Abstract
Temperate woody plants in the Northern Hemisphere have long been known to exhibit high species richness in East Asia and North America and significantly lower diversity in Europe, but the causes of this pattern remain debated. Here, we quantify the roles of dispersal, niche evolution, and extinction in shaping the geographic diversity of the temperate woody plant family Juglandaceae (walnuts and their relatives). Integrating evidence from molecular, morphological, fossil, and (paleo)environmental data, we find strong support for a Boreotropical origin of the family with contrasting evolutionary trajectories between the temperate subfamily Juglandoideae and the tropical subfamily Engelhardioideae. Juglandoideae rapidly evolved frost tolerance when the global climate shifted to ice-house conditions from the Oligocene, with diversification at high latitudes especially in Europe and Asia during the Miocene. Subsequent range contraction at high latitudes and high levels of extinction in Europe driven by global cooling led to the current regional disparity in species diversity. Engelhardioideae showed temperature conservatism while adapting to increased humidity, tracking tropical climates to low latitudes since the middle Eocene with comparatively little diversification, perhaps due to high competition in the tropical zone. The biogeographic history of Juglandaceae shows that the North Atlantic land bridge and Europe played more critical roles than previously thought in linking the floras of East Asia and North America, and showcases the complex interplay among climate change, niche evolution, dispersal, and extinction that shaped the modern disjunct pattern of species richness in temperate woody plants. [Boreotropical origin; climatic niche evolution; disjunct distribution; dispersal; diversity anomaly; extinction; Juglandaceae.]
Current tree diversity in temperate areas of the Northern Hemisphere is characterized by disjunct hotspots in North America and East Asia. This diversity pattern is found consistently across more than 60 genera of temperate trees in the Northern Hemisphere ( Latham and Ricklefs 1993). Its origin and causes have remained a subject of debate for more than a century (Gray 1878; Latham and Ricklefs 1993; Wen 1999; Guo and Ricklefs 2000; Qian and Ricklefs 2000; Manos and Donoghue 2001; Qian and Ricklefs 2001; Wen 2001; Xiang and Soltis 2001; Qian and Ricklefs 2004; Wen et al. 2010). A prevailing view is that small-scale ecological interactions, area-effects, and contemporary habitat heterogeneity in the present are less important than historical evolutionary processes including speciation, extinction and colonization (Latham and Ricklefs 1993; Wiens and Donoghue 2004).
Three main biogeographical hypotheses have been proposed to explain the disjunct distribution of temperate tree biodiversity hotspots. The Arcto-Tertiary hypothesis posits that mixed mesophytic forests of East Asia and North America are relicts of a once continuous high-latitude deciduous forest that existed in the Northern Hemisphere during the Late Cretaceous and Paleogene. This forest then migrated south to the temperate zone as an entire unit due to global climatic cooling during the Neogene and Quaternary (Engler 1882; Chaney 1947, 1959). The Arcto-Tertiary hypothesis implies high-latitude origins of North Temperate forest taxa, with East Asia–North America disjunctions the result of ancestral extinction at high latitudes and in Europe. The Boreotropical hypothesis proposes that a widespread Boreotropical flora developed at midlatitudes of the Northern Hemisphere during the early Paleogene and spread around the globe via the Beringia and North Atlantic land bridges and shores of the Tethys Seaway (Wolfe 1975; Tiffney 1985a,b; Manchester 1999; Wen 1999; Tiffney 2000; Tiffney and Manchester 2001). The mixed mesophytic forests of East Asia and North America developed independently after the disruption of this Boreotropical flora due to extensive extinctions in Europe during the second half of the Cenozoic. In contrast to the Arcto-Tertiary hypothesis, the Boreotropical hypothesis suggests that North Temperate forest taxa originated at middle latitudes, with disjunctions resulting from dispersal throughout the Northern Hemisphere, and extinction at high latitudes and in Europe. Finally, the Out of Asia hypothesis postulates that the majority of North Temperate plant clades originated in Asia, and subsequently dispersed to North America either directly through the Beringia land bridge, or indirectly via Europe and the North Atlantic land bridge (Wen 1999; Donoghue et al. 2001; Xiang and Soltis 2001). The Arcto-Tertiary and Boreotropical hypotheses thus emphasize the latitude of origin during the Paleogene, and the disjunct distributions caused by the contraction of latitude since the global cooling, whereas the Out of Asia hypothesis emphasizes the continent of origin and source of dispersal.
So far, these hypotheses have been mainly tested with either paleobotanical data (Chaney 1947, 1959; Wolfe 1975; Axelrod 1983) or phylogenetic analyses of living taxa (Stanford et al. 2000; Manos and Stanford 2001; Deng et al. 2015; Wen et al. 2016; Yang et al. 2018). Many lineages (e.g., Fagus, Quercus, Castanea, Aesculus, Ulmus, Acer, Alnus, Juglans) have fossil records at middle latitudes that predate their high latitude records, suggesting Boreotropical origins over Arcto-Tertiary origins (Crepet and Daghlian 1980; Manchester 1987, 1994, 2001; Manchester and Dillhoff 2004; Denk and Dillhoff 2005; Wang et al. 2010; Hofmann et al. 2011; Manchester 2011). A few biogeographic studies also suggested Boreotropical origins for genera such as Vitis (Liu et al. 2016) and Toxicodendron (Jiang et al. 2019). Several temperate taxa thought to have originated in East Asia and dispersed to North America, for example, Symplocarpus (Nie et al. 2006), Berberidaceae (Wang et al. 2007), Astilbe (Zhu et al. 2013), and Rhodiola (Zhang et al. 2014). However, rigorously inferring their geographic origins and whether their dispersals between continents mostly occurred across high latitude land bridges remains challenging due to the lack of integration of fossil information. Given the conspicuous modern diversity of temperate tree clades and the likely substantial extent of extinction over tens of millions of years of evolution, a formal assessment of these hypotheses calls for an integration of paleontological and neontological evidence.
Of particular interest is the role of climate change in driving these diversity patterns (Svenning et al. 2015; Meseguer et al. 2018). Many studies of the effects of climate change on biogeographical evolution are based on extant species and their climate niches (e.g., Kozak and Wiens 2010; Quintero and Wiens 2013; Cooney et al. 2016; Liu et al. 2020). However, deep-time effects of climatic change on biodiversity have been shown to be better understood after integrating extant data with paleoclimate and fossils, especially for groups that have experienced large numbers of extinctions (Meseguer et al. 2018; Rolland et al. 2018). Recent studies have shown that integrating fossil and phylogenetic data improves macroevolutionary reconstructions, including the estimation of divergence times (Heath et al. 2014; Zhang et al. 2016), trait evolution (Silvestro et al. 2019), historical biogeography (Mao et al. 2012; Nauheimer et al. 2012; Matzke 2013b; Wood et al. 2013; Zhang et al. 2013; Meseguer et al. 2015; Chen et al. 2017; Xiang et al. 2019), and niche evolution (Meseguer et al. 2018; Rolland et al. 2018). These analyses included fossil species into phylogenetic trees as extinct tips based on morphological data (Mao et al. 2012; Nauheimer et al. 2012; Wood et al. 2012; Zhang et al. 2013; Chen et al. 2017), or used them as calibration constraints at internal nodes in the tree or along stem branches (Slater et al. 2012; Meseguer et al. 2015, 2018; Rolland et al. 2018).
Here, we used the walnut family Juglandaceae as a case study to evaluate different hypotheses about the origin of intercontinental disparities in temperate woody plant diversity. Thanks to the exceptionally rich fossil record of this family, we infer a phylogenetic tree of extinct and extant species and use it to infer their history of dispersal, extinction, and niche evolution. Juglandaceae, a woody family in the order Fagales, contains ca. 60 extant species in three subfamilies: Rhoipteleoideae (1 sp.), Engelhardioideae (ca. 15 spp.), and Juglandoideae (ca. 45 spp.). Rhoipteleoideae and Engelhardioideae species are mainly distributed in subtropical to tropical forests, while Juglandoideae are common elements in temperate deciduous forests in the Northern Hemisphere. Most species of Juglandaceae are found in eastern North America and East Asia with only two species occurring in Europe (Supplementary Fig. S1 available on Dryad at https://doi.org/10.5061/dryad.t1g1jwt1r), thus conforming to the general disjunct pattern of temperate woody plants. Extant Juglandaceae have been extensively studied in a phylogenetic and biogeographic context (e.g., Manos and Stone 2001; Song et al. 2019; Mu et al. 2020). Additionally, several studies have clarified phylogenetic relationships, hybridization, and historical biogeography within Juglandaceae’s most diverse genera Juglans (e.g., Stanford et al. 2000; Aradhya et al. 2006, 2007; Zhang et al. 2019) and Carya (e.g., Zhang et al. 2013). For instance, Zhang et al. (2019) used genomic data to infer phylogenetic relationships and discover hybrid origins of species in Juglans that likely occurred as the result of climate-induced range shifts in Eurasia in the late Pliocene. Zhang et al. (2013) combined fossils and phylogeny to infer that Carya originated in North America, migrated to Eurasia during the early Tertiary and evolved a disjunct distribution as a result of global cooling in the late Tertiary.
Juglandaceae is exceptionally well represented in the fossil record, with well-preserved samples spanning 60 Myr, from the Paleocene to the Pleistocene, including many occurrences outside of its modern range, especially across Europe and at high latitudes (Supplementary Fig. S1 available on Dryad; Manchester 1987; Manos et al. 2007). This presents an excellent opportunity to study the origins of disjunct biodiversity patterns in the temperate woody flora of the Northern Hemisphere by integrating fossil and extant species.
In this study, we analyze the evolutionary history of Juglandaceae to assess the roles of diversification, extinction, and dispersal in establishing its current disjunct distribution. We integrate molecular, morphological, and (paleo)environmental data in a comprehensive analytical framework, including a new Bayesian model for inferring time-variable trends in climatic niche evolution. With the inclusion of extinct lineages, we find robust support for a Boreotropical origin of Juglandaceae and contrasting evolutionary trajectories in its subfamilies showing how Cenozoic climate change played a key role in modulating dispersal, local extinctions and adaptation to changing environments.
Methods
Fossil Data, (Paleo)-coordinates and (Paleo)-climate Data
We compiled a comprehensive data set of fruit macrofossil records of Juglandaceae based on the Cenozoic Angiosperm Database (Xing et al. 2016) and published literature, in which we only considered fruit fossils that allow accurate identification. The final fossil data set included 403 occurrences of 111 recognized species in 19 genera including 13 extinct/morpho-genera, and 9 records identified only to the genus level (Supplementary Table S1 available on Dryad).
To infer the evolution of geographic ranges and climatic niches, we converted modern coordinates of fossil localities to paleo-coordinates using the Getech Group plc plate model (Lunt et al. 2016) according to their geological ages. We then collected the paleoclimate data of fossil sites from published literature. The climate data included six quantitative variables: i) mean annual temperature (MAT,  C), ii) mean temperature in the warmest month (Twarm,
C), ii) mean temperature in the warmest month (Twarm,  C), iii) mean temperature in the coldest month (Tcold,
C), iii) mean temperature in the coldest month (Tcold,  C), iv) mean annual precipitation (MAP, mm), v) mean precipitation in the wettest month (Pwet, mm), and vi) mean precipitation in the driest month (Pdry, mm). In some cases, we used paleoclimate data of close fossil sites of the same age. Data for 11 fossil floras were not available from previous studies and we used the Coexistence Approach (CA) to estimate their climate variables (Supplementary Table S2 available on Dryad; http://www.palaeoflora.de/; Mosbrugger and Utescher 1997; Utescher et al. 2014). All paleoclimate data for each fossil occurrence are listed in Supplementary Table S3 available on Dryad.
C), iv) mean annual precipitation (MAP, mm), v) mean precipitation in the wettest month (Pwet, mm), and vi) mean precipitation in the driest month (Pdry, mm). In some cases, we used paleoclimate data of close fossil sites of the same age. Data for 11 fossil floras were not available from previous studies and we used the Coexistence Approach (CA) to estimate their climate variables (Supplementary Table S2 available on Dryad; http://www.palaeoflora.de/; Mosbrugger and Utescher 1997; Utescher et al. 2014). All paleoclimate data for each fossil occurrence are listed in Supplementary Table S3 available on Dryad.
