Abstract
In cirrhosis with ascites, hepatorenal syndrome (HRS) is a specific prerenal dysfunction unresponsive to fluid volume expansion. Acute-on-chronic liver failure (ACLF) comprises a group of clinical syndromes with multiple organ failure and early high mortality. There are differences in the characterization of ACLF between the Eastern and Western medical communities. Patients with ACLF and acute kidney injury (AKI) have more structural injuries, contributing to confusion in diagnosing HRS-AKI. In this review, we discuss progress in the pathogenesis, diagnosis, and management of HRS-AKI, especially in patients with ACLF. Controversy regarding HRS-AKI in ACLF and acute liver failure, hepatic carcinoma, shock, sepsis, and chronic kidney disease is also discussed. Research on the treatment of HRS-AKI with ACLF needs to be more actively pursued to improve disease prognosis.
Keywords: hepatorenal syndrome, acute-on-chronic liver failure, acute kidney injury, review
Introduction
Hepatorenal syndrome (HRS) is a complication of advanced cirrhosis characterized by an abrupt deterioration in renal function. It is unresponsive to fluid volume expansion and is associated with an extremely poor prognosis [1, 2]. The morbidity of HRS is ∼12%–40% [3–7]. With the deterioration of liver and renal function, the difficulty of treatment and the cost of hospitalization increase significantly [8], and the mortality rises markedly [1–7]. In 2015, the International Club of Ascites (ICA) issued a revised consensus on HRS after 2007 (Table 1) [9, 10]. The revised consensus indicated that the absolute diagnostic value of serum creatinine (sCr) was cancelled and the diagnostic and grade criteria for HRS were adjusted based on the dynamic change in sCr in patients with cirrhosis and ascites according to the recommendation of the Acute Kidney Injury Network group [11], the Acute Dialysis Quality Initiative group for the Risk, Injury, Failure, Loss of renal function, and End-stage renal disease (RIFLE) [12], and the Kidney Disease Improving Global Outcome (KDIGO) [13] for acute kidney injury (AKI) (Table 2) [9]. Through personalized values of sCr, the diagnostic sensitivity of HRS was improved and the occurrence of missed diagnosis was reduced [14]. Reasonable treatment can therefore be given in a timely manner [15, 16]. However, confusion regarding the classification remains. The guidelines for decompensated cirrhosis issued by the European Association for the Study of the Liver (EASL) proposed that type 1 HRS (HRS-1) corresponds to HRS-AKI. Type 2 HRS (HRS-2) should include renal impairment, which fulfills the criteria for HRS but not of AKI, namely non-AKI type of HRS (HRS-NAKI) and HRS-chronic kidney disease (CKD) characterized by renal dysfunction that progresses slowly over 3 months [17]. Angeli et al. [18] further revised the HRS diagnostic criteria to include acute liver failure (ALF) and acute-on-chronic liver failure (ACLF) as preconditions. The classification was ultimately revised considerably, making it easier to understand and operate (Table 3) [18].
Table 1.
Diagnostic criteria for hepatorenal syndrome (HRS) type of acute kidney injury (AKI) in patients with cirrhosis [9]
| HRS-AKI • Diagnosis of cirrhosis and ascites • Diagnosis of AKI according to ICA-AKI criteria • No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin 1 g per kg of body weight • Absence of shock • No current or recent use of nephrotoxic drugs (NSAIDs, aminoglycosides, iodinated contrast media, etc.) • No macroscopic signs of structural kidney injurya, defined as: – absence of proteinuria (>500 mg/day) – absence of microhaematuria (>50 RBCs per high power field) – normal findings on renal ultrasonography |
ICA, International Club of Ascites; NSAIDs, non-steroidal anti-inflammatory drugs; RBCs, red blood cells.
Patients who fulfill these criteria may still have structural damage such as tubular damage. Urine biomarkers will become an important element in making a more accurate differential diagnosis between HRS and acute tubular necrosis.
Reprinted from Journal of Hepatology, Paolo Angeli, Pere Ginès, Florence Wong, Mauro Bernardi, Thomas D. Boyer, Alexander Gerbes, Richard Moreau, Rajiv Jalan, Shiv K. Sarin, Salvatore Piano, Kevin Moore, Samuel S. Lee, Francois Durand, Francesco Salerno, Paolo Caraceni, W. Ray Kim, Vicente Arroyo, et al., Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites, page 0, 2015, with permission from Elsevier.
Table 2.
International Club of Ascites (ICA-AKI) new definitions for the diagnosis and management of AKI in patients with cirrhosis [9]
| Subject | Definition | |||
|---|---|---|---|---|
| Baseline sCr |
A value of sCr obtained in the previous 3 months, when available, can be used as baseline sCr. In patients with more than one value within the previous 3 months, the value closest to the admission time to the hospital should be used In patients without a previous sCr value, the sCr on admission should be used as baseline |
|||
| Definition of AKI | Increase in sCr ≥0.3 mg/dL (≥26.5 µmol/L) within 48 h or a percentage increase in sCr ≥50% from baseline which is known, or presumed, to have occurred within the prior 7 days | |||
| Staging of AKI |
• Stage 1: increase in sCr ≥0.3 mg/dL (26.5 μmol/L) or an increase in sCr ≥1.5-fold to 2-fold from baseline • Stage 2: increase in sCr >2-fold to 3-fold from baseline • Stage 3: increase in sCr >3-fold from baseline or sCr ≥4.0 mg/dL (353.6 μmol/L) with an acute increase ≥0.3 mg/dL (26.5 μmol/L) or initiation of renal replacement therapy |
|||
| Progression of AKI |
Progression Progression of AKI to a higher stage and/or need for RRT |
Regression Regression of AKI to a lower stage |
||
| Response to treatment |
No response No regression of AKI |
Partial response Regression of AKI stage with a reduction of sCr to ≥0.3 mg/dl (26.5 µmol/L) above the baseline value |
Full response Return of sCr to a value within 0.3 mg/dl (26.5 µmol/L) of the baseline value |
|
AKI, acute kidney injury; RRT, renal replacement therapy; sCr, serum creatinine.
Reprinted from Journal of Hepatology, Paolo Angeli, Pere Ginès, Florence Wong, Mauro Bernardi, Thomas D. Boyer, Alexander Gerbes, Richard Moreau, Rajiv Jalan, Shiv K. Sarin, Salvatore Piano, Kevin Moore, Samuel S. Lee, Francois Durand, Francesco Salerno, Paolo Caraceni, W. Ray Kim, Vicente Arroyo, et al., Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites, page 0, 2015, with permission from Elsevier.
Table 3.
New classification of HRS subtypes [18]
| Old classification | New classification | Criteria | |
|---|---|---|---|
| HRS-1a | HRS-AKI |
a) Absolute increase in sCr ≥0.3 mg/dL within 48 h and/or b) Urinary output ≤0.5 mL/kg B.W. ≥6 hb or c) Percent increase in sCr ≥50% using the last available value of outpatient sCr within 3 months as the baseline value |
|
| HRS-2a | HRS-NAKI | HRS-AKD |
a) eGFR <60 mL/min per 1.73 m2 for <3 months in the absence of other (structural) causes b) Percent increase in sCr <50% using the last available value of outpatient sCr within 3 months as the baseline value |
| HRS-CKD | a) eGFR <60 mL/min per 1.73 m2 for ≥3 months in the absence of other (structural) causes |
AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HRS, hepatorenal syndrome; sCr, serum creatinine.
aFulfillment of all the new International Ascites Club criteria for the diagnosis of HRS.
bThe evaluation of this parameter requires a urinary catheter.
Reprinted from Journal of Hepatology, Volume 71, Paolo Angeli, Guadalupe Garcia-Tsao, Mitra K. Nadim, Chirag R. Parikh, News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document, pages 811–22, 2019, with permission from Elsevier.
ACLF is a group of clinical syndromes characterized by acute deterioration of hepatic function in patients with chronic liver disease accompanied by multiple organ failure and early high mortality [19]. However, disputes exist regarding the diagnostic criteria between Eastern and Western medical practice [20–22]. The degree of AKI is the most important factor in the prognosis of ACLF, even in stage 1B AKI [23]. Maiwall et al. [24] conjectured that the kidney is differently affected in patients with cirrhosis with or without liver failure. Moreover, they indicated that patients with ACLF and AKI exhibit more structural injuries, a lower reversal rate, and a higher mortality than patients with acute decompensation of cirrhosis. Davenport et al. [25] reviewed AKI in ACLF and concluded that it is important to distinguish HRS in AKI, but there is no reasonable method for its appropriate identification. In this review, we discuss the latest progress in the pathogenesis, diagnosis, and management of HRS-AKI to increase the understanding of this condition.
Differences in the definition of ACLF
There is a long-standing debate regarding the definition of ACLF (Table 4). The definition of ACLF from the Asian Pacific Association for the Study of the Liver (APASL), which focused on changes in liver function, stated that
Table 4.
| Item | APASL | CMA | EASL/CLIF | NACSELD |
|---|---|---|---|---|
| Circulation | NA | NA | Dopamine <5 or dobutamine or terlipressin | Shock: (MAP <60 or a reduction of 40 mmHg in systolic blood pressure from the baseline) despite adequate fluid resuscitation and cardiac output |
| Coagulation | INR ≥1.5 or PTA <40% | INR ≥1.5 or PTA <40% | INR ≥2.5 or platelet count <20 × 109/L | NA |
| Liver (TBil, mg/dL) | ≥5 | ≥10 or increase 1 mg/dL/d | ≥12 | NA |
| Kidney (sCr, mg/dL) | NA | NA | ≥2.0 | Need for dialysis or other forms of renal replacement therapy |
| Cerebral (HE grade) | ≥ I | NA | III or IV | III or IV |
| Respiratory | NA | NA | PaO2/FiO2: >100 to <200 or SpO2/FiO2: >89 to <214 | Need for mechanical ventilator |
| Definition | TBil ≥5 mg/dL and INR ≥1.5 or PTA <40% complicated by ascites and/ or HE | INR ≥1.5 or PTA <40% and TBil ≥10 or increase 1 mg/dL/d |
Grade 1: (1) only sCr ≥2.0 mg/dL (2) sCr: 1.5–1.9 mg/dL and/or HE I/II with one organ failure (liver, coagulation, circulation, or respiration) (3) sCr: 1.5–1.9 mg/dL and HE III/IV Grade 2: Two organ failures Grade 3: Three organ failures or more |
Two or more organ failures |
| Include chronic liver disease | Yes | Yes | No | No |
| Include compensated cirrhosis | Yes | Yes | Yes | Yes |
| Include decompensated cirrhosis | No | Yes | Yes | Yes |
| Include hepatic carcinoma | No | Yes | No | Disseminated malignancies are excluded |
ACLF, acute-on-chronic liver failure; APASL, Asian Pacific Association for the Study of the Liver; CMA, Chinese Medical Association; EASL, European Association for the Study of the Liver; CLIF, Chronic Liver Failure Consortium; NACSELD, North American Consortium for the Study of End-Stage Liver Disease; NA, not applicable; MAP, mean arterial pressure; INR, international normalized ratio; PTA, prothrombin activity; TBil, total bilirubin; sCr, serum creatinine; HE, hepatic encephalopathy; PaO2; partial pressure of oxygen; FiO2, fraction of inspired oxygen; SpO2, blood oxygen saturation.
“ACLF is an acute hepatic insult manifesting as jaundice (serum bilirubin ≥5 mg/dL [85 μmol/L]) and coagulopathy (international normalized ratio [INR] ≥1.5 or prothrombin activity <40%) complicated within 4 weeks by clinical ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease/cirrhosis, and is associated with a high 28-day mortality” [20].
