Abstract
The diversity of cognitive deficits and neuropathological processes associated with dementias has encouraged divergence in pathophysiological explanations of disease. Here, we review an alternative framework that emphasizes convergent critical features of cognitive pathophysiology. Rather than the loss of ‘memory centres’ or ‘language centres’, or singular neurotransmitter systems, cognitive deficits are interpreted in terms of aberrant predictive coding in hierarchical neural networks. This builds on advances in normative accounts of brain function, specifically the Bayesian integration of beliefs and sensory evidence in which hierarchical predictions and prediction errors underlie memory, perception, speech and behaviour. We describe how analogous impairments in predictive coding in parallel neurocognitive systems can generate diverse clinical phenomena, including the characteristics of dementias. The review presents evidence from behavioural and neurophysiological studies of perception, language, memory and decision-making. The reformulation of cognitive deficits in terms of predictive coding has several advantages. It brings diverse clinical phenomena into a common framework; it aligns cognitive and movement disorders; and it makes specific predictions on cognitive physiology that support translational and experimental medicine studies. The insights into complex human cognitive disorders from the predictive coding framework may therefore also inform future therapeutic strategies.
Keywords: predictive coding, dementia, top-down processing, prediction, neurodegeneration
Kocagoncu et al. propose an alternative framework for interpreting the cognitive deficits observed in dementia. They argue that deficits should be considered in terms of aberrant predictive coding in hierarchical neural networks, rather than as reflecting the loss of functional hubs or singular neurotransmitter systems.
Introduction
Cognitive deficits in neurodegenerative diseases have often been characterized as the loss of core functional modules in distinct brain regions, or specific networks, each serving functionally specialized cognitive systems such as memory, language comprehension or executive function. This approach emphasizes the functional differences between disorders linked to functional anatomical susceptibility and network vulnerability.1 Alongside these functional anatomical differences that contribute to distinct phenotypes, preclinical models and clinical studies suggest convergence in important aspects of the pathophysiology of different dementias, with commonalities for example in terms of loss of synapses, synaptic plasticity and major neurotransmitters.2 The relative contributions of toxic misfolded protein aggregates, neuroinflammation and proteostasis to synaptic impairment vary across dementias, but their physiological consequences overlap, with potential convergence on a core cognitive mechanisms of predictive coding. Here we propose a re-evaluation of the diversity of cognitive features in dementia, in terms of impairments in predictive coding, leading to a trans-diagnostic neuro-computational model that may aid the development of novel therapeutic strategies.
Predictive coding is a core feature of brain function, implementing generative models that ‘explain’ sensory inputs via hierarchical beliefs about the world.3-6 In this review, we reassess clinical deficits in terms of the disruption of predictive coding in precisely tuned neural hierarchies engaged in prediction, prediction error and inference. The predictive coding account of normative brain function integrates cognitive and computational neuroscience to explain perception and action. The central tenet is that the brain acts as an active inference machine that learns statistical regularities of the external world (Box 1) and generates predictions to increase the efficiency of information processing and understanding of the sensorium.3–6
Box 1 Predictive coding and hierarchical networks
Predictive coding is a process by which the brain updates a model of the environment, to explain sensory inputs. The process applies hierarchically over increasingly abstract causes, and over time, forming the basis of diverse cognitive and behavioural functions. It rests the premise that perception is a probabilistic inference. Complex and abstract beliefs are represented in higher levels (e.g. on semantics and social norms) and direct sensory inputs at lower levels. Based on learned statistical dependencies, each level predicts the activity in the level below (‘feedback’). A mismatch between the prediction and the sensory input leads to a prediction error, which is propagated back up the hierarchy (‘feedforward’). The forward and backward connections convey prediction errors and predictions, respectively.7,8
Different biological implementations of predictive coding have been put forward at micro- and macroscopic levels,3–6 but they have multi-level hierarchies of neural circuits in common. There are different algorithmic implementations of the way in which the fit between predictions and sensory data is optimized, and the underlying model updated (e.g. linear estimation of parameters,6 Bayesian inference,9 a review of models).10 There are also alternatives to predictive coding, that nonetheless posit that the brain performs a probabilistic inference in hierarchical networks, and maintains a generative (i.e. explanatory) model of the environment by alternative mechanisms.11 This review does not seek to differentiate these alternative mechanisms, but focus on their commonalities, with the generation of predictions and updating them in response to prediction errors.
A critical feature of predictive coding is the estimation of uncertainty of the predictions and sensory inputs. Both the predictions and prediction errors are relayed with varying ‘precision’ (i.e. the inverse of variance, or uncertainty). This precision determines the relative weighting of the prediction error, whilst priors are updated iteratively, across all levels of the hierarchy.12,13 Precision weighting of the prediction errors is controlled by neuromodulation (Box 2) and postsynaptic gain control at the cellular level. Feedforward propagation of more precise prediction errors will have a greater impact updating beliefs represented in the higher levels (i.e. faster learning). Feedback generation of more precise predictions ‘cancels out’ incoming prediction errors, leading to stable beliefs and behaviour (i.e. slow learning). Healthy cognition requires fine tuning of this process, adjusting relative precision at upper versus lower levels of the hierarchy. The impact of neurodegeneration on the neural mechanisms that regulate precision, and govern the representations within each level, explain diverse cognitive and behavioural phenomena in dementia, and raise new hypotheses about candidate treatment strategies.
Box 2 Precision changes in dementia and neurotransmitters
‘Precision’ represents the level of certainty, and describes the confidence attributed to prediction errors at each level of the cortical hierarchy.3,5 For example, in noisy settings with high levels of uncertainty (e.g. driving on a foggy day, talking during a concert), precision of the sensory prediction errors is reduced while the precision at the higher levels is relatively increased. Neurotransmitters such as acetylcholine,14–18 glutamate,19–21 GABA22–27 and norepinephrine18 have been shown to regulate prediction errors and their precision across different cortical hierarchies. Impairments in the neurotransmitter mediated precision weighting gives rise to diverse clinical representations in dementia depending on the level and the functional domain of the cortical hierarchy where the mechanistic impairment occurs.
An example of the abnormally high precision in the lower levels of the hierarchy comes from Parkinsonian disorders. Akinesia, the poverty of movement, can arise from reduced precision in the higher order sensorimotor prediction errors, and an over-reliance on sensory evidence (Fig. 1D).12,28 Akinesia can be partially improved using the peripheral vibration devices that increase the uncertainty of sensory evidence, thereby reducing the precision.29,30 However precision changes are more commonly observed at the higher levels of the hierarchy. In normal ageing, impairments in vision and hearing, lead to the adaptation of precision weights across the cortical hierarchy,31 where the reliance on ‘inaccurate’ sensory evidence is reduced, and to balance, precision at higher cortical levels are boosted. Similarly, in Parkinson’s disease and Lewy body dementia, in the visual cortical hierarchy, the precision at the higher level prediction errors are up-weighted, albeit abnormally, giving rise to visual hallucinations.32–36
A key modulator of precision is acetylcholine that suppresses prediction errors at the higher order and regulates precision of the sensory prediction errors.14–17 Cholinergic loss can affect ascending sensory precision even in the absence of atrophy. Impaired mismatch negativity responses in Alzheimer’s disease, indicating unsuccessful sensory learning, is partially explained by the widespread degeneration of cholinergic projections.37,38 Similarly, patients with Lewy body dementia who have more severe degeneration of their cholinergic pathways experience more visual hallucinations.39–41 Cholinesterase inhibitors that mediate sensory precision, can amplify the amplitude of the mismatch response in patients with Alzheimer’s disease,42 and alleviate hallucinations in Lewy body dementia.43 Acetylcholine regulates inhibitory activity by suppressing or inactivating GABAergic interneurons.25–27 While slower neurotransmitters like acetylcholine are proposed to compute the precision, faster neurotransmitters like GABA are thought to encode the prediction errors.44 Patients with behavioural variant frontotemporal dementia show reduced mismatch negativity response, as a product of impaired inhibitory connections and reduced GABA concentrations in the frontal cortex.23,24 These patients show reduced precision in higher levels of the auditory hierarchy, leading to errors in encoding of conditional expectations at lower levels.22
The predictive coding account provides a common neurobiological framework to describe diverse cognitive, perceptual and behavioural phenomena. For example, there is evidence for predictive coding in vision,45,46 rhythm perception,47,48 auditory processing,49–53 reward and preferences54 and action control.55,56 The representation of predictions, prediction errors and precision in each system depends on a fine-tuned cortical hierarchy, with laminar-specific connectivity and balanced excitatory-inhibitory neurochemistry (Fig. 1A). Deficits in predictive coding have been proposed to cause domain-specific and domain-general cognitive impairments in neuropsychiatric disorders as diverse as psychosis,57,58 autism59,60 and alien limb.61
Figure 1.
