Ocean acidification and increased ocean heat content has direct and indirect effects on marine organisms such as holothurians (sea cucumbers) that are vulnerable to changes in pH and temperature. These environmental factors have the potential to influence organismal performance and fitness at different life stages. Tropical and temperate holothurians are more vulnerable to temperature and pH than those from colder water environments. The high level of environmental variation observed in the oceans could influence organismal responses and even produce a wide spectrum of compensatory physiological mechanisms. It is possible that in these areas, larval survival will decline by up to 50% in response to a reduction of 0.5 pH units. Such reduction in pH may trigger low intrinsic growth rates and affect the sustainability of the resource. Here we describe the individual and combined effects that temperature and pH could produce in these organisms. We also describe how these effects can scale from individuals to the population level by using age-structured spatial models in which depensation can be integrated. The approach shows how physiology can improve the conservation of the resource based on the restriction of growth model parameters and by including a density threshold, below which the fitness of the population, specifically intrinsic growth rate, decreases.
Keywords: temperature, sea cucumber, population, physiology, pH, Allee effect
Introduction
Marine organisms as we know them today are the result of a natural selection process that has existed during different evolutionary periods (Reich, 2010). Unlike the modifications that occurred over thousands of years, the responses observed in the past century and that continue to occur as the temperature increases and the pH of the oceans decreases are taking place in a relatively short period of time (IPCC, 2011; Murray et al., 2013; Widicombe and Spicer, 2008). Thus, there is a need to better understand the physiological adaptations that could allow organisms to adjust to these conditions and how these would, in turn, affect individual survival and the population.
Sea cucumbers are found in virtually all oceans, from the Arctic to the tropics. Purcell et al., (2016) mentioned that there are more than 70 species commercially exploited in the world, and the compilation of field records by Shiell (2004) shows the presence of 28 species, distributed in 8 Fishing areas of the Food and Agriculture Organization (FAO). In the Eastern Center Pacific, 10 commercially important species have been reported (Astichopus mauritiana, Astichopus echinites, Holothuria leucospilota, Holothuria scabra, Holothuria fucogilva, Holothuria nobilis, Holothuria atra, Stichopus hermanni, Stichopus chloronotus and Thelenota ananas); in the Eastern Central Atlantic, 4 commercially important species have been documented (Astichopus multifidus, Actinopyga agassizzi, Holothuria mexicana and Isostochopus badionotus); in the Central Pacific East, there are records for 2 species (H. atra and Isostichopus fuscus); and in the Eastern areas, 2 species have also been reported (H. scabra and S. chloronotus). These species represent examples of sea cucumbers from tropical waters. The temperate water sea cucumbers are located in the Southwest and Northwest Pacific, in fishing areas where the presence of H. scabra and Apostichopus japonicus have been documented. The cold-water species occur in the Northwestern Atlantic, in areas where the presence of Chiridota laevis, Cucumaria frondosa and Psolus fabricii have been reported (Shiell, 2004). Species of temperate waters such as H. scabra and A. japonicus are distributed in shallow areas, where temperature and salinity can oscillate rapidly between 20°C and 30°C and fall to 20 USP in rainy season (Hu and Li, 2010). Yang et al. (2005) and Yuan et al., (2009) reported having collected juveniles of A. japonicus during the winter season at temperatures of 5°C, while An et al., (2009), Dong et al., (2006) and Dong and Dong (2006) reported to have found them at 15°C. Tropical species such as I. badionotus and H. glaberrima (Pérez-Vega et al., 2013 and Quiñones et al., 2002, respectively) have temperature ranges that fluctuate between 22°C and 24°C. The cold water species (C. frondosa), which is located in areas where the fluctuations of temperature are more stable, have been reported at between 3 and 300 m deep (Kale et al., 2013). The wide range of thermal tolerance that some temperate (A. japonicus and H. scabra) and tropical (I. badionotus, H. mexicana, I. fuscus) sea cucumbers species present, contrasts with the tolerance of cold-water organisms (C. frondosa). Without distinction of its distribution, sea cucumbers, especially those of tropical and temperate areas, have adopted strategies that lead to the development of hypometabolism and involuntary non-pathological response (Gullian, 2013; Gullian & Terrats, 2017; Storey, 2015). Hypometabolism occurs by reduction of the organisms’ aerobic scope, which in turn determines physiological responses that generate dormancy and aestivation (Asha & Muthiah, 2005; Morgan, 2008; Yuan et al., 2009; Quiñones et al., 2002). Involuntary non-pathological responses, such as skin ulceration and evisceration, occur as extreme control mechanisms that seek to reset organismal physiological conditions at the expense of anatomical modifications (Garcia-Arraras and Greenberg, 2001; Quiñones et al., 2002; Zamora & Jeffs, 2012).
For the specific case of temperate and tropical sea cucumbers, which is the focus of this work, published studies indicate that these organisms are highly vulnerable to decreases in pH and increases in temperature, especially during their larval stages (Brander, 2010; Morgan, 2008; Yuan et al., 2015). The pH and the water temperature generate diverse biochemical and physiological adaptations in these organisms (Gullian, 2013; Gullian & Terrats, 2017; Wu et al., 2013; Zamora & Jeffs, 2012) that can change the population abundance and density (Brierley & Kingsford, 2009; Doney et al., 2009; Widicombe and Spicer, 2008). Some of these effects are considered to be direct, while others include interactions at the population level (Brierley & Kingsford, 2009; Doney et al., 2009). Reduction of density in these organisms is critical, as they are gonochoric sedentary species. Their reproductive success depends largely on their gregarious behaviour, spawning synchrony and their chemical communication (Fujiwara et al., 2010; Hamel & Mercier, 1996, 1999; Zacarías-Soto et al., 2013). A reduction in density can affect fertilization and thus produce changes in the population intrinsic growth rate (González-Durán et al., 2018; Hutchings, 2014; Kuparinen & Hutchings, 2014). From this perspective, we explore the responses that tropical and temperate sea cucumbers exhibit throughout their life cycle when exposed to variation in water temperature and pH. Our aim is to identify the ways in which changes in pH and temperature influence holothurians at various critical stages and generate hypotheses to explain these observations. In order to do that, we describe the sublethal effects of pH and temperature on holothurian fitness and how this is reflected at the population level. We conclude by suggesting how these threats can be incorporated into population models. The paper ultimately seeks to demonstrate the importance of considering links between environmental conditions and organismal physiology to inform the management of holothurians.