The EASL-Chronic Liver Failure (EASL-CLIF) Acute-on-Chronic Liver Failure in Cirrhosis (CANONIC) study redefined the ACLF diagnostic criteria by prospectively analysing the data of 1,343 patients with cirrhosis [21]. They focused on the 28-day mortality during the estimation of multiple organ dysfunction. Total bilirubin (TBil) levels (5 vs 12 mg/dL) and INR (1.5 vs 2.5) were not the same with respect to liver function. The North American Consortium for the Study of End-Stage Liver Disease (NACSELD) defines ACLF as cirrhosis in patients who develop infection with at least two types of extrahepatic organ failure [22], but this definition is not widely accepted. Jalan et al. [27] posited that ACLF can be divided into three types, A/B/C, according to chronic liver disease, compensated cirrhosis, and decompensated cirrhosis. However, type C ACLF tends to be with extrahepatic organ failure. Kim et al. [28] retrospectively analysed the data from 1,470 cases and proposed that non-cirrhotic chronic liver disease, previous acute decompensation within 1 year, and extrahepatic organ failure should be included in the ACLF diagnostic criteria. The Chinese Medical Association (CMA) renewed the diagnosis and treatment guidelines for ACLF and required that TBil ≥10 mg/dL or an increase of ≥1 mg/dL/d, except for INR ≥1.5 or prothrombin activity <40%. This may be accompanied by complications including hepatic encephalopathy, ascites, electrolyte disturbance, infection, HRS, hepatopulmonary syndrome, and extrahepatic organ failure. ACLF was also differentiated into A/B/C types according to the degree of chronic liver disease [26]. The APASL did not include AKI in the diagnostic criteria, but sCr was included in the APASL ACLF Research Consortium score that was superior to the Sequential Organ Failure Assessment (SOFA), CLIF-SOFA, and the Model for End-Stage of Liver Disease (MELD) for assessing the severity of ACLF [20]. Despite some variances, all parties agreed that the definition of ACLF should include the following components: (i) status of pre-existing liver disease; (ii) defining the acute hepatic insult that leads to rapid deterioration of liver status; (iii) the time frame during which the acute hepatic insult can lead to rapid deterioration; (iv) the quantification and definition of liver-failure status after deterioration, which will determine the severity of ACLF; and (v) the prediction of the prognosis after analysing the first four components in the short and long term [19]. Duseja et al. [29] refined the ACLF concept:
“ACLF is a syndrome in patients with chronic liver disease with or without cirrhosis, previously diagnosed or undiagnosed, compensated or decompensated, which is characterized by acute hepatic decompensation within 12 weeks of an acute precipitating event, (both hepatic and/or non-hepatic) in the form of ascites and/or hepatic encephalopathy, associated with jaundice (TBil >5 mg/dL) and prolonged INR (>1.5).”
This is currently the most extensive definition of ACLF, but it is not widely accepted.
Epidemiology of HRS in ACLF
According to the CANONIC study [21], AKI is one of the important components of ACLF, but it is graded according to the sCr levels. As the cause of AKI is not involved, the incidence of HRS-AKI remains unclear. D'Souza et al. [30] and Trebicka et al. [31] found that the proportion of patients with HRS-AKI was 3/20 in children and 22/380 in ACLF according to the EASL/CLIF criteria. However, some studies in the Asia-Pacific region have reported the epidemiologic data of HRS-AKI according to the APASL or CMA criteria (Table 5) [14, 24, 32–38].
Table 5.
HRS-AKI data according to the APASL/CMA criteria
| Reference | Country | Patient | Study | Diagnostic criteria | Number of patients | Date | Infection (SBP) | AKI | HRS- AKI |
|---|---|---|---|---|---|---|---|---|---|
| Khatua et al. 2018 [32] | India | ACLF | Prospective | APASL | 113 | October 2016 to February 2018 | ND | 78 | 22 |
| Maiwall R et al. 2017 [33] | India | ACLF | Prospective | APASL | 373 | January 2014 to January 2015 | 159 (ND) | 177 | 129 |
| Arora V et al. 2020 [34] | India | ACLF | Prospective | APASL | 340 | October 2015 to December 2016 | 58 (ND) | 181 | 120 |
| Huang Z et al. 2015 [14] | China | HBV-ACLF | Retrospective | APASL | 439 | January 2004 to December 2011 | 410 (320) | 158 | 56 |
| Yuan W et al. 2017 [35] | China | HBV-ACLF | Retrospective | APASL | 150 | January 2013 to December 2015 | 39 (27) | 90 | 23 |
| Lal BB et al. 2018 [36] | India | Children- ACLF | Retrospective | APASL | 84 | August 2011 to December 2014 | ND (3) | 19 | 6 |
| Jindal A et al. 2016 [37] | India | ACLF | Retrospective | APASL | 241 | August 2010 to April 2013 | ND (16) | ND | 28 |
| Zang H et al. 2016 [38] | China | ACLF | Retrospective | CMA | 1,032 | January 2009 to December 2014 | 286 (119) | 440 | 251 |
| Maiwall R et al. 2015 [24] | India | ACLF | Prospective | APASL | 382 | January 2013 to January 2014 | 259 (105) | 174 | 134 |
HRS, hepatorenal syndrome; APASL, Asian Pacific Association for the Study of the Liver; CMA, Chinese Medical Association; SBP, spontaneous bacterial peritonitis; AKI, acute kidney injury; ACLF, acute-on-chronic liver failure; ND, no description; HBV, hepatitis B virus.
HRS-AKI accounted for 32%–77% of AKI cases (Table 5), but the incidence was underestimated because decompensated cirrhosis was not included according to the APASL definition [20]. Because of different diagnostic criteria for ACLF, none fully accounts for or determines the incidence of HRS-AKI.
Pathogenesis of HRS-AKI in ACLF
Owing to the pathological changes in cirrhosis, the pressure in the portal vein is elevated, endogenous vasodilators are increased, and the splanchnic vasculature is dilated [39]. With renal artery contraction and hypoperfusion, the effective circulation of the kidney is diminished, resulting in HRS-AKI [40–43]. No matter what the diagnostic criteria for ACLF, a common characteristic is the requirement of precipitating factors, whether intrahepatic or extrahepatic. Among the precipitating factors, there are many similar mechanisms underlying HRS-AKI [25]. Owing to the different diagnostic criteria for ACLF, whether these are symbiotic or secondary to HRS-AKI requires further exploration.
Role of systemic inflammation
Systemic inflammation is an important factor of multiple organ dysfunction in patients with decompensated cirrhosis and ACLF [44–47]. Patients with ACLF manifested significantly abnormal levels of cytokines compared with those without ACLF, including vascular cell adhesion molecule (VCAM)-1, vascular endothelial growth factor (VEGF)-A, fractalkine, macrophage inflammatory protein (MIP)-1α, eotaxins, interferon-gamma-induced protein (IP)/CXC–chemokine ligand (CXCL)-10, CC–chemokine ligand (CCL)-5, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-1β, IL-2, intercellular adhesion molecule (ICAM)-1, and monocyte chemoattractant protein (MCP)-1 [48]. Of these, VCAM-1 and VEGF-A exhibited the most notable relationship with ACLF. Functional-enrichment analysis showed that inflammatory markers differently expressed in ACLF patients were enriched in leukocyte migration, particularly monocytes and macrophages, and in the chemotactic pathways [48]. The increase in IP-10 mRNA expression in hepatitis B virus (HBV)-ACLF patients was confirmed [49]. Solé et al. [50] characterized HRS-AKI by an altered cytokine profile compared with the cirrhotic patients without AKI and hypovolemia-induced AKI, and found that higher levels of IP-10 and VCAM-1 were related to the effect of treatment. Moreover, Wu et al. [51] found that plasma IL-6, IL-10, granulocyte colony-stimulating factor (G-CSF), and GM-CSF levels were higher in HBV-ACLF patients, whereas RANTS, CCL2, and CXCL9 were expressed at lower levels in patients without ACLF. Blood transcriptomic analyses showed that genes associated with cell migration and mobility, as well as responses to wounding and bacteria, were expressed at higher levels, while genes involved in lymphocyte-mediated immunity were expressed at lower levels in HBV-ACLF patients relative to non-ACLF patients. “Immune maladjustment” is an important state in ACLF [52–54] and shows a similar degree of cellular immune depression with severe sepsis. Reduced cellular immune function with ACLF might contribute to the increased infectious morbidity of these patients. Neutrophil gelatinase-associated lipocalin (NGAL) is a small protein secreted by neutrophils after activation that was noted to be markedly augmented in patients with ACLF and to be correlated with a poor prognosis [55, 56]. Moreover, NGAL was markedly overexpressed in the liver tissue of ACLF patients [57]. There was also an increase in the level of NGAL in plasma and urine of HRS-AKI patients, suggesting that ACLF and HRS-AKI involve a similar pathogenesis. Inflammation plays an important role in the development of ACLF and HRS-AKI has been found to be closely related to uncontrolled inflammation [58]. However, there are still many mechanisms to be explored regarding the relationships among ACLF, HRS-AKI, and inflammation.
Effect of the intestinal microbiome
Portal hypertension can disrupt the intestinal mucosal barrier and translocate intestinal flora, which not only affects the progress of liver cirrhosis, but also plays an important role in promoting the development of multiple organ failure [59]. In patients with ACLF, the diversity and abundance of the intestinal microbiome were shown to decrease significantly [60]. HBV-ACLF patients were enriched with Moraxellaceae, Sulfurovum, Comamonas, and Burkholderiaceae, but had depleted Actinobacteria, Deinococcus-Thermus, Alphaproteobacteria, Xanthomonadaceae, and Enterobacteriaceae compared with healthy controls [60]. The abundance of Veilonella was positively correlated with TBil and that of Coprococcus was negatively correlated with TBil and INR [61]. An abundance of Enterococcus was associated with progression, while abundant Faecalibacterium was associated with regression of HBV-ACLF [62]. Network analysis revealed a direct positive correlation between Burkholderiaceae and chemokine IP-10 in HBV-ACLF patients [63]. Alterations in circulating microbiota were also associated with systemic inflammation and the clinical outcome in HBV-ACLF [63]. Bacterial translocation, subsequent inflammation, and bacterial DNA in ascitic fluid were implicated in HRS-AKI induction [64]. By initiating an inflammatory reaction, the intestinal microbiome may promote ACLF and alter host immune and metabolic homeostasis, eventually promoting and potentiating the development of AKI.
Role of cardiac dysfunction
The concept of cirrhotic cardiomyopathy was proposed by the World Gastroenterology Organization in Montreal in 2005 [65]. Patients with cirrhosis are in a state of hyperdynamic circulation, which manifests itself as increased cardiac output (CO) and decreased peripheral vascular resistance and mean arterial pressure (MAP). With the deterioration in cardiac function, the first manifestation is systolic dysfunction, followed by diastolic dysfunction [66, 67]. Kumar et al. [68] found no significant changes in cardiac hemodynamics in patients with ACLF compared with those with decompensated cirrhosis. However, with the further deterioration of ACLF, the functional state of the heart worsened because of the loss of compensatory capacity [69]. HRS-AKI occurs in the setting of a significant reduction in MAP, CO, and pulmonary wedge pressure with no changes in peripheral vascular resistance [70]. The data showed that cardiac dysfunction was one of the synergistic factors involved in HRS-AKI and revealed that the stability of the circulatory system is important for the maintenance of renal function.
