Abstract
Background
The data we present are part of the AGRO-ECOSERVICES project (Assessing ecosystem services and disservices provided by arthropod species in Azorean agroecosystems). The project aims to evaluate the relative importance of native and non-native organisms as ecosystem services (ES) and disservices (ED) providers, by combining novel, direct and quantitative tools for monitoring agro-biodiversity. Ecosystem services include evaluation of natural pest control by predation, seed predation on weed plants, pollination, decomposition and ecosystem disservices, herbivory and seed predation on crop plants. Active Aerial Searching (AAS) (only in maize-fields) and pitfall traps were used to sample the arthropod biodiversity (predatory spiders, true-bugs and beetles and main insect pests) on four agricultural habitats of Terceira Island, namely citrus orchards, low and high elevation maize fields and vineyards.
New information
We provided an inventory of all arthropods recorded in four Azorean agroecosystems (citrus orchards, low and high elevation maize fields and vineyards) from Terceira Island. A total of 50412 specimens were collected, belonging to four classes, 20 orders, 81 families and 200 identified species of arthropods. A total of 127 species are considered introduced (n = 22646) and 69 native non-endemic (n = 24117). Four endemic species were recorded with very few specimens (n = 14) and 3635 specimens belong to unidentified taxa recorded only at genus or family level. Five species are new records for Terceira Island, with Lagriahirta (Linnaeus, 1758) (Coleoptera, Tenebrionidae) being also a new record for the Azores. This publication contributes to a better knowledge of the arthropods communities present in agro-ecosystems of Terceira Island and will serve as a baseline for future monitoring schemes targeting the long-term change in arthropod diversity and abundance.
Keywords: Active Aerial Searching (AAS), citrus, dataset, invertebrates, island diversity, Macaronesia, maize, occurrence, orchards, pitfall traps, vineyards.
Introduction
Land-use transformation with associated habitat degradation, is one of the major drivers of biodiversity loss worldwide (Vitousek et al. 1997, Barnosky et al. 2011, Borges et al. 2019a, Harvey et al. 2020). In the case of Azores, since Portuguese colonisation in the 15th century, the original landscape has suffered severe transformations, with the replacement of native forests by exotic tree plantations, pastures, agricultural and urban areas (Gaspar et al. 2008, Borges et al. 2019a, Borges et al. 2019b, Norder et al. 2020).
However, although exotic species have a competitive advantage to colonise new human-altered habitats given that their tolerance to wide range of environmental conditions and habitats (e.g. generalist behaviour) (Rigal et al. 2017), these non-natural habitats also offer opportunities to native biota (McKinney and Lockwood 1999, Blackburn et al. 2004, Sax 2008, Tsafack et al. 2021).
Many species were also introduced because of human settlement (Frutuoso 2011). The current remnants of native forests represent less than 5% of the total area of the archipelago (Gaspar et al. 2008). Currently, the Azorean economy depends greatly on agroecosystems (Gil et al. 2017). Agrosecoystems with the largest area are pastures, followed by maize, with the two crops usually grown in rotation. Due to their long co-existence and close taxonomic relationship between pastures and maize (both are grasses), several pests interact with both crops all year round (P. Monjardino, pers. observ.). These interactions need to be further understood, because of ongoing current significant yield losses in both agroecosystems (P. Monjardino, pers. observ.). Vineyards and citrus orchards are amongst the most important crops on the Azores. Both crops have significant pest and disease problems due to the benign environmental conditions and to improper cultural practices (Lopes et al. 2009).
Azorean terrestrial arthropod fauna have been extensively surveyed in the last two decades. Although most surveys have been conducted in native forests (e.g. Borges et al. 2005, Ribeiro et al. 2005, Borges et al. 2006), several also included anthropogenic habitats, as exotic forest plantations, pastures for cattle grazing and other agricultural areas (Cardoso et al. 2009, Florencio et al. 2015, Rigal et al. 2017, Marcelino et al. 2021, Tsafack et al. 2021).
