 2014). Recent epidemiologic studies suggest a strong link between retinal microvasculature and chronic inflammation, nitric oxide-dependent endothelial dysfunction and impairment of perfusion or oxygenation (Klein et al., 2006; Wong et al., 2006; Daien et al., 2013), as well as the peripheral, cerebral and coronary circulation in general population (Wong et al., 2002; Cheung et al., 2007, 2013; De Silva et al., 2012; Wong et al., 2002). Owing to its non-invasive nature, retinal imaging has become accessible in many populations, including pregnant women and young children, to study their general microcirculation (Li et al., 2011, 2012) and provide additional screening and/or prediction values for chronic diseases (Smith et al., 2004; Cheung et al., 2013, 2014). For example, retinal vascular abnormalities (i.e. arteriolar narrowing, venular widening, sparser fractal, and both narrowing and enlargement in branching angle) have been associated with obesity, hyperglycemia, psychosocial stress, hypertension and even changes in fetal growth (Li et al., 2012, 2013, 2015, 2017; Tapp et al., 2020). Retinal microvascular morphology may, therefore, shed light on the pathophysiology of female fecundability. In a prospective pre-conceptional and pregnancy cohort in Singapore, we explored the novel associations of women’s pre-gravid retinal vascular morphological features, including central vascular equivalence (caliber) and geometry (fractal dimension and branching angle) and TTP over a 1-year follow-up, to understand the role of retinal microvasculature underlying reduced female fecundability and to provide a proof-of-concept.
Materials and methods
Study population
Participants in this study were enrolled in the Singapore PREconception Study of long-Term maternal and child Outcomes (S-PRESTO, ClinicalTrials.gov, NCT03531658) from February 2015 to October 2017. This prospective cohort study was designed to examine the influences of events in the pre-conceptional period and early pregnancy on metabolic and mental health outcomes for both mother and offspring in later life. Women of Chinese, Malay, or Indian ethnicity aged 18–45 years and planning to conceive spontaneously within the next 12 months were recruited during pre-conception period into S-PRESTO. Women with type 1 diabetes or T2D were excluded from S-PRESTO. To facilitate recruitment, a pre-conception clinic was established in KK Women’s and Children’s Hospital (KKH). Public recruitment outside the hospital occurred through a variety of approaches, including distributed brochures, publicly displayed posters at out-patient clinics in general practices, community/religious centers and advertisements on social media platforms and the radio. The cohort profile has been detailed elsewhere (Loo et al., 2021). The study was conducted according to the guidelines under the Declaration of Helsinki and approved by the SingHealth Centralized Institute Review Board (2014/629/D). All participants provided written informed consent at the study entry.
During recruitment, in-person interviews were used to collect information on socio-demographic factors, health history, menstrual characteristics and lifestyle behaviors. Weight, height, skinfold thickness and body fat percentage were measured by trained research staff at the clinic of KKH. Participants were asked to undergo pregnancy testing if their period was 3–4 days late or if 2 weeks had passed after unprotected intercourse at any time, using a urinary pregnancy test kit sensitive to 25 mIU/ml Human Chorionic Gonadotropin (hCG) (Biotron Diagnostics, Hemet, CA, USA), and to inform the research staff of a positive result. Once a pregnancy was reported, the last menstruation period (LMP) date was subsequently documented, and an ultrasound scan was scheduled to confirm clinical pregnancy. Research staff also conducted follow-up phone surveys to track each participant’s pregnancy status at 6, 9 and 12 months after enrollment.
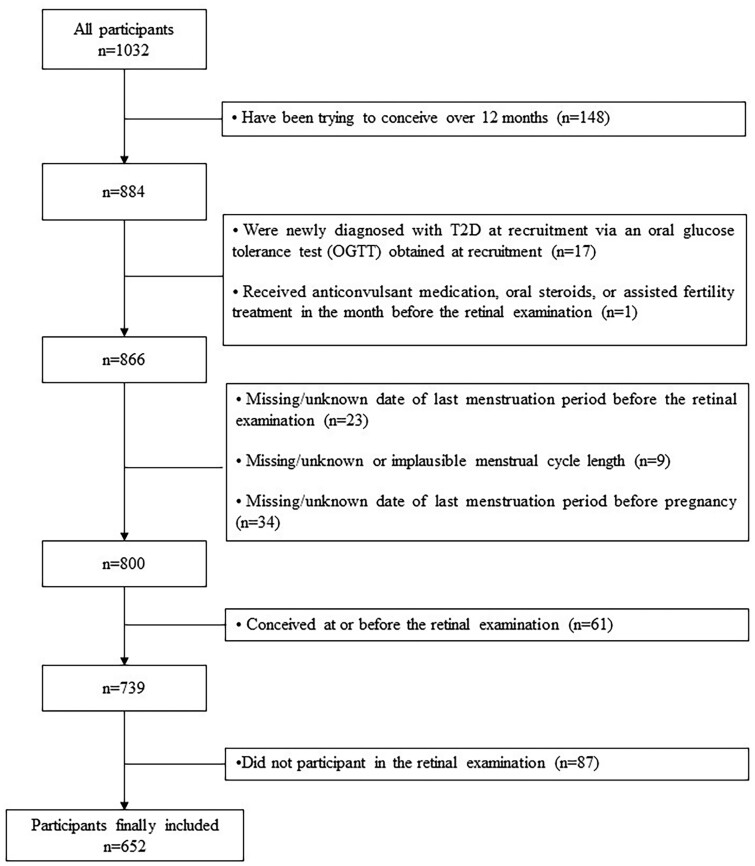
Of the 1032 eligible women in S-PRESTO, 380 were excluded from analysis for one or more of the following reasons: (i) have been trying to conceive for over 12 months (n = 148); (ii) had received anticonvulsant medication, oral steroids or assisted fertility treatment in the month before the retinal examination (n = 1); (iii) were newly diagnosed with T2D at recruitment via an oral glucose tolerance test (OGTT) (n = 17); (iv) had a missing or unknown date of LMP before the retinal examination (n = 23); (v) had missing, unknown or implausible menstrual cycle length (n = 9); (vi) had a missing or unknown date of LMP before pregnancy (n = 34); (vii) had conceived at or before retinal examination (n = 61); or (viii) did not participate in the retinal examination (n = 87). After these exclusions, 652 participants were included for analysis (Fig. 1).
Figure 1.
The flowchart of study participants’ recruitment.
