Abstract
Background:
Infectious diseases are a leading cause of morbidity and mortality worldwide. As of 2018, the total world population of children < 5 years of age was roughly estimated at 679 million. Of these children, an estimated 5.3 million died of all causes in 2018, with an estimated 700,000 who died of vaccine-preventable infectious diseases; 99% of the children who died had lived in low- and middle-income countries. The infectious diseases that remain major causes of mortality for which vaccines have been shown to provide proven preventive success include, in order of prevalence, are those caused by Streptococcus pneumoniae, Rotavirus, Bordetella pertussis, measles virus, Haemophilus influenzae type b and influenza virus.
Objective:
The purpose of the present report was to address the global burden of these six vaccine-preventable infectious diseases in children < 5 years of age, together with implications for the prevention of coronavirus disease 2019 (COVID-19) infection in children.
Methods:
The current immunization strategies for the prevention of the six vaccine-preventable infectious diseases in children are reviewed as a framework for new strategies of vaccine prevention of COVID-19 in children.
Results:
The burden of addressing vaccine prevention of future infectious disease in children can be effectively pursued through knowledge gained from past experiences with vaccine usage in these six vaccine-preventable childhood infectious diseases.
Conclusion:
Issues with regard to the burden of disease mortality, disease transmission, and available vaccines as well as vaccine successes and shortcomings for specific pathogens can serve as important landmarks for effective use of future vaccines. Although much success has been made globally in preventing these childhood deaths, much remains to be done.
Keywords: Streptococcus pneumoniae, Haemophilus influenzae type b, (Hib), rotavirus, Bordetella pertussis, measles virus, influenza virus, COVID-19, vaccine hesitancy
I am both deeply pleased and privileged to present the First Annual Joseph A. Bellanti, M.D. Lectureship honoring an internationally renowned clinician-investigator, educator, and author who in addition to being my mentor, counselor, and adviser for more than 50 years, is and continues to be my friend. 1
Infectious diseases are a leading cause of morbidity and mortality worldwide. As of 2018, the total world population of children < 5 years of age was roughly estimated at 679 million.2 Of these, an estimated 5.3 million children died of all causes in 2018,3 with an estimated 700,000 who died of vaccine-preventable infectious diseases (Table 1)4; 99% of the children who died had lived in low-and middle-income countries. The major issue in developing countries is not the acceptance of vaccines but their cost and availability. The focus of this article is directed to six of these infectious diseases, which remain significant causes of mortality and for which vaccines have been shown to provide proven preventive success. These include, in order of prevalence, infectious diseases caused by Streptococcus pneumoniae, Rotavirus, Bordetella pertussis, measles virus, Haemophilus influenzae type b (Hib), and influenza virus1 (Table 2).
Table 1.
Ways we can improve global immunization rates and decrease child mortality from vaccine preventable infectious2,3

| Infectious diseases are leading causes of morbidity and mortality worldwide |
|---|
| 679 million children < 5 years of age were alive globally in 2018 |
| 5.3 million died of all causes in 2018 |
| 700,000 died of vaccine-preventable infectious disease |
| 99% lived in low-and middle-income countries |
Table 2.
Global estimates of annual vaccine-preventable deaths in children < 5 years of age in order of prevalence of deaths*
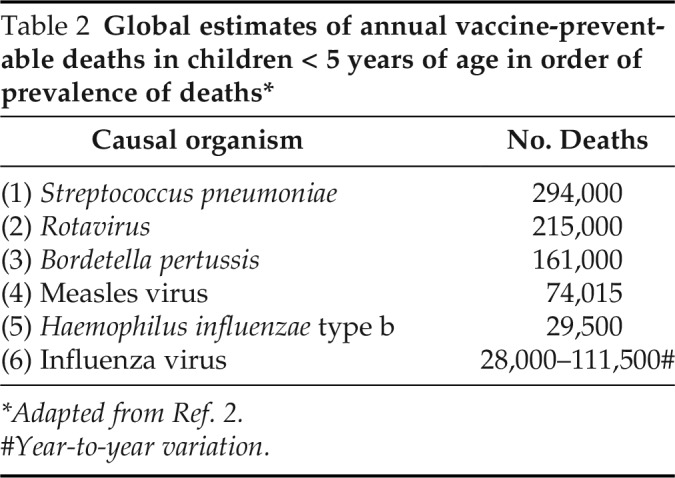
| Causal organism | No. Deaths |
|---|---|
| (1) Streptococcus pneumoniae | 294,000 |
| (2) Rotavirus | 215,000 |
| (3) Bordetella pertussis | 161,000 |
| (4) Measles virus | 74,015 |
| (5) Haemophilus influenzae type b | 29,500 |
| (6) Influenza virus | 28,000–111,500# |
*Adapted from Ref. 2.