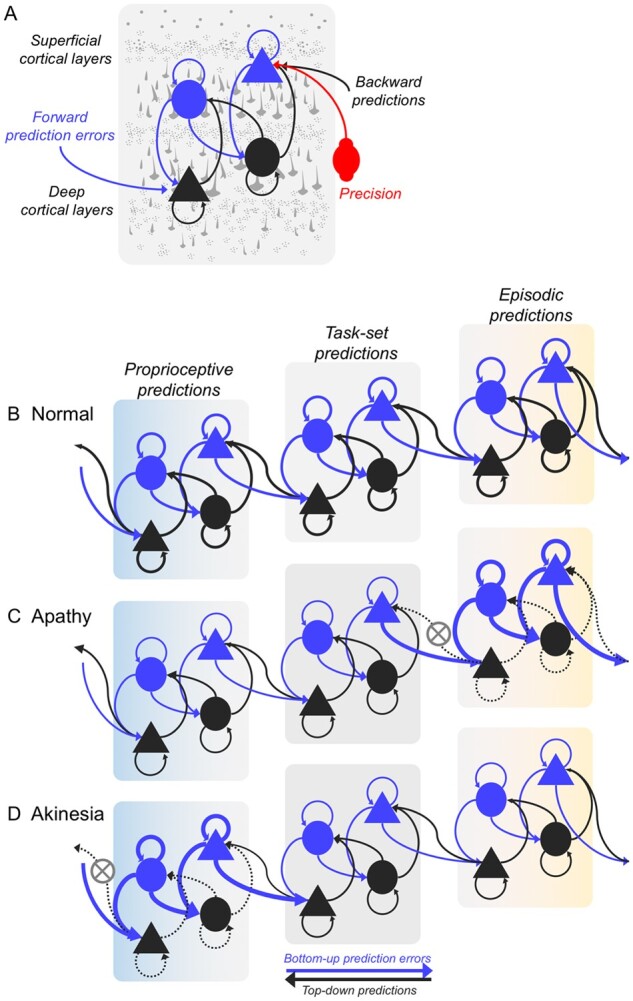
Predictive coding mechanism within the hierarchical brain network. (A) Schematic illustration of the predictive coding mechanism in a single cortical region at one layer in the hierarchy. Top-down predictions are conveyed via the backward connections (black arrows) from state representation units (black nodes) in deep cortical layers. The predictions are compared with conditional expectations at the lower level in the hierarchy by the error units in the superficial cortical layers (blue nodes) to produce prediction errors, which are passed bottom-up (blue arrows) to the higher level to update the predictions. Triangles and circles represent pyramidal neurons and inhibitory interneurons respectively. Precision weighting (red) regulates the postsynaptic gain of the error units, e.g. via neuromodulation. Panels B–D illustrate three layers of a hierarchical network of the behavioural/motor system, with three cortical layers from left (light blue) to right (yellow). Each layer of the hierarchy makes predictions relayed in a top-down fashion. Higher layers of the network make episodic predictions that are multimodal, abstract and span across a longer timescale (e.g. ‘that the city marathon is happening’). Intermediate layers represent medium-term, task-set or context specific predictions (e.g. ‘I am running, and see supporters and water stands’). Lower layers make transient, proprioceptive predictions on the immediate consequences of running action (e.g. ‘position of my limbs’). (B) Healthy state of the hierarchy with optimal control in which top-down predictions are matched by sensory inputs, minimizing prediction errors at each layer. In apathy and akinesia, behavioural impairments arise from a mismatch between the strength of predictions and prediction errors. (C) In apathy, top-down predictions at the higher level are represented with insufficient precision, and are therefore overwhelmed by bottom-up prediction errors from the intermediate hierarchical level. Therefore, high-level priors, representing abstract goals and desires, fail to be translated into specific proprioceptive predictions for movement, and as such there is a loss of goal-directed behaviour. (D) In contrast, with akinesia there is a poverty of movement because predictions at the lowest hierarchical level fail to suppress proprioceptive prediction errors. Even though the absence of behaviour may manifest similarly in apathy and akinesia, the underlying mechanism of impairment arises from predictive mismatch in different levels of the hierarchical network.
We propose that dementias’ effects on memory, perception, language and action control may also arise from a change in predictive coding. In particular, we set out how the effect of neurodegeneration on the ‘precision’ of predictions and prediction error can impair perception, learning and complex behaviours. The symptoms arising from a change in predictive coding are a function of the neural networks that are selectively vulnerable to each specific molecular pathology. The predictive coding account of dementia is therefore not an alternative to network specificity models,1 but instead augments these models by describing the homologous changes in predictive coding arising within each network.
We start with the basic processes of perception and action to introduce the principles predictive coding and the direct evidence is strongest. We then consider higher cognitive disorders, of amnesia and aphasia, and neuropharmacological factors, with examples drawn from studies of Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia and dementia with Lewy bodies.
Perception
In perceiving our environment, one makes use of prior knowledge and context to predict sensory inputs. For example, in a complex auditory scene such as a noisy cocktail party, prior knowledge or experience facilitates the parsing of constituent objects (or speakers) in time and space, making it easy to recognize one’s own name (‘the cocktail party effect’).62 Top-down predictions based on prior experience of the speakers, their language and the topic, facilitate this segregation.63 In vision, context-based predictions likewise aid rapid object recognition under both normal and challenging conditions.4,64 The use of auditory predictions is largely preserved in normal ageing. Indeed, people may become more dependent on their predictions and perceptually less sensitive to the sensorium with age, as the precision of the higher-order prediction errors increases relative to the precision sensory evidence.14,28
This balance is disrupted in mild cognitive impairment and dementia, with degeneration of temporo-parietal cortex from Alzheimer’s disease.65 Accordingly, patients develop greater difficulty following conversations in the presence of background noise, show impairments in segregating, tracking and grouping auditory objects that evolve over time66 and in perceiving sound location and motion.65 They become worse even at automatic prediction of repetitive stimuli and fail to generate a prediction error following unexpected sensory events. This failure to generate a prediction error with Alzheimer’s disease and other dementias is readily seen in the reduced ‘mismatch negativity responses’ in oddball tasks.37,67–69 Alzheimer’s disease similarly impairs higher order precepts such as melodic contours.70 Even otherwise healthy APOE4 carriers (i.e. at an elevated risk of developing Alzheimer’s disease) show impairments in detecting auditory targets using contextual information.71
In the visual domain, hallucinations and illusions commonly occur with cortical Lewy body pathology, in Parkinson’s disease dementia and dementia with Lewy bodies. The perceptual content is commonly influenced by the immediate environment or autobiographical memories, with pareidolic experiences in ambiguous scenes,72 or the perception of familiar people or pets even if known to have died.73 The hallucinations are typically visually complex and familiar.14,15,74 This can be understood as a result of abnormal up-weighting of beliefs (i.e. more precise priors) that establish overly precise predictions relative to down-weighting (i.e. less precise) visual sensory evidence.32–34,58 Note that it is not just the absolute precision that matters, but the relative precision between upper and lower levels in a hierarchy. Note too that the symptoms depend on the anatomical distribution of the network that represents the cognitive hierarchy. The medial temporal and medial prefrontal areas are implicated in the cognitive hierarchy for such misperceptions,75 with hallucinations associated with abnormal activity and connectivity among lower visual cortical regions.35,76–83 The loss of cholinergic modulation of the precision of neural representations is a candidate cause, even in the absence of significant atrophy. Such cholinergic loss reduces the precision of feed-forward prediction errors relative to the precision of feedback predictions from higher level priors.14–17 This accords with the observation that patients who have more severe degeneration of their cholinergic pathways experience more visual hallucinations,39–41 and symptoms are alleviated with cholinesterase inhibitors.43
Action, apathy and behavioural disorder
As Adams et al.84 highlight, perceptual and motor systems are not separate entities, but operate as a single ‘inference machine’ that serves to predict sensory input in all sensory domains and intermediate inferences on the causes of the sensory inputs. The concept of ‘active inference’ posits that prediction errors can be reduced by actively changing sensory inputs through movement. Active inference uses hierarchical predictive coding, with direct evidence coming from the physiology of motor control (Fig. 1A).56
The failure to attenuate proprioceptive prediction errors in the lower levels of a behavioural hierarchy leads to akinesia (Fig. 1D),85 in the context of neurodegenerative movement disorders like Parkinson’s disease. Over-precise priors (in upper levels of a motor control hierarchy, represented by premotor and prefrontal cortex) also explain the alien limb syndrome (that one’s own limb is moving without intention or volition). Specifically, alien limb syndrome is associated with disrupted information flow between medial areas (supplementary motor area) that encode precision of proprioceptive predictions to the lateral pre-motor areas which encode action outcomes.61
There is empirical evidence for active inference at the lower level of the cognitive hierarchy for behaviour, expressed as specific actions. For example, there is ubiquitous ‘sensorimotor attenuation’ in health across the lifespan: a transient down-weighting of the predicted sensory consequences of actions, observed in 98% of healthy adults (Fig. 1B).31 Attenuation facilitates movement and provides a sense of agency.86 In healthy ageing, there is greater reliance on predictions arising from greater precision of prior beliefs, and less on the sensorium.31 In neurodegenerative parkinsonism, deficits in sensorimotor predictions (reduced precision) results in an over-reliance on sensory evidence and poverty of movement.12,28 Such deficits in sensorimotor predictions are linked to disease severity of corticobasal syndromes,28,86 and to atrophy and white matter connectivity of the pre-supplementary motor area—a cortical region that lies at the intermediate level of a spatially embedded cognitive hierarchy for behaviour, between motor cortex and prefrontal cortex.28,40
There are therapeutic implications of active inference. For example, akinesia can be improved by high frequency peripheral vibration which reduces the precision of sensory evidence and increasing the relative precision of sensorimotor predictions (cf. Sweeney et al.29 and Macerollo et al.30). This is in line with suggestions that high-frequency vibration attenuates proprioceptive feedback allowing for greater top-down control.87 A physiological correlate is the decrease of power of beta oscillations at the onset of the vibration, preceding the improved movement. Similar beta desynchronization13,88,89 is essential for movement planning and initiation.90 In bradykinetic disorders, beta power is elevated,91–94 while dopaminergic treatment in Parkinson’s disease enhances beta desynchronization,93,95 alleviates akinesia, and increases sensorimotor attenuation.28,96 Under active inference, beta power may index somatosensory precision and therefore mediate sensorimotor attenuation, modulated by dopamine.28,96
A lack of behaviour can also be caused by apathy, without akinesia. Apathy is common in dementia, including Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia and vascular dementia.97–99 We propose that apathy arises from deficits in the precision of the higher order predictions of goal-states and context rather than proprioception (Fig. 1C). This is analogous to the causes of akinesia, but at a higher level of a cognitive hierarchy for goal-directed behaviour.100 When the relative precision of the goal prior is low, it will fail to propagate through the hierarchy down to effector mechanisms, and the outcome is a lack of behaviour.57,85,101 The failure of active inference thereby shifts from lack of movement (akinesia) to a lack of goal-directed behaviour (apathy) according to the level of the hierarchy in which precision is affected by the cellular and pharmacological effects of each molecular pathology.