Examination of retinal microvasculature
Retinal examination was performed by trained photographers using a 45° non-mydriatic retinal camera (Canon CR-1, 40D SLR digital retinal camera backing, Canon Inc., Tokyo, Japan) at the study entry. Two retinal photographs centered on the optic disc and macula were taken on each eye without pharmacological pupillary dilation. Subsequently, all retinal images were assigned to graders blinded to the participants’ other data, and all retinal microvascular caliber beyond 25 µm crossing through 0.5–2.0 disc diameters (Zone C) from the optic disc margin were assessed according to a standard protocol (Cheung et al., 2015). These features included central retinal arteriolar and venular equivalence (CRAE and CRVE) and vascular geometry (i.e. fractal dimension and branching angle), each evaluated quantitatively using a semi-automated computer-based program (Singapore I Vessel Assessment [SIVA] version 4.0, Singapore Eye Research Institute, Singapore, Singapore). Fractal dimension quantifies the complexity of the branching pattern of the retinal vascular tree, which was calculated from the outlined retinal vessels using the ‘box-counting method’ (Li et al., 2016). Branching angle with its unit (degrees) is defined as the first angle subtended between two daughter vessels at each bifurcation (Li et al., 2016). The inter- and intra-grader intraclass correlation coefficient (ICC) achieved at least 0.8 for all retinal vascular features in a randomly selected 10% subset of re-graded retinal photos.
Assessment of TTP
Pregnancy was determined by a positive urinary pregnancy test and later confirmed by a dating ultrasound scan (>6 weeks of gestation) via the presence of an intrauterine gestational sac. In the event where an ultrasound scan was not available or inconclusive, the diagnosis of pregnancy was made clinically. TTP was defined as the number of menstrual cycles required to achieve a clinical pregnancy over 1-year follow-up, calculated as the total number of cycles at pregnancy risk. The total number of cycles at pregnancy risk was calculated with the following formula:
Total number of cycles at pregnancy risk = INT ()
INT: the integer portion of the quotient
a: number of months attempting to conceive before the study entry
b: average cycle length, i.e.the usual number of days between the start of one menstrual cycle and the start of the next menstrual cycle.
c: number of days between the date of LMP before conception or the most recent follow-up and date of LMP immediately before the study entry.
For women who became pregnant, one more conception cycle was added, assuming that the actual conception took place in a 0.5–1.0 cycle after the documented LMP (Zhao et al., 2019). For example, consider a woman with an average cycle length of 28 days who had been trying to conceive for 3 months at study entry and who successfully conceived during our observational window phase of 1 year. We archived her date of LMP before conception (say, 2 October 2015) and the date of LMP before study entry (say, 2 February 2015). The difference between these two LMPs is 242 days. Her total number of cycles equals to 13.
Covariates
Personal medical history, lifestyle data and socio-economic information were obtained via interviews at study entry. Covariates collected at study entry included: maternal age, ethnicity (Chinese vs Malay vs Indian vs others), college education (yes vs no), parity (nulliparous vs parous), cycle length, cycle regularity (regular vs irregular [variation of menstrual interval >5 days]), age at menarche (<12, 12–13, >13 years), number of months attempting to conceive before study entry, body mass index (BMI) at study entry (underweight [BMI < 18.5 kg/m2] vs normal weight [18.5 kg/m2 ≤ BMI ≤ 22.9 kg/m2] vs. overweight [23.0 kg/m2 ≤ BMI < 27.4 kg/m2] vs obese [BMI ≥ 27.5 kg/m2]) (Consultation, 2004; Loy et al., 2018), systolic blood pressure (SBP) and diastolic blood pressure (DBP), physical activity in the past 7 days classified based on the International Physical Activity Questionnaire guidelines (inactive vs minimally active vs HEPA [health-enhancing physical activity] active) (IPAQ Research Committee, 2005), smoking (never vs past or current), alcohol intake (never vs past or current), micronutrient supplements intake in the past 3 months (yes vs no), anxiety status in the past 7 days (State-Trait Anxiety Inventory [STAI] score within cohort distribution: ≥75th vs <75th percentile) (Teixeira et al., 1999), and depression status in the past 7 days (Edinburgh Postnatal Depression Scale [EPDS] score: ≥14 vs <14 points) (Gibson et al., 2009). A 2-h 75 g two-timepoint OGTT was performed at the clinic during the first pre-conception visit, and the fasting and 2-h plasma glucose concentrations were retrieved from medical records. Fasting glucose level was categorized into two groups by setting the threshold at 5.1 mmol/L, as in our prior publication (Loy et al., 2021).
Statistical analysis
Student’s t-test, Wilcoxon–Mann–Whitney tests, Chi-square test or Fisher’s exact test were used to describe the maternal characteristics: (i) between participants included and excluded in the study, (ii) between eligible participants with complete and missing data of retinal indices, (iii) between pregnant and non-pregnant participants within 1 year, and (iv) across the tertiles of retinal arteriolar fractal dimension and venular branching angle. In order to show the natural pregnancy rate during the 12 months’ follow-up, we applied life-time table method to calculate cumulative percentage, among 270 couples with 0–1 month of attempt time in conception.
Since our analysis was hypothesis-driven based on prior findings (Liew et al., 2011; Cheung et al., 2017; Huang et al., 2020), we used crude models including restricted cubic splines with three knots (10th, 50th and 90th percentiles) to assess non-linear relationships between retinal vascular features and TTP (Steenland and Deddens, 2004). The P-value for linear trend was assessed across tertiles of individual retinal microvascular feature in monotonic association. The discrete-time proportional hazards models were applied to estimate the fecundability odds ratios (FORs) and 95% CIs in all associations with tertiles of retinal microvascular measurements (Spira, 1998; Wilcox, 2010). FOR <1 denotes reduced fecundability (a longer TTP), whereas FOR >1 denotes increased fecundability (a shorter TTP) (Wise et al., 2010). To account for differences in individual durations of attempting to conceive before study entry, the Andersen–Gill data structure was applied to reduce left truncation bias (Wise et al., 2010). For example, if a woman conceived after her 4th cycle and she had two cycles attempting to conceive before study entry, the model used only two cycles (4–2 = 2) for analysis. For participants who did not conceive within 1 year after the baseline recruitment or who were lost to follow-up or initiated fertility treatment, TTP was censored in our model. Covariates were tested in a forward stepwise manner for all associations between retinal microvascular features and TTP. We included all confounders previously reported and then selected a parsimonious model containing confounders that changed the FOR by at least 10%. Of the three adjustment models, Model 1 adjusted for maternal age, ethnicity (Chinese vs Malay vs Indian vs others), college education (yes vs no) and EPDS score (≥14 vs <14); Model 2 additionally adjusted for maternal STAI score (≥75th vs <75th percentile), SBP, physical activity (inactive vs minimally active vs HEPA active) and micronutrient supplements intake (yes vs no); and Model 3 further adjusted for BMI at study entry (underweight vs normal weight vs overweight vs obese) and fasting glucose at study entry (≥5.1 vs <5.1 mmol/L).