Year-to-year variation.
THE PNEUMOCOCCAL AND Hib VACCINES: A SUCCESS STORY
Before the introduction of effective vaccines in the 1980s, Hib was the leading cause of invasive bacterial disease in young children worldwide and infected ∼1 in 200 children ages < 5 years in the United States. Even with the availability of antibiotic treatment, pneumococcal and Hib infection resulted in thousands of deaths annually. By 1985, several pure polysaccharide (PS) vaccines were developed that provided some protection in adults and were subsequently licensed in the United States. However, these pure polysaccharide vaccines were ineffective in children ages < 18 months, the age group most at risk of disease, and failed to induce immunologic memory at any age. In addition, the pure polysaccharide vaccines did not reduce nasopharyngeal carriage of pneumococcus. As with the polysaccharide capsule of S. pneumoniae, the polyribosylribitol phosphate (PRP) of the Hib capsule is a T-independent antigen and not immunogenic when administered as a vaccine in infancy. Because the highest rates of disease occur in the first 2 years of life, efficacious Hib vaccines have been designed by covalently linking the PRP capsule to a carrier protein that recruits T-cell help for the polysaccharide immune response and induces anti-PRP antibody production, even in the first 6 months of life.
S. pneumoniae and Hib are the two pathogens that, when combined, are the leading cause of both acute lower respiratory tract infections and infectious disease mortality in childhood3 and, together, account for 64% of respiratory deaths in children ages < 5 years (Table 3). S. pneumoniae is transmitted via respiratory secretions and is a frequent colonizer of the upper respiratory tract in children. Up to 90% of children in developing countries and 20% in developed countries are colonized by S. pneumoniae, and it is the most frequent cause of community-acquired pneumonia, otitis media, and sinusitis globally. The huge global burden of severe disease caused by S. pneumoniae can be largely attributed to the cost and availability of the pneumococcal vaccines and the subsequent deficient development of herd immunity. Comorbid viral infections, e.g., influenza, can also facilitate the tissue-invasive properties of pneumococcal and Hib infections.
Table 3.
| The whole-cell pertussis vaccine was introduced in developed countries in the 1940s and was soon widely adopted |
|---|
| In the United States, the number of reported pertussis cases per year decreased from a high of 250,000 to a low of 1000 in the late 1980s |
| The whole-cell pertussis vaccine was effective but febrile episodes and seizures occurred in young infants |
| The more reactogenic whole-cell pertussis vaccine was replaced by the less reactogenic and slightly less-effective acellular vaccine in 1997 |
| Unfortunately, in 2012, > 41,000 cases were reported in the United States, the highest in 50 years |
| Immunity from natural disease and immunization begins to wane after ∼10 years* |
*From Ref. 11.
Hib causes pneumonia, bacteremia, meningitis, epiglottitis, septic arthritis, cellulitis, otitis media, and pericarditis. Similar to pneumococcal infection, Hib is transmitted via respiratory secretions and can colonize the respiratory tract, but, unlike S. pneumoniae, colonization does not occur as frequently. Results of studies showed that 81% of children who died of pneumococcal infection had pneumonia as a cause of death and 12% had meningitis, and an additional 7% had sepsis. In developing countries, exclusive breast-feeding for the first 6 months of life has been shown to decrease acute respiratory tract infections by one-third5 and an additional one-fourth of deaths can be reduced by hand washing. These statistics demonstrate how simple interventions can reduce mortality. Polysaccharide-encapsulated bacteria such as Hib and S. pneumoniae can cause serious bacterial infections and have been a deadly scourge on humans for centuries.
The immunogenicity of the polysaccharide, therefore, was increased by conjugating it to a protein carrier with strong antigenic properties, which leads to the first protein–polysaccharide conjugate vaccines for both Hib and S. pneumoniae. The pneumococcal conjugate vaccine (PCV) 7, which contains 7 serotypes, was licensed in 2000, and the PCV13 vaccine, which contains 13 serotypes, was licensed in 2010. The introduction of conjugate vaccines saw a rapid reduction in the number of cases of invasive Hib disease in those countries with good adoption of the pneumococcal and Hib vaccines, and, in the past 3 decades, has saved the lives of millions of infants and children. PCV13 reduced the incidence of pneumococcal bacteremia by 95% in children between 3 months and 3 years of age in the United States.6 After 7 years of use of PCV13 in the United States, protection against pneumococcal disease seems to be minimally affected by the emergence of nonvaccine serotypes (so-called serotype replacement).7 The use of Hib vaccine has essentially eliminated invasive disease in those countries where it is widely used. The success of these vaccines stimulated the development of other conjugate vaccines that target various polysaccharide-encapsulated bacteria, including Neisseria meningitidis, and led to a remarkable prevention of formerly deadly infectious diseases through the application of a basic immunologic principle to clinical relevance.