Responses throughout the life cycle
Temperate and tropical sea cucumbers undergo a series of anatomical modifications that allow them to move from planktonic to benthic lifestyles, to defend themselves against predators and to survive adverse environmental conditions (Hamel & Mercier, 1996). Despite the clear anatomical differences among these stages, the occurrence of evisceration and autotomy in both adults and juveniles, reinforces the hypothesis that the mechanisms involved in temperature perception are similar to those observed in larval stages. In juveniles and adults, the detachment of the sensory fibres of collagen from the body walls (Ferguson, 1982, García-Arrarás and Greenberg, 2001, Wu et al., 2013) suggests that coelomocytes play a greater role in perception. The mechanism is similar in larvae, where all sensitivity depends on coelomocytes (Ferguson, 1982). A plausible mechanism that explains how coelomocytes might respond to temperature could involve the adjustment of their affinity to Ca2+, which activates transient receptor proteins, an important kind of membrane protein that constitute a primary mechanism for detecting heat (Crockett and Landroville, 2006; Takahashi et al., 2011). Even when it is very possible that this mechanism could be present throughout the entire life cycle, some differences related to the ontogeny of the stages need to be considered to understand the direct and indirect effects that temperature and pH generates in these organisms.
Larvae
Some species of sea cucumbers have an indirect development, which means that there are multiple stages of larvae before settlement. In larvae, the effects of temperature are direct and indirect; the former affects the metamorphosis and the development time, while the latter determine the abundance and quality of the food consumed by the organisms (Asha & Muthiah, 2005; Brander, 2010; Yuan et al., 2015). A greater displacement from the optimal value of temperature delays gastrulation, which is the stage in which the digestive tract develops and the feeding activity begins (Smiley, 1986). Greater displacement might limit energy use, resulting in higher physiological costs and slower development, which could increase predatory risk (Dorey et al., 2013). Larvae can delay their development for a few days depending on the availability of food; therefore, food-poor environments can exacerbate their phenotypic plasticity and negatively impact on their survival (Brander, 2010). When analysing the effects of food abundance on auricularia of Apostichopus mollis, Morgan (2008) found that food concentration was a major determinant in larvae development. The effect that different feeding rates (6000, 3000, 600 and 300 cells of Chaetoceros muelleri ml day−1) had on growth was evidenced by smaller larvae in extreme densities and by a better use of food in intermediate levels. These differences could be related to the development of the digestive tract and the size of the mouth, which were longer and bigger, respectively, for larvae that fed in intermediate densities (Morgan, 2008). The viability of the larvae became less evident in organisms that fed 600 and 300 cells ml day−1, suggesting that an early application of natural selection filters, in this case represented by food availability, encouraged a better development and magnified the direct environmental effects.
With respect to pH, several studies conducted with larvae indicate that it is a critical factor (Asha & Muthiah, 2005; Hamel & Mercier, 2008; Yuan et al., 2015). An experiment with Holuthuria spinifera, which maintained auricularia larvae for 12 days at pH values ranging from 6.5 to 9.0, showed that at pH 7.8 the larvae developed faster, grew better and had better survival rates. Additionally, of the total analysed variables (temperature, salinity and pH), larval development and survival were most sensitive to pH, given the small optimal range (Asha & Muthiah, 2005). The experiments showed that extreme alkaline values (pH 9.0) generated malformation and disintegration of individuals, while the reduction of 0.5 pH units from the optimum (pH 8.0) reduced survival rates by 49%. Recently, Yuan et al. (2015) determined the effects that different pH levels (from 7.42 to 8.04) exerted on the post-fertilization success and growth rate of the larval stages of A. japonicus. The success of fertilization decreased linearly with the reduction of pH, which according to Stumpp et al., (2011) could be the consequence of the following: (i) high energy cost associated with the regulation of the redox environment and (ii) lack of feeding activity during larval development. In any case, the consequences of pH on the survival of larvae were also related with direct and indirect effects.
Juvenile and adults
In post-settlement juveniles and adults, pH and temperature most commonly affect growth rates (Dong et al., 2006; Yuan et al., 2009), hypometabolic responses (Storey, 2015; Yuan et al., 2009), aestivation and dormancy (Gullian, 2013; Yang et al., 2006), evisceration (Garcia-Arraras and Greenberg, 2001) and detachment of the body wall (Zamora & Jeffs, 2012). Despite the range of responses, the net effect exerted by the alteration of pH and temperature on the survival of the juveniles is less than those observed in the larvae.
