Abstract
Background
Colorectal cancer (CRC) is one of the most common cancers in China. However, detailed clinical characteristics and survival information are limited. This study aimed to investigate the potential epidemiological and clinical risk factors affecting the survival of CRC patients in southern China.
Methods
Patients with primary CRC between 1994 and 2019 at the First and the Sixth Affiliated Hospitals of Sun Yat-sen University (Guangzhou, China) were included. Clinical characteristics and survival outcomes were collected from medical records. The Kaplan–Meier method was used to estimate overall survival (OS) and progression-free survival (PFS), and Cox’s proportional-hazards regression model was used to estimate hazard ratios and 95% confidence intervals.
Results
Of all 13,328 patients, 60.1% were men; the mean age was 61.3 years; 53.5% had colon cancer. Among all patients, 1,864 (14.0%) were diagnosed with stage IV disease. The 3- and 5-year OS rates were 79.90% and 71.50%, respectively, whereas the 3- and 5-year PFS rates were 70.30% and 63.90%, respectively. The median OS and PFS times were 189 and 149 months, respectively. Among 13,328 patients, 428 (14.0%) patients with poor/undifferentiated cancer suffered recurrence. In patients with stage III and stage IV diseases, the median PFS times of the patients who received chemotherapy were significantly longer than those in patients who had not received chemotherapy (stage III: 147 vs 62 months, P < 0.001; stage IV: 14 vs 9.5 months, P < 0.001).
Conclusions
This retrospective cohort study illustrates the current status of the clinical characteristics of patients with CRC in southern China. Sex, age, family history, location of cancer occurrence, differentiation status, T status, N status, M status, clinical stage, operation, and surgical margin are independent factors associated with the OS of CRC patients.
Keywords: colorectal cancer, overall survival, clinical characteristics, progression
Introduction
Colorectal cancer (CRC) is one of the most common gastrointestinal malignancies worldwide. The latest global statistics showed that there were 19.3 million new CRC cases and 9.9 million CRC-related deaths worldwide in 2020, ranking it as the third most commonly diagnosed cancer and the second leading cause of cancer death worldwide [1]. In China, CRC is the fifth most common type of cancer, with 376,300 new CRC cases and 191,000 CRC-related deaths in 2015; these numbers have been increasing since 2000 [2, 3].
A multicenter epidemiologic survey of CRC shows that an increase in the proportion of late-stage diagnoses presents a challenge for CRC treatment in China [4, 5]. CRC-survival outcome is influenced by many different factors, including clinical characteristics, tumor stage, and treatment strategy. However, information from large CRC studies with follow-up is still limited.
This study reports the clinical characteristics and temporal trends of 13,328 patients with CRC in southern China from 1994 to 2019. Risk factors associated with metastasis, prognosis, and recurrence were analysed, and the benefits of chemotherapy in CRC patients at different clinical stages were evaluated.
Patients and methods
Study population
This retrospective study was conducted at the First and the Sixth Affiliated Hospitals of Sun Yat-sen University (Guangzhou, China), which are both large treatment centers for gastroenterology disease. The following information was retrospectively assessed by trained investigators using medical records: the baseline characteristics (sex and age at diagnosis), tumor-related information (pathological type, tumor topographical site, differentiation status, and pathological stage), and treatment-related information (surgery, radiotherapy, and chemotherapy). Patients who met the following criteria were included: (i) newly diagnosed with CRC between 1 January 1994 and 31 December 2019; (ii) diagnosis and treatment criteria were in accordance with the hospital CRC-treatment guidelines; and (iii) initial diagnosis, surgery, and treatments were performed in the above-mentioned hospitals and clinical records were available. Exclusion criteria were as follows: (i) patients with an absence of cancer-staging data or other essential information; or (ii) those who were lost to follow-up.
Treatment
The extent of tumor invasion, presence or absence of obstruction, presence or absence of regional peritoneal or mesenteric implants, and extent of regional lymph-node metastases were recorded. Resection of the tumor-bearing segment of the rectum or colon was defined as a major resection. If no evidence of metastasis was found in preoperative investigations and no macroscopic or microscopic evidence of residual tumor was found after the operation, the procedure was considered a curative resection. 5-Fluorouracil–based adjuvant chemotherapy was offered to patients according to the institutional treatment guidelines. The study was approved by the Sixth Affiliated Hospital of Sun Yat-sen University Review Board (2021ZSLYEC-100) and the First Affiliated Hospital of Sun Yat-sen University Review Board (2019–102).
Follow-up
Patients were followed until 31 May 2021. Patients were required to visit the hospital and undergo laboratory testing and imaging scans semiannually for the first 3 years. Then, the staff of the hospital database performed follow-up evaluations every 3 months via telephone call or email. The overall survival (OS) time of patients who were alive and censored was calculated from the date of diagnosis of CRC until the last date of follow-up, and the OS time of patients who died was calculated from the date of diagnosis until the patients’ death. The progression-free survival (PFS) time of patients who were alive and without tumor progression was calculated from the date of diagnosis of CRC until the last date of follow-up, and the PFS time of patients who suffered tumor progression, such as recurrence or death, was calculated from the date of diagnosis until the date of tumor progression.
Statistical analysis
All statistical analyses were performed using SPSS software (version 21.0, SPSS Inc., Chicago, IL, USA).
For continuous variables, data in accordance with normal distribution are presented as the means ± standard deviation. Categorical variables are presented as frequencies and were analysed using the χ2 test or Fisher’s exact test.
The 3- and 5-year OS rates, PFS rates, and median survival time were analysed using the Kaplan–Meier method. The log-rank test was used to determine differences between the survival curves.
Cox proportional-hazards regression models were used to calculate the hazard ratio (HR) and 95% confidence interval (CI) in the univariate and multivariate survival analyses.
A multivariate Cox proportional-hazards regression model was performed using the associated risk factors with P-values of <0.05 in univariate analysis. Two-sided P-values of <0.05 were considered statistically significant.