PERTUSSIS
Pertussis is a highly communicable febrile acute respiratory infection caused by Bordetella pertussis. The disease presents in children with common “cold” symptoms, followed by paroxysms of coughing, followed by a “whoop,” which characterizes a distinct clinical syndrome called “whooping cough.” Complications of pertussis include pneumonia and subdural bleeding. Most complications (encephalopathy with seizures, dehydration, pneumonia, hypoxia, and intracranial and conjunctival bleeds) and deaths occur in 0.5% of children < 1 year of age8,9 from pneumonia and/or hypoxia. The recovery can take as long as 10 weeks. Many of the adverse effects of pertussis are toxin related.10 The reasons for the higher incidence of infection and morbidity in young infants include their smaller airways, the greater frequency of viral co-infections in infants, and that young infants have not had time to produce antibodies from previous exposure and maternal transplacental-derived antibody levels have waned and, therefore, are not available to the infant for protection. Immunity is not lifelong after natural infection or vaccination. Pertussis outbreaks occur cyclically worldwide, and effective vaccination strategies are needed to control disease.
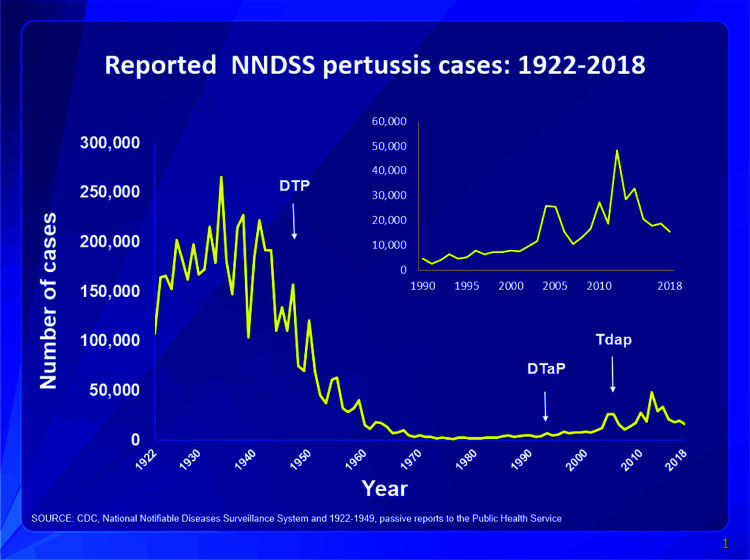
The acquisition of protective immunity to pertussis occurs either following natural infection or following the use of vaccines. Infants are usually infected after exposure to a close contact; ∼50% of children acquire pertussis from parents who were infected but asymptomatic and who believed that they may have had a “common cold”. One strategy to decrease disease is to “cocoon” infants by immunizing their close contacts against pertussis. The history of pertussis vaccines is an interesting trajectory of progression from whole-cell pertussis vaccines which became available in the 1940s5 but which, because of adverse effects, were replaced with less reactogenic acellular pertussis vaccines.2 The current acellular pertussis vaccines contain the modified pertussis toxin combined with modified diphtheria and tetanus toxins and are available in two formulations: 1) an infant formulation, DTaP (Diphtheria, Tetanus, Pertussis], which contains relatively greater content of diphtheria (D), tetanus (T) and pertussis (P) toxins; and, 2) an adult formulation, Tdap, in which the content of diphtheria (d) toxin and pertussis (p) toxins are reduced to minimize reactivity. Some of the historical aspects of the key development of the pertussis vaccines are shown in Table 3 and, in Fig. 1, and contain some practical aspects of an infant pertussis prevention program. Infants do not develop adequate vaccine-induced protection against pertussis until 6 months of age (after their initial DTaP vaccine doses) or unless their mother received a Tdap vaccine during pregnancy.11 Transplacental transfer of immunoglobulin G antibodies from mother to fetus occurs between 27 and 36 weeks of gestation and provides protection for the first 6 months of life. Tdap vaccine is now recommended for every pregnant woman with each pregnancy.
