Abstract
Gastric cancer (GC) is one of the most common malignant tumors of the digestive tract, posing a significant risk to human health. Over the past 10 years, the pathological characteristics and the prognosis of GC have been determined based on the locations of the tumors that were then classified into two types—proximal and distal GC. This review focuses on the differences in epidemiology, etiology, cell source, pathological characteristics, gene expression, molecular markers, manifestations, treatment, prognosis, and prevention between proximal and distal GC to provide guidance and a basis for clinical diagnosis and treatment.
Keywords: gastric cancer, epidemiology, pathology, therapeutics, prognosis
Introduction
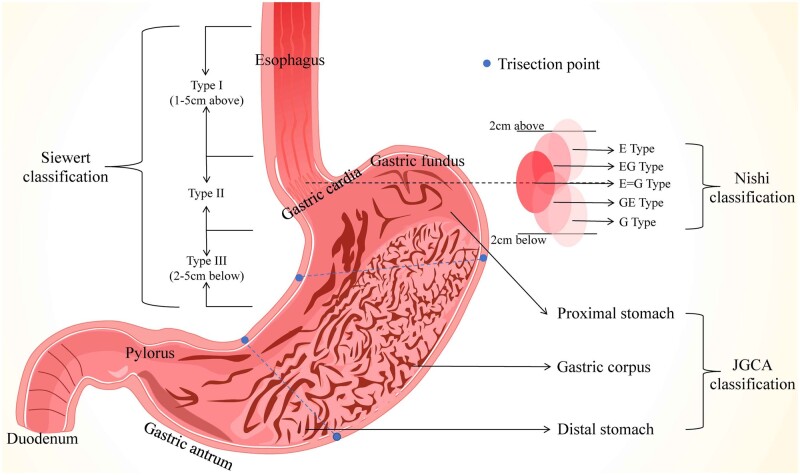
Although gastric cancer (GC) incidence and mortality have declined significantly over the past 70 years, it is still a leading cause of cancer-related deaths. GC was ranked fifth in morbidity and fourth in mortality in the cancer-prone countries [1] and was second in morbidity and third in mortality in China [2]. GC consists of two subtypes—proximal GC (PGC) and distal GC (DGC)—based on its position-specific features. Herein, we define the tumors on the upper third of the stomach (including gastric cardia cancer and gastric fundus cancer) as PGC and those on the lower third of the stomach as DGC (Figure 1), according to the classification of the Japanese Gastric Cancer Association [3]. Previous studies demonstrated that GC locations are associated with the diverse characteristics of epidemiology, etiology, pathology, and symptomatology. Interestingly, different therapies for PGC and DGC may give rise to varied outcomes. Thus, in this review, we summarize the differences between PGC and DGC in epidemiology, etiology, cell source, pathological characteristics, gene expression, molecular markers, manifestations, treatment, prognosis, and prevention, with emphasis on the interactions between non-coding RNAs and GC to guide clinical diagnosis and treatment.
Figure 1.
The anatomy and definitions of gastric cancer. (A) Siewert classification classifies tumors into three types: Type I, adenocarcinoma of the distal part of the esophagus—the tumor center is located 1–5 cm above the gastric cardia; Type II, adenocarcinoma of the real cardia—the tumor center is located 1 cm above or 2 cm below the gastric cardia; Type III, adenocarcinoma of the subcardial stomach—the tumor center is located 2–5 cm below the gastric cardia. (B) Nishi's classification defines five types of EGJ cancer featured by diameters of ≤40 mm and an epicenter within 2 cm proximal or distal from the EGJ, irrespective of histological type. The “E–G” terms of “E,” “EG,” “E = G,” “GE,” and “G” are used to describe the subtype according to the epicenter location at the rostral and caudal portions of the EGJ. (C) JGCA classification has divided the stomach into three portions: the upper (U), middle (M), and lower (L) parts, by the lines connecting the trisected points on the lesser and greater curvatures, which had been separately identified as the proximal stomach, gastric corpus, and distal stomach.
Epidemiology
Regional and time distribution
As shown above, GC has high mortality and morbidity, which drop sharply worldwide, and presents various characteristics depending on regional and time distribution. Asia has witnessed a slow decline in carcinogenesis and cancer-related deaths, especially in developing countries. A similar tendency of regional divergence is observed in China: the incidence of GC in eastern China is relatively high, with a downward trend from the east to the west [4]. The prevalence rate in rural areas, especially Gansu, Henan, Hebei, Shanxi, Shandong, and Shaanxi provinces, is high [5].
The predilection site of GC varies significantly with time and space. DGC, with a decreasing trend worldwide, mainly occurs in developing countries, such as countries in East Asia, East Europe, and South America [6]. On the contrary, PGC, with a rising incidence worldwide, is mainly diagnosed in developed countries. PGC morbidity in developed countries (UK and USA) has increased by 5– to 6-fold in the last 30 years. In northwestern Iran, the morbidity of PGC has been increasing for several years, accounting for 43.7% of GC [7].
The distribution of DGC and PGC is similar with respect to time but varies in space. Typically, the morbidity ratio of DGC/PGC was 1.49:1, according to a survey from 1997 to 2017 [4]. Another survey from 1998 to 2008 encompassed 1,090 GC cases in northern Henan, of which 60% comprised gastric cardia and fundus, and 30% were DGC [8]. Hebei Province has witnessed a similar trend, wherein cardia cancer rose from 54.8% to 75.9%, while the antrum-cancer proportion descended from 17.5% to 7.7% from 1993 to 2006 [9]. However, data from Da Bie Mountain pointed out that DGC has a dominant role, accounting for 47.36% of GC cases, i.e. four times the PGC cases [10]. The leading cause for the above distribution difference might originate from the local lifestyle, economic condition, food preference, and air and water quality. Even without related statistics of other areas, DGC and PGC are specifically featured by space and time distribution.
Population distribution
Age, gender, race, and nationality are essential factors that affect GC population distribution. Based on the relevant statistical data, the peak age of the onset was 40 years. The incidence of GC increases with age and the ratio of male to female patients is about the interval data of 1.5:2.5 vs 1 [11].
GC in various positions showed similar distribution characteristics of the population. Black males with low social status and income comprised the population susceptible to DGC [6]; hence, DGC patients are much younger (P = 0.046) [12]. Concurrently, PGC is usually diagnosed in Caucasians with higher social status, better financial conditions, and a male-to-female ratio of 5:1 [6]. The retrospective study showed that PGC is often diagnosed early in male patients >60 years old without a history of ulcers [13].
Other factors affecting the population distribution that have gradually drawn researchers’ attention include personal cancer history, high body mass index, and environmental toxin exposure, which are the independent risk factors for early PGC and advanced PGC [14]. Conversely, family cancer history was the independent risk factor for early DGC [15].
Etiology and cell sources
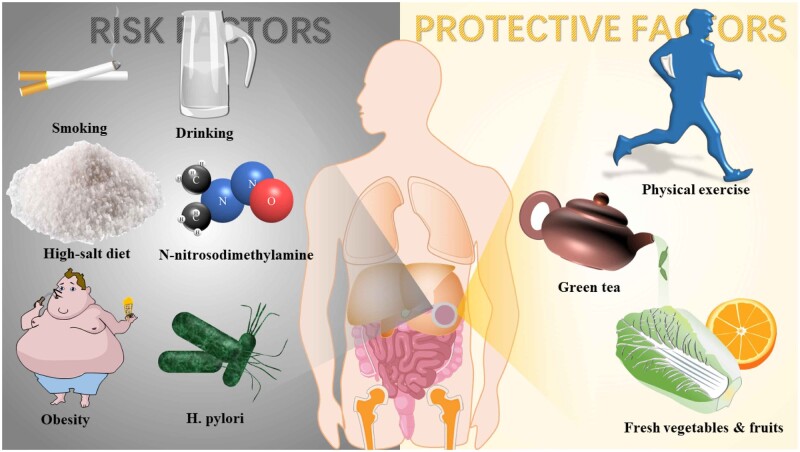
The ultimate pathogenesis of GC is still unclear. Different pathogenesis might lead to DGC, including two main benign gastric diseases: chronic atrophic gastritis (odds ratio [OR] = 3.92) and intestinal metaplasia (IM), led by gastroesophageal reflux disease (GERD) (OR = 10.08) [16]. Moreover, GC is related to various factors that vary with tumor locations (Figure 2).
Figure 2.
Some risk and protective factors of gastric cancer.
Helicobacter pylori infection
This infection has been considered as the class I carcinogen for GC [17]. The correlations between H. pylori and tumor locations are still a hot topic under investigation. The process of non-cardia GC might be linked to the inflammatory-response activation induced by H. pylori, and the mechanisms involving apoptosis promotion, p53-degradation facilitation, and DNA-mutation accretion are stimulated by metabolite accumulation. The developing process of GC—“The cascade of Correa,” followed by chronic superficial gastritis, atrophic gastritis, IM, and atypical hyperplasia—is thus set off. Cytotoxin-associated gene A in H. pylori may significantly increase the risk of atrophic gastritis and DGC [6].
Whether H. pylori infection causes PGC has perplexed scientists for decades. The decreased gastric-acid secretion induced by gastric mucosa atrophy might have several outcomes after H. pylori infection. It facilitates the colonization and reproduction of gastrointestinal microbiota and prevents reflux diseases such as GERD and Barrett’s esophagus (BE), thus decreasing the occurrence rate of PGC to some extent [18]. The eradication of H. pylori might hasten the onset of PGC through the reflux diseases–chronic atrophic gastritis–GC pathway, which has been identified in the developed countries. However, some studies found an opposite trend in the developing countries that H. pylori infection is positively related to PGC, thereby proving that H. pylori cause PGC via mechanisms similar to DGC [19]. However, another study showed that H. pylori are only related to carcinogenesis in metastasis from distant gastric to the gastric body and bottom, while H. pylori merely caused atrophic gastritis instead of cancer while colonizing pylorus [20]. In conclusion, the correlation between H. pylori and PGC is unclear, but those described here may avail the clinicians with an improved treatment plan in the future.
Lifestyle
Lifestyle affects the GC progress in many ways, including smoking, drinking, high-salt food, food with carcinogens, and inadequate physical activity. Smoking is a known risk factor for DGC and may increase PGC risk by 2– to 6-fold [21]. A previous study [22] showed that the combined effect of smoking and drinking promotes the progress of gastric cardia cancer despite a weak linkage between alcohol and PGC. A high-salt diet is an independent risk factor for distal gastric intraepithelial neoplasia [23], damaging the gastric mucosa exposed to the toxic microenvironment and expediting carcinogenesis. Agudo et al. [24] concluded that low-grade chronic inflammation caused by diet habits was positively relevant to PGC by analysing the large samples of patients and the inflammatory score of the diet in Europe. Nitrite and its ramification, N-nitrosodimethylamine, act as indirect carcinogens that cause GC when consumed in high doses [25] and function synergistically with H. Pylori in carcinogenesis.
