Abstract
In the wake of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, an increasing number of patients with neurological disorders, including Guillain-Barré syndrome (GBS), have been reported following this infection. It remains unclear, however, if these cases are coincidental or not, as most publications were case reports or small regional retrospective cohort studies. The International GBS Outcome Study is an ongoing prospective observational cohort study enrolling patients with GBS within 2 weeks from onset of weakness. Data from patients included in this study, between 30 January 2020 and 30 May 2020, were used to investigate clinical and laboratory signs of a preceding or concurrent SARS-CoV-2 infection and to describe the associated clinical phenotype and disease course. Patients were classified according to the SARS-CoV-2 case definitions of the European Centre for Disease Prevention and Control and laboratory recommendations of the World Health Organization. Forty-nine patients with GBS were included, of whom eight (16%) had a confirmed and three (6%) a probable SARS-CoV-2 infection. Nine of these 11 patients had no serological evidence of other recent preceding infections associated with GBS, whereas two had serological evidence of a recent Campylobacter jejuni infection. Patients with a confirmed or probable SARS-CoV-2 infection frequently had a sensorimotor variant 8/11 (73%) and facial palsy 7/11 (64%). The eight patients who underwent electrophysiological examination all had a demyelinating subtype, which was more prevalent than the other patients included in the same time window [14/30 (47%), P = 0.012] as well as historical region and age-matched control subjects included in the International GBS Outcome Study before the pandemic [23/44 (52%), P = 0.016]. The median time from the onset of infection to neurological symptoms was 16 days (interquartile range 12–22). Patients with SARS-CoV-2 infection shared uniform neurological features, similar to those previously described in other post-viral GBS patients. The frequency (22%) of a preceding SARS-CoV-2 infection in our study population was higher than estimates of the contemporaneous background prevalence of SARS-CoV-2, which may be a result of recruitment bias during the pandemic, but could also indicate that GBS may rarely follow a recent SARS-CoV-2 infection. Consistent with previous studies, we found no increase in patient recruitment during the pandemic for our ongoing International GBS Outcome Study compared to previous years, making a strong relationship of GBS with SARS-CoV-2 unlikely. A case-control study is required to determine if there is a causative link or not.
Keywords: Guillain-Barré, syndrome, COVID-19, SARS-CoV-2, preceding infections, clinical phenotype
Luijten et al. report that patients with Guillain-Barré syndrome (GBS) after SARS-CoV-2 infection share uniform neurological features, similar to those previously described in other cases of post-viral GBS. They conclude that SARS-CoV-2 infection may be an occasional trigger for GBS, but that a strong association is unlikely.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has affected the entire world population, either by direct infection or through its social and economic consequences. The severity and impact of this outbreak prompted the World Health Organization (WHO) to declare SARS-CoV-2 a Public Health Emergency of International Concern on 30 January 2020.1 Besides the well known severe respiratory signs, both central and peripheral neurological complications have been reported.2-4 Potential pathophysiological mechanisms for these complications may be either direct viral invasion, indirect damage as a result of the inflammatory response (para-infectious, post-infectious), or hypercoagulability in cases of cardiovascular complications.5 One of the reported neurological disorders is Guillain-Barré syndrome (GBS), an inflammatory polyradiculoneuropathy characterized by rapidly progressive weakness and sensory signs, usually preceded by an infectious trigger.6 Several pathogens have previously been associated with GBS and outbreaks of these infections may lead to an increased incidence of GBS as seen during the Zika virus pandemic in 2015–16.7–9 The clinical phenotype, electrophysiological subtype and disease course of GBS are heterogeneous and may be influenced by the type of preceding infection as a result of differences in antigenic targets. In some of these clinical variants specific antibody responses against gangliosides could be found. For example, a preceding infection with Campylobacter jejuni is associated with antibodies against GM1 and GD1a, and a pure motor axonal variant with a more severe disease course and poor outcome.10
Since the beginning of the recent pandemic, over 90 GBS patients with a possible relation to SARS-CoV-2 have been reported.11–14 However, whether SARS-CoV-2 is another potential infectious trigger or whether the reported cases are coincidental is still unclear. In the current study, we identified GBS cases with a preceding SARS-CoV-2 infection, based on clinical and laboratory features, during the first months of the pandemic within the framework of the International GBS Outcome Study (IGOS), an ongoing prospective observational cohort study which began in 2012.15 We describe in detail the clinical phenotype, electrophysiological subtype, and disease course of these patients.
Materials and methods
Study design and patients
Data from all GBS patients included in IGOS from 30 January until 30 May 2020 were used for this study. IGOS is an international multicentre prospective observational cohort study in which all GBS patients can be included within 2 weeks from the onset of symptoms, independent of the disease severity or clinical variant. Data and biological samples are collected according to a predefined protocol.15 As the first wave of the SARS-CoV-2 pandemic may have caused delays in hospital referral and study inclusion, we allowed 4 weeks from symptom onset for the inclusion of GBS cases. Information on the acute phase of GBS was collected retrospectively in these patients. Patients needed to fulfil the diagnostic criteria for GBS (National Institute of Neurological Disorders and Stroke) or its clinical variants.16,17 Patients with an alternative diagnosis were excluded.
Patient recruitment rates of IGOS from the previous 3 years were compared with the recruitment rate during the first months of the pandemic. Because patient inclusion depends, among other factors, on whether or not study sites are actively recruiting patients, we also looked at inclusion rates in selected countries (China, Italy, Switzerland and The Netherlands) with stable inclusion rates of >10 patients/year in the past years.
Data collection and case definitions
Clinical characteristics
Comprehensive data on demographics, symptoms of preceding infections, co-morbidities, clinical presentation of GBS, CSF examination, nerve conduction studies (NCS), treatment, disease progression, and clinical course were collected prospectively at fixed time points.15 Clinical parameters have been defined in the original IGOS protocol and are described in previous publications.15,18 We interpreted data until a maximum follow-up of 13 weeks. Data collected after 13 weeks will be used for future studies. The clinical variant of GBS was identified by the local investigator at Week 2 and, if missing, at Week 1 or entry. Disease severity was expressed using the GBS disability score (0–6): 0 = healthy, 1 = minor symptoms but capable of running, 2 = able to walk 10 m without assistance but unable to run, 3 = able to walk 10 m with help, 4 = bedridden or chair bound, 5 = requiring assisted ventilation for at least part of the day, 6 = dead.19 Severe GBS was defined as a GBS disability score at nadir ≥ 3, similar to previous studies.20 For patients with Week 13 missing who were able to walk independently at Week 8 or Week 4, this previous visit was used to determine the GBS disability score at Week 13. The electrophysiological subtype was determined according to the Hadden classification, by using the raw data of the first NCS.21 If the raw NCS data were missing, we used the subtype defined by the local investigator.
SARS-CoV-2 suspicion
Additional information regarding the clinical suspicion of SARS-CoV-2 infection was collected with a structured questionnaire, which contained questions on preceding symptoms, laboratory and radiological results, serological evidence of other recent infections, and complications of SARS-CoV-2.
Investigators were asked to test the included patients for SARS-CoV-2 by PCR (on oro/nasopharyngeal, respiratory, or faecal material) and/or serology (IgM and IgG) in the local hospitals. Dutch patients with a probable or confirmed SARS-CoV-2 infection and available material were tested for SARS-CoV-2 serology (Wantai SARS-CoV-2 total Ig and IgM ELISA from Beijing Wantai Biological Pharmacy Enterprise Co., Ltd) at the Erasmus MC University Medical Center.22
Patients were classified according to the SARS-CoV-2 case definitions of the European Centre for Disease Prevention and Control (Box 1).23 Patients were classified as ‘possible’ if they had at least one clinical sign of SARS-CoV-2 infection, ‘probable’ if they had abnormalities on radiological imaging suspicious for SARS-CoV-2 infection, or if they had both clinical signs and an epidemiological link, and ‘confirmed’ if there was laboratory confirmation of SARS-CoV-2 infection. Laboratory confirmation was based on the WHO recommendations and defined as a positive PCR for SARS-CoV-2 or positive serology on repeated samples.24,25 Radiological findings suspicious for SARS-CoV-2 infection on CT-thorax consisted of bilateral infiltrates, uni- or bilateral ground-glass opacities, multifocal consolidation, or bilateral interstitial abnormalities.26
Box 1 Case definitions SARS-CoV-2 infection based on the ECDC criteria and WHO laboratory recommendations
Clinical criteria
Any person with at least one of the following symptoms:
cough
fever
shortness of breath
sudden onset of anosmia, ageusia or dysgeusia
Diagnostic imaging criteria
Radiological evidence showing lesions compatible with COVID-19a
Laboratory criteria
Detection of SARS-CoV-2 nucleic acid in a clinical specimen OR positive serology on repeated serum samplesb
Epidemiological criteria
At least one of the following two epidemiological links:
close contact with a confirmed COVID-19 case in the 14 days prior to onset of symptoms
having been a resident or a staff member, in the 14 days prior to onset of symptoms, in a residential institution for vulnerable people where ongoing COVID-19 transmission has been confirmed
Case classification
Possible case:
Any person meeting the clinical criteria
Probable case:
Any person meeting the clinical criteria with an epidemiological link
OR
Any person meeting the diagnostic imaging criteria
Confirmed case:
Any person meeting the laboratory criteria
In the main analysis, we focused on the clinical phenotype and subtype of GBS patients with a confirmed/probable SARS-CoV-2 infection and compared these patients with the other patients that were included in the same time window (possible and no suspicion combined). We chose this comparison because the patients in the possible group had non-specific symptoms that are also common in respiratory tract infections caused by other pathogens. We also performed three additional analyses. In the first analysis, we aimed to investigate whether the clinical phenotype and subtype of SARS-CoV-2 confirmed/probable cases was specific for SARS-CoV-2 and compared their neurological features with historical control patients matched for region and age (±15 years) that were included in IGOS before the pandemic (2012–17). In the other two additional analyses, we compared the clinical GBS phenotype and disease course of the three subgroups of SARS-CoV-2 suspicion separately (confirmed/probable versus possible versus no suspicion) and excluded the possible patients (confirmed/probable versus no suspicion) as some of them may have had a recent SARS-CoV-2 infection. The additional analyses are provided in the Supplementary material. Significant findings and discrepancies between these analyses and the main analysis are described in the text.
