Abstract
Aims
Lung ultrasound (LUS) relies on detecting artefacts, including A-lines and B-lines, when assessing dyspnoeic patients. A-lines are horizontal artefacts and characterize normal lung, whereas multiple vertical B-lines are associated with increased lung density. We sought to assess the prevalence of A-lines and B-lines in patients with acute heart failure (AHF) and examine their clinical correlates and their relationship with outcomes.
Methods and results
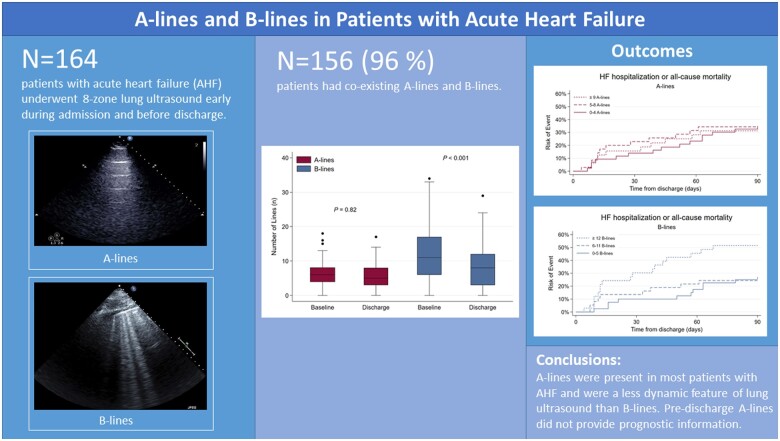
In a prospective cohort study of adults with AHF, eight-zone LUS and echocardiography were performed early during the hospitalization and pre-discharge at an imaging depth of 18 cm. A- and B-lines were analysed separately off-line, blinded to clinical and outcome data. Of 164 patients [median age 71 years, 61% men, mean ejection fraction (EF) 40%], the sum of A-lines at baseline ranged from 0 to 19 and B-line number from 0 to 36. One hundred and fifty-six patients (95%) had co-existing A-lines and B-lines at baseline. Lower body mass index and lower chest wall thickness were associated with a higher number of A-lines (P trend < 0.001 for both). In contrast to B-lines, there was no significant change in the number of A-lines from baseline to discharge (median 6 vs. 5, P = 0.80). While B-lines were associated with 90-day HF readmission or death, A-lines were not [HR 1.67, 95% confidence interval (CI) 1.11–2.51 vs. HR 0.97, 95% CI 0.65–1.43].
Conclusions
A-lines and B-lines on LUS co-exist in the vast majority of hospitalized patients with AHF. In contrast to B-lines, A-lines were not associated with adverse outcomes.
Keywords: Acute heart failure, Lung ultrasound, A-lines, B-lines, Pulmonary congestion
Graphical abstract
Introduction
Lung ultrasonography (LUS) is increasingly used in acute care settings to rapidly assess patients with acute dyspnoea or heart failure (HF).1 Although normal lung tissue cannot be directly imaged with ultrasound due to its high air content, LUS can be used to assess pulmonary congestion by interpreting artefacts, namely A-lines and B-lines.2 A-lines are horizontal reverberation artefacts seen at equal distances reflecting from the pleural line. Current LUS literature associates the presence of A-lines with normal lung surfaces and pulmonary diseases without congestion (e.g. obstructive pulmonary diseases or pneumothorax).3 In addition, the absence of both A- and B-lines may also be seen within one zone in normal lung. B-lines are vertical artefacts that arise from the pleural line and are associated with extravascular lung water but can also be found in other conditions with increases in lung density (e.g. interstitial lung disease).4
While there is a growing body of evidence regarding the diagnostic and prognostic value of B-lines in HF, quantitative data regarding A-line artefacts' diagnostic and prognostic utility in adults are sparse.5–8 Despite this lack of evidence, the diagnostic utility of A-lines has been proposed in several imaging protocols and in the didactic literature on the assessment of acute dyspnoea and management of patients with HF.2,9 Specifically, the presence of A-lines has been described as a necessary finding to rule out pulmonary congestion in the examined area of the lung in patients with suspected HF.9,10 This published information has also led to integrating these assumptions into automated B-line analysis protocols that are being incorporated into ultrasound software.11,12
Anecdotal clinical experience suggests that A-lines can be detected in patients with pulmonary congestion. Therefore, we sought to investigate the prevalence of co-existing A-lines and B-lines in patients with acute heart failure (AHF) and their respective dynamic changes with treatment for HF. Secondary goals were to examine clinical correlates of A-lines and, in exploratory analyses, their relationship to post-discharge outcomes.