H. pylori also promote nitroso flora growth, inhibit vitamin C secretion, and increase gastric nitrite [26]. Some lifestyles may prevent gastric carcinogenesis. Anti-oxygen contained in fresh vegetables and fruits reduce the risk of both DGC and PGC [27]. Physical exercise reduces the risk of PGC (OR = 0.80; 95% confidence interval [CI], 0.63–1.00) and DGC (OR = 0.63; 95% CI, 0.52–0.76) [28]. Especially in females, long-term and high-dose green-tea drinking reduces the risk of DGC (hazard ratio = 0.79; 95% CI, 0.65–0.96) because of the polyphenol and phytoestrogen content in green tea [29].
Precancerous conditions and lesions
Esophageal cancer, PGC, and DGC are three independent cancer subtypes known for precancerous lesions and cell sources. Nevertheless, the overlapping definitions of those precancerous lesions usually create confusion. IM, BE, and GERD have overlapping concepts. IM refers to metaplasia mainly in the stomach and esophagus, where the intestine-resembling epithelium replaces the intrinsic cells. BE refers to the squamous-to-columnar epithelium metaplasia in the esophagus >1 cm, which contains goblet cells and three types of epithelia (specialized columnar, junctional, and atrophic gastric fundic-type epithelium) [30]. BE is a specific IM in the esophagus that is less aggressive than short-segment IM [31]. Although these are parallel to precancerous lesions, GERD is known to be the main reason for IM (especially in the proximal position of the stomach) and BE (especially in the distal location of the esophagus). Even without a direct correlation with PGC or DGC development, discussion of these precancerous conditions and lesions helps to elucidate the progression of carcinogenesis.
The renewal of the concept “gastric cardia” further clarifies the definitions of these concepts. Gastric cardia was considered an intrinsic structure connecting the esophageal squamous epithelium and gastric columnar epithelium, and was redefined by Chandrasoma in 1997 [32]. He pointed out that gastric cardia—the specialized intestinal mucosa lacking goblet cells—is an acquired elongated structure formed by GERD-induced squamous-to-columnar epithelium metaplasia, indicating the severity of GERD. It also acts as a precursor for both proximal gastric IM and distal esophageal BE.
Spasmolytic polypeptide-expressing metaplasia (SPEM) is an untypical mode of metaplasia that contains goblet cells and has gained increasing attention. Based on the features of deep antral gland cells and duodenal Brunner gland cells, SPEM expresses biomarkers, including trefoil factor 2 (TFF2), mucin 6 (MUC6), griffonia simplicifolia lectin II (GSII), cluster-of-differentiation gene 44 variant isoform 9 (CD44v9), and protease inhibitor HE4 (WFDC2) [33]. Histologically, it showed typical features like oxyntic atrophy (loss of corpus chief and parietal cells), surface mucous pit-cell hyperplasia, mucous metaplasia (MM), and pseudo-pyloric metaplasia (PM). Characterized by the TFF2+ in the shape of large foam, SPEM-MM expresses the molecule of CD44 and SOX9, secretes neutral or acidic mucins, and substitutes the function of the lost chief and parietal cells. Moreover, SPEM-PM is poorly differentiated pyloric-gland-like cells that occur after SPEM-MM and serve as the precursors of gastric dysplasia, thereby initiating the process of malignant transformation [34].
Differentiated from gastric glandular isthmus stem cells or matured chief cells, SPEM acts as the precursor of IM and helps the cells enter into transdifferentiation [31]. Whether it is relevant with Lgr5+ chief cells, the original cells of GC, remains unclear [34, 35]. As a reparative mechanism at the beginning of injury, SPEM serves as a highly localized metaplasia that may become malignant with chronic inflammation and subsequent injury.
SPEM can also coordinate with H. pylori, a carcinogen, to strengthen its carcinogenesis function [36]. Sialyl-Lewis-X (sLeX) is widely expressed in the gastric gland cells and spreads from the surface to the bottom of the gastric gland during SPEM. Adhesin SabA is anchored on the surface of H. pylori and is a target of sLeX. When SabA and sLeX combine, H. pylori invades into the bottom of the gastric gland for survival. With the expansion of SPEM, H. pylori migrate from the gastric antrum to the corpus and fundus, which enlarges the ecological niche of H. pylori and finally causes upper GC.
Typically, GC is derived from the abnormal differentiation of stem/progenitor cells under specific conditions, albeit the primary cells of PGC and DGC are still under discussion. Based on the perspective of embryonic development, the entoderm is differentiated into the stratified squamous epithelium, whose basal cells—the P63+ ancestral cells—are primarily distributed in the cardiac sinus. The P63+KRT5+KRT7+ basal progenitor cells and the new transitional columnar epithelium in the esophageal–gastric junction are differentiated into intestine-like cells that reproduce the IM process CDX2 expression [19]; these are the initial cells of PGC [37]. Another study suggested that IM development and tumor progression are two different processes, as CDX2 expression and prognosis are positively correlated. Moreover, Lgr5+CCKBR+ stem cells are partly expressed in gastric cardia, functioning as the original cells of PGC [19].
Interestingly, there are various views on the origin cells for DGC. Most studies proposed that Lgr5+ stem cells located primarily on the fundus of lower gastric foveola were the original cells, as they triggered DGC via the Wnt–β-catenin pathway following APC loss [38]. A few studies regarded this cluster as the original cells of PGC. MIST1+ isthmus stem cells that express Cxcr4 initiate DGC by activating the Cxcl12/Cxcr4 signal axis—an essential precondition of carcinogenesis and a promising target of treatment [39]. Migrating from isthmus to fundus, MIST1+ isthmus stem cells reproduce swiftly with KRAS mutation and cover the stomach [34]. Thus, some studies proved that MIST1+ isthmus stem cells function as the original cells of DGC and GC in all sites [40]. Other cell clusters with unclear functions may also be related to GC, such as intestinal tufted cells with DCLK1 expression.
With the development of single-cell sequencing combined with spatial transcriptomics, the research of GC has entered a new stage. These new tools offer improved methods to explore the cellular components and differentiation between PGC and DGC, and thus reveal the original cells of PGC and DGC.
Other factors
Genetic factors have accounted for 1%–3% of GC, usually in areas with a low morbidity rate. It contained three primary syndromes: hereditary diffuse GC (HDGC), gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS), and familial intestinal GC (FIGC). HDGC, featured by CDH1 or CTNNA1 mutation, presents different manifestations in diverse ethnic groups. In Europe and North America, HDGC presents as lesions in the proximal region (from cardiac to pre-pyloric region) but occurs in the distal area in Maori families [41]. GAPPS is a basal gland polypus of the proximal stomach. Irrelevant to its location, FIGC may increase the onset risk of GC in both sites (16 times for non-cardiac GC and 8 times for cardiac GC) when the patients have H. pylori infection [16].
Chronic inflammation, whether induced by H. pylori or not, also plays a significant role. For example, DNA damage caused by chronic inflammation is the driving force of gastric cardiac cancer via activated mTOR and NF-κB pathways and induces the progression of IM and SPEM [42]. In addition, autoimmune gastritis, in which the autoimmune antibodies attack the parietal cells, may lead to pernicious anemia and increase the onset risk of DGC without reducing PGC [43].
Furthermore, obesity is the second leading factor causing PGC progression by increasing abdominal pressure to promote gastroesophageal reflux and changing the diet habit to alter the endocrine [44]. Fasting blood glucose also affects gastric carcinogenesis without apparent location influence. Hypoglycemia also increases the onset risk of DGC with almost no effect on PGC [45].
Pathological characteristics
Clinical pathology
Clinicopathological characteristics of GC are linked to their locations. In a survey of 438 patients, the features, including general pathology, histological pathology, lymph-node metastasis, and pathological stage, varied in the early stage between PGC and DGC [46]. Compared with DGC, PGC has the following characteristics: a higher percentage of non-depressed type (including type I, protrude type; type IIa, superficial protrude type; type IIb, superficial flat type), a shorter average diameter, a deeper submucosal invasion, better differentiation condition, and less lymph-node metastasis. Histological pathology revealed that papillary adenocarcinoma and some rare types, such as mucinous adenocarcinoma, lymphoid stromal cancer, and neuroendocrine carcinoma, are likely to occur in the proximal locations in contrast to the poorly cohesive cancers. PGC has a short survival time, although it is frequently diagnosed in an earlier pathological stage.
Advanced GC shared some common features despite some differences. Some studies [14] revealed PGC characteristics, such as a higher percentage of Borrmann I and II type, lymph-node metastasis, advanced pathological stages, and lower R0 removal rate compared with DGC. Another study supported the above findings with some new features [12]. Compared with PGC, DGC presented a lower organ-removal rate, shorter operation time, less blood loss during surgery, and a higher 5-year survival rate (Table 1).
Table 1.
The characteristics of DGC and PGC
| Variable | DGC | PGC | Reference |
|---|---|---|---|
| Regional distribution | Mainly in developing countries | Mainly in developed countries | [4–7] |
| Population distribution | People with low social status, especially in young black males | Caucasians with higher social status, especially in old male patients | [11–15] |
| Age (years) | 58.9 ± 12.5 | 64.2 ± 8.1 | [46] |
| Gender (male/ female ratio) | [14, 46] | ||
| Early GC | 2.04:1 | 2.64:1 | |
| Advanced GC | 2.00:1 | 4.10:1 | |
| Etiology | [17–29] | ||
| H. pylori infection | strong correlation | Unclear | |
| Lifestyle |
|
|
|
| Clinical pathology | [12, 14, 46] | ||
| Early GC | Bigger in size, poor differentiation, more poorly cohesive carcinoma, more lymph-node metastasis, better overall survival | Smaller in size, good differentiation, more tubular and papillary adenocarcinoma, less lymph-node metastasis, worse overall survival | |
| Advanced GC | Lower organ-removal rate, shorter operation time, less blood loss during surgery | Higher percentage of Borrmann I and II type, lymph-node metastasis, advanced pathological stages, lower R0 removal rate | |
| Molecular pathology | MSI type | CIN and EBV type; higher expression of TAMs, GR, KLF4, MUC2, G-17 | [47, 48] |
| Manifestations | Regurgitation, eructation, or nausea | Retrosternal pain and progressive dysphagia | [49] |
| Treatment | [50–76] | ||
| Early GC | Endoscopic mucosal resection and endoscopic submucosal dissection | ||
| Advanced GC | Laparoscopic D2 radical gastrectomy with uncut RY anastomosis; FLOT and PF schemes chemotherapy; adjuvant, perioperative, and palliative radiotherapy | Proximal gastrectomy with double-tract reconstruction; neoadjuvant chemoradiotherapy plus adjuvant chemotherapy | |
| Survival | Higher 5-year survival rate | Lower 5-year survival rate | [46] |
GC, gastric cancer; DGC, distal gastric cancer; PGC, proximal gastric cancer; MSI, microsatellite instability; CIN, chromosomal instability; EBV, Epstein-Barr virus; TAMs, tumor-associated macrophages; GR, gastrin receptor; KLF4, Kruppel-like factor 4; MUC2, Mucin 2; G-17, gastrin-17; RY, Roux-en-Y.