Of the total study sample, 583 (89.4%) were complete cases. Most of the missing data were anxiety and depression scores (8.6%). Considering the potential bias due to missingness, we performed multiple imputation (MI) and addressed the issue of missing covariates (10.6%) in our cohort. We created 20 imputed datasets to reduce sampling variability from the imputation process. We included all covariates from the analysis model (maternal age, ethnicity, college education, EPDS score, STAI score, SBP, physical activity, micronutrient dietary supplements intake, BMI and fasting plasma glucose at study entry) in the imputation model. The results were pooled over 20 imputed datasets using Rubin’s rules (Rubin, 2008). In a sensitivity analysis, we repeated the analysis above with data on complete cases (n = 583).
Potential interactions between retinal vascular features and major confounders in our model (i.e. age, ethnicity, education, BMI, EPDS score, STAI score, SBP, physical activity, micronutrient supplements intake and fasting glucose) on TTP were also examined in the fully adjusted model.
All statistical analyses used IBM SPSS software version 25.0 (SPSS, IBM, Chicago, IL, USA) and STATA software version 14.0 (StataCorp, College Station, TX, USA), based on two-tailed significant P-values of 0.05.
Results
Maternal characteristics
Supplementary Table SI shows the maternal characteristics between women included (n = 652) and excluded (n = 380) from our analysis. Most baseline variables were comparable between the two groups except for age, micronutrient supplement intake, fasting plasma glucose and conception rate within 1 year. Supplementary Table SII shows the maternal characteristics between eligible participants with complete data of retinal indices (n = 739) and missing data of retinal indices (n = 87). Most of the characteristics were similar between the two groups, except physical activity and micronutrient supplement intake.
The mean (SD) was 1.24 (0.05) Df for retinal arteriolar dimension fraction and 78.45 (9.79) degrees for retinal venular branching angle, respectively. Table I compares maternal characteristics across the tertiles of retinal arteriolar fractal dimension and retinal venular branching angle. Maternal characteristics including age, ethnicity, blood pressure and EPDS score were different across the tertiles of retinal arteriolar fractal dimension and venular branching angle.
Table I.
Comparison of maternal characteristics across the tertiles of retinal arteriolar fractal dimension and retinal venular branching angle.
| Characteristics | DF-a T1 (N = 217) |
DF-a T2
(N = 216) |
DF-a T3
(N = 219) |
P † | BA-v T1 (N = 217) |
BA-v T2
(N = 218) |
BA-v T3
(N = 217) |
P † |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) or N (%) | Mean (SD) or N (%) | Mean (SD) or N (%) | Mean (SD) or N (%) | Mean (SD) or N (%) | Mean (SD) or N (%) | |||
| Age (years) | 31.2 (3.4) | 30.6 (3.6) | 30.1 (3.9) | 0.01 | 30.3 (3.6) | 30.4 (3.4) | 31.1 (4.0) | 0.04 |
| Ethnicity | 0.02 | 0.02 | ||||||
| Chinese | 179 (82.5%) | 163 (75.5%) | 151 (68.9%) | 170 (78.3%) | 161 (73.9%) | 162 (74.7%) | ||
| Malay | 17 (7.8%) | 25 (11.6%) | 41 (18.7%) | 22 (10.2%) | 38 (17.4%) | 23 (10.6%) | ||
| Indian | 13 (6.0%) | 22 (10.2%) | 19 (8.7%) | 16 (7.4%) | 18 (8.3%) | 20 (9.2%) | ||
| Others | 8 (3.7%) | 6 (2.7%) | 8 (3.7%) | 9 (4.1%) | 1 (0.5%) | 12 (5.5%) | ||
| Maternal college degree | 0.02 | 0.65 | ||||||
| Yes | 160 (73.7%) | 139 (64.4%) | 134 (61.2%) | 149 (68.7%) | 144 (66.1%) | 140 (64.5%) | ||
| BMI at study entry | 0.27 | 0.85 | ||||||
| <18.5 kg/m2 | 109 (50.5%) | 98 (45.6%) | 114 (52.1%) | 112 (51.6%) | 108 (49.6%) | 101 (47.0%) | ||
| 18.5–22.9 kg/m2 | 21 (9.7%) | 18 (8.4%) | 25 (11.4%) | 22 (10.1%) | 21 (9.6%) | 21 (9.8%) | ||
| 23.0–27.4 kg/m2 | 50 (23.1%) | 67 (31.1%) | 45 (20.5%) | 54 (24.9%) | 50 (22.9%) | 58 (27.0%) | ||
| ≥27.5 kg/m2 | 36 (16.7%) | 32 (14.9%) | 35 (16.0%) | 29 (13.4%) | 39 (17.9%) | 35 (16.2%) | ||
| SBP, mmHg | 105.5 (10.1) | 104.7 (8.5) | 102.7 (8.9) | 0.004 | 103.5 (9.1) | 103.6 (8.6) | 105.7 (9.8) | 0.02 |
| DBP, mmHg | 69.3 (8.8) | 68.5 (7.4) | 66.3 (7.3) | <0.001 | 67.7 (8.0) | 67.6 (7.6) | 68.7 (8.3) | 0.25 |
| Fasting plasma glucose level at study entry ≥5.1 mmol/L | 0.64 | 0.35 | ||||||
| Yes | 34 (15.7%) | 41 (19.0%) | 36 (16.6%) | 38 (17.5%) | 31 (14.3%) | 42 (19.4%) | ||
| Physical activity | 0.70 | 0.44 | ||||||
| Inactive | 36 (16.6%) | 31 (14.5%%) | 33 (15.1%) | 32 (14.8%) | 41 (18.9%) | 27 (12.5%) | ||
| Minimally active | 115 (53.0%) | 108 (50.5%) | 105 (48.2%) | 112 (51.9%) | 106 (48.8%) | 110 (50.9%) | ||
| HEPA active | 66 (30.4%) | 75 (35.0%) | 80 (36.7%) | 72 (33.3%) | 70 (32.3%) | 79 (36.6%) | ||
| Micronutrient dietary supplements intake | 0.61 | 0.63 | ||||||
| Yes | 145 (67.1%) | 146 (67.6%) | 139 (63.5%) | 138 (63.6%) | 147 (67.7%) | 145 (66.8%) | ||
| Anxiety (STAI score ≥ 75th percentile) | 0.23 | 0.47 | ||||||
| Yes | 47 (23.5%) | 46 (22.7%) | 57 (29.5%) | 56 (28.0%) | 44 (22.7%) | 50 (24.8%) | ||
| Depression (EPDS score ≥14) | 0.01 | 0.98 | ||||||
| Yes | 15 (7.4%) | 13 (6.3%) | 29 (14.8%) | 19 (9.4%) | 18 (9.2%) | 20 (9.8%) | ||
T1, the lowest tertile; T2, the middle tertile; T3, the highest tertile; SBP, systolic blood pressure; DBP, diastolic blood pressure; HEPA, health enhancing physical activity; STAI, state trait anxiety inventory; EPDS, Edinburgh postnatal depression scale; DF-a, fractal dimension-arteriole; BA-v, branching angle-venule.
†Student’s t-test for parametric test while Wilcoxon rank-sum test for non-parametric.
