Abstract
Aims
With the explosion of anticancer drugs, an emerging concern is the risk for drug-induced sudden death (SD) via ventricular arrhythmias (VA).
Methods and results
We used the international pharmacovigilance database VigiBase (n = 18 441 659 reports) to compare drug-induced long QT (diLQT, n = 18 123) and VA (n = 29 193) including torsade de pointes (TdP, n = 8163) reporting for 663 anticancer drugs vs. all other drugs until 01/01/2019. The analysis used the 95% lower-end credibility interval of the information component (IC025), an indicator for disproportionate Bayesian reporting; significant when IC025 >0. There were 2301 reports (13.8% fatal) for 40 anticancer drugs significantly associated with diLQT (with 27 also associated with VA or SD) and 9 drugs associated with VA without diLQT. Half of these (46.9%, 23/49) were associated with SD. Most (41%, 20/49) were kinase inhibitors, 8% (4/49) were hormonal therapies, 6% (3/49) were immunotherapies, 24% (12/49) were cytotoxics, and 20% (10/49) were miscellaneous. In VigiBase, reports of diLQT, TdP, or VA increased from 580 in the period 1967–83 to 15 070 in 2014–18 with the proportion related to anticancer drugs increasing from 0.9% (5/580) to 14.0% (2115/15 070) (P < 0.0001). Concordance between these VigiBase signals and data concerning diLQT and VA/TdP identified in CredibleMeds or US Food and Drug Administration (FDA) labels was moderate (κ = 0.47 and 0.40, P < 0.0001). Twenty-three drugs represent new signals, while 24 flagged by CredibleMeds or FDA had no signal in VigiBase. A three-level SD risk stratification relying on isolated long QT (low risk), associated with VA without SD (moderate risk), and VA with SD (high risk) is proposed.
Conclusion
This list of liable anticancer drugs may prove useful for physicians and regulatory authorities to re-evaluate cardiac monitoring requirements.
Clinical trial registration
Keywords: Disproportionality analysis, Anticancer drugs, Long QT, Ventricular arrhythmias, Pharmacovigilance, Torsade de pointes
Graphical Abstract
See the editorial comment for this article ‘QT prolongation and cancer therapeutics: a coming Tempest or Much Ado About Nothing?’, by L. Garg and M. G. Fradley, doi:10.1093/eurheartj/ehab483.
Introduction
The development of cancer therapeutics has resulted in a better prognosis and long-term survival for patients with many malignancies.1 Anticancer drugs may also lead to severe cardiovascular adverse drug reactions (ADR) carrying a high morbidity burden and can be fatal.2 This interplay between cancer and heart conditions is the subject of the booming field of cardio-oncology. Cardiac ADR of cytotoxic anticancer drugs have been identified for decades, such as anthracycline-induced heart failure or acute myocardial infarction with anti-metabolites.2 , 3 With the exponential development of new classes of anticancer drugs [including immunotherapy and kinase inhibitors (KI)], other heart-related ADR have emerged and represent an important concern for regulatory agencies, companies, and patient care providers. A striking example is the increased reporting of myocarditis (fatality rate ∼30–50%) occasionally induced by immune checkpoint inhibitors, which are breakthrough therapeutics approved in a wide variety of cancers.4 , 5 Cardiac arrhythmias are another emerging and poorly characterized concern of anticancer drugs with an increasing number of targeted therapies such as KI and anti-hormonal agents prolonging QT interval, a well-recognized marker of increased risk for cardiac arrhythmias and sudden death (SD).6 , 7
The QT interval on the electrocardiogram (ECG), corrected for heart rate (QTc), is a measure of the duration of ventricular repolarization and is a widely used proxy of the drug-induced ventricular arrhythmia (VA) risk.8 It remains the recommended standard surrogate used in human studies, despite its well-recognized limitations.9 Many drugs slightly prolong the QT interval, but in some patients, this prolongation can be exaggerated and provoke the morphologically distinctive polymorphic VA torsade de pointes (TdP). Symptoms associated with TdP include syncope and SD if the arrhythmia is prolonged or degenerates into ventricular fibrillation. More recently, it has been reported that some anticancer drugs, such as ibrutinib (a Bruton KI), can also lead to fatal VA without prolonging QT.10
Using VigiBase, the World Health Organization’s (WHO) global pharmacovigilance database, we aimed to better define and risk stratify these severe cardiac arrhythmia ADR to improve patient safety and facilitate monitoring guidelines after the administration of anticancer drugs. We also sought to identify new drugs with signals for VA, long QT, and TdP, which were not previously identified during clinical trials.
Methods
Study design and data sources
This observational, retrospective, pharmacovigilance study is a disproportionality analysis based on ADR reported in VigiBase, the WHO deduplicated database of individual case safety reports (i.e. reports thereafter).11 VigiBase is managed by the Uppsala Monitoring Centre (UMC, Uppsala, Sweden) and contains ∼19 million reports (through January 2019) submitted by national pharmacovigilance centres since 1967. The use of confidential, electronically processed patient data was approved by the Vanderbilt University Medical Center institutional review board (# 181337, USA).
Procedures
This study included all drug-induced long QT (diLQT), TdP, or VA classified by group queries according to the Medical Dictionary for Regulatory Activities (Supplementary material online, Table S1), between inception on 14 November 1967 and 1 January 2019. DiLQT, TdP, or VA specifically assessed in the analysis were those reported as suspected to be caused by a drug (vs. concomitant use). Each report contains general administrative information (country of origin, date of reporting, and reporter qualification), patient characteristics (sex, age), drugs (indication, start and end dates of administration, dosage regimen, and route of administration), and reactions or events (reported terms, onset and end date, seriousness, and final outcome). A severe ADR was defined as causing death, being life-threatening, requiring hospital stay (initial or prolonged), or leading to persistent or clinically significant disability, congenital anomaly, birth defect, or any other medically important conditions.
Statistical analysis
VigiBase allows disproportionality analysis (also known as case–non-case analysis), which we used to assess whether suspected diLQT, TdP and VA were differentially reported with each drug (663 individual molecules pertaining to the anticancer drugs) vs. the full database of 20 222 drugs (Supplementary material online, Figure S1 for the flow chart). Disproportionality analyses compare the proportion of a selected specific ADR reported for a single drug with the proportion of the same ADR for a control group of drugs (i.e. full database with all drugs). The denominator in these analyses is the total number of ADR reported for each group of drugs. If the proportion of cases associated with a specific drug is greater than in patients without this ADR (non-cases), there is a disproportional association (signal identification) between the ADR and the drug.5 We calculated a Bayesian disproportionality estimate suitable when taking the full database as comparator, i.e. the information component (IC). IC compares observed and expected number of reports for drug-ADR pairs. The IC025 is the lower end of the 95% credibility interval for the IC so a positive value of the IC025 is deemed significant. More information concerning calculation of the IC/IC025 is provided in the Supplementary material online, Methods, and these methods have been recently used in similar settings and detailed elsewhere.5 , 10 , 12 , 13 Since this work focused on identifying culprit anticancer drugs, we further performed a sensitivity analysis and estimated the frequentist disproportionality association [reporting odds ratio (ROR)] with diLQT, TdP, VA, and SD for each anticancer drug already flagged with positive IC025, restricting the background database to reports associated with at least one anticancer drugs (defined as drugs pertaining to the anatomical therapeutic classification L: antineoplastic and immunomodulating agents). ROR was calculated by Chi2 test, and the 95% confidence interval (CI95%) was estimated, as previously described.5 , 14 A lower end of the ROR CI95% ≥1 is considered statistically significant.
Characteristics of reports in VigiBase were described in terms of means ± standard deviation or medians and interquartile range [IQR] for quantitative variables, and in terms of numbers and proportion for qualitative ones. Comparisons were performed by Chi2 test and Wilcoxon test with Dunn’s post-tests, as appropriate. P < 0.05 was deemed statistically significant.
Concordance (agreement) between the data describing liability of anticancer drugs to induce cardiac arrhythmias according to VigiBase vs. US Food and Drug Administration (FDA) labels (accessible at https://www.accessdata.fda.gov/scripts/cder/daf/) and CredibleMeds® (accessible at www.crediblemeds.org) was computed using the Cohen kappa coefficient.
Results
Trends in anticancer drug-associated cardiac arrhythmia reporting over decades
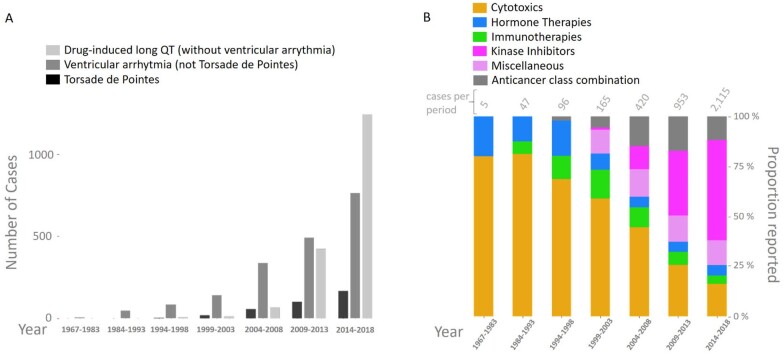
The study included 42 462 reports of diLQT, TdP, or VA from VigiBase inception, through 1 January 2019. The number increased from 580 in the period 1967–83 to 15 070 for 2014–18 (Supplementary material online, Figure S2). The corresponding proportion related to anticancer drugs increased from 0.9% (5/580) to 14.0% (2115/15 070) (P < 0.00001). Anticancer drugs were divided into five subgroups: cytotoxic treatments (CT, including antimetabolites and anthracyclines), hormone therapies (HT), immunotherapies (IT, including immune-related cell therapies), KI (including any drug interacting directly with a kinase protein or its ligands), and other therapies [miscellaneous (Misc)]. The majority of this increase in reporting over years was in the KI group (Graphical abstract) representing 51.6% (1091/2115) of these cardiac arrhythmia reports associated with anticancer drugs within the 2014–18 period vs. 14.7% (311/2115) with CT, 5.9% (124/2115) with HT, 2.5% (52/2115) with IT, and 12.5% (265/2115) with a combination of any of these anticancer classes (Combo; i.e. one drug or more pertaining to at least two of these classes: CT, HT, IT, KI, Misc) (P < 0.00001).
