Abstract
Background:
Vaccine hesitancy has been defined as a delay in acceptance or refusal of vaccines, despite the availability of vaccine services. In the past, despite an impressive record of vaccine effectiveness in the United States, several factors have contributed to a decreased acceptance of vaccines that has resulted in outbreaks of infectious diseases, e.g., measles. More recently, vaccine hesitancy has spread to coronavirus disease 2019 (COVID-19) vaccines. There are many causes of vaccine hesitancy, such as misinformation, fallacies, and myths, that have contributed to vaccine hesitancy.
Objective:
The purpose of the present report is to address the many causes of vaccine hesitancy and to suggest ways that the allergist/immunologist can be involved in the promotion of vaccine acceptance.
Methods:
The current COVID-19 vaccines were reviewed, together with their mechanisms(s) of action and adverse reactions to them.
Results:
The many causes of vaccine hesitancy include many doubts and concerns related to COVID-19 vaccines as well as a diminished level of confidence and trust by segments of the public in the nation's leaders in government, medical, and business communities, that those groups once enjoyed.
Conclusion:
Vaccination with COVID-19 vaccines is the only way that COVID-19 will be eliminated or at least controlled today, and vaccine hesitancy is the potential nemesis. The present report describes how the allergist/immunologist not only plays a major role in the delivery of specialized therapy of COVID-19 but also in educating the public with regard to the importance of COVID-19 vaccines, in dispelling misinformation, and in promoting trust for vaccine acceptance but must be informed with the most accurate and current information to do so.
Keywords: EUA = Emergency Use Authorization, adv = adenovirus, mRNA vaccines = messenger RNA vaccines, SARS-CoV-2-transcribed DNA vaccines utilize the SARS-CoV-2-transcribed DNA delivered within non-replicating recombinant adenovirus (Adv) vector systems
In a previous publication, we described the struggle with coronavirus disease 2019 (COVID-19) in which the world is currently engaged as a war on an infectious disease.1 Despite the availability of several safe and effective COVID-19 vaccines, there are segments of the public who are reluctant to be immunized, which impedes the attainment of sufficient herd immunity to extinguish the pandemic and to impede the development of variants. Vaccine hesitancy has been defined as a delay in acceptance or a refusal of vaccines despite availability of vaccine services and information. In the past, despite an impressive record of vaccine safety and effectiveness in the United States, several factors have contributed to a decreased acceptance of vaccines that has resulted in outbreaks of infectious diseases, e.g, measles.2 More recently, vaccine hesitancy has spread to COVID-19 vaccines.3–5 There are many causes of vaccine hesitancy, such as misinformation, fallacies, and myths, that have been promoted by an active “anti-vax” movement. The purpose of the present report is to address the many causes of vaccine hesitancy and to suggest ways that the allergist/immunologist can be involved in the promotion of vaccine acceptance by providing the most current information related to COVID-19 vaccines.
EMERGENCY USE AUTHORIZATION AND COVID-19 VACCINES
An Emergency Use Authorization (EUA) is a mechanism used by the U.S. Food and Drug Administration (FDA) to facilitate the availability and use of medical countermeasures, including vaccines, during public health emergencies, such as the current COVID-19 pandemic. Under an EUA, the FDA may allow the use of unapproved medical products or unapproved uses of approved medical products in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, including that there are no adequate approved and available alternatives. With input from the FDA, manufacturers decide whether and when to submit an EUA request to the FDA.
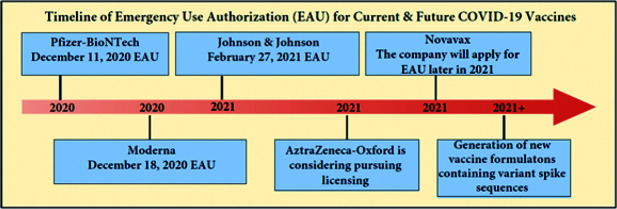
The currently available COVID-19 vaccines that have been given EUA approval include the Pfizer-BioNTech (235 East 42nd Street, New York City, U.S.) and Moderna (Global Headquarters, 200 Technology Square, Cambridge, MA 02139) vaccines, which have been available in the United States since December 2020, and the Johnson & Johnson vaccine developed by Janssen Pharmaceuticals (One Johnson & Johnson Plaza, New Brunswick, New Jersey, U.S.), which was authorized in late February 2021. AstraZeneca (1 Francis Crick Avenue, Cambridge, UK), working in collaboration with Oxford University, was expected to request EUA from the FDA for their vaccine in early 2021 but, more recently, is considering pursuing application for full-fledged licensing. Novavax (Gaithersburg, Maryland, U.S.) is expected to follow shortly with its EUA application later in 2021. Shown in Fig. 1 are the timeline landmarks of the EUAs for current and future COVID-19 vaccines.
Figure 1.