We extracted the climate data for each extant species from the WorldClim database (http://www.worldclim.org) at a spatial resolution of 10 minutes using the R package “raster” (Hijmans et al. 2015) based on the coordinates of all natural occurrences from the Global Biodiversity Information Facility (GBIF, https://www.gbif.org/) by manually removing outlier distribution points (e.g., occurrences in the sea), introduced occurrences, areas out of the native distribution, etc. The final filtered data set included 11,716 occurrences for Juglandaceae representing all 9 extant genera and 47 species out of ca. 60 described species in the family (the occurrence number for each species was listed in Supplementary Table S4 available on Dryad). For each species, we recorded the mean values of geographic coordinates and each climate variable across all specimen records.
Phylogenetic Relationships and Divergence Times of Juglandaceae
We downloaded sequences for four plastid genes (matK, atpB–rbcL, psbA–trnH, and trnL–trnF) and one nuclear ribosomal DNA (ITS) from GenBank of 46 species of Juglandaceae representing 77% of all currently recognized diversity (Supplementary Table S5 available on Dryad). We then assembled a character matrix of 73 floral, vegetative, and fruit characters by incorporating the matrix from Manos et al. (2007) and 10 additional characters for 160 species (47 extant species and 113 extinct species), 127 newly added taxa which were absent from the data set of Manos et al. (2007) (Supplementary Table S6 available on Dryad).
We aligned all sequences using MAFFT v.7.308 (Katoh and Standley 2013) implemented in Geneious v.11.1.2 (Kearse et al. 2012) and manually edited if necessary. We performed phylogenetic analyses based on partitioned plastid (2,697 bp) and ITS (698 bp) and nonpartitioned (total 2,779 bp) data sets to compare the resulting topologies. We inferred phylogenetic trees of extant species using maximum likelihood (ML) for the partitioned and nonpartitioned molecular data sets in raxmlGUI 2.0 (Stamatakis 2014; Edler et al. 2020) to assess the effect of partitioning the sequence data on tree topology and support, but we did not run additional analyses on those ML trees. We then used total-evidence dating based on the nonpartitioned molecular data set and morphological matrix to estimate a calibrated phylogenetic tree of extant and extinct species under the fossilized birth–death process (Heath et al. 2014) in BEAST v.2.6.1 (Bouckaert et al. 2014) available on the CIPRES Science Gateway 3.3 (Miller et al. 2010). We used the mean values of the maximum and the minimum age of fossil assemblages for the extinct species (Supplementary Table S7 available on Dryad). We selected the GTR substitution model based on results from j-Modeltest v2.1.10 (Darriba et al. 2012), and used Mk models (Lewis 2001) for morphological traits. We ran two independent MCMC analyses for 100 million generations, sampling every 5000 generations. We checked the convergence of the Markov chains in Tracer v.1.7.1 (Rambaut et al. 2018) and combined the chains after removing a burn-in of the first 10% generations. The effective sample sizes exceeded 200 for all parameters.
Biogeographic Analysis
We defined seven geographical regions based on floristic regions of living and fossil species: A) Asian High Latitudes, B) Europe, C) North American High Latitudes, D) East Asia, E) North America, F) Southeast Asia, and G) South America. We used the Dispersal-Extinction-Cladogenesis model (Ree et al. 2005; Ree and Smith 2008) to infer geographical range evolution with three dispersal-constrained time slices (T1: before 30 Ma; T2: 30-5 Ma, and T3: 5-0 Ma, Supplementary Table S8 available on Dryad) in BioGeoBEARS (Matzke 2013a), which reflected the main changes of continental connectivity and climate through the history of Juglandaceae. Before the Oligocene (T1), Earth was in a hot-house climatic phase (Zachos et al. 2001) and the Tethys ocean remained a barrier between Europe and Asia. Therefore, we set the dispersal rate between the two continents to a relatively low value (0.01). The climate between the Oligocene and late Miocene (T2) was relatively stable (Zachos et al. 2001). During this time, the Central American Seaway remained a barrier between North and South America and the role of the North Atlantic land bridge became weaker compared with the early Paleogene. Thus, we set the dispersal between North and South America to 0.75 and the dispersal between North American High Latitudes and Europe to 0.5 during this time frame (30 to 5 Ma). After the late Miocene (T3), the global climate became substantially colder and more seasonal (Zachos et al. 2001). North America and Europe were farther apart than in previous time slices and the connectivity between North America and Asia was weaker, therefore, the dispersal rate between North American High Latitudes and Europe was set to 0.25 and the dispersal between North American and Asian High Latitudes was constrained to 0.5. Dispersal rates between nonadjacent continents were set to 0.01 across all three time slices. We reconstructed ancestral ranges on 100 trees randomly sampled from the posterior distribution of BEAST trees. We constrained the maximum number of areas allowed for the ancestors of Juglandaceae to two areas, reflecting the maximum number of occupied areas observed in modern species.
We then computed the number of lineages through time within each geographic range. To this end, we first extracted the ages and ancestral areas at the start and end of each internode in the tree. We then mapped the geographic range along each branch by randomly sampling the time of transitions (if any) between the starting and ending ranges from a uniform distribution spanning the length of the internode. We then counted the number of lineages occurring in each area within 1-Myr time bins. We summarized these counts across trees and computed the 95% confidence intervals and mean values to produce lineage through time plots. We used the same counts to calculate the median number of dispersal and extinction events during five geological periods (i.e., Cretaceous–Early Eocene, Middle–Late Eocene, Oligocene, Early–Middle Miocene, and Late Miocene–Present).
Modeling Latitudinal Range and Climatic Niche Evolution
We developed a new model of trait evolution to infer the evolution of latitudinal range and climatic niche in Juglandaceae based on the fossilized Brownian motion model implemented in fossilBM (Silvestro et al. 2019). The fossilBM program implements a joint Bayesian estimation of the ancestral states of a trait and the parameters of a Brownian evolutionary model (BM) along a phylogeny of extinct and extant taxa. The original model included a rate parameter and a trend parameter, quantifying the presence of a positive or negative bias in the random walk used to model trait evolution. Under a BM model with trend (hereafter the BMT model), the expected trait value 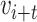 after a time
after a time 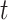 is normally distributed:
is normally distributed:
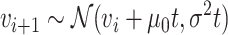 |
(1) |
where 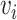 is the ancestral value,
is the ancestral value, 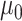 is the trend parameter, and
is the trend parameter, and 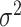 is the evolutionary rate. Here, we further expand this model by allowing the trend parameter
is the evolutionary rate. Here, we further expand this model by allowing the trend parameter 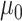 to vary linearly as a function of time. This model with time-variable trend (hereafter the BMVT model) adds a single parameter
to vary linearly as a function of time. This model with time-variable trend (hereafter the BMVT model) adds a single parameter 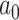 quantifying the slope of the linear trend, whereas
quantifying the slope of the linear trend, whereas 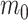 represents the intercept at time
represents the intercept at time 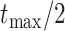 , where
, where 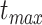 is the root age of the tree. Thus, the trend parameter is expressed as a function of time under this model:
is the root age of the tree. Thus, the trend parameter is expressed as a function of time under this model:
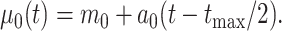 |
(2) |
Under this formulation, 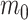 corresponds to the trend parameter at the middle time between the root and the tip ages, thus representing the mean trend through time. This formulation results in a much more efficient sampling of the parameters via MCMC as compared with one where
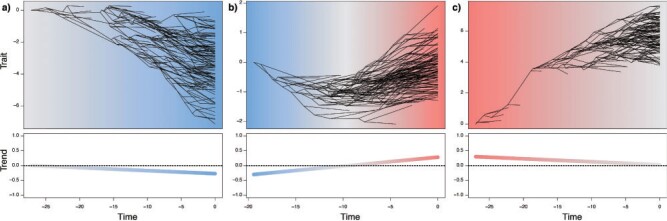
corresponds to the trend parameter at the middle time between the root and the tip ages, thus representing the mean trend through time. This formulation results in a much more efficient sampling of the parameters via MCMC as compared with one where 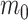 corresponds to the trend at the tips or at the root of the tree. Despite only adding a single parameter, this model allows significant flexibility in terms of expected trait variation through time (Fig. 1). Indeed, the trend parameter can be around 0 at the root age (indicating neutral evolution) and linearly change to, for example, negative, therefore yielding increasingly small expected trait values (Fig. 1a). Alternatively, the trend could start negative, then near 0, and finally become positive, indicating traits evolving toward smaller values first and then returning to larger values (Fig. 1b). We used the 95% credible intervals of m
corresponds to the trend at the tips or at the root of the tree. Despite only adding a single parameter, this model allows significant flexibility in terms of expected trait variation through time (Fig. 1). Indeed, the trend parameter can be around 0 at the root age (indicating neutral evolution) and linearly change to, for example, negative, therefore yielding increasingly small expected trait values (Fig. 1a). Alternatively, the trend could start negative, then near 0, and finally become positive, indicating traits evolving toward smaller values first and then returning to larger values (Fig. 1b). We used the 95% credible intervals of m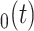 calculated at different times based on the sampled
calculated at different times based on the sampled 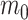 and
and 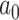 to determine whether the trend was significantly different from 0, and how that changes through time. We implemented this extended BM model within the fossilBM Bayesian framework (available at: https://github.com/dsilvestro/fossilBM). We assigned standard normal priors centered in 0 for both
to determine whether the trend was significantly different from 0, and how that changes through time. We implemented this extended BM model within the fossilBM Bayesian framework (available at: https://github.com/dsilvestro/fossilBM). We assigned standard normal priors centered in 0 for both 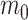 and
and 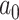 , thus assigning the highest prior probability to a neutral BM process with no trend. The rate and trend parameters are estimated using Metropolis–Hastings updates, while the ancestral states were sampled directly from the posterior using Gibbs updates (Silvestro et al. 2019).
, thus assigning the highest prior probability to a neutral BM process with no trend. The rate and trend parameters are estimated using Metropolis–Hastings updates, while the ancestral states were sampled directly from the posterior using Gibbs updates (Silvestro et al. 2019).
Figure 1.
Three simulated scenarios of the BMVT (Brownian evolution with a time-variable trend) process with neutral to negative trend (a), negative to positive trend (b), and positive to neutral trend (c). The three upper panels show trait evolution across a simulated tree, and the three lower panels show the corresponding trend parameter through time. The blue shades represent a negative trend pushing the trait toward smaller values, gray represents trends around 0, that is, neutral evolution, and red represents a positive trend which favors larger trait values.
We assessed the performance of the BMVT model by analyzing simulated data sets and comparing the estimated rates, trends, and ancestral states with the true values under different Brownian motion models:
1) BM: Brownian evolution without a trend (
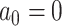 ,
, 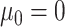 );
);2) BMT: Brownian evolution with a constant nonzero trend (
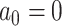 ,
, 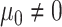 );
);3) BMVT: Brownian evolution with a trend in time-variable direction (
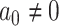 ,
, 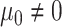 ).
).
We simulated 100 data sets under each model and, for each simulation, generated a complete phylogenetic tree including extinct taxa and with a number of extant taxa randomly sampled between 50 and 150 tips. We simulated the trees under a birth–death process using the R package TreeSim (Stadler 2015; function sim.bd.taxa) with speciation rate 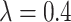 , and extinction rate
, and extinction rate 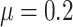 . Trait data were simulated under a BM model with randomly sampled rate
. Trait data were simulated under a BM model with randomly sampled rate 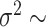 U[0.01, 0.1] and a BMT model with randomly sampled rate
U[0.01, 0.1] and a BMT model with randomly sampled rate 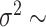 U[0.01, 0.1] and trend
U[0.01, 0.1] and trend 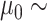 U[0.2, 0.5] *
U[0.2, 0.5] * 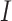 , where
, where 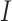 could take a value of either -1 or 1 to simulate positive or negative trends. We also simulated 100 traits under the BMVT model with the same settings for
could take a value of either -1 or 1 to simulate positive or negative trends. We also simulated 100 traits under the BMVT model with the same settings for 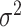 and
and 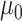 and sampling
and sampling 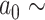 U[0.02, 0.04] *
U[0.02, 0.04] * 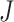 where
where 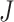 could take a value of either
could take a value of either  1 or 1 to simulate positive or negative change in the trend parameter.