Role of the neurohormonal system
In cirrhosis, neurohormonal mechanisms are activated to counter hypovolemia caused by peripheral vasodilation [71]. The sympathetic nervous system, renin–angiotensin–aldosterone system (RAAS), and arginine-vasopressin (AVP) play significant roles in the progression of cirrhosis. Dysfunction in these systems allows renal hemodynamic imbalance and promotes HRS-AKI [72, 73]. Norepinephrine (NE) from the sympathetic nervous system increases with the severity of ACLF and is closely related to sCr levels [74]. Increased NE levels in end-stage liver disease are also associated with a rightward and downward shift of the renal auto-regulatory curve [72] while activation of the RAAS augments the sympathetic response [75]. Ginès et al. [6] found that plasma NE >544 pg/mL and plasma renin activity >3.5 ng/mL/h were predictors of HRS-AKI development. Patients with ACLF showed significantly higher concentrations of plasma renin than patients with decompensated cirrhosis [46]. The activation of angiotensin and aldosterone under the stimulation of renin leads to the retention of sodium and solute-free water, which results in alterations in renal and systemic hemodynamics. AVP is a hypothalamic neurohormone secreted by the neurohypophysis and is the major physiologic regulator of renal water excretion and blood volume [76]. However, AVP is unstable in serum and difficult to measure. Copeptin is a product of the C-terminal portion of the AVP precursor and is a surrogate marker for AVP. Kerbert et al. [77] found that plasma copeptin levels were markedly higher in patients with ACLF and renal failure relative to ACLF patients without renal failure. This may indicate that elevated plasma copeptin levels not only reflect an increased release of AVP by neurohypophysis, but also a decreased clearance rate of copeptin in patients with ACLF and renal failure. However, how copeptin affects the renal hemodynamic changes remains to be further evaluated. In addition, adrenocortical insufficiency is a potential pathogenic factor in these situations [78]. Patients with adrenocortical insufficiency have a significantly higher incidence of AKI and a significantly higher incidence of new organ failures and ACLF [79]. Thus, a variety of mechanisms promote the occurrence and development of ACLF and may involve the occurrence of AKI, including HRS-AKI.
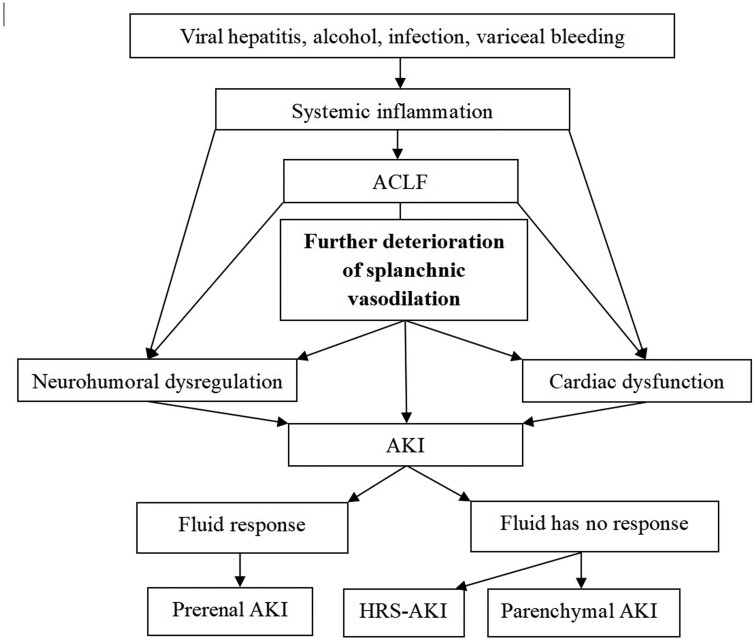
Although the pathogenesis of HRS-AKI in ACLF remains unclear, an excessive inflammatory reaction worsens splanchnic hemodynamics that have been altered by portal hypertension and vascular permeability. Changes in the neurohumoral system and cardiac function then aggravate the insufficient circulation of the kidney. Even if fluid intervention is carried out in a timely way, the rapid deterioration of the disease state may lead to the impairment of renal function, thereby accelerating the progress of the disease (Figure 1).
Figure 1.
Schematic view of the pathogenetic mechanism of the action underlying HRS-AKI in ACLF. ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; HRS, hepatorenal syndrome.
Confusion in the diagnostic criteria for HRS-AKI in ACLF
HRS-AKI is a clinical syndrome rather than a concrete clinical entity. Although there are many new findings in the research of HRS-AKI, many questions linger with regard to clinical application, especially in patients with ACLF.
Does HRS-AKI only apply to patients with cirrhosis?
Ascites is one of the signs of worsening cirrhosis and it is a precondition for diagnosing HRS-AKI. However, ascites and AKI can also be seen in ACLF based on chronic liver disease without cirrhosis, or even ALF. The occurrence of HRS-AKI is one of the outcomes of the persistent deterioration of liver function. If large numbers of hepatocytes are necrotic and swollen, portal vein blood flow will decrease and portal pressure will increase, promoting ascites. The guidelines in China and Spain establish that AKI based on ALF is the same as HRS-AKI [26, 80], yet the EASL and the India national association have pointed out that AKI in ALF patients is similar to AKI in sepsis and multiple organ dysfunction [81, 82]. There is no consensus in this regard. ALF and ACLF were included among conditions linked to HRS-AKI in 1996 and 2007 [9, 81], in keeping with prior reports [83, 84]. This was revised in 2007 and 2015, with only patients with cirrhosis and ascites being redefined. Patients with ALF resulting from acetaminophen overdose exhibited a higher incidence of AKI [85]. Regardless, renal replacement therapy (RRT) should be considered early in patients with ALF and AKI [86]. In a retrospective analysis of 1,604 patients with ALF, 70% of patients had AKI and 30% received RRT [85]. These data indicate that AKI in ALF is more likely to induce parenchymal damage. High-mobility group box protein 1 (HMGB1) is a cytokine mediator of inflammation that is secreted by immune cells. Patients with ALF manifested high HMGB1 levels in their plasma [86, 87]. An ALF mouse model showed that the TNF-α/HMGB1 inflammation signaling pathway may promote AKI [88]. Yang et al. [89] found that HMGB1 linked gut bacterial translocation and systemic inflammation in ALF. Although the role of HMGB1 in ACLF patients with AKI has not been studied, it has been associated with decreased survival rate in cirrhotics with AKI [90]. Together, these findings suggest that there is overlap in the pathogenesis of AKI, especially in the setting of systemic inflammation [82, 91]. Anand et al. [82] conjectured that HRS-AKI may also occur in ALF, especially slowly evolving forms, such as subacute liver failure. Thus, AKI in ALF and ACLF without cirrhosis may have similar pathogenesis to HRS-AKI in cirrhosis and should be treated promptly.
Is it suitable to diagnose HRS-AKI in ACLF patients with hepatic carcinoma?
In hepatic carcinoma (HCC), normal liver tissue is replaced by tumor, which leads to loss of liver function and, in certain instances, liver failure and death. However, HCC is presently excluded in the diagnosis of ACLF [20, 21]. Only the criteria for ACLF defined by NACSELD consider disseminated malignancies [22]. In some studies of HRS-AKI, patients with HCC were not excluded [34, 37, 92, 93] while some patients were excluded beyond the Milan criteria for HCC [38, 94, 95]. Conversely, other studies completely excluded HCC patients [14, 33, 96]. At present, the Milan criteria are more often used to assess prognosis and therapeutic options in patients with HCC, especially those considering liver transplant. However, the criteria are not suitable for the evaluation of liver function. AKI can occur with tumor rupture, surgical operations, or transcatheter arterial chemoembolization. Awareness of these phenomena is important. If AKI is associated with liver failure due to tumor progression, caution should be taken in the diagnosis of HRS-AKI. If it is not, HRS-AKI should be identified and treated as soon as possible. Full consideration of the characteristics of the tumor and other disease factors should be undertaken when a personalized treatment plan conducive to the choice of treatment options is developed.
Does HRS produce only functional damage?
HRS-AKI is a special type of prerenal azotemia that can be treated with vasoconstrictors. However, in HRS-AKI, vasoactive drugs provide therapeutic relief in <50% of patients [97, 98]. Absence of renal parenchymal impairment in HRS-AKI has never been confirmed by renal biopsies. In five cases of HRS-AKI after death, light microscopy revealed severe acute tubular lesions or acute tubular necrosis (ATN). Transmission electron microscopy demonstrated necrosis of the proximal tubules. The rupture of tubular basement membranes and mitochondrial dark bodies suggests ATN due to ischemia or induced by vasoconstrictors [99]. A higher prevalence of granular casts and number of tubular epithelial cells were found in patients with AKI and ACLF than in those with acute decompensation of cirrhosis [24]. In ACLF patients, the renal tubules were damaged with bile cast nephropathy in 32/43 (74.4%) of patients [100], as compared with 25/84 (29.7%) of patients with decompensated cirrhosis [101]. The decrease in tubular aquaporin 2 may explain this phenomenon [102], but dysregulation of arginine metabolism may be the core mechanism underlying this pathology [103]. As patients with ACLF have a higher TBil, they are more likely to have an increased urinary sediment score [104] along with renal structural injury. Jiang et al. [105] concluded that AKI in HBV-ACLF patients is more likely to be associated with structural kidney injury and is more progressive. This may be one of the reasons for the poor response to treatment of ACLF-associated HRS-AKI [25, 106, 107]. Together, this suggests that the diagnostic criteria for HRS-AKI may not reflect the real structural changes of the kidney, especially in ACLF. Acute tubular lesions may be the most important pathological features in patients with irreversible HRS-AKI.
Which diagnosis of shock was suitable for exclusion in HRS-AKI?
In 1996, the absence of shock and ongoing bacterial infection were necessary for the diagnosis of HRS [108]. Shock means a decrease in arterial pressure associated with a reduction in tissue perfusion; however, the definition of shock is not uniform [109]. The European Society of Intensive Care Medicine defines shock as a life-threatening, generalized form of acute circulatory failure associated with inadequate oxygen utilization by cells [110]. The clinical signs of shock typically include arterial hypotension and signs of altered tissue perfusion visible through the three “windows” of the body: the peripheral window (cold, clammy and blue, pale, or discolored skin), the renal window (decreased urine volume, <0.5 mL/kg/h), and the neurologic window (mental symptoms characterized by obtundation, disorientation, and confusion) [111]. However, the presence of arterial hypotension (defined as systolic blood pressure of <90 mmHg, or MAP of <65 mmHg, or decrease of ≥40 mmHg from baseline), while commonly present, should not be required to define shock [110]. Urine output ≤0.5 mL/kg body weight (B.W.) for ≥6 h is one of the diagnostic criteria for HRS-AKI, but it conflicts with the diagnosis of shock. Whether the diagnostic criteria for urine output are reasonable needs to be further studied. A lactate level of >2 mmol/L is a sign of microcirculation disturbance in all cases in which shock is suspected [110]. Elevated serum lactate levels are associated with a higher mortality rate in critically ill patients with cirrhosis and AKI [112]. Yet, liver dysfunction was significantly associated with impaired lactate clearance and normalization [113]. Kruse et al. [114] found that lactate of >2.2 mmol/L was associated with clinical evidence of shock and significant in-hospital mortality in critically ill patients with liver disease. Shock can be associated with four underlying patterns: three associated with a low flow state (hypovolemic, cardiogenic, obstructive) and one associated with a hyperkinetic state (distributive). In 1,679 intensive care unit patients enrolled in the European Sepsis Occurrence in Acutely Ill Patients II trial, septic shock as the main part of distributive shock was the most frequent cause of shock, accounting for 62% of cases, followed by cardiogenic shock (17%) and hypovolemia (16%) [115]. In a recent consensus, septic shock was defined as a vasopressor requirement to maintain a MAP of ≥65 mmHg and lactate of >2 mmol/L in the absence of hypovolemia [116]. Patients with septic shock and end-stage liver disease had higher mortality whether in the emergency department or in the intensive care unit [117, 118]. However, a Sepsis-3 definition reduced the size of the population with shock [119]. Therefore, more tolerant diagnostic criteria for septic shock in ACLF are needed. At present, alterations in the serum lactate level (>2 mmol/L), skin appearance (cold, clammy and blue, pale, or discolored), urine output (<0.5 mL/kg/h for ≥6 h), and mentation (mental alteration characterized by obtundation, disorientation, and confusion) serve as diagnostic criteria for shock. Whether this concept can be extended to cirrhosis or ACLF patients needs to be further studied.