Methods
Patient population
We analysed data from a prospective, single-centre, observational study that enrolled 196 adult patients admitted to an academic hospital in the U.S. for acute heart failure (AHF). Detailed inclusion and exclusion criteria have been described previously.8 Patients who were admitted to an intensive care unit at the time of LUS1, or those with significant pulmonary disease (e.g. cancer, fibrosis, and pneumonia), liver failure, dialysis, or pregnancy were excluded. Clinical, demographic, and laboratory data were extracted from patients’ hospital records by trained investigators. The study complied with the Declaration of Helsinki, written informed consent was obtained from all study subjects, and the local Institutional Review Board (Partners IRB #2014P002618) approved the study.
Lung ultrasound imaging protocol and analysis
Lung ultrasound examination was performed at the time of transthoracic echocardiography in the early phase of the hospitalization (LUS1) and before discharge (LUS2). Patients were examined in a semi-recumbent position using a phased array transducer at an imaging depth of 18 cm in sagittal orientation (perpendicular to the ribs). The chest was divided into eight zones, four on each haemithorax, and 6-s clips were recorded and analysed offline, as previously described.4,8,13
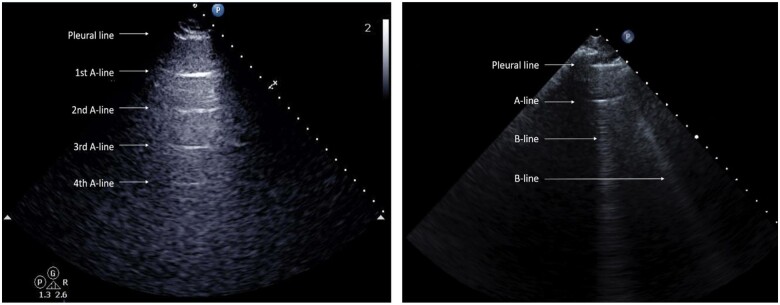
We defined A-lines as horizontal reverberation artefacts seen in a single intercostal space. Based on the characteristics of reverberation artefacts, which are in equal distance from the reflector, the transducer's distance to the pleural line was used to define the distance for the first and subsequent A-lines (Figure 1).14,15 The maximum number of A-lines was counted and summed for each intercostal space across eight zones. We did not count horizontal lines appearing outside the pre-specified distance based on the above definition (n = 10 zones). Zones were considered missing with respect to A-lines when there was one or more missing zone per examination, for instance, due to the presence of a pleural effusion. A single investigator (OJ) performed all A-line measurements (OJ), and a second investigator (EP) assessed inter-rater agreement. Intra-observer and Inter-observer variability were determined by Bland–Altman analyses16 of A-lines measurements in 20 randomly selected patients with an interval of at least 4 weeks between repeated measurements.
Figure 1.
A-line measurement on a lung ultrasound image. The skin to pleural distance defines the distance for the A-line artefact. (Left panel) Lung ultrasound image of an intercostal space showing the pleural line and four subsequent A-lines. (Right panel) Lung ultrasound image showing the pleural line, one A-line, and two B-lines.
B-lines were defined as vertical hyperechoic artefacts that arise from the pleural line, extending towards the edge of the screen and move synchronously with respiration.4 The highest number of B-lines in a single intercostal space was counted and then summed across all eight zones. A- and B-lines were quantified independently by different investigators and blinded to clinical data, the LUS visit (temporal blinding), and outcomes.
In addition, we obtained chest wall measurements from the LUS1 images. The mean chest wall thickness for each LUS zone was calculated from the shortest and longest distance in centimetre from the transducer to the pleural line by a single investigator (V.S.) as previously described.17
Echocardiography
All patients underwent comprehensive transthoracic echocardiography examinations simultaneously with the LUS, with 2–5 MHz phased array transducers and standard ultrasound equipment, as previously described.8 Transthoracic echocardiography cine loops were analysed offline by trained investigators using echocardiographic software (Syngo Dynamics; Siemens, Malvern, PA, USA).
Outcome measures
We used a composite outcome of HF hospitalization and all-cause mortality from discharge to 90 days as the primary endpoint in time-to-first event analyses. Outcome data were collected through follow-up phone calls, contact with patients’ primary care physician or cardiologist, and electronic medical records review.8
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD) or median with interquartile range [IQR]; categorical data as counts and percentages. Due to the lack of established cut-off values in the literature for A-lines and a skewed distribution with high variance, we analysed baseline and outcome data based on tertiles of total A-line number in eight zones (patient-level analysis). For LUS 1: Tertile 1 (0–4 A-lines), Tertile 2 (5–8 A-lines) and Tertile 3 (>8 A-lines), and for LUS2: Tertile 1 (0–4 A-lines), Tertile 2 (5–7 A-lines) and Tertile 3 (>7 A-lines). Similarly, the B-line tertiles for LUS2 were as following: Tertile 1 (0–5 B-lines), Tertile 2 (6–11 B-lines), and Tertile 3 (≥12 B-lines). We assessed trends across the A-line tertiles with the Cuzick nonparametric trend test18, and we assessed the dynamic changes in A-line and B-line artefacts from admission to discharge with the Wilcoxon signed-rank test. NT-proBNP and creatinine were transformed prior to inclusion in models. Given the close association between A-line number and chest wall width, a sensitivity analysis was performed with an A-line score; the number of zones with any A-lines present were counted and summed. Please refer to the Supplementary material online for details regarding missing data and imputations as well as intra- and inter-rater agreement for A-line quantification. In addition to the patient-level analyses, we also report the prevalence of A-lines, B-lines and neither artefact for individual zones for LUS1 and LUS2 in Supplementary material online, Table S4.