Lauren classification is a standard classification of GC based on the tumor locations. It divides GC into two types: intestinal and diffuse. The intestinal type has the following characteristics: (i) mainly found in the cardia and gastric fundus; (ii) mostly occurs in the elderly and male patients; (iii) majority of the cells show medium–high differentiation; (iv) has a relatively early staging with improved prognosis. The diffuse type has the following characteristics: (i) frequently occurs in the gastric antrum; (ii) mostly diagnosed in young women; (iii) has higher regional lymph-node metastasis and distant metastasis rates in the early stage with poor prognosis [77]. In summary, the intestinal type is correlated with PGC, while the diffuse type is associated with DGC. These findings need to be verified further.
Epithelial–mesenchymal transition (EMT) is a hot topic in cancer research. Cancers with EMT embody a high degree of malignancy and poor prognosis. Helicobacter pylori infection, the leading risk factor for DGC, may expedite the progression of cancer. The bacteria increase the expression of some molecules, such as soluble heparin-combined epidermal growth factor (HB-EGF), matrix metalloproteinases 7 (MMP-7), and gastrin (polypeptide hormone secreted by the gastric mucosa), followed by E-cadherin cleavage. The PI3K/PKC/NF-κB pathway activated by gastrin initiates the EMT process and promotes metastasis [78]. Another factor—the tumor parenchymal interstitial ratio—also affects the prognosis of GC. However, the relevance among EMT, tumor parenchymal interstitial ratio, and tumor locations have not yet been clarified. Clinical decision-making and prognosis evaluation may benefit from further exploration in the future.
Molecular pathology
The cancer genome atlas (TCGA) classification
Researchers have put forward a new method of classification—TCGA classification. GC is classified into four types: chromosome instability, microsatellite instability (MSI), genomic stability, and Epstein-Barr virus infection [47]. Accounting for >50% of cancer cases, the chromosome-instability type holds the central position, frequently occurring in the proximal region with RTK–RAS pathway activation and thus causing cancer [14]. MSI primarily occurs in the distal areas, such as the gastric antrum and pylorus, and the majority of the patients are elderly females. DNA hypermethylation is followed by mismatched repair protein MLH1 silencing and inactivation, forming the MSI type, divided into MSI-H and MSI-L subtypes according to the methylation level. Previous studies demonstrated that the MSI-H subtype is presented in the intestinal-type GCs with a better prognosis than the MSI-L and microsatellite-stability subtypes [79]. The lack of cell adhesion caused by a Ras homolog family member A (RHOA) mutation has a mounded stable genomic type and is often diagnosed as diffuse-type cancer with a relatively young population of patients, showing no apparent connection to the tumor location. Epstein-Barr virus-associated GC, caused by methylation-induced CDKN2A silencing, is mainly presented as a lymphoid epithelioma-like carcinoma with a high mutation frequency of PI3K and RAID1a and the overexpression of PD-L1/2. It occurs in the proximal region of young men and shows an improved prognosis [80, 81] (Figure 3).
Figure 3.

The correlation between TCGA classification and tumor locations in the Chinese population. TCGA, The Cancer Genome Atlas; DGC, distal gastric cancer; PGC, proximal gastric cancer; CIN, chromosomal instability; GS, genomic stability; MSI, microsatellite instability; EBV, Epstein-Barr virus.
Genetic alterations
Common genetic alterations, including genetic overexpression, loss of expression, and expressive suppression, occur in GC as in other cancers. Some of these have shown varied expressions along with the change in tumor locations. Specific genes, such as Her-2 and P53, are often overexpressed in PGC, while B-cell translocation gene 1 witnesses expressive suppression in PGC.
Researchers have introduced single-nucleotide polymorphisms and epigenetic modifications underlying GC to investigate the mechanisms of gene-expression changes. Single-nucleotide polymorphisms and single-nucleotide mutation at the genomic level are the most common genetic variations. Some nucleotide mutations are significantly capable of changing cancer susceptibility and location preference. The epigenetic mechanisms and heritable changes without nucleotide mutations also exert some effects. The epigenetic mechanisms involve DNA methylation, histone modification, genomic imprinting, gene silencing, and non-coding RNA dysregulation. DNA methylation is the core of epigenetic mechanisms and plays a dominant role in deciding cancer susceptibility in various locations with different methylation sites. Herein, we listed some genetic alterations that correlate with tumor locations (Table 2).
Table 2.
Genetic alterations and their location preferences in gastric cancer
| Single-nucleotide polymorphic |
DNA methylation |
||||||
|---|---|---|---|---|---|---|---|
| Polymorphic genes | Location preferences | OR (95% CI) | Ref. | Epigenetic mutations | Methylation site | Location preferences | Ref. |
| PRKAA1 (rs10074991) | Distal | 1.18 (1.12–1.26) | [82] | RASSF1A | Promoter | Proximal | [83] |
| NFKBIA (rs696 AA) | Proximal | 2.23 (1.10–4.55) | [84] | HLTF | CpG island | Proximal | [85] |
| NFKBIA (rs2233406 CT) | Distal | 1.66 (1.01–2.75) | TSP1 | Promoter | Proximal | [86] | |
| NFKBIA (rs2233407 CT+TT) | Distal | 1.65 (1.01–2.71) | CAV1 | CpG island and transcription start site (TSS) regions | Proximal | [87] | |
| NFKB1 (rs3755867 GG) | No statistical difference | 1.58 (1.02–2.39) | MEG3 | Promoter | Proximal | [88] | |
| P27(kip1) V/V | Proximal | 2.01 (1.12–3.68) | [89] | C5orf66‐AS1 | TSS regions | Proximal | [90] |
| MTHFR‐ 677TT | Proximal | 2.04 (1.28–3.26) | [91] | Wnt‐antagonist genes | [92] | ||
| ADPRT (Ala/Ala) | Proximal | 2.17 (1.55–3.04) | [93] | sFRP 1 | Promoter | Proximal | |
| XRCC1 (Gln/Gln) | Proximal | 1.61 (1.06–2.44) | sFRP 2 | Promoter | Proximal | ||
| COX‐2 | [94] | sFRP 4 | Promoter | Proximal | |||
| 1195AA | Proximal | 1.50 (1.05–2.13) | sFRP 5 | Promoter | Proximal | ||
| 765GC | Proximal | 2.06 (1.29–3.29) | Wif‐1 | Promoter | Proximal | ||
| 587Arg/Arg | Proximal | 1.67 (1.04–2.66) | Dkk3 | Promoter | Proximal | ||
| MDM2 ‐309 | [95] | E‐cadherin | 5' CpG island | Proximal | [96] | ||
| GG vs TT | Proximal | 2.00 (1.61–2.50) | GATA5 | Promoter | Proximal | [97] | |
| GT vs TT | Proximal | 1.50 (1.20–1.88) | FBXO32 | Promoter | Proximal | [98] | |
| PD‐1 (rs2227982 C > T) | [99] | RKIP | Promoter | Proximal | [100] | ||
| TT vs CC | Proximal | 2.53 (1.11–5.79) | Other genetic alterations | ||||
| TT+CT vs CC | Proximal | 2.04 (1.01–4.13) | Genes | Expression in gastric cancer | Location preferences | Ref. | |
| MYT1L (rs17039396 AG/GG) | Proximal | 0.57(0.40–0.81) | [101] | HER2 | Overexpression | Proximal | [76] |
| XPG (rs751402) | [102] | P53 | Overexpression | Proximal | [103] | ||
| C/T | Proximal | 1.33 (1.00–1.76) | BTG1 | Expressive suppression | Proximal | [104] | |
| T/T | Proximal | 1.77 (1.12–3.30) | hTERT | Overexpression | No statistical difference | [105] | |
| MMP2 − 1306CC | Proximal | 3.36 (2.34–4.97) | [106] | smad4 | Loss of expression | Undefined | |
| FASL‐ 844TT or TC | Proximal | 4.58 (2.07–10.14) | [107] | P16 | Loss of expression | Undefined | |
| FAS‐ 1377AA | |||||||
OR, odds ratio; CI, confidence interval.
Moreover, non-coding RNAs—a group of RNA molecules excluded from “features of protein translation”—have recently gained increasing attention. In addition to the functional types such as transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs), non-coding RNAs also include long non-coding RNAs (lncRNAs), microRNAs (miRNA), circular RNAs (circ-RNAs), small interfering RNAs (siRNAs), piwi-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), extracellular RNA (exRNAs), and small Cajal body-specific RNAs (scaRNAs). These non-coding RNAs may have altered the host-gene expression and promote cancer invasion and metastasis, thereby playing a significant role in carcinogenesis despite their small sizes and qualities. Also, new correlations have been established between some non-coding RNA and tumor locations. Quantitative reverse transcription PCR (RT-qPCR) was applied to detect the expressions of non-coding RNAs in PGC and DGC, which might provide us with more information for the precise treatment for GC in the future (Table 3).
Table 3.
