Abstract
During a verbal conversation, our brain moves through a series of complex linguistic processing stages: sound decoding, semantic comprehension, retrieval of semantically coherent words, and overt production of speech outputs. Each process is thought to be supported by a network consisting of local and long-range connections bridging between major cortical areas. Both temporal and extratemporal lobe regions have functional compartments responsible for distinct language domains, including the perception and production of phonological and semantic components.
This study provides quantitative evidence of how directly connected inter-lobar neocortical networks support distinct stages of linguistic processing across brain development. Novel six-dimensional tractography was used to intuitively visualize the strength and temporal dynamics of direct inter-lobar effective connectivity between cortical areas activated during each linguistic processing stage.
We analysed 3401 non-epileptic intracranial electrode sites from 37 children with focal epilepsy (aged 5–20 years) who underwent extra-operative electrocorticography recording. Principal component analysis of auditory naming-related high-gamma modulations determined the relative involvement of each cortical area during each linguistic processing stage. To quantify direct effective connectivity, we delivered single-pulse electrical stimulation to 488 temporal and 1581 extratemporal lobe sites and measured the early cortico-cortical spectral responses at distant electrodes. Mixed model analyses determined the effects of naming-related high-gamma co-augmentation between connecting regions, age, and cerebral hemisphere on the strength of effective connectivity independent of epilepsy-related factors.
Direct effective connectivity was strongest between extratemporal and temporal lobe site pairs, which were simultaneously activated between sentence offset and verbal response onset (i.e. response preparation period); this connectivity was approximately twice more robust than that with temporal lobe sites activated during stimulus listening or overt response. Conversely, extratemporal lobe sites activated during overt response were equally connected with temporal lobe language sites. Older age was associated with increased strength of inter-lobar effective connectivity especially between those activated during response preparation. The arcuate fasciculus supported approximately two-thirds of the direct effective connectivity pathways from temporal to extratemporal auditory language-related areas but only up to half of those in the opposite direction. The uncinate fasciculus consisted of <2% of those in the temporal-to-extratemporal direction and up to 6% of those in the opposite direction.
We, for the first time, provided an atlas which quantifies and animates the strength, dynamics, and direction specificity of inter-lobar neural communications between language areas via the white matter pathways. Language-related effective connectivity may be strengthened in an age-dependent manner even after the age of 5.
Keywords: high-gamma activity, cortico-cortical evoked potentials, intracranial electroencephalography, dynamic DWI tractography, paediatric epilepsy surgery
Sonoda et al. present a 6D tractography atlas which reveals the dynamics, strength, and direction specificity of neural communication between language areas via the white matter tracts. Age-dependent strengthening of connectivity after age five occurs preferentially between areas supporting lexical retrieval.
Introduction
Human spoken conversations can be considered in two phases: reception and expression. Reception happens in two stages: initially, each sound in the heard sentence is recognized and decoded via phonological processing, followed by integration with higher-order functions to understand the semantic meaning.1-5 The second phase, expression, also consists of two parts. Semantically coherent words and word-specified phonemes are retrieved, followed by overt speech production and monitoring of one’s own speech.5–10 This complex, multistage process is believed to be supported by a cortical network incorporating a combination of local processing and long-range inter-lobar connections. The precise spatial configuration and temporal dynamics of the language network are subject of ongoing research, and multiple competing models exist.2–4,9–12 Common among each model is evidence that long-range white matter tracts from and towards the temporal lobe play a central role in the language network. These inter-lobar pathways are suggested to exchange language information, including mental representation of sentences, and facilitate the translation of sound and semantics into motor representations.11–17 However, previous studies have not directly mapped the rapid neural propagations between language areas via the underlying white matter tracts. Collective evidence indicates that the development of inter-lobar connectivity networks begins early in life and continues in an experience-dependent manner.18,19 Investigators have not reached a consensus on the developmental patterns of effective connectivity between the regions supporting different linguistic processing stages.
We aimed to localize and visualize the strength and dynamics of effective connectivity networks that support direct transfers of neural activity between the temporal and extratemporal lobe neocortices supporting distinct linguistic processing stages. Investigators have proposed models for how the cerebral cortex transfers neural representations of phonological and semantic information. The classical Geschwind model of language-related connectivity did not distinguish separate pathways supporting the phonological and semantic domains.20 Instead, it proposed that the left posterior temporal lobe neocortex transfers the entire linguistic content expressed by each spoken sentence to the left inferior frontal gyrus (IFG) for subsequent speech output. Conversely, most current models propose that neural activity representing phonological and semantic domains is transferred via different pathways in each hemisphere with left-hemispheric dominance.2–4,12 Some suggest that the human brain transfers neural representations directly from the posterior temporal to the extratemporal lobe language areas, mainly via the arcuate fasciculus.3,4,12,13 Another model infers that auditory language information is transferred indirectly via another structure in the temporoparietal junction.2 Another suggests that auditory semantic processing is initially supported by the neural information transfer from the left posterior to the anterior temporal area, which in turn projects to the IFG, presumably via the uncinate fasciculus11; subsequent semantic processing to understand auditory sentences is proposed to be supported by neural transfer to the posterior temporal region mainly via the arcuate fasiculus.11
Convergent evidence across each of these models highlights two general auditory language networks, which exert sound processing and semantic processing/control. The sound processing network is often referred to as the ‘phonological loop’. It is localized mainly in the inferior Rolandic area, superior temporal gyrus (STG), and the surrounding regions of both hemispheres.21–25 The phonological loop is believed to support the transformation of spoken sounds from perceptual to motor representations, short-term buffering of stimuli via subvocal rehearsal, and the monitoring of the volume/pitch of one’s own spoken sounds.7,8,14,21,22
The semantic processing/control network comprises both temporal and extratemporal lobe regions, with left-hemispheric dominance. The crucial brain regions are suggested to include but not limited to the posterior-to-anterior temporal lobe neocortices, IFG, and inferior parietal region.26–31 Linguistic processing is in part sequential.5,32 Phonological process can begin once auditory information is received by the auditory cortex, followed by semantic understanding processes that continue until after the auditory stimulus offset. Lexical retrieval and syllabification take place before an individual initiates an overt response.5,9,10,14 Although each of these processes overlaps during verbal conversations,32 the linear nature of language processing suggests that late neuronal engagement between the offset of auditory sentence stimuli and the onset of overt responses during an auditory naming task reflects, at least in part, retrieval of a relevant word and lexically-specified phonemes.5,33 Interventional evidence supports this notion; high-frequency electrical stimulation of left-hemispheric regions showing such late neuronal engagement often results in transient expressive dysphasia with intact sound repetition.5
Electrocorticography (ECoG) recordings during cognitive tasks can clarify the spatiotemporal distribution of corresponding brain activity. Event-related augmentation of broadband activity, including the high-gamma range at 70–110 Hz, is an excellent surrogate marker for neural activation.5,34–37 An increase in high-gamma amplitude is associated with increased firing rate on a single-neuron recording, haemodynamic response on functional MRI, and cortical metabolism on glucose-metabolism PET.38–40 Naming-related high-gamma augmentation can predict the language areas defined by electrical stimulation mapping as well as postoperative language impairment.41,42
To quantify inter-lobar effective connectivity, we measured cortico-cortical spectral responses (CCSRs) elicited by local single-pulse electrical stimulation (SPES).43 Both CCSRs and cortico-cortical evoked potentials (CCEPs) can quantify the strength and dynamics of effective connectivity between two remote regions.43–47 The two measures generally reflect the same underlying neuronal process but highlight different aspects of the recorded signal. CCSRs comprise a summation of phase-locked and asynchronous responses, whereas CCEPs consist of phase-locked responses alone. CCSRs are agnostic to the polarity of responses, whereas CCEPs may exhibit a variable polarity of responses depending on the structural feature of the underlying cortex.45 Previous studies suggest that early CCSRs roughly correspond to the early CCEP component (also known as N1),48–50 thus reflecting cortical excitation elicited by single-axonal neural propagation from the stimulus site.45,51–53 This notion is supported by the observation that the peak latency of such early responses is correlated with the surface distance and the underlying white matter streamline length on diffusion-weighted imaging (DWI) tractography.54–56 Late, low-frequency band CCSRs, roughly corresponding to the late CCEP component (also known as N2), are suggested to mainly indicate post-excitatory neuronal inhibition43,51,53 and represent a measure of inhibitory networks that govern effective connectivity.
We integrated ECoG high-gamma augmentation during an auditory naming task5 with CCSRs and DWI-based tractography to identify the strength, direction, and anatomical pathway of networks that support each stage of language processing. We estimated the relative involvement of cortical areas in each linguistic processing stage with a principal component analysis (PCA) of the temporal variations in naming-related high-gamma modulations.57 For each PCA-defined linguistic stage, we quantified the strength and dynamics of the inter-lobar effective connectivity towards and from the temporal lobe and localized the corresponding white matter pathways.