In 2019 and 2020, we started the project “Assessing Ecosystem Services and Disservices provided by Arthropod species in Azorean Agroecosystems” (AGRO-ECOSERVICES). This project aims to: (i) initiate the monitoring of terrestrial arthropods in agricultural habitats, (ii) implement novel, direct and quantitative tools to quantify ecosystem services (ES) and disservices (ED) and (iii) evaluate the relative importance of native and non-native organisms as ES/ED providers.
Arthropods, especially insects, support ecosystem stability and functioning (Allan et al. 2015, Bennett et al. 2015). Due to their high species richness and abundance, as well as their importance for several ES and ED (Zhang et al. 2007, Ameixa et al. 2018, Noriega et al. 2018, Ecosystem Services 2019), arthropods play a key role in all terrestrial ecosystems. Evaluating the total effect of arthropods that are providers of both ES and ED is challenging (Shapiro and Báldi 2014). For example, when they prey on pests, generalist predators provide biological control, an ES valued at $400 billion/y (Costanza et al. 1997), while their intraguild predation (Lövei and Ferrante 2017) constitutes an ED. A second great challenge is to assess the role of native vs. exotic biodiversity in providing ES/ED, which is essential to manage sustainable landscapes and an important frontier in theoretical ecology. Exotic species often alter ecological processes and cause severe biodiversity loss (Simberloff et al. 2013). Nevertheless, these species may also provide ES: alien plants can increase microbial activity (Vilà et al. 2011), introduced natural enemies can control pests (Heimpel and Mills 2017) or provide ecological “insurance” after the decline of native species (Stavert et al. 2018).
Oceanic islands have a high proportion of endemic species, being very sensitive to biotic disturbance, such as invasions and land-use changes (Stachowicz and Tilman 2005, Kier et al. 2009) - the perfect setting to test the response of ecological communities to disturbance and its effects on ecosystem processes. Several factors contribute to arthropod decline in the Azores (Borges et al. 2019b), including native forest destruction (Triantis et al. 2010), lack of connectivity between forest patches (Aparício et al. 2018) and climate change (Ferreira et al. 2016).
This publication contributes not only to a better knowledge of the arthropods present in agroecosystems of Terceira Island, but will also contribute as a baseline for future monitoring schemes in Azorean agroecosystems targeting the long-term change in arthropod diversity and abundance.
General description
Purpose
To provide an arthropod inventory of agro-ecosystems from Terceira Island (Azores), based on data collected in four agro-ecosystems, citrus orchards, low and high elevation maize fields and vineyards. This study will contribute to a better knowledge of the arthropods present in agro-ecosystems and will serve as a baseline for future monitoring schemes in Azorean agro-ecosystems targeting the long-term change in arthropod diversity and abundance.
Additional information
The study was conducted between July 2019 and September 2021 in Terceira Island. Active Aerial Searching (only in maize-fields) and pitfall traps were used to sample the arthropod biodiversity (pollinators and predatory spiders, true-bugs and beetles and main insect pests) on four agricultural habitats, namely citrus orchards, vineyards, low elevation maize fields and high elevation maize fields. Information on ecosystem services (ES) and disservices (ED) providers will be the subject of another publication.
Project description
Title
AgEcSe- AGRO-ECOSERVICES - Assessing ecosystem services and disservices provided by arthropod species in Azorean Agroecosystems (ACORES-01-0145-FEDER-000073)
Personnel
Project leaders: Paulo A. V. Borges and António Onofre Soares
Team members: Marco Ferrante, Artur Gil, Marco Girardello, David H. Lopes, Paulo Monjardino, Rui Nunes.
External Consultants: Sven Bacher, Gabor Lövei, François Rigal
Parataxonomists: Jonne Bonnet, Ricardo Costa, Rui Nunes
Darwin Core Database management: Paulo A. V. Borges, Lucas Lamelas-López, Enésima Pereira
Study area description
Terceira Island (area: 400.2 km²; elevation: 1021 m a.s.l.) is located in the central group of the Azores Archipelago (North Atlantic), roughly at 38.638 N and -27.0150 W (Fig. 1). Similar to all islands in Azores, Terceira is volcanic and of recent origin (0.4 Ma, see Florencio et al. 2021). The climate is temperate oceanic, with regular and abundant rainfall, high levels of relative humidity and persistent winds, mainly during the winter and autumn seasons.
Figure 1.
