Abstract
Background
Diethylcarbamazine citrate (DEC) treatment of loiasis is complicated by adverse reactions that are correlated with the number of circulating microfilariae (mf). The cause of these reactions is unknown, but they are accompanied by a dramatic interleukin-5 (IL-5)-dependent increase in eosinophilia and evidence of eosinophil activation.
Methods
To explore the role of IL-5 driven eosinophilia in post-DEC reactions, 8 adults with confirmed loiasis and <5000 mf/mL blood were enrolled in a randomized, double-blind, placebo-controlled trial of the humanized anti-IL-5 antibody, reslizumab, (1.0 mg/kg IV) administered 3 to 7 days prior to initiation of DEC treatment (9 mg/kg/day for 21 days). The primary endpoint was the reduction in absolute eosinophil count (AEC) during the first week of DEC treatment.
Results
Baseline characteristics were comparable between the two groups. Single dose reslizumab lowered the AEC by 77% prior to initiation of DEC therapy (vs. 12% in the placebo group, P < .05). More importantly, AEC remained below baseline in the first week of DEC treatment in all subjects who received reslizumab and in none of the placebo subjects. Mf clearance occurred within 2 days of initiation of DEC in all 7 mf-positive subjects. Mild to moderate adverse events were seen in all 8 subjects and were not significantly different between the groups.
Conclusions
In summary, although reslizumab was able to blunt peripheral eosinophilia post-DEC treatment in subjects with loiasis and had no effect on microfilarial clearance, the reduction in AEC appeared to have been insufficient to prevent post-treatment AEs.
Keywords: Loa loa, diethylcarbamazine, eosinophil, filariasis, interleukin-5
Post-treatment reactions in loiasis are accompanied by an IL-5 driven rise in eosinophil count. Pretreatment with reslizumab (anti-IL-5 antibody) blunted the eosinophil response postdiethylcarbamazine treatment without impeding microfilarial clearance but had no effect on post-treatment clinical manifestations.
Loiasis is a chronic filarial infection affecting approximately 13 million people in Central and West Africa, with a geographic distribution that overlaps considerably with that of Wuchereria bancrofti and Onchocerca volvulus. Microfilarial (mf) counts in the blood range from undetectable to more than 100 000 mf/mL and are remarkably stable in an infected individual over time [1]. Although visitors to endemic areas who acquire infection typically have a wide variety of clinical manifestations (transient migratory angioedema, urticaria, myalgias, and arthralgias) and dramatic eosinophilia, most infected residents are asymptomatic, despite high levels of microfilaremia [2].
Diethylcarbamazine (DEC) has a rapid microfilaricidal and slower macrofilaricidal effect and is the treatment of choice for loiasis in nonendemic countries, as it is curative in the majority (95%) of infected subjects [3, 4]. However, severe, potentially fatal, reactions following DEC treatment can occur in patients with high microfilarial loads, limiting its utility in endemic settings. As previously shown in other filarial infections [5], a transient decrease followed by a dramatic increase in serum interleukin (IL)-5 and peripheral eosinophil counts heralds the appearance of post-DEC symptoms in loiasis [6]. This suggests that the post-treatment reactions observed in Loa-infected subjects likely result from immune responses to released parasite antigens, and that IL-5-driven eosinophilia may be an important factor in this response. Moreover, Loa loa does not harbor the intracellular endosymbiont Wolbachia, found in other human filarial parasites and implicated in the pathogenesis of post-treatment reactions in onchocerciasis and lymphatic filariasis [7, 8].
Whether blocking IL-5 would reduce the post-treatment side effects associated with DEC treatment in loiasis is unknown. One concern in using an immunomodulatory drug is whether it could reduce treatment efficacy by impairing microfilarial clearance, as has been reported with prednisone [9]. Administration of anti-IL5 antibody prior to administration of DEC in a murine model of Brugia malayi microfilaremia did not alter microfilarial clearance (Klion, unpublished data), suggesting that this might not be the case. Reslizumab is a humanized anti-human IL-5 monoclonal antibody that is FDA-approved for the treatment of eosinophilic asthma and has been shown to reduce blood eosinophilia in patients with hypereosinophilic syndrome [10] for up to 12 weeks after a single 1 mg/kg intravenous dose. The aim of the present study was to determine whether administration of a single dose of intravenous reslizumab could prevent the post-treatment eosinophilia and eosinophil activation seen following DEC in patients with loiasis and, if so, whether this would have an effect on the severity of post-treatment reactions or parasite clearance.