How to distinguish sepsis-related AKI and HRS-AKI in ACLF
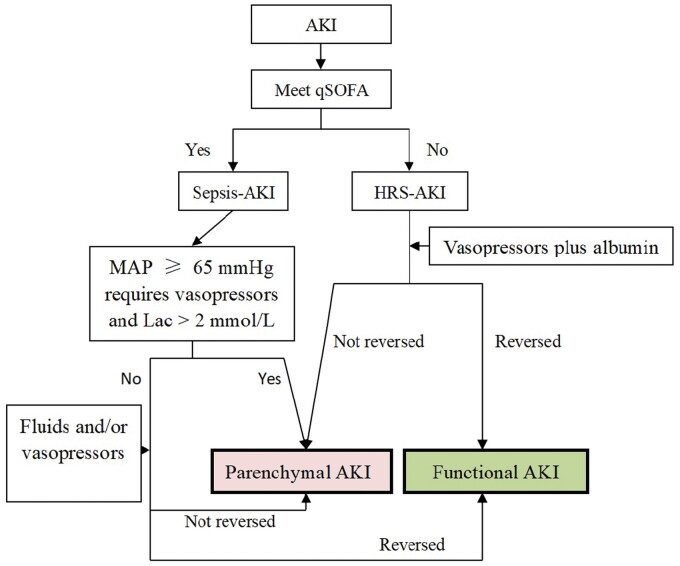
Because of abnormal immune responses, ACLF may present with sepsis-like manifestations [52, 120]. Bacterial infections are also frequent in ACLF [121]. In some studies of HRS-AKI, sepsis-AKI patients were excluded [93], but not in others [34, 92, 94–96, 122]. Sepsis is defined as the presence of an infection combined with an acute change in the SOFA score of 2 points or more (with the baseline assumed to be 0 in patients without any known pre-existing organ dysfunction). A new screening tool for early recognition of sepsis named quick-SOFA (qSOFA), which requires two of three criteria (altered mental status, respiratory rate >22 per min, systolic blood pressure <100 mmHg), was promulgated in non-intensive unit care patients [116]. The SOFA score was replaced by the CLIF-SOFA score in chronic liver disease [123]. HRS-AKI may occur spontaneously with worsening liver function or secondary to a precipitating event such as bacterial infection [10]. A change in sCr is an important component of CLIF-SOFA, but how to distinguish sepsis-related AKI and HRS-AKI in ACLF remains complicated. Many differences between HRS-AKI and sepsis-AKI in ACLF still exist (Table 6). The sepsis-3 criteria are more accurate than systemic inflammatory response syndrome criteria in predicting the severity of infections in patients with cirrhosis [124]. However, Son et al. [125] found that qSOFA had limited utility in predicting adverse outcomes in cirrhosis patients with sepsis. Garofalo et al. [126] reviewed the histopathological changes in sepsis-AKI and found ATN to be less prevalent, whereas apoptosis, interstitial inflammation, and thrombosis were more prevalent. Nevertheless, renal biopsy usually is not feasible in ACLF. The soluble triggering receptor expressed on myeloid cell-1 (sTREM-1) and presepsin are potential biomarkers for the early diagnosis of sepsis in ACLF patients [127]. However, presepsin was not a predictor in patients with cirrhosis [128, 129]. Nonetheless, when the clinical manifestations meet the diagnosis of qSOFA in ACLF with infection, sepsis-AKI should be considered (Figure 2).
Table 6.
Comparison between HRS-AKI and sepsis-AKI in ACLF
| Characteristic | HRS-AKI | Sepsis-AKI |
|---|---|---|
| Infection | Major | All |
| Systemic hemodynamics | Reduction in effective arterial blood volume (+) | Reduction in effective arterial blood volume (+++) |
| Renal blood flow | Reduced | Increased |
| Appearance of renal histology | Normal, acute tubular lesions, bile cast nephropathy |
Apoptosis, interstitial inflammation, thrombosis, acute tubular necrosis |
| Cardiac output | Decreased or normal | Increased |
| Peripheral vascular resistance | Normal or slightly decreased | Decreased |
| Extrarenal organ failure | Common | Common |
| Systemic inflammatory response | Moderate | High |
| Shock | None | Usual |
| Recommended treatment | Vasoactive drugs combined with albumin | Crystalloids and/or renal replacement therapy |
HRS, hepatorenal syndrome; AKI, acute kidney injury; ACLF, acute-on-chronic liver failure.
Figure 2.
Schematic view of the identification of AKI in ACLF with infection
AKI, acute kidney injury; qSOFA, quick Sequential Organ Failure Assessment; HRS, hepatorenal syndrome; MAP, mean arterial pressure; Lac, lactate.
Does HRS-AKI occur on the basis of CKD?
According to the current diagnostic standard for HRS-AKI [9], CKD with structural changes is excluded. However, some studies do not exclude CKD [130]. Angeli et al. [18] pointed out that the new criterion would include cases of known pre-existing structural CKD (e.g. diabetic or hypertensive nephropathy). HBV, hepatitis C virus, and other factors can also lead to various kidney diseases. Red blood cells and protein in the urine can exceed the diagnostic criteria for HRS-AKI. Portal hypertension may accelerate the deterioration of renal function. Whether all CKD cases can be included, they are worth considering. AKI also occurs with the same triggering factors of HRS-AKI. Thus, it is important to consider the diagnosis of HRS-AKI in these patients. However, reasonable methods of evaluation are lacking. The diagnostic criteria for AKI in cirrhosis are clear, but they may not be suitable in CKD, especially when renal dysfunction presents prior to acute exacerbation. An increase in sCr of ≥50% or urinary output of ≤0.5 mL/kg B.W. for ≥6 h may not be suitable. Considering the pathophysiology and progress of HRS-AKI, when HRS-AKI secondary to prerenal factors cannot be excluded, timely intervention is necessary. An absolute increase in sCr of ≥0.3 mg/dL may require intervention to control the progress of CKD.
Does the measured baseline of the sCr value reflect reality?
In patients with cirrhosis and ascites, sCr levels fluctuate because of frequent use of diuretics. In addition, patients show underestimated sCr values because of inadequate intake of calories and protein, and decreased muscle quality [131, 132]. The presence and progress of hyperbilirubinemia can also affect the detection of sCr [133, 134]. In addition, some patients with ACLF did not have regular follow-up, making it difficult to obtain their baseline values [23], which impacts the diagnosis of HRS-AKI. According to the consensus of HRS in 2015, some patients may be diagnosed as stage 2 or stage 3 even if the sCr level does not exceed 1.5 mg/dL [9]. Yet, this may not reflect the reality of renal function. Therefore, other dynamic indicators are needed for the diagnosis and tracking of disease progression. New markers of renal injury, such as NGAL, kidney injury molecule-1 (KIM-1), liver-type fatty acid-binding protein, insulin-like growth factor-binding protein 7, and tissue inhibitor of metalloproteinases 2 [135, 136], have been proposed but require validation. Development of biomarkers may assist in diagnosis and simultaneously provide prognostic information.
Is the scheme of albumin supplementation reasonable?
In patients hospitalized with decompensated cirrhosis, Caraceni et al. [137] found that long-term human albumin administration reduced complications and prolonged overall survival. When prerenal AKI is suspected, 48 h of empirical fluid challenge is required. The fluid infusion consists of 1 g/kg/d of albumin and avoids the use of diuretics. If there is no improvement in renal function, then HRS-AKI should be considered [9, 10]. When the consensus on HRS was first formulated in 1996 [108], the criteria included no improvement with fluid supplementation using 1.5 L of isotonic saline. However, this was then adjusted to albumin supplementation [10]. The theoretical basis was that albumin could improve circulatory function in cirrhosis by expanding central blood volume and increasing CO [138]. A prospective randomized–controlled study showed that albumin infusion reduced the incidence of AKI in patients with spontaneous bacterial peritonitis [139]. Even with the HRS consensus in 2015 and guidelines for the management of decompensated cirrhosis in 2018 [9, 17], no study has verified these conclusions. An official statement [140] was added that one should avoid the use of high concentrations of albumin for volume expansion. EASL recommended that volume replacement should be used in accordance with the cause and severity of fluid losses in decompensated cirrhosis patients with AKI. In the case of AKI without obvious cause, AKI stage >1A, or infection-induced AKI, a 20% albumin solution should be used for 2 consecutive days [17]. China et al. [141] found that increased albumin levels to ≥30 g/L were associated with more severe or life-threatening serious adverse events in hospitalized patients with cirrhosis. However, Caraceni et al. [142] found that serum albumin levels were strongly associated with the survival and occurrence of major complications in patients with cirrhosis and ascites. Achieving a 40-g/L serum albumin concentration is ideal. Regardless, the concentration of albumin should be closely monitored and evaluated. When AKI occurs, it may be a reasonable goal to achieve a serum albumin concentration of >30 g/L as soon as possible. Central venous pressure monitoring can help to optimize the albumin dose and prevent circulatory overload [17]. Central venous pressure of ≤10 cm H2O only was recommended in the Sassari’s Diagnostic Criteria for HRS, which remains controversial [108]. The ideal level of central venous pressure for albumin supplementation needs to be determined by prospective research.
Treatment of HRS-AKI with ACLF
Timing of HRS-AKI treatment
According to the recommendation of the consensus of HRS-AKI, the use of vasoactive drugs should be considered in stage 2 or 3 of AKI [9]. In patients with cirrhosis and spontaneous bacterial peritonitis, vasoactive drugs can significantly improve the reversal rate of HRS-AKI and the 60-day survival rate [143]. The timely and reasonable use of anti-infective drugs has become an important treatment method in ACLF. If AKI occurs with anti-infective treatment, the result is unfavorable [144]. However, there is no evidence that a “one-size-fits-all” approach to AKI in cirrhosis is indicated. A fluid-first treatment algorithm may delay the initiation of vasoconstrictors in patients with HRS-AKI or exacerbate volume overload [135]. The rapid progress of ACLF is aggravated with the onset of AKI. Patients with AKI 1B more often progress to higher AKI stages with significantly lower 28-day and 90-day survival rates [23, 145]. Therefore, it is essential to add vasoactive drugs and albumin in a timely manner, even on the first day when there is no clear evidence of fluid loss or inadequate intake.