Multimodal studies present a unique challenge for intuitive visualization and interpretation of results. Here, the spatiotemporal dynamics are represented in six dimensions: CCSR magnitude, CCSR dynamics, naming task-related high-gamma activity (HGA), and three spatial dimensions. To facilitate interpretation, we generated animations visualizing the strength and dynamics of effective connectivity (i.e. CCSR-based neural propagations) via white-matter tracts directly bridging cortical sites supporting a specific linguistic processing stage. We refer to the corresponding animation-based atlas as six-dimensional (6D) dynamic tractography. We expected that our 6D dynamic tractography would validate or revise existing neurobiological models of language organization and development.2–4,11–13 It is hypothesized that neural circuits that synchronously engage in high-frequency activity are particularly susceptible to use-dependent modifications of synaptic transmission in the developing brain.58 Thus, we determined whether temporal lobe language sites have strengthened direct effective connectivity towards and from extratemporal lobe sites supporting the same linguistic stage. We determined what fasciculi support direct inter-lobar effective connectivity between given language sites in each direction. In this study including participants ranging in age between 5 and 20 years old, we determined whether older age was associated with increased strength of inter-lobar effective connectivity, specifically between sites supporting the semantic/lexical processes rather than the overt motor response.
Materials and methods
Participants
We studied 37 patients with focal seizures (aged 5–20 years; Supplementary Table 1 and Supplementary Fig. 1) who underwent epilepsy surgery at Detroit Medical Center between February 2013 and August 2018. The inclusion criteria included age ≥4 years59 and extra-operative ECoG sampling from temporal and extratemporal lobe regions. The exclusion criteria consisted of a massive structural lesion that renders the landmarks for the central or lateral sulcus unidentifiable,5 an inability to complete the naming task, history of previous epilepsy surgery, and right-hemispheric language dominance estimated based on left-handedness associated with an early epileptogenic lesion in the neocortex of the left hemisphere.42
All procedures were performed as part of our routine presurgical evaluation. The spatial extent and duration of extra-operative ECoG recording and electrical stimulation were determined clinically.22,45,60,61 This study has been approved by the Wayne State University Institutional Review Board. Written informed consent was obtained from the parents or guardians of patients. Written assent was obtained from children older than 13 years, and oral assent was obtained from younger children.
Subdural electrode placement and 3D localization
Patients underwent placement of platinum grid and strip electrodes (10 mm centre-to-centre distance) in the subdural space to localize the boundaries between the epileptogenic zone and eloquent areas.5 Oral anti-epileptic drugs (AEDs) were weaned off on the day of electrode implantation. We created a 3D surface image and determined each electrode’s location on the cortical surface, using the preoperative T1 spoiled gradient-recalled MRI and the FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu, accessed 11 October 2021).5,62–64 We spatially normalized all individual electrode sites’ location to the FreeSurfer template for group-level display and analysis (Fig. 1).5,59
Figure 1.

Spatial distribution of subdural electrode sampling. (A) The surface image presents the spatial distribution of analysed electrodes on each hemisphere. Colour indicates the number of patients available at a given spatial point. A total of 3401 non-epileptic artefact-free electrode sites were included in the analysis. (B) The spatial distribution of 488 stimulus sites in the temporal lobe used for the assessment of CCSR-based temporal to extratemporal lobe connectivity. (C) The spatial distribution of 1581 stimulus sites in the extratemporal lobe used for the assessment of the connectivity in the opposite direction.
Extra-operative ECoG recording
ECoG was recorded at a sampling rate of 1000 Hz using Neurofax Digital System (Nihon Kohden America Inc). To make our results generalizable, we limited the quantitative analysis to non-epileptic electrode sites.65,66 Sites classified as seizure onset zone,60 those showing interictal spikes,35,67 and those on a structural lesion65 were excluded from analysis. Likewise, electrodes affected by artefacts during the naming task or the CCSR acquisition period were excluded from analysis.61,68 After rejecting unusable channels, a total of 3401 non-epileptic sites were included in the analysis. For both high-gamma and CCSR analyses, ECoG signals were analysed using a common average reference.56,61
Linguistic stage categorization using naming-related high-gamma activity
Each patient was assigned a series of auditory question trials and instructed to verbally answer each question (Fig. 2). All question stimuli, starting with either ‘what’, ‘where’, ‘when’, or ‘who’, were sentences designed to draw out answers with nouns. Trials without correct answers were excluded from the analysis.69 The median number of trials was 100 per patient [interquartile range (IQR) = 0; range = 51–107]. The median rate of correct answers was 97.0% (IQR = 8.2; range = 54.5–100.0). The median inter-stimulus interval (interval between two sentences) across patients was 7.13 s (IQR = 1.57; range = 5.41–34.34). The median response time (interval between sentence offset and response onset) was 1.59 s (IQR = 0.66; range = 0.77–2.34).
Figure 2.
Auditory naming task and the time windows incorporated in the PCA. (A) Patients were instructed to give overt verbal answers to each of the auditory sentence questions (median duration of question sentence: 1.8 s; range: 1.2 to 2.4 s). Naming-related high-gamma activity during each 300 ms window was normalized as a relative value to the pre-trial baseline. (B) To determine what linguistic stages a given electrode channel was involved in, we employed a PCA to high-gamma modulations during six 300 ms time windows, that include three 300 ms periods during stimulus listening, two 300 ms periods between stimulus offset and response onset, as well as a 300 ms period immediately after overt response onset.
At each electrode site, we quantified naming-related high-gamma amplitude as a summary measure reflecting the magnitude of neuronal engagement during verbal discourse (Fig. 2).5,61 ECoG signals were transformed into the frequency domain using a complex demodulation technique implemented in BESA EEG V.5.1.8 (BESA GmbH)70,71 in non-overlapping 10 ms time and 5 Hz frequency bins.5,42,72,73 We computed high-gamma augmentation by averaging the amplitude within 70–110 Hz and normalizing to the mean high-gamma amplitude in a 600 to 200 ms pre-stimulus baseline period (Fig. 2A).
The PCA transformed six 300 ms windows (Fig. 2B) into a new dimensional space of orthogonal components (Fig. 3A).57 The first principal components (PCs) explaining 95% of the variance in high-gamma temporal dynamic patterns were extracted and used to categorize the linguistic processing stages (Fig. 3).
Figure 3.

Linguistic stage categorization based on a PCA of naming-related high gamma modulations. (A) This heat map presents the contributions (as reflected by the principal component coefficients) of naming-related high-gamma modulation during each 300 ms window to the principal components (PCs). (B) The first three principal components account for ∼95% of the variance (broken line) in high-gamma temporal patterns (red line). (C) Relative contribution of principal components 1–3 to each time window. (D–F) The spatial distribution of each principal component. A colour is displayed at sites where ≥4 patients’ data were available.
Quantification of direct inter-lobar effective connectivity using early CCSRs
We identified direct effective connectivity between temporal and extratemporal lobe electrode sites by measuring cortical responses to SPES.22,55 We delivered trains of electrical stimuli to a total of 488 electrode sites (314 pairs of adjacent electrodes, 8.5 pairs per patient on average; Fig. 1B) within the temporal lobe neocortex [STG, middle temporal gyrus (MTG), and inferior temporal gyrus] and 1581 extratemporal lobe electrode sites (885 pairs of adjacent electrodes, 24.6 pairs per patient on average; Fig. 1C) at a frequency of 1 Hz for 40 s during sleep. Each stimulus consisted of a biphasic square wave with a pulse width of 0.3 ms and an intensity of 5 mA. The interval between naming and CCSR acquisition sessions was <48 h. No adverse events were noted during the CCSR acquisition period.
Using the Morlet wavelet transformation74 implemented in FieldTrip (http://www.fieldtriptoolbox.org/),75 we transformed ECoG voltage signals into time-frequency bins (2 Hz frequency bins; three cycles for each frequency) sliding in 1 ms steps. The present study excluded recording sites within 1.5 cm of a given stimulus pair from the analysis of inter-lobar effective connectivity.76,77 We measured the per cent change of CCSR amplitude relative to the 50–200 ms pre-stimulus baseline period. The CCSR per cent change was computed for each frequency bin at 20–60 Hz range within 10–50 ms post-stimulus for early CCSRs and between 2 and 20 Hz within 50–300 ms post-stimulus for late CCSRs. The magnitude of the local time-frequency peak that survived a cluster-based permutation test (n = 200; cluster size threshold α = 0.05) was considered the strength of significant effective connectivity from the stimulus towards the recording electrode site (Fig. 4).78 When an electrode site was stimulated multiple times with different pairs, we computed the average connectivity from a given stimulus site (Fig. 1B).
Figure 4.
Workflow to quantify inter-lobar effective connectivity based on CCSRs. (A) CCEPs: Averaged ECoG trace and single-trial ECoG traces are aligned to the onset of SPES. (B) To identify significant CCSRs, a time-frequency transformation followed by a cluster-based permutation test was employed for the 2 to 60 Hz and −50 to +300 ms time-frequency range (pink square).78 (i) Early CCSR: within 20 to 60 Hz and +10 and +50 ms (white dashed square).48,49 This time-frequency range was largely free of stimulation-related broadband artefacts (yellow arrow). (ii) Late CCSR: within 2 and 20 Hz and +50 and +300 ms (white dotted square). (C) Early CCSR peaks were taken as evidence of direct effective connectivity; late CCSRs peaks were taken as evidence of secondary effective connectivity.