Other preceding infections
SARS-CoV-2 probable and confirmed patients were tested locally for other preceding infections associated with GBS including: Campylobacter jejuni, Mycoplasma pneumoniae, Epstein-Barr virus (EBV), cytomegalovirus (CMV) and hepatitis E virus (HEV), when possible. Test results were defined as positive, negative, or inconclusive based on definitions of the local laboratory. In general, evidence of a recent infection was defined via IgM positivity for M. pneumoniae and HEV, IgM or IgA positivity for C. jejuni, IgM positivity with negative IgG or IgG with low avidity for CMV, and virus capsid antigen (VCA) IgM positivity with negative Epstein-Barr nuclear antigen (EBNA) IgG for EBV. See the Supplementary material for a more detailed description of the interpretation of the test results.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics 25. Variables were described using medians (interquartile range, IQR) and numbers (percentage). To compare variables between subgroups, a Mann-Whitney U-test or Kruskal-Wallis test was used for numerical variables and a chi-square test or Fisher’s exact test for categorical variables. A two-sided P-value < 0.05 was considered significant. For the comparison of the SARS-CoV-2 confirmed/probable cases with historical control patients included in IGOS before the pandemic, we used a 1:7 ratio and the cases and matched controls were analysed as two independent groups. In the additional analysis where three subgroups were compared, a Bonferroni correction was used to correct for multiple testing. Therefore, we divided the significance level of 0.05 by the number of possible tests (three groups = three pairwise comparisons), so P-values < 0.017 were considered to be significant for this analysis.
Ethical approval
IGOS was approved by the institutional review boards of the Erasmus MC University Medical Center (MEC-2011-477) and all participating international local site institutes. Written informed consent was obtained from each patient.
Data availability
Data of included patients in IGOS will be used for future studies and may be made available upon reasonable request after consulting the IGOS Steering Committee.
Results
Patient inclusion and classification
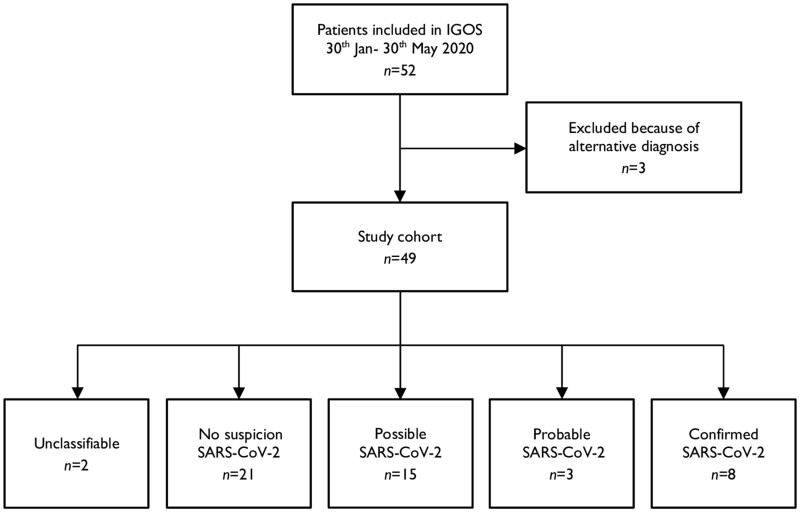
Fifty-two GBS patients were enrolled in IGOS from 30 January to 30 May 2020 (Fig. 1). Three patients were excluded from analysis because of an alternative diagnosis: one patient had myelitis (and a PCR confirmed SARS-CoV-2 infection), one had polyradiculopathy due to a B-cell lymphoma, and one had thiamine deficiency.
Figure 1.
Patient inclusion and SARS-CoV-2 case classification.
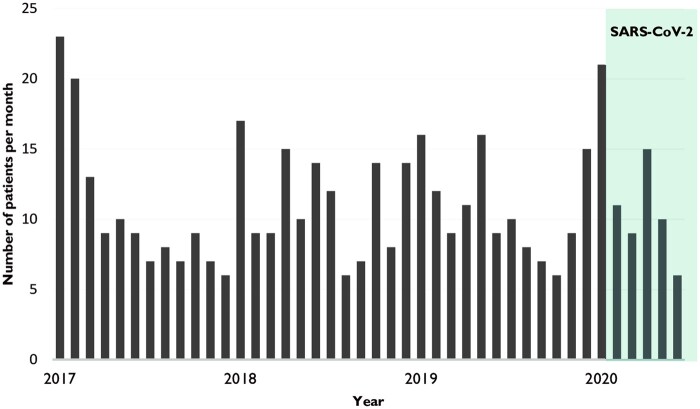
The 49 remaining patients were included in China (n = 6), Denmark (n = 1), France (n = 3), Greece (n = 1), Italy (n = 7), Japan (n = 2), The Netherlands (n = 12), Spain (n = 2), Switzerland (n = 14), and the UK (n = 1). We did not see an increase in the inclusion rate of IGOS during the pandemic compared to the previous 3 years (Fig. 2). When focusing on selected regions (e.g. Switzerland, The Netherlands, China) with more stable inclusion rates, only Switzerland had an increase in patient inclusion in April 2020 (six patients versus an average of one to three inclusions per month in the year before the pandemic).
Figure 2.
Patient inclusion in IGOS between 2017 and 2020. Patient inclusion within IGOS per month from 2017 until 2020. Fluctuations in inclusion rate can be explained by countries that started or stopped recruiting patients.
Forty-eight questionnaires assessing clinical suspicion for SARS-CoV-2 infection were completed (response rate of 98%). Based on the European Centre for Disease Prevention and Control (ECDC) case definitions, two patients (4%) were unclassifiable; 21 (43%) did not have suspicion for SARS-CoV-2; 15 (31%) were classified as possible; three (6%) as probable; and eight (16%) as confirmed cases.
Below we focus on the clinical features and disease course of GBS patients with a probable or confirmed SARS-CoV-2 infection (n = 11) and compare them to the other GBS cases included in the same time period with a possible SARS-CoV-2 infection or without SARS-CoV-2 suspicion (n = 36).
Clinical GBS phenotype in relation to SARS-CoV-2 suspicion
Table 1 summarizes the clinical characteristics of the total cohort and compares the patients with a confirmed/probable SARS-CoV-2 infection to those without. Two patients had an unclassifiable SARS-CoV-2 status and were therefore excluded from this comparison. A more detailed overview of the clinical features of the confirmed and probable SARS-CoV-2 infected patients is shown in Table 2.
Table 1.