Juvenile sea cucumbers respond to suboptimal temperatures by altering their metabolic activity (Yuan et al., 2009). In A. japonicus, temperatures close to the optimum (15.5°C) produced better growth (Dong et al., 2006; Yuan et al., 2009), while higher or lower temperatures decreased food intake, increased metabolism and reduced growth (Zamora & Jeffs, 2012). The main problems associated with suboptimal temperatures are not only tolerance and metabolic energy, but also an increase of diffusion of oxygen and rise of oxygen demands for the maintenance of biological processes (Pörtner & Knust, 2007). Survival at low oxygen levels might be possible through accumulation of high concentrations of CO2 in tissues. This could be achieved by compensation from buffers obtained from the surrounding water, food and their spicules (Occhipinti & Boron, 2015; Pörtner et al., 2004). In sea cucumbers a mechanism could decrease the dissociation of Ca+2 from metalloproteins and reduce eviscerations, in a similar way for decapod crustaceans (Pörtner et al., 2004). This response constitutes a mechanism that stops unnecessary exposure to adverse circumstances (Quiñones et al., 2002), but it is not the only form in which sea cucumbers defend themselves against the exposure to adverse environmental conditions. In addition to evisceration, sea cucumbers can develop aestivation when exposed to hostile parameters. Yang et al. (2006) found aestivation of mature A. japonicus at 20°C, while immature organisms aestivated at 25°C. This difference suggests that temperature reduction causes hibernation and that the presence of endogenous reserves, such as that contained in the gonad, satisfies the energy demands to allow the change. The mechanisms that allow restoration after evisceration and aestivation have been investigated by diverse authors. Zhou et al. (2014) identified the factors responsible for the growth of the intestine of Stichopus japonicus and found the presence of metalloproteins with a maximum activity at pH 5.0 and 50°C. Quiñones et al. (2002) studied the interaction of the epithelial and connective tissues of the extracellular matrix during the process of regeneration of the digestive tract and associated the decrease of collagen fibres with the presence of metalloproteins. These studies concluded that the actions of metalloproteins are effective in reducing the formation of collagen in the intestine, which allows the extracellular matrix to re-establish and retain the mesenteries that support the digestive organs again (Quiñones et al., 2002; Zhou et al., 2014). The authors also indicated that the activity of the metalloproteins were related to the concentration of dissolved oxygen (Quiñones et al., 2002; Wu et al., 2013; Zhou et al., 2014), rather than temperature and pH.
To differentiate evisceration from aestivation, we need to consider that the first develops quickly and produces drastic anatomical changes, while the second develops over longer time intervals and does not produce radical anatomical alterations (Du et al., 2013; Gullian, 2013; Yang et al., 2006). To reverse aestivation, it is necessary to carry out a metabolic adjustment that involves antioxidant activity (Gullian, 2013; Ji et al., 2008).
Irrespective of the type of anatomical changes, most of the aforementioned modifications in adults and juveniles are consequence of direct impacts. This, however, does not mean that indirect effects cannot occur. Sun et al., (2013) investigated the seasonal changes of food supply in A. japonicus. They found that seasonal fluctuations in environmental conditions modified the feeding behaviour of organisms, presumably affecting their physical conditions.
Organized structural response
Temperature and pH produce molecular and physiological responses that generate stress and may influence survival. As seen during aestivation, these parameters increase the presence of reactive oxygen species (ROS) and produce an imbalance in the proportion of antioxidants, which causes oxidative stress (Handy et al., 2009; Shao et al., 2015). The ability to adjust the production of antioxidant enzymes in response to acute temperature fluctuations to avoid the increase of ROS occurs in all eukaryotic cells, and the cells of sea cucumbers are not exception (Davidson & Schiestl, 2001). In these organisms the response might be regulated through adjustment of electron transport in mitochondria (Handy et al., 2009), with production of superoxide anions (O2-•) as minor by-products (Davidson & Schiestl, 2001). As high concentrations of ROS modify protein structure, antioxidant enzyme activity regulates their concentration in mitochondria and cytosol (Candas & Li, 2014). Enzymes such as superoxide (SOD), glutathione peroxidase (GPx), catalases (CAT) and thioredoxins-peroxiredoxin (Trx-Prx) participate in the elimination of O2- by converting it into hydrogen peroxide (H2O2) and water (H2O) (Tomanek, 2015; Vives-Bauza et al., 2007). Some experiments performed with adult sea cucumber A. japonicus, showed that constant increase of temperature from 16°C to 20°C increased the initial SOD activity of the body wall tissues, from 45.2 to 126.5 and 128.2 U mg−1 of protein, respectively, while the increase of exposure time to 25°C for 72 h and 168 h did not produce significant changes in antioxidant activity (Shao et al., 2015).
On the other hand, environmental pH also affects the antioxidant activity of aquatic organisms, especially when this is accompanied by changes in temperature (Matozzo et al., 2013; Wang et al., 2009). When the pH is low, the increase in enzyme production could be a mechanism to cope with oxidative stress and prevent deterioration. For example, in the coelomic fluid of I. badionotus, GPx activity increased when the pH changed from 8.0 to 7.7 (Gullian & Terrats, 2017). Although the effects of temperature and pH are important in oxidative stress, few studies have addressed the interaction of these parameters. Matozzo et al. (2013) reported that gills and digestive gland of Chamelea gallina (clam) and Mytilus gallopronvincialis (mussel) at a temperature of 22°C displayed higher activity of SOD and GST in pH 7.7 than pH 8.1. Byrne et al. (2013) and Gullian and Terrats (2017) reported the physiological responses of two echinoderms (Heliocidaris tuberculata and Isostichopus badioniotus, respectively) to the combined effects of temperature and pH. From the limited publications, the evidence shows that at least in the short term, the pH rather than the temperature appears to be more important when antioxidant activity is considered.