Determination of factors predictive of the strength of direct inter-lobar effective connectivity
Prediction of direct effective connectivity strength between temporal and extratemporal lobe sites was accomplished using a set of mixed model analyses (Fig. 5A and B). Mixed models were conducted using the Matlab Statistics Toolbox (The MathWorks, Inc., Natick, MA, USA), and the significance was set at a false discovery rate (FDR)-corrected P-value of 0.05. The dependent variable was the per cent change of early CCSR-based effective connectivity in a given direction. The fixed-effect predictor variables included: the interaction of high-gamma activity (i.e. multiplication of per cent change) during a given PCA-defined linguistic stage at the stimulus and recording sites, the Euclidean distance (mm) between the stimulus and recording site, patient age (years), the hemisphere of electrode placement (left/right), severity of epilepsy-related burden, estimated by the number of AEDs immediately before the electrode implantation,65,79 naming speed, assessed by the median response time during the auditory naming task (s), the lobe in which the seizure onset zone was identified (i.e. frontal, temporal or parietal/occipital lobe), and the presence of an MRI-visible cortical lesion.65 Intercept and patient were treated as random factors.
Figure 5.
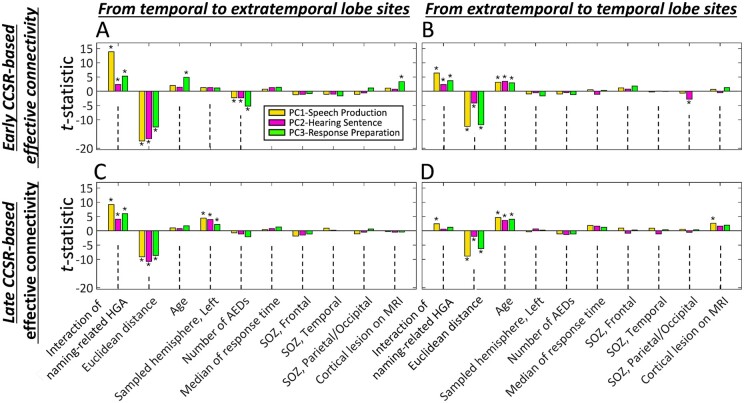
Mixed model-based prediction of the strength of early and late CCSR-defined effective connectivity. The bar graphs show the effects of variables for predicting the strength of (A and B) early CCSR-defined and (C and D) late CCSR-defined inter-lobar effective connectivity between language sites supporting the same linguistic stages. (A and C) Prediction of effective connectivity in the temporal to extratemporal lobe direction. (B and D) Connectivity in the opposite direction. Bars indicate the effect of predictors in each principal component-defined network: Yellow bars: PC1-SpeechProduction. Pink bars: PC2-HearingSentence. Green bars: PC3-ResponsePreparation. *FDR-corrected P-value < 0.05. HGA = high-gamma activity; PC = principal component; SOZ = seizure onset zone.
Two sets of ancillary mixed model analyses were conducted to assess the effect of language proficiency-adjusted age and to predict the strength of late CCSR-defined effective connectivity. Twenty-five patients underwent the Clinical Evaluation of Language Fundamentals-Fourth Edition (CELF-4) before the ECoG recording.80 The mixed model to assess the effect of language proficiency-adjusted age on the strength of early CCSR-based direct effective connectivity was analogous to those described above but incorporated ‘the square root of (patient age × standardized CELF-4 language score)’ instead of ‘patient age’. The second set of ancillary mixed models to predict the strength of late CCSR-based effective connectivity (Fig. 4) were analogous to the above but replaced early CCSR with late CCSR per cent change as the dependent variable (Fig. 5C and D).
6D tractography visualization of rapid neural propagations
Using DWI data acquired from 27 patients, we created animations to visualize, at the group level, the spatial trajectory of stimulation-induced neuronal propagations along with corresponding high-gamma interaction values (Supplementary Videos 1 and 2 at https://doi.org/10.6084/m9.figshare.14766093.v1). The basic dynamic tractography methodology was reported previously.55 Briefly, before the subdural electrode placement, diffusion-weighted images were collected on a 3 T GE Signa MRI scanner, with a multislice single-shot diffusion-weighted echo-planar imaging sequence at repetition time = 12 500 ms, echo time = 88.7 ms, field of view = 24 × 24 cm, 128 × 128 acquisition matrix, 46 contiguous 3 mm thickness axial slices to cover the entire brain using 55 isotropic gradient directions with b = 1000 s/mm2, number of excitations = 1, and single b = 0 image.63 We estimated anatomically-constrained probabilistic DWI tractography,81,82 using the fibre orientation distributions (FOD) image via the iFOD2 algorithm,83 a seed density of 6000 seeds/voxel, minimum/maximum streamline length of 20/250 mm, and a maximum angle of 70°. Electrode coordinates were first moved from the T1 to the DWI space using an affine transformation derived from the DWI b0 images and T1 images,84 then translated from the grey matter surface to the nearest white matter coordinate using FreeSurfer algorithms. We placed a 4 mm radius sphere around each DWI-space electrode coordinate to extract electrode pair-specific streamlines. To estimate the velocity of early CCSR-based neural propagations, we divided the minimum streamline length of each electrode-pair streamline bundle by the local peak latency of the corresponding early CCSR. The resulting movies visualized early CCSR-based neural propagations’ dynamics and strengths along DWI-based streamlines between cortical sites engaged during each PCA-defined linguistic processing stage.
Using open-source DWI data collected from the 1065 healthy participants in the Human Connectome Project (referred to as HCP1065; http://brain.labsolver.org/diffusion-mri-templates/hcp-842-hcp-1021, accessed 11 October 2021),85 we likewise determined the dynamics, strengths, and trajectories of early CCSR-based neural propagations as computed with data from all 37 patients. We previously reported the methodological details of the usage of open-source DWI data.56 In short, we placed a 4 mm radius sphere around each standardized Montreal Neurological Institute (MNI)-space electrode coordinate to extract electrode pair-specific streamlines. Significant streamlines were estimated with the following criteria: a fractional anisotropy threshold of 0.5, minimum/maximum streamline length of 20/250 mm, a maximum angle of 70°, and step size of 0.3 mm. The Pearson correlation determined how well the velocities computed with individual DWI data corresponded to those calculated with open-source DWI data.
We quantified the proportion of each anatomical pathway that supported observed direct inter-lobar effective connectivity between given PCA-based language stages, using the Clopper-Pearson method.86 We used all 37 patients’ CCSR data and open-source HCP1065 DWI data for this analysis. All streamlines satisfying the following criteria were included: naming-related high-gamma activity was augmented at temporal and extratemporal lobe sites during specific PCA-based language stages (Fig. 3), and a given streamline was associated with a significant local peak of early CCSR (Fig. 4). Each included streamline was classified into one of the following anatomical pathways of interest: arcuate fasciculus, cingulum, extreme capsule, frontal aslant tract, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, middle longitudinal fasciculus, parietal aslant tract, superior longitudinal fasciculus, uncinate fasciculus, and vertical occipital fasciculus (http://www.bic.mni.mcgill.ca/ServicesAtlases/ICBM152NLin2009, accessed 11 October 2021).87,88
Data availability
All data and codes are available upon request to the corresponding author.
Results
Linguistic processing stages based on the principal component analysis
The first three principal components explained ∼95% of the variance in naming-related high-gamma modulation patterns (Fig. 3B). The following analyses focus on the effective connectivity between these PCA-based language sites. As shown in the PC coefficient matrix (Fig. 3A and C), PC1 was primarily attributed to high-gamma augmentation elicited during overt speech-response and self-monitoring one’s own voice. Sites showing the PC1-SpeechProduction high-gamma pattern were distributed bilaterally in the Rolandic gyri as well as STG (Fig. 3D). PC2 was attributed to high-gamma augmentation elicited during a sentence stimulus listening period (Fig. 3C); PC2-HearingSentence sites were distributed mainly in the STG and posterior MTG (Fig. 3E). PC3 was attributed to high-gamma augmentation elicited mainly between sentence stimulus offset and response onset (Fig. 3C); PC3-ResponsePreparation sites were distributed predominantly in the left hemisphere, including widespread posterior temporal and frontal neocortical regions (Fig. 3F).
Factors predictive of early CCSR-defined inter-lobar effective connectivity
The magnitude of naming-related coactivation predicted the strength of early CCSR-defined (i.e. direct) inter-lobar effective connectivity. Across all six (three PCA-based language stages and two directions) mixed models, the interaction of naming-related high-gamma activity was a significant predictor of the strength of direct effective connectivity (Fig. 5A and B and Supplementary Tables 2–7). For example, an increased interaction of temporal and extratemporal lobe naming-related high-gamma activity during the PC3-ResponsePreparation stage predicted an increased strength of direct inter-lobar effective connectivity from PC3-ResponsePreparation temporal lobe language sites (mixed model estimate = +106.1%; FDR-corrected P-value < 0.001; t-value = 5.3; DF = 7697) (Fig. 5A and Supplementary Table 4).
Patient age was an independent predictor of the strength of direct effective connectivity between the temporal and extratemporal lobe language sites. Positive associations between age and connectivity strength were observed bidirectionally across the PC3-ResponsePreparation sites (Fig. 5A), whereas across the PC1-SpeechProduction and PC2-HearingSentence networks, this positive association was observed only in the extratemporal-to-temporal direction (Fig. 5B). For example, each 1-year increase in patient age predicted an increased strength of direct inter-lobar effective connectivity from PC3-ResponsePreparation temporal lobe language sites (mixed model estimate = +1.2%/year; FDR-corrected P-value < 0.001; t-value = 4.9; DF = 7697) (Fig. 5A andSupplementary Table 4). However, when language proficiency-adjusted age was used, the relationship to the strength of direct effective connectivity was not replicated (Supplementary Tables 8–10).
