Supplemental digital content is available in the text.
Key Words: AEROBIC FITNESS, CHILDREN, MEDIATION, METABOLIC SYNDROME, OBESITY, ATHEROSCLEROSIS
ABSTRACT
Purpose
This study aimed to determine whether estimated cardiorespiratory fitness (CRF), fat mass (FM), lean mass (LM), and adiponectin bidirectionally associate with arterial function and structure and if CRF mediates the relationship between cardiometabolic health and arterial outcomes in 9- to 11-yr-old children drawn from the Avon Longitudinal Study of Parents and Children birth cohort, United Kingdom.
Methods
Brachial artery flow-mediated dilation (FMD), distensibility coefficient (DC), and carotid–radial pulse wave velocity (PWV) were measured by ultrasonography; CRF was measured during the submaximal ergometer test; total FM, trunk FM, and LM were measured by dual-energy x-ray absorptiometry; plasma adiponectin was measured by enzyme assay; and cardiometabolic health was computed based on the International Diabetes Federation criteria for diagnosing metabolic syndrome. We tested bidirectionality by including CRF, FM, LM, and adiponectin as exposures and FMD, DC, and PWV as outcomes, alternatively.
Results
Among 5566 participants (2816 (51%) girls; median age, 9.75 yr), CRF per body mass0.21 was directly related to DC (β (95% confidence interval) = 0.004 (<0.0001 to 0.008); P = 0.046), whereas CRF per LM0.54 was inversely associated with PWV (−0.034 (−0.063 to −0.003); 0.032) after adjusting for covariates. These associations remained in bidirectional analyses. Total FM, trunk FM, and LM were bidirectionally and positively associated with FMD and DC. Total FM and trunk FM but not LM had bidirectional and inverse associations with PWV. Adiponectin was not related to FMD, DC, or PWV. CRF partially mediated the associations of cardiometabolic health with FMD (1.5% mediation), DC (12.1% mediation), and PWV (3.5% mediation).
Conclusions
Associations of poor cardiometabolic health with adverse arterial structure and function in childhood may be mitigated by increasing CRF. Higher CRF was associated with better arterial structure whereas higher total FM and trunk FM were associated with better arterial function and structure. In the reverse analysis, healthy arterial structure and function were independently associated with increased total FM and trunk FM, suggesting an “arterial paradox.”
Atherosclerotic cardiovascular diseases progress from worsening arterial function (endothelial function) to an altered arterial structure (arterial elasticity and arterial stiffness), beginning in childhood (1–5). The development and progression of atherosclerosis are directly related to modifiable risk factor status among youth and young adults (5,6). Atherosclerotic progression primarily regulated by endothelial changes (7,8) often peaks with cardiovascular morbidity and mortality (9). Hence, identifying factors that could mitigate arterial disease progression from childhood is warranted.
Cardiorespiratory fitness (CRF) predicts cardiovascular health, and poor CRF is related to an elevated risk of cardiometabolic disease in children and adolescents (10–12). CRF was positively associated with arterial elasticity, measured with finger plethysmography, in 329 children after exercise (13). Other studies showed no relationship between CRF and arterial structure, assessed using carotid–radial and carotid–ankle pulse wave velocity (PWV), in 53 boys after exercise (14) or endothelial function, measured as flow-mediated dilatation (FMD) in 129 children (15). However, high CRF was associated with higher PWV estimated from brachial waveform in 646 children (16). These conflicting reports among small- to moderately sized populations require further investigation in a larger cohort while controlling for important covariates like somatic maturation and body composition. Moreover, the associations of CRF with arterial measures in a single pathway (unidirectional) may not inform whether a potential temporal relationship exists (12,17). A multidimensional approach investigating whether the arterial function and structure also relate to CRF (bidirectionality, which is a reverse association) would improve understanding of these associations and inform future intervention programs on target options (CRF, arterial outcomes, or both) to prevent atherosclerosis from early life and to evaluate intervention effects (18).
Low adiponectin levels, a cytokine secreted by adipose tissue, have been directly associated with altered arterial structure and function in adults, and other atherosclerotic risk factors such as insulin resistance and metabolic syndrome in children and adolescents (19–25). Adiponectin measured during childhood predicted adult preclinical carotid atherosclerosis better than other conventional risk factors such as body mass index and systolic blood pressure (26). Adiponectin has been inversely associated with CRF in children, suggesting that higher lean mass–induced CRF in tandem with lower fat mass–enhanced adiponectin may occur concurrently in healthy children (22). Fitter children tend to have higher lean mass and lower fat mass, which may increase adiponectin level leading to improved endothelial function and arterial compliance (22). It is still unclear whether fitness independently influences the associations of adiponectin with arterial structure and function (22). Moreover, whether a temporal relationship exists between adiponectin and arterial function and structure in childhood remains unknown. The association of fat mass with arterial structure (PWV) and function (FMD) in children remains controversial, although this association is likely dependent on lean mass and cardiometabolic health status (27–29). It is still unexamined whether these arterial measures, in turn, associate with lower fat mass and higher lean mass in a temporal fashion.
Low CRF associates with poor cardiometabolic health (10,12), whereas altered arterial structure (PWV) is directly associated with poor cardiometabolic health in adolescence (30). A mediation study reported that the association of increased fat mass with poor cardiometabolic health in children could be decreased by increasing CRF because CRF had a 10% indirect or mediating role (31). Besides, the association of increased fat mass with altered arterial structure (PWV) is influenced by cardiometabolic health (29). It remains unclear whether a higher CRF, reflecting increased muscle metabolism and vasodilation via exercise-induced hyperemia, improves arterial structure and function, thereby attenuating the arterial effects of poor cardiometabolic health, that is, the mediating role of CRF in the relationship between cardiometabolic and arterial health. The Avon Longitudinal Study of Parents and Children (ALSPAC) provides extensive and valid measures on the variables of interest (32). Among a prospective cohort of 5566 children age 9 to 11 yr, we investigated the associations of CRF, fat mass, lean mass, and adiponectin with separate measures of arterial function (FMD) and structure (PWV). Second, we investigated the reverse associations of FMD and PWV with CRF, fat mass, lean mass, and adiponectin. Lastly, we examined the mediating role of CRF on the associations of cardiometabolic health with FMD and PWV. It was hypothesized that 1) CRF, fat mass, lean mass, and adiponectin would have bidirectional relationships with arterial measures, and 2) increased CRF would attenuate the associations of poor cardiometabolic health with altered arterial function and structure.
METHODS
For an unabridged methodology (Methods, Supplemental Digital Content 1, which details measurement of variables and statistical analysis, http://links.lww.com/MSS/C398).
Study design and participants
ALSPAC is a comprehensive and longitudinal birth cohort study that investigates factors influencing childhood development and growth. ALSPAC enrolled 15,541 pregnant women with an expected delivery date from April 1991 to the end of December 1992, representing 85% of the eligible general population in three health authorities in Bristol, United Kingdom. From birth to childhood, a cohort of 14,062 liveborn children was followed up, via questionnaires, thereafter regular annual clinic follow-up began at age 7 yr and continues to date. Exposure variables were measured during the age 9-yr clinic visit from January 18, 2001, to January 11, 2003. Whereas arterial outcomes were measured at age 10-yr clinic visit from February 19, 2002, to March 15, 2003. A detailed description of the cohort and study design is available elsewhere (32,33). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time.
Anthropometric and social measures
The infant’s sex was abstracted from obstetric records and/or birth notifications. The exact age of each child was determined from their birth date and examination date. Height and body weight were measured using standard protocols. The participant’s mother’s socioeconomic status was grouped according to the 1991 British Office of Population and Census Statistics classification. An objective measure of pubertal (somatic) status (time in years to age at peak height velocity) was derived using Superimposition by Translation And Rotation mixed-effects growth curve analysis (34).
Assessments of body composition and other cardiometabolic factors
Total and trunk fat mass and lean mass at age 9 yr were assessed using a Lunar Prodigy DEXA scanner (GE Medical Systems, Madison, WI). Total and trunk fat mass and lean mass indices (FMI and LMI) were computed by dividing fat mass and lean mass by squared height. We categorized participants into age- and population-specific distribution of high total and trunk FMI, and LMI if values are greater than the 75th percentile and low if less than the 25th percentile of the study group values. Moderate FMI and LMI, on the other hand, are the 25th to 75th percentiles of the values. Systolic and diastolic blood pressures were measured with an automated monitor (Dinamap 9301 Vital Signs Monitor). Plasma lipids (total cholesterol, triglycerides, and HDL and LDL cholesterol) were determined by enzymatic reagents (35). All assay coefficients of variation were <5% (29). Total plasma adiponectin was assayed using an enzyme-linked immunosorbent assay (R&D Systems, Abingdon, United Kingdom), and the interassay coefficient of variation was 7% (35). Participants’ plasma adiponectin level was classified in tertile (three equal distributions of low, moderate, and high categories).