1 or 1 to simulate positive or negative change in the trend parameter.
Additionally, we ran a set of simulations to assess whether the parameters of the BMVT model could be accurately estimated in the expected case of the incomplete fossil record. We simulated 190 trees based on a birth–death process (speciation rate 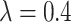 , and extinction rate
, and extinction rate 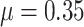 ) from which we dropped 80% of the extinct species. The remaining number of sampled tips ranged from 13 to 89 (median
) from which we dropped 80% of the extinct species. The remaining number of sampled tips ranged from 13 to 89 (median 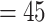 ). We then simulated traits onto these trees under the BMVT model with the same settings used above.
). We then simulated traits onto these trees under the BMVT model with the same settings used above.
We analyzed each simulated data set under the three BM models running 100,000 MCMC iterations, sampling every 100 for BM, BMT models, and 200,000 iterations sampling every 200 for the BMVT model. To summarize the results, we quantified the accuracy of the parameter estimates (posterior mean across the MCMC samples) across all simulations by calculating squared errors between true and estimated values of 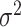 ,
, 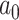 , and
, and 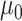 , and the mean squared errors for all ancestral states.
, and the mean squared errors for all ancestral states.
We used the BMVT model to infer the evolution of seven continuous traits that characterize the environmental niche of Juglandaceae, namely the absolute latitude (AbsLat), MAT, Tcold, Twarm, MAP, Pdry, and Pwet. In our empirical analyses, we partitioned the tree in two clades corresponding to the Juglandoideae and the Engelhardioideae so that each clade had its own 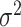 ,
, 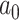 ,
, 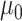 parameters all of which were jointly estimated through MCMC along with the ancestral states in a single analysis. We defined this partition based on the fact that the two subfamilies, while sharing a single ancestor, diverged into a predominantly temperate clade (Juglandoideae) and a predominantly tropical clade (Engelhardioideae). The partitioned analysis thus allowed to fit potentially diverging processes of niche evolution for the temperate and tropical clades. We ran the analyses for 110,000 MCMC iterations sampling every 100 and excluded the first 10,000 iterations as burn-in. The analyses were repeated over a random sample of 100 phylogenetic trees to account for topological and dating uncertainties. We summarized the rate and trend parameters and the inferred ancestral states by computing the mean and 95% and 99% credible intervals after combining the output across 100 replicates.
parameters all of which were jointly estimated through MCMC along with the ancestral states in a single analysis. We defined this partition based on the fact that the two subfamilies, while sharing a single ancestor, diverged into a predominantly temperate clade (Juglandoideae) and a predominantly tropical clade (Engelhardioideae). The partitioned analysis thus allowed to fit potentially diverging processes of niche evolution for the temperate and tropical clades. We ran the analyses for 110,000 MCMC iterations sampling every 100 and excluded the first 10,000 iterations as burn-in. The analyses were repeated over a random sample of 100 phylogenetic trees to account for topological and dating uncertainties. We summarized the rate and trend parameters and the inferred ancestral states by computing the mean and 95% and 99% credible intervals after combining the output across 100 replicates.
Results
Phylogeny, Divergence Times, and Biogeographic history of Juglandaceae
The phylogenetic relationships at the generic level among extant lineages of Juglandaceae inferred from our partitioned and nonpartitioned data sets were largely consistent except for the genus Cyclocarya, which was similar to the result of the most recent study showing that the position of this genus was unresolved based on RAD-Seq and whole chloroplast genome data (Supplementary Figs. S2–S5 available on Dryad; Mu et al. 2020). Our combined analysis of 113 fossil and 47 extant species supports the reciprocal monophyly of the subfamilies Juglandoideae and Engelhardioideae, which is sister to the subfamily Rhoipteleoideae (monotypic genus Rhoiptelea), even though the exact phylogenetic placement of fossil species remains uncertain (Supplementary Fig. S3 available on Dryad; Supplementary materials BEAST resampled trees available on Dryad). The origin of Juglandaceae was estimated in the Late to Middle Cretaceous at 105.14 Ma (95% HPD: 90.54–129.5 Ma; Supplementary Table S9 available on Dryad). The crown age of Juglandoideae was at 101.3 Ma (95% HPD: 77.9–106.94 Ma; Supplementary Table S9 available on Dryad) and Engelhardioideae was at 89.22 Ma (95% HPD: 69.53–99.32 Ma; Supplementary Table S9 available on Dryad). The divergence times among extant genera were estimated between the Late Cretaceous and the late Miocene (Supplementary Table S9 available on Dryad).
Juglandaceae originated in Boreotropical regions (North America or Europe), and the crown ancestor of the subfamilies Juglandoideae and Engelhardioideae originated in North America (Fig. 2; Supplementary Fig. S6 available on Dryad). The Engelhardioideae was diverse at middle latitudes (North America) before the Oligocene, and only later reached the low latitudes (South America and Southeast Asia), in which they occur today (Fig. 2; Supplementary Fig. S6 available on Dryad). The Juglandoideae, today a clade of mainly temperate species, originated in the middle latitudes of North America, subsequently expanded the ranges to high latitudes across continents before the middle Miocene. Their range eventually contracted at midlatitudes after the middle Miocene (Fig. 2; Supplementary Fig. S6 available on Dryad). The crown ancestors of the genera Juglans and Carya, that today mainly occurs in East Asia and North America, originated in North America at middle or high latitudes, then dispersed to East Asia through the high latitudinal regions and Europe. The genera eventually went extinct in Europe (around the Pliocene), thus giving origin to its modern disjunct distribution (Fig. 2; Supplementary Fig. S6 available on Dryad). We found similar patterns in several other genera, such as Platycarya, Cyclocarya, Pterocarya, where modern distributions in Asia result from high-latitude past connectivity among continents followed local extinctions at high latitudes and in North America and Europe (Fig. 2; Supplementary Fig. S6 available on Dryad). Our analyses also demonstrated that such comprehensive biogeographic patterns cannot be derived from biogeographic analysis based only on the living taxa (Supplementary Figs. S7 and S8 available on Dryad).
Figure 2.
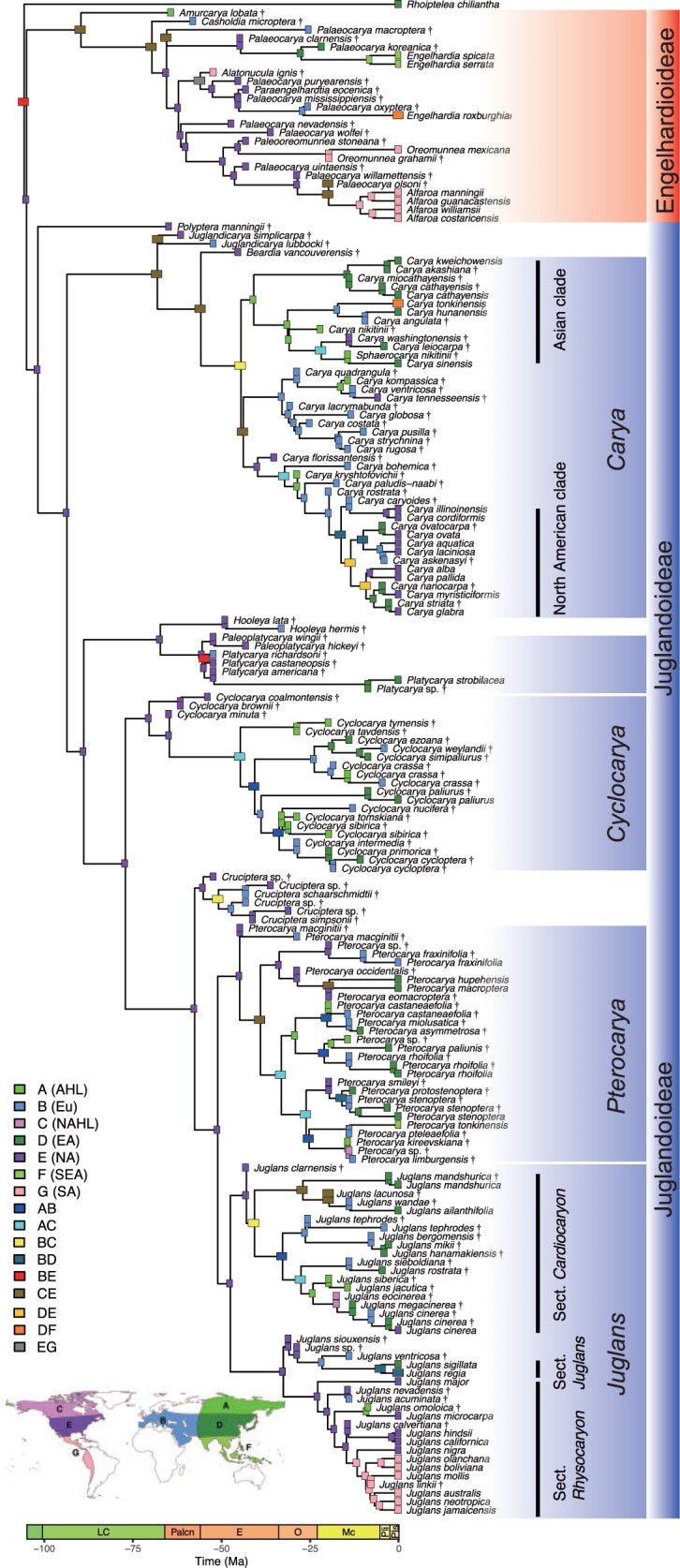
Biogeographic reconstruction of Juglandaceae based on extant and extinct species under Dispersal-Extinction-Cladogenesis model. The probabilities of ancestral states are averaged over 100 trees and plotted on master tree. A  Asian High Latitudes; B
Asian High Latitudes; B  Europe; C
Europe; C  North American High Latitudes; D
North American High Latitudes; D  East Asia; E
East Asia; E  North America; F
North America; F  Southeast Asia; G
Southeast Asia; G  South America. Symbol “† ” represents fossil species. Geological time abbreviations: LC
South America. Symbol “† ” represents fossil species. Geological time abbreviations: LC  Late Cretaceous; Palcn
Late Cretaceous; Palcn  Paleocene; E
Paleocene; E  Eocene; O
Eocene; O  Oligocene; Mc
Oligocene; Mc  Miocene; Pli
Miocene; Pli  Pliocene; Pls
Pliocene; Pls  Pleistocene.
Pleistocene.
Diversity Dynamics in Different Clades and Regions
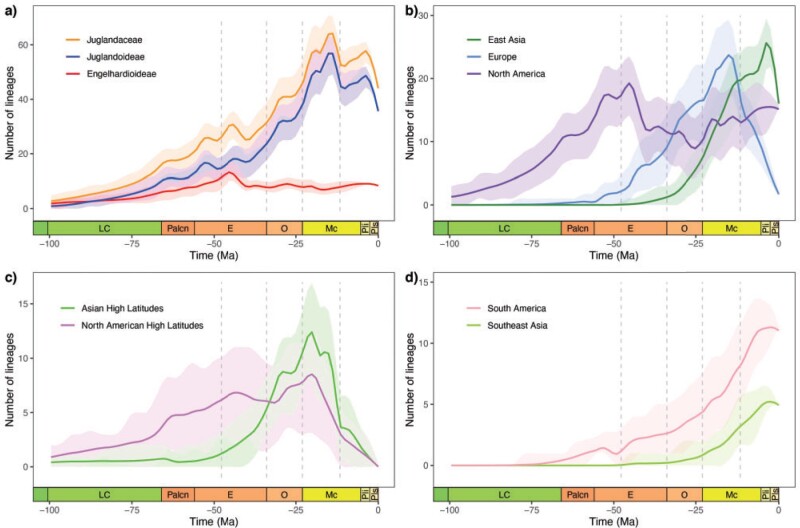
Lineage through time plots showed that the two major clades in Juglandaceae followed starkly different diversity dynamics. Juglandoideae rapidly accumulated a high diversity that peaked in the middle Miocene, followed by a decline that continued through the present resulting in a drop of 40% of its diversity (Fig. 3a). By contrast, Engelhardioideae gradually accumulated a lower diversity of lineages until the middle Eocene, and remained stable for the following 40 Myr (Fig. 3a).