In healthy controls, trait apathy is associated with lower precision of predictions about action outcomes.100 In dementia-related apathy, there is limited direct evidence for higher variance of priors, but indirect support comes from the failure to modulate prefrontal cortical beta oscillations in goal-directed tasks and the correlation between challenging everyday behaviours and beta-power (specifically, the failure of task-related beta-desynchronization).102 We suggest an anatomical correlate of goal priors lies in anterior cingulate and medial prefrontal cortex, with loss of connectivity to motor cortex and the striatum.86,103–105
Disinhibited and impulsive behaviours are common to many dementias,98,106,107 with a predisposition to act out of context, prematurely, or on the basis of little evidence.108 Such behaviours would be explained by impaired precision of high-order predictions which diminish the confidence weighting on the choices or behavioural policies available. This can lead to ‘jumping to conclusions’.109 Dopamine dysregulation may explain some types of impulsivity (e.g. Parkinson’s disease110), but other neurotransmitters such as noradrenaline, GABA, and glutamate, modulate behavioural control and are also deficient in many neurodegenerative disorders.2 For example, noradrenaline regulates impulsive behaviour via widespread projections from the locus coeruleus to the cortex,111–113 in response to salient cues that trigger shifts in behaviour.114 In the predictive coding framework, the locus coeruleus noradrenergic signals update predictions at higher levels mediated by fronto-striatal circuits, in response to prediction error (e.g. ‘surprise’).115 The locus coeruleus is affected by Alzheimer’s disease, Parkinson’s disease and frontotemporal lobar degeneration,111 which has led to noradrenergic treatment strategies to reduce impulsivity.116–118 In active inference terms, behaviours become impulsive and inflexible when the precision of priors is not updated in response to salient behavioural cues.
Memory and learning
Memory deficits and poor learning are prominent features of dementia, including but not limited to Alzheimer’s disease. The degeneration of the medial temporal lobe may affect memory retrieval and associative learning in part because of the disruption of predictive coding in these circuits. The hippocampus encodes expectancies of future events based on the probabilistic consequences of past events,119–121 and hippocampal activity is modulated by the predictability of the future events.122 Hippocampus not only encodes individual episodes but also the ordinal structure of events, a distributed in space, time (time in relation to internal computational demands, not an external clock) or other properties. The representation of ordinal structure may appear as encoding sequences or locations, but it can also be seen as part of a more fundamental generative model of the environment—an ‘inference machine’ engaged in predictive coding.123,124 Such a hippocampal-based hierarchy operates over multiple timescales.
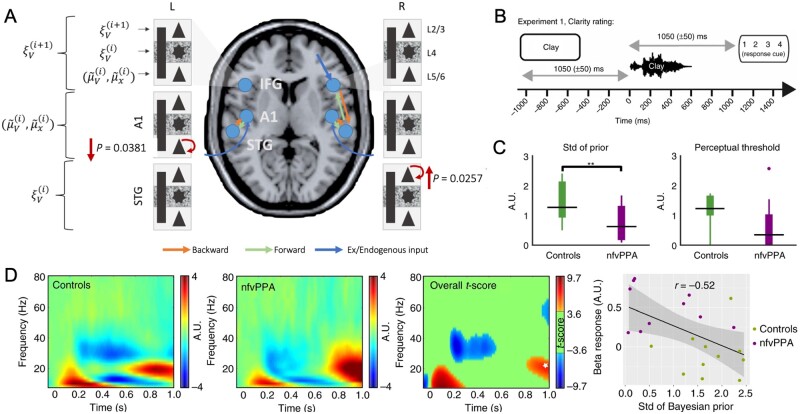
The ability to anticipate events over very short timescales is impaired by many dementias. For example, oddball tasks such as the auditory mismatch negativity paradigm have been interpreted to rely on short term ‘memory traces’ for sensory events. Such tasks have provided some of the strongest direct evidence for predictive coding.14,125–129 The mismatch response indexes the prediction error, that is fed-forward in a frontotemporal hierarchy to update predictions that are in turn fed backwards.126 The active nature of auditory predictions has been corroborated by computational and dynamic causal modelling. Simulations show that the mismatch response is an output of active cortical predictions rather than passive synaptic habituation.128 Omitted events in mismatch paradigms provide an ideal test of cortical hierarchies that actively predict events. Indeed, dynamic causal modelling of omitted events show increased connectivity from and to the prefrontal cortex similar to the connectivity changes observed for the mismatch stimuli.130 In dementia, the mismatch negativity amplitude is reduced,69,131,132 together with impaired frontotemporal connectivity (Fig. 2A).22,69,133,134 Patients with Alzheimer’s disease show larger reductions at longer inter-stimulus intervals37,67,135 in relation to reduced temporal activity and cognitive score of executive function.131,136
Figure 2.
Neurophysiological changes associated with predictive coding impairments. (A) Cortical microcircuit dynamic causal model of the mismatch negativity response in patients with behavioural variant frontotemporal dementia, compared with healthy controls. Local (intrinsic) decreases in self-modulation of the deep pyramidal cells in the primary auditory cortex (A1), and increases in self-modulation of the superficial pyramidal cells in the superior temporal gyrus, lead to failure to establish sensory predictions and thereby reduced mismatch response. (B) Illustration of the MEG paradigm used by Cope et al.,186 in which participants were presented with a written word followed by a noise vocoded spoken word that either matched or mismatched with the written word. Participants rated the clarity of the spoken words. (C) Derived parameters from Bayesian data modelling show that patients with non-fluent primary progressive aphasia (nvPPA) had more precise priors (smaller variance) than controls. A.U. = arbitrary units. (D) Induced responses between the cue offset and spoken word onset: beta power was higher in the nvPPA group after 800 ms and negatively correlated with precision of the prior expectations. A is reprinted from Shaw et al.22 with permission. B–D are reprinted from Cope et al.186 with permission.