Schematic representation of some of the timeline landmarks of Emergency Use Authorization (EUA) for current and future coronavirus disease 2019 (COVID-19) vaccines.
MECHANISM(S) OF ACTION OF COVID-19 VACCINES
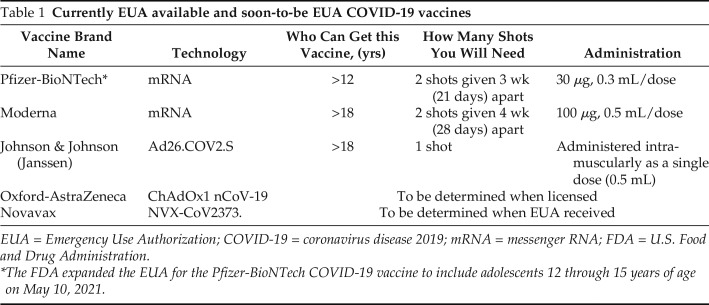
Outstanding advances in cutting-edge vaccine technologies over the past decade have resulted in the development of two types of EUA-approved severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines: (1) messenger RNA (mRNA) and lipid nanoparticle (LNP) delivery systems, and (2) SARS-CoV-2–transcribed DNA delivered within nonreplicating recombinant adenovirus (AdV) vector systems. (Table 1). The Pfizer-BioNTech and the Moderna vaccines use the mRNA and LNP delivery system, and the Johnson & Johnson (Janssen) vaccine uses the SARS-CoV-2–transcribed DNA delivered within a nonreplicating recombinant AdV type 26 vector system to lead to the production of the spike (S) protein by translation. The Oxford-AstraZeneca vaccine uses a SARS-CoV-2–transcribed DNA delivered within nonreplicating recombinant chimpanzee AdV, ChAdOx1 nCoV-19 (chimpanzee adenovirus-vectored vaccine expressing the SARS-CoV-2 spike protein) or AZD1222 (a system that uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein). The Novavax vaccine differs from the Pfizer and Moderna mRNA vaccines and from the Johnson & Johnson (Janssen) AdV vectorized vaccine. The mRNA vaccines, such as Pfizer and Moderna, contain a piece of genetic code called RNA, for which the body creates an immune response. Novavax vaccine is a protein-based coronavirus vaccine system, called NVX-CoV2373, that uses a traditional vaccine approach of using purified pieces of the coronavirus, i.e., the S protein, to stimulate an immune response in the body.
Table 1.
Currently EUA available and soon-to-be EUA COVID-19 vaccines
| Vaccine Brand Name | Technology | Who Can Get this Vaccine, (yrs) | How Many Shots You Will Need | Administration |
|---|---|---|---|---|
| Pfizer-BioNTech* | mRNA | >12 | 2 shots given 3 wk (21 days) apart | 30 μg, 0.3 mL/dose |
| Moderna | mRNA | >18 | 2 shots given 4 wk (28 days) apart | 100 μg, 0.5 mL/dose |
| Johnson & Johnson (Janssen) | Ad26.COV2.S | >18 | 1 shot | Administered intramuscularly as a single dose (0.5 mL) |
| Oxford-AstraZeneca | ChAdOx1 nCoV-19 | To be determined when licensed | ||
| Novavax | NVX-CoV2373. | To be determined when EUA received | ||
EUA = Emergency Use Authorization; COVID-19 = coronavirus disease 2019; mRNA = messenger RNA; FDA = U.S. Food and Drug Administration: .
The FDA expanded the EUA for the Pfizer-BioNTech COVID-19 vaccine to include adolescents 12 through 15 years of age on May 10, 2021.
The Pfizer and Moderna vaccines are based on mRNA technology and LNP delivery systems, whereas the approved formulation by AstraZeneca, Johnson & Johnson (Janssen) uses SARS-CoV-2–transcribed DNA delivered within nonreplicating recombinant AdV type 26 vector system.6 The operative mechanism of action of both the mRNA and the AdV vaccines is brought about by the encoded engagement of transcriptive and translational processes that generate the SARS-CoV-2 S, which subsequently leads to the production of anti-S neutralizing antibodies, the hallmark of protective immunity to COVID-19. The results of phase III clinical trials demonstrated that both the Pfizer/BioNTech (BNT162b2) and Moderna (mRNA-1273) mRNA vaccines achieved 90–95% efficacy in protecting against COVID-19 infection,7,8 whereas the AdV vaccine (ChAdOx1 nCoV-19) showed protection at a slightly lower efficacy, of 70%.9,10
Shown in Fig. 2 is a schematic representation of how mRNA and AdV vector vaccines elicit protective immunity to SARS-CoV-2 by engaging both the innate and adaptive immune systems. To stimulate adaptive immunity, a vaccine requires a pathogen-specific immunogen as well as an adjuvant, the latter stimulates the innate immune system and provides the necessary second signal for T-cell activation. An optimal adjuvant stimulates innate immunity without inducing systemic inflammation, which could elicit severe adverse effects.6 For mRNA vaccines, the mRNA serves as both an immunogen that encodes the viral protein and an adjuvant that activates innate immune responses by creating a local immunocompetent environment (owing to intrinsic immunostimulatory properties of RNA). On entry into cells, the mRNA is recognized by various endosomal and cytosolic innate sensors that form a critical part of the innate immune response to viruses and binds to endosomal Toll-like receptors (TLR) (TLR3 and TLR7) in the endosome and to components of the inflammasome in the cytosol, which results in cellular activation, and production of type I interferon and multiple inflammatory mediators (Fig. 2).11
Figure 2.