As the level of organization increases, the combined effect of temperature and pH causes an increase in energy expenditure, which compromises aerobic metabolism and consequently lead to the development of peius thresholds (conditions even worse than those produced only by temperature) (Pörtner & Langenbuch, 2005). The evidence reported by different authors indicates that these thresholds are not always reached, for example in some marine invertebrates the development of hypercapnia stimulates their thermal tolerance (Byrne et al., 2013; Kroeker et al., 2014; Lanning et al., 2010; Madeira et al., 2014). It is possible that the mechanisms involved in increasing resistance to acidic conditions may be related to the activity of the transient receptor channels whose action increases the concentration of cytosolic Ca2+ according to the temperature. Thus, if Ca2+ increases due to temperature, it could be incorporated to compensate the pH balance, making the organism more resistant to hypercapnia as temperature increases. Of course, this is a hypothesis that needs to be tested.
Incorporation of molecular and physiological responses to pH and temperature to population dynamics
Escalation of molecular and physiological responses that are expected to occur in sea cucumbers as the temperature of the oceans increases and the pH decreases are depicted in Fig. 1. The figure illustrates the production of ROS by mitochondria and the antioxidant response that occurs to reduce the level of reactive species of oxygen and nitrogen (1) (Kalyanaraman, 2013; Quijano et al., 2016). The figure also shows the response of heat shock protein during acute temperature increase (2) (Li C et al., 2021) and the combined effects of co-occurring elevated temperature in the presence of certain contaminants (3) (Li X et al., 2021). As temperature influenced the retention rate of diverse items, such effects are also considered (4) (Iwalaye et al., 2021). The figure shows the influx of calcium ions, which is related to detecting temperatures (5) (Takahashi et al., 2011) and the hypercapnia effects that occur at low levels of pH (6) (Occhipinti & Boron, 2015). Overall, the combined effects modify the physiology and triggers involuntary pathological responses altering the anatomical characteristic of the organisms (Gullian, 2013). Such effects might be lethal in larvae and could impair growth rates in juveniles and adults, compromising reproductive success and reducing recruitment. Sub-lethal effects might include changes in the individual processes such as those described by Pörtner et al. (Pörtner et al., 2004; Pörtner & Langenbuch, 2005), which ultimately affect individual performance (growth, ventilation rate and functional capacity). In the same context, lethal effects could occur when oxidative stress excessively increases hypometabolism, evisceration or detachment of the body wall (7) (García-Arraras and Greenberg, 2001; Gullian & Terrats, 2017; Shao et al., 2015; Zamora & Jeffs, 2012).
Figure 1.
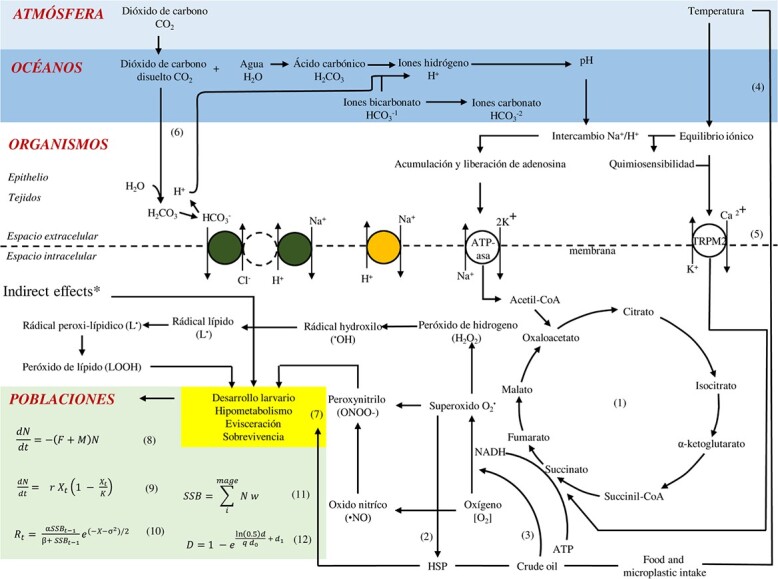
Projection of biochemical pathways and physiological responses into population. Adapted from Seijo et al. (1994), Pörtner et al. (2004), Pörtner & Langenbuch (2005), Dhaka et al. (2006), An and Seijo (2010), Takahashi et al. (2011), Kalyanaraman (2013), (Quijano et al. (2016) and González-Durán et al. (2018). *Although indirect effects constitute an important source of impacts, for the simplicity of the figure they are not considered at this time.
Given that some sea cucumber species are commercially exploited, it is necessary to include the impact of fishing mortality (F) in the analysis (An & Seijo, 2010). In this sense, Fig. 1 assumes that the population size depends on the number of individuals that are incorporated into the population in each period as a result of reproduction or, in other words, recruitment to the population (8–9) (An & Seijo, 2010). This biological process can be modelled with a stock-recruitment function (Beverton & Holt, 1957) (10) that includes two parameters and the spawning biomass. Because the model is asymptotic, the maximum number of recruits produced (a) is analogous to the population’s carrying capacity. Recruitment to the population depends basically on three factors: the amount of eggs spawned, the fertilization rate and the survival of larval stages (11). Furthermore, since the species have external fertilization, spawners’ density is incorporated as a depensatory function that occurs at low abundance (12) (González-Durán et al., 2018). From this perspective, it is clear that the increase in natural or fishing mortality produces a reduction in abundance, which eventually affects the spawning biomass and reduces the success of recruitment. It is also clear that the effects of temperature and pH are exacerbated when the pressure of the fishing industry is added.