Results
Clinical characteristics of the investigated CRC patients
In total, 13,328 patients with primary CRC were included in the current analysis. The cohort was separated into two temporal cohorts: Cohort 1 (1994–2013) and Cohort 2 (2014–2019). Details are provided in Table 1 and Supplementary Figure 1.
Table 1.
Clinical characteristics of the 13,328 investigated colorectal-cancer patients
| No. of patients (%) |
||||
|---|---|---|---|---|
| Characteristic | Total (n = 13,328) | Cohort 1/1994–2013 (n = 3,945) | Cohort 2/2014–2019 (n = 9,383) | P-valuea |
| Sex | 0.010 | |||
| Male | 8,006 (60.1) | 2,303 (58.4) | 5,703 (60.8) | |
| Female | 5,322 (39.9) | 1,642 (41.6) | 3,680 (39.2) | |
| Ageb | <0.001 | |||
| <60 years | 5,589 (41.9) | 1,864 (47.2) | 3,725 (39.7) | |
| ≥60 years | 7,739 (58.1) | 2,081 (52.8) | 5,658 (60.3) | |
| Family history | <0.001 | |||
| No | 12,530 (94.0) | 3,629 (92.0) | 8,901 (94.9) | |
| Yes | 798 (6.0) | 316 (8.0) | 482 (5.1) | |
| Location | 0.099 | |||
| Right-sided colon | 3,087 (23.2) | 954 (24.2) | 2,133 (22.7) | |
| Left-sided colon | 4,034 (30.3) | 1,152 (29.2) | 2,882 (30.7) | |
| Rectum | 6,207 (46.6) | 1,839 (46.6) | 4,368 (46.6) | |
| Differentiation status | <0.001 | |||
| High | 1,539 (11.5) | 338 (8.6) | 1,201 (12.8) | |
| Moderate | 10,246 (76.9) | 3,002 (76.1) | 7,244 (77.2) | |
| Poor/undifferentiated | 1,543 (11.6) | 605 (15.3) | 938 (10.0) | |
| pT category | <0.001 | |||
| T1 | 1,248 (9.3) | 214 (5.4) | 1,034 (11.1) | |
| T2 | 1,821 (13.7) | 610 (15.5) | 1,211 (12.9) | |
| T3 | 6,446 (48.4) | 1,686 (42.7) | 4,760 (50.7) | |
| T4 | 3,813 (28.6) | 1,435 (36.4) | 2,378 (25.3) | |
| pN category | <0.001 | |||
| N0 | 8,036 (60.3) | 2,391 (60.6) | 5,645 (60.2) | |
| N1 | 3,434 (25.8) | 975 (24.7) | 2,459 (26.2) | |
| N2 | 1,858 (13.9) | 579 (14.7) | 1,279 (13.6) | |
| pM category | 0.057 | |||
| M0 | 11,464 (86.0) | 3,428 (86.9) | 8,036 (85.6) | |
| M1 | 1,864 (14.0) | 517 (13.1) | 1,347 (14.4) | |
| Clinical stage | <0.001 | |||
| I | 2,555 (19.1) | 664 (16.8) | 1,891 (20.1) | |
| II | 4,888 (36.7) | 1,523 (38.6) | 3,365 (35.9) | |
| III | 4,021 (30.2) | 1,241 (31.5) | 2,780 (29.6) | |
| IV | 1,864 (14.0) | 517 (13.1) | 1,347 (14.4) | |
| Pathologic subtype | <0.001 | |||
| Adenocarcinoma | 11,744 (88.1) | 3,486 (88.4) | 8,258 (88.0) | |
| MA and SRCC | 1,087 (8.2) | 438 (11.1) | 649 (6.9) | |
| Othersc | 497 (3.7) | 21 (0.5) | 476 (5.1) | |
| Operation | <0.001 | |||
| Radical | 12,554 (94.2) | 3,600 (91.3) | 8,954 (95.4) | |
| Palliative | 774 (5.8) | 345 (8.7) | 429 (4.6) | |
| Surgical margin | 0.121 | |||
| Negative | 12,805 (96.1) | 3,774 (95.7) | 9,030 (96.2) | |
| Positive | 823 (3.9) | 171 (4.3) | 353 (3.8) | |
| Chemotherapy | <0.001 | |||
| No | 7,337 (55.0) | 2,323 (58.9) | 5,014 (53.4) | |
| Yes | 5,991 (45.0) | 1,622 (41.1) | 4,369 (46.6) | |
Comparisons are made between Cohort 1 and Cohort 2.
The mean ± standard deviation age at diagnosis is 61.3 ± 13.4 years for all patients; the range is 16–101 years.
Other pathologic subtypes include gastrointestinal stromal tumors, adenosquamous carcinoma, undifferentiated carcinoma, neuroendocrine carcinoma, malignant melanoma, lymph hematopoietic neoplasms, and gastrointestinal mesenchymal tumors.
MA, mucinous adenocarcinoma; SRCC, signet-ring cell carcinoma.
Of all 13,328 patients with CRC, 8,006 (60.1%) were men (male-to-female ratio, 1.50:1) and the frequency of males increased from 58.4% in Cohort 1 to 60.8% in Cohort 2 (P = 0.010). The overall proportion of patients aged <60 years at diagnosis was 41.9% (5,589/13,328) and this proportion decreased from 47.2% (1,864/3,945) in Cohort 1 to 39.7% (3,725/9,383) in Cohort 2 (P < 0.001). The average proportion of patients with colon cancer was 53.5% (7,121), whereas the average proportion of patients with rectal cancer was 46.6% (6,207). With regard to the T category, the percentage of patients with T3 lesions was the highest, which increased from 42.7% in Cohort 1 to 50.7% in Cohort 2 (P < 0.001). Approximately half of these patients (39.7%) had lymph-node metastasis and 14.0% had distal metastasis. In terms of pathologic subtype, adenocarcinoma was the most common type of CRC (88.1%) over the entire group.