Spearman rho was used to examine the relationship between the sum of A-lines in eight zones and other clinical variables. Given the distribution of A-lines we used unadjusted and adjusted negative binomial regression models to explore determinants of A-lines as a count variable. We reported rate ratios (Ratio) with 95% confidence intervals (95% CIs) to represent the clinical variables' impact on the sum of A-lines. Models were adjusted for potential confounding variables including age, sex, systolic blood pressure, prior HF, chest wall width, New York Heart Association (NYHA) class, log-transformed NT-proBNP, and sum of B-lines in eight zones.
We used unadjusted and adjusted Cox proportional hazard models to explore A-line and B-line tertiles' relationship to post-discharge outcomes. Covariates considered for multivariable models included known predictors of HF events following an AHF hospitalization: age, sex, NYHA class, left ventricular ejection fraction (LVEF), creatinine, and systolic blood pressure.19,20 These analyses were performed in imputed (n = 118) and not imputed datasets (n = 110).
A two-sided P-value of < 0.05 was considered statistically significant for all tests. Stata SE version 14.2 (Statacorp., College Station, TX, USA 2015) was used for all analyses.
Results
Study population
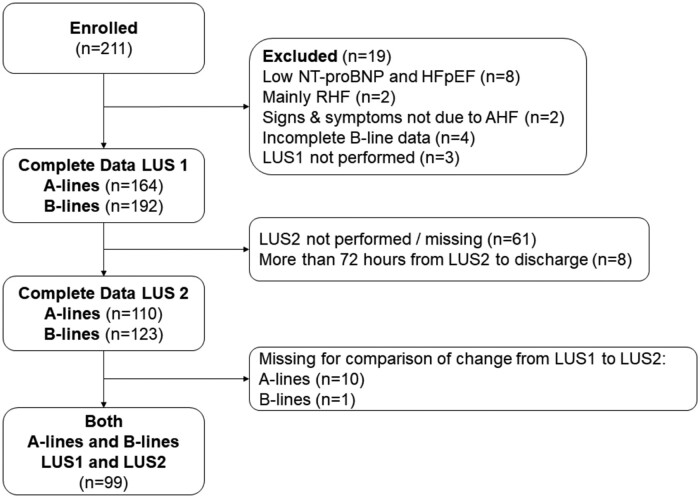
A total of 192 patients enrolled in the study had LUS performed at baseline (LUS1) with interpretable images for B-lines and 123 patients had a LUS2 performed within 3 days before discharge (Figure 2). Only patients with complete LUS data with regards to A-lines were included in the descriptive analysis. The median age of 164 patients was 72 years (range 21–102), 60% were men and the mean LVEF was 40 ± 14%.
Figure 2.
Flow chart. AHF, acute heart failure; HFpEF, heart failure with preserved ejection fraction; LUS, lung ultrasound; LUS1, lung ultrasound at baseline; LUS2, lung ultrasound before discharge; NT-proBNP, N-terminal pro B-type natriuretic peptide; RHF, right heart failure.
Table 1 demonstrates the baseline characteristics of the study population, by number of A-lines at baseline. Patients with the lowest number of A-lines (tertile 1) were more likely to be younger, have a higher BMI and greater chest wall width, higher systolic blood pressure, and were more likely in NYHA Class III to IV. They displayed a higher incidence of prior HF, history of sleep apnoea and were more likely to use insulin at baseline. There were no significant differences in LVEF or B-lines across A-line tertiles.
Table 1.