Among 652 recruited women, the pregnancy proportion within 12 months of follow-up period was 42.3% (n = 276). After restricting the cohort to couples with 0–1 month of pregnancy attempt time at study entry (n = 270), the cumulative pregnancy rate within 12 months was 56.2% (Supplementary Table SIII). Table II compares maternal characteristics between participants who conceived within 1 year of follow-up time and those who did not. Women who conceived within 1 year were younger (30.0 vs 31.0 years) and less likely to have fasting plasma glucose concentration ≥5.1 mmol/l (13.1% vs 19.9%) than those who failed. Other characteristics were similar between these two groups.
Table II.
Comparison of maternal characteristics between pregnant and non-pregnant participants within 1 year.
| Characteristics | Failed to conceive within 1 year (N = 376) | Conceived within 1 year (N = 276) | P † |
|---|---|---|---|
|
Mean (SD) or
N (%) |
Mean (SD) or
N (%) |
||
| Age, (years) | 31.0 (3.9) | 30.0 (3.3) | <0.001 |
| Ethnicity | |||
| Chinese | 286 (76.1%) | 207 (75.0%) | 0.22 |
| Malay | 50 (13.3%) | 33 (12.0%) | |
| Indian | 32 (8.5%) | 22 (8.0%) | |
| Others | 8 (2.1%) | 14 (5.1%) | |
| Maternal college degree | |||
| Yes | 241 (64.1%) | 192 (69.6%) | 0.17 |
| Nulliparity | |||
| Yes | 246 (65.4%) | 166 (60.1%) | 0.19 |
| Cycle length, days | |||
| <28 | 20 (5.3%) | 8 (2.9%) | 0.09 |
| 28–30 | 182 (48.4%) | 120 (43.5%) | |
| >30 | 174 (46.3%) | 148 (53.6%) | |
| Cycle regularity (variation of menstrual interval ≤ 5 days), | |||
| Yes | 247 (65.7%) | 185 (67.0%) | 0.79 |
| Age at menarche, years | 12.5 (1.6) | 12.2 (1.3) | 0.01 |
| Age at menarche | |||
| <12 years | 88 (23.7%) | 86 (31.4%) | 0.05 |
| 12–13 years | 196 (52.8%) | 140 (51.1%) | |
| >13 years | 87 (23.5%) | 48 (17.5%) | |
| BMI at study entry | |||
| <18.5 kg/m2 | 170 (45.3%) | 151 (54.9%) | 0.06 |
| 18.5–22.9 kg/m2 | 44 (11.7%) | 20 (7.3%) | |
| 23.0–27.4 kg/m2 | 100 (26.7%) | 62 (22.5%) | |
| ≥27.5 kg/m2 | 61 (16.3%) | 42 (15.3%) | |
| SBP, mmHg | 104.6 (9.8) | 103.8 (8.3) | 0.24 |
| DBP, mmHg | 68.4 (8.3) | 67.5 (7.4) | 0.14 |
| Fasting plasma glucose level at study entry ≥5.1 mmol/L | |||
| Yes | 75 (19.9%) | 36 (13.1%) | 0.03 |
| Physical activity | |||
| Inactive | 52 (13.9%) | 48 (17.5%) | 0.13 |
| Minimally active | 202 (53.9%) | 126 (46.0%) | |
| HEPA active | 121 (32.3%) | 100 (36.5%) | |
| Micronutrient dietary supplements intake | |||
| Yes | 245 (65.3%) | 185 (67.0%) | 0.71 |
| Smoking exposure | |||
| Past or current | 34 (9.0%) | 20 (7.2%) | 0.50 |
| Alcohol intake | |||
| Past or current | 272 (72.3%) | 186 (67.4%) | 0.20 |
| Anxiety (STAI score ≥ 75th percentile) | |||
| Yes | 88 (26.0%) | 62 (24.0%) | 0.64 |
| Depression (EPDS score ≥14) | |||
| Yes | 37 (10.8%) | 20 (7.7%) | 0.26 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; HEPA, health enhancing physical activity; STAI, state trait anxiety inventory; EPDS, Edinburgh postnatal depression scale.
†Student’s t-test for parametric test while Wilcoxon rank-sum test for non-parametric.
Associations between retinal vascular features and TTP
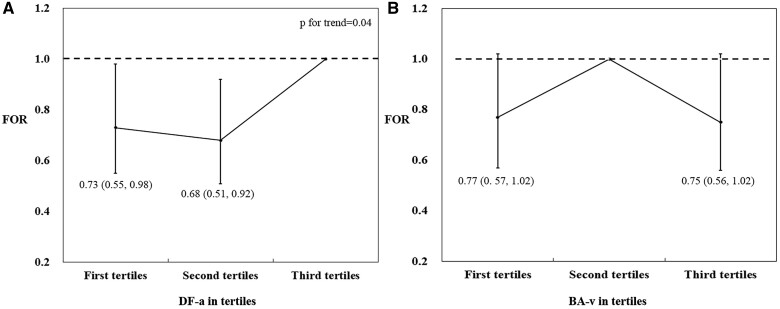
The results after MI showed that women in the second tertile of arteriolar fractal dimension had a lower FOR of 0.70 (95% CI: 0.52–0.94) than women in the highest tertile. This association remained significant after further adjustments in Model 2 (FOR: 0.70; 95% CI: 0.52–0.94) and Model 3 (FOR: 0.68; 95% CI: 0.51–0.92) (Table III and Fig. 2). Women in the lowest tertile of arteriolar fractal dimension also had a lower FOR of 0.73 (95% CI: 0.55–0.98) in Model 3 compared with women in the highest tertile. Owing to the inverted V-shape relationship between retinal venular branching angle and TTP (Supplementary Table SIV and Fig. S1), the middle tertile was set as the group reference for that feature, rather than the highest tertile. Compared with women in the middle tertile, women in the highest tertile had a lower and significant FOR of 0.73 (95% CI: 0.54–0.98), as did women in the lowest tertile (FOR: 0.76, 95% CI: 0.56–1.01). The estimates remained significant for women in the highest tertile in Model 2 (FOR: 0.74; 95% CI: 0.54–0.98) but were attenuated slightly in Model 3 (FOR: 0.75; 95% CI: 0.56–1.02) (Table III and Fig. 2). We observed no significant associations between any other retinal vascular features and TTP (Table III).
Table III.