Choice of treatment methods
Terlipressin (TP) is recommended, but the side effects are considerable [9, 17]. A large-scale prospective study supported this conclusion [146]. Cavallin et al. [94] prospectively compared the administration of TP as a continuous intravenous infusion vs intravenous boluses in the treatment of HRS-AKI and found that the former was more tolerable. Arora et al. [34] used a similar approach in patients with ACLF and AKI, but the side effects did not decrease significantly. In addition, noradrenaline (NE), which improves renal perfusion primarily through vascular smooth muscle contraction via α-adrenergic receptors, is another alternative drug for HRS-AKI. Although meta-analysis showed that both drugs elicited the same therapeutic effect [147], the side effects of the two drugs did not overlap [34]. Meta-analysis revealed that vasopressin combined with catecholamines could significantly reduce the incidence of atrial fibrillation and significantly increase the 28- or 30-day survival rate in patients with distributive shock [148]. The combination of the two drugs also improved the survival rate to 30 days in septic shock [149]. At least one clinical trial assessed TP alone vs TP combined with NE in the treatment of HRS-AKI (Identifier: NCT03822091) [150]. We compared a balanced salt solution vs albumin for reversing AKI in end-stage liver disease (ChiCTR2000034544) [151]. When HRS-AKI was diagnosed, TP combined with NE was employed in the trial [151]. To date, unpublished data showed that adverse effects were reduced with no significant increase in the reversal rate of HRS-AKI. Octreotide can be used in the treatment of HRS-AKI associated with ACLF [38]. However, it is not recommended owing to its poor effectiveness relative to TP [152]. While the current treatment protocol for HRS-AKI suggests a MAP of ≥65 mmHg, or 10 mmHg over baseline, there was no significant difference between high and low blood pressure in the reversal rate of HRS-AKI [153]. Velez et al. [154] found that the magnitude of the MAP rise during HRS-AKI therapy was positively correlated with a reduction in the sCr level. Improving CO and reducing the pressure in the portal vein may represent another direction for therapy.
Application of diuretics
There is no consensus regarding the use of diuretics in combination with vasoactive drugs in HRS-AKI. Triple therapy (the combination of 2 μg/kg/min of dopamine, 0.01 mg/kg/h of furosemide, and 20 g/d of albumin) was similar in effect as combined therapy (0.5 mg of TP every 6 h plus 20 g/day of albumin) in HRS-AKI [96]. Péron et al. [155] found that almost all patients with HRS-AKI needed to receive added diuretics and the patient prognosis was thereafter improved. If the results of TP combined with albumin were poor, the application of diuretics in ACLF did not improve the treatment effect [34]. It is recognized that diuretic use is controversial, but early use of diuretics with TP and albumin should be considered.
Treatment with transjugular intrahepatic portosystemic stent-shunt
Although transjugular intrahepatic portosystemic stent-shunt (TIPS) treatment is employed in HRS patients [156] and meta-analysis shows that it improves renal function and prognosis, there are few high-quality extant studies [157]. Indeed, one study on TIPS in HRS-AKI was very selective in its design, thus limiting the application of the findings [2]. Trebicka et al. [31] found that ACLF patients with acute variceal bleeding benefited from pre-emptive TIPS, but only one patient with HRS-AKI was treated in this manner. Cornman-Homonoff et al. [158] considered several aspects to be crucial and deserving further study, including lower MELD score and ACLF grade. Because of the rapid progress of ACLF patients with HRS-AKI, the benefit of TIPS should be considered cautiously.
Blood-purification treatment
If AKI with ACLF cannot be reversed, continuous RRT (CRRT) is likely required [159–161]. Arora et al. [34] reported that 82 of 120 (68.3%) patients with ACLF and HRS-AKI required RRT at a median of 4 days. Yet, patients with ACLF and AKI requiring CRRT exhibited a poor survival, even with the provision of extracorporeal support therapy [162]. Such intervention appears to only prolong hospital stay [163]. Under the current guidelines, CRRT is not recommended for ACLF with AKI unless there is an acute reversible component or a plan for liver transplantation [20, 164]. A small-sample study consisting of intermittent high-throughput albumin dialysis combined with continuous veno-venous hemodialysis in ACLF patients with AKI improved 28- and 90-day survival [165]. However, no large-scale prospective clinical study has been performed to confirm. A molecular adsorbent recycling system was used in the treatment of HRS-AKI, which improved the 7- and 30-day survival rates [166]. In another report, the molecular adsorbent recycling system reduced the concentration of nitric oxide without other benefit [167]. Patients with HRS-AKI who are not receiving mechanical ventilation may benefit from hemodialysis. Conversely, hemodialysis appears to be futile in patients requiring mechanical ventilation [168]. There is no evidence to support the early application of CRRT in ACLF with HRS-AKI, but regional citrate anticoagulation in liver dysfunctionis a feasible and valuable tool for CRRT in the perioperative care of liver transplant recipients when limitations and pitfalls are adequately considered [169 ].
Liver transplantation or combined liver–kidney transplantation
Patients with ACLF can undergo liver transplantation alone, because HRS-AKI is a functional renal injury [170]. Importantly, the reversal rate of HRS-AKI following liver transplantation can reach 83% [171–174]. The response to TP and albumin reduces the need for RRT and the risk of CKD at 1 year after liver transplantation [175]. In patients with HRS-AKI and ACLF undergoing liver transplantation, MELD >36 or hyponatremia (≤126 mEq/L) is closely related to higher mortality [155]. Whether simultaneous liver–kidney transplantation is necessary requires careful evaluation [176–178]. The factors influencing outcome include a higher CLIF-organ failure score, TBil, non-controlled infection, sCr, and INR level [122, 179]. If sCr is decreased, the prognosis of transplantation can be improved [180]. If CRRT therapy was established at the time of liver transplantation, then the prognosis for transplantation alone was worsened [181]. Estimated glomerular filtration rate (GFR) of ≤35 mL/min or measured GFR of ≤25 mL/min for ≥4 weeks was an important criterion for combined liver–kidney transplantation [182]. Thus, liver transplantation should be considered early if ACLF and renal function were not improved. Delayed implantation of kidney grafts in combined liver–kidney transplantation (with the implantation of kidneys delayed for >48 h) was associated with improved kidney function and improved patient and graft survival [183]. This procedure has been confirmed in the USA and Europe [184], and needs to be extended.
Prevention of HRS-AKI
Although therapy for HRS-AKI is continuously updated, the survival rate is not significantly improved. How to prevent the occurrence of HRS-AKI must be considered. Rifaximin and pentoxifylline reduced the incidence of cirrhosis-related complications, including HRS-AKI [185–187]. At present, the most effective treatment for the prevention of HRS-AKI is intermittent albumin infusion, which prevents the occurrence of AKI in decompensated cirrhosis and ACLF [137, 139, 188]. However, Fernández et al. [189] found that albumin exerted little effect on renal function except to improve CO. In addition, the use of non-selective β receptor blockers significantly reduced the incidence of HRS-AKI after a reduction in the hepatic venous pressure gradient [190]. In the absence of a more reasonable and effective treatment, it is of paramount importance to undertake active preventive measures to improve the prognosis with respect to ACLF. ACLF can be identified based to patients’ characteristics, but close monitoring and evaluation, early detection, and appropriate treatment are still the mainstays of care.
Conclusions
HRS-AKI is an important factor that adversely affects the prognosis of ACLF. The current diagnostic criteria for HRS-AKI do not reflect the true status of the kidney, especially in ACLF. Uncontrolled inflammation aggravates the hemodynamic state of patients with ACLF. Pathological injury of the kidney may be common, contributing to the difficulty in reversing this process. At present, HRS-AKI in ACLF should be diagnosed cautiously when the serum lactate level is >2 mmol/L. Urine output of <0.5 mL/kg/h for ≥6 h cannot be relied upon exclusively to resolve clinical ambiguities. QSOFA is useful in distinguishing sepsis-AKI from HRS-AKI in ACLF with infection. When HRS-AKI is present, more active strategies of vasoactive drugs combined with albumin should be considered. If therapy is not effective, liver transplantation should be performed as soon as possible. Many questions concerning diagnosis and treatment are still unanswered. Future research may help to clarify the diagnosis of HRS-AKI and improve outcomes [191].
Authors’ Contributions
S.L. and J.Z. contributed to the concept and design of the review and the writing of the manuscript. Q.M. focused on researching the new-therapy section. Y.X. focused on the epidemiology and diagnosis aspects of the study. All authors read and approved the final manuscript.
Funding
This research was supported by the Scientific Research Project of Beijing You’an Hospital [CCMU 2019] and the China Primary Health Care Foundation–Youan Foundation of Liver Disease and AIDS [BJYAYY-GG2019-01 ].
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance and scientific consultation during the preparation of this manuscript.
Conflict of Interest
None declared .
References
- 1. Ginès P, Guevara M, Arroyo V et al. Hepatorenal syndrome. Lancet 2003;362:1819–27. [DOI] [PubMed] [Google Scholar]
- 2. Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ 2020;370:m2687. [DOI] [PubMed] [Google Scholar]
- 3. Carvalho GC, Regis CA, Kalil JR et al. Causes of renal failure in patients with decompensated cirrhosis and its impact in hospital mortality. Ann Hepatol 2012;11:90–5. [PubMed] [Google Scholar]
- 4. Rey RM, Delgado AF, De Zubiria A et al. Prevalence and short-term outcome of hepatorenal syndrome: a 9-year experience in a high-complexity hospital in Colombia. PLoS One 2020;15:e0239834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thapa P, Kc S, Hamal AB et al. Prevalence of acute kidney injury in patients with liver cirrhosis. JNMA J Nepal Med Assoc 2020;58:554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ginès A, Escorsell A, Ginès P et al. Incidence, predictive factors, and prognosis of hepatorenal syndrome in cirrhosis. Gastroenterology 1993;105:229–36. [DOI] [PubMed] [Google Scholar]
- 7. Bashir MH, Iqbal S, Miller R et al. Management and outcomes of hepatorenal syndrome at an urban academic medical center: a retrospective study. Eur J Gastroenterol Hepatol 2019;31:1545–9. [DOI] [PubMed] [Google Scholar]
- 8. Jamil K, Huang X, Lovelace B et al. The burden of illness of hepatorenal syndrome (HRS) in the United States: a retrospective analysis of electronic health records. J Med Econ 2019;22:421–9. [DOI] [PubMed] [Google Scholar]
- 9. Angeli P, Ginès P, Wong F et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol 2015;62:968–74. [DOI] [PubMed] [Google Scholar]
- 10. Salerno F, Gerbes A, Ginès P et al. Diagnosis, prevention and treatment of the hepatorenal syndrome in cirrhosis a consensus workshop of the International Ascites Club. Gut 2007;56:1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehta RL, Kellum JA, Shah SV et al. ; Acute Kidney Injury Network. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bellomo R, Ronco C, Kellum J et al. ; Acute Dialysis Quality Initiative workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) Group. Crit Care 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. [DOI] [PubMed] [Google Scholar]
- 14. Huang Z, Lin C, Fang J et al. Acute kidney injury in hepatitis B-related acute-on-chronic liver failure without preexisting liver cirrhosis. Hepatol Int 2015;9:416–23. [DOI] [PubMed] [Google Scholar]
- 15. Piano S, Rosi S, Maresio G et al. Evaluation of the acute kidney injury network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol 2013;59:482–9. [DOI] [PubMed] [Google Scholar]
- 16. Belcher JM, Garcia-Tsao G, Sanyal AJ et al. ; for the TRIBE-AKI Consortium. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology 2013;57:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.. European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60. [DOI] [PubMed] [Google Scholar]
- 18. Angeli P, Garcia-Tsao G, Nadim MK et al. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol 2019;71:811–22. [DOI] [PubMed] [Google Scholar]