The reduction of density, the main adverse impact that environment produced on sea cucumbers, have been known by several authors as a natural processes of population growth (Hutchings, 2014; Keith & Huchings, 2012; Lierman & Hilborn, 2001). In ecology, such response is known as the Allee effect, which defines a positive relationship between any component of individual fitness and population density (Hutchings, 2014; Stephens et al., 1999). Critical densities below which the collapse of the population is expected have been used to include depensatory mortality in the models of other species (Courchamp et al., 2008; Gascoigne & Lipcius, 2004). Commonly, the incorporation of the Allee effect has been achieved through the addition of exponents in the parameters of the recruitment function (Lierman & Hilborn, 2001); however, the disadvantage is that these exponents do not have a simple biological interpretation (Lierman & Hilborn, 1997). Recently, a model developed for holothurians showed how external factors such as fishing could reduce the spawning stock biomass down to levels, under which reproductive fitness is no longer possible (González-Durán et al., 2018). To include the Allee effect, the authors set the recruitment as a function of density and included an additional term to shift the origin (threshold) to the left; this point represents the moment when all recruitment ceased (12).
Until now, the inclusion of adverse environmental effects has been done considering probable scenarios of change using decision theory and adding stochasticity based on expected environmental trends. Seijo et al., (2016) determined the bioeconomic effect of ocean acidification in the fisheries of calcified species. These authors developed an age-structured dynamic model linked to decision tables with alternative decision criteria in the absence of probability of occurrence of three alternative pH states of nature reported by as climate change scenarios by the Intergovernmental Panel on Climate Change (IPCC). The authors built dynamic functions for natural mortality M (an increasing non-linear function of pH) and K of Von Bertalanffy growth equation (a decreasing non-linear function of pH), dependent on the possible dynamic trajectories of pH reported by IPCC scenarios. Parameter values for the above-mentioned functions used experimental results reported in the literature. Another analysis that has been used to include the effects of environmental variability on fisheries is the sensitivity analysis of reference points. Defeo and Seijo (1999) produced variation of the parameters using the bootstrap technique and subsequently carried out risk analysis according to the maxi-min, mini-max and maxi-max criteria. On the other hand, Hare et al., (2010) developed a coupled climate population model for Micropongonias undulatus based on the hypothesis that the recruitment of juveniles was affected by temperature. The authors considered the decadal variation of temperature to simulate the behaviour of 100 hypothetical populations, introducing stochasticity in the M and F parameters, as well as variability in recruitment. Punt et al., (2015) considered different levels of tolerance to ocean acidification and developed pre- and post-recruitment models for Chionoecetes bairdi. These authors used a stage-structured population model to forecast the change over time in recruitment to the first size class in the post-recruitment model; in this model, recruited male were modelled and their biomass was used as a proxy for fertilized egg production. The approach was similar to the vector tracking projections carried out by Seijo et al. (2016). Co et al. (2015) linked three models (biogeochemical, biological and socioeconomic) into an integrated assessment model that simulate oceanographic and population dynamics and the socioeconomic relationship for the fishery of Placopecten megallanicus. Several studies conclude that ocean acidification will have negative consequences on exploited wild populations.
The studies summarized above reveal how the environment could be considered when estimating population growth responses. Doing so has the potential to illustrate the importance of physiological tools and modelling for exploring different environmental scenarios. From this perspective, a complete understanding of the life cycle of the species including its critical stages, as well as the recognition of the demographic patterns that define its distribution and fitness and the identification of appropriate procedures to include responses at the population level, are fundamental aspects to enhance the conservation of the resource.
Conclusions
Given the importance of pH and temperature for the survival of many marine populations, it is extremely important to understand the mechanisms that allow them to cope with changes in these parameters and develop logical procedures to expand these responses. In temperate and tropical sea cucumbers, the direct and indirect effects of high variation of pH and temperature result in sublethal and lethal responses, depending on the stage of their life cycle. The critical stage of holothurians to the adverse effects of temperature and pH appears to be the recruitment phase. In larvae, a pH reduction of 0.5 units can lead to elevated mortality with the potential to impact population growth. On the other hand, juveniles and adults display a wider spectrum of responses in some cases associated with anatomical modifications. Thus, the mechanisms that define holothurian responses to changes in pH and temperature occur at different biological levels of organization, from the molecular to the entire organism. In this way, the responses can be incorporated at the population level through the parameters of the population model equations and create realistic scenarios associated with different climate change projections.
Acknowledgements
The authors thank the Consejo Nacional de Ciencia y Tecnologia de Mexico, the Marist University of Mérida and the Autonomous University of Campeche for the facilities provided to complete this work.