Patients with Alzheimer’s disease have difficulty encoding and processing novel information (e.g. high rates of false recognition of novel items,137,138 reduced primacy,139,140 von Restorff effect141) associated with reduced functional connectivity between hippocampus, temporal and frontal areas.142 Asymptomatic APOE4 carriers compared to non-carriers, show reduced prediction errors to novel words, and elevated hippocampal activity to subsequently remembered words.143 In those at risk of familial Alzheimer’s disease, PSEN1 and APP mutation carriers who approach the familial age of diagnosis, show elevated blood oxygenation level-dependent response in the middle temporal gyri during novelty encoding.144 These impairments in novelty processing are consistent with impaired predictive processing in a hippocampal hierarchy. Larger prediction errors generated after encountering novel or contextually unexpected items (e.g. ‘the butcher in the office’), drive stronger episodic encoding compared to expected items (e.g. ‘the butcher in the butcher shop’).145,146 Unsuccessful learning could therefore result from smaller prediction errors arising from relatively low precision weighting of the prediction error.145,147
At the cellular level, the modulation of the precision of a hippocampal prediction error in memory tasks is dependent on both cholinergic and dopaminergic modulation of NMDA receptor plasticity14,148–151 Impaired mismatch response in Alzheimer’s disease is partially explained by the degeneration of cholinergic projections, in the presence of relatively preserved top-down propagation of predictions from intact higher level priors.136 Cholinergic agents partially restore the mismatch response in Alzheimer’s disease,42 enhancing feed-forward signalling by precision of the sensory evidence weighting.14,152 Similarly, dopamine is proposed to modulate saliency of the stimuli in hippocampus in response to novelty and facilitate encoding of the new information via its connections with the ventral tegmental area and substantia nigra.150,151,153–155 Supporting this, administration of dopamine agonists, accelerates the processing speed of novel information,156 and enhances recollection.157 GABAergic modulation of feedback predictions and feedforward prediction errors may also contribute to the impairment of predictive coding from frontotemporal lobar degeneration.23,24
Speech and language
In health, language comprehension shows remarkable speed and resistance to noisy environments. This is enabled by predictive coding at multiple levels of linguistic representation: phonological,158–160 semantic,161–166 syntactic167–169 and discourse context.170 In neurodegenerative aphasias, poor comprehension arises from the impact of lesions on the frontotemporal and temporo-parietal networks which support top-down propagation and updating of predictions. For example, people with non-fluent variant primary progressive aphasia show particular vulnerability to processing deficits and delays at the lexical level when speech inputs are degraded171,172 or ambiguous.173–175 This arises from degeneration of frontal and perisylvian cortex, with reduction of top-down control used to optimize perception and production of speech,176–179 leading to speech production deficits and agrammatism,180–182 In contrast, damage to the temporo-parietal junction leads to speech repetition deficits183,184 arising from disrupted mapping between priors for speech representations and proprioceptive articulatory predictions in the ventral motor cortex and inferior frontal cortex.84,185
Cope et al.186 showed that in the presence of intact temporal cortex, frontal lobe neurodegeneration from non-fluent variant primary progressive aphasia causes overly precise contextual priors, together with reduced frontal-to-temporal directional connectivity in the beta frequency range (Fig. 2B–D). This combination leads to delayed resolution of speech inputs by the temporal cortex, and impaired perception of degraded speech input. The reliance on overly precise priors explains the paradoxical relative advantage for patients as noise increases (in contrast to healthy adults). The patients’ speech comprehension deficit was more severe in quiet settings. Overly precise priors may also affect speech production in primary progressive aphasia: whereas delayed auditory feedback in healthy controls reduces fluency and accuracy of speech,187,188 delayed feedback does not impair fluency. This suggests a reliance on internal models of speech and relative weakness of the precision of sensory representations.189
Efficient reading requires top-down signalling from higher order language areas, to disambiguate visually confusable words.190 While damage to the left medial occipito-temporal areas causes alexia and object agnosia with spared central language abilities and orthographic knowledge,191,192 reading deficits are often more severe than object recognition deficits. Lesions of inferior frontal cortex cause auditory agnosias and pure word deafness.193,194 Woodhead et al.195 showed that whole-word training to improve reading was associated with stronger feedback connectivity from the inferior frontal gyrus to the occipital areas, and bidirectional connectivity between ventral occipito-temporal and occipital areas. This suggests stronger top-down priors aid prediction of the words in reading.
Semantic processing of words in context is similarly dependent on top-down signalling, as contextual information and prior knowledge is used to predict forthcoming words.165,196,197 The N400 is an electrophysiological index of the prediction error, reflecting the degree of mismatch between semantic priors and sensory input.198 In semantic dementia differentiation of concepts that belong to the same semantic category is impaired, such as ‘giraffe’ and ‘zebra’ (i.e. taxonomic blurring). The N400 is absent for mismatches of the same semantic category,199 suggesting that semantic priors are under-specified (i.e. imprecise). Furthermore, disambiguating meaningful objects (but not meaningless shapes) in difficult viewing conditions is also impaired,200 suggesting a domain-general deficit of top-down semantic control, thought to depend on intact connectivity within the larger fronto-temporo-parietal network.201
Conclusion
We propose a reformulation of cognitive deficits in dementia away from specific localized functional-anatomical impairments towards a generalized framework of aberrant Bayesian inference, within cortical hierarchies. Predictive coding principles can be generalized to account for multiple cognitive and perceptual impairments observed in neurodegenerative diseases, arising from diverse molecular aetiologies. The cognitive deficits and related neurophysiological abnormalities, can be understood in terms of altered precision in the normally finely-balanced feedforward and feedback pathways in cortical hierarchies. There are multiple potential cellular and molecular pathological routes to disrupt the precision of predictions and prediction errors, including localized cell loss (atrophy), and changes in acetylcholine, dopamine, and noradrenaline, that weight the importance (i.e. precision) of predictions and gain function of prediction errors. The predictive coding framework provides a unifying framework to understand the effects of these changes, in different hierarchical functional brain networks, as the basis for different dementia syndromes. It is a powerful trans-diagnostic framework that can aid better understanding of the mechanisms of disease across the lifespan and in turn facilitate new therapeutic strategies for dementia. New analytical methods enable new experimental medicine studies with techniques like dynamic causal modelling that can inform the efficacy and mechanism of candidate therapies. We therefore hope that this Update on predictive coding stimulates progress towards a new form of precision medicine, defined in terms of the precise cognitive and physiological consequences of disease.
Funding
E.K. is funded by the Dementias Platform UK and Alzheimer’s Research UK (RG94383/RG89702). J.B.R. is supported by the Wellcome Trust (103838) and Medical Research Council (SUAG/051 G101400) and the National Institute for Health Research Cambridge Biomedical Research Centre. L.E.H. is funded by the Wellcome Trust (103838). A.K.-G. is funded by the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant (798971).
Competing interests
J.B.R. received research funding from Janssen, Lilly, AstraZeneca and GSK in the past 3 years, and is acting as a consultant for SV Health, Astex, Biogen, UCB, Curasen, Asceneuron, and Alzheimer Research UK, none related to the current work.