MATERIALS AND METHODS
Study Population
Otherwise healthy, nonpregnant individuals between 18 and 65 years of age with a compatible exposure history were recruited and screened for Loa loa microfilaremia by nuclepore filtration of 1 mL of blood drawn between 10 am and 2 pm and Loa loa PCR [11]. Subjects with Loa loa microfilaremia between 0 and 5000 mf/mL, a positive PCR for Loa loa DNA, or a history of documented eyeworm in the absence of detectable Loa loa mf underwent additional screening procedures in order to determine eligibility for the interventional portion of the study. Potential participants were excluded if they were HIV-positive, actively infected with Onchocerca volvulus (as determined by skin snips and ophthalmological examination), had used any investigational or immunosuppressive agent within the past 30 days, had a history of allergic reaction to antibody therapy or DEC, or had chronic liver or kidney disease or any condition that the investigator felt placed them at undue risk (see flow diagram in Supplementary Figure 1).
Study Objectives
The primary objective of this study was to assess the effect of reslizumab compared to placebo in reducing eosinophilia following DEC treatment of Loa loa infection in subjects with 0–5000 mf/mL blood. Secondary objectives included determination of the effect of reslizumab on the severity of post-treatment adverse events (AE), markers of eosinophil activation, drug efficacy (microfilarial clearance and disease recurrence), and antifilarial immune responses.
Study Design
Subjects were block randomized 1:1 in groups of 4 to receive reslizumab (Teva Branded Pharmaceutical Products R&D, Inc.) or saline placebo. Following baseline assessment, including targeted history and physical examination, pregnancy testing, microfilarial count, complete blood count (CBC), routine chemistries and urinalysis, and serologic testing for strongyloidiasis and schistosomiasis [performed at the Centers for Disease Control (CDC), Atlanta, GA], subjects received a single dose of reslizumab (1 mg/kg) or saline placebo by intravenous infusion at day 0. Three to 7 days later, subjects were admitted to the NIH clinical center for initiation of DEC treatment at the standard dose of 3 mg/kg orally 3 times a day for 21 days. DEC was provided by the CDC under an Investigational New Drug protocol for treatment of filariasis and chemoprophylaxis of loiasis. Subjects were followed daily as inpatients for a minimum of 3 days and as outpatients at days 7, 14, and 28 and months 3, 6, 12, 18, and 24 postinitiation of DEC treatment for the assessment of post-treatment AEs, microfilarial counts, and collection of research samples. New or worsening symptoms, physical examination findings, or laboratory abnormalities were considered AEs and were scored using the Common Terminology Criteria for AEs v4.0. Subjects and study staff were blinded to the identity of the study drug and to the results of serial CBCs and any other research tests that could unblind the absolute eosinophil count (AEC) for an individual subject until 6 months after the final subject initiated DEC treatment. The study was approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases (NCT01111305), and informed consent was obtained from all participants.
Serum Mediator Analysis
Serum levels of eosinophil granule proteins, cytokines, and chemokines were measured by suspension array multiplex immunoassays, using a previously described assay [12] and Millipore kits (EMD Millipore, Burlington, MA) according to the manufacturer’s instructions. Mediators measured and minimal detectable levels are provided in the Supplementary Methods.
Whole Blood Flow Cytometry
Surface expression of CD25, CD69, and IL-5 receptor α on peripheral blood eosinophils was assessed by whole blood flow cytometry, as previously described [13]. The normal ranges for surface receptor expression represent the 95% prediction intervals for expression on eosinophils from healthy blood bank volunteers.
Statistical Analysis
Although this pilot study was designed as a proof-of-principle, and the small sample size was dictated by the difficulty in recruiting infected patients with the appropriate microfilarial levels, the power calculation estimate for the primary endpoint is provided in the Supplementary Methods. Changes over time were assessed using one-sample t-tests on the log percent change to test for significant change and determine confidence intervals on the geometric mean ratios. For continuous measures (eg, markers of eosinophil activation, percent change in eosinophil count), the placebo arm was compared to the reslizumab arm using an exact 2-sided Wilcoxon-Mann-Whitney test. Time until clearance of mf was compared between arms using an exact log-rank test (the subject without baseline mf was excluded from this analysis).