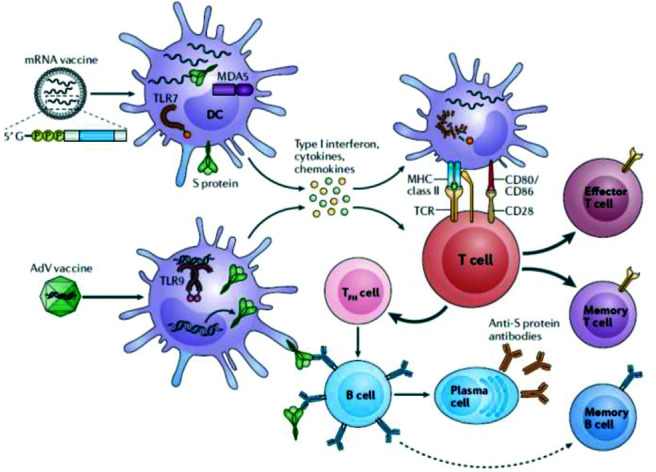
How mRNA and AdV vector vaccines elicit immunity to SARS-CoV-2. The two vaccine formulations, i.e., mRNA vaccine(s) and the AdV vectorized vaccine gain entry into DCs, which results in the production of high levels of S protein. In addition, innate sensors are triggered by the intrinsic adjuvant activity of the vaccines, which results in production of type I interferon and multiple pro-inflammatory cytokines and chemokines. RNA sensors, such as TLR7 and MDAS are triggered by the mRNA vaccines, and TLR9 is the major double-stranded DNA sensor for the AdV vaccine. The resultant activated DCs present antigen and costimulatory molecules to S protein–specific naive T cells, which become activated and differentiated into T cytotoxic effector cells or T-helper lymphocytes. TFH cells help S protein–specific B cells to differentiate into antibody-secreting plasma cells and promote the production of high-affinity anti–S protein antibodies. Reproduced with permission from Ref. 6. mRNA = Messenger RNA; AdV = adenovirus; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; DC = dendritic cell; S = spike; TLR = Toll-like receptor; MDAS = melanoma differentiation-associated protein 5; TFH = T follicular helper.
The AdV vaccines also contain inherent adjuvant properties, although these reside with the virus particle that encases the DNA encoding the immunogen. After injection, the AdV particles target innate immune cells, such as dendritic cells and macrophages, and stimulate innate immune responses by engaging multiple pattern-recognition receptors, including those that bind double-stranded DNA, in particular, TLR9, to induce type I interferon secretion.12 Unlike AdV vectors, mRNA vaccines do not engage TLR9, but both vaccine formulations converge on the production of type I interferon (Fig. 2).
In the case of mRNA vaccines, the resultant activated dendritic cells present antigen and costimulatory molecules to S protein–specific naive T cells, which become activated and differentiated into effector cells to form cytotoxic T lymphocytes or helper T cells. In the case of AdV vaccines, T follicular helper cells help S protein–specific B cells differentiate into antibody-secreting plasma cells and promote the production of high-affinity anti–S protein antibodies, and the B cells can also differentiate into memory B cells. After vaccination with either the mRNA or AdV vaccines, S protein–specific memory T cells and B cells develop and circulate along with high-affinity SARS-CoV-2 antibodies, which together help prevent subsequent infection with SARS-CoV-2 or limit the severity of illness?
WHY IS THERE VACCINE HESITANCY?