GBS patient characteristics in relation to SARS-CoV-2 infection
| SARS-CoV-2 confirmed/probablea |
||||
|---|---|---|---|---|
| Total (n = 49) | Yes (n = 11) | No (n = 36) | P-value | |
| Demographics | ||||
| Median age (IQR) | 56 (37–67) | 63 (60–67) | 53 (32–66) | 0.035* |
| Males/females (ratio) | 31/18 (1.7) | 7/4 (1.8) | 23/13 (1.8) | 0.99 |
| Preceding symptoms (%) | ||||
| Fever | 22/48 (46) | 10 (91) | 12 (33) | 0.001* |
| Respiratoryb | 14/47 (30) | 9 (82) | 5 (14) | <0.001* |
| Gastro-intestinal | 14 (29) | 3 (27) | 11 (31) | 0.84 |
| None | 17 (35) n = 48 | 1 (9) | 16 (44) | 0.039* |
| Days before onset GBS (IQR) | 13 (6–22) n = 31 | 16 (12–22) n = 10 | 12 (5–23) n = 20 | 0.40 |
| Clinical GBS variant (%) | ||||
| Sensorimotor | 35/47 (75) | 8 (73) | 27/34 (79) | 0.69 |
| Pure motor | 3/47 (6) | 0 (0) | 3/34 (9) | 0.57 |
| MFS | 2/47 (4) | 0 (0) | 1/34 (3) | – |
| MFS-GBS overlap syndrome | 3/47 (6) | 2 (18) | 1/34 (3) | 0.14 |
| Ataxic | 4/47 (9) | 1 (9) | 2/34 (6) | 0.71 |
| Neurological deficits at entryc | ||||
| Cranial nerve involvement (%) | 16/47 (34) | 5 (46) | 10/34 (29) | 0.46 |
| Oculomotor | 6 (13) | 1 (9) | 4 (12) | 0.81 |
| Facial | 12 (26) | 4 (36) | 8 (24) | 0.45 |
| Bulbar | 10 (21) | 3 (27) | 6 (17) | 0.67 |
| Median MRC sum score (IQR) | 52 (41–60) n = 45 | 51 (22–54) | 51 (41–59) n = 32 | 0.58 |
| Tetraparesis (%) | 30 (67) | 8 (73) | 22 (69) | 0.73 |
| Paraparesis (%) | 3 (7) | 0 (0) | 3 (9) | 0.57 |
| Sensory deficits (%) | 32/45 (71) | 9 (82) | 21/32 (66) | 0.46 |
| Pain (%) | 22/48 (46) | 3 (27) | 18/35 (51) | 0.16 |
| Ataxia(%) | 15/37 (41) | 3/9 (33) | 11/27 (41) | 0.69 |
| Autonomic dysfunctiond (%) | 11/47 (23) | 4 (36) | 7/34 (21) | 0.42 |
| Days onset GBS-entry (IQR) | 5 (3–10) n = 48 | 9 (3–11) | 5 (2–9) n = 35 | 0.25 |
| Clinical severity of GBS | ||||
| Lowest MRC sum score (IQR) | 47 (33–56) n = 46 | 44 (2–52) n = 11 | 46 (34–55) n = 33 | 0.39 |
| Highest GBS disability score (%) | ||||
| 0–2 | 8/47 (17) | 0 (0) | 8/34 (24) | 0.17 |
| 3–4 | 30/47 (64) | 7 (64) | 21/34 (62) | 0.91 |
| 5 | 9/47 (19) | 4 (36) | 5/34 (15) | 0.19 |
| CSF | ||||
| Leucocyte count (IQR) | 2 (1–3) n = 42 | 1 (1–3) n = 9 | 2 (1–4) n = 31 | 0.50 |
| Protein level, g/l (IQR) | 1.01 (0.49–1.55) | 1.50 (0.85–1.87) | 0.80 (0.45–1.51) | 0.16 |
| Elevated, >0.45 g/l (%) | 32 (76) | 8 (89) | 23 (74) | 0.65 |
| Days onset GBS–spinal tap (IQR) | 4 (2–8) | 4 (2–9) | 4 (2–9) | 0.69 |
| Electrophysiological subtype (%) | ||||
| Demyelinating | 23/39 (59) | 8/8 (100) | 14/30 (47) | 0.012* |
| Axonal | 3/39 (8) | 0 (0) | 3/30 (10) | 0.59 |
| Equivocal | 13/39 (33) | 0 (0) | 13/30 (43) | 0.034* |
| Treatment (%) | ||||
| Intravenous immunoglobulins | 39/47 (83) | 10/11 (91) | 28/34 (82) | 0.66 |
| Plasma exchange | 3/47 (6) | 1/11 (9) | 2/34 (6) | 0.71 |
| Corticosteroidse | 2/47 (4) | 0 (0) | 2/34 (6) | – |
| None | 5/47 (11) | 0 (0) | 4/34 (12) | 0.56 |
Results were given as median (25th–75th percentile) or as counts (percentage). MRC sum score = sum of Medical Research Council scores for muscle groups for shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension and ankle dorsiflexion of both limbs.
Significant values (P < 0.05).
Classification by the ECDC case definitions. Unclassifiable patients (n = 2) are not shown.
Respiratory symptoms included cough and or dyspnoea.
Parameters that could not be examined were coded as missing values.
Autonomic dysfunction included disturbances in blood pressure and cardiac, gastro-intestinal or bladder dysfunction.
Additional to intravenous immunoglobulins.
Table 2.
Characteristics of GBS patients with a confirmed or probable SARS-CoV-2 infection
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Date onset GBS | 22 March | 29 March | 26 March | 8 April | 8 April | 7 April | 8 April | 9 April | 24 April | 17 April | 18 April |
| Country | IT | IT | CH | CH | CH | NL | NL | NL | NL | UK | ES |
| Age/sex | 80/Male | 78/Male | 52/Female | 63/Female | 61/Female | 67/Male | 50/Male | 63/Male | 60/Female | 64/Male | 66/Male |
| Preceding symptoms | |||||||||||
| Fever | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Respiratorya | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Gastro-intestinal | No | No | No | No | Yes | No | No | Yes | No | Yes | No |
| Days before onset GBS | 4 | 24 | 13 | 13 | 21 | Undefined | 28 | 12 | 18 | 12 | 21 |
| Neurological deficitsb | |||||||||||
| CNI | IX, X | No | No | No | VII, IX, X | III, VII | VII | VII | VII | III, VII, IX, X | VII |
| Lowest MRC sumc | 2 | 60 | 22 | 60 | 49 | 0 | 48 | 0 | 44 | 40 | 52 |
| Sensory deficits | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ataxia | No | Yes | No | Yes | No | No | Yes | No | No | Yes | No |
| Auto. dysfunction | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Clinical GBS variant | Sensorimotor | Ataxic | Sensorimotor | Sensorimotor | Sensorimotor | Sensorimotor | MFS-GBS | Sensorimotor | Sensorimotor | MFS-GBS | Sensorimotor |
| Clinical severity of GBS | |||||||||||
| Highest GBS-DS | 6 | 3 | 5 | 4 | 4 | 6 | 4 | 5 | 4 | 4 | 3 |
| ICU admission | Yes | No | Yes | No | Yes | Yes | No | Yes | No | Yes | No |
| Needed MV | Yes | No | Yes | No | No | Yes | No | Yes | No | No | No |
| CSF | |||||||||||
| Leucocytes, cells/µl | 1 | 20 | 3 | 2 | 1 | N.t. | 1 | N.t. | 3 | 0 | 1 |
| Protein level, g/l | 1.75 | 1.06 | 5.91 | 0.39 | 1.5 | N.t. | 0.64 | N.t. | 1.11 | 1.8 | 1.93 |
| PCR SARS-CoV-2 | N.t. | Neg. | Neg. | N.t. | N.t. | N.t. | Neg. | N.t. | Neg. | N.t. | N.t. |
| NCS subtype | AIDP | AIDP | AIDP | AIDP | AIDP | N.t. | AIDP | N.t. | AIDP | N.t. | AIDP |
| Brighton classification | Level 1 | Level 4 | Level 1 | Level 2 | Level 1 | Level 3 | Level 1 | Level 3 | Level 1 | Level 2 | Level 1 |
| SARS-CoV-2 suspicion | Probable | Confirmed | Confirmed | Confirmed | Confirmed | Confirmed | Confirmed | Probable | Confirmed | Probable | Confirmed |
| PCR (times tested) | Negative (4×) | Positive | Positive | Positive | Positive | Positive | Positive | Negative (5×) | Positive | Negative (2×) | Negative (2×) |
| Serologyd | N.t. | N.t. | IgM+ IgG+ | IgM+ IgG− | N.t. | IgM+ Ig+ | IgM+ Ig+ | IgM+ Ig+ | IgM− Ig+ | IgG+ | IgM+ IgG+ |
| CT-thorax suspected | Yes | Yes | Yes | N.t. | Yes | Yes | N.t. | Yes | N.t. | Yes | No |
| Complications | Pneumonia, ARDS, sepsis | Pneumonia | Pneumonia | Pneumonia | Pneumonia | Pneumonia, PE, cardial | None | Pneumonia, sepsis, cardial | Cervical myelitis | Pneumonia | None |
| Other infections | |||||||||||
| Positive | – | CJE | CJE | – | – | – | – | – | – | – | – |
| Negativee | CJE, MP, EBV, CMV, HEV | MP, EBV, CMV, HEV | MP, EBV, CMV, HEV | CJE, MP, EBV, CMV, HEV | CJE, EBV, CMV | CJE, MP, EBV, CMV, HEV | CJE, MP, EBV, CMV, HEV | CJE, MP, EBV, CMV, HEV | CJE, MP, EBV, CMV, HEV | CJE, MP, EBV, CMV, HEV | CJE, EBV, CMV |
Auto. dysfunction = autonomic dysfunction; AIDP = acute inflammatory demyelinating polyradiculoneuropathy; CH = Switzerland; CJE = C. jejuni; CNI = cranial nerve involvement; ES = Spain; GBS-DS = GBS disability score; HEV = hepatitis E virus; IT = Italy; MRC sum = sum of Medical Research Council scores for muscle groups for shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension and ankle dorsiflexion of both limbs; MFS-GBS = MFS-GBS overlap; MP = M. pneumoniae; MV = mechanical ventilation; NL = Netherlands; N.t. = not tested; PE = pulmonary embolism.