Graphical Abstract.
Evolution of reporting for drug-induced long QT, ventricular arrhythmias, and torsade de pointes associated with anticancer drugs (A) as a function of their classes (B) in VigiBase from inception (1967) to January 2019.
Anticancer drugs associated with long QT and VA including TdP and SD
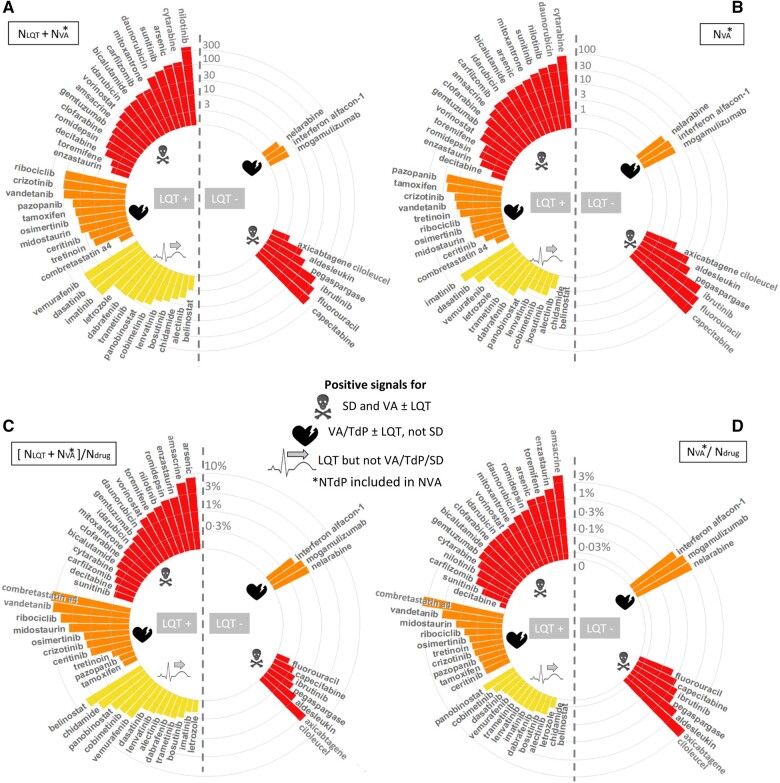
Forty anticancer drugs were significantly associated with diLQT (including 27 also associated with VA or SD) and 9 with VA without diLQT when taking as background either the full database (n = 18 441 659; IC025 >0) or when restricting the database to cases involving at least one anticancer drug used (n = 4 197 602; ROR CI95% ≥1) (Table 1). Most (41%, 20/49) were KI, 24% (12/49) were CT, 8% (4/49) were HT, 6% (3/49) were IT, and 20% (10/49) were Misc. Details regarding the magnitude of the association by drug and per subtype of arrythmia (diLQT, VA and TdP) and signals for SD are shown in Table 1. Details concerning the year for which these anticancer drugs were first significantly associated with any of these cardiac arrhythmias are shown in Supplementary material online, Figure S2. Details concerning number of reports per year of these cardiac arrhythmias are shown in Figure 1. To further evaluate the seriousness of these cardiac events (diLQT, VA including TdP), we stratified the 49 drugs of interest as a function of the presence or not of a significant association with drug-induced SD (Table 2). Half of these anticancer drugs (46.9%, 23/49) were associated with SD. We generated a three-level SD risk stratification (Figure 2) constituted of drugs associated with only isolated diLQT without VA nor SD (low risk, n = 13), drugs associated with VA without SD (moderate risk, n = 13), and drugs associated with VA and SD (high risk, n = 23). Among anticancer drugs with moderate and high risk for SD, most were also associated with diLQT (75%, 27/36) but not all (25%, 9/36). The top three drugs with the highest disproportional association (Table 1 and Supplementary material online, Figure S3, using IC025) with diLQT were vandetanib (KI, n = 97, IC025 = 5.8, year of FDA approval 2011), arsenic trioxide (Misc, n = 115, IC025 = 5.5, year of FDA approval 2000), and ribociclib (KI, n = 105, IC025 = 5.3, year of FDA approval 2017). This was concordant with arsenic trioxide (n = 14, IC025 = 2.7), vandetanib (n = 10, IC025 = 2.5), and vorinostat (Misc, n = 6, IC025 = 1.2, year of FDA approval 2006) carrying the highest association with TdP. The top three drugs associated with VA were amsacrine (CT, n = 14, IC025 = 3.1), arsenic trioxide (n = 25, IC025 = 2.4), and daunorubicin (CT, n = 52, IC025 = 1.8, year of FDA approval 1979). The top drugs in terms of absolute number of reports were respectively nilotinib for diLQT (KI, n = 369, IC025 = 0.4, year of FDA approval 2007), TdP (n = 18, IC025 = 0.4), and capecitabine for VA (CT, n = 161, IC025 = 0.8, year of FDA approval 1998) (Table 1 and Figure 1). The major mechanisms of action of these drugs are detailed in Table 2.
Table 1.
Anticancer drugs associated with at least one of the following adverse drug reactions: drug-induced long QT syndrome, torsade de pointes, and ventricular arrhythmias based on disproportionality analysis in VigiBase (through 01 January 2019 )
| Drug | Class | N drug | diLQT (N
effect
a = 3036) |
TdPb (N
effect
a = 761) |
VA (N
effect
a = 3748) |
SD (N
effect
a = 13 288) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N obs | IC025 | rOR [CI95%] | N obs | IC025 | rOR [CI95%] | N obs | IC025 | rOR [CI95%] | N obs | IC025 | rOR [CI95%] | |||
| Amsacrined | CT | 287 | 5 | 1.3 | 25 [10–59] | 3 | 0.4 | 58 [19–183] | 14 | 3.1 | 58 [34–99] | 10 | 1.5 | 11.4 [6.1–21.4] |
| Capecitabinec ,d | CT | 49 174 | 161 | 0.8 | 3.8 [3.2–4.4] | 319 | 0.3 | 2.1 [1.9–2.3] | ||||||
| Clofarabinec ,d | CT | 2216 | 5 | 3.1 [1.3–7.5] | 2 | 5 [1.2–20] | 11 | 0.5 | 5.6 [3.1–10] | 57 | 2.0 | 8.3 [6.4–10.9] | ||
| Combretastatin a4 | CT | 25 | 3 | 0.7 | 189 [56–630] | 1 | 230 [31–1703] | 1 | 47 [6.3–345] | |||||
| Cytarabinec ,d | CT | 26 300 | 52 | 0.6 | 2.8 [2.1–3.6] | 14 | 3 [1.8–5] | 89 | 0.8 | 3.9 [3.1–4.8] | 358 | 1.4 | 4.4 [4–4.9] | |
| Daunorubicinc ,d | CT | 6655 | 28 | 1.4 | 5.9 [4.1–8.5] | 9 | 0.4 | 7.5 [3.9–15] | 52 | 1.8 | 8.9 [6.8–12] | 149 | 2.0 | 7.3 [6.2–8.6] |
| Decitabinec ,d | CT | 2894 | 10 | 0.6 | 4.8 [2.6–8.9] | 22 | 2.4 [1.6–3.7] | |||||||
| Fluorouracilc ,d | CT | 65 547 | 138 | 0.2 | 2.4 [2–2.9] | 383 | 0.2 | 1.9 [1.7–2.1] | ||||||
| Idarubicinc ,d | CT | 3539 | 12 | 0.7 | 4.7 [2.7–8.3] | 5 | 7.8 [3.3–19] | 18 | 0.9 | 5.7 [3.6–9.1] | 42 | 0.9 | 3.8 [2.8–5.1] | |
| Mitoxantronec ,d | CT | 4847 | 12 | 0.3 | 3.4 [1.9–6.1] | 9 | 0.8 | 10 [5.4–20] | 28 | 1.2 | 6·5 [4.5–9.5] | 60 | 1.0 | 4 [3.1–5.1] |
| Nelarabinec ,d | CT | 370 | 4 | 0.3 | 12 [4.6–33] | |||||||||
| Pegaspargasec ,d | CT | 4481 | 22 | 0.9 | 5.5 [3.6–8.4] | 146 | 2.5 | 10.7 [9.1–12.6] | ||||||