Determining cardiometabolic health
We categorized participants as having good or poor cardiometabolic health if they had or did not have three or more cardiometabolic risk factors according to the modified National Cholesterol Education Program and International Diabetes Federation. These guidelines stipulate that children and adolescents who have at least three of systolic or diastolic blood pressure >75th percentile, HDL <25th percentile, triglycerides >75th percentile, and/or body mass index >75th percentile meet the criteria for diagnosing metabolic syndrome (36). We classified children as having poor cardiometabolic health if their systolic blood pressure, triglycerides, and/or FMI lies above the 75th percentile and HDL falls below the 25th percentile of the studied population values. Children with good cardiometabolic health had systolic blood pressure, triglycerides, and/or FMI below the 75th percentile and HDL above the 25th percentile. We used total FMI in place of body mass index because it provided a more precise measure of adiposity in this age group (37).
Assessment of CRF
We assessed CRF at a heart rate of 170 bpm via physical work capacity (PWC170), measured in watts (W) (38), on an electronically braked cycle ergometer (Lode Rechor P). PWC170 is moderately correlated (r = 0.49–0.54, P ≤ 0.01) with absolute peak oxygen uptake (39). After a 1-min warm-up period, children pedaled at 55–65 rpm for 3 min at each workload (20, 40, and 60 W), interspersed with a 2-min rest. The workload required to elicit a heart rate of 170 bpm was predicted from the mean heart rate at the end of each stage by regression models. At a heart rate of ≥150 bpm in the final stage, children were considered to exercise at about the predicted value of 170 bpm. We computed a log-linear regression model with sex and body mass or lean mass as independent variables and CRF as the dependent variable (40,41) and expressed CRF as absolute CRF (W), allometrically scaled CRF/body mass (W·kg BM−0.21) and allometrically scaled CRF/lean mass (W·kg LM−0.54) (Supplemental Digital Content 1, http://links.lww.com/MSS/C398). We also categorized participants’ CRF allometrically scaled by body mass or lean mass in tertile categories of low, moderate, and high groups.
Assessment of arterial stiffness, elasticity, and endothelial function
During a regular clinic visit that lasted over 3 h, arterial measurements were acquired in approximately 40 min by six trained research technicians. Of these arterial measures, carotid–radial PWV was first assessed then brachial artery distensibility and, lastly, brachial endothelial function testing, using high-resolution ultrasound techniques (8,42). A high-fidelity micromanometer (SPC-301; Millar Instruments, Houston, TX) measured pressure–pulse waveforms from the radial and carotid pulse. The electrocardiogram signal provided an R-timing reference. Integrated software analyzed the mean time difference between R-wave and pressure wave on a beat-to-beat basis over 10 s. We calculated carotid–radial PWV as a ratio of the arterial path length between the two recording points to the meantime difference for the pulse waveform (SphygmoCorversion 7.1; Scanmed, England, United Kingdom). We assessed brachial artery elasticity on the arterial segment using an ALOKA 5500 high-resolution ultrasound system with a 5- to 10-MHz linear array probe (Keymed, England, United Kingdom). A pediatric cuff was used for arm circumference <25 cm and an adult cuff for arm circumference >25 cm when blood pressure was measured at the time of image acquisition. Real-time B-mode images were recorded and saved on SVHS video for 20 s, which were later analyzed offline at the Vascular Physiology Laboratory Unit. The luminal diameter excursion from diastole to systole was computed as the elasticity of the artery. We calculated the distensibility coefficient (DC) from the distension, and the pulse pressure was expressed as a mean percent change in cross-sectional area per unit change in blood pressure. Using high-resolution ultrasound (ALOKA 5500) at 5–10 cm above the antecubital fossa, the right brachial artery was scanned after a 5-min inflation of a pneumatic cuff to 200 mm Hg (8). The edge-detection software was used in measuring the brachial artery diameter (Brachial Tools, MIA, IA) from electrocardiogram-triggered ultrasound images captured at 3-s intervals throughout the 11-min recording protocol. We expressed FMD as the maximum percentage change in vessel diameter from baseline. We did not allometrically scale FMD for baseline diameter because the scaling exponent was close to 1.0; hence, our results relate to unscaled FMD adjusted for baseline diameter.
Interrater and intraparticipant reproducibility of arterial measures
Over a 2-yr study period, the within- and between-technician reproducibility for the acquisition of arterial measures was assessed at three different stages (8). During this study period, healthy staff volunteers (i.e., adults who were not ALSPAC participants) age 18–30 yr were studied at the beginning (n = 23), middle (n = 25), and end (n = 10). All the participants underwent assessment of vascular measures on at least two occasions. The coefficients of variation between technicians for FMD, carotid–radial PWV, and DC measures were 10.5, 4.6, and 6.6 at the beginning of the study; 7.7, 3.4, and 6.6 at the middle of the study; and 7.7, 4.1, and 10 at the end, respectively (8). Technician performance explained <5% of the variation in the individual vascular measures. The intraparticipant variability of the measures was assessed by collection of repeat vascular measures among randomly selected 231 children (3% of the cohort) within 6 wk of their initial visit (8). The coefficients of intraparticipant variation for FMD, carotid–radial PWV, and DC were 10.9, 8.7, and 18, respectively. This intraparticipant variability outlined the influence of temperature, time of day, position of the ultrasound probe, tonometer, and physiological hemodynamic such as blood flow, vessel size, and reactive hyperemia, although these parameters accounted for minimal variation in vascular measures (R2 = 0.02 for FMD, 0.03 for carotid–radial PWV, and 0.006 for DC).
Dealing with missing data and multiple imputations
Altogether, 7706 children attended the clinic visit at 9 yr of age and 6972 children were present at the age 10-yr clinic visit. We restricted study participants to those who had all arterial outcomes (FMD, DC, and carotid–radial PWV) at age 10 yr (n = 5566). Children who were excluded for incomplete arterial outcomes had similar CRF, arterial, and metabolic measures, but trivially different body composition compared with complete cases (Table S1, Supplemental Digital Content 2, characteristics of participants excluded from the study, http://links.lww.com/MSS/C399). However, eligible sample size for the present study varied by vascular measures that was conducted at the age 10-yr clinic visit (Table S2, Supplemental Digital Content 3, listwise exclusion of missing data among study participants, http://links.lww.com/MSS/C400). Among 5566 children with complete vascular variables, exclusions via listwise deletion of missing values ranged from 4.7% to 51.8% for predictors and 0.2% to 54.9% for covariates. The complete case sample sizes for predictors were 2813 (51%) for CRF, 5304 (95%) for total and trunk fat mass and lean mass, and 3767 (68%) for adiponectin. We conducted Little’s missing completely at random test to ascertain data missingness (42) (Little’s missing completely at random test, χ2 = 724.274, df = 346, P < 0.0001). Because the P value is statistically significant, we conclude that the variables are not missing completely at random. We then conducted linear regression–type multiple imputations (29) using SPSS version 25 (IBM SPSS Statistics; IBM Corporation, Armonk, NY) to minimize selection bias (see Table S3, Supplemental Digital Content 4 for imputed variables, http://links.lww.com/MSS/C401). Constraints for the imputation process were the observed minimum and maximum values; for skewed variables, the logarithmic transformation was used in the imputation model. Twenty cycles of imputation via regression model produced 20 imputed data sets. Outcomes from these imputed data were pooled using the multiple imputation module in SPSS. Given the percentage of missing values, we estimated that 20 imputation cycles would be sufficient: the variable with the highest missing value (54.9%, mother’s socioeconomic status) had an estimate that was 99% efficient after 20 cycles of imputations (computed using Rubin’s formula) (43). We examined the histogram’s normality curves for imputed variables ensuring that the distributions of predictors and covariates had the same pattern as in the observed data. Variable distributions after imputation (mean, SD, and percentages) were congruent with the observed data (Table S4, Supplemental Digital Content 5, characteristics of cohort participants with imputed data, http://links.lww.com/MSS/C402). We presented results in the main article as tables and figures from the pooled outcomes of the imputation, and the same analyses using the observed (nonimputed) are also presented (Tables S5–8, Supplemental Digital Content 6–9, complete case analyses of associations between predictors and outcomes, http://links.lww.com/MSS/C403, http://links.lww.com/MSS/C404, http://links.lww.com/MSS/C405, http://links.lww.com/MSS/C406). Where multiple imputations have been conducted, presenting imputed results is preferred to presenting nonimputed results (44).