The aforementioned significant effects were all independent of those of inter-electrode Euclidean distance as well as epilepsy-related factors. For example, longer Euclidean distance between stimulus and recording sites was associated with a decreased strength of direct effective connectivity across all PCA-defined language networks. The number of AEDs was found to negatively predict the connectivity strength in the temporal-to-extratemporal direction across all three PCA-defined language networks (Fig. 5A and B and Supplementary Tables 2–7).
Factors predictive of late CCSR-defined inter-lobar effective connectivity
The mixed model analyses confirmed the positive relationship between the magnitude of naming-related high-gamma activity interaction and the strength of late CCSR-defined inter-lobar effective connectivity from the temporal to the extratemporal lobe sites in all three PCA-defined language networks (Fig. 5C). The high-gamma interaction effect in the extratemporal-to-temporal direction, however, was significant only across the PC1-SpeechProduction sites (Fig. 5D). In contrast to early CCSR-defined effective connectivity, which did not show any hemispheric differences, a bias towards the left hemisphere was observed for late CCSR-defined effective connectivity projecting from all PCA-defined language sites in the temporal lobe (mixed model estimate = 18.0–32.5%; Fig. 5C andSupplementary Tables 11–13). Older age was associated with an increased strength of late CCSR-defined effective connectivity from all extratemporal lobe PCA-defined language sites (Fig. 5D andSupplementary Tables 14–16).
Direct inter-lobar effective connectivity across sites supporting overlapping linguistic stages
Figure 6 summarizes the statistical significance and effect size of the PCA-defined linguistic stages at temporal and extratemporal lobe sites on the strength of direct inter-lobar effective connectivity in each direction. The mixed model analysis demonstrated the evidence of direct effective connectivity between temporal and extratemporal lobe sites supporting different linguistic stages (Fig. 6A and B). Nonetheless, extratemporal lobe sites supporting the PC3-ResponsePreparation stage (Fig. 3C) had the strongest effective connectivity from the PC3-ResponsePreparation temporal lobe sites; this connectivity was approximately twice more robust than that with the PC1-SpeechProduction or PC2-HearingSentence temporal lobe sites (Fig. 6C). Likewise, the PC3-ResponsePreparation temporal lobe sites had the strongest effective connectivity from the PC3-ResponsePreparation extratemporal lobe sites (Fig. 6C). Conversely, extratemporal lobe sites supporting the PC1-SpeechProduction linguistic stage were non-specifically connected with PC1-SpeechProduction, PC2-HearingSentence, and PC3-ResponsePreparation temporal lobe sites (Fig. 6C).
Figure 6.

Direct inter-lobar effective connectivity across sites supporting overlapping linguistic stages. (A and B) The statistical effect (i.e. mixed model t-values) of naming-related high-gamma activity (HGA) interaction between sites supporting the same and different principal component (PC) language sites on the strength of direct inter-lobar effective connectivity. *FDR-corrected P < 0.05. (C) The effect size (i.e. significant mixed model estimate value) of naming-related high-gamma activity interaction on the strength of direct inter-lobar effective connectivity is indicated by the arrowhead length and the arrow width (a–n). Connectivity direction is presented by arrow colour: white = a temporal-to-extratemporal direction; purple = the opposite direction. For example, effective connectivity to PC3-ResponsePreparation extratemporal language sites was strongest from the PC3-ResponsePreparation temporal lobe sites (white arrow e; effect size = +106.1%) while small from the PC1-SpeechProduction temporal lobe sites (white arrow i; effect size = +61.0%) and the PC2-HearingSentence temporal lobe sites (white arrow c; effect size = +48.2%). Effective connectivity to the PC3-ResponsePreparation temporal lobe sites was strongest from the PC3-ResponsePreparation extratemporal lobe sites (purple arrow f; effect size = +254.3%) while moderate from the PC1-SpeechProduction extratemporal lobe sites (purple arrow h; effect size = +99.5%) and the PC2-HearingSentence extratemporal lobe sites (purple arrow d; effect size = +169.4%).
6D dynamic tractography
Supplementary Videos 1 and 2 (https://doi.org/10.6084/m9.figshare.14766093.v1) best demonstrate the behavioural mode of early CCSR-based neural propagations across PCA-defined language stages. We localized streamline estimates of white matter pathways directly connecting regions supporting PCA-defined linguistic stages using DWI data acquired from 27 patients. Readers will find that red streamlines are associated with large moving dots. The video snapshots (Fig. 7) present the anatomical clustering of ‘collaborative’ (high-gamma co-augmentation at the stimulus and the recording sites; red streamlines) and ‘uncollaborative’ (high-gamma augmentation at the stimulus site but high-gamma attenuation at the recording end point; blue streamlines) relationships. For example, the PC1-SpeechProduction temporal lobe language sites collaborated mainly with the precentral gyrus bilaterally (Fig. 7A), whereas the PC3-ResponsePreparation temporal lobe language sites collaborated with widespread regions in the left hemisphere (Fig. 7C).
Figure 7.
Snapshots of 6D dynamic tractography. The snapshots of each animation demonstrate the trajectories of rapid neural propagations elicited by SPES and its relationship to naming-related high-gamma modulations at the stimulus and recording sites. Each plot displays group-level effective connectivity data derived from 27 patients. The streamline colour reflects the interaction (i.e. multiplication) of high-gamma activity (HGA) at temporal and extratemporal lobe sites during a given linguistic stage. Thus, red streamlines reflect high-gamma co-augmentation at both stimulus and recording sites, whereas blue streamlines indicate high-gamma augmentation at the stimulus sites but attenuation at the recording sites. The size of each white dot reflects the strength of effective connectivity rated by the magnitude of significant early CCSR local peak. Each white dot indicates the location of stimulation-induced neural propagation estimated by the propagation velocity. To optimize visibility, each movie delineates the effective connectivity pathways satisfying the following criteria: (i) stimulus sites with naming-related high-gamma augmentation during a specific PCA-based linguistic stage; (ii) the interaction of naming-related high-gamma activity was ≥|±200|; and (iii) a given streamline was associated with a significant early CCSR. (A–C) Temporal to extratemporal lobe propagations (Supplementary Video 1). (D–F) Extratemporal to temporal lobe propagations (Supplementary Video 2). (A and D) Neural propagations elicited by stimulation of PC1-SpeechProduction language sites. (B and E) PC2-HearingSentence. (C and F) PC3-ResponsePreparation. Supplementary Videos 1 and 2 are available at https://doi.org/10.6084/m9.figshare.14766093.v1.
The additional analysis using open-source DWI scans of healthy individuals and CCSR-based effective connectivity derived from all 37 study patients provided generalizable language-network models (Supplementary Fig. 2). Before using the DWI data to analyse the anatomical distribution of inter-lobar effective connectivity pathways between language sites, we first verified that, where the open-source DWI data and the individual data overlapped, they had similar relationships to the CCSR data. Specifically, we found that the velocity of CCSR-based neural propagations was highly correlated between individual and open-source DWI data (r = 0.81, P-value < 0.001 in the 338 commonly available streamlines in the temporal-to-extratemporal direction; r = 0.80; P-value < 0.001 in the 189 streamlines in the extratemporal-to-temporal direction). Assessment of all 37 patients’ CCSR data using the open-source DWI data indicated that the arcuate fasciculus allowed approximately two-thirds of the temporal-to-extratemporal lobe neural propagations; the uncinate fasciculus was used for <2% of such propagations (Table 1 and Supplementary Fig. 2). Conversely, the arcuate fasciculus was used for up to half of the extratemporal-to-temporal lobe neural propagations; the uncinate fasciculus was used for up to 6% of such propagations (Table 1 and Supplementary Fig. 2).
Table 1.