RESULTS
Baseline Characteristics of the Study Population
Baseline demographic and clinical characteristics were similar between the patients in the reslizumab and placebo groups (Table 1). All patients had detectable blood microfilariae with the exception of one patient in the reslizumab group who met entry criteria on the basis of a clinically documented eyeworm. Although all patients had positive serology for strongyloidiasis, none had larvae detected in stool samples. The single patient with positive serology for S. haematobium had eggs documented on stool examination. Eosinophil counts were increased (≥500/μL) at baseline in 7/8 patients and markedly elevated (≥1500/μL) in 2 patients in the reslizumab group and 1 in the placebo group. There were no significant differences in baseline leukocyte counts or levels of T, B, or NK cells between the two groups (Supplementary Figure 2).
Table 1.
Baseline Characteristics of the Study Population
| Reslizumab (n = 4) | Placebo (n = 4) | |
|---|---|---|
| Gender (male/female) | 3/1 | 3/1 |
| Median age in years (range) | 36 (22–44) | 29 (20–55) |
| Country of origin | ||
| Cameroon | 2 | 3 |
| Dem. Rep. Congo | 2 | 0 |
| Equatorial Guinea | 0 | 1 |
| GM Loa loa mf/mLa (range) | 212 (0–2120) | 357 (178–810) |
| GM AEC x109/L (range) | 1.35 (.53–3.13) | .71 (.56–1.59) |
| Clinical presentation | ||
| Eyeworm | 2 | 3 |
| Calabar swelling | 2 | 2 |
| Asymptomatic | 2 | 1 |
| Concomitant helminth infectionb | ||
| Positive stool examination | 0 | 1c |
| Positive serology for schistosomiasis | 0 | 1 |
| Positive Strongyloides serology | 4 | 4 |
Abbreviations: AEC, absolute eosinophil count; Dem. Rep., Democratic Republic; GM, geometric mean; M. perstans, xxx; MF, microfilaria; n, number; O. volvulus, Onchocerca volvulus; PCR, xxx; S. haematobium, xxx; W. bancrofti, xxx.
aIn the calculation of the GM, .1 was used instead of 0 for the one patient without detectable mf.
bNo subjects had M. perstans in the blood detected by filtration or PCR, positive filarial circulating antigen (W. bancrofti), positive skin snips or Ov16 serology for O. volvulus.
c S. haematobium eggs confirmed by stool examination.
Effect of Reslizumab on Eosinophilia
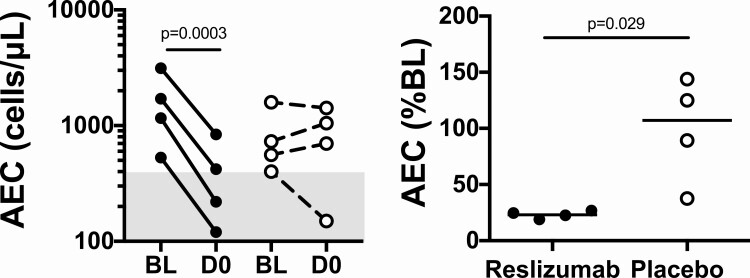
Prior to DEC treatment, reslizumab treatment led to a dramatic decrease in AEC in all 4 subjects [geometric mean (GM) AEC 1347 to 311, P = .0003, Figure 1A]. In contrast, the AEC rose or was stable in 3 of the 4 subjects in the placebo group (GM 714 to 629, P = .70, Figure 1A). Expressed as a percent of the baseline value, the GM on Day 0 (D0) AEC was 23% of the baseline (95% CI: 18%, 29%) in the reslizumab group prior to DEC treatment and was significantly lower than the 88% of baseline (95% CI: 34%, 230%) in the placebo group, Mann-Whitney test P = .029, Figure 1B).
Figure 1.
Reslizumab lowers absolute eosinophil counts (AEC) in patients with loiasis prior to diethylcarbamazine therapy. A shows the AEC at baseline (BL) and at Day 0 (D0). There is a significant decline in the reslizumab group (P = .0003), but not in the placebo group (P = .70). B shows the AEC on D0 expressed as a percent of the baseline AEC (P = .029, Mann-Whitney test). Subjects who received reslizumab are indicated by closed circles, and those who received placebo by open circles. The horizontal lines in panel B indicate geometric means for each group. The gray shading denotes the normal range for AEC (A) and values representing a percent decrease from baseline AEC (B).