Clinical characteristics of the study population stratified by number of A-lines at baseline (n = 164)
| Tertile 1: 0–4 A-lines (n = 59) | Tertile 2: 5–8 A-lines (n = 65) | Tertile 3: >8 A-lines (n = 40) | P trend * | |
|---|---|---|---|---|
| A-lines (n) | 3 [1–4] | 6 [5–7] | 11 [10–12] | – |
| Clinical characteristics | ||||
| Age (years) | 71 [54–78] | 72 [63–81] | 72 [64–83] | 0.045 |
| Male | 35 (59) | 40 (62) | 24 (60) | 0.92 |
| White race | 46 (78) | 49 (75) | 31 (78) | 0.92 |
| BMI (kg/m2) | 33.1 [27.8–39.5] | 27.1 [24.3–32.6] | 23.6 [21.7–26.5] | <0.001 |
| Chest wall width (cm)a | 4.0 ± 1.2 | 3.0 ± 0.8 | 2.4 ± 0.5 | <0.001 |
| Heart rate (beats/min) | 81 ± 17 | 78 ± 16 | 76 ± 14 | 0.14 |
| Systolic BP (mmHg) | 126 ± 20 | 121 ± 20 | 118 ± 22 | 0.05 |
| Diastolic BP (mmHg) | 67 ± 11 | 67 ± 11 | 68 ± 11 | 0.65 |
| NYHA Class III—IV | 47 (80) | 50 (77) | 23 (57) | 0.021 |
| Past medical history | ||||
| Heart failure | 51 (86) | 50 (77) | 26 (65) | 0.013 |
| Diabetes | 28 (47) | 28 (43) | 11 (28) | 0.06 |
| Hypertension | 47 (80) | 55 (85) | 32 (8) | 0.89 |
| Myocardial infarction | 15 (25) | 23 (35) | 14 (35) | 0.27 |
| PCI/CABG | 23 (39) | 31 (48) | 9 (23) | 0.16 |
| Atrial fibrillation/flutter | 20 (34) | 13 (20) | 11 (28) | 0.41 |
| Pacemaker | 10 (17) | 12 (18) | 11 (28) | 0.22 |
| CRT | 6 (10) | 6 (9) | 5 (13) | 0.51 |
| OSA | 16 (27) | 8 (12) | 5 (13) | 0.042 |
| COPD/asthma | 21 (36) | 14 (22) | 8 (20) | 0.07 |
| Pre-admission medications | ||||
| ACEi, ARB, or ARNI | 29 (49) | 25 (38) | 21 (52) | 0.89 |
| Beta-blockers | 52 (88) | 49 (75) | 30 (75) | 0.85 |
| MRAs | 15 (25) | 9 (14) | 7 (19) | 0.25 |
| Calcium channel blocker | 15 (25) | 10 (15) | 9 (23) | 0.61 |
| Insulin | 20 (36) | 16 (36) | 3 (15) | 0.018 |
| Diuretics | 38 (64) | 46 (71) | 25 (63) | 0.94 |
| Digoxin | 5 (8) | 3 (5) | 7 (18) | 0.19 |
| Warfarin/NOAC/other anti-coagulant | 24 (41) | 30 (46) | 23 (57) | 0.11 |
| Laboratory values | ||||
| NT-proBNP (pg/mL)a | 3476 [1835–7010] | 6192 [2743–11 206] | 6492 [2712–12 782] | 0.007 |
| Sodium (mmol/L) | 139 [137–141] | 138 [135–141] | 140 [136–141] | 0.39 |
| Blood urea nitrogen (mg/dL) | 24 [17–50] | 26 [19–54] | 28 [21–42] | 0.39 |
| Creatinine (mg/dL) | 1.28 [1.04–2.36] | 1.42 [1.06–2.26] | 1.34 [1.13–1.88] | 0.85 |
| Haemoglobin (g/dL) | 11.6 ± 2.1 | 11.2 ± 2.3 | 11.8 ± 2.3 | 0.80 |
| Ultrasound measures | ||||
| B-lines (n) | 10 [5–18] | 12 [5–18] | 12 [7–16] | 0.50 |
| LVEF (%) | 41 ± 14 | 39 ± 13 | 39 ± 16 | 0.42 |
The P-value expresses the trend across the tertiles calculated by Cuzick test.18
Chest wall width (n = 157), NT-proBNP (n = 153).
ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; LUS, lung ultrasound; LVEF, left ventricular ejection fraction; MRA, mineral corticoid receptor antagonist; NOAC, non-vitamin K antagonist oral anticoagulants; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; OSA, obstructive sleep apnoea; PCI, percutaneous coronary intervention.
Prevalence of A-lines and B-lines at baseline and pre-discharge
All patients displayed either A-lines or B-lines at baseline and discharge. The sum of A-lines in 8 zones ranged from 0 to 19 (median 6; IQR 4–8) and the sum of B-lines in 8 zones ranged from 0 to 36 (median 11; IQR 6–17). For LUS1, 156 patients (95%) had co-existing A-lines and B-lines. Five patients (3%) demonstrated A-lines without the presence of any B-lines and three patients (1%) displayed B-lines without any A-lines. Nineteen (12%) patients required intravenous inotropes, other than digoxin, underwent left ventricular assist device placement, were admitted to an intensive care unit or died during the hospitalization following LUS1 at baseline. These patients had a higher number of B-lines: 18 B-lines (IQR 16–21) vs. 10 B-lines (IQR 5–17); P < 0.001 on LUS1. In contrast, there was no significant difference in A-line number on LUS1: 5 A-lines (IQR 4–10) vs. 6 A-lines (IQR 4–8), P = 0.95.