Figure 3.
Lineage accumulation through time in different clades and regions of extant and extinct species of Juglandaceae. Geological time abbreviations: LC  Late Cretaceous; Palcn
Late Cretaceous; Palcn  Paleocene; E
Paleocene; E  Eocene; O
Eocene; O  Oligocene; Mc
Oligocene; Mc  Miocene; Pli
Miocene; Pli  Pliocene; Pls
Pliocene; Pls  Pleistocene. The lines are mean values and the shaded areas represent 95% credible intervals.
Pleistocene. The lines are mean values and the shaded areas represent 95% credible intervals.
Juglandaceae diversified first in the New World (North America, North American High Latitudes, and South America) during the Late Cretaceous and Paleocene, with subsequent expansion and diversification in Europe and Asia High Latitudes in the early Eocene, and East Asia and Southeast Asia in the Oligocene (Fig. 3). Species diversity in North America increased rapidly until the early Eocene, after which it remained relatively stable (Fig. 3b). Lineages in Europe and those at high latitudes of North America and Asia followed similar trends with a peak diversity in the middle Miocene, followed by a rapid decline that left only two species in Europe and the complete disappearance of the clade at high latitudes (Fig. 3b,c). However, the diversity dynamics of Europe and high latitudes of North America and Asia cannot be presented in the analysis of extant species only (Supplementary Fig. S9 available on Dryad). Juglandaceae diversity in East Asia accumulated in the Oligocene with a rapid increase until the Pliocene, followed by a decrease in the last 3 myr (Fig. 3b). In Southeast Asia and South America, lineages accumulated gradually throughout the late Paleogene and Neogene (Fig. 3d).
Dispersal and Extinction Patterns
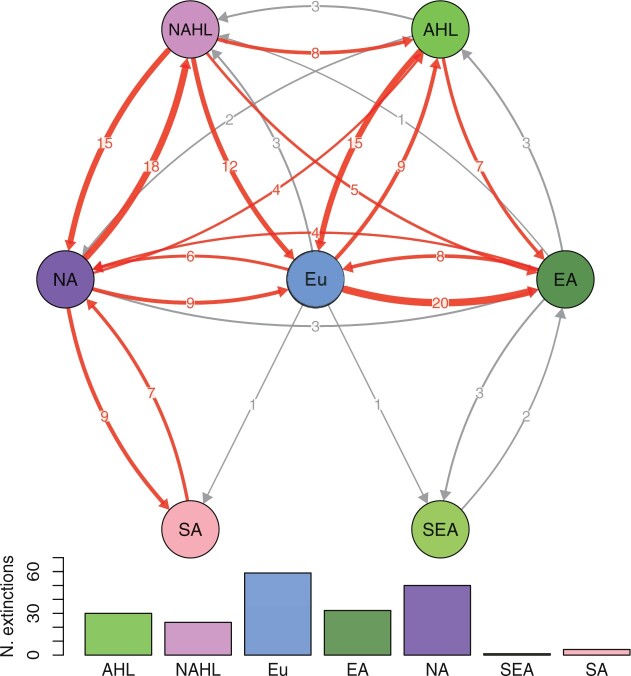
The overall count of estimated dispersal events in our biogeographic inferences shows that North America served as the main source of Juglandaceae dispersal, with 43 emigration events (Fig. 4; Supplementary Table S10 available on Dryad). Europe was a critical stepping stone connecting the New and Old Worlds, being both a major source (40 emigration events) and sink (44 immigration events), and was the region of the highest estimated number of (local) extinctions (59 events; Fig. 4; Supplementary Table S10 available on Dryad). East Asia was an important sink (37 immigration events) and played a relatively smaller role as a source (19 emigration events), while harboring a relatively large number of extinctions (32 events; Fig. 4; Supplementary Table S10 available on Dryad). Dispersals between the New and the Old Worlds may have primarily occurred via the North Atlantic land bridge (15 dispersal events between North American High Latitudes and Europe; 11 dispersal events between North American High Latitudes and Asian High Latitudes; Fig. 4).
Figure 4.
Dispersal and extinction events of extant and extinct species of Juglandaceae during the entire Cenozoic. Bar plots show the number of local extinction events during the entire Cenozoic. Region abbreviations: AHL  Asian High Latitudes; NAHL
Asian High Latitudes; NAHL  North American High Latitudes; Eu
North American High Latitudes; Eu  Europe; EA
Europe; EA  East Asia; NA
East Asia; NA  North America; SEA
North America; SEA  Southeast Asia; SA
Southeast Asia; SA  South America. The thickness of the arrows represents the number of dispersal events. Red arrows indicate that the dispersal events are more than or equal to four events.
South America. The thickness of the arrows represents the number of dispersal events. Red arrows indicate that the dispersal events are more than or equal to four events.
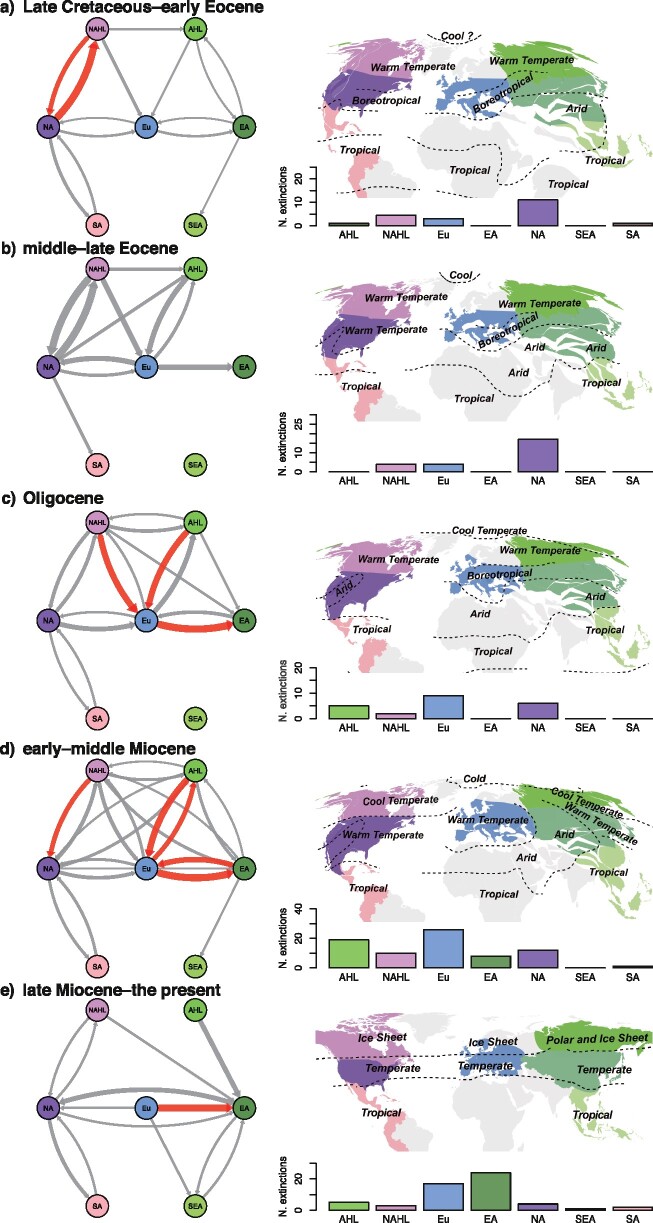
Across different time frames, North America was a major nexus of interchange, with 20 emigration and 13 immigration events inferred from the Late Cretaceous to the late Eocene, with relatively high extinction (28 estimated events; Fig. 5a,b; Supplementary Table S10 available on Dryad). Between the early Oligocene and the middle Miocene, Europe became the center of the interchanges (55 dispersal events), with lineage movement from North American and Asian High Latitudes and to East Asia. This was also coupled with relatively high (local) extinctions (35 events; Fig. 5c,d; Supplementary Table S10 available on Dryad). Finally, from the late Miocene through the Quaternary, Europe was a major source of emigration (7 events) associated with high extinctions (17 events), and East Asia was a major target of immigration (11 events) accompanied by high extinctions (24 events; Fig. 5e; Supplementary Table S10 available on Dryad). During the Neogene, extinctions in Europe (43 events), North American High Latitudes (13 events) and Asian High Latitudes (24 events) generated disjunct distributions at middle and low latitudes in the Americas and Asia (Fig. 5d,e; Supplementary Table S10 available on Dryad).
Figure 5.
Dispersal and extinction events of extant and extinct species of Juglandaceae across five time bins. a) Late Cretaceous–early Eocene, b) middle–late Eocene, c) Oligocene, d) early–middle Miocene, and e) late Miocene–the present. Networks depict dispersal events during different time bins. Bar plots show the number of local extinction events during different time bins. The paleoclimate maps for a–d) are modified from Boucot et al. (2013). The paleoclimate map for e) is modified from Ray and Adams (2001). Region abbreviations: AHL  Asian High Latitudes; NAHL
Asian High Latitudes; NAHL  North American High Latitudes; Eu
North American High Latitudes; Eu  Europe; EA
Europe; EA  East Asia; NA
East Asia; NA  North America; SEA
North America; SEA  Southeast Asia; SA
Southeast Asia; SA  South America. The thickness of the arrows represents the number of dispersal events. Red arrows indicate that the dispersal events are more than or equal to four events.
South America. The thickness of the arrows represents the number of dispersal events. Red arrows indicate that the dispersal events are more than or equal to four events.
Time-variable Trend Model Validation
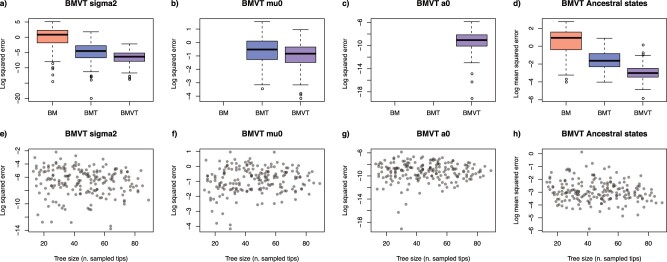
Our simulations showed that the three BM models performed similarly well when the trait evolution is neutral, that is, in the absence of a trend (Supplementary Fig. S10a–d available on Dryad). The squared errors between estimated and true parameter values were almost equally low across the three models, indicating that the additional parameters included in the BMT and BMVT models do not cause substantial overparameterization. In simulations performed under the BMT model (with constant trend) parameter estimated under BMT and BMVT strongly outperform BM estimates, as expected, while BMT and BMVT models perform similarly well (Supplementary Fig. S10e–h available on Dryad). Finally, when the trend is varying through time, the squared errors of rates, trend parameters, and ancestral states are orders of magnitude smaller under the BMVT model than under BM and BMT models (Fig. 6a–d; Supplementary Fig. S10i–l available on Dryad). The results indicate that the BMVT model strongly improves the accuracy of estimated trait evolution in the presence of a time-variable trend, while showing negligible effects of overparameterization when traits evolved under simpler models (Fig. 6a–d; Supplementary Fig. S10 available on Dryad). Our simulations also demonstrate that the parameters are identifiable, and that tree size does not have a strong effect on their accuracy (Fig. 6e–h; Supplementary Fig. S11 available on Dryad). This indicates that even in comparatively small trees including extinct lineages (e.g.,  40 tips) the BMVT model can be robustly estimated.
40 tips) the BMVT model can be robustly estimated.
Figure 6.