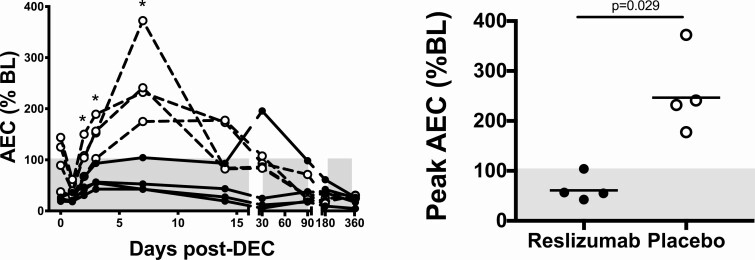
As expected, the AEC decreased transiently post-DEC (from D0 at D1) in all 4 placebo subjects and in 3 of the 4 reslizumab subjects (Figure 2A). The AEC subsequently increased in all 8 subjects (Figure 2B), but remained at or below baseline (prereslizumab or placebo) levels during the first 2 weeks postinitiation of DEC treatment in all 4 subjects in the reslizumab group compared to 0 of the 4 patients in the placebo group (Figure 2A). The geometric mean peak AEC expressed as a% of baseline level was significantly lower in the reslizumab group (Mann-Whitney test P = .029, Figure 2B). The AEC began to decrease from peak values in all subjects by D14 and, with the exception of one subject in the reslizumab group who had an unexplained transient rise in AEC at D30 to 2270/mL, continued to decline steadily after that time (Figure 2A). For all subjects, AEC reached normal levels (≤500 eosinophils/μL) by 1-year post-treatment.
Figure 2.
Reslizumab blunts post-treatment eosinophilia. A shows the percent of baseline AEC as a function of time. The gray shaded area indicates levels that are below baseline. *P < .05, Mann Whitney test, reslizumab vs. placebo. B shows the peak AEC during the first week of DEC treatment expressed as a percent of the baseline AEC (P = .029, Mann-Whitney test). The horizontal lines indicate the geometric mean for each group and the gray shading denotes the normal range for AEC. In both panels, subjects who received reslizumab are indicated by closed circles and those who received placebo by open circles. Abbreviations: AEC, absolute eosinophil counts; DEC, diethylcarbamazine citrate.
Reslizumab Selectively Blocks IL-5 Production
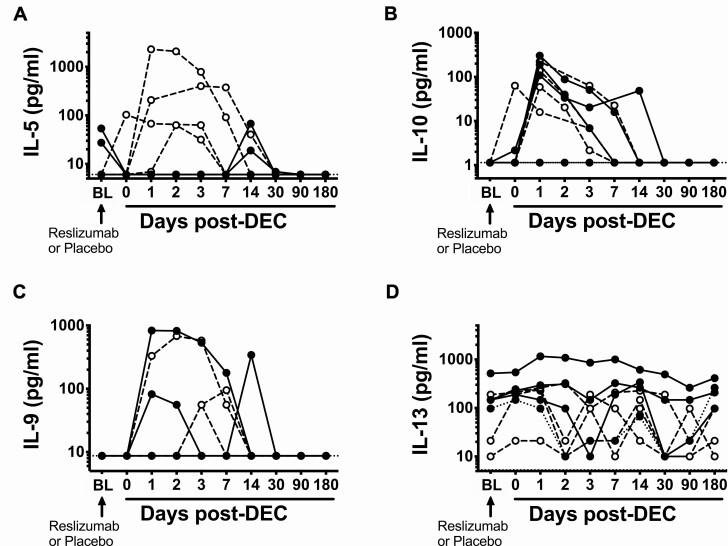
Serum IL-5 levels were undetectable following reslizumab administration and remained suppressed for 7 days following DEC administration in all 4 subjects in the reslizumab group (Figure 3A). In contrast, post-DEC serum IL-5 levels increased in all 4 placebo subjects, peaking at 3–7 days before returning to the normal range by day 30. Eosinophil surface expression of IL5RA typically correlates negatively with serum levels of IL-5 [13]. Eosinophil expression of IL5RA increased prior to DEC administration as expected in 2 of the 3 subjects who received reslizumab and for whom baseline IL5RA data were available (and remained elevated above baseline for 3–7 days after initiation of DEC) but in none of the 4 subjects who received placebo (Supplementary Figure 3). Serum IL-10 and IL-9 levels were unaffected by pretreatment with reslizumab and increased following DEC treatment in nearly all subjects (Figure 3B, C). Serum levels of IL-13 were measurable in most patients but showed no clear pattern in response to reslizumab or DEC treatment (Figure 3D).