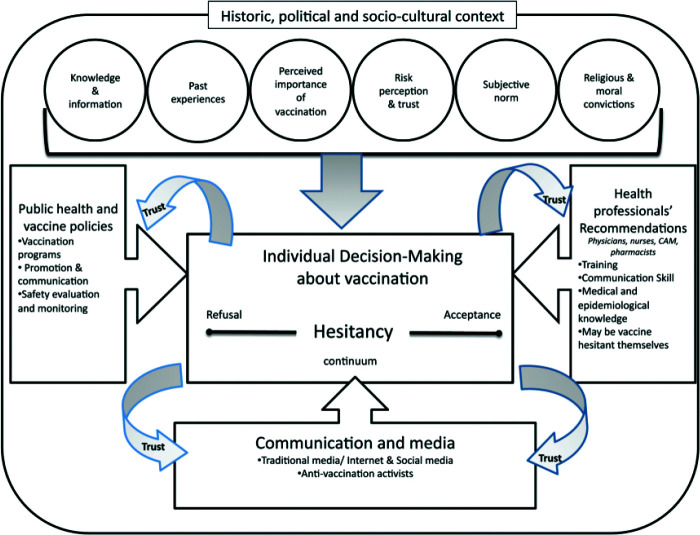
In a previous publication, we described some of the factors that contribute to vaccine hesitancy and act as barriers of acceptance of COVID-19 vaccines.1 The many causes of vaccine hesitancy not only include misinformation, fallacies, and myths surrounding the vaccine but also a diminished level of confidence and trust by segments of the public in the nation's leaders in government, medical, and business communities that these groups once enjoyed. In a recent publication by Dubé et al.,2 the many historic and sociocultural factors that contribute to vaccine hesitancy are presented; these are summarized in Fig. 3. These include knowledge and information, past experiences, perceived importance of vaccination and associated risk perception and trust, subjective norms, and religious and moral convictions.
Figure 3.
Conceptual model of vaccine hesitancy that shows the many historic, political, and sociocultural contributing factors. Reproduced with permission from Ref. 2.
In a study by SteelFisher et al.3 designed to specifically identify reasons for refusal to take COVID-19 vaccines, a system of polls was used. Among those who said they did not plan to get a vaccine against the coronavirus when it becomes available, the following responses were elicited: “I would be concerned about side effects from the vaccine, I'm concerned about the rapidity of development and approval process, I would be concerned about getting infected with the coronavirus from the vaccine, I'm not concerned about getting seriously ill from the coronavirus, the coronavirus outbreak is not as serious as some people say it is, I don't think vaccines work very well, I don't like needles, I am allergic to vaccines, I won't have time to get vaccinated.”
VACCINE HESITANCY VERSUS VACCINE APATHY
A new term has been added to the COVID-19 lexicon, referred to as vaccine apathy to be distinguished from vaccine hesitancy. In a recent article by Wood and Schulman,13 they note that, whereas vaccine hesitancy is a mindful emotional and/or cognitive response to assessing the risks and benefits of vaccination, vaccine apathy, in contrast, is a condition of disinterest characterized by weak attitudes and little time spent in considering vaccination. The investigators argue that what persuades people differs by their involvement level in the decision and that the less involved a person is with a choice, paradoxically, the less persuaded he or she will be by strong arguments based on logical or fact-filled appeals, e.g., those that are directed to the promotion of vaccine acceptance.13 Recognition of vaccine apathy adds another dimension to vaccine promotion by clinicians and attention to low involvement populations (the “vaccine-apathetic”) will require the development of different and newer forms of vaccine-promotion messaging to help overcome impediments to vaccine acceptance for achieving national vaccination goals.
ADVERSE REACTIONS TO COVID-19 VACCINES
The subject of vaccine safety and issues associated with adverse reactions to COVID-19 vaccines are among the most pressing challenges that face the allergist/immunologist today.14–17 The most common question raised by patients is “should I get the COVID-19 vaccine if I have allergies?” To answer this question, the allergist/immunologist must be optimally informed with the most accurate and current information that deals with these issues. Reassuring the patient that COVID-19 vaccines are safe and effective, and that serious allergic reactions are rare events should be the first step in allaying concerns.
To decide which procedures are most appropriate for patient testing of adverse reactions, knowledge of the components of COVID-19 vaccines is crucial.18 The following ingredients are found in the vaccines: mRNA, Ad26.COV2.S, lipids, polyethylene glycol (PEG), and polysorbates. Of these, it is believed that PEG and possibly polysorbates (a.k.a. excipients) may be a cause of the allergic reactions.16 However, the dilemma raised by deciding on PEG as the major constituent for testing is that it is not only unclear whether PEG is the causative agent of the sensitivity and whether some of the reactions are caused by other vaccine components but also if non–immunoglobulin E (IgE) mechanisms are involved as well. The most common COVID-19 vaccine adverse effects include localized findings of pain, redness, and swelling at the site of vaccine administration, and, less commonly, more generalized symptoms of malaise, fatigue, myalgia, low-grade fever, which last for only a day or two. More severe allergic reactions and anaphylaxis are extremely rare, and, after administration of millions of doses of the three authorized vaccines, adverse vaccine reactions reported to the Vaccine Adverse Event Reporting System occurred in approximately two to five people per million vaccinated in the United States.19 If a serious allergic reaction or anaphylaxis occurs within minutes after receiving the first shot of a COVID-19 vaccine, then the Centers for Disease Control and Prevention (CDC) recommends deferring a second shot.