Recently study shows that the incidence of early-onset CRC (patients aged ≤40 years) has been increasing worldwide [6]. We therefore further analysed the clinical characteristics of the CRC patients categorized by age (aged ≤40 and >40 years). The proportion of CRC patients at an early age (aged ≤40 years) with a CRC-related family history was slightly lower than that of CRC patients aged >40 years (P = 0.010). For patients with poor/undifferentiated diseases, the proportion of patients aged ≤40 years was 17.7% (176), which was higher than that of patients aged >40 years (11.1%, 1,367). In addition, the proportion of patients aged ≤40 years with mucinous adenocarcinoma and signet-ring cell carcinoma was 13.0%, which was higher than that of patients aged >40 years (P < 0.001). Further details are shown in Supplementary Table 1 and Supplementary Figure 2.
Analysis of factors associated with distal metastasis
We further analysed the potential factors associated with metastasis and found that 62.5% of patients with metastasis were men, whereas only 59.7% of patients without metastasis were men (P = 0.018). Among the patients with metastasis, 54.9% were older (≥60 years); the proportion of older patients was higher than that of patients without metastasis (58.6%, P = 0.003). Compared to those diagnosed with colon cancer, patients diagnosed with rectal cancer had a lower risk of metastasis. As the T or N category increased in CRC patients, the risk of metastasis also increased (P < 0.001). With regard to pathological subtypes, CRC patients with mucinous adenocarcinoma and signet-ring cell carcinoma were much easier to diagnose together with metastasis (P < 0.001). Further details are provided in Supplementary Table 2.
OS analysis of the CRC patients
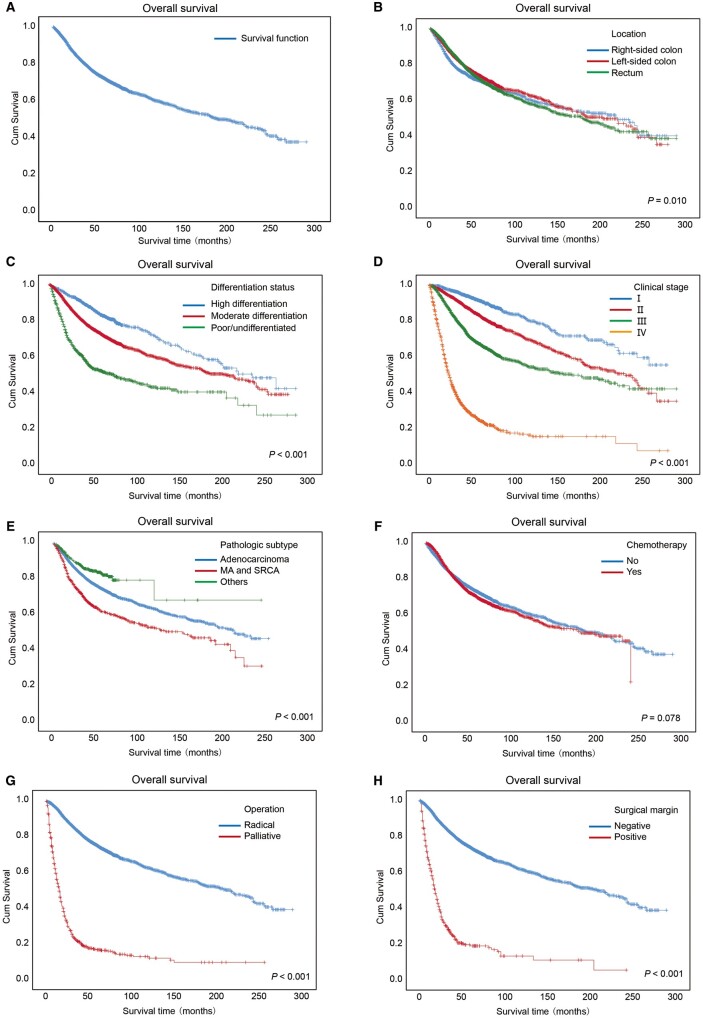
The 3- and 5-year OS rates of all patients were 79.90% (95% CI, 79.12%–80.68%) and 71.50% (95% CI, 70.52%–72.48%), respectively (Figure 1A). The median survival time of females was significantly longer than that of males (238.00 vs 167.00 months, P < 0.001).
Figure 1.
Kaplan–Meier overall survival curves of the CRC patients. Kaplan–Meier overall survival curves of all of the CRC patients (A) and the patients with several clinical characteristics (B–F), including (B) locations at which cancer occurred, (C) differentiation status, (D) clinical stages, (E) pathological subtypes, (F) chemotherapy, (G) operation, and (H) surgical margin. Log-rank analysis was used to test for significance. MA, mucinous adenocarcinoma; SRCC, signet-ring cell carcinoma.
Figure 1B shows OS curves for CRC patients stratified by tumor location. The median survival time of patients with rectal cancer (179 months) was shorter than that of patients with colon cancer (right-sided colon: 220 months; left-sided colon: 203 months; P = 0.010). The OS curves for the CRC patients stratified by differentiation status are shown in Figure 1C. A poorer differentiation status of patients was significantly associated with lower OS rates or shorter median survival times (P < 0.001). Moreover, a higher clinical stage accounted for a lower OS rate or shorter median survival time (P < 0.001), as shown in Figure 1D. With regard to pathologic subtype, as shown in Figure 1E and Supplementary Table 3, the 3- and 5-year OS rates of patients with mucinous adenocarcinoma and signet-ring cell carcinoma were 68.30% and 58.20%, respectively, which were lower than those of patients with adenocarcinoma or other types of diseases (P < 0.001).
The 3- and 5-year OS rates of the CRC patients receiving chemotherapy were not significantly different from those of patients not receiving chemotherapy, as shown in Figure 1F. As shown in Figure 1G, the patients receiving palliative surgery had much worse OS than those receiving radical surgery (3-year OS rate, 22.40% vs 83.4%; 5-year OS rate, 17.00% vs 74.8%; median survival time, 15 vs 217 months; P < 0.001). Moreover, as shown in Figure 1H, the median survival time of the patients with positive surgical margins was significantly shorter than that of the patients with negative surgical margins (18 vs 208 months, P < 0.001). Further details are shown in Supplementary Table 3.