| Bicalutamidec ,d | HT | 4802 | 13 | 0.5 | 3.8 [2.2–6.5] | 10 | 1.0 | 12 [6.2–22] | 23 | 0.9 | 5.4 [3.6–8.2] | 24 | 1.6 [1.1–2.4] | |
| Letrozolec ,d | HT | 15 564 | 25 | 0.1 | 2.2 [1.5–3.3] | |||||||||
| tamoxifenc ,d | HT | 18 567 | 28 | 0.0 | 2.1 [1.4–3] | 8 | 2.4 [1.2–4.8] | |||||||
| Toremifenec ,d | HT | 258 | 2 | 11 [2.7–43] | 1 | 21 [3–153] | 4 | 0.6 | 18 [6.6–47] | 8 | 1.2 | 10.1 [5–20.4] | ||
| Aldesleukinc ,d | IT | 1553 | 15 | 1.6 | 11 [6.6–18] | 29 | 1.4 | 6 [4.2–8.7] | ||||||
| Axicabtagene ciloleucelc ,d | IT | 117 | 4 | 1.0 | 40 [15–108] | 3 | 8.3 [2.6–26.1] | |||||||
| Interferon alfacon-1e | IT | 742 | 5 | 0.2 | 7.6 [3.2–18] | |||||||||
| Alectinibc ,d | KI | 1346 | 5 | 0.1 | 5.2 [2.1–12] | |||||||||
| Bosutinibc ,d | KI | 2927 | 9 | 0.4 | 4.3 [2.2–8.2] | |||||||||
| Ceritinibc ,d | KI | 1747 | 21 | 2.6 | 17 [11–26] | 2 | 6.3 [1.6–25] | |||||||
| Cobimetinibc ,d | KI | 1496 | 20 | 2.7 | 19 [12–29] | |||||||||
| Crizotinibc ,d | KI | 7614 | 102 | 3.4 | 19 [16–24] | 9 | 0.2 | 6.6 [3.4–13] | ||||||
| Dabrafenibc ,d | KI | 7612 | 27 | 1.2 | 5 [3.4–7.2] | |||||||||
| Dasatinibc ,d | KI | 19 654 | 94 | 1.9 | 6.8 [5.6–8.4] | |||||||||
| Enzastaurin | KI | 138 | 1 | 10 [1.4–72] | 3 | 0.2 | 25 [7.9–78] | 2 | 4.6 [1.1–18.7] | |||||
| Ibrutinibc ,d | KI | 21 110 | 99 | 1.3 | 5.4 [4.4–6.6] | 126 | 0.1 | 1.9 [1.6–2.3] | ||||||
| Imatinibc ,d | KI | 44 671 | 64 | 0.2 | 2 [1.6–2.6] | |||||||||
| Lenvatinibc ,d | KI | 2555 | 11 | 1 | 6 [3.3–11] | |||||||||
| Midostaurinc ,d | KI | 1001 | 34 | 4 | 49 [35–69] | 2 | 11 [2.8–44] | 4 | 4.5 [1.7–12] | |||||
| Nilotinibc ,d | KI | 17 471 | 369 | 4.2 | 34 [30–38] | 18 | 0.4 | 5.8 [3.6–9.3] | 46 | 0.3 | 3 [2.2–4] | 92 | 1.7 [1.4–2.1] | |
| Osimertinibc ,d | KI | 2423 | 37 | 3.2 | 22 [16–30] | 4 | 9.2 [3.4–24] | |||||||
| Pazopanibc ,d | KI | 19 816 | 40 | 0.5 | 2.8 [2.1–3.9] | 14 | 4 [2.3–6.7] | |||||||
| Ribociclibc ,d | KI | 1738 | 105 | 5.3 | 92 [75–112] | 4 | 0.1 | 13 [4.8–34] | ||||||
| Sunitinibc ,d | KI | 29 774 | 71 | 0.9 | 3.4 [2.7–4.2] | 12 | 2.2 [1.3–4] | 130 | 1.4 [1.2–1.6] | |||||
| Trametinibc ,d | KI | 7538 | 26 | 1.1 | 4.8 [3.3–7.1] | |||||||||
| Vandetanibc ,d | KI | 971 | 97 | 5.8 | 158 [128–196] | 10 | 2.5 | 58 [31–109] | 10 | 1.3 | 12 [6.3–22] | |||
| Vemurafenibc ,d | KI | 8322 | 106 | 3.3 | 18 [15–22] | |||||||||
| Arsenic trioxidec ,d | Misc | 1642 | 115 | 5.5 | 108 [89–131] | 14 | 2.7 | 48 [28–82] | 25 | 2.4 | 17 [12–26] | 20 | 0.6 | 3.9 [2.5–6] |
| Belinostatc ,d | Misc | 61 | 3 | 0.6 | 72 [22–228] | |||||||||
| Carfilzomibc ,d | Misc | 8109 | 19 | 0.5 | 3.3 [2.1–5.1] | 18 | 2.5 [1.6–4] | 59 | 0.3 | 2.3 [1.8–3] | ||||
| Chidamide | Misc | 238 | 7 | 2.1 | 42 [20–89] | |||||||||
| Gemtuzumab ozogamicinc ,d | Misc | 1729 | 9 | 1 | 7.2 [3.8–14] | 3 | 9.6 [3.1–30] | 8 | 0.2 | 5.2 [2.6–10] | 34 | 1.5 | 6.3 [4.5–8.9] | |
| Mogamulizumabc ,d | Misc | 497 | 5 | 0.6 | 11 [4.7–27] | |||||||||
| Panobinostatc ,d | Misc | 1483 | 24 | 3 | 23 [15–34] | |||||||||
| Romidepsinc | Misc | 462 | 11 | 2.6 | 34 [19–62] | 2 | 24 [6–97] | 4 | 0.1 | 9.8 [3.7–26] | 4 | 2.8 [1–7.4] | ||
| Tretinoinc ,d | Misc | 5250 | 14 | 0.4 | 3.7 [2.2–6.3] | 3 | 3.2 [1–9.8] | |||||||
| Vorinostatc ,d | Misc | 1378 | 23 | 3 | 24 [16–36] | 6 | 1.2 | 24 [11–54] | 7 | 0.2 | 5.7 [2.7–12] | 24 | 1.2 | 5.6 [3.7–8.4] |
Associations were deemed significant when the lower end of the 95% credibility interval was positive (IC025 >0) for analysis vs. full database (n = 18 441 659); or when the lower end of the 95% confidence interval of the reporting odds ratio was >1 (rOR025 >1) for analysis restricted to reports with anticancer drugs as background (n = 4 197 602). Non-significant associations are not represented. Results with SD are also represented for these latter drugs.
ADR, adverse drug reactions; CI95%, 95% confidence interval; CT, chemotherapy; diLQT, drug-induced long QT syndrome; HT, hormonotherapy; IT, immunotherapy; KI, kinase inhibitor; Misc, miscellaneous; N drug, number of reports for the drug; N obs, number of reports observed for the ADR with the drug of interest; SD, sudden death; TdP, torsade de pointes; VA, ventricular arrhythmias.
N effect refers to the number of reports for the ADR of interest in the anticancer group. The N effect in the full database background is 18 123 for diLQT; 8163 for TdP; 29 193 for VA; and 85 350 for SD.
Data of disproportionality for TdP breakdown (vs. VA) was considered only for drugs with a positive signal for diLQT.
Available on the US market.
Available on the European market.
Withdrawn from the US market,
Figure 1.
Evolution of the absolute number of long QT syndrome and/or ventricular arrhythmias including torsade de pointe reports over time for each of the 49 culprit anticancer drugs identified using VigiBase (see Table 1). For each drug, the year of Food and Drug Administration approval was added when available (otherwise, NA stands for not available).
Table 2.