More delayed large local reactions to the mRNA vaccine have been reported by Blumenthal et al.20 These reactions have been reported mainly in women after they received the Moderna mRNA vaccine and consisted of large erythematous swollen lesions at the site of vaccine injection, which occurred after the first dose in 12 patients, with a median onset on day 8 (range, 4–11 days); 10 of 12 patients were women; 8 of 12 had a history of allergic reactions.20 Shown in Fig. 4 is a typical delayed reaction seen in a 51-year-old patient 8 days after receiving the Moderna vaccine.
Figure 4.
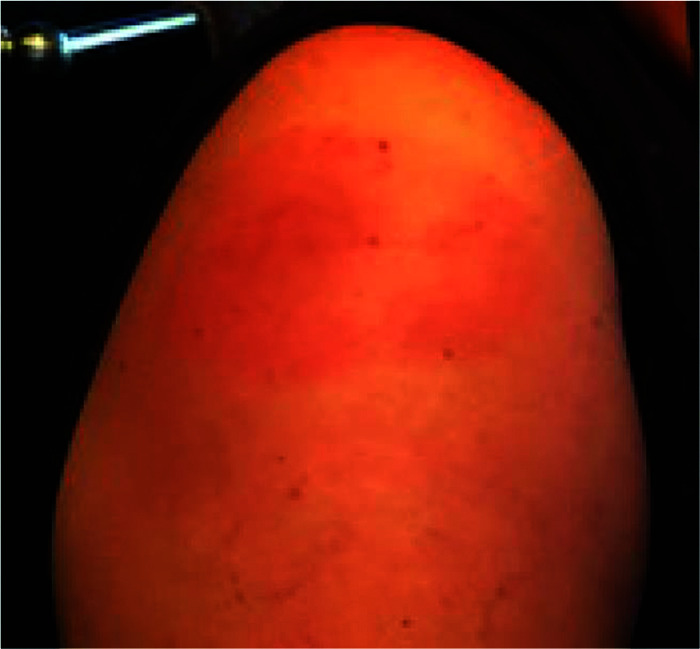
A 51-year-old woman who experienced a delayed reaction at her arm at the immunization site 8–9 days after receiving the first dose of the Moderna coronavirus disease 2019 (COVID-19) vaccine.
THROMBOSIS WITH THROMBOCYTOPENIA SYNDROME AFTER JOHNSON & JOHNSON (JANSSEN) COVID-19 VACCINATION
After issuance of an EUA of the Johnson & Johnson (Janssen) COVID-19 vaccine, which was granted February 27, 2021, its use was paused from April 12 to 23, 2021, after detection of six cases of cerebral venous sinus thrombosis that occurred in subjects who had received the Janssen COVID-19 vaccine.21 As of May 11, 2021, >9 million doses of the Johnson & Johnson (Janssen) COVID-19 vaccine have been given in the United States. Through continuous safety monitoring, the CDC and FDA identified 28 confirmed reports of people who got the Johnson & Johnson (Janssen) COVID-19 vaccine and later developed thrombosis with thrombocytopenia syndrome (TTS). For all women, this is a rare adverse event. For women ≥ 50 years and men of all ages, this adverse event is even rarer.
Thrombosis can also be a complication of COVID-19. In a recent a meta-analysis and systematic review by Nannoni et al.22 of >100,000 patients with COVID-19, they found the incidence of stroke to be 1.4%. Patients with COVID-19 who developed stroke compared with those who did not were older, more likely to have hypertension, diabetes mellitus coronary artery disease, or severe infection.22 The review of TTS reports and analysis of all available data at this time show that the known and potential benefits of the Johnson & Johnson (Janssen) COVID-19 vaccine outweigh its known and potential risks for those recommended to receive it. However, women < 50 years old especially should be aware of the rare but increased risk of TTS. There are other COVID-19 vaccine options available for which this risk has not been seen, i.e., the Pfizer-BioNTech and Moderna mRNA vaccines.
MYOCARDITIS AND PERICARDITIS AFTER COVID-19 VACCINATION
Another recently described set of rare adverse effects associated with the mRNA vaccines are myocarditis and pericarditis that occur in young adults, which may increase vaccine hesitancy in this population.23 As of June 21, 2021, Vaccine Adverse Event Reporting System has received 616 reports of myocarditis or pericarditis among people ages ≤ 30 years who received COVID-19 vaccine. Most cases have been reported after mRNA COVID-19 vaccination (Pfizer-BioNTech or Moderna), particularly in male adolescents and young adults ages ≥ 16 years, more often after receiving the second dose. Typically, these reactions occur within several days after COVID-19 vaccination Through follow-up, including medical record reviews, the CDC and FDA confirmed 393 reports of myocarditis or pericarditis and are investigating these reports to assess whether there is a relationship to COVID-19 vaccination. Most patients who received care responded well to treatment and rest. The CDC continues to recommend COVID-19 vaccination for everyone ages ≥ 12 years, given the greater risk of complications due to COVID-19 illness than from the vaccines.