Non-coding RNAs and their location preferences in gastric cancer
| Long non-coding RNAs | Expression changes | Location preferences | Ref. | Micro RNAs | Expression changes | Location preferences | Ref. | Circular RNAs | Expression changes | Location preferences | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C5orf66‐AS1 | Downregulated | Proximal | [90] | miR‐770 | Downregulated | Proximal | [88] | hsa_circ_002059 (circ_KIAA0907) | Downregulated | Undefined | [108] |
| LOC100130476 | Downregulated | Proximal | [109] | miR‐141 | Downregulated | Proximal | [110] | hsa_circ_0000745 (circ_SPECC1) | Downregulated | Undefined | [111] |
| ASHG19A3A028863 | Upregulated | Proximal | [112] | miR‐203a | Downregulated | Proximal | [113] | hsa_circ_0000181 (circ_TATDN3) | Downregulated | Undefined | [114] |
| ASHG19A3A040903 | Upregulated | Proximal | miR‐107 (rs2296616 TC/CC) | Upregulated | Proximal | [115] | hsa_circ_0074362 (circ_ARHGAP26) | Downregulated | Undefined | [116] | |
| ASHG19A3A041865 | Upregulated | Proximal | miR‐3656 | Downregulated | Proximal | [117] | hsa_circ_0003159 (circ_CACNA2D1) | Downregulated | Undefined | [118] | |
| ASHG19A3A018727 | Upregulated | Proximal | miR‐378c | Downregulated | Proximal | hsa_circ_0000190 (circ_CNIH4) | Downregulated | Undefined | [119] | ||
| ASHG19A3A052295 | Upregulated | Proximal | miR‐628‐3p | Downregulated | Proximal | circ_KIAA1244 | Downregulated | Undefined | [120] | ||
| GUST‐20‐P1426265844 | Upregulated | Proximal | miR‐US33‐3p | Downregulated | Proximal | hsa_circ_0047905 (circ_SERPINB5) | Downregulated | Undefined | |||
| ASHG19A3A041043 | Upregulated | Proximal | miR‐148a‐3p | Downregulated | Proximal | hsa_circ_0138960 (circ_GDA) | Downregulated | Undefined | |||
| ASHG19A3A033911 | Upregulated | Proximal | miR‐H10 | Downregulated | Proximal | hsa_circ_769015 | Downregulated | Undefined | |||
| ASHG19A3A026346 | Upregulated | Proximal | miR‐638 | Downregulated | Proximal | hsa_circ_0013048 (hsa_circ_100269) | Downregulated | Undefined | [121] | ||
| ASHG19A3A007184 | Downregulated | Proximal | miR‐483‐5p | Downregulated | Proximal | hsa_circ_001569 | Upregulated | Undefined | [122] | ||
| ASHG19A3A018598 | Downregulated | Proximal | miR‐675‐5p | Downregulated | Proximal | hsa_circ_0017639 (circ-SFMBT2) | Upregulated | Undefined | [123] | ||
| ASHG19A3A038967 | Downregulated | Proximal | miR‐1184 | Downregulated | Proximal | hsa_circ_0001821 (circPVT1) | Upregulated | Undefined | [124] | ||
| ASHG19A3H0000023 | Downregulated | Proximal | miR‐299‐5p | Downregulated | Proximal | hsa_circ_0000284 (circHIPK3) | Upregulated | Undefined | [125] | ||
| ASHG19A3A018662 | Downregulated | Proximal | miR‐4285 | Downregulated | Proximal | hsa_circ_0001946 (ciRS-7) | Upregulated | Undefined | |||
| ASHG19A3A007413 | Downregulated | Proximal | miR‐3665 | Downregulated | Proximal | hsa_circ_0064644 (circRBMS3) | Upregulated | Undefined | |||
| ASHG19A3A011053 | Downregulated | Proximal | miR‐H25 | Downregulated | Proximal | hsa_circ_0056618 | Upregulated | Undefined | |||
| ASHG19A3A035937 | Downregulated | Proximal | miR‐H17 | Downregulated | Proximal | hsa_circ_0027599 | Downregulated | Undefined | |||
| ASHG19A3A055173 | Downregulated | Proximal | miR‐3195 | Downregulated | Proximal | hsa_circ_0007766 (circ_ERBB2) | Upregulated | Undefined | |||
| ASHG19A3A0001119 | Downregulated | Proximal | miR‐518e‐5p | Downregulated | Proximal | hsa_circ_0000267 | Upregulated | Undefined | |||
| HOTAIR | Upregulated | Undefined | [126] | miR‐3196 | Downregulated | Proximal | hsa_circ_0089548 (circ-NOTCH1) | Upregulated | Undefined | ||
| H19 | Upregulated | Undefined | [127] | miR‐30d‐5p | Downregulated | Proximal | hsa_circ_0089547 (circ-NOTCH1) | Upregulated | Undefined | ||
| CCAT1 | Upregulated | Proximal | [128] | miR‐3124‐5p | Downregulated | Proximal | hsa_circ_0067997 | Upregulated | Undefined | ||
| AP001631.9 | Upregulated | Undefined | [129] | miR‐196a‐5p | Upregulated | Proximal | hsa_circ_0004771 (circ_NRIP1) | Upregulated | Undefined | ||
| ATB | Upregulated | Undefined | [130] | miR‐135b‐5p | Upregulated | Proximal | hsa_circ_0017728 | Upregulated | Undefined | ||
| GACAT1 | Upregulated | No statistical difference | [131] | miR‐2355‐3p | Upregulated | Proximal | hsa_circ_0081143 | Upregulated | Undefined | ||
| FENDRR | Downregulated | Undefined | [132] | miR‐4307 | Upregulated | Proximal | hsa_circ_0042881 (circNF1) | Upregulated | Undefined | ||
| FER1L4 | Downregulated | Undefined | [133] | miR‐1244 | Upregulated | Proximal | hsa_circ_0032627 (circDLST) | Upregulated | Undefined | ||
| MALAT1 | Upregulated | Undefined | [134] | miR‐892a | Upregulated | Proximal | hsa_circ_0093398 (circPDSS1) | Upregulated | Undefined | ||
| HULC | Upregulated | No statistical difference | [135] | miR‐20a‐5p | Upregulated | Proximal | hsa_circ_0010522 (ciRS-133) | Upregulated | Undefined | ||
| ZNFX1-AS1 | Upregulated | No statistical difference | miRPlusA1087 | Upregulated | Proximal | hsa_circ_0092303 (circCACTIN) | Upregulated | Undefined | |||
| HOXA | Downregulated | Undefined | [136] | miR‐93‐5p | Upregulated | Proximal | hsa_circ_0008035 | Upregulated | Undefined | ||
| HOTTIP | Upregulated | Undefined | [137] | miR‐455‐3p | Upregulated | Proximal | hsa_circ_0008365 (circ-SERPINE2) | Upregulated | Undefined | ||
| HOXA13 | Upregulated | Undefined | miR‐105‐5p | Upregulated | Proximal | hsa_circ_0090410 (circUBA1) | Upregulated | Undefined | |||
| MSTO2P | Upregulated | No statistical difference | [138] | miR‐764 | Upregulated | Proximal | hsa_circ_0005075 (circ-EIF4G3) | Upregulated | Undefined | ||
| AFAP1-AS1 | Upregulated | Undefined | [139] | miR‐130b‐5p | Upregulated | Proximal | hsa_circ_0008549 (circOSBPL10) | Upregulated | Undefined | ||
| ANRIL | Upregulated | Undefined | miR‐506‐3p | Upregulated | Proximal | hsa_circ_0000199 (circAKT3) | Upregulated | Undefined | |||
| CASC15 | Upregulated | Undefined | miR‐454‐3p | Upregulated | Proximal | hsa_circHECTD1 | Upregulated | Undefined | |||
| GAPLINC | Upregulated | Undefined | miR‐142‐3p | Upregulated | Proximal | hsa_circ_0009109 (circ-DCAF6) | Upregulated | Undefined | |||
| LINC00673 | Upregulated | Undefined | miR‐3591‐3p | Upregulated | Proximal | hsa_circ_0031250 (circ-PRMT5) | Upregulated | Undefined | |||
| PANDAR | Upregulated | Undefined | miR‐196b‐5p | Upregulated | Proximal | hsa_circ_0003855 (circDUSP16) | Upregulated | Undefined | |||
| PVT1 | Upregulated | Undefined | miR‐3664‐5p | Upregulated | Proximal | hsa_circ_0069086 (circMAN2B2) | Upregulated | Undefined | |||
| Sox2ot | Upregulated | Undefined | miR‐636 | Upregulated | Proximal | hsa_circ_0058147 (circFN1) | Upregulated | Undefined | |||
| UCA1 | Upregulated | Undefined | miR-1 | Upregulated | Undefined | [140–142] | hsa_circ_0000467 | Upregulated | Undefined | ||
| XIST | Upregulated | Undefined | miR-34 | Upregulated | Undefined | hsa_circ_0003221 (circPTK2) | Upregulated | Undefined | |||
| ZEB1-AS1 | Upregulated | Undefined | miR-423-5p | Upregulated | Undefined | hsa_circ_0063526 (circ-RanGAP1) | Upregulated | Undefined | |||
| ZFAS1 | Upregulated | Undefined | miR-20a | Upregulated | Undefined | hsa_circ_0066436 (circATXN7) | Upregulated | Undefined | |||
| miR-17-5p | Upregulated | Undefined | hsa_circ_0040809 (circBANP) | Upregulated | Undefined | ||||||
| miR-21 | Upregulated | Undefined | hsa_circ_0077736 (circ-CEP85L) | Downregulated | Undefined | ||||||
| miR-106a | Upregulated | Undefined | hsa_circ_00074444 (circRHOBTB3) | Downregulated | Undefined | ||||||
| miR-106b | Upregulated | Undefined | hsa_circ_101057 (circLARP4) | Downregulated | Undefined | ||||||
| miR-1233 | Downregulated | Undefined | hsa_circ_0000096 | Downregulated | Undefined | ||||||
| miR-593-5p | Downregulated | Undefined | [143] | circMLLT10 circNHSL1 circCCDC9 |
Upregulated Upregulated Downregulated |
Undefined Undefined No statistical difference |
[144] [145] [146] |
Biomarkers
CEA, CA19-9, CA72-4, and CA125 are the classic biomarkers of GC for diagnosis and prognosis evaluation. Some studies discovered that CEA is highly expressed in DGC with the diagnostic values of other traditional biomarkers [147]. These biomarkers are easily detectable and are promising candidates in identifying tumor locations but necessitating further research.
A lack of specificity and differential diagnostic values have restricted the applications of these traditional biomarkers. Thus, finding novel biomarkers for GC is an urgent requisite. In addition to the gene expression mentioned above, some biomarkers, such as proteins and factors, might indicate the tumor locations and thus function in tumor detection and surveillance. Herein, we listed some typical biomarkers that have a strong diagnostic specificity for GC.
1. Tumor-associated macrophages (TAMs) . TAMs are inflammatory cells located in tumor stroma and indicate poor prognosis. TAMs usually occur in gastric cardia, characterized by poor differentiation, deep infiltration, and an advanced stage. However, some viewpoints have objected to this relevance [148].
2. Gastrin receptor (GR). GR is a protein expressed mainly in enterochromaffin and parietal cells, and promotes tumor development through several pathways. The expression of GR is significantly higher in PGC [149].
3. Kruppel-like factor 4 (KLF4) and specificity protein 1 (SP1) . KLF4 is a transcription factor belonging to the KLF family. It is usually combined with SP1 to inhibit gene expression. The high expression of KLF4 indicates a high onset risk for PGC, while SP1 expression indicates a high onset risk for DGC [150].
4. Mucin 2 (MUC2) and mucin 6 (MUC6) . MUC2 and MUC6 are specific mucins expressed in the gastrointestinal tract with differential diagnostic values for PGC and DGC. These mucins are secreted in different parts of the stomach. The higher expression of mucin indicates the diseased location (distal or proximal) of the stomach. Goblet cells in the IM structure secrete MUC2, which is highly expressed in PGC, while MUC6, secreted by gland cells of the gastric corpus and antrum, is highly expressed in DGC [151].
5. Pepsinogen I/Pepsinogen II ratio (PGR) and Gastrin-17 (G-17) . PGR and G17 are indicators for tumor locations. Stored in the gastric wall, pepsinogen is converted into pepsin by gastric acid. Several studies have found that the PGR level drops sharply in PGC, while the G-17 level is elevated in PGC compared with DGC [48].