- 19. Anand AC, Dhiman RK. Acute on chronic liver failure : what is in a “definition”? J Clin Exp Hepatol 2016;6:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sarin SK, Choudhury A, Sharma MK et al. Acute on chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int 2019;13:353–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreau R, Jalan R, Gines P et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–37, 1437.e1-9. [DOI] [PubMed] [Google Scholar]
- 22. Bajaj JS, O'Leary JG, Reddy KR et al. ; North American Consortium for the Study of End-Stage Liver Disease (NACSELD). Survival in sepsis-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huelin P, Piano S, Solà E et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin Gastroenterol Hepatol 2017;15:438–45.e5. [DOI] [PubMed] [Google Scholar]
- 24. Maiwall R, Kumar S, Chandel SS et al. AKI in patients with acute on chronic liver failure is different from acute decompensation of cirrhosis. Hepatol Int 2015;9:627–39. [DOI] [PubMed] [Google Scholar]
- 25. Davenport A, Sheikh MF, Lamb E et al. Acute kidney injury in acute-on-chronic liver failure: where does hepatorenal syndrome fit? Kidney Int 2017;92:1058–70. [DOI] [PubMed] [Google Scholar]
- 26.Liver Failure and Artificial Liver Group; Chinese Society of Infectious Diseases; Chinese Medical Association; Severe Liver Disease and Artificial Liver Group; Chinese Society of Hepatology; Chinese Medical Association. Guideline for diagnosis and treatment of liver failure. Zhonghua Gan Zang Bing Za Zhi 2019;27:18–26. [DOI] [PubMed] [Google Scholar]
- 27. Jalan R, Yurdaydin C, Bajaj JS et al. ; World Gastroenterology Organization Working Party. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology 2014;147:4–10. [DOI] [PubMed] [Google Scholar]
- 28. Kim TY, Song DS, Kim HY et al. ; Korean Acute-on-Chronic Liver Failure (KACLiF) Study Group. Characteristics and discrepancies in acute-on-chronic liver failure: need for a unified definition. PLoS One 2016;11:e0146745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duseja A, Singh SP. Toward a better definition of acute-on-chronic liver failure. J Clin Exp Hepatol 2017;7:262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D’Souza R, Grammatikopoulos T, Pradhan A et al. Acute-on-chronic liver failure in children with biliary atresia awaiting liver transplantation. Pediatr Transplant 2019;23:e13339. [DOI] [PubMed] [Google Scholar]
- 31. Trebicka J, Gu W, Ibáñez-Samaniego L et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol 2020;73:1082–91. [DOI] [PubMed] [Google Scholar]
- 32. Khatua CR, Panigrahi S, Mishra D et al. Acute kidney injury at admission is a better predictor of mortality than its persistence at 48 h in patients with acute-on-chronic liver failure. J Clin Transl Hepatol 2018;6:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maiwall R, Kumar G, Bharadwaj A et al. AKI persistence at 48 h predicts mortality in patients with acute on chronic liver failure. Hepatol Int 2017;11:529–39. [DOI] [PubMed] [Google Scholar]
- 34. Arora V, Maiwall R, Rajan V et al. Terlipressin is superior to noradrenaline in the management of acute kidney injury in acute on chronic liver failure. Hepatology 2020;71:600–10. [DOI] [PubMed] [Google Scholar]
- 35. Yuan W, Zhang YY, Zhang ZG et al. Risk factors and outcomes of acute kidney injury in patients with hepatitis B virus-related acute-on-chronic liver failure. Am J Med Sci 2017;353:452–8. [DOI] [PubMed] [Google Scholar]
- 36. Lal BB, Alam S, Sood V et al. Profile, risk factors and outcome of acute kidney injury in paediatric acute-on-chronic liver failure. Liver Int 2018;38:1777–84. [DOI] [PubMed] [Google Scholar]
- 37. Jindal A, Bhadoria AS, Maiwall R et al. Evaluation of acute kidney injury and its response to terlipressin in patients with acute-on-chronic liver failure. Liver Int 2016;36:59–67. [DOI] [PubMed] [Google Scholar]
- 38. Zang H, Liu F, Liu H et al. Incidence, risk factors and outcomes of acute kidney injury (AKI) in patients with acute-on-chronic liver failure (ACLF) of underlying cirrhosis. Hepatol Int 2016;10:807–18. [DOI] [PubMed] [Google Scholar]
- 39. Martin PY, Gines P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med 1998;339:533–41. [DOI] [PubMed] [Google Scholar]
- 40. Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009;361:1279–‐90. [DOI] [PubMed] [Google Scholar]
- 41. Maroto A, Gines A, Salo J et al. Diagnosis of functional kidney failure of cirrhosis with Doppler sonography: prognostic value of resistive index. Hepatology 1994;20:839–44. [DOI] [PubMed] [Google Scholar]
- 42. Ginès P, Solà E, Angeli P et al. Hepatorenal syndrome. Nat Rev Dis Primers 2018;4:23. [DOI] [PubMed] [Google Scholar]
- 43. Mindikoglu AL, Dowling TC, Wong-You-Cheong JJ et al. A pilot study to evaluate renal hemodynamics in cirrhosis by simultaneous glomerular filtration rate, renal plasma flow, renal resistive indices and biomarkers measurements. Am J Nephrol 2014;39:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weichselbaum L, Gustot T. The organs in acute-on-chronic liver failure. Semin Liver Dis 2016;36:174–80. [DOI] [PubMed] [Google Scholar]
- 45. Trebicka J, Amoros A, Pitarch C et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol 2019;10:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Claria J, Stauber RE, Coenraad MJ et al. ; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology 2016;64:1249–64. [DOI] [PubMed] [Google Scholar]
- 47. Choudhury A, Kumar M, Sharma BC et al. ; for the APASL ACLF working party. Systemic inflammatory response syndrome in acute-on-chronic liver failure: relevance of “golden window”: a prospective study. J Gastroenterol Hepatol 2017;32:1989–97. [DOI] [PubMed] [Google Scholar]
- 48. Solé C, Solà E, Morales-Ruiz M et al. Characterization of inflammatory response in acute-on-chronic liver failure and relationship with prognosis. Sci Rep 2016;6:32341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang XL, Chen XJ, Ye HH et al. Association of mRNA expression level of IP-10 in peripheral blood mononuclear cells with HBV-associated acute-on-chronic liver failure and its prognosis. Curr Med Sci 2017;37:755–60. [DOI] [PubMed] [Google Scholar]
- 50. Solé C, Solà E, Huelin P et al. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int 2019;39:1246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu W, Yan H, Zhao H et al. Characteristics of systemic inflammation in hepatitis B-precipitated ACLF: differentiate it from No-ACLF. Liver Int 2018;38:248–57. [DOI] [PubMed] [Google Scholar]
- 52. Wasmuth HE, Kunz D, Yagmur E et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol 2005;42:195–201. [DOI] [PubMed] [Google Scholar]
- 53. Martin-Mateos R, Alvarez-Mon M, Albillos A. Dysfunctional immune response in acute-on-chronic liver failure: it takes two to Tango. Front Immunol 2019;10:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weiss E, de la Grange P, Defaye M et al. Characterization of blood immune cells in patients with decompensated cirrhosis including ACLF. Front Immunol 2020;11:619039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lu J, Lin L, Ye C et al. Serum NGAL is superior to cystatin C in predicting the prognosis of acute-on-chronic liver failure. Ann Hepatol 2019;18:155–64. [DOI] [PubMed] [Google Scholar]
- 56. Grønbaek H, Møller HJ, Saliba F et al. Improved prediction of mortality by combinations of inflammatory markers and standard clinical scores in patients with acute-on-chronic liver failure and acute decompensation. J Gastroenterol Hepatol 2021;36:240–8. [DOI] [PubMed] [Google Scholar]
- 57. Ariza X, Graupera I, Coll M et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol 2016;65:57–65. [DOI] [PubMed] [Google Scholar]
- 58. Thabut D, Massard J, Gangloff A et al. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology 2007;46:1872–82. [DOI] [PubMed] [Google Scholar]
- 59. Dominguez JA, Coopersmith CM. Can we protect the gut in critical illness? The role of growth factors and other novel approaches. Crit Care Clin 2010;26:549–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen Y, Guo J, Qian G et al. Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J Gastroenterol Hepatol 2015;30:1429–37. [DOI] [PubMed] [Google Scholar]
- 61. Yao X, Yu H, Fan G et al. Impact of the gut microbiome on the progression of hepatitis B virus related acute-on-chronic liver failure. Front Cell Infect Microbiol 2021;11:573923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang K, Zhang Z, Mo ZS et al. Gut microbiota as prognosis markers for patients with HBV-related acute-on-chronic liver failure. Gut Microbes 2021;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Y, Zhao R, Shi D et al. Characterization of the circulating microbiome in acute-on-chronic liver failure associated with hepatitis B. Liver Int 2019;39:1207–16. [DOI] [PubMed] [Google Scholar]
- 64. El-Naggar MM, Khalil EA, El-Daker MAM et al. Bacterial DNA and its consequences in patients with cirrhosis and culture-negative, non-neutrocytic ascites. J Med Microbiol 2008;57:1533–8. [DOI] [PubMed] [Google Scholar]
- 65. Moller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut 2008;57:268–78. [DOI] [PubMed] [Google Scholar]
- 66. Krag A, Bendtsen F, Henriksen JH et al. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut 2010;59:105–10. [DOI] [PubMed] [Google Scholar]
- 67. Premkumar M, Devurgowda D, Vyas T et al. Left ventricular diastolic dysfunction is associated with renal dysfunction, poor survival and low health related quality of life in cirrhosis. J Clin Exp Hepatol 2019;9:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kumar A, Das K, Sharma P et al. Hemodynamic studies in acute-on-chronic liver failure. Dig Dis Sci 2009;54:869–78. [DOI] [PubMed] [Google Scholar]
- 69. Liu H, Lee SS. Acute-on-chronic liver failure: the heart and systemic hemodynamics. Curr Opin Crit Care 2011;17:190–4.48. [DOI] [PubMed] [Google Scholar]
- 70. Ruiz-del-Arbol L, Monescillo A, Arocena C et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology 2005;42:439–47. [DOI] [PubMed] [Google Scholar]
- 71. Liu PMF, de Carvalho ST, Fradico PF et al. Hepatorenal syndrome in children: a review. Pediatr Nephrol 2020;36:2203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stadlbauer V, Wright GA, Banaji M et al. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology 2008;134:111–9. [DOI] [PubMed] [Google Scholar]
- 73. Bernardi M, Trevisani F, Gasbarrini A et al. Hepatorenal disorders: role of the renin-angiotensin-aldosterone system. Semin Liver Dis 1994;14:23–34. [DOI] [PubMed] [Google Scholar]
- 74. Mehta G, Mookerjee RP, Sharma V et al. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int 2015;35:724–34. [DOI] [PubMed] [Google Scholar]
- 75. Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol 2003;38(Suppl 1):S69–89. [DOI] [PubMed] [Google Scholar]
- 76. Gassanov N, Semmo N, Semmo M et al. Arginine vasopressin (AVP) and treatment with arginine vasopressin receptor antagonists (vaptans) in congestive heart failure, liver cirrhosis and syndrome of inappropriate antidiuretic hormone secretion (SIADH). Eur J Clin Pharmacol 2011;67:333–46. [DOI] [PubMed] [Google Scholar]
- 77. Kerbert AJC, Verspaget HW, Navarro ÀA et al. ; CANONIC Study Investigators of the EASL-CLIF Consortium. Copeptin in acute decompensation of liver cirrhosis: relationship with acute-on-chronic liver failure and short-term survival. Crit Care 2017;21:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Acevedo J, Fernández J, Prado V et al. Relative adrenal insufficiency in decompensated cirrhosis: relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death. Hepatology 2013;58:1757–65. [DOI] [PubMed] [Google Scholar]
- 79. Piano S, Favaretto E, Tonon M et al. Including relative adrenal insufficiency in definition and classification of acute-on-chronic liver failure. Clin Gastroenterol Hepatol 2020;18:1188–96.e3. [DOI] [PubMed] [Google Scholar]
- 80. Escorsell À, Castellote J, Sánchez-Delgado J et al. Management of acute liver failure: clinical guideline from the Catalan Society of Digestology. Gastroenterol Hepatol 2019;42:51–64. [DOI] [PubMed] [Google Scholar]
- 81.European Association for the Study of the Liver. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047–81. [DOI] [PubMed] [Google Scholar]
- 82. Anand AC, Nandi B, Acharya SK et al. Indian national association for the study of the liver consensus statement on acute liver failure (Part 1): epidemiology, pathogenesis, presentation and prognosis. J Clin Exp Hepatol 2020;10:339–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wilkinson SP, Blendis LM, Williams R. Frequency and type of renal and electrolyte disorders in fulminant hepatic failure. Br Med J 1974;1:186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Akriviadis E, Botla R, Briggs W et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000;119:1637–48. [DOI] [PubMed] [Google Scholar]