Map of the Azores Archipelago location in mid-Atlantic with the studied island TER - Terceira, marked in black (Credit: Enésima Pereira).
Design description
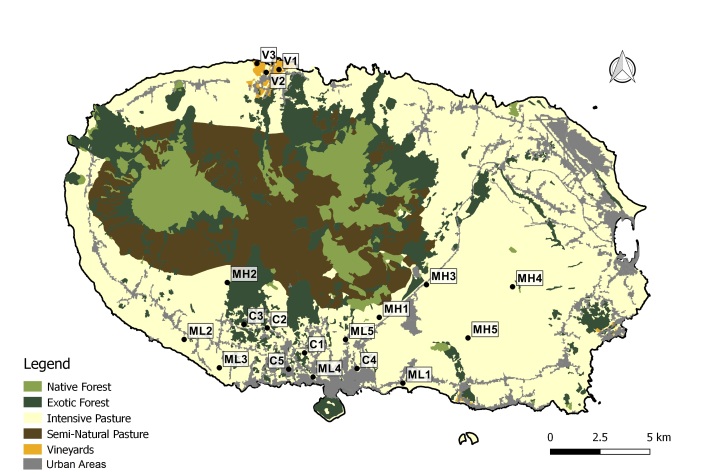
The sampled habitats included citrus orchards, vineyards and low elevation maize fields, all located at low elevation areas and high elevation maize fields (Fig. 2, Table 1). The two types of maize fields differ not only in the elevation, but principally in crop management, the low elevation being an annual rotation of maize and Italian ryegrass and the high elevation (located at intermediate elevation in the Island) being a perennial rotation of maize and perennial ryegrass.
Figure 2.
Map of the study area (Terceira Island, Azores). Codes of sites as in Table 1. Maize fields are located in intensive pasture since they are only operating in summer, with the two crops usually grown in rotation (Land-use data extracted from Cruz et al. 2007).
Table 1.
Description of the habitat, locality, elevation and coordinates of the 18 sampled sites on Terceira Island, Azores.
| Code Site | Habitat | Location ID | Locality | Elevation (m a.s.l.) | Latitude | Longitude |
| C1 | Citrus | TER_CITRUS_T1_T206 | Pico da Urze | 117 | 38.66989 | -27.24047 |
| C2 | Citrus | TER_CITRUS_T2_T207 | Qt. Rosário | 158 | 38.68111 | -27.26206 |
| C3 | Citrus | TER_CITRUS_T3_T208 | S. Bartolomeu | 189 | 38.6827 | -27.27555 |
| C4 | Citrus | TER_CITRUS_T4_T209 | S. Bento | 66 | 38.66287 | -27.21019 |
| C5 | Citrus | TER_CITRUS_T5_T210 | S. Carlos | 69 | 38.6625 | -27.24961 |
| ML1 | Maize Low | TER_MAIZE_LOW_T2_T221 | Atalaia | 111 | 38.65631 | -27.18368 |
| ML2 | Maize Low | TER_MAIZE_LOW_T1_T220 | Cinco Ribeiras | 90 | 38.6758 | -27.30998 |
| ML3 | Maize Low | TER_MAIZE_LOW_T3_T222 | S. Mateus | 42 | 38.66304 | -27.28962 |
| ML4 | Maize Low | TER_MAIZE_LOW_T4_T223 | Universidade dos Açores - Campus do Pico da Urze | 36 | 38.659 | -27.23555 |
| ML5 | Maize Low | TER_MAIZE_LOW_T5_T224 | Vinha Brava | 167 | 38.67593 | -27.21684 |
| MH1 | Maize High | TER_MAIZE_HIGH_T1_T215 | Casa da Mina | 314 | 38.68602 | -27.1974 |
| MH2 | Maize High | TER_MAIZE_HIGH_T2_T216 | Escampadouro | 309 | 38.70159 | -27.2852 |
| MH3 | Maize High | TER_MAIZE_HIGH_T3_T217 | Granja | 385 | 38.70083 | -27.17019 |
| MH4 | Maize High | TER_MAIZE_HIGH_T4_T218 | Juncal | 321 | 38.69996 | -27.12048 |
| MH5 | Maize High | TER_MAIZE_HIGH_T5_T219 | Poejo | 275 | 38.6768 | -27.14616 |
| V1 | Vineyards | TER_VINE_F1_T211 | Biscoitos Vinha_F1 | 23 | 38.79793 | -27.25567 |
| V2 | Vineyards | TER_VINE_F2_T212 | Biscoitos Vinha_F2 | 52 | 38.79664 | -27.26302 |
| V3 | Vineyards | TER_VINE_F3_T213 | Biscoitos Vinha_F3 | 28 | 38.80066 | -27.26842 |
Funding
This work was financed by FEDER (European Regional Development Fund) in 85% and by Azorean Public funds by 15% through the Operational Program Azores 2020, under the project AGRO-ECOSERVICES (ACORES-01-0145-FEDER-000073).