REFERENCES
- An Z, Dong Y, Dong S (2009) A high-performance temperature-control scheme: growth of sea cucumber Apostichopus japonicus with different modes of diel temperature fluctuation. Aquacult Int 17: 459–467. [Google Scholar]
- Anderson L, Seijo JC (2010) Bioeconomics of Fisheries Management. Wiley Blackwell, 2121 State avenue, Ames, Iowa, USA, 305 pp. [Google Scholar]
- Asha PS, Muthiah P (2005) Effects of temperature, salinity and pH on larval growth, survival and development of the sea cucumber Holothuria spinifera Theel. Aquaculture 250: 823–829. [Google Scholar]
- Beverton RJH, Holt SJ (1957) On the dynamics of exploited fish populations. Fish Inv Ser II 167: 101–113. [Google Scholar]
- Brander K (2010) Impacts of climate change on fisheries. J Mar Syst 79: 389–402. [Google Scholar]
- Brierley A, Kingsford M (2009) Impacts of climate change on marine organisms and ecosystems. Curr Biol 19: R602–R614. [DOI] [PubMed] [Google Scholar]
- Byrne M, Foo S, Soars N, Wolfe K, Nguyen H, Hardy N, Dwojanyn S (2013) Ocean warming will mitigate the effects of acidification on calcifying sea urchin larvae (Heliocidaris tuberculata) from the Australian global warming hot spot. J Exp Mar Biol Ecol 448: 250–257. [Google Scholar]
- Candas D, Li J (2014) MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antiox Red Sign 20: 1599–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley S, Rheuban J, Hart D, Luu V, Glover D, Hare J, Doney S (2015) An integrated assessment model for helping the United State sea scallop (Placopecten megallanicus) fishery plan ahead for ocean acidification and warming. PLoS One 10: e0124145,1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchamp F, Clutton-Brock T, Grenfell B (2008) Inverse density dependence and the Allee effect. Trends Ecol Evolut 14: 405–410. [DOI] [PubMed] [Google Scholar]
- Crockett EL, Londraville RL (2006) Temperature. In DH Evans, JB Claiborne, eds, The Physiology of Fishes, Ed3rd. CRC Press, Boca Raton, FL, pp. 231–269. [Google Scholar]
- Davidson J, Schiestl R (2001) Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae. Mol Cel Biol 21: 8483–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeo O, Seijo JC (1999) Yield-mortality models: a precautionary bioeconomic approach. Fish Res 40: 7–16. [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A (2006) TRP ion channels and temperature sensation. Annu. Rev. Neurosci 29: 135–161. [DOI] [PubMed] [Google Scholar]
- Doney S, Fabry V, Feely R, Kleypas J (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sci 1: 169–192. [DOI] [PubMed] [Google Scholar]
- Dong Y, Dong D (2006) Growth and oxygen consumption of the juvenile sea cucumber Apostichopus japonicus (Selenka) at constant and fluctuating water temperatures. Aqua Res 37: 1327–1333. [Google Scholar]
- Dong Y, Dong S, Tian X, Wang F, Zhang M (2006) Effects of diel temperature fluctuations on growth, oxygen consumption and proximate body composition in the sea cucumber Apostichopus japonicus Selenka. Aquaculture 255: 514–521. [Google Scholar]
- Dorey N, Lancon P, Thorndyke M, Dupont S (2013) Assessing physiological tipping point of sea urchin larvae exposed to a broad range of pH. Glob Chang Biol 18: 3355–3367. [DOI] [PubMed] [Google Scholar]
- Du R, Zang Y, Tian X, Dong S (2013) Growth, metabolism and physiological response of the sea cucumber, Apostichopus japonicus Selenka during periods of inactivity. J Ocean Univ China 12: 146–154. [Google Scholar]
- Ferguson JC (1982) Nutrient translocation. In Echinoderm Nutrition (Jangoux y Lawrence, 1982). AA Balkema, Rotterdam, pp. 373–394. [Google Scholar]
- Fujiwara A, Yamano K, Ohno K, Yoshikuni M (2010) Spawning induced by cubifrin in the Japanese common sea cucumber Apostichopus japonicus. Fish Sci 76: 795–801. [Google Scholar]
- Garcia-Arrarás J, Greenberg M (2001) Visceral regeneration in holothurians. Microscopy ResTech 55: 438–451. [DOI] [PubMed] [Google Scholar]
- Gascoigne J, Lipcius R (2004) Allee effects driven by predation. J Appl Ecol 41: 801–810. [Google Scholar]
- González-Durán E, Hernández-Flores A, Seijo JC, Cuevas-Jiménez A, Moreno-Enriquez A (2018) Bioeconomics of the Allee effect in fisheries targeting sedentary resources. ICES J Mar Sci 75: 1362–1373. [Google Scholar]
- Gullian M (2013) Physiological and immunological condition of the sea cucumber Isostichopus badionotus (Selenka, 1867) during dormancy. J Exp Mar Biol Ecol 444: 31–37. [Google Scholar]
- Gullian M, Terrats M (2017) Effect of pH on temperature controlled degradation of reactive oxygen species, heat shock protein expression, and mucosal immunity in the sea cucumber, Isostichopus badionotus. PLoS One 12, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel J, Mercier A (1996) Early development, settlement, growth, and spatial distribution of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea). Can J Fish Aqua Sci 53: 253–271. [Google Scholar]
- Hamel J, Mercier A (1999) Mucus as a mediator of gametogenic synchrony in the sea cucumber Cucumaria frondosa (Holothuroidea: Echinodermata). J Mar Biol Assoc UK 79: 121–129. [Google Scholar]
- Hamel J, Mercier A (2008) Precautionary management of Cucumaria frondosa in Newfoundland and Labrador, Canada. In V Toral-Granda, A Lovatelli, M Vasconcellos, eds, Sea Cucumbers. A Global Review of Fisheries and Trade.. FAO Fisheries and Aquaculture Technical Paper 516, pp. 293–306.