Classification of the 49 anticancer drugs as a function of the signals identified in VigiBase for drug-induced long QT syndrome, ventricular arrhythmias including torsade de pointes, and sudden death
| Drug | Class | Subclass | Target/mechanism |
|---|---|---|---|
| diLQT | |||
| VA or TdP and sudden death | |||
| Amsacrine | CT | Anthracycline and derivatives | Topoisomerase II |
| Daunorubicin | CT | Anthracycline and derivatives | Topoisomerase II |
| Idarubicin | CT | Anthracycline and derivatives | Topoisomerase II |
| Mitoxantrone | CT | Anthracycline and derivatives | Topoisomerase II |
| Clofarabine | CT | Antimetabolite | Purine analog |
| Cytarabine | CT | Antimetabolite | Cytidine analog |
| Decitabinea | CT | Antimetabolite | Hypomethylating agent/cytidine analog |
| Bicalutamide | HT | Antiandrogen | Androgen receptor |
| Toremifene | HT | SERM | Estrogen receptor |
| Enzastaurin | KI | Kinase inhibitor | PKCβ, AuroraA/B, Chk1/2, URACα, and PI3Kα |
| Nilotinib | KI | Kinase inhibitor | BCR-ABL, PDGFR, KIT, CSF-1R, and DDR1 |
| Sunitinib | KI | Kinase inhibitor | VEGFR1/2/3, PDGFRα/β, KIT, FLT3, CSF-1R, and RET |
| Arsenic trioxide | Misc | Other small molecule | PML/RAR-alpha |
| Carfilzomib | Misc | Other small molecule | Proteasome inhibitors |
| Romidepsin | Misc | Epigenetic inhibitor | Histone deacetylase |
| Vorinostat | Misc | Epigenetic inhibitor | Histone deacetylase |
| Gemtuzumab ozogamicin | Misc | Antibody drug conjugate | CD33 |
| VA or TdP without sudden death | |||
| Combretastatin a4 | CT | Vascular disruptive agent | Vascular endothelial cells |
| Tamoxifen | HT | SERM | Estrogen receptor |
| Ceritinib | KI | Kinase inhibitor | ALK, IGF-1R, InsR, and ROS1 |
| Crizotinib | KI | Kinase inhibitor | ALK, ROS1, Met, RON |
| Midostaurin | KI | Kinase inhibitor | FLT3, KIT ,PDGFRα/β, VEGFR2, and PKC |
| Osimertinib | KI | Kinase inhibitor | EGFRm (19 indel, L858R, T790M) |
| Pazopanib | KI | Kinase inhibitor | VEGFR1/2/3, PDGFRα/β, KIT, FGFR1/3, ITK, LCK, FMS |
| Ribociclib | KI | Kinase inhibitor | CDK4/6 |
| Vandetanib | KI | Kinase inhibitor | EGFR, VEGFR, RET, BRK, TIE2, EPHR, and SRC |
| Tretinoin | Misc | Other small molecule | PML/RAR-alpha |
| No VA, TdP, nor sudden death | |||
| Letrozole | HT | Aromatase inhibitor | Estrogen receptor |
| Alectinib | KI | Kinase inhibitor | ALK and RET |
| Bosutinib | KI | Kinase inhibitor | BCR-ABL, Src, Lyn, and Hck |
| Cobimetinib | KI | Kinase inhibitor | MEK1, MEK2 |
| Dabrafenib | KI | Kinase inhibitor | BRAF V600/wt, CRAF, SIK1, NEK11, and LIMK1 |
| Dasatinib | KI | Kinase inhibitor | BCR-ABL, SRC, LCK, YES, FYN, c-KIT, EPHA2, and PDGFRβ |
| Imatinib | KI | Kinase inhibitor | BCR-ABL, PDGFR, SCF, and KIT |
| Lenvatinib | KI | Kinase inhibitor | VEGFR1/2/3, PDGFRα, KIT, FGFR1/2/3/4, and RET |
| Trametinib | KI | Kinase inhibitor | MEK1, MEK2 |
| Vemurafenib | KI | Kinase inhibitor | BRAF, CRAF, ARAF, SRMS, ACK1, MAP4K5, and FGR |
| Belinostat | Misc | Epigenetic inhibitor | Histone deacetylase |
| Chidamide | Misc | Epigenetic inhibitor | Histone deacetylase |
| Panobinostat | Misc | Epigenetic inhibitor | Histone deacetylase |
| No diLQT | |||
| VA with sudden death | |||
| Capecitabine | CT | Antimetabolite | Uracil analogue |
| Fluorouracil | CT | Antimetabolite | Uracil analogue |
| Pegaspargase | CT | Other protein-based therapies | l-Asparagine |
| Aldesleukin | IT | Cytokine | Interleukin-2 |
| Axicabtagene ciloleucel | IT | CAR T cell | CD19 |
| Ibrutinib | KI | Kinase inhibitor | BTK |
| VA without sudden death | |||
| Nelarabine | CT | Antimetabolite | Guanosine analogue |
| Interferon alfacon-1 | IT | Cytokine | Interferon |
| Mogamulizumab | Misc | Chemokine receptor inhibitor | CCR4 |
CAR, chimeric antigen receptor; CT, chemotherapy; diLQT, drug-induced long QT syndrome; HT, hormonotherapy; IT, immunotherapy; KI, kinase inhibitor; MAB, monoclonal antibody; Misc, miscellaneous; SD, sudden death; SERM, selective oestrogen receptor modulator; VA, ventricular arrhythmias; TdP, torsade de pointes.
Decitabine was significantly associated with diLQT and SD, but not with TdP nor VA.
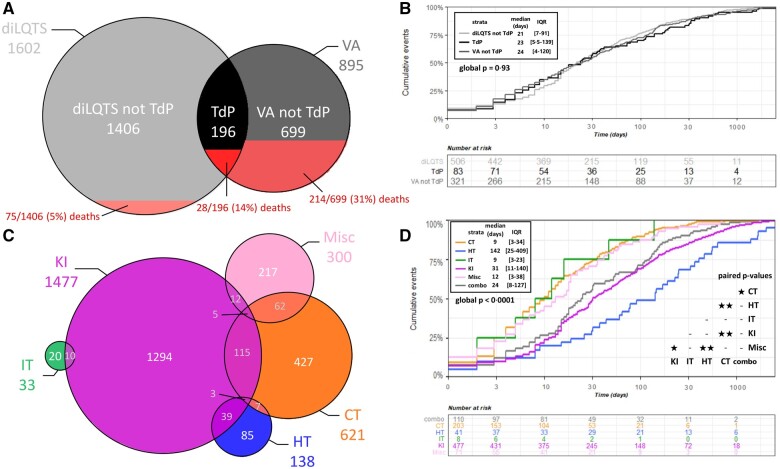
Figure 2.
Three-level sudden death risk stratification for the 49 liable anticancer drugs associated with isolated drug-induced long QT (low risk), ventricular arrhythmias without sudden death (moderate risk), and ventricular arrhythmias with sudden death (high risk) identified in VigiBase (through 1 January 2019). Absolute number of ventricular arrhythmias (N VA) including torsade de pointes (associated (N LQT + N VA) or not with diLQT reports by drug and sudden death risk level is displayed in (A) and (B), respectively. The corresponding proportion of such cases (N VA or N LQT + N VA) over the total number of overall adverse drug reactions per drug (N drug) are displayed in (C) and (D), respectively.
Of note, we further validated this disproportionality method using positive and negative controls in terms of drugs at known risk of diLQT and TdP [dofetilide, sotalol, ibutilide with IC025 values among the highest (4.9–5.82)] vs. protective for diLQT and TdP (progesterone, levonorgestrel, and testosterone carrying among the lowest IC025 values).7 , 9 , 15 These data are shown in Supplementary material online, Figure S3 and the top 25 highest and lowest IC025 values for diLQT among all drugs available in VigiBase are shown in Supplementary material online, Tables S2 and S3.
Clinical features of cardiac arrhythmias associated with anticancer drugs in VigiBase
Clinical characteristics derived from the 2301 reports (diLQT without TdP, n = 1406; TdP, n = 196, and VA without TdP, n = 699) associated with the 49 anticancer drugs of interest are displayed in Table 3 and in Supplementary material online, Table S4. Overlap between culprit anticancer drug classes within these reports is represented in Figure 3. The median age was 63 years (IQR 51–71). Male predominance was found in VA reports excluding TdP (64.9%, 431/664), contrasting with female predominance in diLQT and TdP reports (51.4%, 698/1359, P < 0.0001). Most reports were in the last 5 years (1434/2301, 62%) and were by healthcare professionals (1701/1943, 88%) in America (1038/2301, 45%) or Europe (762/2301, 33%). Most reports involved at least one culprit KI (64%, 1477/2301). A majority of reports were considered serious (94%, 1946/2078). All-cause fatality was 13.8% (317/2301) and 10% reported SD (228/2301, Table 3). The final outcome after stopping the culprit anticancer drug was available for 397 reports, of which 326/397 (82%) resolved. Most patients (49%, 766/1555) had hematological diseases, particularly chronic myeloid leukaemia (23%, 363/1555) or other leukaemia (17%, 272/1555). Among solid tumors, the most represented were colorectal, lung, breast, and kidney cancers (8.1%, 126/1555; 7.3%, 114/1555; 6.7%, 104/1555; and 6.1%, 95/1555, respectively).
Table 3.