- 85. Tujios SR, Hynan LS, Vazquez MA et al. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol 2015;13:352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Anand AC, Nandi B, Acharya SK et al. Indian national association for the study of liver consensus statement on acute liver failure (Part-2): management of acute liver failure. J Clin Exp Hepatol 2020;10:477–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hu Y, Hu D, Fu R. Correlation between High mobility group box-1 protein and chronic hepatitis B infection with severe hepatitis B and acute-on-chronic liver failure: a meta-analysis. Minerva Medica 2016;108:268–76. [DOI] [PubMed] [Google Scholar]
- 88. Wang Y, Zhang H, Chen Q et al. TNF-α/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif 2020;53:e12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang R, Zou X, Tenhunen J et al. HMGB1 and extracellular histones significantly contribute to systemic inflammation and multiple organ failure in acute liver failure. Mediators Inflamm 2017;2017:5928078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. de Oliveira Gomes CG, de Andrade MVM, Guedes LR et al. Evaluation of the biomarkers HMGB1 and IL-6 as predictors of mortality in cirrhotic patients with acute kidney injury. Mediators Inflamm 2020;2020:2867241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Moore JK, Love E, Craig DG et al. Acute kidney injury in acute liver failure: a review. Expert Rev Gastroenterol Hepatol 2013;7:701–12. [DOI] [PubMed] [Google Scholar]
- 92. Cavallin M, Kamath PS, Merli M et al. ; Italian Association for the Study of the Liver Study Group on Hepatorenal Syndrome. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology 2015;62:567–74. [DOI] [PubMed] [Google Scholar]
- 93. Boyer TD, Sanyal AJ, Wong F et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology 2016;150:1579–89.e2. [DOI] [PubMed] [Google Scholar]
- 94. Cavallin M, Piano S, Romano A et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology 2016;63:983–92. [DOI] [PubMed] [Google Scholar]
- 95. Piano S, Schmidt HH, Ariza X et al. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol 2018;16:1792–800.e3. [DOI] [PubMed] [Google Scholar]
- 96. Srivastava S, Vishnubhatla S, Shalimar et al. Randomized controlled trial comparing the efficacy of terlipressin and albumin with a combination of concurrent dopamine, furosemide, and albumin in hepatorenal syndrome. J Clin Exp Hepatol 2015;5:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang H, Liu A, Bo W et al. Terlipressin in the treatment of hepatorenal syndrome: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Thomson MJ, Taylor A, Sharma P et al. Limited progress in hepatorenal syndrome (HRS) reversal and survival 2002-2018: a systematic review and meta-analysis. Dig Dis Sci 2020;65:1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mandal AK, Lansing M, Fahmy A. Acute tubular necrosis in hepatorenal syndrome: an electron microscopy study. Am J Kid Ney Dis 1982;2:363–74. [DOI] [PubMed] [Google Scholar]
- 100. van Slambrouck CM, Salem F, Meehan SM et al. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int 2013;84:192–7. [DOI] [PubMed] [Google Scholar]
- 101. Nayak SL, Kumar M, Bihari C et al. Bile cast nephropathy in patients with acute kidney injury due to hepatorenal syndrome: a postmortem kidney biopsy study. J Clin Transl Hepatol 2017;XX:1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bräsen JH, Mederacke YS, Schmitz J et al. Cholemic nephropathy causes acute kidney injury and is accompanied by loss of aquaporin 2 in collecting ducts. Hepatology 2019;69:2107–19. [DOI] [PubMed] [Google Scholar]
- 103. Varga ZV, Erdelyi K, Paloczi J et al. Disruption of renal arginine metabolism promotes kidney injury in hepatorenal syndrome in mice. Hepatology 2018;68:1519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Poloni JAT, Perazella MA, Keitel E et al. Utility of a urine sediment score in hyperbilirubinemia/hyperbilirubinuria. Clin Nephrol 2019;92:141–50. [DOI] [PubMed] [Google Scholar]
- 105. Jiang QQ, Han MF, Ma K et al. Acute kidney injury in acute-on-chronic liver failure is different from in decompensated cirrhosis. World J Gastroenterol 2018;24:2300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Barreto R, Fagundes C, Guevara M et al. Type-1 hepatorenal syndrome associated with infections in cirrhosis: natural history, outcome of kidney function, and survival. Hepatology 2014;59:1505–13. [DOI] [PubMed] [Google Scholar]
- 107. Nazar A, Pereira GH, Guevara M et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology 2010;51:219–26. [DOI] [PubMed] [Google Scholar]
- 108. Arroyo V, Ginès P, Gerbes A et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology 1996;23:164–76. [DOI] [PubMed] [Google Scholar]
- 109. Millham FH. A brief history of shock. Surgery 2010;148:1026–37. [DOI] [PubMed] [Google Scholar]
- 110. Cecconi M, De Backer D, Antonelli M et al. Consensus on circulatory shock and hemodynamic monitoring: Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vincent JL, Ince C, Bakker J. Clinical review: circulatory shock—an update: a tribute to Professor Max Harry Weil. Crit Care 2012;16:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sun DQ, Zheng CF, Lu FB et al. Serum lactate level accurately predicts mortality in critically ill patients with cirrhosis with acute kidney injury. Eur J Gastroenterol Hepatol 2018;30:1361–7. [DOI] [PubMed] [Google Scholar]
- 113. Sterling SA, Puskarich MA, Jones AE. The effect of liver disease on lactate normalization in severe sepsis and septic shock: a cohort study. Clin Exp Emerg Med 2015;2:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kruse JA, Zaidi SA, Carlson RW. Significance of blood lactate levels in critically ill patients with liver disease. Am J Med 1987;83:77–82. [DOI] [PubMed] [Google Scholar]
- 115. De Backer D, Biston P, Devriendt J et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779–89. [DOI] [PubMed] [Google Scholar]
- 116. Singer M, Deutschman CS, Seymour CW et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Okonkwo E, Rozario N, Heffner AC. Presentation and outcomes of end stage liver disease patients presenting with septic shock to the emergency department. Am J Emerg Med 2020;38:1408–13. [DOI] [PubMed] [Google Scholar]
- 118. Baudry T, Hernu R, Valleix B et al. Cirrhotic patients admitted to the ICU with septic shock: factors predicting short and long-term outcome. Shock 2019;52:408–13. [DOI] [PubMed] [Google Scholar]
- 119. Vasques F, Duscio E, Romitti F et al. Septic shock-3 vs 2: an analysis of the ALBIOS study. Crit Care 2018;22:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hensley MK, Deng JC. Acute on chronic liver failure and immune dysfunction: a mimic of sepsis. Semin Respir Crit Care Med 2018;39:588–97. [DOI] [PubMed] [Google Scholar]
- 121. Wong F, Piano S, Singh V et al. Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure. J Hepatol 2021;74:330–9. [DOI] [PubMed] [Google Scholar]
- 122. Rodríguez E, Elia C, Solà E et al. Terlipressin and albumin for type-1 hepatorial syndrome associated with sepsis. J Hepatol 2014;60:955–61. [DOI] [PubMed] [Google Scholar]
- 123. Philips CA, Ahamed R, Rajesh S et al. Update on diagnosis and management of sepsis in cirrhosis: current advances. World J Hepatol 2020;12:451–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Piano S, Bartoletti M, Tonon M et al. Assessment of sepsis-3 criteria and quick SOFA in patients with cirrhosis and bacterial infections. Gut 2018;67:1892–9. [DOI] [PubMed] [Google Scholar]
- 125. Son J, Choi S, Huh JW et al. The quick sepsis-related organ failure score has limited value for predicting adverse outcomes in sepsis patients with liver cirrhosis. Korean J Intern Med 2020;35:861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Garofalo AM, Lorente-Ros M, Goncalvez G et al. Histopathological changes of organ dysfunction in sepsis. Intensive Care Med Exp 2019;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen J, Huang ZB, Li H et al. Early diagnostic biomarkers of sepsis for patients with acute-on-chronic liver failure: a multicenter study. Infect Dis Ther 2021;10:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ferrarese A, Frigo AC, Mion MM et al. Diagnostic and prognostic role of presepsin in patients with cirrhosis and bacterial infection. Clin Chem Lab Med 2020;23:cclm-2020-1212. [DOI] [PubMed] [Google Scholar]
- 129. Fischer P, Grigoras C, Bugariu A et al. Are presepsin and resistin better markers for bacterial infection in patients with decompensated liver cirrhosis? Dig Liver Dis 2019;51:1685–91. [DOI] [PubMed] [Google Scholar]
- 130. Huelin P, Solà E, Elia C et al. Neutrophil gelatinase-associated lipocalin for assessment of acute kidney injury in cirrhosis: a prospective study. Hepatology 2019;70:319–33. [DOI] [PubMed] [Google Scholar]
- 131. Yoo JJ, Kim SG, Kim YS et al. Estimation of renal function in patients with liver cirrhosis: impact of muscle mass and sex. J Hepatol 2019;70:847–54. [DOI] [PubMed] [Google Scholar]
- 132. Francoz C, Glotz D, Moreau R et al. The evaluation of renal function and disease in patients with cirrhosis. J Hepatol 2010;52:605–13. [DOI] [PubMed] [Google Scholar]
- 133. Spencer K. Analytical reviews in clinical biochemistry: the estimation of creatinine. Ann Clin Biochem 1986;23(Pt 1):1–25. [DOI] [PubMed] [Google Scholar]
- 134. Badiou S, Dupuy AM, Descomps B et al. Comparison between the enzymatic Vitros assist for creatinine determination and three other methods adapted on the Olympus analyzer. J Clin Lab Anal 2003;17:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Allegretti AS, Solà E, Ginès P. Clinical application of kidney biomarkers in cirrhosis. Am J Kidney Dis 2020;76:710–9. [DOI] [PubMed] [Google Scholar]
- 136. Endre ZH, Pickering JW. Acute kidney injury: cell cycle arrest biomarkers win race for AKI diagnosis. Nat Rev Nephrol 2014;10:683–5. [DOI] [PubMed] [Google Scholar]
- 137. Caraceni P, Riggio O, Angeli P et al. ; ANSWER Study Investigators. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018;391:2417–29. [DOI] [PubMed] [Google Scholar]
- 138. Brinch K, Moller S, Bendtsen F et al. Plasma volume expansion by albumin in cirrhosis. Relation to blood volume distribution, arterial compliance and severity of disease. J Hepatol 2003;39:24–31. [DOI] [PubMed] [Google Scholar]
- 139. Fernandez J, Navasa M, Garcia-Pagan JC et al. Effect of intravenous albumin on systemic and hepatic hemodynamics and vasoactive neurohormonal systems in patients with cirrhosis and spontaneous bacterial peritonitis. J Hepatol 2004;41:384–90. [DOI] [PubMed] [Google Scholar]
- 140. Brochard L, Abroug F, Brenner M et al. ; ATS/ERS/ESICM/SCCM/SRLF Ad Hoc Committee on Acute Renal Failure. An official ATS/ERS/ESICM/SCCM/SRLF statement: prevention and management of acute renal failure in the ICU patient: an international consultations conference in intensive care medicine. Am J Respir Crit Care Med 2010;181:1128–55. [DOI] [PubMed] [Google Scholar]
- 141. China L, Freemantle N, Forrest E et al. ; ATTIRE Trial Investigators. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med 2021;384:808–17. [DOI] [PubMed] [Google Scholar]
- 142. Caraceni P, Tufoni M, Zaccherini G et al. ; ANSWER Study Investigators. On-treatment serum albumin level can guide long-term treatment in patients with cirrhosis and uncomplicated ascites. J Hepatol 2021;74:340–9. [DOI] [PubMed] [Google Scholar]
- 143. Rivero M, Rodríguez-Gandía M, Serradilla R et al. A prospective comparative study of terlipressin and albumin for type-1 hepatorenal syndrome (HRS) in patients with cirrhosis and spontaneous bacterial peritonitis (SBP) VS cirrhosis alone. J Hepatol 2010;52:S332. [Google Scholar]
- 144. Piano S, Schmidt HH, Ariza X et al. Impact of acute-on-chronic liver failure on response to treatment with terlipressin and albumin in patients with type 1 hepatorenal syndrome. Dig Liver Dis 2017;49:E54. [Google Scholar]
- 145. Khatua CR, Sahu SK, Barik RK et al. Validation of international club of ascites subclassification of stage 1 acute kidney injury in chronic liver disease. JGH Open 2019;3:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wong F, Pappas SC, Curry MP et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med 2021;384:818–28. [DOI] [PubMed] [Google Scholar]
- 147. Mattos ÂZ, Mattos AA, Ribeiro RA. Terlipressin versus noradrenaline in the treatment of hepatorenal syndrome: systematic review with meta-analysis and full economic evaluation. Eur J Gastroenterol Hepatol 2016;28:345–51. [DOI] [PubMed] [Google Scholar]
- 148. McIntyre WF, Um KJ, Alhazzani W et al. Association of vasopressin plus catecholamine vasopressors vs catecholamines alone with atrial fibrillation in patients with distributive shock: a systematic review and meta-analysis. JAMA 2018;319:1889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Prakash V, Choudhury AK, Sarin SK. To assess the efficacy of early introduction of a combination of low dose vasopressin analogue in addition to noradrenaline as a vasopressor inpatients of cirrhosis with septic shock. Hepatology 2017;66:138A. [Google Scholar]
- 150. Singh V. Terlipressin infusion alone vs terlipressin with noradrenaline infusion in the treatment of hepatorenal syndrome type 1. https://clinicaltrials.gov/ct2/show/NCT03822091?cond=TERLIPRESSIN&draw=5&rank=35.