Accuracy of parameter estimation summarized across 190 simulated trees with traits evolving under a BMVT model. To simulate the incompleteness of the fossil record, 80% of the extinct tips were dropped from the complete trees prior to their analysis. a–d) The log-transformed squared errors of parameter estimates based obtained from fitting BM, BMT, and BMVT models (when BMVT is true). e–h) The log squared errors of the estimated parameters plotted against tree size (after subsampling the extinct tips) under BMVT model. For ancestral states (d, h), we report the log-transformed mean squared error across all internal nodes of the tree.
Climate Niche Evolution in Two Subfamilies
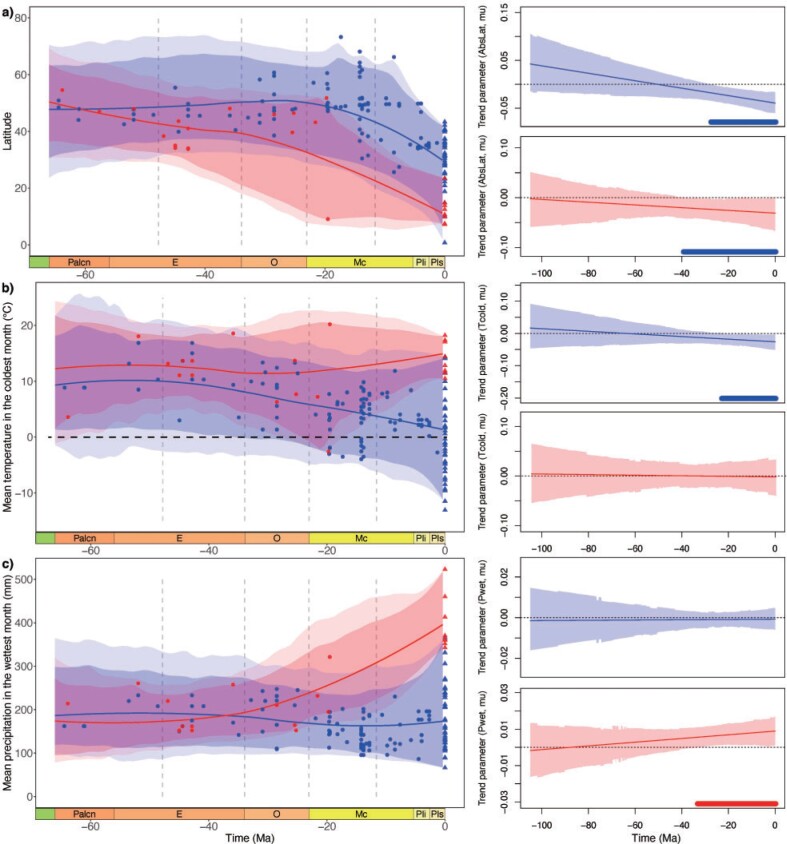
Juglandoideae had higher estimated rates of evolution than Engelhardioideae in all traits (Supplementary Fig. S12 and Table S11 available on Dryad). The sister-clade subfamilies of Juglandaceae originated in semiarid to humid and warm environments at midlatitudes (Supplementary Fig. S13 and Table S12 available on Dryad). The geographic range of Juglandoideae subsequently expanded to high latitudes before the middle Miocene, followed by a range contraction to mid–low latitudes after the middle Miocene. This switch in the evolution of the latitudinal range is captured by a trend parameter that is significantly negative from 27.7 Ma to the present (Fig. 7a; Supplementary Table S11 available on Dryad). This subfamily showed a significant negative trend in Tcold between 23.1 Ma and the present, a positive trend in Twarm between 19.5 Ma and the present, as well as constant MAT, Pwet, Pdry, and MAP during the whole period (Fig. 7b,c; Supplementary Figs. S14 and S15 and Table S11 available on Dryad). Thus, the subfamily experienced an adaptation to cooler winters and warmer summers with increasing tolerance for a wider temperature range, while maintaining a preference for relatively dry environments. The 95% credible interval based on the ancestral states inferred for Tcold show that lineages within Juglandoideae evolved frost tolerance between the Oligocene and the early Miocene (Fig. 7b).
Figure 7.
Latitude and climate niche evolution of mean temperature in the coldest month and mean precipitation in the wettest month for subfamilies Juglandoideae and Engelhardioideae during the Cenozoic. Geological time abbreviations: LC  Late Cretaceous; Palcn
Late Cretaceous; Palcn  Paleocene; E
Paleocene; E  Eocene; O
Eocene; O  Oligocene; Mc
Oligocene; Mc  Miocene; Pli
Miocene; Pli  Pliocene; Pls
Pliocene; Pls  Pleistocene. Lines indicate mean values and the shaded areas represent 95% and 99% credible intervals. Blue represents the clade of Juglandoideae and the red represents Engelhardioideae. Circles represent fossil occurrences and the triangles represent extant species. The frost tolerance is shown below the dotted black line (0
Pleistocene. Lines indicate mean values and the shaded areas represent 95% and 99% credible intervals. Blue represents the clade of Juglandoideae and the red represents Engelhardioideae. Circles represent fossil occurrences and the triangles represent extant species. The frost tolerance is shown below the dotted black line (0 in (b). The right column shows the estimated trend parameters for subfamilies Juglandoideae (blue) and Engelhardioideae (red). Thin lines indicate mean values and the shaded areas represent 95% credible intervals. Bold lines at the bottom of the plots indicate the periods of time with significantly negative (blue) or positive (red) estimated trends. AbsLat
in (b). The right column shows the estimated trend parameters for subfamilies Juglandoideae (blue) and Engelhardioideae (red). Thin lines indicate mean values and the shaded areas represent 95% credible intervals. Bold lines at the bottom of the plots indicate the periods of time with significantly negative (blue) or positive (red) estimated trends. AbsLat  Absolute Latitude; Tcold
Absolute Latitude; Tcold  mean temperature of the coldest month; Pwet
mean temperature of the coldest month; Pwet  mean precipitation of the wettest month.
mean precipitation of the wettest month.
The geographic range of Engelhardioideae shifted southward toward current tropical zones, shown by a significantly negative trend parameter between 39.5 Ma and the present (Fig. 7a; Supplementary Table S11 available on Dryad). This subfamily maintained similar temperature preferences throughout their evolution indicating that Engelhardioideae tracked warm temperatures toward the tropics while the Earth was cooling (Fig. 7b; Supplementary Fig. S14 available on Dryad). However, the trend parameter for MAP in Engelhardioideae was significantly positive between 40.6 Ma and the present, and Pwet was significantly positive between 33.5 Ma and the present, indicating that Engelhardioideae showed a significant increase in tolerance to more humid or seasonally humid environments in order to adapt to wetter tropical environments (Fig. 7c; Supplementary Fig. S15a and Table S11 available on Dryad).
Discussion
Diversification of Juglandaceae and Its General Significance to Temperate Trees
Juglandaceae originated in semiarid and humid warm environments at middle latitudes in North America or Europe and expanded to high latitudes across the Northern Hemisphere and East Asia. Subsequent extinction at high latitudes and in Europe yielded disjunct distributions in East Asia and North America. In a recent study, Song et al. (2019) also inferred a North American and European origin of Juglandaceae by constraining the ancestral states of some lineages based on the fossil record (node constraints). These results favor the Boreotropical hypothesis over the Arcto-Tertiary hypotheses. This is also in contrast with the Out-of-Asia hypothesis that shows other temperate groups originated in East Asia and then dispersed to North America or Europe (Donoghue et al. 2001; Donoghue and Smith 2004; Wen et al. 2010). However, most of the studies of East Asian origins were based on extant lineages only and did not include fossil evidence in their biogeographic analyses. In some cases, origination in East Asia is at odds with fossils that document early occurrence in North America and Europe. For instance, Symplocos (Symplocaceae; Wang et al. 2004), Castanea (Fagaceae; Lang et al. 2007), Aralia (Araliaceae; Wen et al. 2010), and Zelkova (Ulmaceae; Denk and Dillhoff 2005; Zhang et al. 2017) were all inferred to have originated in Asia based on extant taxa, but their earliest known fossil occurrences are found in North America or Europe (Xing et al. 2016). Similarly, we found that biogeographic analysis of extant Juglandaceae excluding fossils suggests an Asian or South American origin, which is substantially different from the results that do include fossils (Fig. 2; Supplementary Figs. S6–S8 available on Dryad). Additionally, Manchester et al. (2009) reviewed the fossil records for East Asian endemic genera and found that the majority of the woody genera formerly occurred in North America or Europe rather than Asia. We predict that greater integration of extinct taxa into biogeographic analyses of these clades will increase general support for Boreotropical origins of temperate woody plants in North America and Europe.
The temperate clade Juglandoideae expanded from North America into high latitudes in the Old and New Worlds, reaching a high diversity during a global warming phase which peaked at the Middle Miocene Climate Optimum until around 17–15 Ma, the last major global warming event. Subsequent extinction at high latitudes and in Europe resulted in the disjunct diversity pattern characteristic of temperate woody plants in the Northern Hemisphere (Figs. 2, 5, and 7a; Supplementary Fig. S6 available on Dryad; Donoghue and Moore 2003). This inferred history is consistent with previous studies of genera within Juglandoideae, for example, Carya (Zhang et al. 2013), Pterocarya (Song et al. 2020), and Juglans (Zhang et al. 2019). More generally, the angiosperm fossil record suggests that a widespread geographic range spanning high latitudes and Europe which is then decimated through severe extinction is not unique to Juglandaceae, but common to many clades across north temperate woody plants. For example, Betulaceae, Salicaceae, Sapindaceae (mainly Acer), Altingiaceae, Hamamelidaceae, Ulmaceae (mainly Ulmus and Zelkova), Symplocaceae, Magnoliaceae (Liriodendron), Eucommiaceae, and Sabiaceae (Xing et al. 2016) all show wide fossil distributions at high latitudes and in Europe, outside their current centers of diversity. Thus, revisiting the biogeographic evolution of these clades in a phylogenetic framework informed by extinction might confirm the generality of the history of Juglandaceae.
The Effects of Continental Connectivity and Barriers
Historical availability of high-latitude dispersal corridors between continents played a central role in reconciling the tradeoffs between dispersal and evolutionary change (Donoghue 2008). Expansion of Boreotropical Juglandaceae from North America to Europe and Asia was facilitated by the Beringia land bridge and North Atlantic land bridge. These land bridges facilitated migration between the New and Old Worlds especially during the Eocene and early to middle Miocene (Figs. 2, 4, and 5; Supplementary Fig. S6 available on Dryad; Tiffney 1985a; Tiffney and Manchester 2001). Whereas previous studies emphasized woody plant dispersal between East Asia and North America through the Beringia land bridge (Donoghue et al. 2001; Donoghue and Smith 2004; Wen et al. 2010, 2016), our results show that the North Atlantic land bridge and Europe were key to many dispersal events in Juglandaceae and therefore played crucial roles in linking the New and Old Worlds during most of the Cenozoic era (Figs. 2, 4, and 5; Supplementary Fig. S6 available on Dryad).
Other geological events also likely had profound effects on dispersal and diversity. For instance, the retreat of the Turgai seaway and the closure of the Tethys Ocean within Eurasia likely improved the connectivity between Europe and East Asia and facilitated increased migration from Europe to East Asia since the Oligocene, leading to a rapid rise of diversity in East Asia (Figs. 2, 3b, and 5c,d,e; Supplementary Fig. S6 available on Dryad; Tiffney 1985a; Tiffney and Manchester 2001).
In contrast to northern high latitudes, the relatively poor connectivity between continents at low and middle latitudes limited the dispersal opportunities for thermophilic plants. For example, North and South America are connected only by a narrow Panama corridor which formed between the Miocene and Pliocene (Bacon et al. 2015) and the middle latitudes in the Old and New Worlds have been separated by oceans throughout the Cenozoic. The rapid decline in Juglandaceae diversity in Europe during the global cooling culminating in the Quaternary glacial cycles may have been exacerbated by the isolation from suitable regions in the South because of geographic barriers such as the Mediterranean Sea and the Sahara region (Fig. 3b; Tiffney 1985b; Milne and Abbott 2002).