PFS analysis of CRC patients
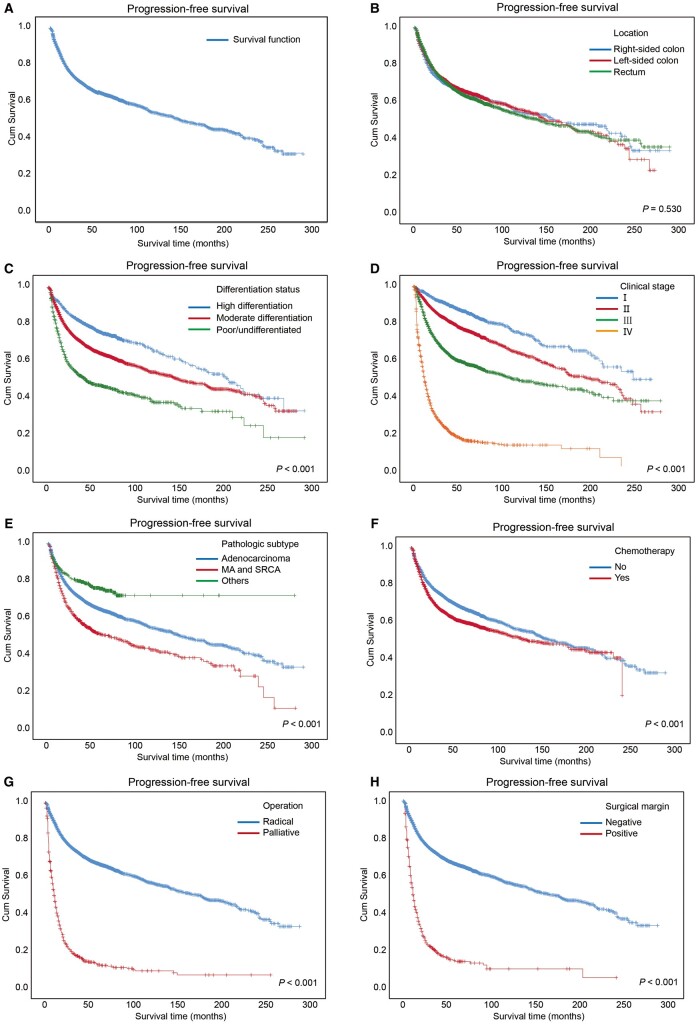
We further analysed the PFS of CRC patients and, as shown in Supplementary Table 4, the 3- and 5-year PFS rates of all patients were 70.30% (95% CI, 69.52%–71.08%) and 63.90% (95% CI, 62.92%–64.88%), respectively. The Kaplan–Meier PFS curve of all patients is shown in Figure 2A.
Figure 2.
Kaplan–Meier progression-free survival curves of the CRC patients. Kaplan–Meier progression-free survival curves of all of the CRC patients (A) and the patients with several clinical characteristics (B–F), including (B) locations at which cancer occurred, (C) differentiation status, (D) clinical stages, (E) pathological subtypes, (F) chemotherapy, (G) operation, and (H) surgical margin. Log-rank analysis was used to test for significance. MA, mucinous adenocarcinoma; SRCC, signet-ring cell carcinoma.
There were no significant differences among the PFS rates and the median PFS time in terms of the location of cancer (P = 0.530), as shown in Figure 2B. The PFS curves for the CRC patients stratified by differentiation status are shown in Figure 2C.
Moreover, an advanced clinical stage accounted for a lower PFS rate or a shorter median PFS time (P < 0.001), as shown in Figure 2D. Patients with mucinous adenocarcinoma and signet-ring cell carcinoma had worse PFS than those with adenocarcinoma or other types of diseases (Figure 2E). The median PFS time of the CRC patients receiving chemotherapy was lower than that of patients receiving no chemotherapy (P < 0.001), as shown in Figure 2F. As shown in Figure 2G, the patients receiving palliative surgery had much worse PFS than those receiving radical surgery (P < 0.001). Moreover, as shown in Figure 2H, the median PFS time of the patients with positive surgical margins was significantly shorter than that of the patients with negative surgical margins (P < 0.001). Further details are shown in Supplementary Table 4.
Univariate and multivariate analysis of the association between clinical characteristics and OS or PFS
Univariate analysis showed that the factors associated with OS were sex, age, family history, location of cancer, differentiation status, T status, N status, M status, clinical stage, pathologic subtype, operation, and surgical margin (Table 2), whereas the factors associated with PFS were sex, age, family history, differentiation status, T status, N status, M status, clinical stage, pathologic subtype, operation, surgical margin, and chemotherapy (Table 3).
Table 2.