Respiratory symptoms included cough and or dyspnoea.
Neurological deficits during acute phase GBS (from study entry to 4 weeks).
Lowest MRC sum score observed during study period, which is not necessarily equal to the MRC sum score at disease nadir.
Patient 11 was tested repeatedly and therefore classified as a confirmed case: first sample IgG positive, IgM not tested; second sample IgM positive, IgG not tested. Patient 8 was tested only once for SARS-CoV-2 serology, and therefore not classified as a confirmed case.
Patients 5, 6 and 8 were tested on serum samples obtained after treatment with intravenous immunoglobulins, because no pretreatment material was available.
The median age of the total cohort was 56 years (IQR 37–67). The patients with a confirmed/probable SARS-CoV-2 infection were significantly older than the remaining patients [63 years (IQR 60–67) versus 53 years (IQR 32–66), P = 0.035]. The median time from onset of weakness until study entry was 5 days (IQR 3–10). Three patients entered the study between 2 and 4 weeks after onset of neurological symptoms, due to a delay in hospital admission or a delay in informed consent due to the pandemic. Preceding respiratory symptoms and fever occurred more frequently in patients with a confirmed/probable SARS-CoV-2, which was expected as the classification according to the ECDC case definitions is partly based on such symptoms.
The majority of SARS-CoV-2 confirmed/probable patients had a sensorimotor GBS variant (8/11, 73%), although Miller Fisher syndrome (MFS) GBS overlap (2/11, 18%) and an ataxic variant (1/11, 9%) were also reported. All patients with a confirmed/probable SARS-CoV-2 infection had a severe form of GBS (GBS disability score at nadir ≥ 3). Common early neurological features were: facial weakness in 7/11 (64%), sensory deficits in 9/11 (82%), and autonomic dysfunction in 7/11 (64%), although not significantly different compared to the other patients.
Electrophysiological examination was performed in 39/49 (80%) patients, with raw data available in 37 (including all patients with a confirmed/probable SARS-CoV-2 infection). The data for these 37 patients were independently assessed and classified according to the Hadden classification. For the other two patients, the classification of the local investigator was used. All confirmed and probable SARS-CoV-2 patients who underwent NCS had a demyelinating subtype, which was more frequent than in the other GBS patients [8/8 (100%) versus 14/30 (47%), P = 0.012]. After excluding the patients from Asia (n = 8), this association was still statistically significant (P = 0.047). Both SARS-CoV-2 confirmed/probable cases and the other patients underwent extensive electrophysiological examination as approximately four motor and three sensory nerves were examined in both groups.
In 42/49 (86%) patients, a spinal tap was performed with a median leucocyte count of 2 cells/µl (IQR 1–3) and protein level of 1.01 g/l (IQR 0.49–1.55). CSF examination was performed in 9/11 (82%) confirmed/probable SARS-CoV-2 patients of whom only one had an increased (>5 cell/µl) leucocyte count of 20 cells/µl. This patient was negative for SARS-CoV-2 PCR in CSF and other diagnoses (e.g. myelitis, infectious causes) were excluded after extensive investigation. The demyelinating features on NCS further confirmed the diagnosis of GBS in this patient. All SARS-CoV-2 confirmed/probable patients received immunomodulatory treatment: 10/11 (91%) intravenous immunoglobulins and 1/11 (9%) plasma exchange. None received additional treatment with steroids. Treatment did not differ between subgroups. Antiganglioside antibodies were tested in two SARS-CoV-2 infected patients and were negative in both.
Based on the Brighton Collaboration criteria, 6/11 SARS-CoV-2 confirmed/probable patients had a Level 1 certainty of GBS, 2/11 a Level 2, 2/11 a Level 3 (no CSF and NCS performed due to SARS-CoV-2 restrictions), and 1/11 a Level 4 (ataxic variant). Notably, one patient (Level 1) also had cervical myelitis with two short-segment foci on spinal cord MRI, one lateral and one central, which did not fully explain all of the observed neurological features such as facial palsy. The CSF of this patient showed a normal leucocyte count (3 cells/µl) and increased protein level (1.11 g/l) with a negative SARS-CoV-2 PCR in CSF. Electrophysiological examination in this patient showed a sensorimotor demyelinating polyneuropathy, which further supported the diagnosis of GBS.
Features of SARS-CoV-2 infection
All 11 GBS patients with a confirmed and probable SARS-CoV-2 developed neurological symptoms between 22 March and 24 April. These patients were included in Italy (n = 2), The Netherlands (n = 4), Spain (n = 1), Switzerland (n = 3), and the UK (n = 1) (Table 2). Three have been described in previously published case reports and another two were included and analysed in a retrospective cohort study.13,27,28 Common preceding infectious symptoms were fever 10/11 (91%), cough 7/11 (64%), and dyspnoea 5/11 (45%). Other reported symptoms were diarrhoea 3/11 (27%), fatigue 3/11 (27%), anosmia 3/11 (27%) and ageusia 2/11 (18%). The median time between preceding infectious symptoms and GBS symptoms was 16 days (IQR 12–22), which did not significantly differ when compared with the patients without a probable or confirmed SARS-CoV-2 infection (Table 1).
PCR SARS-CoV-2 testing was performed in 26/49 (53%) patients with a median time of 14 (IQR 5–28) days after onset of infectious symptoms. One patient tested positive for SARS-CoV-2 with PCR during rehabilitation 2 months after onset of GBS symptoms, and was therefore not included in the SARS-CoV-2 confirmed/probable group. Of the eight patients with a confirmed SARS-CoV-2 infection, seven had a positive PCR on an oro/nasopharyngeal swab during the acute phase of GBS or in the 3 weeks before, and in one a recent infection was confirmed by paired serology. All three patients with a probable SARS-CoV-2 infection had suspicious findings on CT-thorax in the setting of a negative PCR: in one serology was not performed, one had positive IgM and positive total Ig, and one had a positive IgG, but these were not confirmed in a paired test. SARS-CoV-2 PCR in CSF was performed in four of eight patients who had a positive oro/nasopharyngeal swab for SARS-CoV-2 and was negative in all four. A CT-thorax was performed in 8/11 (73%) of the confirmed/probable cases, seven of whom had abnormalities suspicious for SARS-CoV-2 infection. Four had a bilateral interstitial pneumonia and three had bilateral or unilateral ground glass opacities.
At hospital admission, 8/11 (73%) confirmed/probable SARS-CoV-2 patients had increased inflammatory markers (C-reactive protein and/or erythrocyte sedimentation rate). Other frequent laboratory abnormalities were increased liver enzymes (aspartate aminotransferase and/or alanine transaminase) 9/10 (90%), lymphocytopaenia 4/10 (40%), increased lactate dehydrogenase 4/8 (50%), and increased ferritin and creatinine kinase in 2/3 (67%). Pneumonia was the most common complication of SARS-CoV-2 infection and was present in 8/11 (73%) patients. Additionally, one patient suffered from acute respiratory distress syndrome and sepsis, one from pulmonary embolism and myocardial infarction, one from sepsis and atrial fibrillation, and one had a cervical myelitis.
Two SARS-CoV-2 confirmed/probable patients had serological evidence of a recent C. jejuni infection. One patient had a positive PCR for CMV on respiratory material with a negative IgM antibody response, indicating a reactivation of CMV.
Clinical course and short-term outcome of GBS patients with SARS-CoV-2 infection
Twenty-eight (57%) patients had a follow-up duration of 8 weeks or longer. Three (7%) patients died (Table 3): two from SARS-CoV-2 pneumonia or related complications (pulmonary embolism), and one SARS-CoV-2 negative patient died from a Pseudomonas aeruginosa pneumonia. Of the SARS-CoV-2 confirmed/probable patients, 6/11 (55%) needed to be admitted to the intensive care unit (ICU), and 4/11 (36%) required mechanical ventilation. All patients admitted to the ICU had CT thorax abnormalities and complications related to SARS-CoV-2 infection (pneumonia, acute respiratory distress syndrome, sepsis, pulmonary embolism), and five had a severe tetraparesis, of whom four with cranial nerve involvement. Three of the six patients admitted to the ICU also underwent NCS and had a demyelinating GBS subtype. GBS disability score and Medical Research Council (MRC) sum score at Weeks 4 and 13 did not differ between subgroups.
Table 3.