Figure 3.
Reslizumab selectively inhibits IL-5 following diethylcarbamazine administration. Serum (A) IL-5, (B) IL-10, (C) IL-9, and (D) IL-13 levels over time in patients treated with reslizumab (closed circles, solid lines) or placebo (open circles, dashed lines). X-axis labels do not accurately reflect elapsed time. Values are plotted for all time points for all subjects (no missing data). Horizontal dotted lines represent the limit of detection for the assay. Abbreviations: DEC, diethylcarbamazine citrate; IL, interleukin.
Reslizumab Decreases Eosinophil Activation
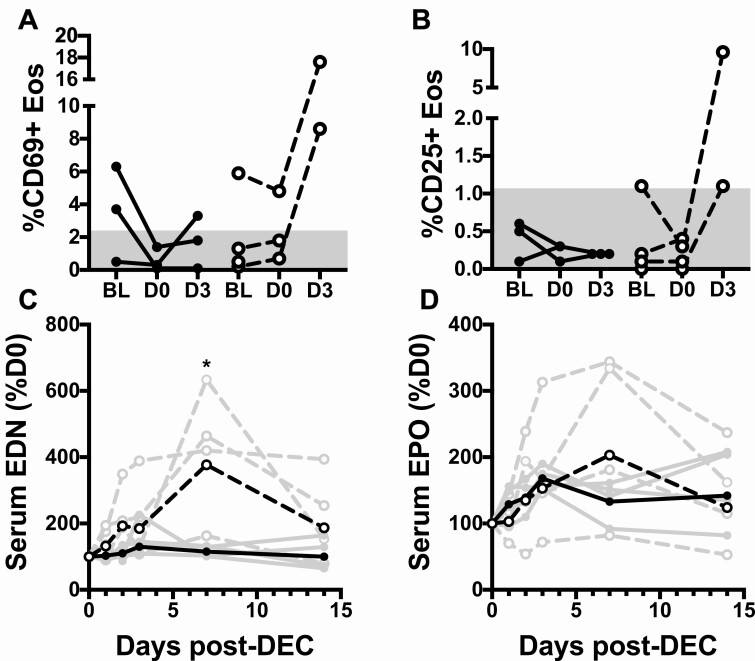
Eosinophil expression of the activation markers, CD69 and CD25, were measured at baseline, D0 (prior to DEC) and D3. The percent of eosinophils expressing CD69 was increased in 3 of the 7 subjects with available data at baseline (Figure 4A). Whereas %CD69 expression normalized to ≤2.2% by D0 in both subjects who received reslizumab, this downmodulation of eosinophil activation was not observed in the placebo subject (Figure 4A). Moreover, %CD69 expression increased dramatically following DEC in both placebo patients with available data but remained below or near baseline in all 3 subjects who received reslizumab. A similar pattern was observed for %CD25 expression (Figure 4).
Figure 4.
Reslizumab blocks eosinophil activation. The percent of eosinophils expressing CD69 (panel A) or CD25 (panel B) was assessed by whole blood flow cytometry at baseline, D0 (before DEC) and D3 in subjects who received reslizumab (closed circles, solid lines) or placebo (open circles, dashed lines). The gray areas indicate the normal ranges in healthy controls. Serum levels of EDN (panel C) and EPO (panel D) are shown over time as a percent of D0 value for subjects who received reslizumab (closed circles, solid lines) or placebo (open circles, dashed lines). Individual subject data is shown in gray and geometric mean data in black. *P = .029, Mann Whitney test. Abbreviations: DEC, diethylcarbamazine citrate; EDN, eosinophil-derived neurotoxin; Eos, xxx; EPO, xxx.
Serum levels of eosinophil-derived neurotoxin (EDN) and eosinophil peroxidase (EPO) were measured at baseline, D0 (prior to DEC), D1, D2, D3, D7, and D14. Serum EDN and EPO levels decreased between baseline and D0 in all 4 subjects who received reslizumab with average values of 44% baseline (95% CI: 16%, 120%; P = .08) for EDN and 48% baseline (95% CI: 27%, 88%; P = .03) for EPO (Supplementary Figure 4). Although serum EDN and EPO levels also decreased in 2 of the 4 subjects who received placebo, neither decrease was statistically significant with average values of 68% baseline (95% CI: 40%, 117%, P = .11) for serum EDN and 94% of baseline (95% CI: 59%, 148%, P = .68) for EPO (Supplementary Figure 4).