- Handy D, Lubos E, Yang Y, Galbraith J, Kelly N, Zhang Y, Leopold J, Loscalzo J (2009) Glutathione peroxidase−1 regulates mitochondrial function to modulate redox-dependent cellular response. J Biol Chem 284: 11913–11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J, Alexander M, Fogarty M, William E, Scott J (2010) Forecasting the dynamics of a coastal fishery species using a coupled climate-population model. Ecol Appl 20: 452–464. [DOI] [PubMed] [Google Scholar]
- Hu M, Li Q, Li L (2010) Effect of salinity and temperature on salinity tolerance of the sea cucumber Apostichopus japonicus. Fish Sci 76: 267–273. [Google Scholar]
- Hutchings J (2014) Renaissance of a caveat: Allee effects in marine fish. Contribution to the special issue: commemorating 100 years since Hjort’s 1914 treatise on fluctuations in the great fisheries of northern Europe. Review article, ICES J Mar Sci 71: 2152–2157. [Google Scholar]
- IPCC (2011) In CB Field, V Barros, TF Stocker, D Qin, KJ Mach, GK Plattner, MD Mastrandrea, M Tignor, KL Ebi, eds, Workshop Report of the Intergovernmental Panel on Climate Change Workshop on Impacts of Ocean Acidification on Marine Biology and Ecosystems. IPCC Working Group II Technical Support Unit, Carnegie Institution, Stanford, California, United States of America, p. 164 [Google Scholar]
- Iwalaye O, Moodley G, Robertson-Andersson D (2021, 2021) Water temperature and microplastic concentration influenced microplastic ingestion rates in sea cucumber (Holothuria cinerascens Brandt, 1835). Ocean Sci J 56: 1–15. [Google Scholar]
- Ji T, Dong Y, Dong S (2008) Growth and physiological response in the sea cucumber Apostichopus japonicus Selenka: aestivation and temperatura. Aquaculture 283: 180–187. [Google Scholar]
- Kale V, Freysdottir J, Paulsen B, Fridjónsson H, Hreggvidsson G, Omarsdottir S (2013) Suphated polysaccharide from the sea cucumber Cucumaria frondosa affect maturation of human dendritic cells and their activation of allogeneic CD4(+) T cells in vitro. Bio Carb Diet Fib 2, 2: 108–117. [Google Scholar]
- Kalyanaraman B (2013) Teaching the basic of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol 1: 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DM, Huchings JA (2012) Population dynamics of marine fishes at low abundance. Can J Fish Aqua Sci 69: 1150–1163. [Google Scholar]
- Kroeker K, Gaylord B, Hill T, Hosfelt J, Miller S, Sanford E (2014) The role of temperature in determining species’vulnerability to ocean acidification: a case study using Mytilus galloprovincialis. PLoS One 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuparinen A, Hutchings J (2014) Increased natural mortality at low abundance can generate Allee effect in a marine fish. R Soc Open Sci 1: 140075, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanning G, Eilers S, Pörtner H, Sokolova I, Bock C (2010) Impact of ocean acidification on energy metabolism of oyster Crassotrea gigas – changes in metabolic pathways and thermal response. Mar Drug 8: 2318–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhao W, Qin C, Yu G, Ma Z, Guo Y, Pan W, Fu Z, Huang X, Chen J (2021) Comparative transcriptome analysis reveals changes in gene expression in sea cucumber (Holothuria leucospilota) in response to acute temperature stress. Comp Bioch Physiol D Genom Proteom 40: 1–10. [DOI] [PubMed] [Google Scholar]
- Li X, Wang C, Li N, Gao Y, Ju Z, Liao G, Xiong D (2021) Combined effects of elevated temperature and crude oil pollution on oxidative stress and apoptosis in sea cucumber (Apostichopus japonicus, Selenka). Int J Environ Res Pub Health 18: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lierman M, Hilborn R (1997) Depensation in fish stocks: a hierarchic Bayesian meta-analysis. Can J Fish Aqua Sci 54: 1976–1984. [Google Scholar]
- Lierman M, Hilborn R (2001) Depensation: evidence, models and implications. Fish Fish 2: 33–58. [Google Scholar]
- Madeira D, Narciso L, Diniz M, Vinagre C (2014) Synergy of environmental variables alters the thermal window and heat shock response: an experimental test with the crab Pachygrapsus marmoratus. Mar Environ Res 98: 21–28. [DOI] [PubMed] [Google Scholar]
- Matozzo V, Chinellato A, Munari M, Bressan M, Marin M (2013) Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar Pollut Bull 72: 34–40. [DOI] [PubMed] [Google Scholar]
- Morgan A (2008) The effect of food availability on phenotypic plasticity in larvae of the temperate sea cucumber Australostichopus mollis. J Exp Mar Biol and Ecol 363: 89–95. [Google Scholar]
- Murray F, Widdicombe S, McNeil L, Solan M (2013) Consequences of a simulated rapid ocean acidification event for benthic ecosystem processes and functions. Mar Pollut Bull 73: 435–442. [DOI] [PubMed] [Google Scholar]
- Occhipinti R, Boron W (2015) Mathematical modelling of acid-base physiology. Progress in Biop and Mol Biol 117: 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Vega J, Olivera-Castillo L, Gómez-Ruiz A, Hernández-Ledesma B (2013) Release of multifunctional peptides by gastrointestinal digestion of sea cucumber (Isostichopus badionotus). J Func Food 5: 869–877. [Google Scholar]
- Pörtner H, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315: 95–97. [DOI] [PubMed] [Google Scholar]
- Pörtner H, Langenbuch M, Reipschlager A (2004) Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J Ocean 60: 705–718. [Google Scholar]
- Pörtner H, Langenbuch R (2005) Synergistic effects of temperature extremes, hypoxia and increase in CO2 on marine animals: from earth history to global change. J Geoph Res 110: 1–15. [Google Scholar]