Statistical analyses
We summarized descriptive characteristics as means with SD or medians with interquartile ranges, and frequencies in percentages (%), and sex differences were explored using independent t-tests or Mann–Whitney U tests for normally distributed or skewed variables, χ2 test was used for dichotomous variable, and one-way analysis of variance for multicategorical variable. The normal distribution of variables was assessed by histogram and Kolmogorov–Smirnov tests. We separately examined the associations of absolute CRF, CRF allometrically scaled for body mass, CRF allometrically scaled for lean mass, adiponectin, total fat mass, trunk fat mass, and lean mass with FMD, DC, and carotid–radial PWV via multivariable linear regression, first exploring adjustment for age and sex (model 1); adjusted for age, sex, total fat mass, or CRF allometrically scaled for lean mass (model 2); adjusted for age, sex, and lean mass or CRF allometrically scaled for lean mass (model 3); additional adjustment of model 2 for puberty and diameter of brachial artery (model 4); and adjusted model 4 for maternal socioeconomic status, LDL cholesterol, systolic blood pressure, and time (years) difference between measurement of exposure and outcome variable (model 5). We presented five models to provide extensive details regarding how different covariates influenced the associations. All covariates were selected based on previous studies and biological plausibility (2,8,10,29,45). A logarithm-transformed total and trunk fat mass, adiponectin, and DC variables were used in the analysis, but logged DC variable was back-transformed by exponentiation. To correct for multiple testing, we investigated the associations of categories of CRF allometrically scaled for body mass, CRF allometrically scaled for lean mass, adiponectin, LMI, total, and trunk FMI with arterial outcomes using generalized linear models. All analyses were corrected for multiple testing using the Sidak correction method. The results are presented as estimated marginal means. The Sidak-corrected multivariate analyses yields similar results to those of the unidirectional analysis, thus also serving as sensitivity analysis. We also examined the mediating effects of CRF allometrically scaled for lean mass on the associations of cardiometabolic health with FMD, DC, and carotid–radial PWV through bootstrapped (10,000 samples) (46–48) linear regression analysis that was adjusted for age, sex, brachial artery diameter, puberty, and maternal socioeconomic status. There were three equations per regression model: linear regression on the association of CRF allometrically scaled for lean mass with cardiometabolic health (equation 1); linear regression on the association of CRF allometrically scaled for lean mass with FMD, DC, and carotid–radial PWV (equation 2); and linear regression on the association of cardiometabolic health with FMD, DC, and carotid–radial PWV (equation 3), and equation 3′ accounted for the mediating role of CRF allometrically scaled for lean mass. The proportion of mediating roles was estimated as the ratio of the difference between equation 3 and equation 3′ divided by equation 3 and expressed in percentage. A mediating or indirect role is confirmed when there are statistically significant associations between (a) the predictor and mediator, (b) the predictor and outcome, and (c) the mediator and outcome, and when (d) the association between the predictor and outcome variable was attenuated upon inclusion of the mediator (46,47). We tested the hypothesis of a significant mediating effect using the Sobel test and reported the z scores and P values. We also reported the point estimates and 95% confidence interval (CI) for the mediating effect of CRF allometrically scaled for lean mass. To test for bidirectional associations (where the relationship between the independent and dependent variable is analyzed in a reversed pattern), we performed multivariable regression analyses but modeled arterial outcomes (FMD, DC, and carotid–radial PWV) as the predictor, and absolute CRF, CRF allometrically scaled for body mass, CRF allometrically scaled for lean mass, adiponectin, total and trunk fat mass, and lean mass as outcomes, separately. Model 1 was unadjusted, whereas model 2 was adjusted for all covariates detailed in unidirectional analyses. Differences and associations with P values <0.05 from two-sided tests were considered statistically significant. Analyses involving 50% of a sample of 10,000 ALSPAC children at 80% statistical power, an α value of 0.05, and a two-sided P value would show a minimum detectable effect size of 0.048 SD if they had relevant exposure for a normally distributed quantitative variable (49). For a primary outcome, our sensitivity analysis revealed that a fixed sample size of 5566 participants yielded Cohen’s f2 effect size of 0.003 at 90% power and a two-sided α threshold of 0.05. We performed all statistical analyses using SPSS statistics software, version 25.0 (IBM Corp, Armonk, NY) and mediation analyses by PROCESS SPSS script Version 3.5 (48).
RESULTS
Of the 7706 children who participated in the ALSPAC study “focus @ 9” clinic examination, 5566 (2816 (51%) girls; median age, 9.75 yr) children had complete data for outcome (arterial) variables and were included in the analysis. We presented our participant’s demography in Table 1, and there are sex differences in nearly all variables except for age, maternal socioeconomic status, and DC.
TABLE 1.
Characteristics of cohort participants.
| Variable | All Participants | Boys | Girls | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | P for Sex Difference | |
| Agea (yr) | 9.75 (0.33) | 5566 | 9.7 (0.33) | 2750 | 9.75 (0.33) | 2816 | 0.967 |
| Somatic maturation (puberty) by time to aPHV, n (%) | 22 (0.5) | 4203 | 0 | 2003 | 22 (1) | 2200 | <0.0001 |
| Socioeconomic status based on mother’s occupation | |||||||
| I: Professional (%) | 5.1 | 127 | 5.9 | 75 | 4.2 | 52 | 0.857 |
| II: Managerial and technical (%) | 36.3 | 910 | 35.6 | 453 | 37.0 | 457 | |
| IIIa: Skilled nonmanual (%) | 39.1 | 981 | 38.6 | 491 | 39.6 | 490 | |
| IIIb: Skilled manual (%) | 1.7 | 43 | 1.5 | 19 | 1.9 | 24 | |
| IV: Partly skilled (%) | 14.7 | 370 | 15.8 | 201 | 13.7 | 169 | |
| V: Unskilled (%) | 3.1 | 78 | 2.7 | 34 | 3.6 | 44 | |
| Anthropometry and fat measure | |||||||
| Body height (m) | 1.40 (0.06) | 5510 | 1.40 (0.06) | 2729 | 1.39 (0.06) | 2781 | 0.003 |
| Weighta (kg) | 33.0 (8.6) | 5555 | 32.8 (8.0) | 2743 | 33.40 (9.20) | 2812 | 0.022 |
| Body mass indexa (kg·m−2) | 16.95 (3.32) | 5506 | 16.74 (3.09) | 2727 | 17.22 (3.54) | 2779 | <0.0001 |
| Lean mass (kg) | 24.52 (3.19) | 5304 | 25.49 (2.93) | 2622 | 23.57 (3.14) | 2682 | <0.0001 |
| Lean mass indexed for squared height (kg·m−2) | 12.56 (0.99) | 5255 | 13.00 (0.86) | 2606 | 12.12 (0.91) | 2649 | <0.0001 |
| Total fat massa (kg) | 7.14 (6.06) | 5304 | 5.67 (5.16) | 2622 | 8.41 (6.04) | 2682 | <0.0001 |
| Total fat mass indexed for squared heighta (kg·m−2) | 3.67 (2.94) | 5255 | 2.94 (2.55) | 2606 | 4.37 (2.92) | 2649 | <0.0001 |
| Trunk fat massa (kg) | 2.65 (2.68) | 5304 | 2.04 (2.17) | 2622 | 3.28 (2.81) | 2682 | <0.0001 |
| Trunk fat mass indexed for squared heighta (kg·m−2) | 1.37 (1.34) | 5255 | 1.04 (1.07) | 2606 | 1.70 (1.39) | 2649 | <0.0001 |
| Metabolic profile | |||||||
| HDL (mmol·L−1) | 1.40 (0.31) | 3769 | 1.43 (0.31) | 1902 | 1.37 (0.30) | 1867 | <0.0001 |
| LDL (mmol·L−1) | 2.35 (0.60) | 3769 | 2.26 (0.58) | 1902 | 2.45 (0.61) | 1867 | <0.0001 |
| Triglyceridea (mmol·L−1) | 1.00 (0.60) | 3769 | 0.97 (0.60) | 1902 | 1.04 (0.59) | 1867 | <0.0001 |
| Adiponectina (μg·mL−1) | 12.35 (7.02) | 3767 | 11.88 (6.38) | 1900 | 13.04 (7.37) | 1867 | <0.0001 |
| Fitness measure | |||||||
| CRF (W) | 63.92 (9.07) | 2813 | 67.13 (8.25) | 1179 | 61.61 (8.94) | 1634 | <0.0001 |
| CRF per BM (W·kg BM−1) | 1.92 (0.38) | 2810 | 2.07 (0.38) | 1178 | 1.82 (0.34) | 1632 | <0.0001 |
| CRF per BM0.21 (W·kg BM−0.21) | 31.31 (4.31) | 2810 | 33.04 (3.93) | 1178 | 30.06 (4.14) | 1632 | <0.0001 |
| CRF per LM0.54 (W·kg LM−0.54) | 11.51 (1.46) | 2683 | 11.90 (1.36) | 1122 | 11.21 (1.46) | 1561 | <0.0001 |
| Vascular measure | |||||||
| Pulse rate (bpm) | 79 (11) | 5512 | 77 (10) | 2722 | 81 (11) | 2790 | <0.0001 |
| Systolic blood pressure (mm Hg) | 103 (9) | 5511 | 102 (9) | 2722 | 103 (10) | 2790 | 0.049 |
| Diastolic blood pressure (mm Hg) | 57 (6) | 5512 | 57 (6) | 2722 | 58 (6) | 2790 | 0.002 |
| Brachial artery diameter (mm) | 2.67 (0.30) | 5566 | 2.76 (0.29) | 2750 | 2.59 (0.29) | 2816 | <0.0001 |
| Flow-mediated dilation (%) | 8.07 (3.37) | 5566 | 7.72 (3.25) | 2750 | 8.41 (3.44) | 2816 | <0.0001 |
| Flow-mediated dilation absolute (mm) | 0.21 (0.08) | 5566 | 0.21 (0.09) | 2750 | 0.22 (0.09) | 2816 | 0.029 |
| Carotid-radial PWV (m·s−1) | 7.54 (1.22) | 5566 | 7.63 (1.24) | 2750 | 7.46 (1.20) | 2816 | <0.0001 |
| Distensibility coefficienta (% per mm Hg) | 11.51 (6.91) | 5566 | 11.66 (6.88) | 2750 | 11.35 (6.96) | 2816 | 0.310 |
The values are means (SD) and amedian (interquartile range) except for maturation status and social economic status in percentage. Differences between girls and boys were tested using Student’s t-test for normally distributed continuous variables, Mann–Whitney U test for skewed continuous variables, χ2 test for dichotomous variable, and one-way analysis of variance for multicategorical variable. A two-sided P value <0.05 is considered statistically significant.