CURRENT GUIDANCE RECOMMENDATIONS FOR PREVENTION OF COVID-19 VACCINE ALLERGIC REACTIONS: ARE THEY USEFUL OR WISHFUL?
The recent issuance of EUAs of the Pfizer-BioNTech, Moderna, and Johnson & Johnson (Janssen) COVID-19 vaccines by the FDA that have shown both high vaccine efficacy and relative safety, has brought hope to millions of Americans longing for the termination of the relentless restrictions placed on their lives and the prevention of this century's worst infectious disease. To provide guidance during widespread global vaccination, the FDA has recommended that these vaccines not be administered to individuals with a known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the COVID-19 vaccine and have appropriately called on allergists to be among the prime health-care specialists to offer clear guidance to individuals based on the best information available but also in accordance with the broader recommendations of regulatory agencies.
In a recent report, Banerji et al.16 described an approach to the management and prevention of allergic reactions from mRNA vaccines, which consisted of a risk stratification schema guide care for (1) individuals with different allergy histories to safely receive their first mRNA COVID-19 vaccine, and (2) individuals who develop a reaction to their first dose of mRNA COVID-19 vaccine. As part of their guidance, PEG skin testing was recommended for evaluation of those patients in the ‘‘higher risk’’ category for potential reactions to vaccine. In the same issue of the journal in which the Banerji et al.16 report was published, there appeared a letter to the editor by Stone24 and a more detailed editorial by Greenhawt et al.,25 which questioned the recommendation of PEG skin testing for evaluation of those patients in the ‘‘higher risk’’ category for potential reactions to COVID-19 vaccine.
Greenhawt et al.,25 raised the following four points when approaching patients who are concerned about allergy risk and COVID-19 vaccination:
First, when considering the many uncertainties in COVID-19 vaccine adverse reactions, one thing is certain; it is unlikely that all the reported reactions to the mRNA vaccines are IgE mediated.
Second, although there is speculation, there is no clear proof that PEG is the causative allergen, which, in the United Kingdom, Canada, and the United States, has led to the restriction of individuals with a history of previous PEG reactions from parenteral medication from receiving the COVID-19 mRNA vaccines.
Third, even with assuming that PEG is the allergen, there is historical difficulty in consistently and accurately assessing anti-PEG IgE skin testing results. The literature on PEG allergy Greenhawt et al.,25 states is scant, albeit growing, but the one commonality in all the small number of case reports has been that skin testing does not always produce a wheal and flare in recipients with true PEG allergy.
Fourth, although prevaccination risk-stratification questionnaires can provide a framework to address some stakeholder concerns, they also add medical complexity that could create confusion and dilute vaccination messaging. As Banerji et al.16 admit, there is no clear evidence that a history of anaphylaxis, medication allergy, food allergy, asthma, allergic rhinitis, or family history produces a risk for an adverse reaction of any kind to COVID-19 vaccination.
The major criticism of the PEG skin testing recommendation is the uncertainty that PEG is the causative allergen and, even if it were, these putative allergic reactions may be caused by non-IgE mechanisms, which would not be detected by immediate IgE-mediated skin testing. So, although the screening questions presented to patients for risk assessment before initial vaccination may generally be helpful in establishing an atopic predisposition, they may have no value as a predictive biomarker of susceptibility to allergic reactions of the mRNA COVID-19 vaccines. In addition, there is a potential ethics concern in performing diagnostic testing that has uncertain scientific validity. The American College of Allergy, Asthma and Immunology recently published the following statement that concerns the allergic reactions to mRNA SARS-CoV-2 vaccines26:
“The American College of Allergy, Asthma, and Immunology (ACAAI) does not currently endorse any testing protocol for PEG, polysorbate, or the mRNA COVID-19 vaccines. There are testing protocols for the above that have been published in the medical literature (Banerji et al.16) However, at present, there are no established predictive values and safety data for the proposed skin testing procedures.”
In a recent communication by Troelnikov et al.,27 the investigators suggest that skin testing with the BNT162b2 (Comirnaty, Pfizer, NY) vaccine itself and not to PEG-containing surrogates is required for a diagnosis of BNT162b2 hypersensitivity. They also provide in vitro evidence, when using basophil activation testing, that PEGylated lipids within nanoparticles, but not PEG in its native state, were able to efficiently induce degranulation.