Clinical course and outcome of GBS in relation to SARS-CoV-2 infection
| SARS-CoV-2 confirmed/probablea |
||||
|---|---|---|---|---|
| Total (n = 49) | Yes (n = 11) | No (n = 36) | P-value | |
| Mortality (%) | 3/46 (7) | 2 (18) | 1/34 (3) | 0.14 |
| Mechanical ventilation (%) | 9/47 (19) | 4 (36) | 5/34 (15) | 0.19 |
| Days of ventilation (IQR) | 17 (10–31) n = 7 | 31 (17–31) n = 2 | 11 (7–25) n = 5 | 0.25 |
| ICU admission (%) | 13/47 (28) | 6 (55) | 7/34 (21) | 0.05 |
| Days in ICU (IQR) | 14 (3–31) n = 12 | 28 (7–40) n = 5 | 3 (3–24) n = 7 | 0.09 |
| Week 4 | ||||
| MRC sum score (IQR) | 54 (32–60) n = 30 | 49 (21–60) n = 8 | 55 (41–60) n = 22 | 0.28 |
| GBS disability score (%) | ||||
| 0–2 | 16/31 (52) | 3/8 (38) | 13/23 (57) | 0.43 |
| 3–4 | 12/31 (39) | 3/8 (38) | 9/23 (39) | 0.94 |
| 5 | 3/31 (10) | 2/8 (25) | 1/23 (4) | 0.16 |
| Week 13 | ||||
| GBS disability score (%) | ||||
| 0–2 | 21/30 (70) | 4/8 (50) | 16/21 (76) | 0.20 |
| 3–4 | 6/30 (20) | 2/8 (25) | 4/21 (19) | 0.72 |
| 5 | 0 (0) | 0 (0) | 0 (0) | – |
| 6 | 3/30 (10) | 2/8 (25) | 1/21 (5) | 0.18 |
Results are given as median (25th–75th percentile) or as counts (percentage). MRC sum = sum of Medical Research Council scores for muscle groups for shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension and ankle dorsiflexion of both limbs.
Significant values (P < 0.05).
Classification by the ECDC case definitions. Unclassifiable patients (n = 2) are not shown.
Additional analyses of the SARS-CoV-2 cases
In the previous analysis, we compared the clinical GBS phenotype of the patients with a confirmed/probable SARS-CoV-2 infection to the patients with no/possible SARS-CoV-2 suspicion that were included in the same time window. Additional analyses on the GBS phenotype and disease course of the SARS-CoV-2 confirmed/probable cases are provided in the Supplementary material. Compared to historical control patients included in IGOS before the pandemic (between 2012 and 2017), matched for region and age (Supplementary Table 1), SARS-CoV-2 confirmed/probable patients had significantly more often a demyelinating NCS subtype [8/8 (100%) versus 23/44 (52%), P = 0.016] and a higher CSF protein level [1.50 g/l (IQR 0.85–1.87) versus 0.65 g/l (0.4–1.11), P = 0.014]. The timing of the lumbar puncture after onset of weakness was similar with a median of 4 days (IQR 2–9) versus 5 days (IQR 2–7) (P = 0.47). Other clinical features, including sex, clinical GBS variant, neurological deficits at study entry, time between onset GBS and study entry, MRC sum score and GBS disability score at nadir, and CSF leucocyte count, did not significantly differ between the SARS-CoV-2 cases and historical controls.
Patients with a confirmed/probable SARS-CoV-2 infection had significantly more often a demyelinating NCS subtype compared to the other subgroups of SARS-CoV-2 suspicion, also after excluding the possible patients (Supplementary Table 2). No other significant differences between subgroups were found (Supplementary Tables 2 and 3).
Discussion
In this international prospective cohort study, 22% of the GBS patients included during the first 4 months of the pandemic had a preceding SARS-CoV-2 infection. Eight (16%) had a confirmed and three (6%) a probable infection. These patients were all ≥50 years of age and had a demyelinating electrophysiological subtype. The most common GBS variant in this group was the sensorimotor (73%), and patients frequently had facial palsy (64%). All GBS patients with a SARS-CoV-2 infection had a severe form of GBS as none of them could walk independently at nadir (GBS disability score ≥3). We cannot determine whether their recovery was worse compared to the other GBS patients as patient numbers were small and their disease course may have also been affected by the severity and complications of SARS-CoV-2 infection.
Similar phenotypic features were found in previously published SARS-CoV-2 related cases, in which a sensorimotor and demyelinating GBS was predominant, although some variants, such as MFS and axonal subtypes, have also been reported.11,29–31 Our study cohort also contained two confirmed/probable SARS-CoV-2 infected patients with MFS-GBS overlap syndrome, but no patients with a pure motor variant or an axonal electrophysiological subtype. A sensorimotor demyelinating GBS with facial palsy has also been described in relation to other viral triggers of GBS, including CMV, Zika virus, HEV and varicella zoster virus.7,32–34 This is therefore the expected clinical and electrophysiological phenotype in virus-related GBS, although its presence in the vast majority of SARS-CoV-2 infected GBS patients does not provide evidence of a causal effect. Should SARS-CoV-2 indeed be able to trigger GBS, our data are consistent with a post-infectious disease mechanism rather than direct viral invasion, as the time between onset of SARS-CoV-2 symptoms and GBS ranged from 2 to 3.5 weeks, none of the tested patients were positive for SARS-CoV-2 PCR in the CSF, and all but one patient had a normal leucocyte count in the CSF. Our findings are consistent with the other published SARS-CoV-2-related GBS case reports, from which in only the first published case the possibility of direct viral invasion was hypothesized.12
When comparing the GBS features of SARS-CoV-2 confirmed/probable patients with the other patients that were included in our study during the same time window we found no significant differences, except for a higher age and a higher frequency of a demyelinating subtype in the SARS-CoV-2 confirmed/probable cases. The latter might be partially explained by the fact that the Asian patients in our cohort were all in a group with no/possible SARS-CoV-2 suspicion and all GBS patients with confirmed/probable SARS-CoV-2 infection came from Europe, where the sensorimotor (demyelinating) variant is generally the most common subtype.18 However, this finding remained significant after excluding the patients from Asia and also cannot be explained by the timing of NCS during disease course or extensiveness of NCS, which were equal in both groups. This suggests that a demyelinating NCS might be a specific feature for GBS following a SARS-CoV-2 infection, although this is no proof for an association, and is supported by the fact that also compared to historical matched control patients included in IGOS before the pandemic, a demyelinating subtype was more frequent in the GBS patients with a confirmed/probable infection. On the other hand, an equivocal subtype, which signifies a group in which the distinction between demyelinating and axonal cannot be accurately made, was more common in the patients without a confirmed/probable SARS-CoV-2 infection, and could therefore have caused a lower proportion of demyelinating variant in this group.
Other preceding infections associated with GBS were tested in all SARS-CoV-2 probable/confirmed cases and were absent in 9/11 (82%). Two SARS-CoV-2 confirmed patients had serological evidence of a recent C. jejuni infection. In addition, one patient had a positive CMV PCR in respiratory material with a negative IgM antibody response, which is a common finding in patients with respiratory illness and is considered to be a sign of reactivation rather than a primary infection.35 The C. jejuni infection could have played a role in the induction of GBS, in which concurrent infection with SARS-CoV-2 may or may not have been contributory. Alternatively, it may have been a coincident infection not related to the induction of GBS, or a false-positive result due to polyclonal B cell bystander activation during the cytokine storm induced by SARS-CoV-2. Furthermore, previous studies reported that approximately one-third of patients with GBS have no known infectious trigger, either symptomatic or serological.6 Either way, these findings show the importance of testing for other infections that are known to trigger GBS when trying to establish the relation between emerging infectious diseases and GBS. Previous SARS-CoV-2 related GBS case reports often did not perform such serological testing.12,30,31
In accordance with the Brighton Collaboration criteria, 8/11 GBS patients with a confirmed/probable SARS-CoV-2 infection reached a Level 1 or 2 diagnostic certainty.17 We considered the presence of an ICU-related (critical illness) polyneuropathy unlikely because five of six patients admitted to the ICU had cranial nerve involvement. Interestingly, one patient with a confirmed SARS-CoV-2 infection had both GBS with facial weakness, limb weakness and a demyelinating NCS and a myelitis with sensory level and urinary/defecation disturbances and a cervical myelopathy. The combination of GBS and myelitis has previously been described in relation to Zika virus.36 Another patient with myelitis after SARS-CoV-2 infection was initially included in this study, but ultimately excluded because the diagnosis of GBS was subsequently refuted. Other cases of myelitis following SARS-CoV-2 infection have also been published in the past year.37 This underlines the importance of being aware of myelitis as a potential mimic of GBS.