aPHV, age at peak height velocity; BM, body mass; CRF, cardiorespiratory fitness; LM, lean mass; PWV, pulse wave velocity; W, watt.
Associations of CRF and adiponectin with arterial function and structure
CRF per body mass0.21 was directly associated with DC (β (95% CI)) = 0.004 (<0.0001 to 0.008); P = 0.046), whereas CRF per lean mass0.54 was inversely related to carotid–radial PWV (−0.034 (−0.063 to −0.003); P = 0.032) after full model adjustment (Table 2, model 5). However, CRF was not associated with FMD. There was a statistically significant difference between categories (low, moderate, and high) of either CRF per body mass0.21 or CRF per lean mass0.54 in relation to DC (Fig. 1 and Table S6, Supplemental Digital Content 7, http://links.lww.com/MSS/C404). Plasma adiponectin was not related to FMD, DC, and carotid–radial PWV, just as there was no difference in the association of adiponectin categories with FMD, DC, and carotid–radial PWV.
TABLE 2.
Multivariable-adjusted associations of cardiorespiratory fitness, fat mass, lean mass, and adiponectin with endothelial function, arterial elasticity, and arterial stiffness.
| n = 5566 | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Flow-mediated dilation (%) | ||||||||||
| CRF (W) | −0.008 (−0.021 to 0.005) | 0.216 | −0.008 (−0.021 to 0.005) | 0.232 | −0.001 (−0.015 to 0.012) | 0.827 | 0.002 (−0.011 to 0.014) | 0.791 | 0.002 (−0.011 to 0.014) | 0.806 |
| CRF per BM0.21 (W·kg BM−0.21) | −0.011 (−0.039 to 0.017) | 0.427 | −0.012 (−0.040 to 0.016) | 0.408 | −0.008 (−0.036 to 0.020) | 0.579 | <0.0001 (−0.027 to 0.027) | 0.999 | −0.001 (−0.028 to 0.026) | 0.953 |
| CRF per LM0.54 (W·kg LM−0.54) | 0.010 (−0.071 to 0.091) | 0.813 | 0.009 (−0.072 to 0.090) | 0.826 | −0.005 (−0.086 to 0.076) | 0.912 | −0.006 (−0.084 to 0.072) | 0.888 | −0.010 (−0.088 to 0.069) | 0.811 |
| Adiponectina (μg·mL−1) | −0.045 (−0.298 to 0.208) | 0.726 | −0.048 (−0.303 to 0.206) | 0.708 | −0.113 (−0.370 to 0.143) | 0.384 | −0.188 (−0.439 to 0.062) | 0.139 | −0.183 (−0.431 to 0.064) | 0.146 |
| Total fat massa (kg) | −0.032 (−0.197 to 0.134) | 0.708 | −0.030 (−0.196 to 0.136) | 0.719 | 0.178 (−0.011 to 0.368) | 0.065 | 0.535 (0.366 to 0.704) | <0.0001 | 0.585 (0.401 to 0.769) | <0.0001 |
| Trunk fat massa (kg) | 0.013 (−0.128 to 0.154) | 0.859 | 0.014 (−0.128 to 0.155) | 0.848 | 0.200 (0.039 to 0.361) | 0.015 | 0.489 (0.344 to 0.634) | <0.0001 | 0.535 (0.377 to 0.693) | <0.0001 |
| Lean mass (kg) | −0.064 (−0.094 to −0.034) | <0.0001 | −0.080 (−0.114 to −0.045) | <0.0001 | −0.064 (−0.094 to −0.034) | <0.0001 | 0.041 (0.006 to 0.077) | 0.022 | 0.047 (0.011 to 0.084) | 0.011 |
| Distensibility coefficienta (% per mm Hg) | ||||||||||
| CRF (W) | 0.002 (<0.0001 to 0.004) | 0.085 | 0.002 (<0.0001 to 0.004) | 0.109 | 0.001 (−0.001 to 0.003) | 0.160 | 0.002 (<0.0001 to 0.004) | 0.053 | 0.002 (<0.0001 to 0.004) | 0.057 |
| CRF per BM0.21 (W·kg BM−0.21) | 0.003 (−0.001 to 0.007) | 0.093 | 0.004 (<0.0001 to 0.008) | 0.076 | 0.003 (−0.001 to 0.007) | 0.111 | 0.004 (<0.0001 to 0.008) | 0.044 | 0.004 (<0.0001 to 0.008) | 0.046 |
| CRF per LM0.54 (W·kg LM−0.54) | 0.009 (−0.003 to 0.021) | 0.144 | 0.009 (−0.003 to 0.021) | 0.129 | 0.010 (−0.002 to 0.022) | 0.109 | 0.009 (−0.003 to 0.020) | 0.156 | 0.009 (−0.003 to 0.021) | 0.151 |
| Adiponectina (μg·mL−1) | −0.006 (−0.039 to 0.026) | 0.697 | −0.005 (−0.038 to 0.027) | 0.759 | −0.003 (−0.036 to 0.030) | 0.868 | −0.010 (−0.043 to 0.022) | 0.528 | −0.009 (−0.042 to 0.024) | 0.589 |
| Total fat massa (kg) | 0.014 (−0.009 to 0.037) | 0.222 | 0.015 (−0.008 to 0.038) | 0.192 | 0.006 (−0.020 to 0.032) | 0.649 | 0.037 (0.013 to 0.061) | 0.003 | 0.043 (0.017 to 0.070) | 0.001 |
| Trunk fat massa (kg) | 0.013 (−0.006 to 0.033) | 0.170 | 0.014 (−0.005 to 0.033) | 0.145 | 0.007 (−0.015 to 0.029) | 0.528 | 0.033 (0.012 to 0.053) | 0.002 | 0.038 (0.016 to 0.060) | 0.001 |
| Lean mass (kg) | 0.004 (<0.0001 to 0.008) | 0.084 | 0.003 (−0.002 to 0.008) | 0.201 | 0.004 (<0.0001 to 0.008) | 0.060 | 0.009 (0.004 to 0.014) | 0.001 | 0.009 (0.004 to 0.014) | 0.001 |
| Carotid-radial pulse wave velocity (m·s−1) | ||||||||||
| CRF (W) | −0.009 (−0.015 to −0.004) | 0.002 | −0.005 (−0.010 to <0.0001) | 0.031 | −0.005 (−0.011 to <0.0001) | 0.040 | −0.004 (−0.009 to 0.001) | 0.096 | −0.004 (−0.009 to 0.001) | 0.096 |
| CRF per BM0.21 (W·kg BM−0.21) | −0.006 (−0.016 to 0.004) | 0.248 | −0.011 (−0.021 to −0.001) | 0.031 | −0.004 (−0.014 to 0.006) | 0.455 | −0.010 (−0.020 to −0.001) | 0.064 | −0.009 (−0.019 to 0.001) | 0.073 |
| CRF per LM0.54 (W·kg LM−0.54) | −0.024 (−0.054 to 0.006) | 0.120 | −0.032 (−0.062 to −0.003) | 0.033 | −0.033 (−0.064 to −0.003) | 0.032 | −0.034 (−0.064 to −0.005) | 0.024 | −0.033 (−0.063 to −0.003) | 0.032 |
| Adiponectina (μg·mL−1) | 0.10 (0.012 to 0.189) | 0.026 | 0.065 (−0.021 to 0.151) | 0.138 | 0.060 (−0.028 to 0.149) | 0.179 | 0.047 (−0.038 to 0.132) | 0.279 | 0.049 (−0.036 to 0.135) | 0.254 |
| Total fat massa (kg) | −0.369 (−0.429 to −0.310) | <0.0001 | −0.373 (−0.433 to −0.313) | <0.0001 | −0.341 (−0.410 to −0.272) | <0.0001 | −0.299 (−0.362 to −0.236) | <0.0001 | −0.324 (−0.393 to −0.256) | <0.0001 |
| Trunk fat massa (kg) | −0.305 (−0.356 to −0.254) | <0.0001 | −0.308 (−0.359 to −0.257) | <0.0001 | −0.279 (−0.337 to −0.221) | <0.0001 | −0.245 (−0.299 to −0.191) | <0.0001 | −0.265 (−0.323 to −0.206) | <0.0001 |
| Lean mass (kg) | −0.040 (−0.051 to −0.030) | <0.0001 | −0.011 (−0.023 to 0.002) | 0.085 | −0.042 (−0.052 to 0.031) | <0.0001 | 0.005 (−0.008 to 0.018) | 0.445 | 0.002 (−0.011 to 0.016) | 0.765 |
The regression coefficient (β) quantifies the association between dependent and independent variables. A two-sided P value <0.05 was considered statistically significant and is bolded.
Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, and total fat mass or cardiorespiratory fitness per lean mass, when the predictor variable is total fat mass. Model 3: adjusted for age, sex, lean mass, or cardiorespiratory fitness per lean mass when the predictor variable is lean mass. Model 4: additional adjustment of model 2 for puberty and brachial artery diameter. Model 5: additional adjustment of model 4 for LDL, systolic blood pressure, maternal socioeconomic status, and time (years) between fat mass, lean mass, adiponectin, cardiorespiratory fitness data collection, and measurement of arterial function and structure.
aLogarithm transformation of skewed variables was used in the analyses.
BM, body mass; CI, confidence interval; CRF, cardiorespiratory fitness; LM, lean mass; W, watt.
FIGURE 1.
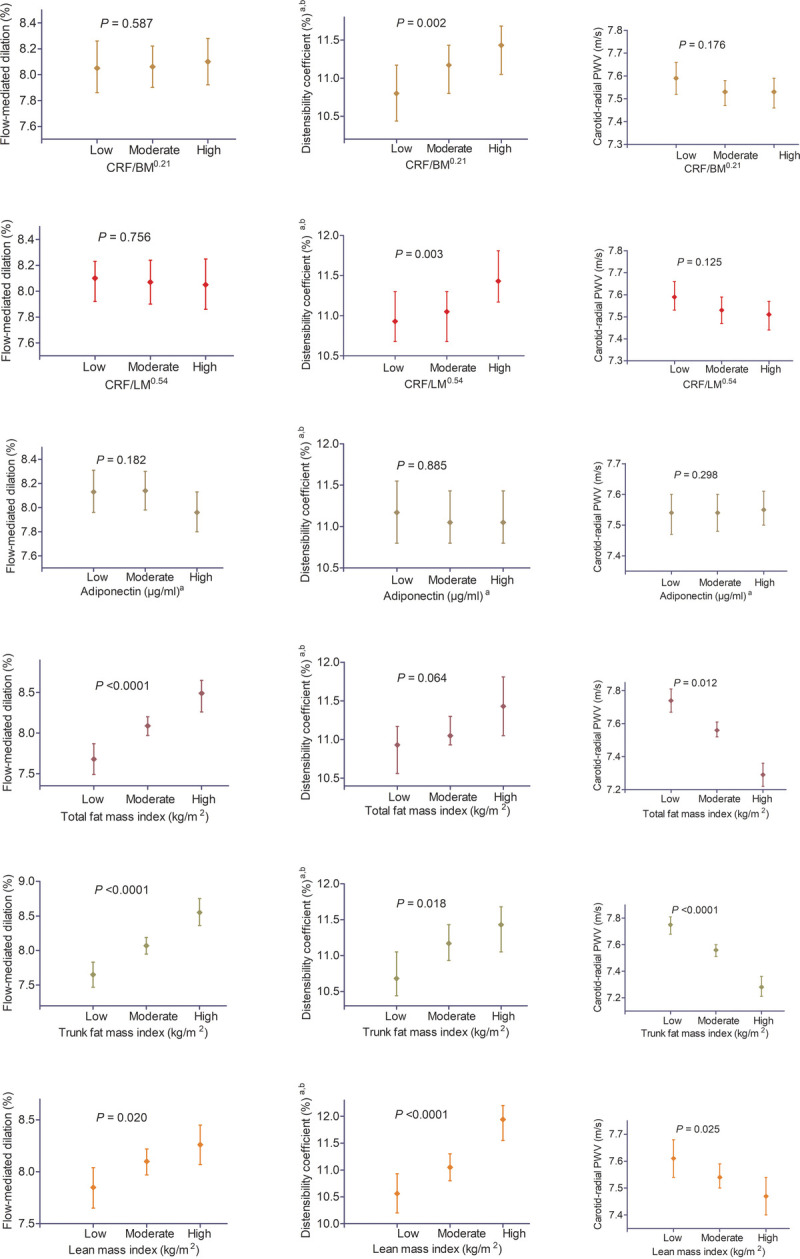
Associations of cardiorespiratory fitness, fat mass, lean mass, and adiponectin categories with endothelial function, arterial elasticity, and arterial stiffness in 5566 children age 9–11 yr. Estimated marginal means were computed from general linear models of exposure categories in relation to outcome variables with Sidak correction adjusted for age, sex, puberty, systolic blood pressure, mother’s socioeconomic status, total fat mass or cardiorespiratory fitness scaled by lean mass, LDL, diameter of the brachial artery, and time between measures of exposure and outcome variable. aThese variables were log transformed by using natural log. bThis logged variable was back-transformed by exponentiation, and values are geometric mean. Associations with P value <0.05 were considered statistically significant. A significant P value indicates that at least one category significantly differs from other tertile groups. Categories of total fat mass, trunk fat mass, and lean mass are high if values are greater than 75th percentile and low if less than 25th percentile. Whereas moderate category lies between the 25th and 75th percentiles of the values. Cardiorespiratory fitness and adiponectin were classified in tertile (three equal distributions of low, moderate, and high groups). BM, body mass; CRF, cardiorespiratory fitness; LM, lean mass.
Associations of total and trunk fat mass and lean mass with arterial function and structure
Total fat mass, trunk fat mass, and lean mass were directly associated with FMD and DC after full model adjustments (Table 2, model 5). Total fat mass (−0.324 (−0.393 to −0.256); P < 0.0001) and trunk fat mass (−0.265 (−0.323 to −0.206); P < 0.0001) but not lean mass were inversely associated with carotid–radial PWV following similar adjustment strategy. Participants with high levels of total FMI, trunk FMI, and LMI had statistically significant higher FMD and lower carotid–radial PWV (Fig. 1 and Table S6, Supplemental Digital Content 7, http://links.lww.com/MSS/C404).
Mediating effects of CRF on the associations of cardiometabolic health and arterial function and structure
CRF per lean mass0.54 mediated the relationships of cardiometabolic health with FMD, DC, and carotid–radial PWV by 1.5%, 12.1%, and 3.5%, respectively, after adjusting for age, sex, diameter of the brachial artery, puberty, and mother’s socioeconomic status (Fig. 2).
FIGURE 2.
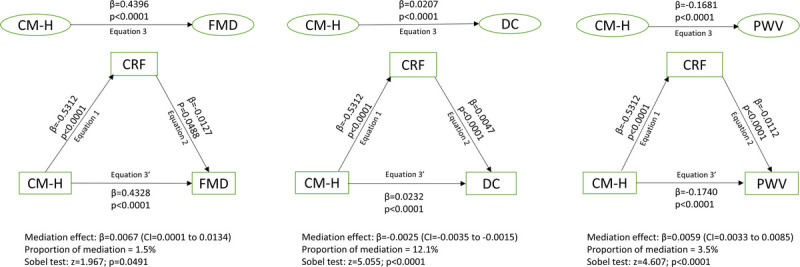
Mediating effect of cardiorespiratory fitness on the associations of cardiometabolic health with endothelial function, arterial elasticity, and arterial stiffness in 5566 children 9–11 yr of age. Analyses were adjusted for age, sex, diameter of the brachial artery, puberty, and mother’s socioeconomic status. Associations with P value <0.05 were considered statistically significant. β, unstandardized regression coefficients, CI, confidence interval; CM-H, cardiometabolic health; CRF, cardiorespiratory fitness allometrically scaled by lean mass; DC, distensibility coefficient, which measures arterial structure; FMD, flow-mediated dilation, which measures endothelial function; PWV, pulse wave velocity, which measures arterial structure.