Among 123 patients with complete images for B-lines and 110 patients with complete images for A-lines for LUS2, the total A-line count ranged from 0 to 17, (median 5: IQR: 3–8) while B-lines ranged from 0 to 29 (median 8, IQR 3–12). Ninety-eight (89%) had co-existing A-lines and B-lines, while nine (8%) had A-lines without any B-lines and four (4%) had B-lines without A-lines present.
Figure 3 demonstrates the dynamic changes of A-lines and B-lines for 99 (60%) patients with complete data for both LUS1 and LUS2. There was no significant change in the median number of A-lines (6 vs. 5, respectively, P = 0.82). In contrast, there was a decrease in the number of B-lines (11 vs. 8, respectively, P < 0.001) between baseline and pre-discharge.
Figure 3.
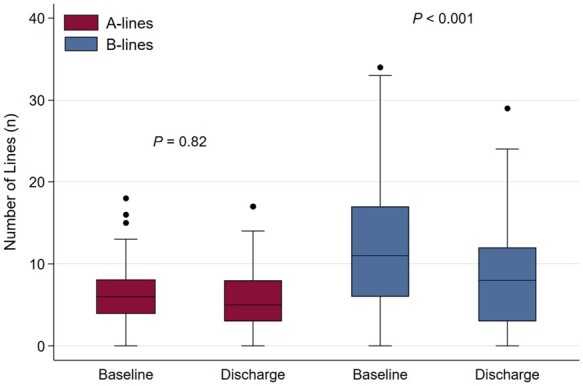
Box plot displaying dynamic changes in A-lines and B-lines artefacts from baseline to discharge (n = 99). Baseline = LUS1, Discharge = LUS2.
To explore possible determinants of A-lines, we used the sum of imputed A-lines as the dependent variable in univariate and multivariable regression (Supplementary material online, Table S1).
We found an inverse relationship between number of A-lines and BMI (Spearman’s rho = −0.57, ratio = 0.82, 95% CI 0.78–0.86, P < 0.001) and for chest wall width (Spearman’s rho = −0.66, ratio = 0.66, 95% CI: 0.61–0.71, P < 0.001). The Ratio represents an 18% decrease in A-lines per 5 unit increase in BMI and a 34% decrease in A-lines per centimetre increase in chest wall width. BMI was removed in the multivariable analysis due to high collinearity with chest wall width (Supplementary material online, Figure S1). The association between higher numbers of A-lines and NT-proBNP in the univariate analysis did not remain significant when we adjusted for chest wall width.
To test the hypothesis that B-lines erase A-lines, we assumed an inverse relationship between these two artefacts and that number of B-lines would predict the number of A-lines. We found a weak inverse relationship between A-lines and B-lines at baseline, which was only detected after adjusting for chest wall width (unadjusted Ratio = 1.00, 95% CI 0.97–1.04, adjusted Ratio = 0.96, 95% CI 0.93–0.99) (Supplementary material online, Table S1). In a sensitivity analysis with the outcome as the number of zones with A-lines present this inverse association between A-lines and B-lines was not statistically significant (Ratio = 0.99, 95% CI 0.95–1.01, P = 0.09).
A-lines and B-lines as predictors of longer-term outcomes
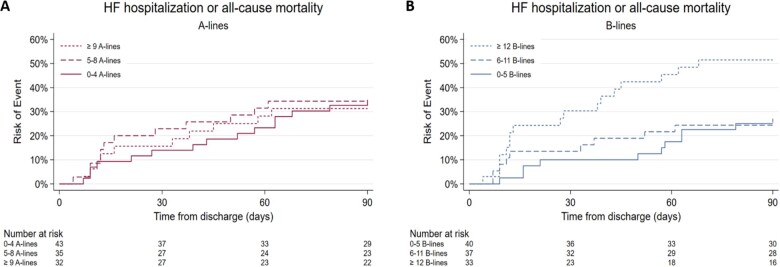
There were 37 events among 110 patients with available LUS2 data during the 90 days following hospital discharge (Tables 2 and 3). There was no significant association between higher A-line number in tertiles and HF hospitalization or death [unadjusted HR for trend across three tertiles was 0.97 (95% CI 0.65–1.43, P = 0.88)] (Figure 4B). The results from the adjusted models were similar. In contrast, there was a trend towards increased risk to experience an event in higher B-line tertiles (unadjusted HR 1.67, 95% CI 1.11–2.52, P = 0.014) (Figure 4A). The HR, after adjusting for sex, creatinine, systolic blood pressure, was 1.48 (95% CI 1.00–2.19, P = 0.05). Age, LVEF, and NYHA class were not statistically significant in multivariable analyses and were excluded from the final model to avoid overfitting. We reproduced these findings for A-line tertiles and B-line tertiles in the imputed dataset.
Table 2.