Univariate and multivariate analysis of the association between clinical characteristics and overall survival of colorectal-cancer patients
| Characteristic | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Sex | <0.001 | 0.014 | ||
| Male | 1.00 | 1.00 | ||
| Female | 0.86 (0.80–0.92) | 0.92 (0.86–0.98) | ||
| Age | <0.001 | <0.001 | ||
| <60 years | 1.00 | 1.00 | ||
| ≥60 years | 1.46 (1.36–1.57) | 1.87 (1.74–2.01) | ||
| Family history | 0.003 | <0.001 | ||
| No | 1.00 | 1.00 | ||
| Yes | 0.79 (0.68–0.92) | 0.73 (0.63–0.85) | ||
| Location | 0.010 | <0.001 | ||
| Right-sided colon | 1.00 | 1.00 | ||
| Left-sided colon | 0.87 (0.79–0.95) | 0.93 (0.84–1.02) | ||
| Rectum | 0.92 (0.84–0.99) | 1.24 (1.14–1.35) | ||
| Differentiation status | <0.001 | <0.001 | ||
| High | 1.00 | 1.00 | ||
| Moderate | 1.67 (1.47–1.88) | 1.12 (0.99–1.27) | ||
| Poor/undifferentiated | 3.51 (3.06–4.04) | 1.65 (1.42–1.92) | ||
| pT category | <0.001 | <0.001 | ||
| T1 | 1.00 | 1.00 | ||
| T2 | 1.90 (1.50–2.40) | 1.52 (1.19–1.95) | ||
| T3 | 3.50 (2.85–4.31) | 2.19 (1.64–2.92) | ||
| T4 | 7.11 (5.77–8.75) | 3.25 (2.43–4.35) | ||
| pN category | <0.001 | <0.001 | ||
| N0 | 1.00 | 1.00 | ||
| N1 | 2.06 (1.90–2.23) | 1.19 (1.04–1.36) | ||
| N2 | 4.14 (3.82–4.50) | 1.89 (1.66–2.16) | ||
| pM category | <0.001 | <0.001 | ||
| M0 | 1.00 | 1.00 | ||
| M1 | 6.57 (6.11–7.06) | 4.43 (3.45–5.70) | ||
| Clinical stage | <0.001 | <0.001 | ||
| I | 1.00 | 1.00 | ||
| II | 1.87 (1.63–2.15) | 1.00 (0.78–1.27) | ||
| III | 3.55 (3.10–4.07) | 1.45 (1.12–1.87) | ||
| IV | 14.47 (12.61–16.60) | 4.43 (3.45–5.70) | ||
| Pathologic subtype | <0.001 | 0.611 | ||
| Adenocarcinoma | 1.00 | 1.00 | ||
| MA and SRCC | 1.64 (1.48–1.82) | 1.06 (0.94–1.18) | ||
| Othersa | 0.66 (0.53–0.81) | 1.04 (0.84–1.30) | ||
| Operation | <0.001 | <0.001 | ||
| Radical | 1.00 | 1.00 | ||
| Palliative | 7.65 (6.99–8.37) | 2.38 (2.11–2.68) | ||
| Surgical margin | <0.001 | <0.001 | ||
| Negative | 1.00 | 1.00 | ||
| Positive | 6.45 (5.78–7.19) | 1.29 (1.13–1.48) | ||
| Chemotherapy | 0.078 | |||
| No | 1.00 | |||
| Yes | 1.06 (0.99–1.14) | |||
Other pathologic subtypes include gastrointestinal stromal tumors, adenosquamous carcinoma, undifferentiated carcinoma, neuroendocrine carcinoma, malignant melanoma, lymph hematopoietic neoplasms, and gastrointestinal mesenchymal tumors.
MA, mucinous adenocarcinoma; SRCC, signet-ring cell carcinoma.
Table 3.
Univariate and multivariate analysis of the association between clinical characteristics and progression-free survival of colorectal-cancer patients
| Characteristic | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Sex | <0.001 | 0.001 | ||
| Male | 1.00 | 1.00 | ||
| Female | 0.86 (0.81–0.92) | 0.91 (0.85–0.96) | ||
| Age | <0.001 | <0.001 | ||
| <60 years | 1.00 | 1.00 | ||
| ≥60 years | 1.23 (1.15–1.30) | 1.42 (1.33–1.51) | ||
| Family history | 0.008 | 0.015 | ||
| No | 1.00 | 1.00 | ||
| Yes | 0.84 (0.73–0.96) | 0.85 (0.74–0.97) | ||
| Location | 0.530 | |||
| Right-sided colon | 1.00 | |||
| Left-sided colon | 0.97 (0.89–1.05) | |||
| Rectum | 1.01 (0.93–1.08) | |||
| Differentiation status | <0.001 | <0.001 | ||
| High | 1.00 | 1.00 | ||
| Moderate | 1.53 (1.38–1.70) | 1.10 (0.98–1.22) | ||
| Poor/undifferentiated | 2.72 (2.41–3.07) | 1.37 (1.20–1.57) | ||
| pT category | <0.001 | <0.001 | ||
| T1 | 1.00 | 1.00 | ||
| T2 | 1.79 (1.48–2.17) | 1.56 (1.28–1.91) | ||
| T3 | 3.20 (2.71–3.78) | 2.10 (1.65–2.66) | ||
| T4 | 5.59 (4.72–6.61) | 2.78 (2.18–3.53) | ||
| pN category | <0.001 | <0.001 | ||
| N0 | 1.00 | 1.00 | ||
| N1 | 2.10 (1.96–2.25) | 1.24 (1.10–1.41) | ||
| N2 | 3.92 (3.64–4.22) | 1.87 (1.65–2.11) | ||
| pM category | <0.001 | <0.001 | ||
| M0 | 1.00 | 1.00 | ||
| M1 | 6.08 (5.70–6.48) | 4.81 (3.89–5.95) | ||
| Clinical stage | <0.001 | <0.001 | ||
| I | 1.00 | 1.00 | ||
| II | 1.74 (1.55–1.96) | 1.05 (0.86–1.29) | ||
| III | 3.35 (2.99–3.75) | 1.65 (1.33–2.05) | ||
| IV | 12.60 (11.22–14.15) | 4.81 (3.89–5.95) | ||
| Pathologic subtype | <0.001 | 0.676 | ||
| Adenocarcinoma | 1.00 | 1.00 | ||
| MA and SRCC | 1.49 (1.35–1.63) | 1.05 (0.95–1.16) | ||
| Othersa | 0.66 (0.55–0.79) | 1.01 (0.84–1.23) | ||
| Operation | <0.001 | <0.001 | ||
| Radical | 1.00 | 1.00 | ||
| Palliative | 5.51 (5.06–6.00) | 1.71 (1.52–1.91) | ||
| Surgical margin | <0.001 | 0.030 | ||
| Negative | 1.00 | 1.00 | ||
| Positive | 4.82 (4.34–5.34) | 1.15 (1.01–1.31) | ||
| Chemotherapy | <0.001 | <0.001 | ||
| No | 1.00 | 1.00 | ||
| Yes | 1.27 (1.20–1.35) | 0.74 (0.69–0.97) | ||
Other pathologic subtypes include gastrointestinal stromal tumors, adenosquamous carcinoma, undifferentiated carcinoma, neuroendocrine carcinoma, malignant melanoma, lymph hematopoietic neoplasms, and gastrointestinal mesenchymal tumors.