Associations of arterial function and structure with CRF and adiponectin
DC was directly associated with CRF per body mass0.21 (0.242 (0.033 to 0.451); P = 0.023), whereas carotid–radial PWV was inversely associated with CRF per lean mass0.54 (−0.030 (−0.060 to −0.001); P = 0.043) after full model adjustments (Table 3, model 2). FMD had no relationship with CRF measures. FMD, DC, and carotid–radial PWV had no association with adiponectin.
TABLE 3.
Association of endothelial function, arterial elasticity, and arterial stiffness with cardiorespiratory fitness, fat mass, lean mass, and adiponectin.
| n = 5566Variable | CRF (W) | CRFper BM0.21 (W·kg BM−0.21) | CRFper LM0.54 (W·kg LM−0.54) | Adiponectina (μg·mL−1) | Total Fat Massa (kg) | Trunk Fat Massa (kg) | Lean Mass (kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Model 1 | ||||||||||||||
| Flow-mediated dilation (%) | −0.099 (−0.161 to −0.037) | 0.002 | −0.038 (−0.066 to −0.009) | 0.010 | 0.003 (−0.008 to 0.013) | 0.615 | <0.001 (−0.004 to 0.004) | 0.979 | 0.003 (−0.001 to 0.008) | 0.187 | 0.005 (<0.001 to 0.010) | 0.059 | −0.081 (−0.106 to −0.056) | <0.0001 |
| Distensibility coefficienta (% per mm Hg) | 0.570 (0.109 to 1.031) | 0.015 | 0.214 (−0.001 to 0.429) | 0.051 | 0.058 (−0.019 to 0.135) | 0.137 | −0.006 (−0.036 to 0.024) | 0.695 | 0.018 (−0.015 to 0.051) | 0.283 | 0.025 (−0.014 to 0.063) | 0.211 | 0.178 (−0.007 to 0.362) | 0.059 |
| Carotid–radial pulse wave velocity (m·s−1) | −0.362 (−0.534 to −0.189) | <0.0001 | −0.017 (−0.097 to 0.062) | 0.668 | −0.016 (−0.045 to 0.013) | 0.267 | 0.010 (<0.001 to 0.020) | 0.044 | −0.081 (−0.093 to −0.069) | <0.0001 | −0.095 (−0.109 to −0.080) | <0.0001 | −0.192 (−0.262 to −0.123) | <0.0001 |
| Model 2 | ||||||||||||||
| Flow-mediated dilation (%) | 0.019 (−0.043 to 0.081) | 0.549 | 0.003 (−0.027 to 0.032) | 0.862 | −0.003 (−0.014 to 0.008) | 0.558 | −0.004 (−0.008 to <0.0001) | 0.056 | 0.012 (0.008 to 0.016) | <0.0001 | 0.016 (0.011 to 0.020) | <0.0001 | 0.027 (0.007 to 0.047) | 0.009 |
| Distensibility coefficienta (% per mm Hg) | 0.594 (0.156 to 1.031) | 0.008 | 0.242 (0.033 to 0.451) | 0.023 | 0.050 (−0.025 to 0.125) | 0.193 | −0.008 (−0.039 to 0.022) | 0.585 | 0.004 (0.002 to 0.006) | <0.0001 | 0.005 (0.002 to 0.007) | <0.0001 | 0.229 (0.088 to 0.369) | 0.001 |
| Carotid–radial pulse wave velocity (m·s−1) | −0.148 (−0.316 to 0.020) | 0.083 | −0.076 (−0.155 to 0.004) | 0.063 | −0.030 (−0.060 to −0.001) | 0.043 | 0.005 (−0.005 to 0.015) | 0.350 | −0.050 (−0.060 to −0.039) | <0.0001 | −0.057 (−0.070 to −0.045) | <0.0001 | 0.013 (−0.040 to 0.067) | 0.628 |
The regression coefficient (β) quantifies the association between dependent and independent variables. A two-sided P value <0.05 was considered statistically significant and is bolded.
Model 1: unadjusted associations of arterial function and structure with cardiorespiratory fitness, fat mass, lean mass, and adiponectin. Model 2: associations were adjusted for age, sex, puberty, systolic blood pressure, mother’s socioeconomic status, total fat mass or cardiorespiratory fitness/lean mass, LDL, diameter of the brachial artery, and time between measures of exposure and outcome variable.
aLogarithm transformation of skewed variables was used in analyses.
BM, body mass; CI, confidence interval; CRF, cardiorespiratory fitness; LM, lean mass; W, watt.
Associations of arterial function and structure with total and trunk fat mass and lean mass
FMD and DC were directly associated with total fat mass, trunk fat mass, and lean mass after adjusting for all unidirectional covariates (Table 3, model 2). However, carotid–radial PWV was inversely associated with total fat mass (−0.050 (−0.060 to −0.039); P < 0.0001) and trunk fat mass (−0.057 (−0.070 to −0.045); P < 0.0001) but not lean mass, after covariate adjustment.
DISCUSSION
We showed the bidirectional associations of CRF allometrically scaled for lean mass, CRF allometrically scaled for body mass, total fat mass, trunk fat mass, and lean mass with arterial function (FMD) and structure (DC and carotid–radial PWV) in a large cohort of 9- to 11-yr-olds, after controlling for cardiometabolic and lifestyle factors (Tables 2, 3). Another novel finding was that CRF allometrically scaled for lean mass partly mediated the relationships of cardiometabolic health with FMD, DC, and carotid–radial PWV (Fig. 2). We also reported that poor cardiometabolic health was related to low CRF allometrically scaled for lean mass in addition to other studies that have reported an inverse association between CRF and cardiometabolic health (10,12), suggesting temporality. Because fat mass mediates the association of cardiometabolic health with arterial stiffness in adolescence (29), improving CRF in childhood may be clinically important to preventing metabolic syndrome, cardiovascular diseases, and preclinical atherosclerosis (5,12,13). Together, these findings suggest a protective effect of CRF and the physiological adaptation of arterial function and structure to body composition and cardiometabolic health in childhood.
Recent studies in children and adolescents have reported no relationships between CRF and carotid–radial PWV (14), and between CRF and FMD (15), an association of CRF with DC but not with arterial stiffness (13), an association between CRF and carotid artery elasticity (6), and a higher CRF in relation to high PWV (16). Some of these studies (13–16), with small to moderate sample sizes, did not account for important covariates such as sex (8), brachial artery diameter (8), blood pressure (28), lean mass (28) or total fat mass (29) and puberty. In our study, controlling for these covariates revealed that allometrically scaled CRF relates to arterial structure independent of body composition and other risk factors and that arterial health is associated with CRF, although with some inconsistencies. We also report that these associations are bidirectional, suggesting that healthy arterial structure may enhance CRF plausibly via increased vital capacity and tidal volume, improved oxygenation for muscle contractility, better oxidative phosphorylation, and cardiac compliance (50). Evidence suggests that altered arterial structure may lead to diminished lung function because the loss of small arteries elasticity results in decreased elastic recoil of the lung tissue (50,51). Endothelial dysfunction may result in the proliferation of pulmonary vascular endothelial cells and type II pneumocytes leading to a cascade of increased proinflammatory cytokines, arterial stiffness, and diminished lung function (50,51). On the other hand, an optimal CRF may improve endothelial function, vasculogenesis, aortic compliance, and nitric oxide production, and decrease arterial stiffness via sympathetic control, endothelium-mediated flow-induced vasodilation, metabolic vasodilation, and myogenic control (50). Small conduit and resistance arteries respond more to endothelium-dependent vasodilation, whereas smaller arterioles have a more myogenic response to increased perfusion pressure and metabolic control (50). The oxygen/metabolic sensor coupled to vascular smooth muscle cell enhances the metabolic control of resistance arteries by increasing the production of the vasodilatory prostaglandins in response to decreased oxygen and tissue acidosis from increased carbon dioxide, leading to an erythrocyte ATP-induced vasodilation (50).
A systematic review and meta-analysis concluded that obesity was associated with higher arterial stiffness in children, but studies that address confounders, such as age, sex, and puberty, are needed (2). A recent study reported an association between high total fat mass but not trunk fat mass at 9 yr and high carotid-femoral PWV at 17 yr (29). On the contrary, we found that high total and trunk fat mass were associated with lower carotid–radial PWV at 9–11 yr in both directions. Our result suggests that the pattern of fat distribution and the quantity of fat mass are both important determinants of arterial health in 9- to 11-yr-olds. Although it may be argued that the carotid–radial PWV assesses muscular arteries rather than elastic arteries, evidence suggests that atherosclerotic changes occur in the former during early life (1,8,45). Besides, additional analyses showed that a 1-unit increase in carotid–radial PWV (arterial stiffness) resulted in a 3-unit decrease in arterial elasticity or compliance (DC), suggesting the importance of carotid–radial PWV measure in early childhood, independent of sex and brachial wall diameter. Our findings in comparison with previous studies revealed that the influence of fat mass on arterial stiffness in children varies across elastic and muscular arteries (27,29).