Association between retinal microvasculature (in tertiles) and time to pregnancy after multiple imputation.
| Retinal vascular features | Time to pregnancy |
||
|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
|
| FOR (95% CI) | FOR (95% CI) | FOR (95% CI) | |
| Retinal arteriolar features | |||
| CRAE | |||
| T1 | 0.96 (0.71, 1.28) | 0.95 (0.70, 1.29) | 0.93 (0.69, 1.27) |
| T2 | 0.93 (0.70, 1.25) | 0.92 (0.69, 1.24) | 0.91 (0.68, 1.23) |
| T3 | Ref. | Ref. | Ref. |
| P for trend | 0.76 | 0.75 | 0.66 |
| DF-a | |||
| T1 | 0.76 (0.57, 1.02) | 0.76 (0.57, 1.02) | 0.73 (0.55, 0.98) |
| T2 | 0.70 (0.52, 0.94) | 0.70 (0.52, 0.94) | 0.68 (0.51, 0.92) |
| T3 | Ref. | Ref. | Ref. |
| P for trend | 0.06 | 0.07 | 0.04 |
| BA-a | |||
| T1 | 0.84 (0.63, 1.13) | 0.84 (0.63, 1.13) | 0.81 (0.60, 1.08) |
| T2 | 0.89 (0.66, 1.18) | 0.88 (0.66, 1.18) | 0.85 (0.63, 1.13) |
| T3 | Ref. | Ref. | Ref. |
| P for trend | 0.25 | 0.25 | 0.15 |
| Retinal venular features | |||
| CRVE | |||
| T1 | Ref. | Ref. | Ref. |
| T2 | 1.05 (0.78, 1.41) | 1.04 (0.77, 1.40) | 1.02 (0.76, 1.38) |
| T3 | 1.17 (0.87, 1.58) | 1.18 (0.87, 1.58) | 1.17 (0.87, 1.59) |
| P for trend | 0.29 | 0.29 | 0.30 |
| DF-v | |||
| T1 | 0.84 (0.62, 1.13) | 0.85 (0.63, 1.15) | 0.83 (0.61, 1.12) |
| T2 | 1.03 (0.78, 1.38) | 1.04 (0.78, 1.38) | 0.96 (0.72, 1.28) |
| T3 | Ref. | Ref. | Ref. |
| P for trend | 0.25 | 0.31 | 0.30 |
| BA-v | |||
| T1 | 0.76 (0.56, 1.01) | 0.76 (0.57, 1.01) | 0.77 (0.57, 1.02) |
| T2 | Ref. | Ref. | Ref. |
| T3 | 0.73 (0.54, 0.98) | 0.73 (0.54, 0.98) | 0.75 (0.56, 1.02) |
FOR, fecundability odds ratio; T1, the lowest tertile; T2, the middle tertile; T3, the highest tertile; CRAE, central retinal arteriolar equivalent value; CRVE, central retinal venular equivalent value; DF-a, fractal dimension-arteriole; DF-v, fractal dimension-venule; BA-a, branching angle-arteriole; BA-v, branching angle-venule, value.
Model 1: Adjusted for maternal age, ethnicity (Chinese vs Malay vs Indian vs Others), college education (yes vs no) and EPDS score (≥14 vs <14) collected at study entry.
Model 2: Model 1 and maternal STAI score (≥75th vs <75th percentile), systolic blood pressure, physical activity (inactive vs minimally active vs HEPA active) and micronutrient dietary supplements intake (yes vs no) at study entry.
Model 3: Model 2 and additionally adjusted for BMI at study entry (underweight vs normal weight vs overweight vs obese) and fasting plasma glucose (≥5.1 vs <5.1 mmol/L) at study entry.
Figure 2.
Association between retinal vascular features in tertiles and fecundability odds ratio. (A) and (B) show the fully adjusted association between DF-a in tertiles, BA-v in tertiles, and FOR, respectively. The reference level for FOR is the first tertile in DF-a and the second tertile in BA-v. DF-a, retinal arteriolar fractal dimension; BA-v, retinal venular branching angle; FOR, fecundability odds ratio.
No significant interactions between retinal vascular features were observed in any of our analyses (Supplementary Table SV). In our sensitivity analysis based on complete cases without missing data, the associations between retinal vascular geometric indexes and TTP were similar (Supplementary Table SVI).
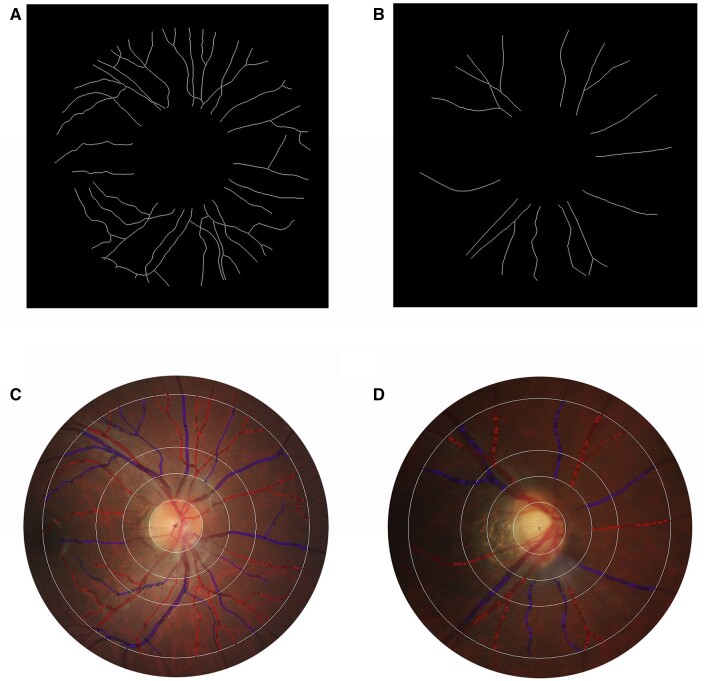
For illustrative purposes, retinal images of two subjects one who conceived and one who failed to conceive within 1 year are shown in Fig. 3. The subject who failed to conceive showed a lower retinal arteriolar fractal dimension (11.1 vs 13.5 × 10−1 Df) and a larger retinal venular branching angle (86.2 vs 74.6 degrees) than the subject who conceived on the 314th days after the study entry.
Figure 3.
Examples of retinal fundus photographs from a participant who conceived and a participant who failed to conceive within one year. (A) and (C) respectively show a participant’s retinal arteriolar fractal dimension as 13.5 × 10−1 Df and retinal venular branching angle as 74.2°. This participant conceived on the 314th days after the study entry. (B) and (D) show a participant’s retinal arteriolar fractal dimension as 11.1 × 10−1 Df and retinal venular branching angle as 86.2°. This participant did not conceive within 1 year.
Discussion
In this prospective pre-conceptional cohort, among 652 multi-ethnic Asian women in Singapore, we found that women with a sparser retinal arteriolar fractional dimension and a relatively larger retinal venular branching angle had a longer TTP. After further adjusting for pre-pregnancy BMI and pre-conception fasting plasma glucose, the association of retinal arteriolar fractal dimension and TTP remained significant, while that of retinal venular branching angle and TTP attenuated.