Figure 1.
This graph illustrates the number of pertussis cases reported to Centers for Disease Control and Prevention from 1922 to 2018. After the introduction of pertussis vaccines in the 1940s when case counts frequently exceeded 100,000 per year, reports declined dramatically, to < 10,000, by 1965. During the 1980s pertussis reports began increasing gradually, and, by 2018, >15,000 cases were reported nationwide. Source: CDC. National Notifiable Diseases Surveillance System and 1922–1949. Passive reports to the Public Health Service.
INFLUENZA
A and B strains of Influenza cause annual epidemics, with an attack rate of 20% to 30% in children, and are characterized by rapid onset, high fever, chills, myalgia, and headache, followed a day later by cough and other symptoms of upper and lower respiratory tract involvement.13,14 Neurologic complications include febrile seizures and encephalopathy. Mortality due to influenza is associated with pneumonia, cardiomyopathy secondary bacterial infections, and rare neurologic events; 99% of deaths occur in low-income countries. Influenza accounts for 7–10% of all severe Acute respiratory tract infections (ARTIs), globally. Children who are immunologically naive have higher attack rates and more severe disease.
Variable mortality from season to season depends on genetic drift and shift of the virus, which results in changes in viral surface antigens (hemagglutinin [HA] and neuraminidase [N]). A shift is a major change in the genetic makeup of the influenza virus and is reflected in major changes in the cell surface antigens. This results in the failure of the circulating antibodies of the infected host to no longer recognizing the shifted virus and the host having more severe disease. A minor change in the antigenic makeup with a limited change in the cell surface HA and N antigens of the invading influenza strain often still allows some protective effect of the circulating antibodies from previous influenza infections.
MATERNAL INFLUENZA
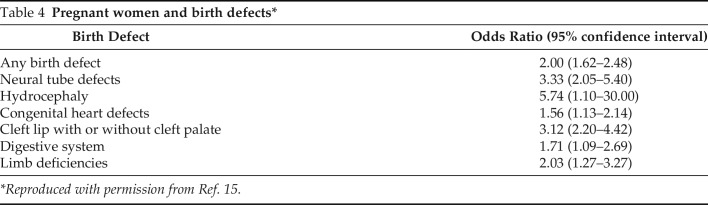
Pregnant women with influenza have increased rates of cardiopulmonary complications, hospitalization, death, stillbirth, and low birth weight. Maternal influenza immunization protects infants for 6 months after birth in a fashion analogous to the protection afforded by maternal pertussis immunization.11 Importantly, first-trimester maternal influenza infection during gestation has been reported to result in birth defects (Table 4).15
Table 4.
Pregnant women and birth defects*
| Birth Defect | Odds Ratio (95% confidence interval) |
|---|---|
| Any birth defect | 2.00 (1.62–2.48) |
| Neural tube defects | 3.33 (2.05–5.40) |
| Hydrocephaly | 5.74 (1.10–30.00) |
| Congenital heart defects | 1.56 (1.13–2.14) |
| Cleft lip with or without cleft palate | 3.12 (2.20–4.42) |
| Digestive system | 1.71 (1.09–2.69) |
| Limb deficiencies | 2.03 (1.27–3.27) |
*Reproduced with permission from Ref. 15.
INFLUENZA VACCINES
Inactivated influenza vaccine was first available in 195716; the cold-adapted live-attenuated vaccine (LAIV) was licensed in the United States in 2003.17 As described previously, influenza vaccines are focused on production of antibodies against the HA and N surface antigens. Three classes of vaccines have been available: inactivated, live attenuated, and, recently, recombinant HA. The trivalent inactivated vaccine currently contains H1N1 and H3N2 subtypes of influenza A, along with the predicted dominant lineage of influenza B for the upcoming season. The quadrivalent vaccine adds a second lineage of influenza B. The live-attenuated temperature-sensitive influenza vaccine (LAIV)16 is administered intranasally but regretfully has had efficacy problems.18,19 In 2016, the Centers for Disease Control and Prevention (CDC) recommended that LAIV not be used for the 2016–2017 flu season and, instead, another type of influenza vaccine be used. This was due to the poor effectiveness of this type of vaccine between 2013 and 2016, and ineffective during the 2015–2016 season. However, in February 2018, the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices reinstated the use of LAIV for the 2018–2019 flu season and again for the 2020–2021 flu season.18,20 The third licensed vaccine is a baculovirus vectored recombinant HA vaccine produced in insect cell culture by using the baculovirus expression system.21 It is available as a trivalent and quadrivalent vaccine but is only approved for adults.