LESSONS LEARNED FROM HYPERSENSITIVITY REACTIONS TO RADIOGRAPHIC CONTRAST MEDIA
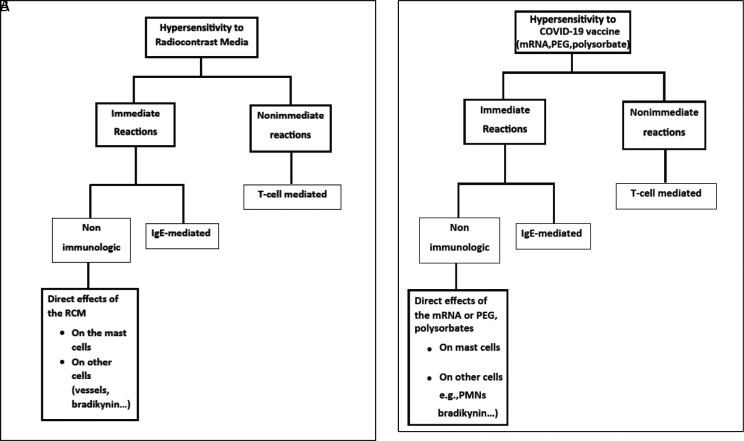
Previously, the diagnostic challenge of determining the risk for developing immediate or delayed hypersensitivity reactions to radiocontrast media (RCM)28 has posed a dilemma for the allergist/immunologist, similar to determining the best risk assessment for COVID-19 vaccines. Shown in Fig. 5A are two types of hypersensitivity reactions to RCM that have been recognized: immediate and nonimmediate (delayed).29 Immediate reactions can be caused by IgE and non-IgE mechanisms. Immediate, anaphylaxis-like reactions may be caused by an IgE-mediated mechanism or by the direct effect of the RCM on the mast cell membrane or, possibly, by direct complement activation, which leads to mediator release. In a similar manner, hypersensitivity reactions to COVID-19 vaccines may have a similar pathogenesis based on postulated reactions to mRNA or excipients, e.g., PEG, polysorbates, which involve immediate and nonimmediate (delayed) reactions as well as immediate reactions that can be caused by IgE and non-IgE mechanisms (Fig. 5B).
Figure 5.
(A) Mechanisms of hypersensitivity reactions to radiocontrast media (RCM). (B) Postulated mechanisms of hypersensitivity reactions to COVID-19 mRNA vaccines. Modified with permission from Ref. 28.
In attempting to reconcile the controversy that concerns the different views expressed related to the use of skin testing with mRNA or excipients, e.g., PEG, polysorbates, it is possible to offer a King Solomon–like resolution and to conclude that, if the postulated dual IgE and non-IgE mechanisms of injury are correct, then the use of PEG skin testing as suggested by Banerji et al.16 may have partial validity in supporting an IgE mechanism but, because non-IgE mechanisms cannot be detected by this procedure, it will require the development of new assays that measure these innate immune-mediated reactions, which then can, it is hoped, complete the validation of the causality of adverse reactions caused by COVID-19 vaccines. In a recent comprehensive systematic report by Greenhawt et al.,30 the risk of allergic reactions to SARS-CoV-2 vaccines is reviewed by a team of national and international authorities, together with a set of useful recommended evaluation and management guidelines.
DISCUSSION
Although every specialty of medicine has been affected by the COVID-19 pandemic and is responding to it in the best possible manner, the field of allergy/immunology holds a unique place in the battle against this modern-day scourge. Because of the specialized training in allergy, clinical immunology, and inflammatory diseases, the allergist/immunologist is uniquely poised to play a major role, not only in the delivery of specialized therapy but also in educating the public with regard to the importance of COVID-19 vaccines, in dispelling misinformation, and in promoting vaccine acceptance; however, to do so, the allergist/immunologist must be informed with the most accurate and current information with concern to adverse reactions to the vaccines that impact decision-making.
The several reasons why COVID-19 has posed a particularly unique challenge for the allergist/immunologist in his or her communication with the public are described in the present communication. The public now has instant access to information (both good and bad) with concern to COVID-19 through the news and social media, and interest in medical research is now soaring with the public. Researchers and clinicians, however, seldom receive training on how to best communicate with the public outside of their academic and clinical domains. Traditional approaches to public communication often lack meaningful engagement and the education of the public for COVID-19, in particular, needs to move beyond the paternalistic views of communication when professionals tend to speak at the public rather than listening to and engaging the public in an active dialog known as shared decision-making. The traditional model of public education, therefore, is counter-productive to effective messaging and, at times, can discourage public involvement and trust in research and public health endeavors and the need for acceptance of COVID-19 vaccines.
Instead, professionals should be pursuing an ongoing dialog that empowers the public to be actively involved in science and medicine as key stakeholders rather than passive recipients. As part of this process, the professional must take into consideration the multiple factors that are contributing to COVID-19 vaccine hesitancy, which include the various doubts and concerns as well as the diminished level of confidence and trust by segments of the public in the nation's leaders in government, medical, and business communities, that those groups once enjoyed. In this way allergist/immunologists can best serve their role in mitigating vaccine hesitancy and in promoting COVID-19 vaccine acceptance. With the most accurate and current information about adverse reactions to the vaccines in hand the allergist/immunologist will best be able to help patients to reach evidence informed and value congruent medical decisions with concern to COVID-19 vaccines, the essence of shared decision-making.