- Punt A, Foy A, Dalton M, Long W, Swiney K (2015) Effects of long-term exposure to ocean acidification on future southern tanner crab (Chionoecetes bairdi) fisheries management. ICES J Mar Sci 73: 849–864. [Google Scholar]
- Purcell SW, Conand C, Uthicke S, Byrne M (2016) Ecological roles of exploited sea cucumbers. Oceanogr Mar Biol 54: 357–386. [Google Scholar]
- Quijano C, Trujillo M, Castro L, Trostchansky A (2016) Interplay between oxidant species and energy metabolism. Redox Biol 8: 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones J, Rosa R, Ruiz D, Garcia-Arrarás J (2002) Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Develop Biol 250: 181–197. [DOI] [PubMed] [Google Scholar]
- Reich M (2010) The early evolution and diversification of holothurians (Echinozoa) in Echinoderms. In L Harris, S Böttger, C Walker, M Lesser, eds, Proceedings of the 12th International Echinoderm Conference, Durham, 7–11 August 2006. Taylor and Francis Group London, Durham, New Hampshire, USA, pp. 55–59. [Google Scholar]
- Seijo J, Caddy J, Euan j (1994) Space-time dynamics in marine fisheries: A bieconomic software package for sedentary species. FAO computarized information series (fisheries) No. 6 Rome, FAO, 116 p. [Google Scholar]
- Seijo J, Villanueva-Poot R, Charles A (2016) Bioeconomics of ocean acidification effects on fisheries targeting calcifier species: a decision theory approach. Fish Res 176: 1–14. [Google Scholar]
- Shao Y, Li C, Chen X, Zhang X, Li Y, Li T, Jiang J (2015) Metabolomic responses of sea cucumber Apostichopus japonicus to thermal stresses. Aquaculture 435: 390–397. [Google Scholar]
- Shiell G (2004) Field observation of juvenile sea cucumbers. SPC Beche de Mer Inform Bull 20: 6–11. [Google Scholar]
- Smiley S (1986) Metamorphosis of Stichopus californicus (Echinodermata: Holothuroidea) and its phylogenetic implications. Biol Bull 171: 611–631. [DOI] [PubMed] [Google Scholar]
- Stephens P, Sutherland W (1999) Consequences of the Allee effect for behaviour, ecology and conservation. Tree 14: 401–405. [DOI] [PubMed] [Google Scholar]
- Stephens P, Sutherland W, Freckleton R (1999) What is the Allee effect? Oikos 87: 185–190. [Google Scholar]
- Storey K (2015) Regulation of hypometabolism: insigths into epigenetic controls. J Exp Biol 218: 150–159. [DOI] [PubMed] [Google Scholar]
- Stumpp M, Dupont S, Thorndyke M, Melzner F (2011) CO2 induced seawater acidification impacts sea urchin larval development II: gene expression patterns in pluteus larvae. Comp Biochem Physiol A 160: 320–330. [DOI] [PubMed] [Google Scholar]
- Sun Z, Gao Q, Dong S, Shin P, Wang F (2013) Seasonal changes in food uptake by the sea cucumber Apostichopus japonicus in a farm pond: evidence from C and N isotopes. J Ocean Univ China 12: 160–168. [Google Scholar]
- Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y (2011) Roles of TRPM2 in oxidative stress. Cell Calcium 50: 279–287. [DOI] [PubMed] [Google Scholar]
- Tomanek L (2015) Proteomic response to environmentally induced oxidative stress. J Exp Biol 218: 1867–1879. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Starkov A, García-Arumi E (2007) Measurement of the antioxidant enzyme activities of superoxide dismutase, catalase, and glutathione peroxidase. Methods Cell Biol 80: 379–393. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhou J, Wang P, Tian T, Zheng Y, Liu Y, Mai W, Wang A (2009) Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp Biochem Physiol C 150: 428–435. [DOI] [PubMed] [Google Scholar]
- Widicombe S, Spicer J (2008) Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J Exp Mar Biol and Ecol 366: 187–197. [Google Scholar]
- Wu H, Li D, Zhu B, Sun J, Zheng J, Wang F, Konno K, Jiang X (2013) Proteolysis of noncollagenous proteins in sea cucumber, Stichopus japonicus, body wall: characterization and the effects of cysteine protease inhibitors. Food Chem 141: 1287–1294. [DOI] [PubMed] [Google Scholar]
- Yang H, Yuan X, Zhou Y, Mao Y, Zhang T, Liu Y (2005) Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (Selenka) with special reference to aestivation. Aqua Res 36: 1085–1092. [Google Scholar]
- Yang H, Zhou Y, Zhang T, Yuan X, Li X, Liu Y, Zhang F (2006) Metabolic characteristic of sea cucumber Apostichopus japonicus (Selenka) during aestivation. J Exp Mar Biol and Ecol 330: 505–510. [Google Scholar]
- Yuan X, Shao S, Dupont S, Meng L, Liu Y, Wang L (2015) Impact of CO2-driven acidification on the development of the sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Mar Pollut Bull 95: 195–199. [DOI] [PubMed] [Google Scholar]
- Yuan X, Yang H, Wang L, Zhou Y, Gabr H (2009) Bioenergetic responses of sub-adult sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea) to temperature with special discussion regarding its southernmost distribution limit in China. J Therm Biol 34: 315–319. [Google Scholar]
- Zacarías-Soto M, Olvera-Novoa M, Pensamiento-Villarauz S, Sánchez-Tapia I (2013) Spawning and larval development of the four-sided sea cucumber, Isostichopus badionotus (Selenka 1867), under controlled conditions. J World Aquac Soc 44: 694–705. [Google Scholar]
- Zamora L, Jeffs A (2012) Feeding, metabolism and growth in response to temperature in juveniles of the Australasian Sea cucumber, Australostichopus mollis. Aquaculture 358: 92–97. [Google Scholar]
- Zhou D, Chang X, Bao S, Song L, Zhu B, Dong X, Zong Y, Li M, Zhang M, Liu Y et al. (2014) Purification and partial characterization of a cathepsin L-like proteinase from sea cucumber (Stichopus japonicus) and its tissue distribution in body wall. Food Chem 158: 192–199. [DOI] [PubMed] [Google Scholar]