References
- 1. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD.. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murley AG, Rowe JB.. Neurotransmitter deficits from frontotemporal lobar degeneration. Brain. 2018;141(5):1263–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friston KJ. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11(7):280–289. [DOI] [PubMed] [Google Scholar]
- 5. Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36(3):181–204. [DOI] [PubMed] [Google Scholar]
- 6. Rao RP, Ballard DH.. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2(1):79–87. [DOI] [PubMed] [Google Scholar]
- 7. Barlow HB. What is the computational goal of the neocortex? In: Koch C, Davis JL, eds. Large-scale neuronal theories of the brain. MIT Press; 1994:1–22. [Google Scholar]
- 8. Mumford D. On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biol Cybern. 1992;66(3):241–251. [DOI] [PubMed] [Google Scholar]
- 9. Friston K, Kiebel S.. Predictive coding under the free-energy principle. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spratling MW. A review of predictive coding algorithms. Brain Cogn. 2017;112:92–97. [DOI] [PubMed] [Google Scholar]
- 11. Aitchison L, Lengyel M.. With or without you: Predictive coding and Bayesian inference in the brain. Curr Opin Neurobiol. 2017;46:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown H, Adams RA, Parees I, Edwards M, Friston K.. Active inference, sensory attenuation and illusions. Cogn Process. 2013;14(4):411–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmer CE, Auksztulewicz R, Ondobaka S, Kilner JM.. Sensorimotor beta power reflects the precision-weighting afforded to sensory prediction errors. NeuroImage. 2019;200:59–71. [DOI] [PubMed] [Google Scholar]
- 14. Moran RJ, Campo P, Symmonds M, Stephan KE, Dolan RJ, Friston KJ.. Free energy, precision and learning: The role of cholinergic neuromodulation. Randomized Controlled Trial Research Support, Non-U.S. Gov’t. J Neurosci. 2013;33(19):8227–8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collerton D, Perry E, McKeith I.. Why people see things that are not there: A novel perception and attention deficit model for recurrent complex visual hallucinations. Behav Brain Sci. 2005;28(6):737–757; discussion 757–794. [DOI] [PubMed] [Google Scholar]
- 16. O'Callaghan C, Kveraga K, Shine JM, Adams RB, Bar M.. Predictions penetrate perception: Converging insights from brain, behaviour and disorder. Conscious Cogn. 2017;47:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diederich NJ, Goetz CG, Stebbins GT.. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: Focused review and a new integrative model. Mov Disord. 2005;20(2):130–140. [DOI] [PubMed] [Google Scholar]
- 18. Yu AJ, Dayan P.. Uncertainty, neuromodulation, and attention. Neuron. 2005;46(4):681–692. [DOI] [PubMed] [Google Scholar]
- 19. Rosch RE, Auksztulewicz R, Leung PD, Friston KJ, Baldeweg T.. Selective prefrontal disinhibition in a roving auditory oddball paradigm under N-methyl-D-aspartate receptor blockade. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(2):140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosburg T, Kreitschmann-Andermahr I.. The effects of ketamine on the mismatch negativity (MMN) in humans—a meta-analysis. Clin Neurophysiol. 2016;127(2):1387–1394. [DOI] [PubMed] [Google Scholar]
- 21. Schmidt A, Diaconescu AO, Kometer M, Friston KJ, Stephan KE, Vollenweider FX.. Modeling ketamine effects on synaptic plasticity during the mismatch negativity. Cereb Cortex. 2013;23(10):2394–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shaw AD, Hughes LE, Moran R, Coyle-Gilchrist I, Rittman T, Rowe JB.. In vivo assay of cortical microcircuitry in frontotemporal dementia: A platform for experimental medicine studies. Cereb Cortex. 2021;31(3):1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adams NE, Hughes LE, Phillips HN, et al. GABA-ergic dynamics in human frontotemporal networks confirmed by pharmaco-magnetoencephalography. J Neurosci. 2020;40(8):1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams N, Hughes L, Rouse M, et al. GABAergic cortical network physiology in frontotempoal lobar degeneration. Brain. 2021;144(7):2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiang Z, Huguenard JR, Prince DA.. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281(5379):985–988. [DOI] [PubMed] [Google Scholar]
- 26. Buia C, Tiesinga P.. Attentional modulation of firing rate and synchrony in a model cortical network. J Comput Neurosci. 2006;20(3):247–264. [DOI] [PubMed] [Google Scholar]
- 27. Baldeweg T, Wong D, Stephan KE.. Nicotinic modulation of human auditory sensory memory: Evidence from mismatch negativity potentials. Int J Psychophysiol. 2006;59(1):49–58. [DOI] [PubMed] [Google Scholar]
- 28. Wolpe N, Zhang J, Nombela C, et al. ; Cam-CAN. Sensory attenuation in Parkinson’s disease is related to disease severity and dopamine dose. Sci Rep. 2018;8(1):15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sweeney D, Quinlan L, Browne P, Richardson M, Meskell P, ÓLaighin G.. A technological review of wearable cueing devices addressing freezing of gait in Parkinson’s disease. Sensors (Basel). 2019;19(6):1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Macerollo A, Palmer C, Foltynie T, et al. High-frequency peripheral vibration decreases completion time on a number of motor tasks. Eur J Neurosci. 2018;48(2):1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolpe N, Ingram JN, Tsvetanov KA, et al. ; Cam-CAN. Ageing increases reliance on sensorimotor prediction through structural and functional differences in frontostriatal circuits. Nat Commun. 2016;7:13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friston KJ. Hallucinations and perceptual inference. Behav Brain Sci. 2005;28(6):764–766. [Google Scholar]
- 33. Sterzer P, Adams RA, Fletcher P, et al. The predictive coding account of psychosis. Biol Psychiatry. 2018;84(9):634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR.. Hallucinations and strong priors. Trends Cogn Sci. 2019;23(2):114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Callaghan C, Hall JM, Tomassini A, et al. Visual hallucinations are characterized by impaired sensory evidence accumulation: Insights from hierarchical drift diffusion modeling in Parkinson’s disease. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(8):680–688. [DOI] [PubMed] [Google Scholar]
- 36. Zarkali A, Adams RA, Psarras S, Leyland LA, Rees G, Weil RS.. Increased weighting on prior knowledge in Lewy body-associated visual hallucinations. Brain Commun. 2019;1(1):fcz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pekkonen E, Hirvonen J, Jääskeläinen IP, Kaakkola S, Huttunen J.. Auditory sensory memory and the cholinergic system: Implications for Alzheimer’s disease. Neuroimage. 2001;14(2):376–382. [DOI] [PubMed] [Google Scholar]
- 38. Pekkonen E. Mismatch negativity in aging and in Alzheimer’s and Parkinson’s diseases. Audiol Neurootol. 2000;5(3-4):216–224. [DOI] [PubMed] [Google Scholar]
- 39. Ballard C, Piggott M, Johnson M, et al. Delusions associated with elevated muscarinic binding in dementia with Lewy bodies. Ann Neurol. 2000;48(6):868–876. [PubMed] [Google Scholar]
- 40. Halliday G. Clarifying Lewy-body parkinsonism with visual hallucinations. Lancet Neurol. 2005;4(10):588–589. [DOI] [PubMed] [Google Scholar]
- 41. Harding AJ, Broe GA, Halliday GM.. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125(Pt 2):391–403. [DOI] [PubMed] [Google Scholar]
- 42. Engeland C, Mahoney C, Mohr E, Ilivitsky V, Knott VJ.. Acute nicotine effects on auditory sensory memory in tacrine-treated and nontreated patients with Alzheimer’s disease: An event-related potential study. Pharmacol Biochem Behav. 2002;72(1-2):457–464. [DOI] [PubMed] [Google Scholar]
- 43. Mori S, Mori E, Iseki E, Kosaka K.. Efficacy and safety of donepezil in patients with dementia with Lewy bodies: Preliminary findings from an open-label study. Psychiatry Clin Neurosci. 2006;60(2):190–195. [DOI] [PubMed] [Google Scholar]
- 44. Corlett PR, Honey GD, Krystal JH, Fletcher PC.. Glutamatergic model psychoses: Prediction error, learning, and inference. Neuropsychopharmacology. 2011;36(1):294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hosoya T, Baccus SA, Meister M.. Dynamic predictive coding by the retina. Nature. 2005;436(7047):71–77. [DOI] [PubMed] [Google Scholar]
- 46. Hohwy J, Roepstorff A, Friston K.. Predictive coding explains binocular rivalry: An epistemological review. Cognition. 2008;108(3):687–701. [DOI] [PubMed] [Google Scholar]
- 47. Vuust P, Ostergaard L, Pallesen KJ, Bailey C, Roepstorff A.. Predictive coding of music–brain responses to rhythmic incongruity. Cortex. 2009;45(1):80–92. [DOI] [PubMed] [Google Scholar]
- 48. Vuust P, Witek MA.. Rhythmic complexity and predictive coding: A novel approach to modeling rhythm and meter perception in music. Front Psychol. 2014;5:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wicha NY, Moreno EM, Kutas M.. Anticipating words and their gender: An event-related brain potential study of semantic integration, gender expectancy, and gender agreement in Spanish sentence reading. J Cogn Neurosci. 2004;16(7):1272–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dikker S, Pylkkänen L.. Predicting language: MEG evidence for lexical preactivation. Brain Lang. 2013;127(1):55–64. [DOI] [PubMed] [Google Scholar]
- 51. Lewis AG, Bastiaansen M.. A predictive coding framework for rapid neural dynamics during sentence-level language comprehension. Cortex. 2015;68:155–168. [DOI] [PubMed] [Google Scholar]