MA, mucinous adenocarcinoma; SRCC, signet-ring cell carcinoma.
Multivariate analysis revealed that the independent factors associated with OS were sex, age, family history, location of cancer occurrence, differentiation status, T status, N status, M status, clinical stage, operation, and surgical margin (Table 2), whereas the independent factors associated with PFS were sex, age, family history, differentiation status, T status, N status, M status, clinical stage, operation, surgical margin, and chemotherapy (Table 3). The forest plots of risk factors related to OS and PFS are shown in Supplementary Figures 3 and 4.
Analysis of factors associated with recurrence
The proportion of CRC patients who exhibited recurrence was 22.8% (3,039/13,328). As shown in Table 4, the risk factors associated with recurrence included sex, age, family history, location, differentiation status, T status, N status, M status, clinical stage, pathologic subtype, operation, surgical margin, and chemotherapy. Among the patients with recurrence, 50.2% (1,525/3,039) were diagnosed with rectal cancer, whereas in the patients without recurrence, 45.5% (4,682/10,289) were diagnosed with rectal cancer (P < 0.001). As expected, the poorer the differentiation status, the higher the risk of recurrence (P < 0.001). In comparison to the patients with adenocarcinoma or other pathological subtypes, the CRC patients with mucinous adenocarcinoma and signet-ring cell carcinoma had a higher risk of recurrence (P < 0.001).
Table 4.
Risk factors associated with recurrence
| Characteristic | Recurrence (n = 3,039) | No recurrence (n = 10,289) | P-value |
|---|---|---|---|
| Sex | 0.003 | ||
| Male | 1,896 (62.4) | 6,110 (59.4) | |
| Female | 1,143 (37.6) | 4,179 (40.6) | |
| Age | 0.757 | ||
| <60 years | 1,267 (41.7) | 4,322 (42.0) | |
| ≥60 years | 1,772 (58.3) | 5,967 (58.0) | |
| Family history | 0.068 | ||
| No | 2,878 (94.7) | 9,652 (93.8) | |
| Yes | 161 (5.3) | 637 (6.2) | |
| Location | <0.001 | ||
| Right-sided colon | 631 (20.7) | 2,456 (23.9) | |
| Left-sided colon | 883 (29.1) | 3,151 (30.6) | |
| Rectum | 1,525 (50.2) | 4,682 (45.5) | |
| Differentiation status | <0.001 | ||
| High | 267 (8.8) | 1,272 (12.4) | |
| Moderate | 2,344 (77.1) | 7,903 (76.8) | |
| Poor/undifferentiated | 428 (14.1) | 1,115 (10.8) | |
| pT category | <0.001 | ||
| T1 | 101 (3.3) | 1,147 (11.1) | |
| T2 | 231 (7.6) | 1,590 (15.5) | |
| T3 | 1,575 (51.8) | 4,871 (47.3) | |
| T4 | 1,132 (37.2) | 2,681 (26.1) | |
| pN category | <0.001 | ||
| N0 | 1,220 (40.1) | 6,816 (66.2) | |
| N1 | 1,030 (33.9) | 2,404 (23.4) | |
| N2 | 789 (26.0) | 1,069 (10.4) | |
| pM category | <0.001 | ||
| M0 | 2,112 (69.5) | 9,352 (90.9) | |
| M1 | 927 (30.5) | 937 (9.1) | |
| Clinical stage | <0.001 | ||
| I | 219 (7.2) | 2,336 (22.7) | |
| II | 739 (24.3) | 4,149 (40.3) | |
| III | 1,154 (38.0) | 2,867 (27.9) | |
| IV | 927 (30.5) | 937 (9.1) | |
| Pathological subtypes | <0.001 | ||
| Adenocarcinoma | 2,652 (87.3) | 9,092 (88.4) | |
| MA and SRCCa | 293 (9.6) | 794 (7.7) | |
| Othersb | 94 (3.1) | 403 (3.9) | |
| Surgical margin | <0.001 | ||
| Negative | 2,885 (94.9) | 9,919 (96.4) | |
| Positive | 154 (5.1) | 370 (3.6) | |
| Chemotherapy | <0.001 | ||
| No | 1,295 (42.6) | 6,042 (58.7) | |
| Yes | 1,744 (57.4) | 4,247 (41.3) |
Mucinous adenocarcinoma and signet-ring cell carcinoma.
Other pathologic subtypes included gastrointestinal stromal tumors, adenosquamous carcinoma, undifferentiated carcinoma, neuroendocrine carcinoma, malignant melanoma, lymph hematopoietic neoplasms, and gastrointestinal mesenchymal tumors.
Effects of chemotherapy on OS and PFS categorized by clinical stage
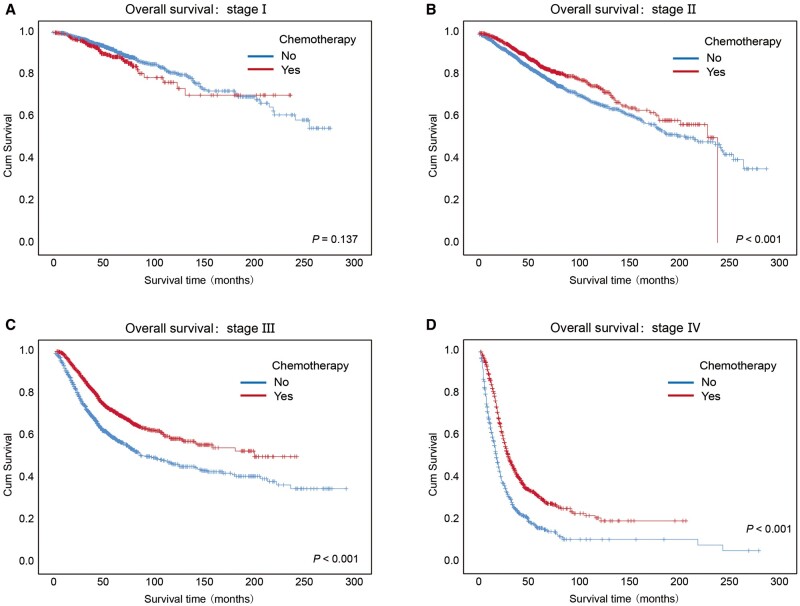
As shown in Figures 1F and 2F, when all CRC subjects were considered, regardless of clinical stage, we did not observe differences in OS or PFS between patients treated with chemotherapy and those who did not receive chemotherapy. Therefore, we further analysed the effects of chemotherapy on OS and PFS categorized by clinical stage.