MEASLES
Measles is a highly contagious infectious disease with respiratory transmission. The disease is manifest with a characteristic clinical presentation, including cough, coryza, conjunctivitis, an erythematous macular heterogeneous rash, high fever, malaise, and a pathognomonic oral enanthema (Koplik spots).22 The primary causes of death in children are respiratory compromise (with croup), pneumonia, encephalitis, and bacterial superinfections. Measles disease is more severe in children who are malnourished. Despite the availability of a safe and highly effective measles vaccine since 1963, measles remains a major killer of unimmunized young children globally, with an estimated 74,015 deaths in children < 15 years of age in 2016.23
MEASLES VACCINES
Inactivated (killed) measles vaccine was available in the United States between 1963 and 1967 but, because of limited efficacy and adverse effects, such as subsequent atypical measles disease, the vaccine was withdrawn. After 1967, the killed measles vaccine was replaced by the live measles vaccine and then combined as the measles, mumps, rubella combined vaccine. Despite the availability of a safe and highly effective measles vaccine since 1963, measles remains a major killer of unimmunized young children globally. However, a drop of 84% in measles deaths between 2000 and 2016 prevented an estimated 20.4 million deaths worldwide. Lifelong immunity occurs after natural infection or immunization.5
More than a decade ago, an unethical scientist published fraudulent data that linked autism with measles immunization.24,25 This resulted in dramatically increased measles vaccine hesitancy and refusal, especially in high-income countries, which leaves those individuals and their unimmunized contacts susceptible to community-wide outbreaks of measles disease. As is often the case, such setbacks affect immunization rates for an uncomfortably long period of time. Regretfully, in the United States, this has now resulted in a markedly elevated number of outbreaks in religious communities where parents have refused to allow their children to be immunized.5 These cases might threaten the celebrated “elimination” of measles in the United States in 2000. The one potential positive result of this situation is that it may lead to the elimination of nonmedical exemptions for immunizations before preschool and school attendance.26,27
ROTAVIRUS
Rotavirus is a genus of double-stranded RNA viruses in the family Reoviridae and the most common cause of diarrheal disease among infants and young children.2 Nearly every child in the world is infected with a Rotavirus at least once by the age of 5 years.3 Rotavirus is a highly infectious enteritis, which begins with an acute onset of fever and vomiting, followed a day or two later with severe watery diarrhea. Symptoms may last for a week or more and may progress to dehydration, electrolyte abnormalities, acidosis, and even death. Basic medical care usually in a hospital where oral or intravenous fluids and monitoring of electrolytes etc., can be accomplished may prevent death. It is the most common cause of gastroenteritis, which causes 30% of diarrheal deaths globally in children < 5 years of age and up to 85% of diarrheal deaths in low-income countries.28 Infectious Rotavirus is found in high quantities for as long as a week before the onset of symptoms.29 In addition to oral-fecal spread, there is evidence that it can be spread as a fomite, such as on toys and surfaces that come in contact with infants and children. This latter mode of transmission, rather than by water or food, is thought to partly explain clusters of disease in day-care settings.28 It is also thought to explain the appreciable incidence in children in upper- and middle- as well as lower-income countries.29
Poor nutrition is associated with decreased vaccine effectiveness, and breast-feeding does not affect vaccine response.30 Two live attenuated oral Rotavirus vaccines (RV) were licensed in 2006; RotaTeq (RV 5) (Merck and Company, Readington Township, NJ) is a three-dose pentavalent bovine-human reassortant RV and Rotarix (RV 1) (GSK Biologics, GLAXOSMITHKLINE US Corporate Headquarters, Philadelphia, USA) is a two-dose monovalent human RV. The World Health Organization estimates that, globally, 23% of eligible infants received a full course of RV in 2015, with coverage nearly fourfold less than the estimated global coverage for polio and Diphtheria, Tetanus, Pertussis (DTP) vaccines (86%).31 This is in contrast to the United States, where, in 73% who were fully immunized against Rotavirus, the reduction in Rotavirus hospitalizations ranged from 49 to 92% in high-income countries, 54 to 59% in middle-income countries, and 69 to 81% in low-income countries.30 Of interest, are data, analysis of which suggests that decreased Rotavirus disease in infants and children also decreases the incidence of disease in older individuals.32 Regretfully, for unexplained reasons, the RVs are not as effective in low-income countries.33,34 Malnutrition, zinc deficiency, vitamin deficiency, co-infections, differences in gut microbiota, and genetic factors may all play a role.33 Serotype replacements have not proven to be an issue.30 Studies demonstrated that RVs have reduced disease caused by both homotypic and heterotypic strains.30
CORONAVIRUS DISEASE 2019
Since its initial description in December 2019 in Wuhan, China, coronavirus disease 2019 (COVID-19) has rapidly progressed into a worldwide pandemic, which has affected millions of lives. Unlike the disease in adults, the vast majority of children with COVID-19 have mild symptoms and are largely spared from severe respiratory disease. However, there are children who have significant respiratory disease, and some may develop a hyperinflammatory response similar to that seen in adults, referred to as multi-system inflammatory syndrome in children.35 The recent global spread of the severe acute respiratory syndrome coronavirus-2, the cause of COVID-19, has led to a disruption of human life, with dimensions unwitnessed with any infectious disease in more than a century.