Composition proportion of naming-related pathways from and to the temporal lobe based on the open-source DWI data
| Stim. | Rec. | n | AF | C | EMC | FAT | IFOF | ILF | MdLF | PAT | SLF | UF | VOF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95%CI: LL–UL (%) | LL–UL (%) | LL–UL (%) | LL–UL (%) | LL–UL (%) | LL–UL (%) | LL–UL (%) | LL–UL (%) | LL–UL (%) | LL–UL (%) | LL–UL (%) | |||
| Temporal to extratemporal | |||||||||||||
| PC1 | PC1 | 229 | 74.6–85.3 | 0–1.6 | 0–1.6 | 0.01–2.4 | 1.2–6.2 | 0.01–2.4 | 0–1.6 | 0–1.6 | 0.3–3.8 | 0–1.6 | 0–1.6 |
| PC2 | 204 | 52.7–66.6 | 0–1.8 | 0–1.8 | 0–1.8 | 2.4–8.8 | 1.1–6.3 | 0.01–2.7 | 0.1–3.5 | 0.1–3.5 | 0–1.8 | 0–1.8 | |
| PC3 | 372 | 72.3–81.1 | 0–1.0 | 0–1.0 | 0.01–1.5 | 1.7–5.6 | 0.01–1.5 | 0.01–1.5 | 0.01–1.5 | 0.3–2.7 | 0–1.0 | 0–1.0 | |
| PC2 | PC1 | 384 | 72.8–81.4 | 0–1.0 | 0–1.0 | 1.3–4.7 | 1.4–5.1 | 0.4–3.0 | 0–1.0 | 0.2–2.3 | 1.6–5.4 | 0–1.0 | 0–1.0 |
| PC2 | 330 | 53.3–64.2 | 0–1.1 | 0–1.1 | 0–1.1 | 1.7–5.9 | 1.9–6.3 | 0.1–2.2 | 1.1–4.7 | 0.1–2.2 | 0–1.1 | 0–1.1 | |
| PC3 | 642 | 68.5–75.6 | 0–0.6 | 0–0.6 | 0.6–2.6 | 1.7–4.4 | 0.9–3.1 | 0–0.9 | 0.9–3.1 | 1.2–3.6 | 0–0.6 | 0–0.6 | |
| PC3 | PC1 | 276 | 70.6–81.0 | 0–1.3 | 0–1.3 | 1.3–5.6 | 1.0–5.2 | 0.2–3.1 | 0–1.3 | 0.2–3.1 | 2.5–7.9 | 0–1.3 | 0–1.3 |
| PC2 | 237 | 53.8–66.6 | 0–1.5 | 0–1.5 | 0–1.5 | 2.3–8.2 | 2.3–8.2 | 0–1.5 | 0.7–4.9 | 0.1–3.0 | 0–1.5 | 0–1.5 | |
| PC3 | 463 | 68.7–77.0 | 0–0.8 | 0–0.8 | 0.5–2.8 | 1.4–4.5 | 0.6–3.1 | 0.01–1.2 | 0.9–3.7 | 2.0–5.6 | 0–0.8 | 0–0.8 | |
| Extratemporal to temporal | |||||||||||||
| PC1 | PC1 | 55 | 27–54.1 | 0–6.5 | 0–6.5 | 0–6.5 | 0.4–12.5 | 0.4–12.5 | 0–6.5 | 0.4–12.5 | 0–6.5 | 0–6.5 | 0–6.5 |
| PC2 | 107 | 40.6–60.3 | 0–3.4 | 0–3.4 | 0–3.4 | 0–3.4 | 0–3.4 | 0–3.4 | 0.6–8.0 | 0–3.4 | 0–3.4 | 0–3.4 | |
| PC3 | 100 | 45.7–65.9 | 0–3.6 | 0–3.6 | 0–3.6 | 0.2–7.0 | 0.2–7.0 | 0–3.6 | 0.2–7.0 | 0–3.6 | 0–3.6 | 0–3.6 | |
| PC2 | PC1 | 64 | 8.9–28.7 | 0–5.6 | 0–5.6 | 0–5.6 | 0.04–8.4 | 1.7–15.2 | 0.4–10.8 | 8.9–28.7 | 0–5.6 | 0–5.6 | 0–5.6 |
| PC2 | 114 | 21.6–39.1 | 0–3.2 | 0–3.2 | 0–3.2 | 1.0–8.7 | 1.44–9.9 | 0.6–7.5 | 9.6–23.8 | 0–3.2 | 0–3.2 | 0–3.2 | |
| PC3 | 90 | 21.8–41.7 | 0–4.0 | 0–4.0 | 0–4.0 | 0.3–7.8 | 1.8–12.5 | 0.7–9.4 | 10.5–27.3 | 0–4.0 | 0–4.0 | 0–4.0 | |
| PC3 | PC1 | 75 | 26.4–49.3 | 0–4.8 | 0–4.8 | 0–4.8 | 0.8–11.3 | 0.83–11.3 | 0–4.8 | 4.7–19.9 | 0–4.8 | 0–4.8 | 0–4.8 |
| PC2 | 147 | 40.0–56.7 | 0–2.5 | 0–2.5 | 0–2.5 | 0.4–5.9 | 0.2–4.8 | 0–2.5 | 5.8–16.3 | 0–2.5 | 0–2.5 | 0–2.5 | |
| PC3 | 132 | 44.2–61.8 | 0–2.8 | 0–2.8 | 0–2.8 | 0.5–6.5 | 0.5–6.5 | 0–2.8 | 3.7–13.5 | 0–2.8 | 0–2.8 | 0–2.8 | |
Each pathway from or towards the temporal lobe sites supporting a specific principal component-defined linguistic stage was classified into one of the following anatomical pathways: (i) AF = arcuate fasciculus; (ii) C = cingulum; (iii) EMC = extreme capsule; (iv) FAT = frontal aslant tract; (v) IFOF = inferior fronto-occipital fasciculus; (vi) ILF = inferior longitudinal fasciculus; (vii) MdLF = middle longitudinal fasciculus; (viii) PAT = parietal aslant tract; (ix) SLF = superior longitudinal fasciulus; (x) UF = uncinate fasciculus; and (xi) VOF = vertical occipital fasciculus. The Clopper-Pearson method computed the 95% confidence interval of the averaged proportion.82 LL = lower limit of 95% confidence interval; PCA = principal component; PC1 = PC1-SpeechProduction; PC2 = PC2-HearingSentence; PC3 = PC3-ResponsePreparation; UL = upper limit.
Discussion
Novelty
We quantitatively determined the strength, dynamics, direction, and anatomical distribution of inter-lobar effective connectivity between language areas in the developing brain. Our 6D tractography animations visualized the trajectory of inter-lobar CCSR-based neural propagations from specific language-related sites, along with correlated metrics. Extratemporal lobe sites supporting response preparation had direct effective connectivity preferentially from and towards temporal lobe sites supporting the same linguistic stage. Conversely, extratemporal lobe sites activated during overt responses had direct effective connectivity with temporal lobe language sites regardless of the supporting linguistic stages. Age-dependent strengthening of temporal-to-extratemporal lobe direct effective connectivity preferentially took place between the language areas supporting response preparation.
Categorization of linguistic processing stages
Cortical regions classified as PC1-SpeechProduction were characterized by neural activation during overt response (Fig. 3). The PC1-SpeechProduction sites were distributed mainly in the bilateral Rolandic gyri and STG, which are suggested to be an integral part of the ‘phonological loop’.7,8,14,21–25 High-frequency electrical stimulation of cortical areas in these regions has been shown to result in speech arrest, face motor symptoms, or auditory hallucination.5,10 We interpreted the PC1-SpeechProduction network to be highly engaged in supporting motor output and interpreting auditory perceptions, in particular the monitoring of one’s own vocal sounds.
The PC2-HearingSentence sites were characterized by neural activation mainly during the sentence listening period (Fig. 3C) and distributed mostly in the bilateral STG and posterior MTG (Fig. 3E). High-frequency stimulation of these areas was reported to elicit auditory hallucination, impaired syllable discrimination, or receptive aphasia.5,89 Prior ECoG, functional MRI, and lesion-deficit studies have suggested that these anatomical regions map acoustic information onto phonological representations.90–93 We interpret the PC2-HearingSentence sites as engaged in support of perceptual processing of auditory sentence stimuli and semantic understanding of each word. In contrast, some investigators suggest that much of the higher-order processing to understand the entire semantic context occurs, mainly after a full sentence is heard.94,95
The PC3-ResponsePreparation sites were characterized by neural activation between sentence offset and response onset (Fig. 3C) and distributed predominantly in the left hemisphere, including widespread posterior temporal and frontal neocortical regions (Fig. 3F). We previously reported that electrical stimulation mapping at these regions frequently elicited expressive aphasia characterized by the inability to generate a relevant answer on time while the patient could repeat heard sounds and recall the heard sentence.5 We interpret the PC3-ResponsePreparation sites as engaged for retrieval of semantically coherent words and lexically-specified phonemes.9
Proposed model of the inter-lobar network dynamics supporting auditory naming
Our multimodal study has expanded the existing models suggesting that the arcuate fasciculus consists of a central pathway allowing temporal-to-extratemporal communications essential for auditory naming.3,4,12,13 The novel aspects of our study include the quantification of the functional specificity and direction dependency of the underlying white matter fasciculi. We propose that extratemporal lobe regions supporting response preparation have direct effective connectivity from widespread temporal lobe language regions but twice more preferentially from those supporting the same linguistic stage (Fig. 6C). Conversely, extratemporal lobe regions supporting overt response have effective connectivity uniformly from the temporal lobe language regions. Our results support the models suggesting that much of the language-related neural propagations from the temporal lobe occur directly through the arcuate fasciculus.4,13 In contrast, we suggest that neural propagations towards the temporal lobe originate from more diffuse extratemporal regions, including those outside the arcuate fasciculus territory such as the anterior IFG, parietal, and occipital regions (Table 1). Verbal discourse is suggested to require self-assessments and monitoring of voice pitch, semantic coherence of answers, and contextual appropriateness.7,8,96,97 Further studies are warranted to determine if the extratemporal-to-temporal effective connectivity outside the arcuate fasciculus in each hemisphere would be involved in the audio-visual integration supporting the pragmatic aspect of language.98,99
Our results do not support a central role for temporal-to-extratemporal connectivity along the uncinate fasciculus in auditory naming.11 Previous studies reported unimpaired language function during high-frequency stimulation of the uncinate fasciculus100 and after left anterior temporal lobectomy.101 Our auditory naming-related network dynamics reported in the present study should not be generalized to those enabling visual language. It remains unknown how much the uncinate fasciculus would contribute to inter-lobar effective connectivity between visual language areas. We previously reported that picture naming, compared to auditory naming, elicited more intense high-gamma augmentation in the anterior fusiform and entorhinal cortices.69 These ventral temporal lobe structures are anatomically connected to the anterior IFG via the uncinate fasciculus.102
Our study by no means disproves the presence of indirect connectivity pathways between temporal and extratemporal lobe sites. We observed early CCSRs between the STG and inferior parietal lobe, which might contribute to indirect information transfer between the STG and the frontal lobe.2,54
Proposed model of the inter-lobar connectivity development
We demonstrated that older age increased the strength of direct temporal-to-extratemporal lobe effective connectivity specifically between the PC3-ResponsePreparation sites (Fig. 5A and Supplementary Table 4). Thus, age-dependent strengthening of inter-lobar effective connectivity after the age of 5 may preferentially occur between language areas supporting retrieval of semantically-coherent words and lexically-specified phonemes.