- 52. Lewis AG, Wang L, Bastiaansen M.. Fast oscillatory dynamics during language comprehension: Unification versus maintenance and prediction? Brain Lang. 2015;148:51–63. [DOI] [PubMed] [Google Scholar]
- 53. Kumar S, Sedley W, Nourski KV, et al. Predictive coding and pitch processing in the auditory cortex. J Cogn Neurosci. 2011;23(10):3084–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O'Doherty JP, Buchanan TW, Seymour B, Dolan RJ.. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49(1):157–166. [DOI] [PubMed] [Google Scholar]
- 55. Ramnani N, Miall RC.. A system in the human brain for predicting the actions of others. Nat Neurosci. 2004;7(1):85–90. [DOI] [PubMed] [Google Scholar]
- 56. Kilner JM. More than one pathway to action understanding. Trends Cogn Sci. 2011;15(8):352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Friston KJ, Stephan KE, Montague R, Dolan RJ.. Computational psychiatry: The brain as a phantastic organ. Lancet Psychiatry. 2014;1(2):148–158. [DOI] [PubMed] [Google Scholar]
- 58. Fletcher PC, Frith CD.. Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10(1):48–58. [DOI] [PubMed] [Google Scholar]
- 59. Pellicano E, Burr D.. When the world becomes ‘too real’: A Bayesian explanation of autistic perception. Trends Cogn Sci. 2012;16(10):504–510. [DOI] [PubMed] [Google Scholar]
- 60. Lawson RP, Rees G, Friston KJ.. An aberrant precision account of autism. Front Hum Neurosci. 2014;8:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wolpe N, Hezemans FH, Rowe JB.. Alien limb syndrome: A Bayesian account of unwanted actions. Cortex. 2020;127:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bregman AS. Auditory scene analysis: The perceptual organization of sound. MIT Press; 1990. [Google Scholar]
- 63. Griffiths TD, Warren JD.. The planum temporale as a computational hub. Trends Neurosci. 2002;25(7):348–353. [DOI] [PubMed] [Google Scholar]
- 64. Summerfield C, de Lange FP.. Expectation in perceptual decision making: Neural and computational mechanisms. Nat Rev Neurosci. 2014;15(11):745–756. [DOI] [PubMed] [Google Scholar]
- 65. Golden HL, Nicholas JM, Yong KX, et al. Auditory spatial processing in Alzheimer’s disease. Brain. 2015;138(Pt 1):189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goll JC, Kim LG, Ridgway GR, et al. Impairments of auditory scene analysis in Alzheimer’s disease. Brain. 2012;135(Pt 1):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gaeta H, Friedman D, Ritter W, Cheng J.. Changes in sensitivity to stimulus deviance in Alzheimer’s disease: An ERP perspective. Neuroreport. 1999;10(2):281–287. [DOI] [PubMed] [Google Scholar]
- 68. Laptinskaya D, Thurm F, Küster OC, et al. Auditory memory decay as reflected by a new mismatch negativity score is associated with episodic memory in older adults at risk of dementia. Front Aging Neurosci. 2018;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hughes LE, Rowe JB.. The impact of neurodegeneration on network connectivity: A study of change detection in frontotemporal dementia. J Cogn Neurosci. 2013;25(5):802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Golden HL, Clark CN, Nicholas JM, et al. Music perception in dementia. J Alzheimers Dis. 2017;55(3):933–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zimmermann J, Alain C, Butler C.. Impaired memory-guided attention in asymptomatic APOE4 carriers. Sci Rep. 2019;9(1):8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Uchiyama M, Nishio Y, Yokoi K, et al. Pareidolias: Complex visual illusions in dementia with Lewy bodies. Brain. 2012;135(Pt 8):2458–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Barnes J, David AS.. Visual hallucinations in Parkinson’s disease: A review and phenomenological survey. J Neurol Neurosurg Psychiatry. 2001;70(6):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mosimann UP, Rowan EN, Partington CE, et al. Characteristics of visual hallucinations in Parkinson disease dementia and dementia with Lewy bodies. Am J Geriatr Psychiatry. 2006;14(2):153–160. [DOI] [PubMed] [Google Scholar]
- 75. Pezzoli S, Cagnin A, Bandmann O, Venneri A.. Structural and functional neuroimaging of visual hallucinations in Lewy body disease: A systematic literature review. Brain Sci. 2017;7(12):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yao N, Pang S, Cheung C, et al. Resting activity in visual and corticostriatal pathways in Parkinson’s disease with hallucinations. Parkinsonism Relat Disord. 2015;21(2):131–137. [DOI] [PubMed] [Google Scholar]
- 77. Shine JM, Muller AJ, O'Callaghan C, Hornberger M, Halliday GM, Lewis SJ.. Abnormal connectivity between the default mode and the visual system underlies the manifestation of visual hallucinations in Parkinson’s disease: A task-based fMRI study. NPJ Parkinsons Dis. 2015;1:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Heitz C, Noblet V, Cretin B, et al. Neural correlates of visual hallucinations in dementia with Lewy bodies. Alzheimers Res Ther. 2015;7(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Peraza LR, Kaiser M, Firbank M, et al. fMRI resting state networks and their association with cognitive fluctuations in dementia with Lewy bodies. Neuroimage Clin. 2014;4:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, et al. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord. 2010;25(5):615–622. [DOI] [PubMed] [Google Scholar]
- 81. Perneczky R, Drzezga A, Boecker H, Förstl H, Kurz A, Häussermann P.. Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord. 2008;25(6):531–538. [DOI] [PubMed] [Google Scholar]
- 82. Ramírez-Ruiz B, Martí MJ, Tolosa E, et al. Cerebral atrophy in Parkinson’s disease patients with visual hallucinations. Eur J Neurol. 2007;14(7):750–756. [DOI] [PubMed] [Google Scholar]
- 83. Stebbins GT, Goetz CG, Carrillo MC, et al. Altered cortical visual processing in PD with hallucinations: An fMRI study. Neurology. 2004;63(8):1409–1416. [DOI] [PubMed] [Google Scholar]
- 84. Adams RA, Shipp S, Friston KJ.. Predictions not commands: Active inference in the motor system. Brain Struct Funct. 2013;218(3):611–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Friston KJ, Daunizeau J, Kilner J, Kiebel SJ.. Action and behavior: A free-energy formulation. Biol Cybern. 2010;102(3):227–260. [DOI] [PubMed] [Google Scholar]
- 86. Wolpe N, Moore JW, Rae CL, et al. The medial frontal-prefrontal network for altered awareness and control of action in corticobasal syndrome. Brain. 2014;137(Pt 1):208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Conrad MO, Scheidt RA, Schmit BD.. Effects of wrist tendon vibration on targeted upper-arm movements in poststroke hemiparesis. Neurorehabil Neural Repair. 2011;25(1):61–70. [DOI] [PubMed] [Google Scholar]
- 88. Palmer C, Zapparoli L, Kilner JM.. A new framework to explain sensorimotor beta oscillations. Trends Cogn Sci. 2016;20(5):321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tan H, Wade C, Brown P.. Post-movement beta activity in sensorimotor cortex indexes confidence in the estimations from internal models. Research support, Non-U.S. Gov’t. J Neurosci. 2016;36(5):1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pfurtscheller G, Lopes da Silva FH.. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol. 1999;110(11):1842–1857. [DOI] [PubMed] [Google Scholar]
- 91. Moisello C, Blanco D, Lin J, et al. Practice changes beta power at rest and its modulation during movement in healthy subjects but not in patients with Parkinson’s disease. Brain Behav. 2015;5(10):e00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bizovicar N, Dreo J, Koritnik B, Zidar J.. Decreased movement-related beta desynchronization and impaired post-movement beta rebound in amyotrophic lateral sclerosis. Clin Neurophysiol. 2014;125(8):1689–1699. [DOI] [PubMed] [Google Scholar]
- 93. Levy R, Lozano AM, Lang AE, Dostrovsky JO.. Event-related desynchronization of motor cortical oscillations in patients with multiple system atrophy. Exp Brain Res. 2010;206(1):1–13. [DOI] [PubMed] [Google Scholar]
- 94. Schnitzler A, Gross J.. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6(4):285–296. [DOI] [PubMed] [Google Scholar]
- 95. Brown P, Marsden CD.. Bradykinesia and impairment of EEG desynchronization in Parkinson’s disease. Mov Disord. 1999;14(3):423–429. [DOI] [PubMed] [Google Scholar]
- 96. Macerollo A, Chen JC, Korlipara P, et al. Dopaminergic treatment modulates sensory attenuation at the onset of the movement in Parkinson’s disease: A test of a new framework for bradykinesia. Mov Disord. 2016;31(1):143–146. [DOI] [PubMed] [Google Scholar]
- 97. Chow TW, Binns MA, Cummings JL, et al. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Arch Neurol. 2009;66(7):888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, et al. Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain. 2017;140(6):1792–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tay J, Morris RG, Tuladhar AM, Husain M, de Leeuw FE, Markus HS.. Apathy, but not depression, predicts all-cause dementia in cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2020;91(9):953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hezemans FH, Wolpe N, Rowe JB.. Apathy is associated with reduced precision of prior beliefs about action outcomes. J Exp Psychol Gen. 2020;149(9):1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Parr T, Rikhye RV, Halassa MM, Friston KJ.. Prefrontal computation as active inference. Cereb Cortex. 2019;30:682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hughes LE, Rittman T, Robbins TW, Rowe JB.. Reorganization of cortical oscillatory dynamics underlying disinhibition in frontotemporal dementia. Brain. 2018;141(8):2486–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Passamonti L, Lansdall CJ, Rowe JB.. The neuroanatomical and neurochemical basis of apathy and impulsivity in frontotemporal lobar degeneration. Curr Opin Behav Sci. 2018;22:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Le Heron C, Apps MAJ, Husain M.. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia. 2018;118(Pt B):54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nobis L, Husain M.. Apathy in Alzheimer’s disease. Curr Opin Behav Sci. 2018;22:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Borges LG, Rademaker AW, Bigio EH, Mesulam MM, Weintraub S.. Apathy and disinhibition related to neuropathology in amnestic versus behavioral dementias. Am J Alzheimers Dis Other Demen. 2019;34(5):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nombela C, Rittman T, Robbins TW, Rowe JB.. Multiple modes of impulsivity in Parkinson’s disease. PLoS One. 2014;9(1):e85747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dalley JW, Robbins TW.. Fractionating impulsivity: Neuropsychiatric implications. Nat Rev Neurosci. 2017;18(3):158–171. [DOI] [PubMed] [Google Scholar]