All GBS patients with a confirmed or probable SARS-CoV-2 infection in our cohort were included in Europe over a period of 1 month, late March to late April, which matches the peak of the first wave of the pandemic in Europe. Whether the prevalence of SARS-CoV-2 infection (22%) in our cohort can be solely explained by a large SARS-CoV-2 infection rate in the community, or whether this indicates that SARS-CoV-2 increases the risk of GBS cannot be established in this study. Accurate estimations of the local community infection rates of SARS-CoV-2 infection during these 4 months of the pandemic are lacking, because asymptomatic patients were likely to be missed and because the prevalence varied greatly within short time periods depending on country, city and even neighbourhood. Seroprevalence studies in Europe (Spain >60 000 patients and Switzerland >2500 patients) showed infection rates in the general population of 5–10%, of whom one-third were asymptomatic.38,39 This percentage is probably an underestimation of the actual community prevalence, because 10% of patients with a positive PCR in this study did not (yet) have detectable antibodies.38 Sensitivity and specificity of antibody testing is strongly dependent on the type and timing of the test.22,40 On the other hand, the prevalence of SARS-CoV-2 infection in our cohort could also have been underestimated, because not all patients have been systematically tested by PCR or serology, and samples were often collected days to weeks after the start of the infectious symptoms, which could have led to false-negative results. However, the nasopharyngeal swabs of ‘negatively tested patients’ were not collected at later times than samples of ‘positively tested patients’.
Previous studies reported that the incidence of GBS can increase due to vaccine programmes or infectious disease epidemics. In 1976, a 7-fold increase of GBS cases was noticed in the USA during the national H1N1 swine flu vaccination programme.41 This association resulted in a more active monitoring of the occurrence of GBS during vaccine safety studies, such as during the 2009 H1N1 flu vaccination programme, and the publication of diagnostic criteria for GBS for vaccine safety studies by the Brighton Collaboration in 2011.17 So far, other studies on the relation between influenza vaccines and GBS have either shown no association or an increase in risk of only one GBS case per 1 million vaccinees.42 Another example is the Zika virus pandemic of 2015–16, when two GBS cases per 10 000 Zika virus cases were reported, with an estimated serological community prevalence of Zika virus of 49%, which led to a 1–6 times increase in incidence of GBS.43,44 Based on the previously mentioned serological estimated population prevalence of SARS-CoV-2 infection, we would also have expected an increase in the incidence of GBS during the first months of the pandemic. In the IGOS study, we did not see such an increase in the total inclusion rate (Fig. 2). However, we cannot accurately estimate the global GBS incidence based on these data because IGOS is not designed as a surveillance study. The sample size of our study represents only 0.001% of the expected global GBS cases during the time period of 4 months (∼30 000 cases). The inclusion rate has fluctuated over the past years, as it is dependent on many factors, including the number of centres actively recruiting patients over time. During the SARS-CoV-2 pandemic, inclusion rate could have been decreased due to SARS-CoV-2 restrictions and general public health measures, or biased due to scientific interest in a possible association between GBS and SARS-CoV-2 infection (referral bias). The latter could have led to an overestimation of the SARS-CoV-2 prevalence in our cohort. When focusing on the patient recruitment in selected regions, a small increase in average patient inclusion per month was seen in April 2020 in Switzerland, but not seen in three other regions (Netherlands, China and Italy).
Several other recently published studies have investigated the relationship between SARS-CoV-2 and GBS, reaching varying conclusions. A retrospective multicentre study in two SARS-CoV-2 hotspot regions in Italy found an increase in GBS incidence in March and April 2020 (30 patients) compared to those same months in 2019 (17 patients).13 From these data they concluded that the incidence rate of GBS is 47.9 cases per 100 000 SARS-CoV-2 infections. However, this is likely to be an overestimation due to underestimation of the total number of SARS-CoV-2 infections.13 A retrospective case-control study among patients in Spanish emergency departments also found that during March and April 2020 patients with SARS-CoV-2 infection were 6 times more likely to develop GBS compared to patients without a SARS-CoV-2 infection.45 However, in this study the total number of GBS cases was in fact lower than in the same period in the preceding year.45 Although these findings suggest a possible relationship, they cannot in themselves establish causation. Both studies had small patient numbers, took place in a short period of time, and have numerous potential confounders. An epidemiological study based on the National Immunoglobulin Database in the UK found a reduction in the incidence of GBS during the SARS-CoV-2 pandemic.46 And although this could be explained in part by a reduced exposure to other bacterial or viral pathogens due to the SARS-CoV-2 restrictions, namely social distancing, there was no correlation between the regional incidences of GBS and SARS-CoV-2. In the same study, a subset of 47 patients with GBS was described, representing <25% of the total 219 GBS cases logged with the National Immunoglobulin Database over the same period, of which 25/47 (53%) patients had a confirmed/probable SARS-CoV-2 infection. No phenotypic differences were found when compared to the remaining 22 non-SARS-CoV-2 controls. In April and May 2020, a total of 25 cases of GBS in London were logged with the national database, by which time an estimated 1.5 million Londoners had already made a serological response to SARS-CoV-2. Assuming all 25 cases were SARS-CoV-2 related, the maximum risk could therefore be calculated at one case of GBS for every 60 000 SARS-CoV-2 infections.46 Should the incidence rate calculated in the Italian study (47.9 cases per 100 000 SARS-CoV-2 infections) be correct, there should have been approximately 718 cases of GBS in London in these 2 months. From these observations, the authors concluded that a causative relationship between SARS-CoV-2 and GBS was unlikely. Based on these findings and given the fact that in our study no increase in inclusion rate was found, it seems that the risk of developing GBS following SARS-CoV-2 infection is either non-existent or at most small, and considerably lower when compared with, for example, C. jejuni or Zika virus. Results from a recently published national registry in Singapore showed a decrease in hospital admissions of GBS patients during the pandemic as well.47
IGOS is a prospective cohort study and the inclusion of patients is dependent on the efforts of the local investigator, and can be selective. Our study should therefore be regarded as a case series with the advantage of having a multicentre and prospective collection of data according to a predefined standard protocol. In addition, we used predefined criteria for a confirmed or probable SARS-CoV-2 infection and were able to exclude other infections in a subgroup of patients. Our study also has several important limitations. First, the study design was not appropriate to establish causation or to determine an association between GBS and SARS-CoV-2 in the absence of a matched control group of patients without GBS. Second, as a consequence of the SARS-CoV-2 pandemic, clinical follow-up was limited, and most laboratory tests were performed locally as samples could not be transported to the coordinating centre at the Erasmus MC University Medical Center Rotterdam on short notice due to travel restrictions. This led to non-uniform testing of biological samples for SARS-CoV-2 serology and other preceding infections. Since three samples were collected post-immunoglobulin treatment, we were not able to completely rule out a recent EBV and CMV infection in these patients. Third, we used the ECDC case definitions for SARS-CoV-2 infection to classify our patients, although this classification system was developed for clinical purposes. We chose to focus on the GBS patients with a confirmed/probable SARS-CoV-2 infection, because 10/15 (67%) of the patients with a possible infection were not tested for SARS-CoV-2 by PCR, and in 12/15 (80%) no CT-thorax was done, leading to considerable diagnostic uncertainty in this patient population. Although the WHO criteria are stricter, we decided against this criteria set as CT abnormalities are not included, which we considered a valuable diagnostic tool in our cohort. Lastly, patient subgroups were small and the follow-up was short, making interpretation of findings worthy of some caution.
In conclusion, we were able to identify a confirmed or probable preceding SARS-CoV-2 infection in 11 (22%) GBS patients during the first months of the pandemic in the context of a large, international, prospective cohort study. These patients shared similar features, as they frequently had a sensorimotor phenotype with facial palsy and significantly more often had a demyelinating subtype compared with both the other patients included in the same time window as well as historical control patients. In line with other studies, we did not find an increase in inclusion rate in IGOS, suggesting that a strong association between SARS-CoV-2 and GBS is unlikely. Nevertheless, we cannot exclude that SARS-CoV-2 may be an occasional trigger for GBS. Since our study was not designed to quantify a causative link between GBS and SARS-CoV-2, an unbiased multicentre international case-control study is needed to determine whether there is an association or not.
Supplementary Material
Acknowledgements
We thank Laura de Koning (Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands) for preparing the data for this manuscript and managing the IGOS database. We thank Marieke van Woerkom (Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands) for her efforts in coordinating the study and requesting the requisite data. We also thank Anne Tio-Gillen (Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands), Sandra Scherbeijn (Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands), Marijke Maas (Reinier Haga Medical Diagnostic Center, Delft, The Netherlands) and Enea Pianezzi (Neurocenter of Southern Switzerland, Lugano, Switzerland) for their efforts in testing samples on SARS-CoV-2 and other preceding infections. We thank Prof. Claudio Gobbi (Neurocenter of Southern Switzerland, Lugano, Switzerland), Baasch-Medicus Stiftung, Foundation for the Advancement of Neurology and GBS initiative Schweiz for their support to the study in Switzerland.
Funding
IGOS is financially supported by the GBS-CIDP Foundation International, Gain, Erasmus MC University Medical Center Rotterdam, Glasgow University, CSL Behring, Grifols, Annexon and Hansa Biopharma.