It is plausible to hypothesize that the numerous transfers of neural representations of linguistic information may strengthen inter-lobar effective connectivity via white matter pathways. Children and young adults are estimated to exchange several thousands of spoken sentences a day,103 implying heavy utilization of the networks between the temporal and extratemporal lobe structures. A putative mechanism for the age-dependent increases in connectivity includes a strengthening of myelination. Studies of cultured cells have demonstrated that increased action potential firing strengthens myelination.104–106 A DWI study of 8 to 12-year-old readers reported that a 6-month training including 100 h of intensive remedial reading instruction additively increased the fractional anisotropy (a measure of underlying myelination) of the left-hemispheric cortico-cortical white matter tracts.107
Hemispheric asymmetry of late CCSR-defined effective connectivity from the temporal lobe
One of our novel findings was that the left hemisphere, compared to the right, had increased strength of late CCSR-based effective connectivity from temporal lobe language sites (Fig. 5C). Late CCSRs, characterized by augmentation of low-frequency activity at 4–7 Hz, are suggested to indicate post-excitatory neural inhibition,43,45 as reflected by reduced firing in simultaneous single-neuron recording.51,53 Likewise, in the current study, the average frequency of the significant late CCSR local peaks in temporal-to-extratemporal lobe direction was 6.8 Hz. The observation of left-hemispheric bias in late CCSRs raises the possibility that the left hemisphere may have more robust inhibitory networks governing inter-lobar effective connectivity.
It is possible that the left-hemispheric dominant late CCSRs, which may reflect inhibitory activity, account for the left-hemispheric dominant naming-related high-gamma augmentation during the response preparation (Fig. 3F).5,33 Task-related high-gamma augmentation has been attributed to the firing of inhibitory interneurons at high-gamma frequencies.108–113 Some reported that increased GABA level measured by magnetic resonance spectroscopy (MRS) predicted a high peak frequency and power of task-induced gamma (<70 Hz) activity measured by magnetoencephalography,114,115 whereas others failed to replicate such findings.116
Methodological considerations
Our mixed model analysis effectively controlled for the effect of epilepsy-related factors on CCSR and DWI measures. The reported effects of linguistic processing stages and patient age on the strength of inter-lobar effective connectivity were all independent of epilepsy-related factors as well as the Euclidean distance between stimulus and recording sites. For example, an increased number of oral AEDs was associated with reduced strength of direct effective connectivity from all PCA-defined temporal lobe language sites (Fig. 5A). Either AEDs or severe epilepsy requiring polytherapy may account for this observation. A study of healthy adults reported that sodium-channel blockers, but not GABA-mediated AEDs, elevated the motor threshold on transcranial magnetic stimulation (TMS)-based mapping.117 Sodium-channel blocker monotherapy did not alter the amplitude of short-latency somatosensory evoked potentials.118 Second, a larger number of oral AEDs generally reflect greater severity of seizure burden, including more severe cognitive dysfunction.79
While task-based functional connectivity, CCSR-based effective connectivity, and DWI-based structural connectivity have specific strengths, each method has an inevitable limitation. Studies using Granger causality reported that cortical sites showing task-related high-gamma augmentation during a shared time window could propagate neural activity between them.14,15,22,119 However, Granger causality cannot consistently distinguish direct versus indirect connectivity. Early CCSRs are a valuable measure to quantify the strength of long-range direct effective connectivity,43,45 but do not reveal the anatomical white matter structure, which supports the observed connectivity. Conversely, DWI-based tractography can characterize anatomical white matter networks,13 but does not measure the directionality or function of the pathway. Our 6D dynamic tractography approach integrates all three modalities into a single model and visualization, which revealed the white matter pathways supporting rapid neural propagations between sites engaged to specific linguistic stages. We hypothesize that the dynamic trajectories observable via 6D tractography are more likely to be used for auditory naming if the remote areas connected are simultaneously engaged in the same linguistic stage.14,15,22,119 Indeed, we found that early CCSRs were larger when the stimulus and recording sites showed high-gamma augmentation during the same linguistic stage. It is possible that although a direct connection exists (as evidenced by a CCSR), this is not the pathway being utilized during the task. One cannot blindly presume that all of the dynamic trajectories on the 6D tractography (particularly those via blue streamlines; Fig. 7) are active during auditory naming. SPES forces a cortical site to induce neural propagations, some of which may or may not occur during a cognitive task. A previous study reported that the power (i.e. a measure proportional to the square of the amplitude) of early CCSR was ∼10% greater when SPES was employed during light sleep compared to during wakefulness.50
We believe that the risk of our late CCSRs being contaminated by stimulation-induced pathological/epileptogenic high-frequency oscillations (HFOs) is small,120 as our CCSR analysis was restricted to the non-epileptic sites. The mean latencies of our late CCSRs from temporal lobe sites were <120 ms, whereas stimulation-induced pathological HFOs typically occur ∼250 ms and after.120 TMS-based single-pulse stimulation at a central region in healthy adults was reported to elicit low-frequency responses in remote areas at a latency of roughly 100 ms.121
A benefit but also a limitation of 6D tractography is that it leverages the intersection of data from CCSR and DWI-based tractography. The use of early CCSRs to constrain tractography helps minimize the influence of false positives in tractography results.55 However, the low resolution and signal-to-noise ratio of tractography may mean that false negatives are potentially present. While we can have confidence in the positive results observed in the 6D dynamic tractography, they likely represent a subset of the full network.
We cannot rule out the possibility that the inter-individual variance in the language organization across our study patients was beyond that across healthy individuals. Nonetheless, the velocity of neural propagations was correlated between the datasets derived from individual and open-source DWI (Pearson’s r = 0.8). This observation supports the notion that the anatomical trajectories of streamlines supporting observed effective connectivity were spatially similar between epilepsy patients and healthy participants in the human connectome project.85
We focused on assessing large-scale, inter-lobar connectivity networks from and towards the temporal lobe. We excluded recording sites within 1.5 cm from a given stimulus pair from the analysis.76,77 This analytical approach may have increased false-negative detection of CCSRs but reduced the risk of false-positive detection, taking into account the artefactual signal deflection related to SPES. This study does not propose a model of short-range network dynamics supporting naming. SPES with a smaller intensity, an ECoG amplifier with a higher sampling rate, and increased density of electrode arrays may allow us to quantify the short-range effective connectivity network.
Supplementary Material
Acknowledgements
We are grateful to Alanna Carlson, MS, LLP, Karin Halsey, BS, REEGT., Jamie MacDougall, RN, BSN, CPN at Children’s Hospital of Michigan for the collaboration and assistance in performing the studies described above.