Outcomes by A-line tertiles from discharge to 90 days (n = 110)
| Tertile 1: ≤4A-lines (n = 43) | Tertile 2: 5–7 A-lines (n = 35) | Tertile 3: ≥8 A-lines (n = 32) | P (trend) | |
|---|---|---|---|---|
| Primary composite outcome | 15 (14) | 12 (11) | 10 (9) | — |
| All-cause mortality (%) | 9 (24) | 4 (11) | 5 (14) | — |
| HF hospitalization (%) | 10 (27) | 11 (30) | 7 (19) | — |
| Unadjusted HR (95% CI) | (Reference) | 1.06 (0.49–2.26) | 0.93 (0.42–2.07) | 0.88 |
| Model 1: Adjusted HR (95% CI) | (Reference) | 1.15 (0.53–2.48) | 0.70 (0.30–1.61) | 0.43 |
Model 1: Adjusted for statistically significant variables; sex, baseline systolic blood pressure baseline log creatinine, and sum of B-lines in eight zones.
Harrell’s C-statistic: Unadjusted: 0.52, Model 1: 0.76.
Table 3.
Outcomes by B-line tertiles from discharge to 90 days (n = 110)
| Tertile 1: 0–5 B-lines (n = 40) | Tertile 2: 6–11 B-lines (n = 37) | Tertile 3: ≥ 12 B-lines (n = 33) | P (trend) | |
|---|---|---|---|---|
| Primary composite outcome (%) | 10 (9) | 10 (9) | 17(15) | — |
| All-cause mortality (%) | 3 (8) | 2 (5) | 13 (35) | — |
| 90 day HF hospitalization (%) | 9 (24) | 9 (24) | 10 (27) | — |
| Unadjusted HR (95% CI) | (Reference) | 1.16 (0.48–2.78) | 2.64 (1.21–5.78) | 0.014 |
| Model 1: Adjusted HR (95% CI) | (Reference) | 1.98 (0.77–5.10) | 2.25 (1.00–5.09) | 0.051 |
Model 1: Adjusted for statistically significant variables: sex, baseline systolic blood pressure, and baseline log creatinine.
Harrell’s C-statistic: Unadjusted: 0.62, Model 1: 0.75.
Figure 4.
(A) Cumulative incidence of 90-day events by A-line tertiles (n = 110). (B) Cumulative incidence of 90-day events by B-line tertiles (n = 110).
Discussion
To our knowledge, this is the first study to comprehensively assess the prevalence of A-lines and B-lines in patients hospitalized for acute heart failure (AHF). We found that most patients had detectable A-lines at baseline and discharge and that there were no significant dynamic changes of A-lines number between the two timepoints. In contrast, the number of B-lines was significantly reduced from baseline to discharge. Furthermore, the number of A-lines did not provide prognostic information regarding 90-day HF readmission or death, as opposed to B-lines. These findings have several clinical, technological, and research-related implications.
Literature on the prevalence of A-lines and B-lines in healthy volunteers is sparse. Zoneff et al.21 investigated B-line prevalence in 200 individuals without symptoms or prior pulmonary disease. In this cohort, B-lines were rare findings in individuals without respiratory symptoms. Only 12.5% had B-lines, with a 20% prevalence in the younger and 5% in the older (>50 years) group.21 These findings are consistent with prior research in patients with known or suspected HF identifying B-lines as markers of pulmonary congestion providing diagnostic information in patients presenting to the Emergency Department with undifferentiated dyspnoea.6,22 These LUS findings change dynamically in hospitalized patients with AHF who receive HF treatment and mark patients at increased risk for subsequent adverse outcomes after hospital discharge.8
Research regarding the prevalence of A-lines is sparse.4 In a study comparing LUS patterns in 150 healthy subjects without a history of smoking, cardiac, or pulmonary disease, younger subjects demonstrated significantly more A-lines than the elderly (>65 years). They found that 96% of the younger subjects demonstrated A-lines in every examined lung region vs. 6% of the elderly. Subsequently, a higher number of A-lines were considered as a sign of normal lungs.23 In contrast, we found no association between A-lines and age after adjusting for baseline characteristics.
Despite this lack of evidence supporting A-lines as a normal finding on LUS, these artefacts are nevertheless incorporated into diagnostic algorithms for patients with dyspnoea.9,10 For example, the Bedside Lung Ultrasound in Emergency (BLUE)—protocol,10 uses A-lines and B-lines to assign clinical profiles based on these LUS findings. According to the BLUE-protocol, patients are assigned to the ‘A-profile’ when bilateral anterior A-lines are the predominant artefacts. The ‘A-profile’ is not associated with pulmonary congestion in AHF.9 The BLUE-protocol was derived from patients in an intensive care unit and is used across different clinical settings to assess patients with dyspnoea. A recent study challenged the BLUE-protocol’s diagnostic utility in dyspnoeic Emergency Department patients and found that strict implementation of the BLUE-protocol led to a high number of false-positive results for asthma and COPD.5 A potential explanation for this finding may be an over-reliance on A-lines to rule out pulmonary congestion.