Competing interests
B.C.J. received grants from Grifols, CSL-Behring, Annexon, Prinses Beatrix Spierfonds, Hansa Biopharma, and GBS-CIDP Foundation International and is on the Global Medical Advisory Board of the GBS CIDP Foundation International. P.R. reports grants from Baasch-Medicus Stiftung, GBS initiative Schweiz and Foundation for the Advancement of Neurology (FAN). R.H. reports grants from GBS-CIDP Foundation International, Grifols and Health∼Holland, outside the submitted work.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- CMV
cytomegalovirus
- EBV
Epstein-Barr virus
- GBS
Guillain-Barré syndrome
- IGOS
International GBS Outcome Study
- ICU
intensive care unit
- MFS
Miller Fisher syndrome
- MRC
Medical Research Council
- NCS
nerve conduction study
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Contributor Information
the IGOS consortium:
Bart C Jacobs, Richard A C Hughes, David R Cornblath, Kenneth C Gorson, Hans-Peter Hartung, Susumu Kusunoki, Pieter A van Doorn, Hugh J Willison, Bianca van den Berg, Christine Verboon, Joyce Roodbol, Alex Y Doets, Sonja E Leonhard, Linda W G Luijten, Laura C de Koning, Melissa Mandarakas, Marieke van Woerkom, Samuel Arends, Ricardo C Reisin, Stephen W Reddel, Zhahirul Islam, Quazi Deen Mohammad, Peter van den Bergh, Tom E Feasby, Yuzhong Wang, Thomas Harbo, Yann Péréon, Helmar C Lehmann, Efthimios Dardiotis, Eduardo Nobile-Orazio, Nortina Shahrizaila, Kathleen Bateman, Isabel Illa, Luis Querol, Paolo Ripellino, Sung-Tsang Hsieh, Govindsinh Chavada, Amy Davidson, James M Addington, Henning Andersen, Giovanni Antonini, Senda Ajroud-Driss, Shahram Attarian, Umesh A Badrising, Claudia Balducci, Fabio A Barroso, Isabelita R Bella, Luana Benedetti, Tulio E Bertorini, Ratna Bhavaraju-Sanka, Thomas H Brannagan, Chiara Briani, Jan Bürmann, Mark Busby, Stephen Butterworth, Carlos Casasnovas, Francesca Castellani, Guido Cavaletti, Chi-Chao Chao, Shan Chen, Kristl G Claeys, Maria Eugenia Conti, Jeremy S Cosgrove, Marinos C Dalakas, Miroslawa A Derejko, Mazen M Dimachkie, Charlotte Dornonville de la Cour, Andoni Echaniz-Laguna, Filip Eftimov, Karin G Faber, Raffaella Fazio, Chris Fokke, Toshiki Fujioka, Ernesto A Fulgenzi, Giuliana Galassi, Tania García-Sobrino, Marcel P J Garssen, Francesco Gentile, Cees J Gijsbers, James M Gilchrist, H Job Gilhuis, Jonathan M Goldstein, Namita A Goyal, Volkan Granit, Aude-Marie Grapperon, Stefano Grisanti, Gerardo Gutiérrez-Gutiérrez, Lauri Gutmann, Robert D M Hadden, Jakob V Holbech, James K L Holt, Min Htut, Andrea Humm, Thomas Hundsberger, Korné Jellema, Ivonne Jericó Pascual, Maria C Jimeno Montero, Kenichi Kaida, Summer Karafiath, Hans D Katzberg, Mohammad Khoshnoodi, Lynette Kiers, Kurt Kimpinski, Ruud P Kleyweg, Norito Kokubun, Noah Kolb, Krista Kuitwaard, Thierry Kuntzer, Satoshi Kuwabara, Motoi Kuwahara, Justin Y Kwan, Shafeeq S Ladha, Lisbeth Landschoff Lassen, Agustina M Lascano, Victoria Lawson, Edward Lee Pan, Luciana León Cejas, Armelle Magot, Hadi Manji, Gerola A Marfia, Celedonio Márquez-Infante, Lorena Martin Aguilar, Eugenia Martinez Hernandez, Pilar Massaro Sanchez, Giorgia Mataluni, Marcelo G Mattiazzi, Chris J McDermott, Gregg D Meekins, James A L Miller, Maria Soledad Monges, Germán Morís de la Tassa, Caterina Nascimbene, Velina Nedkova-Hristova, Richard J Nowak, Michael Osei-Bonsu, Julio Pardo, Robert M Pascuzzi, Jane Pritchard, Michael Pulley, Simon Rinaldi, Rhys C Roberts, Iñigo Rojas-Marcos, Stacy A Rudnicki, George M Sachs, Johnny P A Samijn, Lucio Santoro, Olivier Scheidegger, Angelo Schenone, Lenka Schwindling, Maria J Sedano Tous, Kazim A Sheikh, Nicholas J Silvestri, Soren H Sindrup, Claudia L Sommer, Yan Song, Beth Stein, Amro M Stino, Cheng-Yin Tan, Hatice Tankisi, Pinelopi Tsouni, Paul T Twydell, Philip Van Damme, Anneke J van der Kooi, Willem van der Meulen, Taco C van der Ree, Gert W van Dijk, Rinske van Koningsveld, Jay D Varrato, Frederique H Vermeij, Jan J G M Verschuuren, Alex Vicino, Leo H Visser, Michal Vytopil, Waqar Waheed, Christa Walgaard, Paul W Wirtz, Chunye Xing, Yuko Yamagishi, Lan Zhou, and Sasha Zivkovic
Appendix 1
Full details of the IGOS Consortium are available in the Supplementary material.
IGOS consortium
Bart C. Jacobs, Richard A. C. Hughes, David R. Cornblath, Kenneth C. Gorson, Hans-Peter Hartung, Susumu Kusunoki, Pieter A. van Doorn, Hugh J. Willison, Bianca van den Berg, Christine Verboon, Joyce Roodbol, Alex Y. Doets, Sonja E. Leonhard, Linda W. G. Luijten, Laura C. de Koning, Melissa Mandarakas, Marieke van Woerkom, Samuel Arends, Ricardo C. Reisin, Stephen W. Reddel, Zhahirul Islam, Quazi Deen Mohammad, Peter van den Bergh, Tom E. Feasby, Yuzhong Wang, Thomas Harbo, Yann Péréon, Helmar C. Lehmann, Efthimios Dardiotis, Eduardo Nobile-Orazio, Nortina Shahrizaila, Kathleen Bateman, Isabel Illa, Luis Querol, Paolo Ripellino, Sung-Tsang Hsieh, Govindsinh Chavada, Amy Davidson, James M. Addington, Henning Andersen, Giovanni Antonini, Senda Ajroud-Driss, Shahram Attarian, Umesh A. Badrising, Claudia Balducci, Fabio A. Barroso, Isabelita R. Bella, Luana Benedetti, Tulio E. Bertorini, Ratna Bhavaraju-Sanka, Thomas H. Brannagan, Chiara Briani, Jan Bürmann, Mark Busby, Stephen Butterworth, Carlos Casasnovas, Francesca Castellani, Guido Cavaletti, Chi-Chao Chao, Shan Chen, Kristl G. Claeys, Maria Eugenia Conti, Jeremy S. Cosgrove, Marinos C. Dalakas, Miroslawa A. Derejko, Mazen M. Dimachkie, Charlotte Dornonville de la Cour, Andoni Echaniz-Laguna, Filip Eftimov, Karin G. Faber, Raffaella Fazio, Chris Fokke, Toshiki Fujioka, Ernesto A. Fulgenzi, Giuliana Galassi, Tania García-Sobrino, Marcel P. J. Garssen, Francesco Gentile, Cees J. Gijsbers, James M. Gilchrist, H. Job Gilhuis, Jonathan M. Goldstein, Namita A. Goyal, Volkan Granit, Aude-Marie Grapperon, Stefano Grisanti, Gerardo Gutiérrez-Gutiérrez, Lauri Gutmann, Robert D. M. Hadden, Jakob V. Holbech, James K. L. Holt, Min Htut, Andrea Humm, Thomas Hundsberger, Korné Jellema, Ivonne Jericó Pascual, Maria C. Jimeno Montero, Kenichi Kaida, Summer Karafiath, Hans D. Katzberg, Mohammad Khoshnoodi, Lynette Kiers, Kurt Kimpinski, Ruud P. Kleyweg, Norito Kokubun, Noah Kolb, Krista Kuitwaard, Thierry Kuntzer, Satoshi Kuwabara, Motoi Kuwahara, Justin Y. Kwan, Shafeeq S. Ladha, Lisbeth Landschoff Lassen, Agustina M. Lascano, Victoria Lawson, Edward Lee Pan, Luciana León Cejas, Armelle Magot, Hadi Manji, Gerola A. Marfia, Celedonio Márquez-Infante, Lorena Martin Aguilar, Eugenia Martinez Hernandez, Pilar Massaro Sanchez, Giorgia Mataluni, Marcelo G. Mattiazzi, Chris J. McDermott, Gregg D. Meekins, James A. L. Miller, Maria Soledad Monges, Germán Morís de la Tassa, Caterina Nascimbene, Velina Nedkova-Hristova, Richard J. Nowak, Michael Osei-Bonsu, Julio Pardo, Robert M. Pascuzzi, Jane Pritchard, Michael Pulley, Simon Rinaldi, Rhys C. Roberts, Iñigo Rojas-Marcos, Stacy A. Rudnicki, George M. Sachs, Johnny P. A. Samijn, Lucio Santoro, Olivier Scheidegger, Angelo Schenone, Lenka Schwindling, Maria J. Sedano Tous, Kazim A. Sheikh, Nicholas J. Silvestri, Soren H. Sindrup, Claudia L. Sommer, Yan Song, Beth Stein, Amro M. Stino, Cheng-Yin Tan, Hatice Tankisi, Pinelopi Tsouni, Paul T. Twydell, Philip Van Damme, Anneke J. van der Kooi, Willem van der Meulen, Taco C. van der Ree, Gert W. van Dijk, Rinske van Koningsveld, Jay D. Varrato, Frederique H. Vermeij, Jan J. G. M. Verschuuren, Alex Vicino, Leo H. Visser, Michal Vytopil, Waqar Waheed, Christa Walgaard, Paul W. Wirtz, Chunye Xing, Yuko Yamagishi, Lan Zhou, Sasha Zivkovic.