Sampling methods
Study extent
The study was conducted in four agro-ecosystems of Terceira Island (Fig. 2): citrus orchards (Fig. 3), vineyards (Fig. 4), low elevation maize fields (Fig. 5) and high elevation maize fields (Fig. 6). Five citrus orchards were selected, located at low elevation areas. Ten maize fields, five of which are located inland at higher elevation and five other closer to the coast in low elevation areas. Finally, three vineyards located on the coast, north of the Island were sampled (see also Table 1).
Figure 3.
A citrus orchard in Terceira Island (C5 - S. Carlos) (Credit: Rui Nunes).
Figure 4.
The vineyards in Terceira Island (V3 - Biscoitos) (Credit: Rui Nunes).
Figure 5.
A low elevation maize field in Terceira Island (ML3 - S. Mateus) (Credit: Rui Nunes).
Figure 6.
A high elevation maize field in Terceira Island (MH5 -Poejo) (Credit: Rui Nunes).
Sampling description
Active Aerial Searching (AAS) and pitfall traps were used to sample arthropod diversity. The following main functional groups were collected: predatory arthropods (mostly spiders, true-bugs, beetles and bugs), phytophagous insects and saprophagous arthropods (mostly millipedes and beetles).
AAS consists in picking arthropods found above knee-level by hand, using forceps, pooter or brush and immediately transferring them into vials containing ethanol 96%. It was implemented in five low- and five high-elevation maize fields. Four 1-hour samples were obtained during the night when the main predators are more active. Sampling was performed in the summer when the maize plants were at maximum development. Samples were taken by Paulo A. V. Borges and Rui Nunes (two hours each per site).
Pitfall traps were standard 330 ml plastic cups, 8 cm wide at the top and approximately 12 cm deep - European standard plastic cups (Fig. 7), partially filled with propylene glycol. The traps were deployed for 14 consecutive days.
Figure 7.
Detail of a pitfall trap (standard 330 ml plastic cups, 8 cm wide at the top and approximately 12 cm deep) (Credit: Rui Nunes).
In each of five citrus orchards and six (of ten available) maize fields (three in low- and three in high-elevation areas), 16 pitfall traps organised in sets of two connected with a grid (Fig. 8) were deployed, along a transect, from the point closest to the crop edge. The eight sets of two pitfall traps were separated by at least 10 metres. A total of 80 and 96 pitfall traps were deployed on citrus orchards and maize fields, respectively.
Figure 8.

Pitfall traps used in citrus orchards and maize fields (sets of two connected with a grid) (Credit: Rui Nunes).
For vineyards, a different strategy had to be followed since Azorean vineyards are formed by small rocky enclosures (between 6-20 m2) (Fig. 4) and pitfall traps were deployed in the interior of these enclosures. Following a transect, a total of 144 individual pitfall traps were deployed in three vineyards (48 in each site).
Sampling methods used in citrus and vineyards (pitfall traps) only provide information on the soil-related arthropods; most of crop insect pests (canopy associated species) are not sampled by this sampling technique.
Quality control
All sampled specimens were first sorted by trained paratoxonomists (Jonne Bonnet, Ricardo Costa, Rui Nunes). All specimens were allocated to a taxonomic species by Paulo A. V. Borges. Juveniles were also included in the data presented in this paper since the low diversity of species in Azores allows their reliable identification. Colonisation status for each identified species is based on Borges et al. 2010 (END - Endemic; NAT - native non-endemic; INTR -introduced).