Funding
This work was supported by NIH grants NS064033 (to E.A.) and NS089659 (to J.-W.J.).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- AED
anti-epileptic drug
- CCEP
cortico-cortical evoked potential
- CCSR
cortico-cortical spectral response
- DWI
diffusion weighted imaging
- ECoG
electrocorticography
- IFG
inferior frontal gyrus; PCA = principal component analysis
- SPES
single-pulse electrical stimulation
- STG
superior temporal gyrus
References
- 1. Hamberger MJ, Seidel WT, Mckhann GM, Perrine K, Goodman RR.. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128(Pt 11):2742–2749. [DOI] [PubMed] [Google Scholar]
- 2. Hickok G, Poeppel D.. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. [DOI] [PubMed] [Google Scholar]
- 3. Rauschecker JP, Scott SK.. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12(6):718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang EF, Raygor KP, Berger MS.. Contemporary model of language organization: An overview for neurosurgeons. J Neurosurg. 2015;122(2):250–261. [DOI] [PubMed] [Google Scholar]
- 5. Nakai Y, Jeong J, Brown EC, et al. Three- and four-dimensional mapping of speech and language in patients with epilepsy. Brain. 2017;140(5):1351–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Towle VL, Yoon H-A, Castelle M, et al. ECoG gamma activity during a language task: Differentiating expressive and receptive speech areas. Brain. 2008;131(Pt 8):2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pei X, Leuthardt EC, Gaona CM, Brunner P, Wolpaw JR, Schalk G.. Spatiotemporal dynamics of electrocorticographic high gamma activity during overt and covert word repetition. Neuroimage. 2011;54(4):2960–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenlee JDW, Behroozmand R, Larson CR, et al. Sensory-motor interactions for vocal pitch monitoring in non-primary human auditory cortex. PLoS One. 2013;8(4):e60783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Indefrey P, Levelt WJM.. The spatial and temporal signatures of word production components. Cognition. 2004;92(1-2):101–144. [DOI] [PubMed] [Google Scholar]
- 10. Lu J, Zhao Z, Zhang J, et al. Functional maps of direct electrical stimulation-induced speech arrest and anomia: A multicentre retrospective study. Brain. 2021;144(8):2541–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skeide MA, Friederici AD.. The ontogeny of the cortical language network. Nat Rev Neurosci. 2016;17(5):323–332. [DOI] [PubMed] [Google Scholar]
- 12. Hagoort P. The neurobiology of language beyond single-word processing. Science. 2019;366(6461):55–58. [DOI] [PubMed] [Google Scholar]
- 13. Catani M, Mesulam M.. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44(8):953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flinker A, Korzeniewska A, Shestyuk AY, et al. Redefining the role of Broca’s area in speech. Proc Natl Acad Sci U S A. 2015;112(9):2871–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collard MJ, Fifer MS, Benz HL, et al. Cortical subnetwork dynamics during human language tasks. Neuroimage. 2016;135:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kambara T, Brown EC, Jeong J-W, Ofen N, Nakai Y, Asano E.. Spatio-temporal dynamics of working memory maintenance and scanning of verbal information. Clin Neurophysiol. 2017;128(6):882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trimmel K, Graan AL, van Caciagli L, et al. Left temporal lobe language network connectivity in temporal lobe epilepsy. Brain. 2018;141(8):2406–2418. [DOI] [PubMed] [Google Scholar]
- 18. Simmonds DJ, Hallquist MN, Asato M, Luna B.. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blakemore S-J. Imaging brain development: The adolescent brain. Neuroimage. 2012;61(2):397–406. [DOI] [PubMed] [Google Scholar]
- 20. Geschwind N. Disconnexion syndromes in animals and man: Part I. Neuropsychol Rev. 2010;20(2):128–157. [DOI] [PubMed] [Google Scholar]
- 21. Cogan GB, Thesen T, Carlson C, Doyle W, Devinsky O, Pesaran B.. Sensory–motor transformations for speech occur bilaterally. Nature. 2014;507(7490):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishida M, Korzeniewska A, Crone NE, et al. Brain network dynamics in the human articulatory loop. Clin Neurophysiol. 2017;128(8):1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schubotz RI, von CD, Lohmann G.. Auditory what, where, and when: A sensory somatotopy in lateral premotor cortex. Neuroimage. 2003;20(1):173–185. [DOI] [PubMed] [Google Scholar]
- 24. Bangert M, Peschel T, Schlaug G, et al. Shared networks for auditory and motor processing in professional pianists: Evidence from fMRI conjunction. Neuroimage. 2006;30(3):917–926. [DOI] [PubMed] [Google Scholar]
- 25. Pulvermüller F, Huss M, Kherif F, Martin FM, del P, et al. Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci U S A. 2006;103(20):7865–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ojemann G, Ojemann J, Lettich E, Berger M.. Cortical language localization in left, dominant hemisphere: An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71(3):316–326. [DOI] [PubMed] [Google Scholar]
- 27. Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjectsA functional MRI study. Brain. 1999;122(11):2033–2046. [DOI] [PubMed] [Google Scholar]
- 28. McDonald CR, Thesen T, Carlson C, et al. Multimodal imaging of repetition priming: Using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. Neuroimage. 2010;53(2):707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schoffelen J-M, Oostenveld R, Lam NHL, Uddén J, Hultén A, Hagoort P.. A 204-subject multimodal neuroimaging dataset to study language processing. Sci Data. 2019;6(1):17- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ralph MAL, Jefferies E, Patterson K, Rogers TT.. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18(1):42–55. [DOI] [PubMed] [Google Scholar]
- 31. Shimotake A, Matsumoto R, Ueno T, et al. Direct exploration of the role of the ventral anterior temporal lobe in semantic memory: Cortical stimulation and local field potential evidence from subdural grid electrodes. Cereb Cortex. 2015;25(10):3802–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leuthardt EC, Pei X-M, Breshears J, et al. Temporal evolution of gamma activity in human cortex during an overt and covert word repetition task. Front Hum Neurosci. 2012;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ikegaya N, Motoi H, Iijima K, et al. Spatiotemporal dynamics of auditory and picture naming-related high-gamma modulations: A study of Japanese-speaking patients. Clin Neurophysiol. 2019;130(8):1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lachaux J-P, Axmacher N, Mormann F, Halgren E, Crone NE.. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98(3):279–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacobs J, Kahana MJ, Ekstrom AD, Fried I, Ekstrom AD, Fried I.. Brain oscillations control timing of single-neuron activity in humans. J Neurosci. 2007;27(14):3839–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whittingstall K, Logothetis NK.. Frequency-band coupling in surface EEG reflects spiking activity in monkey visual cortex. Neuron. 2009;64(2):281–289. [DOI] [PubMed] [Google Scholar]
- 37. Manning JR, Jacobs J, Fried I, Kahana MJ.. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29(43):13613–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishida M, Juhász C, Sood S, Chugani HT, Asano E.. Cortical glucose metabolism positively correlates with gamma-oscillations in nonlesional focal epilepsy. Neuroimage. 2008;42(4):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS.. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28(45):11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scheeringa R, Fries P, Petersson K-M, et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69(3):572–583. [DOI] [PubMed] [Google Scholar]
- 41. Arya R, Horn PS, Crone NE.. ECoG high-gamma modulation versus electrical stimulation for presurgical language mapping. Epilepsy Behav. 2018;79:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kojima K, Brown EC, Rothermel R, et al. Clinical significance and developmental changes of auditory-language-related gamma activity. Clin Neurophysiol. 2013;124(5):857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Usami K, Korzeniewska A, Matsumoto R, et al. The neural tides of sleep and consciousness revealed by single-pulse electrical brain stimulation. Sleep. 2019;42(6):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koubeissi MZ, Lesser RP, Sinai A, Gaillard WD, Franaszczuk PJ, Crone NE.. Connectivity between perisylvian and bilateral basal temporal cortices. Cereb Cortex. 2012;22(4):918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsumoto R, Kunieda T, Nair D.. Single pulse electrical stimulation to probe functional and pathological connectivity in epilepsy. Seizure. 2017;44:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matsumoto R, Nair DR, LaPresto E, et al. Functional connectivity in the human language system: A cortico-cortical evoked potential study. Brain. 2004;127(Pt 10):2316–2330. [DOI] [PubMed] [Google Scholar]
- 47. Trebaul L, Deman P, Tuyisenge V, et al. Probabilistic functional tractography of the human cortex revisited. Neuroimage. 2018;181:414–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mitsuhashi T, Sonoda M, Iwaki H, Luat AF, Sood S, Asano E.. Effects of depth electrode montage and single-pulse electrical stimulation sites on neuronal responses and effective connectivity. Clin Neurophysiol. 2020;131(12):2781–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sugiura A, Silverstein BH, Jeong J-W, et al. Four-dimensional map of direct effective connectivity from posterior visual areas. Neuroimage. 2020;210:116548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Usami K, Matsumoto R, Kobayashi K, et al. Sleep modulates cortical connectivity and excitability in humans: Direct evidence from neural activity induced by single‐pulse electrical stimulation. Hum Brain Mapp. 2015;36(11):4714–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alarcón G, Martinez J, Kerai SV, et al. In vivo neuronal firing patterns during human epileptiform discharges replicated by electrical stimulation. Clin Neurophysiol. 2012;123(9):1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Keller CJ, Honey CJ, Mégevand P, Entz L, Ulbert I, Mehta AD.. Mapping human brain networks with cortico-cortical evoked potentials. Philos Trans R Soc B Biol Sci. 2014;369(1653):20130528- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Logothetis NK, Augath M, Murayama Y, et al. The effects of electrical microstimulation on cortical signal propagation. Nat Neurosci. 2010;13(10):1283–1291. [DOI] [PubMed] [Google Scholar]
- 54. Matsumoto R, Nair DR, Ikeda A, et al. Parieto‐frontal network in humans studied by cortico‐cortical evoked potential. Hum Brain Mapp. 2012;33(12):2856–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Silverstein BH, Asano E, Sugiura A, Sonoda M, Lee M-H, Jeong J-W.. Dynamic tractography: Integrating cortico-cortical evoked potentials and diffusion imaging. Neuroimage. 2020;215:116763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mitsuhashi T, Sonoda M, Jeong J, et al. Four-dimensional tractography animates propagations of neural activation via distinct interhemispheric pathways. Clin Neurophysiol. 2021;132(2):520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jolliffe IT. Principal Component Analysis, 2nd ed. Springer-Verlag; 2002. [Google Scholar]
- 58. Singer W. The role of oscillations and synchrony in the development of the nervous system. In: Benasich AA, Ribary U, Lupp J, eds. Emergent Brain Dynamics. Prebirth to Adolescence. Strungmann Forum Reports. MIT Press; 2018:15–32. [Google Scholar]