Similar to previously reported data [6], serum levels of EDN rose following initiation of DEC in all 4 placebo subjects, peaking at D7 (Figure 4C). EDN levels showed some variability over time post-DEC in the subjects who received reslizumab; however, there was no clear pattern. Although both groups showed increased geometric mean% baseline EDN levels at day 7 following initiation of DEC, this increase was significantly smaller in the reslizumab compared to placebo group (115% vs 377%, P = .029, Mann-Whitney test; Figure 4C). Serum EPO showed a similar pattern, albeit less pronounced (Figure 4D).
Reslizumab Does Not Affect mf Clearance
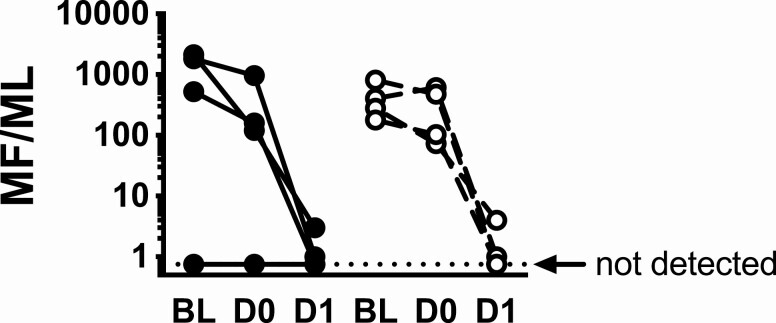
Although mf levels decreased slightly between day -4 and day 0 in 6 of the 7 subjects with detectable mf at baseline, the decrease was comparable between subjects who received reslizumab and those who received placebo (Figure 5). Mf levels decreased dramatically in all 7 subjects following initiation of DEC treatment and became undetectable in 3 subjects by D1and in all subjects from D2 until the end of study (2 years post-treatment), and there was no significant difference between the groups (logrank test, P = 1.0).
Figure 5.
Reslizumab does not affect microfilarial clearance. The microfilarial count per milliliter of blood (MF/mL) is shown at BL (baseline), D0 (prior to DEC) and D1 (post-DEC). Subjects who received reslizumab at baseline are indicated by closed circles and solid lines and those who received placebo by open circles and dashed lines. The horizontal dotted line indicates 0 MF/mL. Abbreviations: D, xxx; DEC, diethylcarbamazine citrate.
Effect of Reslizumab on Adverse Events
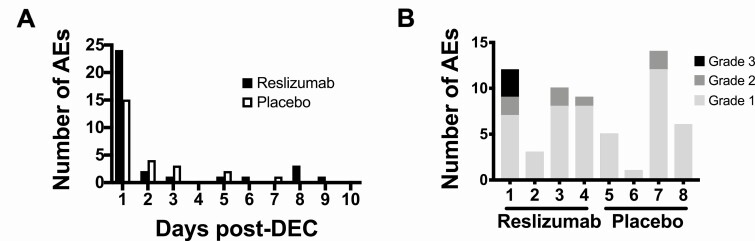
All 8 subjects developed mild to moderate AEs in the week following initiation of DEC therapy (Table 2). Symptoms peaked in the first 24 hours postinitiation of DEC treatment in both groups (Figure 6A). Neither the severity of the side effects nor the number of side effects were appreciably different between the two groups (Figure 6B).
Table 2.
Adverse Events Post-DEC Treatment
| Reslizumab (n = 4) | Placebo (n = 4) | |
|---|---|---|
| Signs and Symptoms | ||
| Fatigue | 1 | 3 |
| Nausea | 2 | 1 |
| Headache | 2 | 2 |
| Calabar swelling | 2 | 1 |
| Pruritus | 1 | 2 |
| Arthralgia | 4 | 0 |
| Low back pain | 2 | 3 |
| Myalgia | 2 | 2 |
| Abdominal pain | 2 | 1 |
| Any sign or symptoms | 4 | 4 |
| Laboratory Abnormalities | ||
| LDH increased | 1 | 2 |
| ALT increased | 2 | 1 |
| AST increased | 3 | 2 |
| Bilirubin increased | 1 | 2 |
| CPK increased | 2 | 1 |
| Hematuria | 2 | 1 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; CPK, creatine phosphokinase; DEC, diethylcarbamazine citrate; LDH, lactate dehydrogenase; n, number.
Figure 6.