In contrast to the disease in adults, COVID-19 in children is not associated with predisposing conditions. Five patterns of COVID-19 in children are recognized: approximately one-third are asymptomatic; approximately one-half present with mild symptoms (such as fever, fatigue and/or myalgia, cough) or with moderate symptoms (including mild pneumonia with abnormal chest imaging); 5% develop more-severe pulmonary symptoms (dyspnea, central cyanosis, hypoxia); and <1% progress to critical disease, which manifests as multisystem inflammatory syndrome in children with acute respiratory failure, shock, and/or multiorgan dysfunction. Symptoms can range from mild, with fever, sore throat, headache, conjunctival injection, abdominal pain, vomiting, and rash, to severe, with necrotizing pneumonia, myocardial dysfunction, shock, kidney injury, coronary artery aneurysms, and death. In a recent study by Feldstein et al,36 the most commonly involved organ systems are the gastrointestinal, cardiovascular, hematologic, mucocutaneous, and respiratory systems. Coronary artery aneurysms were identified in echocardiograms in a smaller percentage of children with a condition referred to as multisystem inflammatory syndrome in children.35
The U.S. Food and Drug Administration (FDA) recently amended the emergency use authorization originally issued on December 11, 2020, for administration in individuals ages ≥ 16 years and, on May 10, 2021, expanded the emergency use authorization for the Pfizer-BioNTech (Pfizer Inc. NY) COVID-19 vaccine to include adolescents 12 through 15 years of age on May 10, 2021. At the present time, children ages < 5 years are becoming sick and dying without the current availability of a preventive vaccine. It is estimated that a vaccine for children 6 months to 11 years of age will have an approved vaccine by the end of 2021. The importance of maternal vaccination against severe acute respiratory syndrome coronavirus-2 is probable, but there are several unanswered issues at this time, as illustrated in Table 5.
Table 5.
COVID-19 vaccine issues
| Mortality rates in children, including those < 5 years of age are not yet well documented |
|---|
| Data with regard to COVID-19 in pregnant women and the fetus remain unclear but analysis of data suggests some adverse maternal effects and increased preterm births |
| The safety and efficacy of immunization against SARS-CoV-2 in children < 5 years of age must be studied in future clinical trials because of the different immune responses in children from those seen in older age groups; these trials are just getting started |
| The Pfizer vaccine clinical trials are underway for adolescents between 12 and 18 years of age and, have resulted in an FDA emergency authorization; thus far COVID-19 vaccines have been found to be safe and effective for adolescents |
COVID-19 = Coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2; FDA = U.S. Food and Drug Administration.
VACCINE HESITANCY
Vaccine hesitancy has been and is a significant problem in the United States and around the world; the pandemic may be increasing this problem.37 It is defined as a delay in acceptance or refusal of safe and effective vaccination because of fear, fallacies, myths, and misinformation, especially fostered by “anti-vax,” media sensationalism, and fraudulent science.38 Vaccine hesitancy can and has resulted in deaths and outbreaks of vaccine-preventable diseases. Some effective responses to this problem include valid information from patients' health professionals and the media, and efforts to build trust in the public health apparatus.39
NONRESPONSIVENESS TO VACCINE IMMUNIZATION
Approximately 2–10% of healthy individuals fail to mount antibody levels to routine vaccines.40 There are two major factors responsible for unresponsiveness to vaccine immunizations. The first is vaccine related, such as failures in vaccine attenuation, vaccination regimens, or administration. The other is host related, in which host genetics, immune status, age, health, or nutritional status can be associated with a lack of or diminished response to vaccines. Nonresponsiveness increases with age, particularly in persons > 65 years, which is associated with a high low- or nonresponder rate, which suggests that vaccine schedules should be modified according to age. To improve immune responsiveness to vaccines in the elderly (ages > 60 years), accelerated schedules, higher vaccine doses, or immune-enhancing adjuvants need to be considered, but this will require more research.41
CAN WE DO BETTER?