Disparity in Diversification for Temperate and Tropical Subfamilies
Our results clearly indicate that the sister clades Engelhardioideae and Juglandoideae originated in a semiarid to humid, warm environment but then diverged in their evolutionary trajectories, with Engelhardioideae gradually adapting to wetter tropical climates and Juglandoideae expanding across temperate regions with increasingly variable and seasonal climate (Fig. 7, Supplementary Figs. S14 and S15 available on Dryad; Eldrett et al. 2009; Eronen et al. 2012).
Because freezing temperatures are a major physiological barrier to plant dispersal (Latham and Ricklefs 1993), the acquisition of frost tolerance in Juglandoideae in the late Oligocene if not earlier was key to its evolutionary success. It enabled the subfamily to reach a wider ecological and geographic range relative to its sister clade Engelhardioideae, while its climatic tolerances for precipitation did not appreciably change (Fig. 7; Supplementary Fig. S15 available on Dryad). A similar pattern was described for the genus Hypericum (Meseguer et al. 2018), which also originated in warm Boreotropical environments in the Northern Hemisphere and evolved towards colder tolerances while continuing on similar precipitation regimes. This could represent a general trend in the adaptation of ancient Boreotropical lineages to temperate environments. Continued adaptation to seasonally colder climates allowed Juglandoideae to reach the Arctic regions, as demonstrated by fossils found in northern Canada and Siberia (Supplementary Fig. S1 available on Dryad). During the Middle Miocene Climatic Optimum, Juglandoideae even at high latitudes lived in an environment with freezing temperatures in winter but warm summers with high diversity (Figs. 3a and 7a,b; Supplementary Fig. S14b available on Dryad). This implied that lineages are able to adapt to changing environments and wider ecological ranges leading to high diversification (Thuiller et al. 2005; Colles et al. 2009; Kozak and Wiens 2010; Lavergne et al. 2013; Rolland et al. 2018). However, as climates became globally colder and drier in the late Neogene and Quaternary, culminating with the onset of the Quaternary ice age, Juglandoideae retreated from high latitudes (Figs. 5d,e and 7a), indicating the limits of its capacity for niche evolution. Additionally, the climate-driven range shifts during the Quaternary climate oscillations likely led to the net loss of walnut diversity in Europe, while facilitating hybridization events that may have resulted in adaptive introgression (Zhang et al. 2019).
In Engelhardioideae, niche evolution was characterized by temperature conservatism such that lineages tracked warm temperatures in the cooling late Cenozoic climate by shifting their latitudinal range toward the tropics, especially in the Neogene. While this latitudinal shift toward the tropics allowed the clade to maintain essentially unaltered temperature tolerances, it involved adaptation to increasingly humid climates with more seasonal precipitation (Fig. 7; Supplementary Figs. S14 and S15 available on Dryad). The low diversity in Engelhardioideae compared to its temperate sister clade may reflect stronger competition for ecological niche space in the tropics, where higher species richness was likely closer to regional carrying capacities (Diamond 1973; Caswell and Cohen 1991; Clark et al. 2004; Cadena 2007; Schemske et al. 2009). Paleobotanical evidence is strongly biased by taphonomy (preservation conditions), sampling effort, and tectonic processes (Adrain and Westrop 2003). Drought-prone tropical habitats (e.g., tropical Africa) offer less favorable conditions for fossil preservation (Looy et al. 2014; Mannion et al. 2014; Xing et al. 2016). Additionally, modern tropical areas are primarily covered with dense forests (e.g., Amazonia, Andes), leading to limited fossil sampling (Xing et al. 2016). In our fossil data set, the tropical Engelhardioideae fossils are far fewer than the temperate fossils. Most of the Engelhardioideae fossils have been found in ancient tropics (subhumid and warm habitats) in the Northern Hemisphere where they can be easily sampled, whereas there are scarce fossils in the recent tropics (Supplementary Fig. S1 available on Dryad). This potential sampling bias may lead to under-representation of Engelhardioideae and obscure an extinction signal. However, a similar imbalance persists in extant species between the temperate clade (ca. 45 spp.) and the tropical one (ca. 15 spp.). Additionally, Engelhardioideae is poorly represented in the rich Cenozoic fossil sites that have been recovered in the subtropical region of China, one of its modern centers of diversity (Huang et al. 2016). Given these observations, we hypothesize that the diversity of Engelhardioideae has been continuously low throughout its evolutionary history (Fig. 3a).
We interpret the contrasting evolutionary trajectories of the two subfamilies as examples of alternative tradeoffs between climatic niche conservatism and adaptability to a changing environment. In Juglandaceae, temperate lineages demonstrated a remarkable adaptive change along the temperature axis of their climatic niche, evolving by as much as 10 degrees their tolerated temperature in the coldest month. However, these lineages did not shift or expand their range of tolerated precipitation. By contrast, lineages distributed today in tropical regions shifted their geographic range rather the adapting to a cooling climate, but this required increased tolerance of seasonal precipitation and a more humid climate.
Modeling Time-Variable Trends from Phylogenies with Fossils
There has been considerable development in comparative methods that allow us to infer trait evolution under a range of Brownian processes, Ornstein–Uhlenbeck (OU) models and other stochastic processes (see Harmon 2019 for a thorough overview). These include BM models with variable rates (Pennell et al. 2014) or with a trend (BMT; Slater et al. 2012; Silvestro et al. 2019) or switching between BM and BMT (Slater et al. 2017).
Here, we have presented an extension of the fossilBM method (Silvestro et al. 2019) which implements a join estimation of ancestral states and the parameters of a model of trait evolution in a Bayesian framework. In our new model, the evolution of a continuous trait follows a random walk with a bias (the trend parameter), which itself can vary over time in a linear fashion. While this model only adds two parameters compared to a neutral BM, it adds a significant amount of flexibility to the patterns that it can generate (Fig. 1). These include OU-like patterns (Fig. 1c), with the mean trait evolving toward a larger or smaller mean value and then stabilizing there, as the trend parameter moves closer to 0. However, as the trend parameter can switch sign during the evolution of a clade, for example, from negative to positive, the BMVT model can also generate more complex patterns that cannot be described by other commonly used models of trait evolution (Fig. 1b). Our empirical analyses show that this model can help describe how lineages may shift their latitudinal ranges in different directions while tracking climate change (Fig. 7a). These models are identifiable due to the inclusion of extinct taxa in the analyses, thus providing further support to the importance of integrating fossil data in phylogenetic comparative methods (Slater and Harmon 2013; Slater et al. 2017; Meseguer et al. 2018; Silvestro et al. 2019).
There are limitations in the inclusion of fossils in comparative analyses starting from the inevitable biases in fossil sampling. For instance, heterogeneous preservation rates may lead fossil taxa to be over- or under-represented in different parts of the tree (e.g., temperate clades vs. tropical clades), or unevenly sampled across geological timescales (Xing et al. 2016). The paleoclimate data used in our study depends on fossil assemblages as well, thus the sampling biases may generate estimate errors in traits related to climatic niche. Yet, several studies show that even a few fossil taxa can drastically improve the ancestral state estimates and paint a more comprehensive picture of the tempo and mode of trait evolution (Slater et al. 2012; Schnitzler et al. 2017; Slater et al. 2017; Silvestro et al. 2019).
Conclusions
The accuracy of biogeographic inference is challenged by extinction. Our Juglandaceae results demonstrate that the explicit integration of extinct lineages within a phylogenetic framework of extant species offers the opportunity to test hypotheses about dispersal and extinction in a more powerful, data-driven way. Our combined analyses also reveal that the movements of Juglandaceae across continents and latitudinal zones are tightly coupled with climatic niche evolution along with different axes, which is also shown in other plant groups with rich fossil records, for example, Hypericum (Hypericaceae; Meseguer et al. 2018). The fossilized Brownian model with time-variable trend presented here contributes to a growing body of approaches combining multiple lines of evidence to produce robust hypotheses about niche and biogeographic evolution (Landis et al. 2020). Meanwhile, the evolutionary consequences of past geographic range shifts, such as ancient hybridization events, are being unraveled by analyses of genomic data (Zhang et al. 2019). Improved models and increasing availability of fossil and genomic data across a wide range of lineages will be crucial to evaluate the processes driving the establishment of disjunct pattern in temperate woody plant diversity.
Acknowledgments
We thank Shufeng Li and Alex Farnsworth for support with the coexistence approach and paleocoordinates and Gregor Kozlowski for feedback on this work. We also thank Hernán López-Fernández, Andrea S. Meseguer, and Susanne Renner for their insightful comments on our study. Finally, we thank DCSR for the computing resources at the University of Lausanne (Lausanne, Switzerland).
Supplementary Material
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.t1g1jwt1r.
Funding
This work was supported by the Strategic Priority Research Program of Chinese Academy of Sciences [XDB31000000], the National Natural Science Foundation of China [No. 31770226] to Q.Z. and Y.X.; the China Scholarship Council [No. 201904910639] to Q.z. the Funding from the Swiss National Science Foundation [PCEFP3_187012; FN-1749] and from the Swedish Research Council [VR: 2019-04739] to D.S.; the Grainger Bioinformatic Center at the Field Museum to R.R., in part.