Finally, A-lines lacked dynamic features compared to B-lines from baseline to hospital discharge in our AHF cohort. If A-lines and B-lines were truly mutually exclusive artefacts, we would expect to observe an increase in A-lines relative to the B-line number in the context of decongestion. This lack of reciprocity suggests that A-lines are a less dynamic feature of LUS than B-lines and may not be as closely associated with the absence of pulmonary congestion as previously thought.
Body mass index and chest wall width were the strongest predictors of A-line number in univariate analysis; one unit increase in chest wall width was associated with a 36% decrease in A-lines. Since A-lines are horizontal imaging artefacts, their presence depends on the distance between the pleural line and the screens’ edge if the imaging depth is standardized. In the sensitivity analysis with A-lines as a score from one to eight based on a binary finding of A-lines in the lung zone, a strong association between chest wall width and A-line score was sustained. Moreover, in our cohort, A-lines did not provide any prognostic information regarding 90-day outcomes when assessed before hospital discharge. Further research in larger cohorts is warranted to determine whether A-lines provide diagnostic or prognostic utility in patients with known or suspected HF.
Patients in the lowest A-line group (Tertile 1) had the highest BMI and accordingly lower NT-proBNP levels but higher prevalence of prior HF and were in higher NYHA classes. Several studies have demonstrated an inverse association between NT-proBNP and BMI in the general population, chronic HF, and AHF.24–26 While we cannot exclude the possibility that obesity may impact the visibility of A-lines on LUS, our analyses suggest that B-line rather than A-line number may represent a graded measure of pulmonary congestion in HF patients.
Although LUS is relatively easy to learn, the acquisition and interpretation of LUS images depend on the sonographer’s knowledge and skills.27 Consequently, there are ongoing efforts to automatize LUS image interpretation through machine learning.11 The results of this current study should be considered when applying machine learning to develop automated LUS algorithms to evaluate patients with dyspnoea or HF with respect to A-lines and B-lines. Our data would suggest that these algorithms focus on detecting and quantifying B-lines irrespective of A-lines' presence and account for the patient's chest wall width.
Limitations
This was a small, though well-characterized sample enrolled at a single centre, which may affect generalizability. Future studies could address this limitation by assessing the presence of A-lines and B-lines in larger cohorts and compare the prevalence of these LUS findings with healthy controls. Pre-discharge NT-proBNP results were only available in a minority of patients. Given the limited sample size of our study cohort and limited number of events in patients with available pre-discharge LUS exams, the relationship between A-lines and post-discharge outcomes should be confirmed in larger cohorts.
Conclusions
A-lines and B-lines commonly co-exist among patients hospitalized for AHF. The A-line number decreased with higher BMI and chest wall thickness and was not related to the number of B-lines. These findings suggest that A-lines and B-lines are not mutually exclusive artefacts, and A-line presence does not rule out pulmonary congestion. In contrast to B-lines, A-lines were not associated with adverse outcomes.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care.
Data availability
The data underlying this article cannot be shared due to privacy regulations from the institutional review board.
Funding
The National Heart, Lung and Blood Institute (K23HL123533 to E.P.). The writing of this manuscript was supported by a grant from the Norwegian Lung Association (O.J.). The sponsors had no input or contribution in the development of the research or manuscript.
Conflict of interest: Ø.J. has received a grant from the Norwegian Lung Association, The Norwegian society for ultrasound in general practice and a grant from Astra-Zeneca via the Norwegian Lung Association. B.C. has consulted for Amgen, Myocardia and Novartis. E.P.'s employer has received support from Novartis for consulting work and she has consulted for scPharmaceuticals outside of the submitted work. She has received research support from the NIH outside of the submitted work. J.D.G. has received research support from Amgen. S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and has consulted for Abbott, Action Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer-Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent. All other authors report no conflict of interest.