In addition to the above biomarkers, the others, such as TFF3, E-cadherin, Catenin, CD44v6, tyrosine kinases, platelet-derived growth factor receptor (PDGFR), S100A6, and Cyclin D1/E2, are promising but require additional investigation. Detecting the differential expressions in the proximal and distal gastric sites of humans or mice models with enzyme-linked immunosorbent assay or immunohistochemical methods might identify valuable biomarkers for GC in the future.
Manifestations
Typically, there are some common symptoms in GC patients, such as dyspepsia, anorexia, emesis, gastralgia, and cachexia. However, the clinical manifestations vary with tumor location. PGC patients present retrosternal pain and progressive dysphagia, occasionally resembling esophageal-cancer patients, while DGC often experiences regurgitation, eructation, or nausea due to tumor obstruction in the pylorus. These non-specific symptoms may help in identifying tumor location.
Treatment and prognosis
Surgical treatment
Early GC
Endoscopic mucosal resection and endoscopic submucosal dissection (ESD) are adopted to treat early GC. Clinicians use endoscopic mucosal resection to treat cancers with small diameters and superficial infiltration [50]. Since ESD has been adopted frequently in cancers invading submucosa layers, it gradually became the first-line treatment for early GC [51]. Patients who underwent ESD could achieve a 94.9% overall resection rate and 97.1% 5-year survival rate, suggesting that it is a safe and effective treatment for early GC [52]. Although it has satisfactory outcomes in both PGC and DGC, the effectiveness is better in treating early PGC.
Advanced GC
Operation-based multidisciplinary treatment has become the therapeutic principle of advanced GC with D2 radical gastrectomy as the primary surgical method. However, how to perform resection and reconstruct the digestive tract is a significant issue for clinicians.
Based on Siewert classification, Western clinicians classified PGC into three types with diverse therapeutic choices. Transthoracic subtotal esophageal resection plus proximal gastrectomy with gastric pull-up reconstruction is adopted to treat type I tumors. Transhiatal extended gastrectomy plus distal esophageal resection with Roux-en-Y (RY) esophagojejunostomy reconstruction is suitable for type III tumors [53]. Transhiatal proximal gastrectomy with double-tract reconstruction might be the best choice for specific type II tumors, with a non-poorly cohesive, intestinal type of Lauren grading 1 or 2 without clinical signs of lymph-node metastasis at the distal stomach [54]. Experts recommended that patients with >3 cm esophageal invasion require transthoracic proximal gastrectomy, which shows fewer risks and better outcomes.
Chinese clinicians prefer proximal gastrectomy (PG) in PGC patients, especially in Ia and Ib stages, since it has a high remission rate and security. Several studies [55] regarded PG as the best surgical choice for PGC, with a better prognosis and shorter resection margins than total gastrectomy [56], despite the complications, such as anastomotic stenosis and reflux esophagitis. Traditional surgical methods, such as esophagogastrostomy, jejunal interposition, jejunal pouch interposition, and double-tract and gastric tubular reconstruction, play significant roles in post-operative digestive-tract reconstruction, albeit with some limitations [57]. For example, the double tract, which has better feasibility and security than others, is now considered the best method for reconstruction [58]. Although new methods, such as the double-flap technique [59], tri-double-flap hybrid method [60], and Cheng’s giraffe reconstruction [61], may have some potential functions, researchers regard proximal gastrectomy with double-tract reconstruction as the best surgical choice for PGC.
Laparoscopic D2 radical gastrectomy showed a similar 3-year disease-free survival rate to the typical open resection, rendering it the best surgical choice for most DGCs [62]. Clinicians recommended laparoscopic surgeries to patients in stages I, II, and IIIa with tumor invasion less than the T4a stage or those needing short-circuit operations in late stages. Open operations are worthy for late-stage patients (situated in stage IV or more than T4a stage). The traditional reconstruction of DGC—Billroth I anastomosis, Billroth II anastomosis, and RY anastomosis is controversial [63]. Due to physiological advantages, Billroth I anastomosis is widely used despite the high risk of anastomotic leakage. If the issue of anastomotic leakage is resolved, Billroth II anastomosis might increase post-operative alkaline reflux gastritis, esophagitis, and anastomotic ulcer. RY anastomosis, another widely used method, usually causes reverse intestinal peristalsis and RY retention syndrome. Uncut RY (U-RY) is a newly proposed reconstruction method that has attracted people’s attention. Gastrojejunostomy, jejunojejunostomy, and input loop blockage based on preserved intestinal continuity improves the shortages of RY. Compared with traditional RY anastomosis, U-RY shortens the operation time, reduces the post-operative complications, delays gastric emptying, and enhances serum albumin levels, thereby proving to be the best choice of reconstruction nowadays [64].
Chemotherapy
Chemotherapies, classified as neoadjuvant chemotherapy, perioperative chemotherapy, and adjuvant chemotherapy by treatment opportunities, are suitable for advanced GC. Patients with advanced PGC are sensitive to more frequently recommended chemotherapy. Clinical research has demonstrated that neoadjuvant chemotherapy increases the R0 resection rate, pathological complete response rate, and 5-year survival rate of PGC patients [65]. Therefore, national guidelines have listed four cycles of preoperative FLOT (docetaxel, oxaliplatin, 5-fluorouracil, and leucovorin) as the first-level neoadjuvant chemotherapy scheme, followed by the second level of neoadjuvant chemotherapy schemes, such as PF (5-fluorouracil and cisplatin), XELOX (oxaliplatin and capecitabine), capecitabine, and FLOFOX (oxaliplatin and 5-fluorouracil). The perioperative FLOT scheme showed more advantages than adjuvant chemotherapy and was recommended as the primary chemotherapy for PGC patients with locoregional advanced potentially resectable tumors in Western countries [66], especially in cT2 or higher stages, according to National Comprehensive Cancer Network (NCCN) guidelines [67].
Advanced DGC, with less sensitivity to chemotherapy, has benefited less from traditional chemotherapy. Thus, national guidelines chose the FLOT and PF schemes as the second-level recommendations, while the XELOX and the FLOFOX schemes are the third-level recommendations [68]. However, some researchers [69] have suggested that taxanes significantly improve the progression-free survival and overall survival of DGC and intestinal GC compared with PGC and diffuse-type. Furthermore, another study demonstrated that the PFtax scheme—a typical PF scheme combined with docetaxel—presented better progression-free survival and overall survival in DGC patients, promising clinical application [70].
Except for the schemes above, the DCF scheme (docetaxel + cisplatin + 5-fluorouracil) presented palliative chemotherapeutic value for late-stage GCs without an operational chance [71].
Radiotherapy
The application of radiotherapy in GC has attracted researchers’ attention since the publication of the INT-0116 research. Patients who have undergone D1 gastrectomy with high-grade GC need adjuvant radiotherapy [72]. Palliative radiotherapy for stage IV is also beneficial [73].
PGC shows better radiotherapeutic value than tumors located in other sites. Therefore, clinicians usually conduct radiotherapy together with chemotherapy, which has more advantages than neoadjuvant chemotherapy. National guidelines regarded preoperative chemoradiotherapy as the third-level recommendation for advanced PGC. Neoadjuvant chemoradiotherapy plus adjuvant chemotherapy is the best choice for PGC patients nowadays [68].
Although neoadjuvant radiotherapy shows no apparent advantages for advanced-DGC patients [74], adjuvant, perioperative, and palliative radiotherapy has some clinical benefits for DGC.
Targeted therapy and immunotherapy
With the discovery of drug targets and the development of targeted drugs, targeted therapy has become a promising treatment for GC. Multiple targeted therapy schemes have been conducted for patients with single/multi-target susceptibility, such as targeted drugs for EGFR, HER2, FGFR, MET, VEGFR2, PD-1, and CLDN18.2. The detection of HER2, PD-L1, MSI, Epstein-Barr virus (EBV), and Tumor Mutation Burden (TMB) is a regular program for those who undergo gastrectomy [68].
The therapeutic strategies vary with tumor locations. For example, Apatinib mesylate (highly selective VEGFR2 inhibitor), nivolumab, and pembrolizumab (immune checkpoint inhibitors) are suitable for late-stage PGC [75]. In addition, since HER2 overexpression significantly occurs in PGC than DGC, anti-HER2 therapy may be beneficial for PGC patients [76]. In conclusion, therapeutic methods targeting different locations of GC deserve additional research.
Nonetheless, additional clinical trials are needed to explore the therapeutic methods and treatment combinations for PGC and DGC. With research development, patients with PGC or DGC might receive better treatment for prolonged survival time and enhanced treatment outcomes.
Prevention
Considering the risk and protective factors discussed above, researchers have recommended the following methods to prevent GC occurrence (Figure 2).
1. Lifestyle transformation . Developing good habits is the most crucial and practical way to prevent GC. On the other hand, bad habits, such as drinking, smoking, and a high-salt diet, are associated with GC development. In contrast, good habits, such as exercising, a regular diet of vegetables and fruits, and drinking green tea, may be beneficial factors [72]. Thus, transforming lifestyles may significantly reduce the onset risk of GCs, irrespective of their locations. Weight control is another valuable method of reducing GERD risks that prevents increased intra-abdominal pressure, improves endocrine disorder, and decreases the onset risk of PGC [152].
2. Helicobacter pylori eradication. Helicobacter pylori eradication is a widely used prevention measure that successfully reduces the incidence of GC and GC-related deaths, especially for DGC. Whether H. pylori eradication is useful in the treatment of PGC is still controversial. Some studies have stated that the bacteria increase the onset risk of PGC because of increasing the GERD possibility. The proton-pump inhibitor (PPI) is a drug conventionally used in eradicating H. pylori. It functions by inhibiting gastric-acid secretion, reducing H. pylori colonization, and controlling related symptoms. However, long-term usage of PPI may lead to hypergastrinemia, thus promoting PGC development [19]. Therefore, proper use of PPI is essential to prevent PGC.
3. Antioxidants. Vitamin C, vitamin E, β-carotene, and lycopene are the mose common antioxidants with antitumor functions. These may reduce the onset risk of PGC, as oxidative stress serves as the risk factor for various gastrointestinal diseases [153], while some adverse opinions have proposed that antioxidants increase the total mortality of GC without a preventive effect [154]. Therefore, its function in GC is still controversial.
4. COX-2 inhibitor. Smoking, acidic condition, and H. pylori infection increase the expression of COX-2, which might cause the atrophy–IM process and finally lead to GC, especially DGC. The COX-2 inhibitor is deemed to reduce the onset risk of DGC; whether it exerts a protective effect in PGC has not yet been verified [155].
5. Ornithine decarboxylase (ODC) inhibitor . BE is the precursor lesion of PGC that is correlated with the ODC activity. The ODC inhibitor exerts a protective effect on PGC. α-Difluoromethyl ornithine and troglitazone are the most common ODC inhibitors that reduce the risk of onset of the disease [6, 156]. However, additional clinical trials are required to demonstrate its safety and effectiveness in clinical use.