Step description
A reference collection for Azorean arthropods (deposited at the Dalberto Teixeira Pombo Insect Collection, University of Azores) started to be prepared in 1999 by one of us (PAVB) and many taxonomists contributed since then in the identification of species. For all the specimens for which adequate identification was not possible, a new "morphospecies code" was created.
Geographic coverage
Description
Terceira Island, Azores, Portugal.
Coordinates
38.638 and 38.814 Latitude; -27.394 and -27.0150 Longitude.
Taxonomic coverage
Description
The following classes and orders of arthropods are covered: Arachnida: Araneae, Opiliones, Pseudoscorpiones; Chilopoda: Geophilomorpha, Lithobiomorpha, Scolopendromorpha, Scutigeromorpha; Diplopoda: Chordeumatida, Julida, Polydesmida; and Insecta: Archaeognatha, Coleoptera, Dermaptera, Hemiptera, Hymenoptera, Lepidoptera, Neuroptera, Orthoptera, Psocoptera, Thysanoptera.
Taxa included
| Rank | Scientific Name | Common Name |
|---|---|---|
| class | Araneae | Spiders |
| class | Opiliones | Opilions |
| class | Pseudoscorpiones | Pseudoscorpions |
| class | Diplopoda | Millipedes |
| class | Chilopoda | Centipedes |
| order | Archaeognatha | Bristletails |
| order | Dermaptera | Earwigs |
| order | Orthoptera | Crickets, Grasshoppers |
| order | Psocoptera | Barklice |
| order | Thysanoptera | Thrips |
| order | Hemiptera | Bugs |
| order | Neuroptera | Lacewings |
| order | Coleoptera | Beetles |
| order | Hymenoptera | Ants |
| order | Lepidoptera | Moths |
Traits coverage
No data available.
Temporal coverage
Notes
16 July 2019 to 9 June 2021
Collection data
Collection name
Entomoteca Dalberto Teixeira Pombo at University of Azores
Collection identifier
DTP
Specimen preservation method
All specimens were preserved in 96% ethanol.
Curatorial unit
Dalberto Teixeira Pombo insect collection at the University of the Azores (Curator: Paulo A. V. Borges)
Usage licence
Usage licence
Creative Commons Public Domain Waiver (CC-Zero)
Data resources
Data package title
Monitoring Arthropods in Azorean Agroecosystems: the project AGRO-ECOSERVICES (AgEcSe)
Resource link
https://www.gbif.org/dataset/822f3765-6950-40c5-9353-1f335599007c
Alternative identifiers
Number of data sets
1
Data set 1.
Data set name
Monitoring Arthropods in Azorean Agroecosystems: the project AGRO-ECOSERVICES
Data format
Darwin Core Archive
Number of columns
56
Download URL
http://ipt.gbif.pt/ipt/resource?r=arthropods_agroecoservices
Data format version
version 1.10
Description
The dataset is available on the Global Biodiversity Information Facility platform, GBIF (Borges et al. 2021). The following data table includes all the records for which a taxonomic identification of the species was possible. The dataset submitted to GBIF is structured as a sample event dataset, with two tables: event (as core) and occurrences (abundance data). The data in this sampling event resource have been published as a Darwin Core Archive (DwCA), which is a standardised format for sharing biodiversity data as a set of one or more data tables. The core data file contains 358 records (eventID) and the occurrences file 5134 records (occurrenceID). This IPT (Integrated Publishing Toolkit) archives the data and thus serves as the data repository. The data and resource metadata are available for download from Borges et al. (2021).