Supplementary Material
References
- 1. Lancellotti P, Price S, Edvardsen T, et al. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2015;4:3–5. [DOI] [PubMed] [Google Scholar]
- 2. Miller A. Practical approach to lung ultrasound. BJA Educ 2016;16:39–45. [Google Scholar]
- 3. Lichtenstein D, Van Hooland S, Elbers P, Malbrain MLNG.. Ten good reasons to practice ultrasound in critical care. Anaesthesiol Intensive Ther 2014;46:323–335. [DOI] [PubMed] [Google Scholar]
- 4. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577–591. [DOI] [PubMed] [Google Scholar]
- 5. Bekgoz B, Kilicaslan I, Bildik F, et al. BLUE protocol ultrasonography in Emergency Department patients presenting with acute dyspnea. Am J Emerg Med 2019;37:2020–2027. [DOI] [PubMed] [Google Scholar]
- 6. Pivetta E, Goffi A, Nazerian P, Castagno D, Tozzetti C, Tizzani P, Tizzani M, Porrino G, Ferreri E, Busso V, Morello F, Paglieri C, Masoero M, Cassine E, Bovaro F, Grifoni S, Maule MM, Lupia E, Paolo B, Alessia B, Giuseppina B, Andrea C, Andrea C, Ottavio D, Paola DR, Andrea E, Paolo FP, Patrizia F, Daniela F, Francesca G, Grazia GM, Sara G, Alda L, Davide L, Franca M, Franco M, Corrado M, Giulia N, Luca P, Sonia P, Paolo Q, Claudia S, Elisa S, Elisabetta S, Flavia S, Fabio S, Michela S, Francesca S, Cristina T, Grazia VM, Marco A, Laura B, Sofia B, Ernesta B, Barbara C, Matteo C, Melisenda C, Beatrice D, Grazia F, Roberto F, Giuseppe G, Chiara G, Simona G, Eriola H, Juri M, Federico M, Andrea N, Maddalena O, Andrea P, Giuseppe P, Stefano P, Michele R, Francesca S, Francesco TP, Federica T, Vanni S, Gabriele V; on behalf of the Study Group on Lung Ultrasound from the Molinette and Careggi Hospitals. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial. Eur J Heart Fail 2019;21:754–766. [DOI] [PubMed] [Google Scholar]
- 7. Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD.. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 2016;37:1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, Lee MMY, Merz AA, Silverman M, Swamy V, Lindner M, Rivero J, Solomon SD, McMurray JJV.. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail 2019;7:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015;147:1659–1670. [DOI] [PubMed] [Google Scholar]
- 10. Lichtenstein DA, Meziere GA.. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008;134:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anantrasirichai N, Hayes W, Allinovi M, et al. Line detection as an inverse problem: application to lung ultrasound imaging. IEEE Trans Med Imaging 2017;36:2045–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brusasco C, Santori G, Bruzzo E, et al. Quantitative lung ultrasonography: a putative new algorithm for automatic detection and quantification of B-lines. Crit Care 2019;23:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Platz E, Jhund PS, Girerd N, et al. Expert consensus document: Reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur J Heart Fail 2019;21:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Picano E, Scali MC, Ciampi Q, Lichtenstein D.. Lung ultrasound for the cardiologist. JACC Cardiovasc Imaging 2018;11:1692–1705. [DOI] [PubMed] [Google Scholar]
- 15. Lichtenstein D. Novel approaches to ultrasonography of the lung and pleural space: where are we now? Breathe (Sheff) 2017;13:100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin Bland J, Altman D.. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. The Lancet 1986;327:307–310. DOI: 10.1016/s0140-6736(86)90837-8. [PubMed] [Google Scholar]
- 17. Brainin P, Claggett B, Lewis EF, Dwyer KH, Merz AA, Silverman MB, Swamy V, Biering‐Sørensen T, Rivero J, Cheng S, McMurray JJV, Solomon SD, Platz E.. Body mass index and B-lines on lung ultrasonography in chronic and acute heart failure. ESC Heart Fail 2020;7:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985;4:87–90. [DOI] [PubMed] [Google Scholar]
- 19. Cleland JG, Chiswell K, Teerlink JR, et al. Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: a report from the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Study. Circ Heart Fail 2014;7:76–87. [DOI] [PubMed] [Google Scholar]
- 20. Felker GM, Hasselblad V, Tang WH, et al. Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail 2012;14:1257–1264. [DOI] [PubMed] [Google Scholar]
- 21. Zoneff ER, Baker K, Sweeny A, Keijzers G, Sanderson J, Watkins S.. The prevalence of lung surface abnormalities in a healthy population as detected by a screening lung ultrasound protocol: comparison between young and older volunteers. Australas J Ultrasound Med 2019;22:129–137. DOI: 10.1002/ajum.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pivetta E, Goffi A, Lupia E, et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multicenter study. Chest 2015;148:202–210. 2015/02/06. DOI: 10.1378/chest.14-2608. [DOI] [PubMed] [Google Scholar]
- 23. Chiesa AM, Ciccarese F, Gardelli G, Regina UM, Feletti F, Bacchi Reggiani ML, Zompatori M.. Sonography of the normal lung: comparison between young and elderly subjects. J Clin Ultrasound 2015;43:230–234. [DOI] [PubMed] [Google Scholar]
- 24. Daniels LB, Clopton P, Bhalla V, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AHB, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA, Maisel AS.. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am Heart J 2006;151:999–1005. [DOI] [PubMed] [Google Scholar]
- 25. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PWF, Vasan RS.. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594–600. [DOI] [PubMed] [Google Scholar]
- 26. Frankenstein L, Remppis A, Nelles M, et al. Relation of N-terminal pro-brain natriuretic peptide levels and their prognostic power in chronic stable heart failure to obesity status. Eur Heart J 2008;29:2634–2640. [DOI] [PubMed] [Google Scholar]
- 27. Pivetta E, Baldassa F, Masellis S, et al. Sources of variability in the detection of B-lines, using lung ultrasound. Ultrasound Med Biol 2018;44:1212–1216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared due to privacy regulations from the institutional review board.