Characteristics of patients receiving at least one of the 49 anticancer drugs associated significantly with drug-induced long QT syndrome, torsade de pointes, or ventricular arrhythmias through 01 January 2019, in VigiBase
| Total N = 2301 | |
|---|---|
| Age at onset (years), median [IQR] | 63 [51–71] N = 1609 available |
| Time to onset (days), median [IQR] | 25 [7–97] N = 776 available |
| Reporting year | |
| 1973–1993 | 22/2301 (1.0%) |
| 1994–1998 | 34/2301 (1.5%) |
| 1999–2003 | 67/2301 (2.9%) |
| 2004–2008 | 191/2301 (8.3%) |
| 2009–2013 | 553/2301 (24%) |
| 2014–2018 | 1434/2301 (62.3%) |
| Notifier | |
| Healthcare professionals | 1701/1943 (88%) |
| Non-healthcare professionals | 242/1943 (12%) |
| Country of reporting | |
| Africa | 1/2301 (0.1%) |
| Americas | 1038/2301 (45.1%) |
| Asia | 423/2301 (18.4%) |
| Europe | 762/2301 (33.1%) |
| Oceania | 77/2301 (3.3%) |
| Sex | |
| Female | 931/2023 (46%) |
| Male | 1092/2023 (54%) |
| Type of report | |
| diLQT without TdP; % including SD | 1406/2301 (61%); 0/1406 (0%) |
| TdP; % including SD | 196/2301 (9%); 44/196 (29%) |
| VA (not TdP); % including SD | 699/2301 (30%); 184/699 (26%) |
| Seriousness | |
| Serious | 1946/2078 (94%) |
| Death | 317/2301 (14%) |
| SD | 228/2301 (10%) |
| Number of anticancer drug suspected/interacting | |
| 1 | 1793/2301 (78%) |
| 2 | 313/2301 (14%) |
| ≥3 | 195/2301 (8%) |
| Type of anticancer drugs suspected/interacting | |
| At least one cytotoxic | 621/2301 (27%) |
| At least one hormonotherapy | 138/2301 (6%) |
| At least one immunotherapy | 33/2301 (1.4%) |
| At least one kinase inhibitor | 1477/2301 (64.2%) |
| At least one miscellaneous drug | 300/2301 (13%) |
| 2 types or more combined | 258/2301 (11.2%) |
| Indications | |
| Chronic myeloid leukaemia (CML) | 363/1555 (23%) |
| Leukaemia other than CML | 272/1555 (17%) |
| Colorectal cancer | 126/1555 (8.1%) |
| Lung cancer | 114/1555 (7.3%) |
| Breast cancer | 104/1555 (6.7%) |
| Kidney cancer | 95/1555 (6.1%) |
| Melanoma | 90/1555 (5.8%) |
| Thyroid cancer | 76/1555 (4.9%) |
| Myeloma | 50/1555 (3.2%) |
| Lymphoma | 47/1555 (3%) |
| Prostate cancer | 24/1555 (1.5%) |
| Cancer other | 90/1555 (5.8%) |
| Cancer no precision | 52/1555 (3.3%) |
| Other hematological diseases or malignancies | 34/1555 (2.2%) |
| Indication other than malignancy (inflammatory or autoimmune diseases) | 18/1555 (1.2%) |
| Concurrent reported drugs at known risk of TdP (in the diLQT and/or TdP reports, n = 1602) | |
| 0 | 1381/1602 (86.2%) |
| 1 | 192/1602 (12%) |
| 2 | 18/1602 (1.1%) |
| ≥3 | 11/1602 (0.7%) |
| Concurrent reported drugs at conditional, possible or known risk of TdP(in the diLQT and/or TdP reports, n = 1602) | |
| 0 | 1157/1602 (72.2%) |
| 1 | 244/1602 (15.2%) |
| 2 | 103/1602 (6.4%) |
| ≥3 | 98/1602 (6.1%) |
| Classes of concurrently reported drugs at conditional, possible or known risk of TdP(in the diLQT and/or TdP reports, n = 1602) | |
| Anti-alpha1-adrenergics | 3/1602 (0.2%) |
| Antiarrhythmic | 41/1602 (2.6%) |
| Antidepressant | 96/1602 (6.0%) |
| Antiemetic | 119/1602 (7.4%) |
| Antihistamine | 36/1602 (2.2%) |
| Anti-infectious | 114/1602 (7.1%) |
| Antipsychotic | 23/1602 (1.4%) |
| Diuretic-potassium lowering agents | 96/1602 (6.0%) |
| Opioid | 73/1602 (4.6%) |
| Proton pump inhibitor | 137/1602 (8.6%) |
| Other cardiovascular drugs | 2/1602 (0.1%) |
| Others | 13/1602 (0.8%) |
| Concurrent reported condition favouring LQT/TdP or VA | |
| None | 1216/2301 (53%) |
| Hypokalemia | 107/2301 (4.7%) |
| Hypocalcemia | 65/2301 (2.8%) |
| Hypomagnesemia | 33/2301 (1.4%) |
| Diabetes mellitus | 39/2301 (1.7%) |
| Uncontrolled hypertension | 92/2301 (4%) |
| Pericarditis or pericardia effusion | 25/2301 (1.1%) |
| Cardiac ischaemia | 183/2301 (8%) |
| Heart failure | 239/2301 (10%) |
| Bradycardia | 94/2301 (4.1%) |
| Tachycardia | 113/2301 (4.9%) |
| Conductive disorders | 174/2301 (7.6%) |
| Atrial fibrillation | 126/2301 (5.5%) |
| Hypotension or shock | 134/2301 (5.8%) |
| Ischaemia or thrombosis (not cardiac nor cerebral) | 75/2301 (3.3%) |
| Acute kidney injury | 133/2301 (5.8%) |
| Acute hepatic injury | 139/2301 (6%) |
| Acute stroke | 43/2301 (1.9%) |
| Epilepsy | 43/2301 (1.9%) |
| Infection (virus, bacteria, fungus, or parasite) | 275/2301 (12%) |
| Inflammation | 160/2301 (7%) |
| Other cardiovascular disorders | 132/2301 (5.7%) |
diLQT, drug-induced long QT syndrome; SD, sudden death; TdP, torsade de pointes; VA, ventricular arrhythmia.
Figure 3.
Overlap and overall fatality rate of drug-induced long QT syndrome, torsade de pointe, and ventricular arrhythmia reports associated with the 49 drugs identified in VigiBase (A) with their respective time to onset (B). Overlap in VigiBase for reports of drug-induced long QT and ventricular arrhythmia including torsade de pointes for these 49 drugs as a function of the underlying drug classes (C) with their corresponding time to onset (D). Differences in median time to onset by groups were compared by Wilcoxon tests and Dunn’s post-test. * and ** stands for P ≤ 0.05 and ≤0.0001, respectively. combo, reports containing at least one culprit anticancer drugs from at least two different anticancer drug classes; CT, cytotoxic therapy; HT, hormone therapy; IT, immunotherapy; KI, kinase inhibitor; Misc, miscellaneous.
Details concerning concurrent drugs and conditions favouring QT prolongation, TdP and VA are shown in Table 3. Most reports 1381/1602 (86%) had no concomitant drugs at known risk of TdP on top of the culprit anticancer drug in the diLQT and/or TdP patients. Among these 1602 reports, the most reported drug classes with molecules concomitantly used at known risk of TdP were proton pomp inhibitors (8.6%; n = 137), antiemetics (7.4%, n = 119), anti-infectious agents (7.1%, n = 114), and antidepressants (6%, n = 96). Reports of concurrent conditions favouring diLQT and VA were frequent with 12% (275/2301) of infection, or cardiac conditions including 10% (239/2301) of heart failure and 8% (183/2301) of cardiac ischaemia (Table 3).
The median time to onset (in days) was not significantly different between patients with diLQT without TdP, TdP, and non-TdP VA (21 [IQR 7–91] vs. 23 [IQR 5·5–139] vs. 24 [IQR 4–120] days, respectively; P = 0·93) (Figure 3). When comparing different drug classes, the median time to onset was variable ranging from 9 days [IQR 3–23] for IT, 9 [IQR 3–34] for CT, 12 [IQR 3–38] for Misc treatments, 31 [IQR 11–140] for KI, to 142 [IQR 25–409] for HT (P < 0.0001). The differences between anticancer drug classes are displayed in Figure 3. The differences between anticancer drug molecules are displayed in Supplementary material online, Table S4.
Concordance of cardiac arrhythmia risk evaluations between VigiBase, CredibleMeds, and FDA
A total of 663 anticancer drugs were referenced in VigiBase (through 1 July 2019), of which 199 were FDA approved at least once and 195 currently approved (through 1 July 2019). Concordance between VigiBase results and data concerning diLQT, TdP, and/or VA available in CredibleMeds database (which aggregates all known drugs prolonging QT) or US FDA labels were moderate (κ = 0.47 [0.34–0.6], P < 0.0001 and 0.40 [0.27–0.54], P < 0.0001, respectively, Supplementary material online, Figure S4). Corresponding concordance between CredibleMeds and US FDA labels was high (κ = 0.74 [0.62–0.85], P < 0.0001). Twenty-three drugs (16 for diLQT or TdP and 14 for VA) were not described in CredibleMeds and/or FDA databases. In contrast, CredibleMeds and/or FDA databases described 24 drugs associated with these ADR, which yielded no significant association in VigiBase. Details concerning the concordance per drug and specific type of cardiac arrhythmia (diLQT, TdP, VA) between these databases are presented in Table 4. Analyses of concordance restricted to the 199 FDA-approved drugs showed similar results (Supplementary material online, Figure S4). The most relevant new signals were those carrying a very high proportion of single suspect culprit drug (SSCD) in the reports (not confounded by the concurrent intake of other liable anticancer drugs) (Supplementary material online, Table S4). Within FDA-approved drugs, these latter were carfilzomib (n = 19, SSCD = 100%, proteasome inhibitor), imatinib (n = 64, SSCD = 73%, KI), alectinib (n = 5, SSCD = 100%, KI), axicabtagene-ciloleucel (n = 4, SSCD = 100%, CAR-T anti-CD19), mogamulizumab (n = 5, SSCD = 100%, C-C chemokine receptor type 4 inhibitor), and bicalutamide (n = 30, SSCD = 93%, androgen receptor antagonist). Interestingly, four drugs were flagged before any FDA approval (amsacrine [CT], combretastatin a4 [CT], chidamide [histone deacetylase inhibitor], and enzastaurin [KI]).
Table 4.