Data set 1.
| Column label | Column description |
|---|---|
| Table of Sampling Events | Table with sampling events data (beginning of table). |
| eventID | Identifier of the events, unique for the dataset. |
| stateProvince | Name of the region of the sampling site. |
| islandGroup | Name of archipelago. |
| island | Name of the island. |
| country | Country of the sampling site. |
| countryCode | ISO code of the country of the sampling site. |
| municipality | Municipality of the sampling site. |
| decimalLongitude | Approximate centre point decimal longitude of the field site in GPS coordinates. |
| decimalLatitude | Approximate centre point decimal latitude of the field site in GPS coordinates. |
| geodeticDatum | The ellipsoid, geodetic datum or spatial reference system (SRS) upon which the geographic coordinates given in decimalLatitude and decimalLongitude are based. |
| coordinateUncertaintyInMetres | Uncertainty of the coordinates of the centre of the sampling plot. |
| coordinatePrecision | Precision of the coordinates. |
| georeferenceSources | A list (concatenated and separated) of maps, gazetteers or other resources used to georeference the Location, described specifically enough to allow anyone in the future to use the same resources. |
| locationID | Identifier of the location. |
| fieldNumber | Code of the sample |
| locality | Name of the locality. |
| minimumElevationInMetres | The lower limit of the range of elevation (altitude, usually above sea level), in metres. |
| habitat | The habitat of the sample. |
| year | Year of the event. |
| month | Month of the event. |
| day | Day of the event. |
| samplingEffort | The amount of effort expended during an Event. |
| eventDate | Date or date range the record was collected. |
| samplingProtocol | The sampling protocol used to capture the species. |
| Occurrence Table | Table with species abundance data (beginning of new table). |
| eventID | Identifier of the events, unique for the dataset. |
| type | Type of the record, as defined by the Public Core standard. |
| licence | Reference to the licence under which the record is published. |
| institutionID | The identity of the institution publishing the data. |
| institutionCode | The code of the institution publishing the data. |
| collectionID | The identity of the collection publishing the data. |
| collectionCode | The code of the collection where the specimens are conserved. |
| datasetName | Name of the dataset. |
| basisOfRecord | The nature of the data record. |
| occurrenceID | Identifier of the record, coded as a global unique identifier. |
| recordedBy | A list (concatenated and separated) of names of people, groups or organisations who performed the sampling in the field. |
| identifiedBy | A list (concatenated and separated) of names of people, groups or organisations who assigned the Taxon to the subject. |
| dateIdentified | The date on which the subject was determined as representing the Taxon. |
| organismQuantity | A number or enumeration value for the quantity of organisms. |
| organismQuantityType | The type of quantification system used for the quantity of organisms. |
| sex | The sex and quantity of the individuals captured. |
| lifeStage | The life stage of the organisms captured. |
| scientificName | Complete scientific name including author and year. |
| scientificNameAuthorship | Name of the author of the lowest taxon rank included in the record. |
| kingdom | Kingdom name. |
| phylum | Phylum name. |
| class | Class name. |
| order | Order name. |
| family | Family name. |
| genus | Genus name. |
| specificEpithet | Specific epithet. |
| infraspecificEpithet | Infrapecific epithet. |
| taxonRank | Lowest taxonomic rank of the record. |
| establishmentMeans | The process of establishment of the species in the location, using a controlled vocabulary: 'native', 'introduced', 'endemic', "unknown". |
| identificationRemarks | Information about morphospecies identification (code in Dalberto Teixeira Pombo Collection). |
Additional information
We collected a total of 50412 specimens, belonging to four classes, 20 orders and 81 families of arthropods. A total of 127 species are considered introduced (n = 22646) and 69 native non-endemic (n = 24117). Four endemic species were recorded with very few specimens (n = 14) and 3635 specimens belong to unidentified taxa recorded only at genus or family level.
Arachnids belonged to three orders, Araneae being the most abundant (95% of arachnid specimens belonged to this order). Chilopoda and Diplopoda classes recorded four and three orders, being Lithobiomorpha and Julida, respectively, the most abundant. Insecta was the most abundant class (n = 39590) recorded in the studied agro-ecosystems, with Coleoptera the most abundant order (38% of specimens).
A total of 200 species were identified (Table 2) and an additional 73 morphospecies need proper identification, totalling potentially 273 species (see Suppl. material 1).
Table 2.