Although chemotherapy did not significantly impact the OS curves of the patients with stage I CRC (P = 0.137), the patients with stage II, stage III, and stage IV cancers who received chemotherapy had higher 3- and 5-year OS rates than those who did not receive chemotherapy (Figure 3A–D and Supplementary Table 5, P < 0.001).
Figure 3.
Kaplan–Meier overall survival curves of CRC patients who received chemotherapy or no chemotherapy at different clinical stages. Kaplan–Meier overall survival curves of the CRC patients received chemotherapy or no chemotherapy at different clinical stages, including (A) stage I, (B) stage II, (C) stage III, and (D) stage IV. Log-rank analysis was used to test for significance.
For the patients with advanced diseases (stage III and stage IV), the patients receiving chemotherapy had better PFS than those not receiving chemotherapy (Supplementary Figure 5A–D and Supplementary Table 6, P < 0.001). Regardless of clinical stage, patients with colon cancer who received chemotherapy had similar OS or PFS to those who did not receive chemotherapy, and the same for those with rectal cancer (Supplementary Figure 6). However, when focusing on stage III colon cancer and stage III rectal cancer, we observed a better OS or PFS in patients receiving chemotherapy (Supplementary Figure 7).
Discussion
Due to the large sample size of patients with CRC, our study could be regarded as a baseline for prescreening or limited the CRC screening of the population in southern China. This study indicated that some characteristics, including tumor location, poorer differentiated status, and advanced stage, served as risk factors associated with distal metastasis and recurrence. Furthermore, the study showed that adjuvant chemotherapy was a protective factor, especially in patients with advanced CRC. These data will be informative for further evaluations of the effectiveness and surveillance of CRC-related, metastasis-related, and recurrence-related screening, diagnosis, and treatment for populations in China.
The male-to-female ratio among patients with CRC in the current study was 1.50, which is comparable with that observed among all patients with CRC in 2020 in China (1.41; GLOBOCAN 2020) [1, 3] and slightly higher than that observed in 2015 in China (1.34; Cancer Statistics in China) [2, 5]. In our study, the average age of CRC patients at diagnosis was 61.3 years, which is 2 years older than the average age reported in a previous multicenter retrospective epidemiologic survey in China from 2005 to 2014 (average age, 59.3 years) [5]. In addition, we observed an increase in the proportion of patients with CRC at ≥60 years of age. To some extent, data from this analysis on age trends over time could not directly support an earlier initiation of CRC screening in China and this viewpoint has been raised by reports from other populations [5, 7, 8]. The latest survey in China from 2005 to 2014 showed that 40.2% of the patients were diagnosed with colon cancer [5] and findings from the present study showed that the average proportion of patients with colon cancer was 53.5%. The proportion of patients with advanced cancer in our study was 44.2% (stage III, 30.2%; stage IV, 14.0%), which was approximately the same as that observed in another study (stage III, 33.4%; stage IV, 14.4%) [5]. In addition, we observed that a large percentage of patients were diagnosed with adenocarcinoma (88.1%) and the proportion of patients with mucinous adenocarcinoma and signet-ring cell carcinoma was 8.2%, which was similar to those observed in China in 2005–2014 (adenocarcinoma, 91.2%; mucinous adenocarcinoma and signet-ring cell carcinoma, 7.0%) [5].
Since the T category is closely related to the duration and extent of CRC development, we observed a decrease in the proportion of patients with T4 lesions over time, which indicated that early screening deserves more effort. However, the proportion of patients with distal metastasis increased over time, possibly due to the promotion of diagnosis techniques, such as positron emission tomography–computed tomography [9]. Further advancement is necessary to improve the early-diagnosis methods for distal metastasis.
Previous studies on CRC in China have mainly focused on clinical characteristics, medical-service utilization, and expenditure [5]. In our study, some characteristics of CRC patients were found to be associated with OS and PFS, such as sex, age, family history, differentiation status, and clinical stage. Zeng et al. [10] observed an improvement in the 5-year survival of CRC patients in China, from 47.2% during 2003–2005 to 56.9% during 2012–2015. The 5-year OS of CRC patients has reached almost 65% in some high-income countries, such as Australia, Canada, the USA, and several European countries [11, 12]. In the current study, the 5-year OS rate of all patients was even higher (71.50%). The 3- and 5-year PFS rates of all patients were 70.30% and 63.90%, respectively.
Bass et al. [13] reported that a history of CRC in a first-degree relative was associated with a significant decrease in survival. However, in our study, the patients with a CRC-related family history had better OS and PFS. One explanation would be that individuals benefit from the application of early screening for patients with a CRC-related family history, which results in earlier diagnosis and treatment [14–16]. In addition, we believe that this observation is partly due to inherited cancers, especially microsatellite instability (MSI) cancers that tend to run in families, which present better prognosis. In a nationwide multicenter retrospective study in Japan, the 3- and 5-year PFS rates of patients with stage III CRC were 70.2% and 66.0%, respectively [17]. The PFS rates of patients with stage III CRC in our study were relatively lower (3-year PFS, 64.40%; 5-year PFS, 57.4%), which should be regarded as motivation for the progression of medical treatment.