Infertility, which is traditionally defined as failing to conceive within 12 months after unprotected sexual intercourse, affects 15–25% couples (Slama et al., 2012). In this S-PRESTO cohort, only 42.3% of women conceived naturally within a 1-year follow-up and it is much lower than the natural conception rate reported in the USA (62.4%) (Wesselink et al., 2017) and Australian (85.3%) (Grieger et al., 2019) studies that monitored pregnancies within 12 menstrual cycles after unprotected intercourse. As mentioned above, many factors (i.e. advanced age, hypertension, diabetes, stress, obesity) have been associated with reduced female fecundability (Loy et al., 2018; Hong et al., 2019; Zhao et al., 2019; Loy et al., 2021) but the pathophysiology underlying these associations is largely unknown.
Vascularization of the human endometrium plays an important role in conception (Roberts and Redman, 1993). Studies in humans and animals have reported that vascular endothelial growth factor (VEGF), a critical angiogenic factor, is associated with changes in ovarian and uterine Doppler blood flow, and its level is reduced in the subjects who conceived (Agrawal et al., 1999; Chiumia et al., 2020). Other studies have observed higher pulsatility index values (i.e. higher vascular resistance) in ovarian arteries and uterine arteries in the luteal phase amongst infertile women compared with healthy/fertile women (Goswamy et al., 1988; Tinkanen et al., 1994). Moreover, a lower endometrial vascularization index and a lower flow index have been reported in women with unexplained infertility compared with parous controls (El-Mazny et al., 2013).
However, it remains unknown how circulatory features might change during implantation, owing to limited accurate measurements of the uterine and ovarian vasculature during this early period of human conception. The retinal microvasculature provides an opportunity to directly visualize the systemic microcirculation and indirectly reflects a range of pathophysiological processes, including endothelial dysfunction, impairment of perfusion or oxidative stress and systemic inflammation (Wong et al., 2006). Suboptimal retinal microcirculation has been associated with maternal obesity, gestational diabetes mellitus (GDM), hypertensive disorders during pregnancy (HDP) and psychological stress in pregnant women, as well as with slower fetal growth during pregnancy and lower birthweights (Li et al., 2012, 2013, 2015, 2017). In other words, the retinal microcirculation may reflect overall female cardio-metabolic health and uteroplacental circulation. We speculate that changes in retinal vascular morphology might mirror the endometrial and ovarian environment that affects female fecundability.
We observed that suboptimal retinal vascular geometry (sparser arteriolar fractal dimension and wider venular bifurcation) was associated with a prolonged TTP. In the retina, a sparser arteriolar fractal dimension indicates rarefaction or collapsing of vessels resulting from hypoxia (Cheung et al., 2012, 2014), which has been associated with hypertension, diabetes and ischemic stroke in previous studies (Li et al., 2012, 2017; Ong et al., 2013). Moreover, we found a non-monotonic relationship between retinal venular branching angle and TTP. Both ends of the retinal venular branching angle showed an association with prolonged TTP. Epidemiological evidence has shown that both wider and narrower retinal venular branching angles are associated with inflammation-related systemic diseases. For instance, a larger retinal venular branching angle has been associated with the risk of diabetic retinopathy in individuals with T2D, which may reflect inflammation and endothelial dysfunction (Cheung et al., 2017). In contrast, a smaller retinal venular branching angle is indicative of increased levels of oxidative stress (Ikram et al., 2004), which has been linked to poorer left ventricular function (i.e. a decrease in peak systolic septal mitral annular velocity) (Huang et al., 2020). Taken together, our findings suggest that microcirculatory abnormalities coupled with reduced blood flow, hypoxia and oxidative stress, as well as inflammation might contribute to reduced female fecundability. Previous studies have suggested that increasingly compromised microcirculation around the leading ovarian follicle could result in reduced oxygen levels in the follicular fluid, which could in turn affect the development of a healthy oocyte for fertilization (Van Blerkom et al., 1997). Additionally, inflammatory-induced changes in blood supply and immune responses might disrupt the circulation and function of the uterus and ovaries, ultimately affecting embryo development and implantation (Smith, 2001; Robertson et al., 2018). Ovarian and endometrial function are important for female fecundability, and may be affected by suboptimal microcirculation and inflammation in vivo.
Retinal imaging-based deep learning algorithms have been used to detect and/or predict a range of cardiovascular risk factors (i.e. hypertension, hyperglycemia, dyslipidemia) and major adverse cardiac events (Poplin et al., 2018; Cheung et al., 2021). In the future, in addition to conventional ultrasonographic evaluation of ovarian and uterine physiological function, assessing the retinal microvasculature might be useful for assessment of ovarian age, fertility prediction and endometrial evaluation before assisted reproductive techniques for fertility treatments. The association between the retinal microvasculature and circulation in female reproductive organs should be explored in future studies.
To our knowledge, ours is the first epidemiological study to explore the relationship of retinal microvascular features with fecundability, as measured by TTP, among women planning for a pregnancy. Retinal microvascular features and all covariate data were comprehensively and systematically collected according to standardized protocols. Nevertheless, our study is not without limitations. First, we did not record the exact number of each participant’s menstrual cycles. Instead, we calculated an estimated cycle count through the couple’s actual waiting days of pregnancy divided by the average menstrual cycle length. Although this is a common approach for studying TTP, some misclassification may have occurred, especially among women with irregular menses. Second, we were unable to adjust other potential confounding factors such as female sexual function (timing and frequency of intercourse), which might introduce residual confounding bias. Third, about 12% of eligible women did not participate in the retinal examination due to religious considerations, time commitments or other reasons. Although we cannot rule out selection bias, we found no significant differences in baseline characteristics between participants with and without retinal imaging (Supplementary Table SII). Fourth, even though this is a prospective cohort design, our findings can only identify the temporal relationship instead of a causal inference between maternal microvasculature and TTP. Fifth, our analyses were performed in Asian women with relatively high socio-economic status and educational attainment, so our results might not be generalizable to other populations. Lastly, we did not correct for multiple comparisons (corresponding to multiple features of the retinal vasculature) since all investigated associations were hypothesis-driven based on prior evidence on retinal microvasculature and systemic disease (Li et al., 2016). However, since our findings on retinal vascular features in relation to TTP were marginal, further exploration and validation with such research schemes are warranted. In summary, suboptimal retinal vascular geometric morphology (sparser arteriolar fractal dimension and larger venular bifurcation) was associated with reduced female fecundability in a cohort of multi-ethnic Asian women in Singapore. Future studies should be conducted to confirm our findings and may help provide novel assessment of female reproductive potential and suggest additional treatment indicators for female infertility.
In summary, suboptimal retinal vascular geometric morphology (sparser arteriolar fractal dimension and larger venular bifurcation) was associated with reduced female fecundability in a cohort of multi-ethnic Asian women in Singapore. Future studies should be conducted to confirm our findings that retinal examination may be a useful marker to predict female fecundability.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author. Any required links or identifiers for the study data are present in the manuscript as described or provide edits with the required information.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the S-PRESTO study.