While examining the varied associations of fat mass and lean mass with arterial function and structure, a study found that controlling for lean mass yielded a negative but nonsignificant relationship between fat mass and carotid–radial PWV (27). They opined that lean mass was the main reason for the association between fat mass and arterial indices (27). The authors also reported a positive association between FMI and FMD in 8- to 9-yr-olds suggesting an adaptive response of endothelial-dependent dilation to increased fat mass (27), although only about 4% of our study participants are obese (42). Interestingly, we found that controlling for lean mass had minimal influence on the positive associations of fat mass with FMD and DC or the inverse relationship of fat mass with carotid–radial PWV. We observed a bidirectional relationship between fat mass and late childhood/early adolescents’ arterial health. Taken together, our findings suggest that better arterial function and structure may influence the accumulation of fat mass, just as excess fat mass may alter arterial function and structure (2,27,29,45). An efficient arterial network may enhance adipogenesis and white adipose tissue browning, lipid droplet formation, low macrophage activation, and effective fat deposit, which can be described as an “arterial paradox” (52). We also noted that high lean mass independent of total fat mass was associated with high FMD and DC in either direction, but no relations with carotid–radial PWV. However, additional analysis revealed that high LMI was significantly related to lower carotid–radial PWV, suggesting that an increase in lean mass may be protective of arterial structure (27).
A key finding was that CRF allometrically scaled for lean mass partially mediated the association of cardiometabolic health with FMD, DC, and carotid–radial PWV in this population. There is a mediating or indirect role when there are statistically significant associations between (a) an independent variable and a mediator, (b) the independent and the dependent variable, (c) the mediator and dependent variable, and (d) the diminished association between the independent and dependent variable when the mediator is included in the model (46,47). We defined cardiometabolic health as poor if children had three or more cardiometabolic risk factors such as systolic or diastolic blood pressure >75th percentile, HDL <25th percentile, triglycerides >75th percentile, and/or FMI >75th percentile. Although CRF allometrically scaled for lean mass seems to associate with FMD in this mediation analysis, it must be noted that, because of the components of cardiometabolic health, we avoided dual adjustments for the same variables. Plausible explanations may be that an increased blood pressure stimulates higher collagen deposits, which alters the arterial wall elastin–collagen ratio. Similarly, dyslipidemia and increasing fat deposits result in disarrayed endothelium, friable elastin molecules, lower levels of adiponectin, and elevated inflammatory markers such as cytokines, macrophages, and growth factors that ultimately stiffen the arteries (50,53,54). However, elevated CRF may increase vessel compliance via higher pulsatile perfusion, decreased late pulse pressure augmentation, higher ventricular ejection during exercise that enhances nitric oxide production, and activation of calcium-sensitive K+ channels linked to endothelial-derived hyperpolarizing factor (13,50,53,54). Thus, poor cardiometabolic health in children may indirectly result in arterial stiffness through diminished CRF. It should be noted that the mediation role of CRF is circa 4% and 12% for carotid–radial PWV and DC, measures of arterial structure, respectively. This CRF mediation role may have a large public health and clinical significance because our population is young, 9–11 yr old, and with most having good cardiometabolic health and arterial structure and function. Our finding reinforces the need for primordial prevention of cardiometabolic-induced arterial diseases from childhood by improving CRF.
Although low childhood adiponectin has been described as a novel pediatric risk factor in better predicting early signs of atherosclerosis in adulthood in comparison to other risk factors such as body mass index and systolic blood pressure (26), we found no association between adiponectin and arterial function and structure in childhood. This result is in consonance with a normal-weight adolescents’ study, where adiponectin was not related to FMD or DC (25). Perhaps, the influence of childhood adiponectin on altered arterial function and structure in adulthood (26) may remain undetected in childhood and adolescence, particularly in normal-weight individuals, because there is an ongoing vascular adaptation to growth and development. Moreover, less than 10% of our cohort participants had poor cardiometabolic health, which might be protective of severe atherosclerosis. Therefore, adiponectin may not predict altered arterial structure and function in these apparently healthy children. Future studies investigating this question may compare a similarly healthy population with those having higher cardiometabolic risks.
This study had several strengths such as a comprehensive measure of cardiometabolic, fat mass, lean mass, and vascular variables in a large well-characterized cohort (ALSPAC) and an objective measure of pubertal status (34). With sufficient statistical power (49), we provided novel evidence on the mediating effect of CRF allometrically scaled for lean mass on cardiometabolic health and arterial outcomes and the bidirectional associations of CRF and body composition with arterial measures in children. From a clinical perspective, we computed cardiometabolic health using diagnostic guidelines (36) rather than compute a cardiometabolic risk score as often used in epidemiological studies (10). This approach improves the clinical relevance of our findings and makes for easier interpretation, particularly among pediatric health care providers. The submaximal measure of CRF, although moderately correlated with maximal aerobic capacity (39), might have decreased its predictive ability. Nonetheless, we adequately controlled this CRF measure for body composition by allometric scaling (40). Almost half of our participants had missing CRF data, and a complete case analysis involving these participants only would be bias to selection and decrease the precision and power of the study (43,44,55). We addressed this limitation by conducting 20 cycles of multiple imputations in line with established practices in epidemiological studies and presented both imputed and complete case results, which were similar (29,43,44,55). The mechanistic basis for our findings would require longitudinal studies to distinguish between early life preclinical atherosclerosis and adaptive effects in relation to growth and development. We may not extrapolate our results to other ethnic groups because we studied mostly Caucasian children. Lastly, our observational study precludes us from inferring causality.
CONCLUSIONS
Our findings suggest that CRF, fat mass, and lean mass associate with arterial function and structure in 9- to 11-yr-olds and may have temporal or bidirectional relationships. We found no evidence associating adiponectin with arterial outcomes in our apparently healthy children. The effective prevention of preclinical markers of atherosclerosis and cardiometabolic diseases from childhood may focus on improving CRF because we have demonstrated that allometrically scaled CRF for lean mass mediates the associations of cardiometabolic health with arterial function and structure.
Supplementary Material
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
The UK Medical Research Council and Wellcome (grant reference no. 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors, and A. O. A., A. R. B., and T.-P. T. will serve as guarantors for the contents of this article and have nothing to disclose.
Comprehensive list of grants funding is available on the ALSPAC Web site (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf); This research (Dr. Agbaje) was specifically funded by the Doctoral Program in Clinical Research, Institute of Public Health and Clinical Nutrition, Faculty of Health Sciences, University of Eastern Finland; the Jenny and Antti Wihuri Foundation (grant no. 00180006); and the North Savo regional and central Finnish Cultural Foundation (grant nos. 65191835 and 00200150). Open access fee was provided by the University of Eastern Finland.
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and the results of the present study do not constitute endorsement by the American College of Sports Medicine.
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third party–maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study Web site contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
ALAN R. BARKER, Email: A.R.Barker@exeter.ac.uk.
TOMI-PEKKA TUOMAINEN, Email: tomi-pekka.tuomainen@uef.fi.