Having addressed the global burden of vaccine-preventable infectious diseases in children < 5 years of age, it may now be possible to provide some recommendations on ways we can together improve public health resources in underdeveloped nations for the promotion of protective immunity against COVID-19 and other vaccine-preventable diseases.
Decrease vaccine hesitancy by education to correct misinformation.
Encourage breast-feeding and maternal immunization in both developed and underdeveloped countries.
Encourage governmental agencies to promote and provide access to immunization.
Improve the efficacy of immunizing agents with established efficacy (influenza, pneumococcal, pertussis, and rotavirus vaccines).
Encourage the public to continue receiving all recommended immunizations during the COVID-19 pandemic.
Careful assessment of nonmedical exemptions for immunizations required for preschool and school attendance.
CONCLUSION
Issues with regard to the burden of disease mortality, disease transmission, and available vaccines as well as vaccine successes and shortcomings for specific pathogens can serve as important landmarks for effective use of future vaccines. Although much success has been made globally in preventing these childhood deaths, much remains to be done.
Footnotes
Presented at the First Annual Joseph A. Bellanti, M.D. Lectureship at Pediatric Grand Rounds, Departments of Pediatrics and Microbiology-Immunology, Georgetown University Medical Center, Washington, D.C., May 7, 2021
No external funding sources reported
The author has no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Frenkel LD, Joseph A. Bellanti, MD, FAAP, FACAAI, FAAAAI. Ann Allergy Asthma Immunol. 2016; 117:1–8. [DOI] [PubMed] [Google Scholar]
- 2. Frenkel LD. Infectious diseases as a cause of global childhood mortality and morbidity: Progress in recognition, prevention, and treatment. Adv Pediatr Res. 5:14. doi: 10.24105/apr.2018.5.14. [DOI] [Google Scholar]
- 3. Lucia H, David S, Danzhen Y. Levels and Trends in Child Mortality Report 2019. UNICEF. https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2019.
- 4. Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011; 378:1917–1930. [DOI] [PubMed] [Google Scholar]
- 5. Selvaraj K, Chinnakali P, Majumdar A, et al. Acute respiratory infections among under-5 children in India: a situational analysis. J Nat Sci Biol Med. 2014; 5:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whitney CG. Pneumococcal and Haemophilus influenzae type b disease: moving numbers in the right direction. Lancet Glob Health. 2018; 6:e706–e707. [DOI] [PubMed] [Google Scholar]
- 7. Feldman C, Anderson R. Recent advances in the epidemiology and prevention of Streptococcus pneumoniae infections. F1000Res. 2020; 9:F1000 Faculty Rev-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gabutti G, Azzari C, Bonanni P, et al. Pertussis. Hum Vaccin Immunother. 2015; 11:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cherry JD. Pertussis in young infants throughout the world. Clin Infect Dis. 2016; 63(suppl 4):S119–S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hewlett EL, Burns DL, Cotter PA, et al. Pertussis pathogenesis–what we know and what we don't know. J Infect Dis. 2014; 209:982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frenkel LD. The underappreciated need for maternal and neonatal immunization: call to action. O J Gyn Obstet Res. 2019; 1:13–18. https://www.google.com/search?sxsrf=ALeKk02xgWJUvA4BGT_KTIn_VjFsxWZvkA:1627955139192&q=frenke. [Google Scholar]
- 12. Klein NP, Bartlett J, Rowhani-Rahbar A, et al. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012; 367:1012–1019. [DOI] [PubMed] [Google Scholar]
- 13. Ruf BR, Knuf M. The burden of seasonal and pandemic influenza in infants and children. Eur J Pediatr. 2014; 173:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005; 5:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luteijn JM, Brown MJ, Dolk H. Influenza and congenital anomalies: a systematic review and meta-analysis. Hum Reprod. 2014; 29:809–823. [DOI] [PubMed] [Google Scholar]
- 16. Francis T, Jr. Vaccination against influenza. Bull World Health Organ. 1953; 8:725–741. [PMC free article] [PubMed] [Google Scholar]
- 17. Belshe RB, Ambrose CS, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2–7 years of age. Vaccine. 2008; 26(suppl 4):D10–D16. [DOI] [PubMed] [Google Scholar]
- 18. Piedra PA. Live attenuated influenza vaccine: will the Phoenix rise again? Pediatrics. 2019; 143:e20183290. [DOI] [PubMed] [Google Scholar]