Inventory of arthropods collected in four agroecosystems in Terceira Island (Azores, Portugal) following an elevation gradient: vineyards (Vine), citrus orchards (Citrus), maize fields at low elevation (Maize L) and at high elevation (Maize H). The list includes only the specimens identified at species-level. Class, order, family, scientific name follow alphabetical sequence. Colonisation status based on Borges et al. 2010 (Origin: END - Endemic; NAT - native non-endemic; INTR - introduced) and abundance per habitat type are provided. Bold scientific names constitute new records for Terceira Island. * - New record for Azores.
| class | order | family | scientificName | Origin | VINE | CITRUS | MAIZE L | MAIZE H | Total |
| Arachnida | Araneae | Agelenidae | Tegenariadomestica (Clerck, 1757) | INTR | 1 | 1 | |||
| Arachnida | Araneae | Agelenidae | Tegenariapagana C.L. Koch, 1840 | INTR | 3 | 3 | |||
| Arachnida | Araneae | Araneidae | Agalenatearedii (Scopoli, 1763) | INTR | 7 | 2 | 9 | ||
| Arachnida | Araneae | Araneidae | Araneusangulatus Clerck, 1757 | INTR | 30 | 30 | |||
| Arachnida | Araneae | Araneidae | Argiopebruennichi (Scopoli, 1772) | NAT | 37 | 50 | 87 | ||
| Arachnida | Araneae | Araneidae | Gibbaraneaoccidentalis Wunderlich, 1989 | END | 1 | 1 | |||
| Arachnida | Araneae | Araneidae | Mangoraacalypha (Walckenaer, 1802) | INTR | 1 | 1 | |||
| Arachnida | Araneae | Araneidae | Neosconacrucifera (Lucas, 1838) | INTR | 2 | 2 | 4 | ||
| Arachnida | Araneae | Araneidae | Zygiellax-notata (Clerck, 1757) | INTR | 6 | 12 | 18 | ||
| Arachnida | Araneae | Clubionidae | Clubionaterrestris Westring, 1851 | INTR | 2 | 2 | |||
| Arachnida | Araneae | Clubionidae | Porrhoclubionadecora (Blackwall, 1859) | NAT | 25 | 4 | 29 | ||
| Arachnida | Araneae | Clubionidae | Porrhoclubionagenevensis (L. Koch, 1866) | INTR | 1 | 1 | |||
| Arachnida | Araneae | Dictynidae | Lathysdentichelis (Simon, 1883) | NAT | 1 | 1 | |||
| Arachnida | Araneae | Dictynidae | Nigmapuella (Simon, 1870) | INTR | 3 | 3 | |||
| Arachnida | Araneae | Dysderidae | Dysderacrocata C.L. Koch, 1838 | INTR | 4 | 70 | 20 | 15 | 109 |
| Arachnida | Araneae | Gnaphosidae | Marinarozeloteslyonneti (Audouin, 1826) | INTR | 15 | 15 | 30 | ||
| Arachnida | Araneae | Linyphiidae | Agynetadecora (O. Pickard-Cambridge, 1871) | INTR | 1 | 1 | |||
| Arachnida | Araneae | Linyphiidae | Agynetafuscipalpa (C. L. Koch, 1836) | INTR | 28 | 7 | 396 | 18 | 449 |
| Arachnida | Araneae | Linyphiidae | Erigoneatra Blackwall, 1833 | INTR | 1 | 3 | 3 | 13 | 20 |
| Arachnida | Araneae | Linyphiidae | Erigoneautumnalis Emerton, 1882 | INTR | 1 | 309 | 333 | 95 | 738 |
| Arachnida | Araneae | Linyphiidae | Erigonedentipalpis (Wider, 1834) | INTR | 2 | 176 | 484 | 662 | |
| Arachnida | Araneae | Linyphiidae | Mermessusbryantae (Ivie & Barrows, 1935) | INTR | 2 | 3 | 2 | 7 | |
| Arachnida | Araneae | Linyphiidae | Mermessusfradeorum (Berland, 1932) | INTR | 117 | 7 | 53 | 177 | |