References
- Adrain J.M., Westrop S.R. 2003. Paleobiodiversity: we need new data. Paleobiology 29:22–25. [Google Scholar]
- Aradhya M.K., Potter D., Simon C.J. 2006. Cladistic biogeography of Juglans (Juglandaceae) based on chloroplast DNA intergenic spacer sequences. Darwin’s harvest: new approaches to the origins, evolution and conservation of crops. New York: Columbia University Press. p. 143–170. [Google Scholar]
- Aradhya M.K., Potter D., Gao F., Simon C.J. 2007. Molecular phylogeny of Juglans (Juglandaceae): a biogeographic perspective. Tree Genet. Genomes 3:363–378. [Google Scholar]
- Axelrod D.I. 1983. Biogeography of oaks in the Arcto-Tertiary province. Ann. Mo. Bot. Gard. 70:629–657. [Google Scholar]
- Bacon C.D., Silvestro D., Jaramillo C., Smith B.T., Chakrabarty P., Antonelli A. 2015. Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proc. Natl. Acad. Sci. USA 112:6110–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert R., Heled J., Kühnert D., Vaughan T., Wu C.-H., Xie D., Suchard M.A., Rambaut A., Drummond A.J. 2014. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 10:e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucot A.J., Xu C., Scotese C.R., Morley R.J. 2013. Phanerozoic paleoclimate: an atlas of lithologic indicators of climates: SEPM (Society for Sedimentary Geology), concepts in sedimentology and paleontology, No. 11, Map Folio, p. 487. [Google Scholar]
- Cadena C.D. 2007. Testing the role of interspecific competition in the evolutionary origin of elevational zonation: an example with Buarremon brush-finches (Aves, Emberizidae) in the Neotropical mountains. Evolution 61:1120–1136. [DOI] [PubMed] [Google Scholar]
- Caswell H., Cohen J.E. 1991. Disturbance, interspecific interaction and diversity in metapopulations. Biol. J. Linn. Soc. 42:193–218. [Google Scholar]
- Chaney R.W. 1947. Tertiary centers and migration routes. Ecol. Monogr. 17:139–148. [Google Scholar]
- Chaney R.W. 1959. Miocene floras of the Columbia Plateau. Carnegie Inst. Wash. Publ. 617:1–229. [Google Scholar]
- Chen Y.S., Meseguer A.S., Godefroid M., Zhou Z., Zhang J.W., Deng T., Kim J.H., Nie Z.L., Liu Y.S., Sun H. 2017. Out-of-India dispersal of Paliurus (Rhamnaceae) indicated by combined molecular phylogenetic and fossil evidence. Taxon 66:78–90. [Google Scholar]
- Clark J.S., LaDeau S., Ibanez I. 2004. Fecundity of trees and the colonization-competition hypothesis. Ecol. Monogr. 74:415–442. [Google Scholar]
- Colles A., Liow L.H., Prinzing A. 2009. Are specialists at risk under environmental change? Neoecological, paleoecological and phylogenetic approaches. Ecol. Lett. 12:849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney C.R., Seddon N., Tobias J.A. 2016. Widespread correlations between climatic niche evolution and species diversification in birds. J. Anim. Ecol. 85:869–878. [DOI] [PubMed] [Google Scholar]
- Crepet W.L., Daghlian C.P. 1980. Castaneoid inflorescences from the Middle Eocene of Tennessee and the diagnostic value of pollen (at the subfamily level) in the Fagaceae. Am. J. Bot. 67:739–757. [Google Scholar]
- Darriba D., Taboada G.L., Doallo R., Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9:772–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T., Nie Z.-L., Drew B.T., Volis S., Kim C., Xiang C.-L., Zhang J.-W., Wang Y.-H., Sun H. 2015. Does the Arcto-Tertiary biogeographic hypothesis explain the disjunct distribution of northern hemisphere herbaceous plants? The case of Meehania (Lamiaceae). PLoS One 10:e0117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk T., Dillhoff R.M. 2005. Ulmus leaves and fruits from the Early–Middle Eocene of northwestern North America: systematics and implications for character evolution within Ulmaceae. Botany 83:1663–1681. [Google Scholar]
- Denk, T., Grimm, G.W. 2005. Phylogeny and biogeography of Zelkova (Ulmaceae sensu stricto) as inferred from leaf morphology, ITS sequence data and the fossil record. Bot. J. Linn. Soc. 147:129–157. [Google Scholar]
- Diamond J.M. 1973. Distributional ecology of New Guinea birds: recent ecological and biogeographical theories can be tested on the bird communities of New Guinea. Science 179:759–769. [DOI] [PubMed] [Google Scholar]
- Donoghue M.J. 2008. A phylogenetic perspective on the distribution of plant diversity. Proc. Natl. Acad. Sci. USA 105:11549–11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M.J., Bell C.D., Li J. 2001. Phylogenetic patterns in Northern Hemisphere plant geography. Int. J. Plant Sci. 162:S41–S52. [Google Scholar]
- Donoghue M.J., Moore B.R. 2003. Toward an integrative historical biogeography. Integr. Comp. Biol. 43:261–270. [DOI] [PubMed] [Google Scholar]
- Donoghue M.J., Smith S.A. 2004. Patterns in the assembly of temperate forests around the Northern Hemisphere. Philos. Trans. R. Soc. Lond. B 359:1633–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler D., Klein J., Antonelli A., Silvestro D. 2020. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2041–210X.13512–5. [Google Scholar]
- Eldrett J.S., Greenwood D.R., Harding I.C., Huber M. 2009. Increased seasonality through the Eocene to Oligocene transition in northern high latitudes. Nature 459:969–973. [DOI] [PubMed] [Google Scholar]
- Engler A. 1882. Versuch einer Entwicklungsgeschichte der Pflanzenwelt. II. Teil. Die extratropischen Gebiete der südlichen Hemisphäre und die tropischen Gebiete. Leipzig: W. Engelmann. [Google Scholar]
- Eronen J.T., Fortelius M., Micheels A., Portmann F., Puolamäki K., Janis C.M. 2012. Neogene aridification of the Northern Hemisphere. Geology 40:823–826. [Google Scholar]
- Gray A. 1878. Forest geography and archaeology. Am. J. Sci. 16:183–196. [Google Scholar]
- Guo Q., Ricklefs R.E. 2000. Species richness in plant genera disjunct between temperate eastern Asia and North America. Bot. J. Linn. Soc. 134:401–423. [Google Scholar]
- Harmon L.J., 2019. Phylogenetic comparative methods. Independent Publisher. [Google Scholar]
- Heath T.A., Huelsenbeck J.P., Stadler T. 2014. The fossilized birth–death process for coherent calibration of divergence-time estimates. Proc. Natl. Acad. Sci. USA 111:E2957–E2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans R.J., Van Etten J., Cheng J., Mattiuzzi M., Sumner M., Greenberg J.A., Lamigueiro O.P., Bevan A., Racine E.B., Shortridge A. 2015. Package “raster”: geographic data analysis and modeling. In: R Package Version 2.5-2. Available from: https://github.com/rspatial/raster.
- Hofmann C.-C., Mohamed O., Egger H. 2011. A new terrestrial palynoflora from the Palaeocene/Eocene boundary in the northwestern Tethyan realm (St. Pankraz, Austria). Rev. Palaeobot. Palynol. 166:295–310. [Google Scholar]
- Huang Y., Jia L., Wang Q., Mosbrugger V., Utescher T., Su T., Zhou Z. 2016. Cenozoic plant diversity of Yunnan: a review. Plant Divers. 38:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Gao M., Meng Y., Wen J., Ge X.-J., Nie Z.-L. 2019. The importance of the North Atlantic land bridges and eastern Asia in the post-Boreotropical biogeography of the Northern Hemisphere as revealed from the poison ivy genus (Toxicodendron, Anacardiaceae). Mol. Phylogenet. Evol. 139:106561. [DOI] [PubMed] [Google Scholar]
- Kozak K.H., Wiens J.J. 2010. Accelerated rates of climatic-niche evolution underlie rapid species diversification. Ecol. Lett. 13:1378–1389. [DOI] [PubMed] [Google Scholar]
- Landis M.J., Edwards E.J., Donoghue M.J. 2020. Modeling phylogenetic biome shifts on a planet with a past. Syst. Biol. 70:86–107. [DOI] [PubMed] [Google Scholar]
- Lang, P., Dane, F., Kubisiak, T.L. and Huang, H., 2007. Molecular evidence for an Asian origin and a unique westward migration of species in the genus Castanea via Europe to North America. Mol. Phylogenet. Evol. 43:49–59. [DOI] [PubMed] [Google Scholar]
- Latham R.E., Ricklefs R.E. 1993. Continental comparison of temperate-zone tree species richness. In: Ricklefs R.E., Schluter D., editors. Species diversity in ecological communities: historical and geographical perspectives. Chicago, IL: The University of Chicago Press. p. 294–314. [Google Scholar]
- Lavergne S., Evans M.E., Burfield I.J., Jiguet F., Thuiller W. 2013. Are species’ responses to global change predicted by past niche evolution? Philos. Trans. R. Soc., Biol. Sci. 368:20120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.O. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50:913–925. [DOI] [PubMed] [Google Scholar]
- Liu H., Ye Q., Wiens J.J. 2020. Climatic-niche evolution follows similar rules in plants and animals. Nat. Ecol. Evol. 4:753–763. [DOI] [PubMed] [Google Scholar]
- Liu X.-Q., Ickert-Bond S.M., Nie Z.-L., Zhou Z., Chen L.-Q., Wen J. 2016. Phylogeny of the Ampelocissus–Vitis clade in Vitaceae supports the New World origin of the grape genus. Mol. Phylogenet. Evol. 95:217–228. [DOI] [PubMed] [Google Scholar]
- Looy C., Kerp H., Duijnstee I., DiMichele, B. 2014. The late Paleozoic ecological-evolutionary laboratory, a land-plant fossil record perspective. Sediment. Rec. 12:4–18. [Google Scholar]
- Lunt D.J., Farnsworth A., Loptson C., L Foster G., Markwick P., O’Brien C.L., Pancost R.D., Robinson S.A., Wrobel N. 2016. Palaeogeographic controls on climate and proxy interpretation. Clim. Past 12:1181. [Google Scholar]
- Mai D. 1991. Palaeofloristic changes in Europe and the confirmation of the Arctotertiary-Palaeotropical geofloral concept. Rev. Palaeobot. Palynol. 68:29–36. [Google Scholar]
- Manchester S.R. 1987. The fossil history of the Juglandaceae. Monogr. Syst. Bot. Missouri Bot. Gard. 21:1–137. [Google Scholar]
- Manchester S.R. 1994. Fruits and seeds of the Middle Eocene nut beds flora, Clarno Formation, Oregon. Palaeontogr. Am. 58:1–205. [Google Scholar]
- Manchester S.R. 1999. Biogeographical relationships of North American tertiary floras. Ann. Mo. Bot. Gard. 472–522. [Google Scholar]
- Manchester S.R. 2001. Leaves and fruits of Aesculus (Sapindales) from the Paleocene of North America. Int. J. Plant Sci. 162:985–998. [Google Scholar]
- Manchester S.R. 2011. Fruits of Ticodendraceae (Fagales) from the Eocene of Europe and North America. Int. J. Plant Sci. 172:1179–1187. [Google Scholar]
- Manchester S.R., Chen Z.D., Lu A.M., Uemura K. 2009. Eastern Asian endemic seed plant genera and their paleogeographic history throughout the Northern Hemisphere. J. Syst. Evol. 47:1–42. [Google Scholar]
- Manchester S.R., Dillhoff R.M. 2004. Fagus (Fagaceae) fruits, foliage, and pollen from the Middle Eocene of Pacific northwestern North America. Can. J. Bot. 82:1509–1517. [Google Scholar]
- Mannion P.D., Upchurch P., Benson R.B., Goswami A. 2014. The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. 29:42–50. [DOI] [PubMed] [Google Scholar]
- Manos P.S., Donoghue M.J. 2001. Progress in Northern Hemisphere phytogeography: an introduction. Int. J. Plant Sci. 162:S1–S2. [Google Scholar]
- Manos P.S., Stone D.E. 2001. Evolution, phylogeny, and systematics of the Juglandaceae. Ann. Mo. Bot. Gard. 88:231–269. [Google Scholar]
- Manos P.S., Soltis P.S., Soltis D.E., Manchester S.R., Oh S.-H., Bell C.D., Dilcher D.L., Stone D.E. 2007. Phylogeny of extant and fossil Juglandaceae inferred from the integration of molecular and morphological data sets. Syst. Biol. 56:412–430. [DOI] [PubMed] [Google Scholar]
- Manos P.S., Stanford A.M. 2001. The historical biogeography of Fagaceae: tracking the tertiary history of temperate and subtropical forests of the Northern Hemisphere. Int. J. Plant Sci. 162:S77–S93. [Google Scholar]
- Mao K., Milne R.I., Zhang L., Peng Y., Liu J., Thomas P., Mill R.R., Renner, S.S.. 2012. Distribution of living Cupressaceae reflects the breakup of Pangea. Proc. Natl. Acad. Sci. USA 109:7793–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke N.J. 2013a. BioGeoBEARS: BioGeography with Bayesian (and Likelihood) Evolutionary Analysis in R Scripts. R package, version 0.2.1. 2013. Available from: http://CRAN.R-project.org/package=BioGeoBEARS.
- Matzke N.J. 2013b. Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 5:242–248. [Google Scholar]
- Meseguer A.S., Aldasoro J.J., Sanmartin I. 2013. Bayesian inference of phylogeny, morphology and range evolution reveals a complex evolutionary history in St. John’s wort (Hypericum). Mol. Phylogenet. Evol. 67:379–403. [DOI] [PubMed] [Google Scholar]
- Meseguer A.S., Lobo J.M., Ree R., Beerling D.J., Sanmartín I. 2015. Integrating fossils, phylogenies, and niche models into biogeography to reveal ancient evolutionary history: the case of Hypericum (Hypericaceae). Syst. Biol. 64:215–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer A.S., Lobo J.M., Cornuault J., Beerling D., Ruhfel B.R., Davis C.C., Jousselin E., Sanmartín I. 2018. Reconstructing deep-time palaeoclimate legacies in the clusioid Malpighiales unveils their role in the evolution and extinction of the boreotropical flora. Glob. Ecol. Biogeogr. 27:616–628. [Google Scholar]
- Miller M., Pfeiffer W., Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Gateway Computing Environments Workshop (GCE). p.1–8. Available from: https://www.phylo.org.