Comparison of VigiBase signals (IC025 >0 vs. full database; or rOR025 >1 vs. anticancer drug background) for drug-induced long QT, torsade de pointes, and ventricular arrhythmias with information retrieved in CredibleMeds® website and US Food and Drug Administration labels (through 1 July 2019)
| Drug | Signal in VigiBase |
CredibleMeds TdP riska | Signal in FDA label |
New signal | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| diLQT | TdP | VA | SD | diLQT | TdP | VA | SD | |||
| Abarelix | Possible | In text | ||||||||
| Aldesleukin | Yes | Yes | BW | |||||||
| Alectinib | Yes | Yes | ||||||||
| Amsacrine | Yes | Yes | Yes | Yes | NA | NA | NA | NA | Yes | |
| Apalutamide | Possible | In text | ||||||||
| Arsenic trioxide | Yes | Yes | Yes | Yes | Known | BW | BW | BW | BW | |
| Axicabtagene ciloleucel | Yes | Yes | Yes | |||||||
| Belinostat | Yes | Yes | ||||||||
| Bendamustine | Possible | |||||||||
| Bicalutamide | Yes | Yes | Yes | Yes | ||||||
| Bortezomib | Yes | Possible | In text | |||||||
| Bosutinib | Yes | Possible | ||||||||
| Cabozantinib | Possible | |||||||||
| Capecitabine | Yes | Yes | Possible | |||||||
| Carfilzomib | Yes | Yes | Yes | Yes | ||||||
| Ceritinib | Yes | Yes | Possible | Warning | In text | In text | In text | |||
| Chidamide | Yes | NA | NA | NA | NA | Yes | ||||
| Clofarabine | Yes | Yes | Yes | Yes | Yes | |||||
| Cobimetinib | Yes | Possible | ||||||||
| Combretastatin a4 | Yes | Yes | Yes | NA | NA | NA | NA | Yes | ||
| Crizotinib | Yes | Yes | Possible | Warning | ||||||
| Cyclophosphamide | Yes | In text | ||||||||
| Cytarabine | Yes | Yes | Yes | Yes | Yes | |||||
| Dabrafenib | Yes | Possible | ||||||||
| Dasatinib | Yes | Possible | Warning | |||||||
| Daunorubicin | Yes | Yes | Yes | Yes | Yes | |||||
| Decitabine | Yes | Yes | Yes | |||||||
| Degarelix | Possible | Warning | ||||||||
| Encorafenib | Possible | Warning | ||||||||
| Enzastaurin | Yes | Yes | Yes | NA | NA | NA | NA | Yes | ||
| Epirubicin | Possible | |||||||||
| Eribulin | Possible | Warning | ||||||||
| Fluorouracil | Yes | Yes | Possible | |||||||
| Gemtuzumab ozogamicin | Yes | Yes | Yes | Yes | In text | Yes | ||||
| Gilteritinib | Possible | Warning | ||||||||
| Glasdegib | Possible | Warning | In text | |||||||
| Goserelin | Warning | |||||||||
| Histrelin | Warning | In text | Warning | |||||||
| Ibrutinib | Yes | Yes | Warning | |||||||
| Idarubicin | Yes | Yes | Yes | Yes | Yes | |||||
| Ifosfamide | Warning | |||||||||
| Imatinib | Yes | Yes | ||||||||
| Inotuzumab | Possible | Warning | ||||||||
| Interferon alfacon-1 | Yes | In text | ||||||||
| Ivosidenib | Possible | Warning | In text | |||||||
| Lapatinib | Possible | Warning | In text | |||||||
| Lenvatinib | Yes | Possible | Warning | |||||||
| Letrozole | Yes | Yes | ||||||||
| Leuprorelin | Possible | Warning | Warning | |||||||
| Midostaurin | Yes | Yes | Yes | Possible | In text | |||||
| Mitoxantrone | Yes | Yes | Yes | Yes | Yes | |||||
| Mogamulizumab | Yes | Yes | ||||||||
| Necitumumab | Possible | |||||||||
| Nelarabine | Yes | Yes | ||||||||
| Nilotinib | Yes | Yes | Yes | Yes | Possible | BW | BW | |||
| Osimertinib | Yes | Yes | Possible | Warning | ||||||
| Oxaliplatin | Yes | Known | In text | In text | In text | |||||
| Panobinostat | Yes | Possible | Warning | |||||||
| Pazopanib | Yes | Yes | Possible | Warning | Warning | In text | ||||
| Pegaspargase | Yes | Yes | Yes | |||||||
| Ribociclib | Yes | Yes | Possible | Warning | ||||||
| Romidepsin | Yes | Yes | Yes | Yes | Possible | Warning | ||||
| Sorafenib | Possible | Warning | In text | |||||||
| Sunitinib | Yes | Yes | Yes | Possible | Warning | Warning | In text | |||
| Tamoxifen | Yes | Yes | Possible | |||||||
| Tipiracil-trifluridine | Possible | |||||||||
| Toremifene | Yes | Yes | Yes | Yes | Possible | BW | BW | BW | ||
| Trametinib | Yes | Yes | ||||||||
| Tretinoin | Yes | Yes | Yes | |||||||
| Triptorelin | Warning | Warning | ||||||||
| Vandetanib | Yes | Yes | Yes | Known | BW | BW | In text | BW | ||
| Vemurafenib | Yes | Possible | Warning | In text | In text | |||||
| Vorinostat | Yes | Yes | Yes | Yes | Possible | |||||
Among the 663 anticancer drugs screened (full list in Supplementary material online, Table S3), only those with evidence of association with diLQT, TdP, and VA mentioned in one of these three reference sources are shown. For these latter drugs, information concerning SD is also represented.
BW, box warning; diLQT, drug-induced long QT syndrome; FDA, Food and Drug Administration; NA: Not available; SD, sudden death; TdP, torsade de pointes; VA, ventricular arrhythmias.
Signals accounted when drugs were flagged at possible or known risk for TdP (conditional risk not accounted).
Discussion
In this worldwide pharmacovigilance study that included almost 19 million reports, disproportionality analyses yielded significant association between 49 anticancer drugs and cardiac arrhythmias, including diLQT, TdP, and VA. This detailed report summarizes all available data addressing drug-induced cardiac arrhythmias extracted from US FDA labels, CredibleMeds and VigiBase. We believe our data can serve as a compendium for all clinicians using anticancer drugs and considering their potential arrhythmic risk (Table 4). FDA labels mainly summarize data systematically gathered and analysed during drug development (thorough and concentration QT studies, clinical trials), and in some cases updates arising from post-marketing evaluation. Consensus achieved by experts from academia of these available data is found in the widely recognized CredibleMeds website for TdP risk.31 VigiBase is a complementary source, which assembles data from real-life surveillance with spontaneous post-marketing reporting mainly arising from healthcare professionals. VigiBase has previously been utilized to describe other cardiovascular sequelae from anticancer therapies and has allowed a better appreciation of the magnitude of these toxicities.5 , 7 , 10 Interestingly, in our work, 23 drugs represented new signals, while 24 flagged by Crediblemeds or FDA had no signal in VigiBase. These findings may guide clinicians and regulatory institutions to conduct further research to re-evaluate cardiac monitoring requirements focusing on these specific drugs. Moreover, information generally contained in FDA labels focus on the magnitude of QT prolongation identified in QT studies but does not provide information concerning VA and TdP risk as such, because these events are often too rare and not adjudicated in cancer-focused clinical trials. CredibleMeds website only assesses TdP risk in the context of QTc prolongation. Herein, we were able to identify three levels of SD risk profile with anticancer drugs only associated with isolated long QT (low risk), associated with VA without SD (moderate risk), and VA with SD (high risk). This SD risk stratification may prove useful to clinicians when confronted to difficulties in the risk/benefit assessment of pursuing a liable anticancer drug with a possible overall benefit for the patient. Importantly, we have identified a novel group that has not been particularly well flagged previously: drugs associated with potentially fatal VA but not mediated by QTc prolongation. This group is important to recognize in clinical situations where arrhythmias are suspected with a normal QTc on ECG. Lastly, this work provides a quantitative magnitude of disproportional association of 663 anticancer drugs with diLQT, TdP, and VA. These data may prove useful for translational cardio-oncology researches seeking at identifying new pathways involved in arrhythmias, as we recently showed with ibrutinib and identification of kinase-dependent off-target inhibition leading to atrial fibrillation.16
To date, this study is the most extensive, analysing over 42 000 suspected drug-induced cardiac arrhythmia events internationally reported from healthcare professionals. The evolution of reporting in VigiBase has been marked in the last decade by the introduction of new drug classes, KI, and IT; they currently represent the majority of reported drug-induced cardiac arrhythmias. As expected, CT have been associated with these ADR for far longer. In VigiBase, the first treatment to yield a significant association with cardiac arrhythmias was an anthracycline, idarubicin, in 1995. Of note, an average QTc >500 ms (normal <450 ms for men and <460 ms for women) or a >60-ms QTc change from baseline is considered as of particular concern (grade 3) according to the Common Terminology Criteria for Adverse Events, the grading mostly used in oncology trials. Anthracycline-related increase in QTc >60 ms vs. baseline has been reported with an incidence up to 14% with doxorubicin, and relates to their propensity to induce cardiomyocyte injury via overproduction of free radicals and alteration of cardiac ion currents, notably via I Ks channel blockade and intra-cellular calcium dysregulation.3 , 6 , 17 Our study also supports multiple observations previously reported in the literature, highlighting the robustness of the methodology with positive controls (e.g. arsenic trioxide, nilotinib, vandetanib, vorinostat, ribociclib).6 , 17–19 The anticancer drug most reported with long QT in VigiBase was arsenic trioxide, a drug used against some leukaemia, and significantly associated with long QT/TdP since 2002 in VigiBase. The most comprehensive QT study included 99 patients with advanced malignancies who received 170 courses of arsenic trioxide. Of them, 35/99 (35.4%) developed increase in QTc >60 ms vs. baseline, and one developed asymptomatic TdP.18 Nilotinib, a second-generation BCR-ABL inhibitor, has been previously linked to moderate increase in QTc (average QTc prolongation of 5–15 ms).6 In studies, 2.5–4% of patients exhibited QTc prolongation >60 ms on nilotinib, and in one study, 1.2% of patients showed QTc >500 ms.6 , 17 , 20 Similarly, vandetanib, a vascular endothelial growth factor receptor inhibitor, has been associated with long QT in a meta-analysis including nine phase II–III trials, which found a significant risk of QTc prolongation (all-grade according to the National Cancer Institute Common Toxicity Criteria v.2.0 or 3.0), 123/2552 (4.82%) in treated patients vs. 6/2204 (0.27%) in control group (relative-risk 7.90, CI95% [4.03–15.50]).21 Vorinostat, a histone deacetylase inhibitor used in the treatment of cutaneous T-cell lymphoma, was associated with QTc prolongation (>470 ms or delta >60 ms from baseline) in 5/116 (4.3%) patients in a retrospective review including phase I–II trials.22 Ribociclib, a CDK 4/6 inhibitor used in breast cancer, was associated with QTc prolongation (>480 ms) in 11/334 (3.3%) of ribociclib-treated patients vs. 1/334 (0.3%) in the placebo arm, in its landmark randomized controlled trial.23