- 19. Chung JR, Flannery B, Ambrose CS, et al. Live attenuated and inactivated influenza vaccine effectiveness. Pediatrics. 2019; 143:e20182094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2020–21 influenza season. MMWR Recomm Rep. 2020; 69:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cox MMJ, Izikson R, Post P, et al. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther Adv Vaccines. 2015; 3:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frenkel LD, Bellanti JA. Immunology of measles, mumps, and rubella viruses. In: Nahmias AJ, O'Reilly RJ. (Eds). Immunology of Human Infection. Boston: Springer US, 135–163, 1982. [Google Scholar]
- 23. Worldwide measles deaths climb 50% from 2016 to 2019 claiming over 207 500 lives in 2019 12 November 2020 WHO. Available online at https://www.who.int/news/item/12-11-2020-worldwide-measles-deaths-climb-50-from-2016-to-2019-claiming-over-207-500-lives-in-2019#:∼:text=EPA,Worldwide%20measles%20deaths%20climb%2050%25%20from%202016%20to%202019%20claiming,207%20500%20lives%20in%202019&text=Measles%20surged%20worldwide%20in%202019,reported%20cases%20in%2023%20years; Accessed November 12, 2020.
- 24. Wakefield AJ, Murch SH, Anthony A, et al. Retracted article. Ileal lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998; 351:637–641. [DOI] [PubMed] [Google Scholar]
- 25. Eggertson L. Lancet retracts 12-year-old article linking autism to MMR vaccines. CMAJ. 2010; 182:E199–E200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis MM, Shah SK. Outbreaks of vaccine-preventable diseases responding to system failure with national vaccination requirements. JAMA. 2019; 322:33–34. [DOI] [PubMed] [Google Scholar]
- 27. Yang YT, Silverman RD. Legislative prescriptions for controlling nonmedical vaccine exemptions. JAMA. 2015; 313:247–248. [DOI] [PubMed] [Google Scholar]
- 28. Tate JE, Burton AH, Boschi-Pinto C, et al. Global, regional, and national estimates of rotavirus mortality in children <5 years of age. Clin Infect Dis. 2016; 62(suppl 2):S96–S105. [DOI] [PubMed] [Google Scholar]
- 29. Lopman B, Vicuña Y, Salazar F, et al. Household transmission of rotavirus in a community with rotavirus vaccination in Quininde, Ecuador. PLoS One. 2013; 8:e67763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burnett E, Yen C, Tate JE, et al. Rotavirus vaccines: current global impact and future perspectives. Future Virol. 2016; 11:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization. Immunization coverage. Available online at www.who.int/mediacentre//factsheet; accessed https://www.who.int/news-room/fact-sheets/detail/immunization-coverage mmunization.
- 32. Atchison CJ, Stowe J, Andrews N, et al. Rapid declines in age group-specific rotavirus infection and acute gastroenteritis among vaccinated and unvaccinated individuals within 1 year of rotavirus vaccine introduction in England and Wales. J Infect Dis. 2016; 213:243–249. [DOI] [PubMed] [Google Scholar]
- 33. Lopman BA, Pitzer VE, Sarkar R, et al. Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PLoS One. 2012; 7:e41720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Desselberger U. Differences of rotavirus vaccine effectiveness by country: likely causes and contributing factors. Pathogens. 2017; 6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frenkel L, Gomez F, Bellanti JA. COVID-19 in children: pathogenesis and current status. Allergy Asthma Proc. 2021; 42:8–15. [DOI] [PubMed] [Google Scholar]
- 36. Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021; 325:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson MG, Ballinger EA, Benjamin D, et al. A clinical perspective of the U.S. anti-vaccination epidemic: considering marginal costs and benefits, CDC best practices guidelines, free riders, and herd immunity. Vaccine. 2020; 38:7877–7879. [DOI] [PubMed] [Google Scholar]
- 38. Bellanti JA. COVID-19 vaccines and vaccine hesitancy: role of the allergist/immunologist in promotion of vaccine acceptance. Allergy Asthma Proc. 2021; 42(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:591–593. [DOI] [PubMed] [Google Scholar]
- 40. Wiedermann U, Garner-Spitzer E, Wagner A. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother. 2016; 12:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boraschi D, Italiani P. Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunol Lett. 2014; 162(pt B):346–353. [DOI] [PubMed] [Google Scholar]