- 109. FitzGerald TH, Schwartenbeck P, Moutoussis M, Dolan RJ, Friston K.. Active inference, evidence accumulation, and the urn task. Neural Comput. 2015;27(2):306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Averbeck BB, Djamshidian A, O'Sullivan SS, Housden CR, Roiser JP, Lees AJ.. Uncertainty about mapping future actions into rewards may underlie performance on multiple measures of impulsivity in behavioral addiction: Evidence from Parkinson’s disease. Behav Neurosci. 2013;127(2):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Betts MJ, Kirilina E, Otaduy MCG, et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain. 2019;142(9):2558–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Berridge CW, Waterhouse BD.. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42(1):33–84. [DOI] [PubMed] [Google Scholar]
- 113. Holland N, Robbins T, Rowe J.. The role of noradrenaline in cognition and cognitive disorders. Brain. 2021;144(8):2243–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dayan P, Yu AJ.. Phasic norepinephrine: A neural interrupt signal for unexpected events. Network. 2006;17(4):335–350. [DOI] [PubMed] [Google Scholar]
- 115. Sales AC, Friston KJ, Jones MW, Pickering AE, Moran RJ.. Locus Coeruleus tracking of prediction errors optimises cognitive flexibility: An active inference model. PLoS Comput Biol. 2019;15(1):e1006267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rahman S, Robbins TW, Hodges JR, et al. Methylphenidate (‘Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. 2006;31(3):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kehagia AA, Housden CR, Regenthal R, et al. Targeting impulsivity in Parkinson’s disease using atomoxetine. Randomized Controlled Trial Research Support, Non-U.S. Gov’t. Brain. 2014;137(7):1986–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rae CL, Nombela C, Rodriguez PV, et al. Atomoxetine restores the response inhibition network in Parkinson’s disease. Brain. 2016;139(Pt 8):2235–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Harrison LM, Duggins A, Friston KJ.. Encoding uncertainty in the hippocampus. Neural Netw. 2006;19(5):535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Strange BA, Duggins A, Penny W, Dolan RJ, Friston KJ.. Information theory, novelty and hippocampal responses: Unpredicted or unpredictable? Neural Netw. 2005;18(3):225–230. [DOI] [PubMed] [Google Scholar]
- 121. Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H.. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23(2):209–226. [DOI] [PubMed] [Google Scholar]
- 122. Weiler JA, Suchan B, Daum I.. Foreseeing the future: Occurrence probability of imagined future events modulates hippocampal activation. Hippocampus. 2010;20(6):685–690. [DOI] [PubMed] [Google Scholar]
- 123. Friston K, Buzsáki G.. The functional anatomy of time: What and when in the brain. Trends Cogn Sci. 2016;20(7):500–511. [DOI] [PubMed] [Google Scholar]
- 124. Buzsáki G, Llinás R.. Space and time in the brain. Science. 2017;358(6362):482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T, Kilner JM.. The functional anatomy of the MMN: A DCM study of the roving paradigm. Neuroimage. 2008;42(2):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Garrido MI, Kilner JM, Kiebel SJ, Friston KJ.. Dynamic causal modeling of the response to frequency deviants. J Neurophysiol. 2009;101(5):2620–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. David O, Kiebel SJ, Harrison LM, Mattout J, Kilner JM, Friston KJ.. Dynamic causal modeling of evoked responses in EEG and MEG. Neuroimage. 2006;30(4):1255–1272. [DOI] [PubMed] [Google Scholar]
- 128. Wacongne C, Changeux JP, Dehaene S.. A neuronal model of predictive coding accounting for the mismatch negativity. J Neurosci. 2012;32(11):3665–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Phillips HN, Blenkmann A, Hughes LE, et al. Convergent evidence for hierarchical prediction networks from human electrocorticography and magnetoencephalography. Cortex. 2016;82:192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chennu S, Noreika V, Gueorguiev D, Shtyrov Y, Bekinschtein TA, Henson R.. Silent expectations: Dynamic causal modeling of cortical prediction and attention to sounds that weren’t. J Neurosci. 2016;36(32):8305–8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jiang S, Yan C, Qiao Z, et al. Mismatch negativity as a potential neurobiological marker of early-stage Alzheimer disease and vascular dementia. Neurosci Lett. 2017;647:26–31. [DOI] [PubMed] [Google Scholar]
- 132. Brønnick KS, Nordby H, Larsen JP, Aarsland D.. Disturbance of automatic auditory change detection in dementia associated with Parkinson’s disease: A mismatch negativity study. Neurobiol Aging. 2010;31(1):104–113. [DOI] [PubMed] [Google Scholar]
- 133. Stam CJ, Jones BF, Manshanden I, et al. Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer’s disease. Neuroimage. 2006;32(3):1335–1344. [DOI] [PubMed] [Google Scholar]
- 134. Beste C, Mückschel M, Rosales R, et al. Striosomal dysfunction affects behavioral adaptation but not impulsivity-Evidence from X-linked dystonia-parkinsonism. Mov Disord. 2017;32(4):576–584. [DOI] [PubMed] [Google Scholar]
- 135. Pekkonen E, Jousmäki V, Könönen M, Reinikainen K, Partanen J.. Auditory sensory memory impairment in Alzheimer’s disease: An event-related potential study. Neuroreport. 1994;5(18):2537–2540. [DOI] [PubMed] [Google Scholar]
- 136. Ruzzoli M, Pirulli C, Mazza V, Miniussi C, Brignani D.. The mismatch negativity as an index of cognitive decline for the early detection of Alzheimer’s disease. Sci Rep. 2016;6:33167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Budson AE, Michalska KJ, Sullivan AL, Rentz DM, Daffner KR, Schacter DL.. False recognition in Alzheimer disease: Evidence from categorized pictures. Cogn Behav Neurol. 2003;16(1):16–27. [DOI] [PubMed] [Google Scholar]
- 138. Belleville S, Ménard MC, Lepage E.. Impact of novelty and type of material on recognition in healthy older adults and persons with mild cognitive impairment. Neuropsychologia. 2011;49(10):2856–2865. [DOI] [PubMed] [Google Scholar]
- 139. Howieson DB, Mattek N, Seeyle AM, et al. Serial position effects in mild cognitive impairment. J Clin Exp Neuropsychol. 2011;33(3):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cunha C, Guerreiro M, de Mendonça A, Oliveira PE, Santana I.. Serial position effects in Alzheimer’s disease, mild cognitive impairment, and normal aging: Predictive value for conversion to dementia. J Clin Exp Neuropsychol. 2012;34(8):841–852. [DOI] [PubMed] [Google Scholar]
- 141. Vitali P, Minati L, Chiarenza G, et al. The Von Restorff effect in ageing and Alzheimer’s disease. Neurol Sci. 2006;27(3):166–172. [DOI] [PubMed] [Google Scholar]
- 142. Brueggen K, Kasper E, Dyrba M, et al. The primacy effect in amnestic mild cognitive impairment: associations with hippocampal functional connectivity. Front Aging Neurosci. 2016;8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Evans S, Dowell NG, Tabet N, King SL, Hutton SB, Rusted JM.. Disrupted neural activity patterns to novelty and effort in young adult. Brain Behav. 2017;7(2):e00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Braskie MN, Medina LD, Rodriguez-Agudelo Y, et al. Increased fMRI signal with age in familial Alzheimer’s disease mutation carriers. Neurobiol Aging. 2012;33(2):424.e11–424.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Henson RN, Gagnepain P.. Predictive, interactive multiple memory systems. Hippocampus. 2010;20(11):1315–1326. [DOI] [PubMed] [Google Scholar]
- 146. van Kesteren MT, Ruiter DJ, Fernández G, Henson RN.. How schema and novelty augment memory formation. Trends Neurosci. 2012;35(4):211–219. [DOI] [PubMed] [Google Scholar]
- 147. Clark A. A nice surprise? Predictive processing and the active pursuit of novelty. Phenomenol Cogn Sci. 2018;17(3):521–534. [Google Scholar]
- 148. Miasnikov AA, Chen JC, Weinberger NM.. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiol Learn Mem. 2008;90(2):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Carbajal GV, Malmierca MS.. The neuronal basis of predictive coding along the auditory pathway: From the subcortical roots to cortical deviance detection. Trends Hear. 2018;22:2331216518784822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Düzel E, Bunzeck N, Guitart-Masip M, Düzel S.. NOvelty-related motivation of anticipation and exploration by dopamine (NOMAD): Implications for healthy aging. Neurosci Biobehav Rev. 2010;34(5):660–669. [DOI] [PubMed] [Google Scholar]