Pretreatment with reslizumab did not reduce the number or severity of post-treatment adverse events (AE). The number of AEs reported in each group during the first 10 days post-treatment is shown in A. B shows the severity of these post-treatment reactions in individual subjects who received reslizumab (subjects 1–4) or placebo (subjects 5–8). Abbreviation: DEC, diethylcarbamazine citrate.
Discussion
Post-treatment reactions in loiasis pose a significant problem for individual infected patients and for endemic communities, where mass drug administration programs with ivermectin have been interrupted in areas co-endemic for loiasis due to these reactions. Although the pathophysiology of these reactions is incompletely understood, reaction severity is clearly related to microfilarial load [14], and several studies have demonstrated a temporal association between post-treatment reactions and IL-5-driven eosinophilia and eosinophil activation, suggesting that eosinophils may play an important role [6, 15].
Historically, antihistamines and anti-inflammatory agents, including corticosteroids, have been used to mitigate post-treatment reactions in filariasis. Whereas corticosteroids and antihistamines have demonstrated some efficacy in reducing the intensity of some post-DEC symptoms in onchocerciasis [16] and lymphatic filariasis [17], neither appears to prevent severe post-treatment adverse events, including encephalopathy, in loaisis [18, 19]. This lack of protective effect was confirmed in a study of postivermectin reactions in baboons with high levels of Loa loa microfilaremia [20]. Reduction of microfilarial loads with apheresis prior to treatment has proven effective in preventing severe post-treatment reactions in humans with microfilaremic loiasis [21] but is impractical in most settings.
A better understanding of the pathogenesis of post-treatment reactions in loiasis could lead to novel approaches for the prevention of post-treatment reactions. Given the data supporting a role for IL-5-driven eosinophilia, the present study was designed as a proof-of-concept. As predicted, reslizumab selectively blocked the post-DEC rise in serum IL-5 levels and significantly blunted post-treatment eosinophilia and eosinophil activation. Importantly, this effect was not due to impaired microfilaricidal activity, as the rate and extent of microfilarial clearance following DEC treatment were comparable in the reslizumab and placebo groups. Unfortunately, post-treatment adverse events were also similar between the two groups. Although these data appear to contradict the hypothesis that eosinophils play a central role in post-treatment reactions in loiasis, several limitations in the study design likely decreased the potential to detect a difference between the two groups. These include the relatively low dose of reslizumab (which lowered but did not completely suppress the post-treatment eosinophilia and eosinophil activation), the exclusion of patients with >5000 mf/mL (ie, most likely to develop significant post-treatment reactions) and the small sample size, due in part to the difficulty in identifying and recruiting patients with loiasis and low levels of microfilaremia in a nonendemic area.
Despite the limitations, this study met its predefined primary endpoint (reduction in peak eosinophil count measured during the first 7 days of DEC treatment as a percent of baseline count) and definitively demonstrated for the first time that IL-5 is the predominant driver of pre- and post-treatment eosinophilia and eosinophil activation in a human helminth infection. Conversely, the lack of effect of reslizumab on pretreatment microfilarial levels suggests that, despite the association between high eosinophil counts and low microfilarial levels observed in prior clinical studies, eosinophils do not play a major role in the control of steady state microfilarial levels. These findings illustrate the power of targeted biologics as a tool to dissect human immune responses in parasitic infection. Future studies using higher doses of anti-IL5 antibody or other agents that more completely suppress eosinophilia, such as the afucosylated antibody to IL-5 receptor, benralizumab, may be useful in further elucidating what role, if any, eosinophils play in post-treatment reactions in loiasis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the many members of the NIH Clinical Center staff and the study subjects without whom this study would not have been possible.