References
- 1.World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report - 11. 31 January 2020. Accessed 1 March 2021. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200131-sitrep-11-ncov.pdf?sfvrsn=de7c0f7_4
- 2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China . JAMA Neurol. 2020;77(6):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G.. Neurological involvement of coronavirus disease 2019: A systematic review. J Neurol. 2020;267(11):3135–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guidon AC, Amato AA.. COVID-19 and neuromuscular disorders. Neurology. 2020;94(22):959–969. [DOI] [PubMed] [Google Scholar]
- 5. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willison HJ, Jacobs BC, van Doorn PA.. Guillain-Barré syndrome. Lancet. 2016;388(10045):717–727. [DOI] [PubMed] [Google Scholar]
- 7. Leonhard SE, Bresani-Salvi CC, Lyra Batista JD, et al. Guillain-Barré syndrome related to Zika virus infection: A systematic review and meta-analysis of the clinical and electrophysiological phenotype. PLoS Negl Trop Dis. 2020;14(4):e0008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parra B, Lizarazo J, Jiménez-Arango JA, et al. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375(16):1513–1523. [DOI] [PubMed] [Google Scholar]
- 9. Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet. 2016;387(10027):1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobs BC, van Doorn PA, Schmitz PI, et al. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barre syndrome. Ann Neurol. 1996;40(2):181–187. [DOI] [PubMed] [Google Scholar]
- 11. De Sanctis P, Doneddu PE, Viganò L, Selmi C, Nobile-Orazio E.. Guillain Barré Syndrome associated with SARS-CoV-2 infection. A systematic review. Eur J Neurol. 2020;27(11):2361–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao H, Shen D, Zhou H, Liu J, Chen S.. Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020;19(5):383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Filosto M, Cotti Piccinelli S, Gazzina S, et al. Guillain-Barré syndrome and COVID-19: An observational multicentre study from two Italian hotspot regions. J Neurol Neurosurg Psychiatry. 2021;92(7):751–756. [DOI] [PubMed] [Google Scholar]
- 14. Hasan I, Saif-Ur-Rahman KM, Hayat S, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: A systematic review and individual participant data meta-analysis. J Peripher Nerv Syst. 2020;25(4):335–343. [DOI] [PubMed] [Google Scholar]
- 15. Jacobs BC, van den Berg B, Verboon C, et al. ; IGOS Consortium. International Guillain-Barré Syndrome Outcome Study: Protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain-Barré syndrome. J Peripher Nerv Syst. 2017;22(2):68–76. [DOI] [PubMed] [Google Scholar]
- 16. Asbury AK, Cornblath DR.. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27 Suppl:S21–4. [DOI] [PubMed] [Google Scholar]
- 17. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barré syndrome and Fisher syndrome: Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599–612. [DOI] [PubMed] [Google Scholar]
- 18. Doets AY, Verboon C, van den Berg B, et al. Regional variation of Guillain-Barré syndrome. Brain. 2018;141(10):2866–2877. [DOI] [PubMed] [Google Scholar]
- 19. Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM.. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978;2(8093):750–753. [DOI] [PubMed] [Google Scholar]
- 20. van Koningsveld R, Steyerberg EW, Hughes RA, Swan AV, van Doorn PA, Jacobs BC.. A clinical prognostic scoring system for Guillain-Barré syndrome. Lancet Neurol. 2007;6(7):589–594. [DOI] [PubMed] [Google Scholar]
- 21. Hadden RD, Cornblath DR, Hughes RA, et al. Electrophysiological classification of Guillain-Barré syndrome: Clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Ann Neurol. 1998;44(5):780–788. [DOI] [PubMed] [Google Scholar]
- 22. GeurtsvanKessel CH, Okba NMA, Igloi Z, et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11(1):3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Centre for Disease Prevention and Control. Case definition for coronavirus disease 2019 (COVID-19), as of 29 May 2020. Accessed 1 September 2020. https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition
- 24.World Health Organization. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases. Accessed 1 September 2020. https://www.who.int/publications/i/item/10665-331501
- 25.World Health Organization. Use of laboratory methods for SARS diagnosis. Accessed 1 September 2020. https://www.who.int/health-topics/severe-acute-respiratory-syndrome/technical-guidance/laboratory/use-of-laboratory-methods-for-sars-diagnosis
- 26. Ye Z, Zhang Y, Wang Y, Huang Z, Song B.. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur Radiol. 2020;30(8):4381–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lascano AM, Epiney JB, Coen M, et al. SARS-CoV-2 and Guillain-Barré syndrome: AIDP variant with favorable outcome. Eur J Neurol. 2020;27(9):1751–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kilinc D, Pasch S, Doets AY, Jacobs BC, Vliet J, Garssen MPJ.. Guillain-Barré syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020;27(9):1757–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uncini A, Vallat JM, Jacobs BC.. Guillain-Barré syndrome in SARS-CoV-2 infection: An instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry. 2020;91(10):1105–1110. [DOI] [PubMed] [Google Scholar]
- 30. Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. [DOI] [PubMed] [Google Scholar]
- 31. Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orlikowski D, Porcher R, Sivadon-Tardy V, et al. Guillain-Barré syndrome following primary cytomegalovirus infection: A prospective cohort study. Clin Infect Dis. 2011;52(7):837–844. [DOI] [PubMed] [Google Scholar]
- 33. Islam B, Islam Z, GeurtsvanKessel CH, et al. Guillain-Barré syndrome following varicella-zoster virus infection. Eur J Clin Microbiol Infect Dis. 2018;37(3):511–518. [DOI] [PubMed] [Google Scholar]
- 34. van den Berg B, van der Eijk AA, Pas SD, et al. Guillain-Barré syndrome associated with preceding hepatitis E virus infection. Neurology. 2014;82(6):491–497. [DOI] [PubMed] [Google Scholar]
- 35. Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300(4):413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Román GC, Anaya JM, Mancera-Páez Ó, Pardo-Turriago R, Rodríguez Y.. Concurrent Guillain-Barré syndrome, transverse myelitis and encephalitis post-Zika: A case report and review of the pathogenic role of multiple arboviral immunity. J Neurol Sci. 2019;396:84–85. [DOI] [PubMed] [Google Scholar]
- 37. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. [DOI] [PubMed] [Google Scholar]
- 41. Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol. 1979;110(2):105–123. [DOI] [PubMed] [Google Scholar]
- 42. Sejvar JJ, Pfeifer D, Schonberger LB.. Guillain-Barré syndrome following influenza vaccination: Causal or coincidental? Curr Infect Dis Rep. 2011;13(4):387–398. [DOI] [PubMed] [Google Scholar]
- 43. Mier Y-RL, Delorey MJ, Sejvar JJ, Johansson MA.. Guillain-Barré syndrome risk among individuals infected with Zika virus: a multi-country assessment. BMC Med. 2018;16(1):67- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aubry M, Teissier A, Huart M, et al. Zika virus seroprevalence, French polynesia, 2014-2015. Emerg Infect Dis. 2017;23(4):669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fragiel M, Miró Ò, Llorens P, et al. ; SIESTA (Spanish Investigators in Emergency Situations Team) network. Incidence, clinical, risk factors and outcomes of Guillain-Barré in Covid-19. Ann Neurol. 2021;89(3):598–603. [DOI] [PubMed] [Google Scholar]
- 46. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144(2):682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Umapathi T, Er B, Koh JS, Goh YH, Chua L.. Guillain-Barré syndrome decreases in Singapore during the COVID-19 pandemic. J Peripher Nerv Syst. 2021;26(2):235–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of included patients in IGOS will be used for future studies and may be made available upon reasonable request after consulting the IGOS Steering Committee.