- 151. Lisman JE, Grace AA.. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. [DOI] [PubMed] [Google Scholar]
- 152. Yu AJ, Dayan P.. Acetylcholine in cortical inference. Neural Netw. 2002;15(4-6):719–730. [DOI] [PubMed] [Google Scholar]
- 153. Lisman J, Grace AA, Duzel E.. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34(10):536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bunzeck N, Guitart-Masip M, Dolan RJ, Duzel E.. Pharmacological dissociation of novelty responses in the human brain. Cereb Cortex. 2014;24(5):1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Schultz W, Dayan P, Montague PR.. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. [DOI] [PubMed] [Google Scholar]
- 156. Eckart C, Bunzeck N.. Dopamine modulates processing speed in the human mesolimbic system. Neuroimage. 2013;66:293–300. [DOI] [PubMed] [Google Scholar]
- 157. Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, Düzel E.. Dopamine modulates episodic memory persistence in old age. J Neurosci. 2012;32(41):14193–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ettinger A, Linzen T, Marantz A.. The role of morphology in phoneme prediction: Evidence from MEG. Brain Lang. 2014;129:14–23. [DOI] [PubMed] [Google Scholar]
- 159. Monsalve IF, Bourguignon M, Molinaro N.. Theta oscillations mediate pre-activation of highly expected word initial phonemes. Sci Rep. 2018;8(1):9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gagnepain P, Henson RN, Davis MH.. Temporal predictive codes for spoken words in auditory cortex. Curr Biol. 2012;22(7):615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. DeLong KA, Urbach TP, Kutas M.. Probabilistic word pre-activation during language comprehension inferred from electrical brain activity. Nat Neurosci. 2005;8(8):1117–1121. [DOI] [PubMed] [Google Scholar]
- 162. Lau EF, Holcomb PJ, Kuperberg GR.. Dissociating N400 effects of prediction from association in single-word contexts. J Cogn Neurosci. 2013;25(3):484–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lau EF, Nguyen E.. The role of temporal predictability in semantic expectation: An MEG investigation. Cortex. 2015;68:8–19. [DOI] [PubMed] [Google Scholar]
- 164. Maess B, Mamashli F, Obleser J, Helle L, Friederici AD.. Prediction signatures in the brain: Semantic pre-activation during language comprehension. Front Hum Neurosci. 2016;10:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Klimovich-Gray A, Tyler LK, Randall B, Kocagoncu E, Devereux B, Marslen-Wilson WD.. Balancing prediction and sensory input in speech comprehension: the spatiotemporal dynamics of word recognition in context. J Neurosci. 2019;39(3):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang L, Kuperberg G, Jensen O.. Specific lexico-semantic predictions are associated with unique spatial and temporal patterns of neural activity. Elife.12: 2018;7:e39061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Fonteneau E. Structural syntactic prediction measured with ELAN: Evidence from ERPs. Neurosci Lett. 2013;534:211–216. [DOI] [PubMed] [Google Scholar]
- 168. Wlotko EW, Federmeier KD.. Time for prediction? The effect of presentation rate on predictive sentence comprehension during word-by-word reading. Cortex. 2015;68:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Henderson JM, Choi W, Lowder MW, Ferreira F.. Language structure in the brain: A fixation-related fMRI study of syntactic surprisal in reading. Neuroimage. 2016;132:293–300. [DOI] [PubMed] [Google Scholar]
- 170. Otten M, Van Berkum JJ.. Discourse-based word anticipation during language processing: Prediction of priming? Discourse Process. 2008;45(6):464–496. [Google Scholar]
- 171. Utman JA, Blumstein SE, Sullivan K.. Mapping from sound to meaning: Reduced lexical activation in Broca’s aphasics. Brain Lang. 2001;79(3):444–472. [DOI] [PubMed] [Google Scholar]
- 172. Moineau S, Dronkers NF, Bates E.. Exploring the processing continuum of single-word comprehension in aphasia. J Speech Lang Hear Res. 2005;48(4):884–896. [DOI] [PubMed] [Google Scholar]
- 173. Hagoort P. Impairments of lexical-semantic processing in aphasia: Evidence from the processing of lexical ambiguities. Brain Lang. 1993;45(2):189–232. [DOI] [PubMed] [Google Scholar]
- 174. Swaab TY, Brown C, Hagoort P.. Understanding ambiguous words in sentence contexts: Electrophysiological evidence for delayed contextual selection in Broca’s aphasia. Neuropsychologia. 1998;36(8):737–761. [DOI] [PubMed] [Google Scholar]
- 175. Grindrod CM, Baum SR.. Hemispheric contributions to lexical ambiguity resolution in a discourse context: Evidence from individuals with unilateral left and right hemisphere lesions. Brain Cogn. 2005;57(1):70–83. [DOI] [PubMed] [Google Scholar]
- 176. Pickering MJ, Garrod S.. Do people use language production to make predictions during comprehension? Trends Cogn Sci. 2007;11(3):105–110. [DOI] [PubMed] [Google Scholar]
- 177. Pickering MJ, Garrod S.. How tightly are production and comprehension interwoven? Front Psychol. 2013;4:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Park H, Ince RA, Schyns PG, Thut G, Gross J.. Frontal top-down signals increase coupling of auditory low-frequency oscillations to continuous speech in human listeners. Curr Biol. 2015;25(12):1649–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Sohoglu E, Davis MH.. Perceptual learning of degraded speech by minimizing prediction error. Research Support, Non-U.S. Gov’t. Proc Natl Acad Sci U S A. 2016;113(12):E1747–E1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hayes RA, Dickey MW, Warren T.. Looking for a location: dissociated effects of event-related plausibility and verb-argument information on predictive processing in aphasia. Am J Speech Lang Pathol. 2016;25(4S):S758–S775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Henry ML, Wilson SM, Babiak MC, et al. Phonological processing in primary progressive aphasia. J Cogn Neurosci. 2016;28(2):210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Baldo JV, Klostermann EC, Dronkers NF.. It’s either a cook or a baker: Patients with conduction aphasia get the gist but lose the trace. Brain Lang. 2008;105(2):134–140. [DOI] [PubMed] [Google Scholar]
- 184. Buchsbaum BR, Baldo J, Okada K, et al. Conduction aphasia, sensory-motor integration, and phonological short-term memory—an aggregate analysis of lesion and fMRI data. Brain Lang. 2011;119(3):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Parr T, Rees G, Friston KJ.. Computational neuropsychology and bayesian inference. Front Hum Neurosci. 2018;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Cope TE, Sohoglu E, Sedley W, et al. Evidence for causal top-down frontal contributions to predictive processes in speech perception. Nat Commun. 2017;8(1):2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Huang X, Chen X, Yan N, et al. The impact of Parkinson’s disease on the cortical mechanisms that support auditory-motor integration for voice control. Hum Brain Mapp. 2016;37(12):4248–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lin IF, Mochida T, Asada K, Ayaya S, Kumagaya S, Kato M.. Atypical delayed auditory feedback effect and Lombard effect on speech production in high-functioning adults with autism spectrum disorder. Front Hum Neurosci. 2015;9:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hardy CJD, Bond RL, Jaisin K, et al. Sensitivity of speech output to delayed auditory feedback in primary progressive aphasias. Front Neurol. 2018;9:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Price CJ, Devlin JT.. The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn Sci. 2011;15(6):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Damasio AR, Damasio H.. The anatomic basis of pure alexia. Neurology. 1983;33(12):1573–1583. [DOI] [PubMed] [Google Scholar]
- 192. Binder JR, Mohr JP.. The topography of callosal reading pathways. A case-control analysis. Brain. 1992;115 (6):1807–1826. [DOI] [PubMed] [Google Scholar]
- 193. Confavreux C, Croisile B, Garassus P, Aimard G, Trillet M.. Progressive amusia and aprosody. Arch Neurol. 1992;49(9):971–976. [DOI] [PubMed] [Google Scholar]
- 194. Otsuki M, Soma Y, Sato M, Homma A, Tsuji S.. Slowly progressive pure word deafness. Eur Neurol. 1998;39(3):135–140. [DOI] [PubMed] [Google Scholar]
- 195. Woodhead ZV, Penny W, Barnes GR, et al. Reading therapy strengthens top-down connectivity in patients with pure alexia. Brain. 2013;136(Pt 8):2579–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kocagoncu E, Clarke A, Devereux BJ, Tyler LK.. Decoding the cortical dynamics of sound-meaning mapping. J Neurosci. 2017;37(5):1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Lyu B, Choi HS, Marslen-Wilson WD, Clarke A, Randall B, Tyler LK.. Neural dynamics of semantic composition. Proc Natl Acad Sci U S A. 2019;116(42):21318–21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kutas M, Federmeier KD.. Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP). Annu Rev Psychol. 2011;62:621–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Hurley RS, Paller KA, Rogalski EJ, Mesulam MM.. Neural mechanisms of object naming and word comprehension in primary progressive aphasia. J Neurosci. 2012;32(14):4848–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Cumming TB, Patterson K, Verfaellie M, Graham KS.. One bird with two stones: Abnormal word length effects in pure alexia and semantic dementia. Cogn Neuropsychol. 2006;23(8):1130–1161. [DOI] [PubMed] [Google Scholar]
- 201. Ralph MA, Jefferies E, Patterson K, Rogers TT.. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18(1):42–55. [DOI] [PubMed] [Google Scholar]