Distinct from drugs prolonging QT, several drugs were associated with VA without long QT. They included ibrutinib, and CAR-T. Indeed, although ibrutinib has not been associated with long QT (studies reported concentration-dependent QTc shortening),24 it has been associated with atrial and VA and SD.10 , 25 As described previously, it may correspond to a short-coupled variant of polymorphic ventricular tachycardia, which is thought to involve alteration in cardiac sarcoplasmic reticulum Ca2+ homeostasis associated with cardiac ryanodine receptor-calmodulin-dependent protein kinase pathways.10 , 25 In our study, which found a few cases of CAR-T (axicabtagene-ciloleucel) related VA, there was also a signal towards association with VA but not long QT. In a retrospective study, 54% of tested patients who received CAR-T showed myocardial injury with troponin elevation and 12% developed a cardiac ADR (including heart failure, arrhythmias, and cardiac deaths).26
Our study also yielded new signals requiring further investigations to confirm the causality of the association, its magnitude and mechanisms at play. Alectinib, an ALK inhibitor, was not previously associated with VA nor QT modification, but it was associated with mild sinus bradycardia.27 Carfilzomib, a proteasome inhibitor approved for the treatment of multiple myeloma, was known for its risk of cardiac failure, but not long QT and SD.28 We also found imatinib associated with long QT, while imatinib was considered so far as relatively safe from a cardiovascular standpoint, as compared to other BCR-ABL inhibitors including nilotinib, ponatinib, and dasatinib.6 , 17 , 29 In our study, a key element strengthening the association between these drugs and cardiac arrhythmias is the fact that in most reports alectinib (100%), carfilzomib (100%), and imatinib (73.4%) was the only anticancer drug suspect involved in the appearance of these cardiac ADR. Notably, we also observed hormone therapies blocking testosterone association with long QT and TdP, such as bicalutamide, an androgen receptor antagonist used in prostate cancer. In a translational study combining pharmacoepidemiological and mechanistic studies using iPSC cardiomyocytes, we recently confirmed the causal association between androgen deprivation and long QT and TdP.7 Interestingly, our analysis showed that in four instances, signals of association between an incriminated drug under development and long QT, VA, and TdP, in VigiBase appeared prior to FDA approval of those drugs (namely, amsacrine, chidamide, combretastatin a4, and enzastaurin). In the specific field of drug-induced QT prolongation and cardiac arrhythmias, the agreement between the FDA labels, CredibleMeds, and VigiBase remains modest at best and emphasizes the complementarity of a multimodal approach to apprehend the toxicity of a specific drug.
The variety of anticancer drug classes associated with long QT highlights the heterogeneity of the mechanisms, which may underlie cardiac arrhythmias related to these agents. The generally accepted common mechanism whereby drugs prolong QT is a block of a key cardiac repolarizing potassium current, IKr.8 While some anticancer drugs associated with diLQT and TdP have been shown to inhibit IKr, recent works focusing on anticancer drugs prolonging QT identified new pathways.8 The in vitro effects of some KI to prolong cardiac action potentials (the cellular correlate of QT) can be rescued by intracellular phosphatidylinositol 3,4,5-trisphosphate, the downstream effector of phosphoinositide-3-kinase. This finding supports a role for inhibition of this enzyme, either directly or by inhibition of upstream kinases, to prolong QTc through mechanisms that are being investigated but include enhanced inward ‘late’ sodium current (INaL) activation during the plateau of the action potential.8 These observations emphasize the need to better explore the kinome in general, and in particular, the effects of kinases located up- and downstream to that of phosphoinositide-3-kinase, to better understand their influence on cardiac electrophysiology.30
We acknowledge several inherent limitations to our results related to pharmacovigilance studies.12 First, the exact denominator of patients exposed to anticancer drugs cannot be evaluated; hence, the true incidence of the events cannot be computed, and all values are expressed as relative to each other, with the basis that VigiBase aggregates millions of reports, and hence may allow for a generalization of the findings. The second bias stems from the observational and declarative nature of the reports with variable degree of exhaustivity. Third, the number of reports for a particular medicinal product may be influenced by the extent of use of the product, publicity, the nature of the reactions, and other bias.12 Fourth, some drugs specifically annotated in Supplementary material online, Table S4 (low SSCD) were systematically used in association, which require cautious interpretation of the results when one of these were known to be associated with cardiac arrhythmias (i.e letrozole, often asssociated with ribociclib with ribociclib being a well indentified liable drug). Finally, in the specific context of anticancer drugs, the added risk due to these drugs is difficult to assess, as end-stage cancers may be associated with cardiac arrhythmias; however, this has been partially mitigated by our sensitivity disproportionality analysis restricted to patients on anticancer drugs. While these limitations are numerous, the added value of pharmacovigilance studies has already been demonstrated in various settings.5 , 10 , 14 , 15 Nevertheless, they are only to be taken as signal-generating studies and all hypotheses generated require validation by translational mechanistic or prospective studies.7 , 10 Indeed, while randomized clinical trials are mandatory to establish efficacy, their power to detect ADR may be lower, due to the rarer incidence in these events and the fact that ‘real-world population’ may differ from included patients in the said clinical trial. Translational experimental studies specifically designed to answer a question of cardiotoxicity in oncology remain the most comprehensive design available, yet.7 16
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The supplied data from VigiBase come from various sources. The likelihood of a causal relationship is not the same in all reports. The information does not represent the opinion of World Health Organization. We thank the custom searches team at the Uppsala Monitoring Centre (Uppsala, Sweden) research section, without whom this study would not have been possible.
Funding
Funding sources had no role in study design, collection, analysis, or interpretation of the data, in the writing of the manuscript, and in the decision to submit it for publication. The corresponding author had full access to all of the data and the final responsibility to submit for publication. Javid Moslehi is supported by grants from National Institute of Health (R01HL141466, R01HL155990, R01HL156021).
Author contributions
J.-E.S. and P.G. were involved in study design. P.G. did the literature search. P.G. and J.-E.S. made the figures. P.G., J.-E.S., and B.L.-V. were involved in data collection. P.G. and J.-E.S. analysed the data. P.G., L.S.N., and J.-E.S. were involved in data interpretation. P.G., L.S.N., J.-E.S., J.J.M., C.F.-B., S.E., A.C., D.R., and B.L.-V. were involved in the writing of the manuscript. All authors edited the manuscript.
Conflict of interest: J.-E.S. had paid lecture fees from AstraZeneca and BMS unrelated to this work and have patents pending and issued related to methods for detecting the risk of torsade de pointes. J.J.M. had consultancy fees from BMS, AstraZeneca, Deciphera, Janssen, Takeda, Cytokinetics, Audentes, Boston Biomedical, and Myovant unrelated to this work. All other authors have nothing to disclose.
Data availability
Data are available upon request to VigiBase (https://www.who-umc.org/vigibase/vigibase/).
Contributor Information
Joe-Elie Salem, INSERM, CIC-1901, Sorbonne Université, AP-HP, Pitié-Salpêtrière Hospital, Department of Pharmacology, CLIP² Galilée, Regional Pharmacovigilance Center, UNICO-GRECO Cardio-Oncology Program, Paris 75013, France; Cardio-Oncology Program, Department of Medicine and Pharmacology, Vanderbilt University Medical Center, Nashville, TN, USA.
Lee S Nguyen, INSERM, CIC-1901, Sorbonne Université, AP-HP, Pitié-Salpêtrière Hospital, Department of Pharmacology, CLIP² Galilée, Regional Pharmacovigilance Center, UNICO-GRECO Cardio-Oncology Program, Paris 75013, France; Research & Innovation of CMC Ambroise Paré, Neuilly-sur-Seine 92200, France.