- 151. Liu ST. Non-inferior study of balanced salt solution versus human albumin solution in reversing end-stage liver disease be complicated of acute kidney injury. http://www.chictr.org.cn/showproj.aspx?proj=54611.
- 152. Best LM, Freem An SC, Sutton AJ et al. Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev 2019;9:CD013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Varajic B, Cavallazzi R, Mann J et al. High versus low mean arterial pressures in hepatorenal syndrome: a randomized controlled pilot trial. J Crit Care 2019;52:186–92. [DOI] [PubMed] [Google Scholar]
- 154. Velez JC, Kadian M, Taburyanskaya M et al. Hepatorenal acute kidney injury and the importance of raising mean arterial pressure. Nephron 2015;131:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Péron JM, Bureau C, Gonzalez L et al. Treatment of hepatorenal syndrome as defined by the international ascites club by albumin and furosemide infusion according to the central venous pressure: a prospective pilot study. Am J Gastroenterol 2005;100:2702–7. [DOI] [PubMed] [Google Scholar]
- 156. Rossle M. TIPS: 25 years later. J Hepatol 2013;59:1081–93. [DOI] [PubMed] [Google Scholar]
- 157. Song T, R€ Ossle M, He F et al. Transjugular intrahepatic portosystemic shunt for hepatorenal syndrome: a systematic review and meta-analysis. Dig Liver Dis 2018;50:323–30. [DOI] [PubMed] [Google Scholar]
- 158. Cornman-Homonoff J, Madoff DC. Commentary on: “Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS.” AJR Am J Roentgenol 2021;216:878. [DOI] [PubMed] [Google Scholar]
- 159. Gonwa TA, Wadei HM. The challenges of providing renal replacement therapy in decompensated liver cirrhosis. Blood Purif 2012;33:144–8. [DOI] [PubMed] [Google Scholar]
- 160. Cardoso FS, Gottfried M, Tujios S et al. ; For the US Acute Liver Failure Study Group. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology 2018;67:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Elizabeth Parsons C, Nelson R, Book LS et al. Renal replacement therapy in infants and children with hepatorenal syndrome awaiting liver transplantation: a case-control study. Liver Transpl 2014;20:1468–74. [DOI] [PubMed] [Google Scholar]
- 162. Saraiva IE, Ortiz-Soriano VM, Mei X et al. Continuous renal replacement therapy in critically ill patients with acute on chronic liver failure and acute kidney injury: a retrospective cohort study. Clin Nephrol 2020;93:187–94. [DOI] [PubMed] [Google Scholar]
- 163. Zhang Z, Maddukuri G, Jaipaul N et al. Role of renal replacement therapy in patients with type 1 hepatorenal syndrome receiving combination treatment of vasoconstrictor plus albumin. J Crit Care 2015;30:969–74. [DOI] [PubMed] [Google Scholar]
- 164. Nadim MK, Kellum JA, Davenport A et al. ; ADQI Workgroup. Hepatorenal syndrome: the 8th international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2012;16:R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Niewinski G, Raszeja-Wyszomirska J, Hrenczuk M et al. Intermittent high-flux albumin dialysis with continuous venovenous hemodialysis for acute-on-chronic liver failure and acute kidney injury. Artif Organs 2020;44:91–9. [DOI] [PubMed] [Google Scholar]
- 166. Mitzner SR, Stange J, Klammt S et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl 2000;6:277–86. [DOI] [PubMed] [Google Scholar]
- 167. Wong F, Raina N, Richardson R. Molecular adsorbent recirculating system is ineffective in the management of type 1 hepatorenal syndrome in patients with cirrhosis with ascites who have failed vasoconstrictor treatment. Gut 2010;59:381–6. [DOI] [PubMed] [Google Scholar]
- 168. Witzke O, Baumann M, Patschan D et al. Which patients benefit from hemodialysis therapy in hepatorenal syndrome? J Gastroenterol Hepatol 2004;19:1369–73. [DOI] [PubMed] [Google Scholar]
- 169. Sponholz C, Settmacher U, Bauer M et al. Regional citrate anticoagulation for continuous renal replacement therapy in the perioperative care of liver transplant recipients: a single center experience. Ther Apher Dial 2015;19:8–15. [DOI] [PubMed] [Google Scholar]
- 170. Iwatsuki S, Popovtzer MM, Corman JL et al. Recovery from "hepatic syndrome" after orthotopic liver transplantation. N Engl J Med 1973;289:1155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. MacDonald AJ, Nadim MK, Durand F et al. Acute kidney injury in cirrhosis: implications for liver transplantation. Curr Opin Crit Care 2019;25:171–8. [DOI] [PubMed] [Google Scholar]
- 172. Utako P, Emyoo T, Anothaisintawee T et al. Clinical outcomes after liver transplantation for hepatorenal syndrome: a systematic review and meta-analysis. Biomed Res Int 2018;2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Gonwa TA, McBride MA, Anderson K et al. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant 2006;6:2651–9. [DOI] [PubMed] [Google Scholar]
- 174. Xu X, Ling Q, Zhang M et al. Outcome of patients with hepatorenal syndrome type 1 after liver transplantation: Hangzhou experience. Transplantation 2009;87:1514–9. [DOI] [PubMed] [Google Scholar]
- 175. Piano S, Gambino C, Vettore E et al. Response to terlipressin and albumin is associated with improved liver transplant outcomes in patients with hepatorenal syndrome. Hepatology 2021;73:1909–19. [DOI] [PubMed] [Google Scholar]
- 176. Sung RS, Wiseman AC. Simultaneous liver-kidney transplant: too many or just enough? Adv Chronic Kidney Dis 2015;22:399–403. [DOI] [PubMed] [Google Scholar]
- 177. Pham PT, Lunsford KE, Bunnapradist S et al. Simultaneous liver-kidney transplantation or liver transplantation alone for patients in need of liver transplantation with renal dysfunction. Curr Opin Organ Transplant 2016;21:194–200. [DOI] [PubMed] [Google Scholar]
- 178. Singal AK, Ong S, Satapathy SK et al. Simultaneous liver kidney transplantation. Transpl Int 2019;32:343–52. [DOI] [PubMed] [Google Scholar]
- 179. Wong F, Boyer TD, Sanyal AJ et al. Reduction in acute kidney injury stage predicts survival in patients with type-1 hepatorenal syndrome. Nephrol Dial Transplant 2020;35:1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Boyer TD, Sanyal AJ, Garcia-Tsao G et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol 2011;55:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Tinti F, Mitterhofer AP, Umbro I et al. Combined liver-kidney transplantation versus liver transplant alone based on KDIGO stratification of estimated glomerular filtration rate: data from the United Kingdom Transplant Registry—a retrospective cohort study. Transpl Int 2019;32:918–32. [DOI] [PubMed] [Google Scholar]
- 182. Fernandez-Lorente L, Martin-Moreno PL, Arteaga J. Acute kidney failure in the cirrhotic patient: management, kidney biopsy and dual kidney liver transplantation indication. Nephrol Dial Transplant 2020;35:408–10. [DOI] [PubMed] [Google Scholar]
- 183. Ekser B, Mangus RS, Fridell W et al. A novel approach in combined liver and kidney transplantation with long-term outcomes. Ann Surg 2017;265:1000–8. [DOI] [PubMed] [Google Scholar]
- 184. Ekser B, Goggins WC. Delayed kidney transplantation in combined liver-kidney transplantation. Curr Opin Organ Transplant 2021;26:153–9. [DOI] [PubMed] [Google Scholar]
- 185. Flamm SL, Mullen KD, Heimanson Z et al. Rifaximin has the potential to prevent complications of cirrhosis. Therap Adv Gastroenterol 2018;11:175628481880030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Dong T, Aronsohn A, Gautham Reddy K et al. Rifaximin decreases the incidence and severity of acute kidney injury and hepatorenal syndrome in cirrhosis. Dig Dis Sci 2016;61:3621–6. [DOI] [PubMed] [Google Scholar]
- 187. Lee YS, Kim HJ, Kim JH et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017;51:364–77. [DOI] [PubMed] [Google Scholar]
- 188. Sort P, Navasa M, Arroyo V et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999;341:403–9. [DOI] [PubMed] [Google Scholar]
- 189. Fernández J, Clària J, Amorós A et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology 2019;157:149–62. [DOI] [PubMed] [Google Scholar]
- 190. Turco L, Villanueva C, La Mura V et al. Lowering portal pressure improves outcomes of patients with cirrhosis, with or without ascites: a meta-analysis. Clin Gastroenterol Hepatol 2020;18:313–27.e6. [DOI] [PubMed] [Google Scholar]
- 191. Oshima G, Shinoda M, Tanabe M et al. Increased plasma levels of high mobility group box 1 in patients with acute liver failure. Eur Surg Res 2012;48:154–62. [DOI] [PubMed] [Google Scholar]