Authors’ roles
L.H. conducted the statistical analysis and drafted the manuscript; S.L.L. supervised the statistical analysis and critically revised the manuscript; W.-Q.C. critically revised the manuscript; J.G.E. acquired the data and conducted the research; Y.S.C. designed and conducted the study; Z.H. critically revised the manuscript; J.K.Y.C. designed and conducted the study; T.Y.W. supervised the study design and critically revised the manuscript; M.K. supervised the statistical analysis and critically revised the manuscript; C.Z. supervised the statistical analysis and revised the manuscript; L.-J.L. designed and conducted the study, supervised the statistical analysis and critically revised the manuscript.
Funding
This research was supported by the Singapore National Research Foundation (NRF) under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC) (Singapore-NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014) and Singapore National Medical Research Council Transition Award (NMRC TA/0027/2014).
Conflict of interest
The authors have nothing to disclose.
References
- Abulafia O, Sherer DM.. Angiogenesis of the ovary. Am J Obstet Gynecol 2000;182:240–246. [DOI] [PubMed] [Google Scholar]
- Agrawal R, Conway GS, Sladkevicius P, Payne NN, Bekir J, Campbell S, Tan SL, Jacobs HS.. Serum vascular endothelial growth factor (VEGF) in the normal menstrual cycle: association with changes in ovarian and uterine Doppler blood flow. Clin Endocrinol (Oxf) 1999;50:101–106. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Ikram MK, Klein R, Wong TY.. The clinical implications of recent studies on the structure and function of the retinal microvasculature in diabetes. Diabetologia 2015;58:871–885. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Ong S, Ikram MK, Ong YT, Chen CP, Venketasubramanian N, Wong TY.. Retinal vascular fractal dimension is associated with cognitive dysfunction. J Stroke Cerebrovasc Dis 2014;23:43–50. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Sabanayagam C, Law AK, Kumari N, Ting DS, Tan G, Mitchell P, Cheng CY, Wong TY.. Retinal vascular geometry and 6 year incidence and progression of diabetic retinopathy. Diabetologia 2017;60:1770–1781. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Tay WT, Ikram MK, Ong YT, De Silva DA, Chow KY, Wong TY.. Retinal microvascular changes and risk of stroke: the Singapore Malay Eye Study. Stroke 2013;44:2402–2408. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Thomas GN, Tay W, Ikram MK, Hsu W, Lee ML, Lau QP, Wong TY.. Retinal vascular fractal dimension and its relationship with cardiovascular and ocular risk factors. Am J Ophthalmol 2012;154:663–674.e661. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Xu D, Cheng CY, Sabanayagam C, Tham YC, Yu M, Rim TH, Chai CY, Gopinath B, Mitchell P. et al. A deep-learning system for the assessment of cardiovascular disease risk via the measurement of retinal-vessel calibre. Nat Biomed Eng 2021;5:498–508. [DOI] [PubMed] [Google Scholar]
- Cheung N, Bluemke DA, Klein R, Sharrett AR, Islam FM, Cotch MF, Klein BE, Criqui MH, Wong TY.. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol 2007;50:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiumia D, Hankele AK, Groebner AE, Schulke K, Reichenbach HD, Giller K, Zakhartchenko V, Bauersachs S, Ulbrich SE.. Vascular endothelial growth factor A and VEGFR-1 change during preimplantation in Heifers. Int J Mol Sci 2020;21:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- Daien V, Carriere I, Kawasaki R, Cristol JP, Villain M, Fesler P, Ritchie K, Delcourt C.. Retinal vascular caliber is associated with cardiovascular biomarkers of oxidative stress and inflammation: the POLA study. PLoS One 2013;8:e71089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva DA, Woon FP, Manzano JJ, Liu EY, Chang HM, Chen C, Wang JJ, Mitchell P, Kingwell BA, Cameron JD. et al. ; behalf of the Multi-Centre Retinal Stroke Study Collaborative Group. The relationship between aortic stiffness and changes in retinal microvessels among Asian ischemic stroke patients. J Hum Hypertens 2012;26:716–722. [DOI] [PubMed] [Google Scholar]
- Dickey RP. Doppler ultrasound investigation of uterine and ovarian blood flow in infertility and early pregnancy. Hum Reprod Update 1997;3:467–503. [DOI] [PubMed] [Google Scholar]
- El-Mazny A, Abou-Salem N, Elshenoufy H.. Doppler study of uterine hemodynamics in women with unexplained infertility. Eur J Obstet Gynecol Reprod Biol 2013;171:84–87. [DOI] [PubMed] [Google Scholar]
- Fong DS, Aiello LP, Ferris FL, Klein R.. Diabetic retinopathy. Diabetes Care 2004;27:2540–2553. [DOI] [PubMed] [Google Scholar]
- Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R.. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand 2009;119:350–364. [DOI] [PubMed] [Google Scholar]
- Goswamy RK, Williams G, Steptoe PC.. Decreased uterine perfusion–a cause of infertility. Hum Reprod 1988;3:955–959. [DOI] [PubMed] [Google Scholar]
- Grieger JA, Grzeskowiak LE, Smithers LG, Bianco‐Miotto T, Leemaqz SY, Andraweera P, Poston L, McCowan LM, Kenny LC, Myers J. et al. Metabolic syndrome and time to pregnancy: a retrospective study of nulliparous women. BJOG: Int J Obstet Gynecol 2019;126:852–862. [DOI] [PubMed] [Google Scholar]
- Hong X, Zhao J, Huang K, Dai Q, Zhang H, Xuan Y, Wu J, Fang S, Wang Q, Shen H. et al. Pre-conception blood pressure and time to pregnancy among couples attempting to conceive their first pregnancy. Am J Obstet Gynecol 2019;221:470.e471–470.e410. [DOI] [PubMed] [Google Scholar]
- Huang L, Chen WQ, Aris IM, Teo LLY, Wong TY, Koh AS, Li LJ.. Associations between cardiac function and retinal microvascular geometry among Chinese adults. Sci Rep 2020;10:14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, de Jong PT.. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 2004;45:2129–2134. [DOI] [PubMed] [Google Scholar]
- IPAQ Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ). 2005. https://www.physio-pedia.com/images/c/c7/Quidelines_for_interpreting_the_IPAQ.pdf.