- 59. Ghosh SS, Kakunoori S, Augustinack J, et al. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11years of age. Neuroimage. 2010;53(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Asano E, Juhász C, Shah A, Sood S, Chugani HT.. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132(Pt 4):1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kambara T, Sood S, Alqatan Z, et al. Presurgical language mapping using event-related high-gamma activity: The Detroit procedure. Clin Neurophysiol. 2018;129(1):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stolk A, Griffin S, R van der M, et al. Integrated analysis of anatomical and electrophysiological human intracranial data. Nat Protoc. 2018;13(7):1699–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jeong J, Asano E, Brown EC, Tiwari VN, Chugani DC, Chugani HT.. Automatic detection of primary motor areas using diffusion MRI tractography: Comparison with functional MRI and electrical stimulation mapping. Epilepsia. 2013;54(8):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pieters TA, Conner CR, Tandon N.. Recursive grid partitioning on a cortical surface model: An optimized technique for the localization of implanted subdural electrodes: Clinical article. J Neurosurg. 2013;118(5):1086–1097. [DOI] [PubMed] [Google Scholar]
- 65. Motoi H, Jeong J-W, Juhász C, et al. Quantitative analysis of intracranial electrocorticography signals using the concept of statistical parametric mapping. Sci Rep. 2019;9(1):17385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Frauscher B, von EN, Zelmann R, et al. Atlas of the normal intracranial electroencephalogram: Neurophysiological awake activity in different cortical areas. Brain. 2018;141(4):1130–1144. [DOI] [PubMed] [Google Scholar]
- 67. Zijlmans M, Jacobs J, Kahn YU, Zelmann R, Dubeau F, Gotman J.. Ictal and interictal high frequency oscillations in patients with focal epilepsy. Clin Neurophysiol. 2011;122(4):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Uematsu M, Matsuzaki N, Brown EC, Kojima K, Asano E.. Human occipital cortices differentially exert saccadic suppression: Intracranial recording in children. Neuroimage. 2013;83:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nakai Y, Sugiura A, Brown EC, et al. Four‐dimensional functional cortical maps of visual and auditory language: Intracranial recording. Epilepsia. 2019;60(2):255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Papp N, Ktonas P.. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- 71. Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M.. BESA Source Coherence: A New Method to Study Cortical Oscillatory Coupling. Brain Topogr. 2004;16(4):233–238. [DOI] [PubMed] [Google Scholar]
- 72. Kojima K, Brown EC, Matsuzaki N, et al. Gamma activity modulated by picture and auditory naming tasks: Intracranial recording in patients with focal epilepsy. Clin Neurophysiol. 2013;124(9):1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kojima K, Brown EC, Rothermel R, et al. Multimodality language mapping in patients with left-hemispheric language dominance on Wada test. Clin Neurophysiol. 2012;123(10):1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tallon-Baudry C, Bertrand O, Delpuech C, Permier J.. Oscillatory gamma-band (30-70 Hz) activity induced by a visual search task in humans. J Neurosci. 1997;17(2):722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oostenveld R, Fries P, Maris E, Schoffelen J-M.. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011(1):156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Swann NC, Cai W, Conner CR, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage. 2012;59(3):2860–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Prime D, Woolfe M, O’Keefe S, Rowlands D, Dionisio S.. Quantifying volume conducted potential using stimulation artefact in cortico-cortical evoked potentials. J Neurosci Meth. 2020;337(108639):108639- [DOI] [PubMed] [Google Scholar]
- 78. Maris E, Oostenveld R.. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Meth. 2007;164(1):177–190. [DOI] [PubMed] [Google Scholar]
- 79. Kwan P, Brodie MJ.. Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet. 2001;357(9251):216–222. [DOI] [PubMed] [Google Scholar]
- 80. Paslawski T. The Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4). Can J Sch Psychology. 2005;20(1-2):129–134. [Google Scholar]
- 81. Patenaude B, Smith SM, Kennedy DN, Jenkinson M.. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang Y, Brady M, Smith S.. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE T Med Imaging. 2001;20(1):45–57. [DOI] [PubMed] [Google Scholar]
- 83. Tournier J, Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. In: 18th Annual Meeting for Proceedings of International Society for Magnetic Resonance in Medicine (ISMRM). Stockholm; 2010.
- 84. Avants BB, Epstein CL, Grossman M, Gee JC.. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yeh F-C, Panesar S, Fernandes D, et al. Population-averaged atlas of the macroscale human structural connectome and its network topology. Neuroimage. 2018;178:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Johnson NL, Kemp AW, Kortz S.. Univariate Discrete Distributions. Wiley-Interscience; 1993. [Google Scholar]
- 87. Fonov V, Evans AC, Botteron K, et al. Brain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fonov V, Evans A, McKinstry R, Almli C, Collins D.. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47:S102- [Google Scholar]
- 89. Boatman D, Lesser RP, Gordon B.. Auditory Speech Processing in the Left Temporal Lobe: An Electrical Interference Study. Brain Lang. 1995;51(2):269–290. [DOI] [PubMed] [Google Scholar]
- 90. Chang EF, Rieger JW, Johnson K, Berger MS, Barbaro NM, Knight RT.. Categorical speech representation in human superior temporal gyrus. Nat Neurosci. 2010;13(11):1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Desai R, Liebenthal E, Waldron E, Binder JR.. Left Posterior Temporal Regions are Sensitive to Auditory Categorization. J Cognitive Neurosci. 2008;20(7):1174–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rogalsky C, Basilakos A, Rorden C, et al. The Neuroanatomy of Speech Processing: A Large-Scale Lesion Study. bioRxiv. [Preprint] doi:10.1101/2020.04.02.022822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mirman D, Chen Q, Zhang Y, et al. Neural organization of spoken language revealed by lesion–symptom mapping. Nat Commun. 2015;6(1):6762- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wickens DD. Encoding categories of words: An empirical approach to meaning. Psychol Rev. 1970;77(1):1–15. [Google Scholar]
- 95. Baddeley A. The episodic buffer: A new component of working memory? Trends Cogn Sci. 2000;4(11):417–423. [DOI] [PubMed] [Google Scholar]
- 96. Prutting CA, Kirchner DM.. A clinical appraisal of the pragmatic aspects of language. J Speech Hear Disord. 1987;52(2):105–119. [DOI] [PubMed] [Google Scholar]
- 97. Yoshinaga-Itano C, Sedey AL, Mason CA, Wiggin M, Chung W.. Early intervention, parent talk, and pragmatic language in children with hearing loss. Pediatrics. 2020;146(Suppl 3):S270–S277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Calvert GA, Hansen PC, Iversen SD, Brammer MJ.. Detection of audio-visual integration sites in humans by application of electrophysiological criteria to the BOLD effect. Neuroimage. 2001;14(2):427–438. [DOI] [PubMed] [Google Scholar]
- 99. Chen T, Rao RR.. Audio-visual integration in multimodal communication. Proc IEEE. 1998;86(5):837–852. [Google Scholar]
- 100. Duffau H, Gatignol P, Moritz-Gasser S, Mandonnet E.. Is the left uncinate fasciculus essential for language? J Neurol. 2009;256(3):382–389. [DOI] [PubMed] [Google Scholar]
- 101. Parker GJM, Luzzi S, Alexander DC, Wheeler-Kingshott CAM, Ciccarelli O, Ralph MAL.. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24(3):656–666. [DOI] [PubMed] [Google Scholar]
- 102. Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M.. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48(1):82–96. [DOI] [PubMed] [Google Scholar]
- 103. Mehl MR, Vazire S, Ramírez-Esparza N, Slatcher RB, Pennebaker JW.. Are women really more talkative than men? Science. 2007;317(5834):82. [DOI] [PubMed] [Google Scholar]
- 104. Demerens C, Stankoff B, Logak M, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93(18):9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ishibashi T, Dakin KA, Stevens B, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stevens B, Porta S, Haak LL, Gallo V, Fields RD.. Adenosine a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36(5):855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Keller TA, Just MA.. Altering cortical connectivity: Remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Averkin RG, Szemenyei V, Bordé S, Tamás G.. Identified cellular correlates of neocortical ripple and high-gamma oscillations during spindles of natural sleep. Neuron. 2016;92(4):916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bartos M, Vida I, Jonas P.. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. [DOI] [PubMed] [Google Scholar]
- 110. Rangel LM, Rueckemann JW, Riviere PD, et al. Rhythmic coordination of hippocampal neurons during associative memory processing. Elife. 2016;5:e09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Suffczynski P, Crone NE, Franaszczuk PJ.. Afferent inputs to cortical fast-spiking interneurons organize pyramidal cell network oscillations at high-gamma frequencies (60–200 Hz). J Neurophysiol. 2014;112(11):3001–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Varga C, Oijala M, Lish J, et al. Functional fission of parvalbumin interneuron classes during fast network events. Elife. 2014;3:e04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Whittington MA, Roopun AK, Traub RD, Davies CH.. Circuits and brain rhythms in schizophrenia: A wealth of convergent targets. Curr Opin Pharmacol. 2011;11(5):508–514. [DOI] [PubMed] [Google Scholar]
- 114. Gaetz W, Edgar JC, Wang DJ, Roberts TPL.. Relating MEG measured motor cortical oscillations to resting γ-Aminobutyric acid (GABA) concentration. Neuroimage. 2011;55(2):616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD.. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106(20):8356–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cousijn H, Haegens S, Wallis G, et al. Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Proc Natl Acad Sci U S A. 2014;111(25):9301–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W.. Effects of antiepileptic drugs on motor cortex excitability in humans: A transcranial magnetic stimulation study. Ann Neurol. 1996;40(3):367–378. [DOI] [PubMed] [Google Scholar]
- 118. Borah NC, Matheshwari MC.. Effect of antiepileptic drugs on short‐latency somatosensory evoked potentials. Acta Neurol Scand. 1985;71(4):331–333. [DOI] [PubMed] [Google Scholar]
- 119. Arya R, Ervin B, Wilson JA, et al. Development of information sharing in language neocortex in childhood‐onset drug‐resistant epilepsy. Epilepsia. 2019;60(3):393–405. [DOI] [PubMed] [Google Scholar]
- 120. van 't Klooster MA, Zijlmans M, Leijten FSS, Ferrier CH, van Putten MJAM, Huiskamp GJM.. Time–frequency analysis of single pulse electrical stimulation to assist delineation of epileptogenic cortex. Brain. 2011;134(10):2855–2866. [DOI] [PubMed] [Google Scholar]
- 121. Hallett M, Di Iorio R, Rossini PM, et al. Contribution of transcranial magnetic stimulation to assessment of brain connectivity and networks. Clin Neurophysiol. 2017;128(11):2125–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and codes are available upon request to the corresponding author.