Financial support. This work was supported by the Division of Intramural Research of the National Institute for Allergy and Infectious Diseases at the US National Institutes of Health. There were no sponsors for the study design, collection, and analysis of data, or writing of this report.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Garcia A, Abel L, Cot M, et al. Longitudinal survey of Loa loa filariasis in southern Cameroon: long-term stability and factors influencing individual microfilarial status. Am J Trop Med Hyg 1995; 52:370–5. [DOI] [PubMed] [Google Scholar]
- 2. Klion AD, Massougbodji A, Sadeler BC, Ottesen EA, Nutman TB. Loiasis in endemic and nonendemic populations: immunologically mediated differences in clinical presentation. J Infect Dis 1991; 163:1318–25. [DOI] [PubMed] [Google Scholar]
- 3. Klion AD, Ottesen EA, Nutman TB. Effectiveness of diethylcarbamazine in treating loiasis acquired by expatriate visitors to endemic regions: long-term follow-up. J Infect Dis 1994; 169:604–10. [DOI] [PubMed] [Google Scholar]
- 4. Herrick JA, Makiya MA, Holland-Thomas N, Klion AD, Nutman TB. Infection-associated immune perturbations resolve one year following treatment for Loa loa. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Limaye AP, Ottesen EA, Kumaraswami V, et al. Kinetics of serum and cellular interleukin-5 in posttreatment eosinophilia of patients with lymphatic filariasis. J Infect Dis 1993; 167:1396–400. [DOI] [PubMed] [Google Scholar]
- 6. Herrick JA, Legrand F, Gounoue R, et al. Posttreatment reactions after single-dose diethylcarbamazine or ivermectin in subjects with Loa loa infection. Clin Infect Dis 2017; 64:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cross HF, Haarbrink M, Egerton G, Yazdanbakhsh M, Taylor MJ. Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood. Lancet 2001; 358:1873–5. [DOI] [PubMed] [Google Scholar]
- 8. Turner PF, Rockett KA, Ottesen EA, Francis H, Awadzi K, Clark IA. Interleukin-6 and tumor necrosis factor in the pathogenesis of adverse reactions after treatment of lymphatic filariasis and onchocerciasis. J Infect Dis 1994; 169:1071–5. [DOI] [PubMed] [Google Scholar]
- 9. Schofield FD, Rowley RE. The effect of prednisone on persistent microfilaremia during treatment with diethylcarbamazine. Am J Trop Med Hyg 1961; 10:849–54. [DOI] [PubMed] [Google Scholar]
- 10. Klion AD, Law MA, Noel P, Kim YJ, Haverty TP, Nutman TB. Safety and efficacy of the monoclonal anti-interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood 2004; 103:2939–41. [DOI] [PubMed] [Google Scholar]
- 11. Fink DL, Kamgno J, Nutman TB. Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia. PLoS Negl Trop Dis 2011; 5:e1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makiya MA, Herrick JA, Khoury P, Prussin CP, Nutman TB, Klion AD. Development of a suspension array assay in multiplex for the simultaneous measurement of serum levels of four eosinophil granule proteins. J Immunol Methods 2014; 411:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson TM, Maric I, Shukla J, et al. IL-5 receptor α levels in patients with marked eosinophilia or mastocytosis. J Allergy Clin Immunol 2011; 128:1086–92.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chippaux JP, Boussinesq M, Gardon J, Gardon-Wendel N, Ernould JC. Severe adverse reaction risks during mass treatment with ivermectin in loiasis-endemic areas. Parasitol Today 1996; 12:448–50. [DOI] [PubMed] [Google Scholar]
- 15. Herrick JA, Metenou S, Makiya MA, et al. Eosinophil-associated processes underlie differences in clinical presentation of loiasis between temporary residents and those indigenous to Loa-endemic areas. Clin Infect Dis 2015; 60:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stingl P, Pierce PF, Connor DH, et al. Does dexamethasone suppress the Mazzotti reaction in patients with onchocerciasis? Acta Trop 1988; 45:77–85. [PubMed] [Google Scholar]
- 17. March HN, Laigret J. The effect of cortisone and prednisone on bullous reactions following treatment of filariasis with diethylcarbamazine. Am J Trop Med Hyg 1958; 7:185–6. [DOI] [PubMed] [Google Scholar]
- 18. Carme B, Boulesteix J, Boutes H, Puruehnce MF. Five cases of encephalitis during treatment of loiasis with diethylcarbamazine. Am J Trop Med Hyg 1991; 44:684–90. [DOI] [PubMed] [Google Scholar]
- 19. van Bogaert L, Dubois A, Janssens PG, Radermecker J, Tverdy G, Wanson M. Encephalitis in loa-loa filariasis. J Neurol Neurosurg Psychiatry 1955; 18:103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wanji S, Eyong EE, Tendongfor N, et al. Parasitological, hematological and biochemical characteristics of a model of hyper-microfilariaemic loiasis (Loa loa) in the baboon (Papio anubis). PLoS Negl Trop Dis 2015; 9:e0004202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muylle L, Taelman H, Moldenhauer R, Van Brabant R, Peetermans ME. Usefulness of apheresis to extract microfilarias in management of loiasis. Br Med J (Clin Res Ed) 1983; 287:519–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.