Javid J Moslehi, Cardio-Oncology Program, Department of Medicine and Pharmacology, Vanderbilt University Medical Center, Nashville, TN, USA.
Stéphane Ederhy, INSERM, Sorbonne Université, Department of Cardiology, AP-HP, Saint Antoine Hospital, UNICO-GRECO Cardio-oncology program, Paris, France.
Bénédicte Lebrun-Vignes, INSERM, CIC-1901, Sorbonne Université, AP-HP, Pitié-Salpêtrière Hospital, Department of Pharmacology, CLIP² Galilée, Regional Pharmacovigilance Center, UNICO-GRECO Cardio-Oncology Program, Paris 75013, France; UPEC EA EpiDermE, 7379, France.
Dan M Roden, Cardio-Oncology Program, Department of Medicine and Pharmacology, Vanderbilt University Medical Center, Nashville, TN, USA; Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, TN, USA.
Christian Funck-Brentano, INSERM, CIC-1901, Sorbonne Université, AP-HP, Pitié-Salpêtrière Hospital, Department of Pharmacology, CLIP² Galilée, Regional Pharmacovigilance Center, UNICO-GRECO Cardio-Oncology Program, Paris 75013, France.
Paul Gougis, INSERM, CIC-1901, Sorbonne Université, AP-HP, Pitié-Salpêtrière Hospital, Department of Pharmacology, CLIP² Galilée, Regional Pharmacovigilance Center, UNICO-GRECO Cardio-Oncology Program, Paris 75013, France.
References
- 1. Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust T, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, De P, McClure C, Ramanakumar AV, Stuart-Panko H, Engholm G, Walsh PM, Jackson C, Vernon S, Morgan E, Gavin A, Morrison DS, Huws DW, Porter G, Butler J, Bryant H, Currow DC, Hiom S, Parkin DM, Sasieni P, Lambert PC, Møller B, Soerjomataram I, Bray F. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol 2019;20:1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 3. Levis BE, Binkley PF, Shapiro CL. Cardiotoxic effects of anthracycline-based therapy: what is the evidence and what are the potential harms? Lancet Oncol 2017;18:e445–e456. [DOI] [PubMed] [Google Scholar]
- 4. Geraud A, Gougis P, Vozy A, Anquetil C, Allenbach Y, Romano E, Funck-Brentano E, Moslehi JJ, Johnson DB, Salem JE. Clinical pharmacology and interplay of immune checkpoint agents: a Yin-Yang balance. Annu Rev Pharmacol Toxicol 2021;61:85–112. [DOI] [PubMed] [Google Scholar]
- 5. Salem J-E, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano J-P, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexandre J, Moslehi JJ, Bersell KR, Funck-Brentano C, Roden DM, Salem JE. Anticancer drug-induced cardiac rhythm disorders: current knowledge and basic underlying mechanisms. Pharmacol Ther 2018;189:89–103. [DOI] [PubMed] [Google Scholar]
- 7. Salem JE, Yang T, Moslehi JJ, Waintraub X, Gandjbakhch E, Bachelot A, Hidden-Lucet F, Hulot JS, Knollmann BC, Lebrun-Vignes B, Funck-Brentano C, Glazer AM, Roden DM. Androgenic effects on ventricular repolarization: a translational study from the international pharmacovigilance database to iPSC-cardiomyocytes. Circulation 2019;140:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roden DM. A current understanding of drug-induced QT prolongation and its implications for anticancer therapy. Cardiovasc Res 2019;115:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vicente J, Zusterzeel R, Johannesen L, Mason J, Sager P, Patel V, Matta MK, Li Z, Liu J, Garnett C, Stockbridge N, Zineh I, Strauss DG. Mechanistic model-informed proarrhythmic risk assessment of drugs: review of the "CiPA" initiative and design of a prospective clinical validation study. Clin Pharmacol Ther 2018;103:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salem JE, Manouchehri A, Bretagne M, Lebrun-Vignes B, Groarke JD, Johnson DB, Yang T, Reddy NM, Funck-Brentano C, Brown JR, Roden DM, Moslehi JJ. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol 2019;74:1667–1678. [DOI] [PubMed] [Google Scholar]
- 11. Lindquist M. VigiBase, the WHO Global ICSR Database System: basic facts. Drug Inf J 2008;42:409–419. [Google Scholar]
- 12. Bihan K, Lebrun-Vignes B, Funck-Brentano C, Salem JE. Uses of pharmacovigilance databases: an overview. Therapie 2020;75:591–598. [DOI] [PubMed] [Google Scholar]
- 13. Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, De Freitas RM. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 1998;54:315–321. [DOI] [PubMed] [Google Scholar]
- 14. Grouthier V, Lebrun-Vignes B, Glazer AM, Touraine P, Funck-Brentano C, Pariente A, Courtillot C, Bachelot A, Roden DM, Moslehi JJ, Salem JE. Increased long QT and torsade de pointes reporting on tamoxifen compared with aromatase inhibitors. Heart 2018;104:1859–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salem JE, Dureau P, Bachelot A, Germain M, Voiriot P, Lebourgeois B, Tregouet DA, Hulot JS, Funck-Brentano C. Association of oral contraceptives with drug-induced QT interval prolongation in healthy nonmenopausal women. JAMA Cardiol 2018;3:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiao L, Salem JE, Clauss S, Hanley A, Bapat A, Hulsmans M, Iwamoto Y, Wojtkiewicz G, Cetinbas M, Schloss MJ, Tedeschi J, Lebrun-Vignes B, Lundby A, Sadreyev RI, Moslehi J, Nahrendorf M, Ellinor PT, Milan DJ. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation 2020;142:2443–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buza V, Rajagopalan B, Curtis AB. Cancer treatment-induced arrhythmias: focus on chemotherapy and targeted therapies. Circ Arrhythm Electrophysiol 2017;10:e005443. [DOI] [PubMed] [Google Scholar]
- 18. Barbey JT, Pezzullo JC, Soignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol 2003;21:3609–3615. [DOI] [PubMed] [Google Scholar]
- 19. Porta-Sánchez A, Gilbert C, Spears D, Amir E, Chan J, Nanthakumar K, Thavendiranathan P. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J Am Heart Assoc 2017;6:e007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 2006;354:2542–2551. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y, Liu Y, Fan ZW, Li J, Xu GG. Meta-analysis of the risks of hypertension and QTc prolongation in patients with advanced non-small cell lung cancer who were receiving vandetanib. Eur J Clin Pharmacol 2015;71:541–547. [DOI] [PubMed] [Google Scholar]
- 22. Lynch DR Jr., Washam JB, Newby LK. QT interval prolongation and torsades de pointes in a patient undergoing treatment with vorinostat: a case report and review of the literature. Cardiol J 2012;19:343–438. [PubMed] [Google Scholar]
- 23. Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Petrakova K, Hart LL, Villanueva C, Chan A, Jakobsen E, Nusch A, Burdaeva O, Grischke E-M, Alba E, Wist E, Marschner N, Favret AM, Yardley D, Bachelot T, Tseng L-M, Blau S, Xuan F, Souami F, Miller M, Germa C, Hirawat S, O’Shaughnessy J. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738–1748. [DOI] [PubMed] [Google Scholar]
- 24. Schaffer M, Chaturvedi S, Davis C, de Jong J, Aquino R, Oki Y, Fourneau N, Younes A, Balasubramanian S. Ibrutinib does not prolong the corrected QT interval in healthy subjects: results from a thorough QT study. Cancer Chemother Pharmacol 2020;25:100235–101237. [Google Scholar]
- 25. Lampson BL, Yu L, Glynn RJ, Barrientos JC, Jacobsen ED, Banerji V, Jones JA, Walewska R, Savage KJ, Michaud GF, Moslehi JJ, Brown JR. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood 2017;129:2581–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, Lee DH, Zlotoff DA, Zhang L, Drobni ZD, Hassan MZO, Bassily E, Rhea I, Ismail-Khan R, Mulligan CP, Banerji D, Lazaryan A, Shah BD, Rokicki A, Raje N, Chavez JC, Abramson J, Locke FL, Neilan TG. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol 2019;74:3099–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morcos PN, Bogman K, Hubeaux S, Sturm-Pellanda C, Ruf T, Bordogna W, Golding S, Zeaiter A, Abt M, Balas B. Effect of alectinib on cardiac electrophysiology: results from intensive electrocardiogram monitoring from the pivotal phase II NP28761 and NP28673 studies. Cancer Chemother Pharmacol 2017;79:559–568. [DOI] [PubMed] [Google Scholar]
- 28. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; Group ESCSD. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 29. Manouchehri A, Kanu E, Mauro MJ, Aday AW, Lindner JR, Moslehi J. Tyrosine kinase inhibitors in leukemia and cardiovascular events: from mechanism to patient care. Arterioscler Thromb Vasc Biol 2020;40:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fleuren ED, Zhang L, Wu J, Daly RJ. The kinome ‘at large’ in cancer. Nat Rev Cancer 2016;16:83–98. [DOI] [PubMed] [Google Scholar]
- 31. Woosley RL, Heise CW, Gallo T, Tate J., Woosley D., Romero KA. www.CredibleMeds.org, QTdrugs List, [Accession Date], AZCERT, Inc. 1457 E. Desert Garden Dr., Tucson, AZ 85718. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to VigiBase (https://www.who-umc.org/vigibase/vigibase/).