For patients with advanced cancer, the 3- and 5-year OS rates or PFS rates of patients who received chemotherapy were actually much higher than those of patients who did not receive chemotherapy, which indicated that patients who received chemotherapy had advanced cancer and that these patients did benefit from chemotherapy. It has been reported that the total mesorectal excision (TME) alone significantly reduces the local recurrence rate [18] and chemotherapy also serves as an important method to prevent local recurrence and peritoneal metastases in patients with CRC [19]. Watch and wait is a novel management strategy in patients with rectal cancer who have a clinically complete response after neoadjuvant chemoradiotherapy [20, 21]. More studies are needed to discover better treatment strategies for CRC [22].
One limitation of this study is that the lack of lymph-node counts might result in inaccurate stage II and stage III information. In future studies, we will record the number of lymph nodes with cancer metastasis. Since a majority of patients received chemotherapy at a local hospital, detailed information about chemotherapy remains unclear. Besides, this study covers data in 25 years, among which the treatment strategy of CRC changed a lot. Hence, the lack of information on the treatment guidelines used over the years affects the accuracy of some outcomes, to a certain extent.
In conclusion, this retrospective cohort study illustrates the current status and time trends (1994–2019) of the clinical characteristics of patients with CRC in southern China. Independent factors associated with CRC-related OS and PFS were clearly identified in the current study. The analysis of the indicated risk factors provides some frameworks for preventing CRC distal metastasis and relapse. We expect that the findings could act as guidance for enhancing the awareness of CRC prevention and for improving the clinical diagnosis and treatment of CRC patients and could assist authorities in seeking to develop better surveillance strategies to increase the survival and quality of life of CRC patients.
Supplementary Data
Supplementary data is available at Gastroenterology Report online.
Authors’ Contributions
L.F. and C.C. conceived of and designed the project. L.F., Z.Y., and M.Z. collected the data. L.F., Z.Y., M.Z., M.M., J. F., and C.C. analysed and interpreted the data. L.F., Z.Y., and C.C. drafted the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China [81773098, 82111530099], Guangdong Special Young Talent Plan of Scientific and Technological Innovation [2019TQ05Y510], the Guangdong International Joint Research Program [2020A0505100027], Nature Science Foundation of Guangzhou [202102080387], Guangdong Public Health Project [2021-58] and National Key Clinical Discipline.
Supplementary Material
Acknowledgements
The authors would like to thank the Department of Clinical Database of the First and the Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) for clinical data collection.
Conflict of Interest
None declared.
References
- 1. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- 3. Xie Y, Shi L, He X et al. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep (Oxf) 2021;9:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou M, Wang H, Zeng X et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi JF, Wang L, Ran JC et al. Clinical characteristics, medical service utilization, and expenditure for colorectal cancer in China, 2005 to 2014: overall design and results from a multicenter retrospective epidemiologic survey. Cancer 2021;127:1880–93. [DOI] [PubMed] [Google Scholar]
- 6. Akimoto N, Ugai T, Zhong R et al. Rising incidence of early-onset colorectal cancer—a call to action. Nat Rev Clin Oncol 2021;18:230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altieri MS, Thompson H, Pryor A et al. Incidence of colon resections is increasing in the younger populations: should an early initiation of colon cancer screening be implemented? Surg Endosc 2021;35:3636–41. [DOI] [PubMed] [Google Scholar]
- 8. Feletto E, Yu XQ, Lew JB et al. Trends in colon and rectal cancer incidence in Australia from 1982 to 2014: analysis of data on over 375,000 cases. Cancer Epidemiol Biomarkers Prev 2019;28:83–90. [DOI] [PubMed] [Google Scholar]
- 9. Tsilimigras DI, Brodt P, Clavien PA et al. Liver metastases. Nat Rev Dis Primers 2021;7:27. [DOI] [PubMed] [Google Scholar]
- 10. Zeng H, Chen W, Zheng R et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555–67. [DOI] [PubMed] [Google Scholar]
- 11. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet (London, England) 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- 12. Bosetti C, Levi F, Rosato V et al. Recent trends in colorectal cancer mortality in Europe. Int J Cancer 2011;129:180–91. [DOI] [PubMed] [Google Scholar]
- 13. Bass AJ, Meyerhardt JA, Chan JA et al. Family history and survival after colorectal cancer diagnosis. Cancer 2008;112:1222–9. [DOI] [PubMed] [Google Scholar]
- 14. Beebe-Dimmer JL, Kapron AL, Fraser AM et al. Risk of prostate cancer associated with familial and hereditary cancer syndromes. JCO 2020;38:1807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song M, Emilsson L, Roelstraete B et al. Risk of colorectal cancer in first degree relatives of patients with colorectal polyps: nationwide case-control study in Sweden. BMJ 2021;373:n877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor DP, Burt RW, Williams MS et al. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology 2010;138:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shida D, Inoue M, Tanabe T et al. Prognostic impact of primary tumor location in Stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: a nationwide multicenter retrospective study. J Gastroenterol 2020;55:958–68. [DOI] [PubMed] [Google Scholar]
- 18. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet (London, England) 1986;327:1479–82. [DOI] [PubMed] [Google Scholar]
- 19. Sugarbaker PH. Update on the prevention of local recurrence and peritoneal metastases in patients with colorectal cancer. World J Gastroenterol 2014;20:9286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernandez LM, Sao Juliao GP, Figueiredo NL et al. ; International Watch & Wait Database Consortium. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: a retrospective, international, multicentre registry study. Lancet Oncol 2021;22:43–50. [DOI] [PubMed] [Google Scholar]
- 21. Bahadoer RR, Dijkstra EA, van Etten B et al. ; RAPIDO orative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29–42. [DOI] [PubMed] [Google Scholar]
- 22. Fotheringham S, Mozolowski GA, Murray EMA et al. Challenges and solutions in patient treatment strategies for stage II colon cancer. Gastroenterol Rep (Oxf) 2019;7:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.