- Milne R.I., Abbott R.J. 2002. The origin and evolution of tertiary relict floras. Adv. Bot. Res. 38:281–314. [Google Scholar]
- Mosbrugger V., Utescher T. 1997. The coexistence approach–a method for quantitative reconstructions of tertiary terrestrial palaeoclimate data using plant fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 134:61–86. [Google Scholar]
- Mu X.-Y., Tong L., Sun M., Zhu Y.-X., Wen J., Lin Q.-W., Liu B. 2020. Phylogeny and divergence time estimation of the walnut family (Juglandaceae) based on nuclear RAD-Seq and chloroplast genome data. Mol. Phylogenet. Evol. 147:106802. [DOI] [PubMed] [Google Scholar]
- Nauheimer L., Metzler D., Renner, S.S. 2012. Global history of the ancient monocot family Araceae inferred with models accounting for past continental positions and previous ranges based on fossils. New Phytol. 195:938–950. [DOI] [PubMed] [Google Scholar]
- Nie Z.-L., Sun H., Li H., Wen J. 2006. Intercontinental biogeography of subfamily Orontioideae (Symplocarpus, Lysichiton, and Orontium) of Araceae in eastern Asia and North America. Mol. Phylogenet. Evol. 40:155–165. [DOI] [PubMed] [Google Scholar]
- Pennell M.W., Eastman J.M., Slater G.J., Brown J.W., Uyeda J.C., FitzJohn R.G., Alfaro M.E., Harmon L.J. 2014. geiger v2. 0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics 30:2216–2218. [DOI] [PubMed] [Google Scholar]
- Qian H., Ricklefs R.E. 2000. Large-scale processes and the Asian bias in species diversity of temperate plants. Nature 407: 180–182. [DOI] [PubMed] [Google Scholar]
- Qian H., Ricklefs R.E. 2001. Diversity of temperate plants in east Asia. Nature 413:130–130. [DOI] [PubMed] [Google Scholar]
- Qian H., Ricklefs R.E. 2004. Geographical distribution and ecological conservatism of disjunct genera of vascular plants in eastern Asia and eastern North America. J. Ecol. 92:253–265. [Google Scholar]
- Quintero I., Wiens J.J. 2013. Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol. Lett. 16:1095–1103. [DOI] [PubMed] [Google Scholar]
- Rambaut A., Drummond A.J., Xie D., Baele G., Suchard M.A. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray N., Adams J. 2001. A GIS-based vegetation map of the world at the last glacial maximum (25,000–15,000 BP). Internet Archaeol. 11:37–38. [Google Scholar]
- Ree R.H., Moore B.R., Webb C.O., Donoghue M.J. 2005. A likelihood framework for inferring the evolution of geographic range on phylogenetic trees. Evolution 59:2299–2311. [PubMed] [Google Scholar]
- Ree R.H., Smith S.A. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 57:4–14. [DOI] [PubMed] [Google Scholar]
- Rolland J., Silvestro D., Schluter D., Guisan A., Broennimann O., Salamin N. 2018. The impact of endothermy on the climatic niche evolution and the distribution of vertebrate diversity. Nat. Ecol. Evol. 2:459–464. [DOI] [PubMed] [Google Scholar]
- Schemske D.W., Mittelbach G.G., Cornell H.V., Sobel J.M., Roy K. 2009. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40:245–269. [Google Scholar]
- Schnitzler J., Theis C., Polly P.D., Eronen J.T. 2017. Fossils matter–understanding modes and rates of trait evolution in Musteloidea (Carnivora). Evol. Ecol. Res. 18:187–200. [Google Scholar]
- Silvestro D., Tejedor M.F., Serrano-Serrano M.L., Loiseau O., Rossier V., Rolland J., Zizka A., Höhna S., Antonelli A., Salamin N. 2019. Early arrival and climatically-linked geographic expansion of New World monkeys from tiny African ancestors. Syst. Biol. 68:78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater G.J., Harmon L.J., Alfaro M.E. 2012. Integrating fossils with molecular phylogenies improves inference of trait evolution. Evolution 66:3931–3944. [DOI] [PubMed] [Google Scholar]
- Slater G.J., Harmon L.J. 2013. Unifying fossils and phylogenies for comparative analyses of diversification and trait evolution. Methods Ecol. Evol. 4:699–702. [Google Scholar]
- Slater G.J., Goldbogen J.A., Pyenson N.D. 2017. Independent evolution of baleen whale gigantism linked to Plio-Pleistocene ocean dynamics. Proc. R. Soc. B 284:20170546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.G., Fragnière Y., Meng H.H., Li Y., Bétrisey S., Corrales A., Manchester S., Deng M., Jasiñska A.K., Vãn Sâm H. 2019. Global biogeographic synthesis and priority conservation regions of the relict tree family Juglandaceae. J. Biogeogr. 47:643–657. [Google Scholar]
- Song Y.G., Li Y., Meng H.H., Fragnière Y., Ge B.J., Sakio H., Yousefzadeh H, Bétrisey S, Kozlowski G. 2020. Phylogeny, taxonomy, and biogeography of Pterocarya (Juglandaceae). Plants 9:1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler T. 2015. TreeSim: simulating phylogenetic trees. R package version, 2. Available from: https://cran.r-project.org/src/contrib/Archive/TreeSim/.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post- analysis of large phylogenies. Bioinformatics 30:1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford A.M., Harden R., Parks C.R. 2000. Phylogeny and biogeography of Juglans (Juglandaceae) based on matK and ITS sequence data. Am. J. Bot. 87:872–882. [PubMed] [Google Scholar]
- Svenning J.C., Eiserhardt W.L., Normand S., Ordonez A., Sandel B. 2015. The influence of paleoclimate on present-day patterns in biodiversity and ecosystems. Annu. Rev. Ecol. Evol. Syst. 46:551–572. [Google Scholar]
- Thuiller W., Lavorel S., Araújo M.B. 2005. Niche properties and geographical extent as predictors of species sensitivity to climate change. Glob. Ecol. Biogeogr. 14:347–357. [Google Scholar]
- Tiffney B.H. 1985a. The Eocene North Atlantic land bridge: its importance in tertiary and modern phytogeography of the Northern Hemisphere. J. Arnold Arbor. 66:243–273. [Google Scholar]
- Tiffney B.H. 1985b. Perspectives on the origin of the floristic similarity between eastern Asia and eastern North America. J. Arnold Arbor. 66:73–94. [Google Scholar]
- Tiffney B.H. 2000. Geographic and climatic influence on the cretaceous and tertiary history of Euramerican floristic similarity. Acta Univ. Carol. Geol. 44:5–16. [Google Scholar]
- Tiffney B.H., Manchester S.R. 2001. The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the Northern Hemisphere Tertiary. Int. J. Plant Sci. 162:S3–S17. [Google Scholar]
- Utescher T., Bruch A., Erdei B., François L., Ivanov D., Jacques F., Kern A., Mosbrugger V., Spicer R. 2014. The coexistence approach–theoretical background and practical considerations of using plant fossils for climate quantification. Palaeogeogr. Palaeoclimatol. Palaeoecol. 410:58–73. [Google Scholar]
- Wang Q., Manchester S.R., Li C., Geng B. 2010. Fruits and leaves of Ulmus from the Paleogene of Fushun, northeastern China. Int. J. Plant Sci. 171:221–226. [Google Scholar]
- Wang W., Chen Z.-D., Liu Y., Li R.-Q., Li J.-H. 2007. Phylogenetic and biogeographic diversification of Berberidaceae in the northern hemisphere. Syst. Bot. 32:731–742. [Google Scholar]
- Wang, Y., Fritsch, P.W., Shi, S., Almeda, F., Cruz, B.C., Kelly, L.M. 2004. Phylogeny and infrageneric classification of Symplocos (Symplocaceae) inferred from DNA sequence data. Am. J. Bot. 91:1901–1914. [DOI] [PubMed] [Google Scholar]
- Wen J. 1999. Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Ann. Rev. Ecol. Syst. 30:421–455. [Google Scholar]
- Wen J. 2001. Evolution of eastern Asian–eastern North American biogeographic disjunctions: a few additional issues. Int. J. Plant Sci. 162:S117–S122. [Google Scholar]
- Wen J., Ickert-Bond S., Nie Z.-L., Li R. 2010. Timing and modes of evolution of eastern Asian-North American biogeographic disjunctions in seed plants. In: Darwin’s heritage today: Proceedings of the Darwin 200 Beijing International Conference. Beijing: Higher Education Press.p. 252–269. [Google Scholar]
- Wen J., Nie Z.L., Ickert-Bond S.M. 2016. Intercontinental disjunctions between eastern Asia and western North America in vascular plants highlight the biogeographic importance of the Bering land bridge from late Cretaceous to Neogene. J. Syst. Evol. 54:469–490. [Google Scholar]
- Wiens J.J., Donoghue M.J. 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19:639–644. [DOI] [PubMed] [Google Scholar]
- Wolfe J.A. 1975. Some aspects of plant geography of the Northern Hemisphere during the late Cretaceous and Tertiary. Ann. Mo. Bot. Gard. pp.264–279. [Google Scholar]
- Wood H.M., Matzke N.J., Gillespie R.G., Griswold C.E. 2013. Treating fossils as terminal taxa in divergence time estimation reveals ancient vicariance patterns in the palpimanoid spiders. Syst. Biol. 62:264–284. [DOI] [PubMed] [Google Scholar]
- Xiang Q.-Y., Soltis D.E. 2001. Dispersal-vicariance analyses of intercontinental disjuncts: historical biogeographical implications for angiosperms in the Northern Hemisphere. Int. J. Plant Sci. 162:S29–S39. [Google Scholar]
- Xiang X., Xiang K., Ortiz R.D.C., Jabbour F., Wang W. 2019. Integrating palaeontological and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamelidaceae. J. Biogeogr. 46:2622–2631. [Google Scholar]
- Xing Y., Gandolfo M.A., Onstein R.E., Cantrill D.J., Jacobs B.F., Jordan G.J., Lee D.E., Popova S., Srivastava R., Su T. 2016. Testing the biases in the rich Cenozoic angiosperm macrofossil record. Int. J. Plant Sci. 177:371–388. [Google Scholar]
- Yang T., Lu L.-M., Wang W., Li J.-H., Manchester S.R., Wen J., Chen Z.-D. 2018. Boreotropical range expansion and long-distance dispersal explain two amphi-Pacific tropical disjunctions in Sabiaceae. Mol. Phylogenet. Evol. 124:181–191. [DOI] [PubMed] [Google Scholar]
- Zachos J., Pagani M., Sloan L., Thomas E., Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292:686–693. [DOI] [PubMed] [Google Scholar]
- Zhang B.W., Xu L.L., Li N., Yan P.C., Jiang X.H., Woeste K.E., Lin K., Renner S.S., Zhang D.Y., Bai W.N. 2019. Phylogenomics reveals an ancient hybrid origin of the Persian walnut. Mol. Biol. Evol. 36:2451–2461. [DOI] [PubMed] [Google Scholar]
- Zhang C., Stadler T., Klopfstein S., Heath T. A., Ronquist F. 2016. Total-evidence dating under the fossilized birth–death process. Syst. Biol. 65:228–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.B., Li R.Q., Xiang X.G., Manchester S.R., Lin L., Wang W., Wen J., Chen Z.D. 2013. Integrated fossil and molecular data reveal the biogeographic diversification of the eastern Asian-eastern North American disjunct hickory genus (Carya Nutt.). PLoS one 8:e70449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-Q., Meng S.-Y., Allen G.A., Wen J., Rao G.-Y. 2014. Rapid radiation and dispersal out of the Qinghai-Tibetan Plateau of an alpine plant lineage Rhodiola (Crassulaceae). Mol. Phylogenet. Evol. 77:147–158. [DOI] [PubMed] [Google Scholar]
- Zhang M.L., Wang L., Lei Y., Sanderson S.C. 2017. Cenozoic evolutionary history of Zelkova (Ulmaceae), evidenced from ITS, trnL–trnF, psbA–trnH, and rbcL. Tree Genet. Genomes 13:101. [Google Scholar]
- Zhu W.-D., Nie Z.-L., Wen J., Sun H. 2013. Molecular phylogeny and biogeography of Astilbe (Saxifragaceae) in Asia and eastern North America. Bot. J. Linn. Soc. 171:377–394. [Google Scholar]