6. Endoscopic surveillance. Regular endoscopic surveillance is a frequently used method to diagnose GC in the early stage. Clinicians have recommended regular endoscopic surveillance for the population who are susceptible to GC, especially those suffering from H. pylori infection and precancerous lesions or having familial histories of GC [157].
Conclusion
In summary, we found many differences between PGC and DGC in epidemiological characteristics, etiology, cell source, pathology characteristics, gene expression, molecular markers, manifestations, treatment, prognosis, and prevention. With the development of precision medicine, it is an urgent task to explore the differences among tumor locations. Traditional comparison analysis has helped researchers to discover the apparent diversity of GC, while new techniques, such as artificial-intelligence imaging and single-cell RNA sequencing, might improve the understanding of the process of carcinogenesis and regional differences. These differences would guide diagnosis and treatment, and serve as a promising approach in improving the long-term survival rates and quality of life for patients.
Authors’ Contributions
Y.Z., P.S.Z., and C.H. conceived of and designed the project. Y.Z., P.S.Z., Z.Y.R., and C.H. wrote, reviewed, and revised the manuscript. C.H. supervised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81772526 and 82072662], Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support [grant number 20161425], Shanghai Jiao Tong University Medical Cross Fund [grant number YG2017MS28], Science and Technology Commission Project of Songjiang District [grant numbers 18SJKJGG23 and 19SJKJGG22], Three-year Action Plan for Clinical Skills and Clinical Innovation in Shanghai-level Hospitals [grant number SHDC2020CR4022], and the 2021 Shanghai “Rising Stars of Medical Talent” Youth Development Program: Outstanding Youth Medical Talents.
Acknowledgements
None.
Conflict of Interest
None declared.
References
- 1. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Zhang S, Sun K, Zheng R et al. Cancer incidence and mortality in China, 2015. J Natl Cancer Center 2021;1:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101–12. [DOI] [PubMed] [Google Scholar]
- 4. Zhao L, Huang H, Zhao D et al. Clinicopathological characteristics and prognosis of proximal and distal gastric cancer during 1997-2017 in China National Cancer Center. J Oncol 2019;2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nie Y, Wu K, Yu J et al. A global burden of gastric cancer: the major impact of China. Expert Rev Gastroenterol Hepatol 2017;11:651–61. [DOI] [PubMed] [Google Scholar]
- 6. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Derakhshan MH, Yazdanbod A, Sadjadi AR et al. High incidence of adenocarcinoma arising from the right side of the gastric cardia in NW Iran. Gut 2004;53:1262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jing X, Wang J, Zhou B et al. Clinical epidemiological characteristics of gastric cancer in recent 10 years. Chin J Bases Clin Gen Surg 2010;17:29–33 (in Chinese). [Google Scholar]
- 9. Zhao C, Zhang X, Xue L et al. Analysis of the changing trends of frequency and localization of gastric cancers arising from different sites of the stomach in population of the high incidence area of esophageal and gastric cancers in Hebei province. Zhonghua Zhong Liu Za Zhi 2008;30:817–20 (in Chinese). [PubMed] [Google Scholar]
- 10. Li X, Hui Q. Epidemiologic research progress of pathogenic site changes of gastric cancer. China Med Herald 2014;11:160–2 (in Chinese). [Google Scholar]
- 11. Roder DM. The epidemiology of gastric cancer. Gastric Cancer 2002;5(Suppl 1):5–11. 5- [DOI] [PubMed] [Google Scholar]
- 12. Yu X, Hu F, Li C et al. Clinicopathologic characteristics and prognosis of proximal and distal gastric cancer. Onco Targets Ther 2018;11:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu F, Wang L. Differences of lymph node metastasis between early proximal and distal gastric cancer and indication of endoscopic resection. Chin J Gastroenterol 2018;23:157–60. [Google Scholar]
- 14. Liu K, Zhang W, Chen X et al. Comparison on clinicopathological features and prognosis between esophagogastric junctional adenocarcinoma (Siewert II/III types) and distal gastric adenocarcinoma: retrospective cohort study, a single institution, high volume experience in China. Medicine (Baltimore ) 2015;94:e1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang C, Huang Q, Lu L et al. Risk factors of early proximal gastric carcinoma in Chinese diagnosed using WHO criteria. J Dig Dis 2015;16:327–36. [DOI] [PubMed] [Google Scholar]
- 16. Abdi E, Latifi-Navid S, Zahri S et al. Risk factors predisposing to cardia gastric adenocarcinoma: insights and new perspectives. Cancer Med 2019;8:6114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penta RD, Falco M, Iaquinto G et al. Helicobacter pylori and gastric epithelial cells: from gastritis to cancer. J Exp Clin Cancer Res 2005;24:337–45. [PubMed] [Google Scholar]
- 18. Smolka AJ, Schubert ML. Helicobacter pylori-induced changes in gastric acid secretion and upper gastrointestinal disease. Curr Top Microbiol Immunol 2017;400:227–52. [DOI] [PubMed] [Google Scholar]
- 19. Hayakawa Y, Sethi N, Sepulveda AR et al. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat Rev Cancer 2016;16:305–18. [DOI] [PubMed] [Google Scholar]
- 20. Lai S. A human mode of intestinal type gastric carcinoma. Med Hypotheses 2019;123:27–9. [DOI] [PubMed] [Google Scholar]
- 21. Souza RF, Spechler SJ. Concepts in the prevention of adenocarcinoma of the distal esophagus and proximal stomach. CA Cancer J Clin 2005;55:334–51. [DOI] [PubMed] [Google Scholar]
- 22. Shimazu T, Tsuji I, Inoue M et al. ; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Alcohol drinking and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 2008;38:8–25. [DOI] [PubMed] [Google Scholar]
- 23. Yu Y, Fang C, Peng C et al. Risk factors for gastric intraepithelial neoplasia in Chinese adults: a case-control study. Cancer Manag Res 2018;10:2605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agudo A, Cayssials V, Bonet C et al. Inflammatory potential of the diet and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr 2018;107:607–16. [DOI] [PubMed] [Google Scholar]
- 25. Song P, Wu L, Guan W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients 2015;7:9872–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vermeer IT, Engels LG, Pachen DM et al. Intragastric volatile N-nitrosamines, nitrite, pH, and Helicobacter pylori during long-term treatment with omeprazole. Gastroenterology 2001;121:517–25. [DOI] [PubMed] [Google Scholar]
- 27. Serafini M, Bellocco R, Wolk A et al. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology 2002;123:985–91. [DOI] [PubMed] [Google Scholar]
- 28. Singh S, Edakkanambeth Varayil J, Devanna S et al. Physical activity is associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2014;7:12–22. [DOI] [PubMed] [Google Scholar]
- 29. Inoue M, Sasazuki S, Wakai K et al. ; for the Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut 2009;58:1323–32. [DOI] [PubMed] [Google Scholar]
- 30. Spechler SJ. Cardiac metaplasia: follow, treat, or ignore? Dig Dis Sci 2018;63:2052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Que J, Garman KS, Souza RF et al. Pathogenesis and cells of origin of Barrett's esophagus. Gastroenterology 2019;157:349–64.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chandrasoma P. Pathophysiology of Barrett's esophagus. Semin Thorac Cardiovasc Surg 1997;9:270–8. [PubMed] [Google Scholar]
- 33. Goldenring JR. Pyloric metaplasia, pseudopyloric metaplasia, ulcer-associated cell lineage and spasmolytic polypeptide-expressing metaplasia: reparative lineages in the gastrointestinal mucosa. J Pathol 2018;245:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayakawa Y, Fox JG, Wang TC. Isthmus stem cells are the origins of metaplasia in the gastric corpus. Cell Mol Gastroenterol Hepatol 2017;4:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leushacke M, Tan SH, Wong A et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 2017;19:774–86. [DOI] [PubMed] [Google Scholar]
- 36. Saenz JB, Vargas N, Mills JC. tropism for spasmolytic polypeptide-expressing metaplasia allows helicobacter pylori to expand its intragastric niche. Gastroenterology 2019;156:160–74.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang M, Li H, Zhang Y et al. Transitional basal cells at the squamous-columnar junction generate Barrett's oesophagus. Nature 2017;550:529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barker N, Huch M, Kujala P et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010;6:25–36. [DOI] [PubMed] [Google Scholar]
- 39. Sakitani K, Hayakawa Y, Deng H et al. CXCR4-expressing Mist1(+) progenitors in the gastric antrum contribute to gastric cancer development. Oncotarget 2017;8:111012–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hayakawa Y, Ariyama H, Stancikova J et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 2015;28:800–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oliveira C, Pinheiro H, Figueiredo J et al. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol 2015;16:e60–70. [DOI] [PubMed] [Google Scholar]
- 42. Lin R, Xiao D, Guo Y et al. Chronic inflammation-related DNA damage response: a driving force of gastric cardia carcinogenesis. Oncotarget 2015;6:2856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ye W, Nyren O. Risk of cancers of the oesophagus and stomach by histology or subsite in patients hospitalised for pernicious anaemia. Gut 2003;52:938–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015;18:23–32. [DOI] [PubMed] [Google Scholar]
- 45. Kim TJ, Lee H, Min YW et al. Diabetic biomarkers and the risk of proximal or distal gastric cancer. J Gastroenterol Hepatol 2016;31:1705–10. [DOI] [PubMed] [Google Scholar]
- 46. Huang Q, Fang C, Shi J et al. Differences in clinicopathology of early gastric carcinoma between proximal and distal location in 438 Chinese patients. Sci Rep 2015;5:13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bass AJ, Thorsson V, Shmulevich I et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yingjin L, Su J, Changyan X. Analysis on the value of serum CEA,PGR and G-17 in the diagnosis of gastric cancer. Chin J Lab Diagn 2017;21:1498–501. [Google Scholar]
- 49. Al-Hussaini A, AlGhamdi S, Alsaaran R et al. Gastric adenocarcinoma presenting with gastric outlet obstruction in a child. Case Rep Gastrointest Med 2014;2014:527471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Banks M, Uedo N, Bhandari P et al. EMR achieves similar oncological outcomes as ESD for gastric neoplasia of <1cm, requiring less expertise, training and time. Gut 2019;69:1712–3. [DOI] [PubMed] [Google Scholar]