- Kanagasingam Y, Bhuiyan A, Abramoff MD, Smith RT, Goldschmidt L, Wong TY.. Progress on retinal image analysis for age related macular degeneration. Prog Retin Eye Res 2014;38:20–42. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY.. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol 2006;124:87–94. [DOI] [PubMed] [Google Scholar]
- Li LJ, Aris I, Su LL, Tint MT, Cheung CY, Ikram MK, Gluckman P, Godfrey KM, Tan KH, Yeo G. et al. Associations of maternal retinal vasculature with subsequent fetal growth and birth size. PLoS One 2015;10:e0118250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LJ, Cheung CY, Ikram MK, Gluckman P, Meaney MJ, Chong YS, Kwek K, Wong TY, Saw SM.. Blood pressure and retinal microvascular characteristics during pregnancy: Growing Up in Singapore Towards Healthy Outcomes (GUSTO) Study. Hypertension 2012;60:223–230. [DOI] [PubMed] [Google Scholar]
- Li LJ, Cheung CY, Liu Y, Chia A, Selvaraj P, Lin XY, Chan YM, Varma R, Mitchell P, Wong TY. et al. Influence of blood pressure on retinal vascular caliber in young children. Ophthalmology 2011;118:1459–1465. [DOI] [PubMed] [Google Scholar]
- Li LJ, Ikram MK, Broekman L, Cheung CY, Chen H, Gooley JJ, Soh SE, Gluckman P, Kwek K, Chong YS. et al. Antenatal mental health and retinal vascular caliber in pregnant women. Transl Vis Sci Technol 2013;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LJ, Ikram MK, Wong TY.. Retinal vascular imaging in early life: insights into processes and risk of cardiovascular disease. J Physiol 2016;594:2175–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LJ, Kramer M, Tapp RJ, Man RE, Lek N, Cai S, Yap F, Gluckman P, Tan KH, Chong YS. et al. Gestational diabetes mellitus and retinal microvasculature. BMC Ophthalmol 2017;17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew G, Mitchell P, Rochtchina E, Wong TY, Hsu W, Lee ML, Wainwright A, Wang JJ.. Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur Heart J 2011;32:422–429. [DOI] [PubMed] [Google Scholar]
- Loo EXL, Soh SE, Loy SL, Ng S, Tint MT, Chan SY, Huang JY, Yap F, Tan KH, Chern BSM. et al. ; S-PRESTO Study Group. Cohort profile: Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO). Eur J Epidemiol 2021;36:129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy SL, Cheung YB, Soh SE, Ng S, Tint MT, Aris IM, Bernard JY, Chong YS, Godfrey KM, Shek LP. et al. Female adiposity and time-to-pregnancy: a multi-ethnic prospective cohort. Hum Reprod 2018;33:2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy SL, Ku CW, Lai AEQ, Choo XH, Ho AHM, Cheung YB, Godfrey KM, Chong YS, Gluckman PD, Shek LP. et al. Plasma glycemic measures and fecundability in a Singapore pre-conception cohort study. Fertil Steril 2021;115:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon CJ, Hatch EE, Rothman KJ, Mikkelsen EM, Wesselink AK, Hahn KA, Wise LA.. Body mass index, physical activity and fecundability in a North American pre-conception cohort study. Fertil Steril 2016;106:451–459. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Callender CSKH, Kulikoff XR, Srinivasan V, Abate D, Abate KH, Abay SM, Abbasi N, Abbastabar H, Abdela J. et al. Population and fertility by age and sex for 195 countries and territories, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1995–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong YT, De Silva DA, Cheung CY, Chang HM, Chen CP, Wong MC, Wong TY, Ikram MK.. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke 2013;44:2121–2127. [DOI] [PubMed] [Google Scholar]
- Ou XH, Li S, Wang ZB, Li M, Quan S, Xing F, Guo L, Chao SB, Chen Z, Liang XW. et al. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum Reprod 2012;27:2130–2145. [DOI] [PubMed] [Google Scholar]
- Poplin R, Varadarajan AV, Blumer K, Liu Y, McConnell MV, Corrado GS, Peng L, Webster DR.. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng 2018;2:158–164. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Redman CW.. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 1993;341:1447–1451. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Care AS, Moldenhauer LM.. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest 2018;128:4224–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, 2008. [Google Scholar]
- Sharma R, Biedenharn KR, Fedor JM, Agarwal A.. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol 2013;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singapore Department of Statistics. https://wwwsingstatgovsg/modules/infographics/total-fertility-rate (9 September 2020, date last accessed).
- Slama R, Hansen OK, Ducot B, Bohet A, Sorensen D, Giorgis Allemand L, Eijkemans MJ, Rosetta L, Thalabard JC, Keiding N. et al. Estimation of the frequency of involuntary infertility on a nation-wide basis. Hum Reprod 2012;27:1489–1498. [DOI] [PubMed] [Google Scholar]
- Smith SK. Regulation of angiogenesis in the endometrium. Trends Endocrinol Metab 2001;12:147–151. [DOI] [PubMed] [Google Scholar]
- Smith W, Wang JJ, Wong TY, Rochtchina E, Klein R, Leeder SR, Mitchell P.. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the Blue Mountains Eye Study. Hypertension 2004;44:442–447. [DOI] [PubMed] [Google Scholar]
- Spira A. The use of fecundability in epidemiological surveys. Hum Reprod 1998;13:1753–1756. [DOI] [PubMed] [Google Scholar]
- Steenland K, Deddens JA.. A practical guide to dose-response analyses and risk assessment in occupational epidemiology. Epidemiology 2004;15:63–70. [DOI] [PubMed] [Google Scholar]
- Tapp RJ, Owen CG, Barman SA, Welikala RA, Foster PJ, Whincup PH, Strachan DP, Rudnicka AR; UK Biobank Eye, Vision Consortium. Retinal vascular tortuosity and diameter associations with adiposity and components of body composition. Obesity (Silver Spring) 2020;28:1750–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira JM, Fisk NM, Glover V.. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 1999;318:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkanen H, Kujansuu E, Laippala P.. Vascular resistance in uterine and ovarian arteries: its association with infertility and the prognosis of infertility. Eur J Obstet Gynecol Reprod Biol 1994;57:111–115. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Antczak M, Schrader R.. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod 1997;12:1047–1055. [DOI] [PubMed] [Google Scholar]
- Wesselink AK, Rothman KJ, Hatch EE, Mikkelsen EM, Sorensen HT, Wise LA.. Age and fecundability in a North American pre-conception cohort study. Am J Obstet Gynecol 2017;217:667. e661–667.e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselink AK, Wise LA, Hatch EE, Rothman KJ, Mikkelsen EM, Stanford JB, McKinnon CJ, Mahalingaiah S.. Menstrual cycle characteristics and fecundability in a North American pre-conception cohort. Ann Epidemiol 2016;26: 482–487 e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ. Fertility and Pregnancy: An Epidemiologic Perspective. New York: Oxford University Press; 2010. [Google Scholar]
- Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, Thom SM; BHS Guidelines Working Party, for the British Hypertension Society. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004;328:634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE.. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod 2010;25:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR, Shahar E.. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci 2006;47:2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD.. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 2002;287:1153–1159. [DOI] [PubMed] [Google Scholar]
- Zhao J, Hong X, Zhang H, Dai Q, Huang K, Zhang X, Liu Y, Wu J, Wang Q, Shen H. et al. Pre-pregnancy maternal fasting plasma glucose levels in relation to time to pregnancy among the couples attempting first pregnancy. Hum Reprod 2019;34:1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. Any required links or identifiers for the study data are present in the manuscript as described or provide edits with the required information.