CONCLUSION
Vaccine hesitancy has been defined as a delay in the acceptance or refusal of vaccines despite the availability of vaccine services. Vaccination with COVID-19 vaccines is the only way that COVID-19 will be eliminated or at least controlled today, and vaccine hesitancy is the potential nemesis. The present report describes how the allergist/immunologist not only plays a major role in the delivery of specialized therapy of COVID-19 but also in educating the public with regard to the importance of COVID-19 vaccines, in dispelling misinformation and in promoting trust for vaccine acceptance but must be informed with the most accurate and current information to do so.
Footnotes
Presented as a lecture at the Eastern Allergy Conference, Palm Beach, Florida, June 5, 2021
Funding provided by the Eastern Allergy Conference
Additional funding, in part, by a grant from the Martyn A. Vickers Sr, MD, Endowment Fund, Georgetown University Medical Center (J.A.B.)
REFERENCES
- 1. Bellanti JA, Settipane RA. The war on infectious diseases: COVID-19 vaccines and the public: challenges and solutions. Allergy Asthma Proc. 2021; 42:5–7. [DOI] [PubMed] [Google Scholar]
- 2. Dubé È, Ward JK, Verger P, et al. Vaccine hesitancy, acceptance, and anti-vaccination: trends and future prospects for public health. Annu Rev Public Health. 2021; 42:175–191. [DOI] [PubMed] [Google Scholar]
- 3. SteelFisher GK, Blendon RJ, Caporello H. An uncertain public - encouraging acceptance of Covid-19 vaccines. N Engl J Med. 2021; 384:1483–1487. [DOI] [PubMed] [Google Scholar]
- 4. Rosenbaum L. Escaping catch-22 - overcoming Covid vaccine hesitancy. N Engl J Med. 2021; 384:1367–1371. [DOI] [PubMed] [Google Scholar]
- 5. Quinn SC, Andrasik MP. Addressing vaccine hesitancy in BIPOC communities - toward trustworthiness, partnership, and reciprocity. N Engl J Med. 2021; 385:97. [DOI] [PubMed] [Google Scholar]
- 6. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021; 21:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector–based heterologous prime–boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021; 397:671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018; 17:261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao Y, Jeyanathan M, Haddadi S, et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell. 2018; 175:1634–1650.e17. [DOI] [PubMed] [Google Scholar]
- 13. Wood S, Schulman K. When vaccine apathy, not hesitancy, drives vaccine disinterest. JAMA. 2021; 325:2435–2436. [DOI] [PubMed] [Google Scholar]
- 14. Kounis NG, Koniari I, de Gregorio C, et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines (Basel). 2021; 9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anand P, Stahel VP. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021; 15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021; 9:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021; 384:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ionova Y, Wilson L. Biologic excipients: importance of clinical awareness of inactive ingredients. PLoS One. 2020; 15:e0235076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html, accessed July 27, 2021.
- 20. Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021; 384:1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ionova Y, Wilson L. Biologic excipients: Importance of clinical awareness of inactive ingredients. PLoS One. 2020. June 25; 15(6):e0235076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nannoni S, de Groot R, Bell S, et al. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021; 16:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. CDC. Centers for Disease Control and Prevention. Myocarditis and pericarditis following mRNA COVID-19 vaccination. Updated June 23, 2021. https://www.cdc.gov/coronavirus/2019ncov/vaccines/safety/myocarditis.html?sc_cid=11374:myocarditis%20covid%20vaccine:sem.ga:p:RG:GM:gen:PTN:FY21; accessed July 27, 2021.
- 24. Stone BD. PEG skin testing for COVID-19 vaccine allergy. J Allergy Clin Immunol Pract. 2021; 9:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenhawt M, Abrams EM, Oppenheimer J, et al. The COVID-19 pandemic in 2021: avoiding overdiagnosis of anaphylaxis risk while safely vaccinating the world. J Allergy Clin Immunol Pract. 2021; 9:1438–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy KR, Patel NC, Ein D, et al. Insights from American College of Allergy, Asthma, and Immunology COVID-19 Vaccine Task Force: allergic reactions to mRNA SARS-CoV-2 vaccines. Ann Allergy Asthma Immunol. 2021; 126:319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Troelnikov A, Perkins G, Yuson C, et al. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. J Allergy Clin Immunol. 2021; 148:91–95. [DOI] [PubMed] [Google Scholar]
- 28. Sánchez-Borges M, Aberer W, Brockow K, et al. Controversies in drug allergy: radiographic contrast media. J Allergy Clin Immunol Pract. 2019; 7:61–65. [DOI] [PubMed] [Google Scholar]
- 29. Brockow K. Immediate and delayed reactions to radiocontrast media: is there an allergic mechanism? Immunol Allergy Clin North Am. 2009; 29:453–468. [DOI] [PubMed] [Google Scholar]
- 30. Greenhawt M, Abrams EM, Shaker M, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021; 18:S2213–S2198(21)00671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]