- 51. Shahidi N, Bourke MJ. ESD, not EMR, should be the first-line therapy for early gastric neoplasia. Gut 2020;69:1– 2. [DOI] [PubMed] [Google Scholar]
- 52. Isomoto H, Shikuwa S, Yamaguchi N et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 2009;58:331–6. [DOI] [PubMed] [Google Scholar]
- 53. Berlth F, Hoelscher AH. History of esophagogastric junction cancer treatment and current surgical management in western countries. J Gastric Cancer 2019;19:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hölscher AH, Law S. Esophagogastric junction adenocarcinomas: individualization of resection with special considerations for Siewert type II, and Nishi types EG, E=G and GE cancers. Gastric Cancer 2020;23:3–9. [DOI] [PubMed] [Google Scholar]
- 55. Ushimaru Y, Fujiwara Y, Shishido Y et al. Clinical outcomes of gastric cancer patients who underwent proximal or total gastrectomy: a propensity score-matched analysis. World J Surg 2018;42:1477–84. [DOI] [PubMed] [Google Scholar]
- 56. Sugoor P, Shah S, Dusane R et al. Proximal gastrectomy versus total gastrectomy for proximal third gastric cancer: total gastrectomy is not always necessary. Langenbecks Arch Surg 2016;401:687–97. [DOI] [PubMed] [Google Scholar]
- 57. Shaibu Z, Chen Z, Mzee SAS et al. Effects of reconstruction techniques after proximal gastrectomy: a systematic review and meta-analysis. World J Surg Oncol 2020;18:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fan JY, Qian F, Liu JJ et al. Comparison of clinical efficacy between proximal gastrectomy with double tract reconstruction and total gastrectomy with Roux-en-Y reconstruction for proximal gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2019;22:767–73 (in Chinese). [DOI] [PubMed] [Google Scholar]
- 59. Shoji Y, Nunobe S, Ida S et al. Surgical outcomes and risk assessment for anastomotic complications after laparoscopic proximal gastrectomy with double-flap technique for upper-third gastric cancer. Gastric Cancer 2019;22:1036–43. [DOI] [PubMed] [Google Scholar]
- 60. Omori T, Yamamoto K, Yanagimoto Y et al. A novel valvuloplastic esophagogastrostomy technique for laparoscopic transhiatal lower esophagectomy and proximal gastrectomy for Siewert type II esophagogastric junction carcinoma-the tri double-flap hybrid method. J Gastrointest Surg 2021;25:16–27. [DOI] [PubMed] [Google Scholar]
- 61. Cheng XD, Xu ZY, Du YA et al. Preliminary efficacy analysis of Cheng's Giraffe reconstruction after proximal gastrectomy in adenocarcinoma of esophagogastric junction. Zhonghua Wei Chang Wai Ke Za Zhi 2020;23:158–62 (in Chinese). [DOI] [PubMed] [Google Scholar]
- 62. Hu Y, Huang C, Sun Y et al. Morbidity and mortality of laparoscopic versus open d2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 2016;34:1350–7. [DOI] [PubMed] [Google Scholar]
- 63. Yang D, He L, Tong WH et al. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol 2017;23:6350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun MM, Fan YY, Dang SC. Comparison between uncut Roux-en-Y and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol 2018;24:2628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chadha MK, Kuvshinoff BW, Javle MM. Neoadjuvant therapy for gastric cancer. Oncology (Williston Park) 2005;19:1219–27; discussion 27-8, 31–2. [PubMed] [Google Scholar]
- 66. Greally M, Agarwal R, Ilson DH. Optimal management of gastroesophageal junction cancer. Cancer 2019;125:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ajani JA, D'Amico TA, Almhanna K et al. Gastric cancer, version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286–312. [DOI] [PubMed] [Google Scholar]
- 68. Wang FH, Shen L, Li J et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019;39:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sedef AM, Kose F, Sumbul AT et al. Patients with distal intestinal gastric cancer have superior outcome with addition of taxanes to combination chemotherapy, while proximal intestinal and diffuse gastric cancers do not: does biology and location predict chemotherapy benefit? Med Oncol 2015;32:476. [DOI] [PubMed] [Google Scholar]
- 70. Murat Sedef A, Taner Sumbul FKA, Ayberk Besen A et al. Addition of taxanes to combination chemotherapy in distal intestinal gastric cancer is more beneficial than proximal ones: a multicenter retrospective study of Turkish Oncology Group. J BUON 2019;24:650–5. [PubMed] [Google Scholar]
- 71. Aznab M, Beiki O, Pia KE et al. Evaluation the survival of patients with gastric cancer treated with adjuvant or palliative chemotherapy. J Gastrointest Cancer 2017;48:31–7. [DOI] [PubMed] [Google Scholar]
- 72. Ilson DH. Current progress in the adjuvant treatment of gastric cancer. Surg Oncol Clin North Am 2017;26:225–39. [DOI] [PubMed] [Google Scholar]
- 73. Hashimoto K, Mayahara H, Takashima A et al. Palliative radiation therapy for hemorrhage of unresectable gastric cancer: a single institute experience. J Cancer Res Clin Oncol 2009;135:1117–23. [DOI] [PubMed] [Google Scholar]
- 74. Gumus M, Kaya S, Eris S et al. Neoadjuvant treatment in patients with locally advanced gastric cancer. J Clin Oncol 2019;37:127. [Google Scholar]
- 75. Maron SB, Catenacci DVT. Update on gastroesophageal adenocarcinoma targeted therapies. Hematol Oncol Clin North Am 2017;31:511–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fan XS, Chen JY, Li CF et al. Differences in HER2 over-expression between proximal and distal gastric cancers in the Chinese population. World J Gastroenterol 2013;19:3316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qiu MZ, Cai MY, Zhang DS et al. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J Transl Med 2013;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yin Y, Grabowska AM, Clarke PA et al. Helicobacter pylori potentiates epithelial:mesenchymal transition in gastric cancer: links to soluble HB-EGF, gastrin and matrix metalloproteinase-7. Gut 2010;59:1037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ratti M, Lampis A, Hahne JC et al. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cell Mol Life Sci 2018;75:4151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer (review). Int J Oncol 2015;46:1421–34. [DOI] [PubMed] [Google Scholar]
- 81. Wang Q, Xie Q, Liu Y et al. Clinical characteristics and prognostic significance of TCGA and ACRG classification in gastric cancer among the Chinese population. Mol Med Rep 2020;22:828–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hu N, Wang Z, Song X et al. Genome-wide association study of gastric adenocarcinoma in Asia: a comparison of associations between cardia and non-cardia tumours. Gut 2016;65:1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhou SL, Cui J, Fan ZM et al. Polymorphism of A133S and promoter hypermethylation in Ras association domain family 1A gene (RASSF1A) is associated with risk of esophageal and gastric cardia cancers in Chinese population from high incidence area in northern China. BMC Cancer 2013;13:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li D, Wu C, Cai Y et al. Association of NFKB1 and NFKBIA gene polymorphisms with susceptibility of gastric cancer. Tumour Biol 2017;39:1010428317717107. [DOI] [PubMed] [Google Scholar]
- 85. Guo W, Dong Z, Guo Y et al. Aberrant methylation of the CpG island of HLTF gene in gastric cardia adenocarcinoma and dysplasia. Clin Biochem 2011;44:784–8. [DOI] [PubMed] [Google Scholar]
- 86. Guo W, Dong Z, He M et al. Aberrant methylation of thrombospondin-1 and its association with reduced expression in gastric cardia adenocarcinoma. J Biomed Biotechnol 2010;2010:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guo YL, Zhu TN, Guo W et al. Aberrant CpG Island Shore Region Methylation of CAV1 is associated with tumor progression and poor prognosis in gastric cardia adenocarcinoma. Arch Med Res 2016;47:460–70. [DOI] [PubMed] [Google Scholar]
- 88. Guo W, Dong Z, Liu S et al. Promoter hypermethylation-mediated downregulation of miR-770 and its host gene MEG3, a long non-coding RNA, in the development of gastric cardia adenocarcinoma. Mol Carcinog 2017;56:1924–34. [DOI] [PubMed] [Google Scholar]
- 89. Guo W, Cui YJ, Fang SM et al. Association of polymorphisms of p21cip1 and p27kip1 genes with susceptibilities of esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma [in Chinese]. Ai Zheng 2006;25:194–9. [PubMed] [Google Scholar]
- 90. Guo W, Lv P, Liu S et al. Aberrant methylation-mediated downregulation of long noncoding RNA C5orf66-AS1 promotes the development of gastric cardia adenocarcinoma. Mol Carcinog 2018;57:854–65. [DOI] [PubMed] [Google Scholar]
- 91. Miao X, Xing D, Tan W et al. Susceptibility to gastric cardia adenocarcinoma and genetic polymorphisms in methylenetetrahydrofolate reductase in an at-risk Chinese population. Cancer Epidemiol Biomarkers Prev 2002;11:1454–8. [PubMed] [Google Scholar]
- 92. Guo Y, Guo W, Chen Z et al. Hypermethylation and aberrant expression of Wnt-antagonist family genes in gastric cardia adenocarcinoma. Neoplasma 2011;58:110–7. [DOI] [PubMed] [Google Scholar]
- 93. Miao X, Zhang X, Zhang L et al. Adenosine diphosphate ribosyl transferase and x-ray repair cross-complementing 1 polymorphisms in gastric cardia cancer. Gastroenterology 2006;131:420–7. [DOI] [PubMed] [Google Scholar]
- 94. Zhang XM, Zhong R, Liu L et al. Smoking and COX-2 functional polymorphisms interact to increase the risk of gastric cardia adenocarcinoma in Chinese population. PLoS One 2011;6:e21894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen W, Wu Q, Ren H. Meta-analysis of associations between MDM2 SNP309 polymorphism and gastric cancer risk. Biomed Rep 2014;2:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guo W, Dong Z, Guo Y et al. Detection of promoter hypermethylation of the CpG island of E-cadherin in gastric cardiac adenocarcinoma. Eur J Med Res 2009;14:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang X, Kang GH, Campan M et al. Epigenetic subgroups of esophageal and gastric adenocarcinoma with differential GATA5 DNA methylation associated with clinical and lifestyle factors. PLoS One 2011;6:e25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Guo W, Zhang M, Guo Y et al. FBXO32, a new TGF-β/Smad signaling pathway target gene, is epigenetically inactivated in gastric cardia adenocarcinoma. Neoplasma 2015;62:646–57. [DOI] [PubMed] [Google Scholar]
- 99. Tang W, Chen Y, Chen S et al. Programmed death-1 (PD-1) polymorphism is associated with gastric cardia adenocarcinoma. Int J Clin Exp Med 2015;8:8086–93. [PMC free article] [PubMed] [Google Scholar]
- 100. Guo W, Dong Z, Guo Y et al. Aberrant methylation and loss expression of RKIP is associated with tumor progression and poor prognosis in gastric cardia adenocarcinoma. Clin Exp Metastasis 2013;30:265–75. [DOI] [PubMed] [Google Scholar]