REFERENCES
- 1.Charakida M, Tousoulis D, Stefanadis C. Early atherosclerosis in childhood: diagnostic approaches and therapeutic strategies. Int J Cardiol. 2006;109(2):152–9. [DOI] [PubMed] [Google Scholar]
- 2.Cote AT, Phillips AA, Harris KC, Sandor GG, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35(4):1038–44. [DOI] [PubMed] [Google Scholar]
- 3.Avolio A. Arterial stiffness. Pulse (Basel). 2013;1(1):14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadizar F, Voortman T. Arterial stiffness in childhood: a predictor for later cardiovascular disease? Eur J Prev Cardiol. 2018;25(1):100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattsson N Rönnemaa T Juonala M, et al. Arterial structure and function in young adults with the metabolic syndrome: The Cardiovascular Risk in Young Finns Study. Eur Heart J. 2008;29(6):784–91. [DOI] [PubMed] [Google Scholar]
- 6.Pahkala K Laitinen TT Heinonen OJ, et al. Association of fitness with vascular intima-media thickness and elasticity in adolescence. Pediatrics. 2013;132(1):e77–84. [DOI] [PubMed] [Google Scholar]
- 7.Halcox JP, Deanfield JE. Endothelial cell function testing: how does the method help us in evaluating vascular status? Acta Paediatr Suppl. 2004;93(446):48–54. [DOI] [PubMed] [Google Scholar]
- 8.Donald AE Charakida M Falaschetti E, et al. Determinants of vascular phenotype in a large childhood population: the Avon Longitudinal Study of Parents and Children (ALSPAC). Eur Heart J. 2010;31(12):1502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berenson GS Srinivasan SR Hunter SM, et al. Risk factors in early life as predictors of adult heart disease: the Bogalusa Heart Study. Am J Med Sci. 1989;298(3):141–51. [DOI] [PubMed] [Google Scholar]
- 10.Agbaje AO Haapala EA Lintu N, et al. Peak oxygen uptake cut-points to identify children at increased cardiometabolic risk—the PANIC study. Scand J Med Sci Sports. 2019;29(1):16–24. [DOI] [PubMed] [Google Scholar]
- 11.Barker AR Gracia-Marco L Ruiz JR, et al. Physical activity, sedentary time, TV viewing, physical fitness and cardiovascular disease risk in adolescents: the HELENA study. Int J Cardiol. 2018;254:303–9. [DOI] [PubMed] [Google Scholar]
- 12.Mintjens S, Menting MD, Daams JG, van Poppel MNM, Roseboom TJ, Gemke RJBJ. Cardiorespiratory fitness in childhood and adolescence affects future cardiovascular risk factors: a systematic review of longitudinal studies. Sports Med. 2018;48(11):2577–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agbaje AO Haapala EA Lintu N, et al. Associations of cardiorespiratory fitness and adiposity with arterial stiffness and arterial dilatation capacity in response to a bout of exercise in children. Pediatr Exerc Sci. 2019;31(2):238–47. [DOI] [PubMed] [Google Scholar]
- 14.Farr C Middlebrooke AR Armstrong N, et al. Objectively measured aerobic fitness is not related to vascular health outcomes and cardiovascular disease risk in 9–10 year old children. J Sports Sci Med. 2019;18(3):513–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins ND Stratton G Tinken TM, et al. Relationships between measures of fitness, physical activity, body composition and vascular function in children. Atherosclerosis. 2009;204(1):244–9. [DOI] [PubMed] [Google Scholar]
- 16.Meyer J, Elmenhorst J, Giegerich T, Oberhoffer R, Müller J. Controversies in the association of cardiorespiratory fitness and arterial stiffness in children and adolescents. Hypertens Res. 2017;40(7):675–8. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner L, Weberruß H, Oberhoffer-Fritz R, Schulz T. Vascular structure and function in children and adolescents: what impact do physical activity, health-related physical fitness, and exercise have? Front Pediatr. 2020;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Bey A Ruiz JR Ortega FB, et al. Bidirectional associations between fitness and fatness in youth: a longitudinal study. Scand J Med Sci Sports. 2020;30:1483–96. [DOI] [PubMed] [Google Scholar]
- 19.Arnaiz P Acevedo M Barja S, et al. Adiponectin levels, cardiometabolic risk factors and markers of subclinical atherosclerosis in children. Int J Cardiol. 2010;138(2):138–44. [DOI] [PubMed] [Google Scholar]
- 20.Winer JC Zern TL Taksali SE, et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin Endocrinol Metab. 2006;91(11):4415–23. [DOI] [PubMed] [Google Scholar]
- 21.Beauloye V, Zech F, Tran HT, Clapuyt P, Maes M, Brichard SM. Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab. 2007;92(8):3025–32. [DOI] [PubMed] [Google Scholar]
- 22.Nemet D Wang P Funahashi T, et al. Adipocytokines, body composition, and fitness in children. Pediatr Res. 2003;53(1):148–52. [DOI] [PubMed] [Google Scholar]
- 23.Van De Voorde J, Pauwels B, Boydens C, Decaluwé K. Adipocytokines in relation to cardiovascular disease. Metabolism. 2013;62(11):1513–21. [DOI] [PubMed] [Google Scholar]
- 24.Saarikoski LA Huupponen RK Viikari JS, et al. Adiponectin is related with carotid artery intima-media thickness and brachial flow-mediated dilatation in young adults—The Cardiovascular Risk in Young Finns Study. Ann Med. 2010;42(8):603–11. [DOI] [PubMed] [Google Scholar]
- 25.Jaakkola JM Pahkala K Viitala M, et al. Association of adiponectin with adolescent cardiovascular health in a dietary intervention study. J Pediatr. 2015;167(2):353–60.e1. [DOI] [PubMed] [Google Scholar]
- 26.Saarikoski LA Juonala M Huupponen R, et al. Low serum adiponectin levels in childhood and adolescence predict increased intima-media thickness in adulthood. The Cardiovascular Risk in Young Finns Study. Ann Med. 2017;49(1):42–50. [DOI] [PubMed] [Google Scholar]
- 27.Sletner L Mahon P Crozier SR, et al. Childhood fat and lean mass differing relations to vascular structure and function at age 8 to 9 years. Arterioscler Thromb Vasc Biol. 2018;38(10):2528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiesa ST Charakida M Georgiopoulos G, et al. Determinants of intima-media thickness in the young: the ALSPAC study. JACC Cardiovasc Imaging. 2019;14(2):468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dangardt F Charakida M Georgiopoulos G, et al. Association between fat mass through adolescence and arterial stiffness: a population-based study from The Avon Longitudinal Study of Parents and Children. Lancet Child Adolesc Health. 2019;3(7):474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoner L, Kucharska-Newton A, Meyer ML. Cardiometabolic health and carotid-femoral pulse wave velocity in children: a systematic review and meta-regression. J Pediatr. 2020;218:98–105.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cristi-Montero C Courel-Ibáñez J Ortega FB, et al. HELENA study group . Mediation role of cardiorespiratory fitness on the association between fatness and cardiometabolic risk in European adolescents: the HELENA study. J Sport Health Sci. 2021;10:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyd A Golding J Macleod J, et al. Cohort profile: the ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraser A Macdonald-wallis C Tilling K, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frysz M, Howe LD, Tobias JH, Paternoster L. Using SITAR (SuperImposition by Translation and Rotation) to estimate age at peak height velocity in Avon Longitudinal Study of Parents and Children. Wellcome Open Res. 2018;3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayers A Timpson NJ Sattar N, et al. Adiponectin and its association with bone mass accrual in childhood. J Bone Miner Res. 2010;25(10):2212–20. [Published correction appears in J Bone Miner Res. 2012;27(9):2035. Dosage error in article text]. [DOI] [PubMed] [Google Scholar]
- 36.Magnussen CG Koskinen J Chen W, et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study. Circulation. 2010;122(16):1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child. 2014;99(11):1020–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowland TW, Rambusch JM, Staab JS, Unnithan VB, Siconolfi SF. Accuracy of physical working capacity (PWC170) in estimating aerobic fitness in children. J Sports Med Phys Fitness. 1993;33(2):184–8. [PubMed] [Google Scholar]
- 39.Bland J, Pfeiffer K, Eisenmann JC. The PWC170: comparison of different stage lengths in 11–16 year olds. Eur J Appl Physiol. 2012;112(5):1955–61. [DOI] [PubMed] [Google Scholar]
- 40.Loftin M, Sothern M, Abe T, Bonis M. Expression of VO2peak in children and youth, with special reference to allometric scaling. Sports Med. 2016;46(10):1451–60. [DOI] [PubMed] [Google Scholar]
- 41.Welsman J, Armstrong N. Interpreting aerobic fitness in youth: the fallacy of ratio scaling. Pediatr Exerc Sci. 2019;31(2):184–90. [DOI] [PubMed] [Google Scholar]
- 42.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83(404):1198–202. [Google Scholar]
- 43.Rubin DB. An overview of multiple imputation. Proc Surv Res Methods Sect Am Stat Assoc. 1988;16:79–84. [Google Scholar]
- 44.Mackinnon A. The use and reporting of multiple imputation in medical research—a review. J Intern Med. 2010;268(6):586–93. [DOI] [PubMed] [Google Scholar]
- 45.Charakida M Jones A Falaschetti E, et al. Childhood obesity and vascular phenotypes: a population study. J Am Coll Cardiol. 2012;60(25):2643–50. [DOI] [PubMed] [Google Scholar]
- 46.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. [DOI] [PubMed] [Google Scholar]
- 47.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–31. [DOI] [PubMed] [Google Scholar]
- 48.Hayes A. Introduction to Mediation, Moderation, and Conditional Process Analysis (Methodology in the Social Sciences) (The Guilford Press, New York (NY); 2013. [Google Scholar]
- 49.Golding J Pembrey M Jones R, ALSPAC Study Team . ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15(1):74–87. [DOI] [PubMed] [Google Scholar]
- 50.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122(12):1221–38. [DOI] [PubMed] [Google Scholar]
- 51.Duprez DA Hearst MO Lutsey PL, et al. Associations among lung function, arterial elasticity, and circulating endothelial and inflammation markers: the multiethnic study of atherosclerosis. Hypertension. 2013;61(2):542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonopoulos AS, Tousoulis D. The molecular mechanisms of obesity paradox. Cardiovasc Res. 2017;113(9):1074–86. [DOI] [PubMed] [Google Scholar]
- 53.Högström G, Nordström A, Nordström P. Aerobic fitness in late adolescence and the risk of early death: a prospective cohort study of 1.3 million Swedish men. Int J Epidemiol. 2016;45(4):1159–68. [DOI] [PubMed] [Google Scholar]
- 54.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–43. [DOI] [PubMed] [Google Scholar]
- 55.Sterne JAC White IR Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