- 101. Zhang Y, Zhu H, Zhang X et al. Clinical significance of MYT1L gene polymorphisms in Chinese patients with gastric cancer. PLoS One 2013;8:e71979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhou RM, Niu CX, Wang N et al. XPG gene polymorphisms and the risk of gastric cardia adenocarcinoma. Genet Test Mol Biomarkers 2016;20:432–7. [DOI] [PubMed] [Google Scholar]
- 103. Starzynska T, Markiewski M, Domagala W et al. The clinical significance of p53 accumulation in gastric carcinoma. Cancer 1996;77:2005–12. [DOI] [PubMed] [Google Scholar]
- 104. Kanda M, Oya H, Nomoto S et al. Diversity of clinical implication of B-cell translocation gene 1 expression by histopathologic and anatomic subtypes of gastric cancer. Dig Dis Sci 2015;60:1256–64. [DOI] [PubMed] [Google Scholar]
- 105. Gulmann C, Lantuejoul S, Grace A et al. Telomerase activity in proximal and distal gastric neoplastic and preneoplastic lesions using immunohistochemical detection of hTERT. Dig Liver Dis 2005;37:439–45. [DOI] [PubMed] [Google Scholar]
- 106. Miao X, Yu C, Tan W et al. A functional polymorphism in the matrix metalloproteinase-2 gene promoter (-1306C/T) is associated with risk of development but not metastasis of gastric cardia adenocarcinoma. Cancer Res 2003;63:3987–90. [PubMed] [Google Scholar]
- 107. Liu L, Wu C, Wang Y et al. Association of candidate genetic variations with gastric cardia adenocarcinoma in Chinese population: a multiple interaction analysis. Carcinogenesis 2011;32:336–42. [DOI] [PubMed] [Google Scholar]
- 108. Li P, Chen S, Chen H et al. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta 2015;444:132–6. [DOI] [PubMed] [Google Scholar]
- 109. Guo W, Dong Z, Shi Y et al. Methylation-mediated downregulation of long noncoding RNA LOC100130476 in gastric cardia adenocarcinoma. Clin Exp Metastasis 2016;33:497–508. [DOI] [PubMed] [Google Scholar]
- 110. Li S, Zhu J, Li J et al. MicroRNA-141 inhibits proliferation of gastric cardia adenocarcinoma by targeting MACC1. Arch Med Sci 2018;14:588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Huang M, He YR, Liang LC et al. Circular RNA hsa_circ_0000745 may serve as a diagnostic marker for gastric cancer. World J Gastroenterol 2017;23:6330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang Y, Feng X, Jia R et al. Microarray expression profile analysis of long non-coding RNAs of advanced stage human gastric cardia adenocarcinoma. Mol Genet Genomics 2014;289:291–302. [DOI] [PubMed] [Google Scholar]
- 113. Liu W, Dong Z, Liang J et al. Downregulation of potential tumor suppressor miR-203a by promoter methylation contributes to the invasiveness of gastric cardia adenocarcinoma. Cancer Invest 2016;34:506–16. [DOI] [PubMed] [Google Scholar]
- 114. Zhao Q, Chen S, Li T et al. Clinical values of circular RNA 0000181 in the screening of gastric cancer. J Clin Lab Anal 2018;32:e22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang S, Lv C, Jin H et al. A common genetic variation in the promoter of miR-107 is associated with gastric adenocarcinoma susceptibility and survival. Mutat Res 2014;769:35–41. [DOI] [PubMed] [Google Scholar]
- 116. Xie Y, Shao Y, Sun W et al. Downregulated expression of hsa_circ_0074362 in gastric cancer and its potential diagnostic values. Biomark Med 2018;12:11–20. [DOI] [PubMed] [Google Scholar]
- 117. Gao S, Zhou F, Zhao C et al. Gastric cardia adenocarcinoma microRNA profiling in Chinese patients. Tumor Biol 2016;37:9411–22. [DOI] [PubMed] [Google Scholar]
- 118. Wang J, Lv W, Lin Z et al. Hsa_circ_0003159 inhibits gastric cancer progression by regulating miR-223-3p/NDRG1 axis. Cancer Cell Int 2020;20:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chen S, Li T, Zhao Q et al. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta 2017;466:167–71. [DOI] [PubMed] [Google Scholar]
- 120. Tang W, Fu K, Sun H et al. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol Cancer 2018;17:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang Y, Liu H, Li W et al. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging (Albany NY) 2017;9:1585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shen F, Liu P, Xu Z et al. CircRNA_001569 promotes cell proliferation through absorbing miR-145 in gastric cancer. J Biochem 2019;165:27–36. [DOI] [PubMed] [Google Scholar]
- 123. Sun H, Xi P, Sun Z et al. Circ-SFMBT2 promotes the proliferation of gastric cancer cells through sponging miR-182-5p to enhance CREB1 expression. Cancer Manag Res 2018;10:5725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen J, Li Y, Zheng Q et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett 2017;388:208–19. [DOI] [PubMed] [Google Scholar]
- 125. Pereira AL, Magalhães L, Pantoja RP et al. The biological role of sponge circular RNAs in gastric cancer: main players or coadjuvants? Cancers (Basel) 2020;12:1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Xu ZY, Yu QM, Du YA et al. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci 2013;9:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhou X, Ye F, Yin C et al. The interaction between MiR-141 and lncRNA-H19 in regulating cell proliferation and migration in gastric cancer. Cell Physiol Biochem 2015;36:1440–52. [DOI] [PubMed] [Google Scholar]
- 128. Yang F, Xue X, Bi J et al. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol 2013;139:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cai H, Ye X, He B et al. LncRNA-A. P001631.9 promotes cell migration in gastric cancer. Int J Clin Exp Pathol 2015;8:6235–44. [PMC free article] [PubMed] [Google Scholar]
- 130. Saito T, Kurashige J, Nambara S et al. A long non-coding RNA activated by transforming growth factor-β is an independent prognostic marker of gastric cancer. Ann Surg Oncol 2015;22: 915–22. [DOI] [PubMed] [Google Scholar]
- 131. Tan L, Yang Y, Shao Y et al. Plasma lncRNA-GACAT2 is a valuable marker for the screening of gastric cancer. Oncol Lett 2016;12:4845–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xu TP, Huang MD, Xia R et al. Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression. J Hematol Oncol 2014;7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Xia T, Chen S, Jiang Z et al. Long noncoding RNA FER1L4 suppresses cancer cell growth by acting as a competing endogenous RNA and regulating PTEN expression. Sci Rep 2015;5:13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Xia H, Chen Q, Chen Y et al. The lncRNA MALAT1 is a novel biomarker for gastric cancer metastasis. Oncotarget 2016;7:56209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Xian HP, Zhuo ZL, Sun YJ et al. Circulating long non-coding RNAs HULC and ZNFX1-AS1 are potential biomarkers in patients with gastric cancer. Oncol Lett 2018;16:4689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yang Y, Ma B, Yan Y et al. Long non-coding RNA HOXA transcript at the distal tip as a biomarker for gastric cancer. Oncol Lett 2017;14:1068–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chang S, Liu J, Guo S et al. HOTTIP and HOXA13 are oncogenes associated with gastric cancer progression. Oncol Rep 2016;35:3577–85. [DOI] [PubMed] [Google Scholar]
- 138. Li H, Zhu H, Zhou Y et al. Long non-coding RNA MSTO2P promotes the proliferation and colony formation in gastric cancer by indirectly regulating miR-335 expression. Tumour Biol 2017;39:1010428317705506. [DOI] [PubMed] [Google Scholar]
- 139. Gao S, Zhao ZY, Wu R et al. Prognostic value of long noncoding RNAs in gastric cancer: a meta-analysis. Onco Targets Ther 2018;11:4877–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Liu R, Zhang C, Hu Z et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer 2011;47:784–91. [DOI] [PubMed] [Google Scholar]
- 141. Tsujiura M, Ichikawa D, Komatsu S et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer 2010;102:1174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Li X, Zhang Y, Zhang Y et al. Survival prediction of gastric cancer by a seven-microRNA signature. Gut 2010;59:579–85. [DOI] [PubMed] [Google Scholar]
- 143. Yu H, Wei W, Cao W et al. Regulation of cell proliferation and metastasis by microRNA-593-5p in human gastric cancer. Onco Targets Ther 2018;11:7429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhu Z, Yu Z, Rong Z et al. The novel GINS4 axis promotes gastric cancer growth and progression by activating Rac1 and CDC42. Theranostics 2019;9:8294–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhu Z, Rong Z, Luo Z et al. Circular RNA circNHSL1 promotes gastric cancer progression through the miR-1306-3p/SIX1/vimentin axis. Mol Cancer 2019;18:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Luo Z, Rong Z, Zhang J et al. Circular RNA circCCDC9 acts as a miR-6792-3p sponge to suppress the progression of gastric cancer through regulating CAV1 expression. Mol Cancer 2020;19:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tachibana M, Takemoto Y, Nakashima Y et al. Serum carcinoembryonic antigen as a prognostic factor in resectable gastric cancer. J Am Coll Surg 1998;187:64–8. [DOI] [PubMed] [Google Scholar]
- 148. Park JY, Sung JY, Lee J et al. Polarized CD163+ tumor-associated macrophages are associated with increased angiogenesis and CXCL12 expression in gastric cancer. Clin Res Hepatol Gastroenterol 2016;40:357–65. [DOI] [PubMed] [Google Scholar]
- 149. Lee HK, Lee HJ, Hur K et al. Growth effect of gastrin on gastric cancer and its clinical implications for gastric cancer surgery. Oncol Rep 2005;14:383–8. [PubMed] [Google Scholar]
- 150. Jinfeng C, Chenyan Z, Liyong C. Comparison of KLF4, SP1, and Cyclin D1 expressions between adenocarcinanoma of the esophagogastric junction and distal gastric adenocarcinoma. Chin J Clin Oncol 2014;41:108–12. [Google Scholar]
- 151. Gulmann C, Counihan I, Grace A et al. Cytokeratin 7/20 and mucin expression patterns in oesophageal, cardia and distal gastric adenocarcinomas. Histopathology 2003;43:453–61. [DOI] [PubMed] [Google Scholar]
- 152. Cheng XJ, Lin JC, Tu SP. Etiology and prevention of gastric cancer. Gastrointest Tumors 2016;3:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Myung SK, Kim Y, Ju W et al. Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann Oncol 2010;21:166–79. [DOI] [PubMed] [Google Scholar]
- 154. Bjelakovic G, Nikolova D, Simonetti RG et al. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 2004;364:1219–28. [DOI] [PubMed] [Google Scholar]
- 155. Wang WH, Huang JQ, Zheng GF et al. Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2003;95:1784–91. [DOI] [PubMed] [Google Scholar]
- 156. Barry DP, Asim M, Leiman DA et al. Difluoromethylornithine is a novel inhibitor of Helicobacter pylori growth, CagA translocation, and interleukin-8 induction. PLoS One 2011;6:e17510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yeh JM, Hur C, Kuntz KM et al. Cost-effectiveness of treatment and endoscopic surveillance of precancerous lesions to prevent gastric cancer. Cancer 2010;116:2941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]