| Arachnida | Araneae | Linyphiidae | Nerieneclathrata (Sundevall, 1830) | INTR | 3 | 2 | 2 | 7 | |
| Arachnida | Araneae | Linyphiidae | Oedothoraxfuscus (Blackwall, 1834) | INTR | 4 | 80 | 577 | 661 | |
| Arachnida | Araneae | Linyphiidae | Osteariusmelanopygius (O. Pickard-Cambridge, 1880) | INTR | 1 | 6 | 17 | 24 | |
| Arachnida | Araneae | Linyphiidae | Palliduphantesschmitzi (Kulczynski, 1899) | NAT | 7 | 1 | 1 | 2 | 11 |
| Arachnida | Araneae | Linyphiidae | Pelecopsisparallela (Wider, 1834) | INTR | 32 | 1 | 33 | ||
| Arachnida | Araneae | Linyphiidae | Prinerigonevagans (Audouin, 1826) | INTR | 130 | 229 | 359 | ||
| Arachnida | Araneae | Linyphiidae | Tenuiphantestenuis (Blackwall, 1852) | INTR | 132 | 104 | 177 | 413 | |
| Arachnida | Araneae | Lycosidae | Arctosaperita (Latreille, 1799) | INTR | 1 | 1 | |||
| Arachnida | Araneae | Lycosidae | Pardosaacorensis Simon, 1883 | END | 6 | 3 | 9 | ||
| Arachnida | Araneae | Oecobiidae | Oecobiusnavus Blackwall, 1859 | INTR | 5 | 5 | 10 | ||
| Arachnida | Araneae | Salticidae | Chalcoscirtusinfimus (Simon, 1868) | INTR | 14 | 14 | |||
| Arachnida | Araneae | Salticidae | Heliophanuskochii Simon, 1868 | INTR | 1 | 1 | |||
| Arachnida | Araneae | Salticidae | Macaroerisdiligens (Blackwall, 1867) | NAT | 1 | 2 | 3 | ||
| Arachnida | Araneae | Salticidae | Pseudeuophrysvafra (Blackwall, 1867) | INTR | 3 | 3 | |||
| Arachnida | Araneae | Salticidae | Salticusmutabilis Lucas, 1846 | INTR | 1 | 1 | |||
| Arachnida | Araneae | Salticidae | Synagelesvenator (Lucas, 1836) | INTR | 1 | 1 | |||
| Arachnida | Araneae | Scytotidae | Scytodesthoracica (Latreille, 1802) | INTR | 1 | 1 | |||
| Arachnida | Araneae | Segestriidae | Segestriaflorentina (Rossi, 1790) | INTR | 1 | 1 | |||
| Arachnida | Araneae | Tetragnathidae | Pachygnathadegeeri Sundevall, 1830 | INTR | 1 | 55 | 56 | ||
| Arachnida | Araneae | Theridiidae | Cryptachaeablattea (Urquhart, 1886) | INTR | 5 | 2 | 11 | 18 | |
| Arachnida | Araneae | Theridiidae | Neottiurabimaculata (Linnaeus, 1767) | INTR | 1 | 1 | |||
| Arachnida | Araneae | Theridiidae | Parasteatodatepidariorum (C. L. Koch, 1841) | INTR | 8 | 69 | 77 | ||
| Arachnida | Araneae | Theridiidae | Steatodagrossa (C. L. Koch, 1838) | INTR | 16 | 71 | 87 | ||
| Arachnida | Araneae | Theridiidae | Steatodanobilis (Thorell, 1875) | INTR | 2 | 2 | |||
| Arachnida | Araneae | Theridiidae | Theridionmelanostictum O. Pickard-Cambridge, 1876 | INTR | 1 | 3 | 4 | ||
| Arachnida | Araneae | Theridiidae | Theridionmusivivum Schmidt, 1956 | NAT | 1 | 1 | |||
| Arachnida | Araneae | Thomisidae | Xysticusnubilus Simon, 1875 | INTR | 3 | 3 | |||
| Arachnida | Araneae | Zodariidae | Zodarionatlanticum Pekár & Cardoso, 2005 | INTR | 934 | 7 | 14 | 1 | 956 |
| Arachnida | Opiliones | Phalangiidae | Homalenotuscoriaceus (Simon, 1879) | NAT | 1 | 156 | 20 | 177 | |
| Arachnida | Opiliones | Phalangiidae | Leiobunumblackwalli Meade, 1861 | NAT | 7 | 12 | 19 | ||
| Arachnida | Pseudoscorpiones | Chthoniidae | Chthoniusischnocheles (Hermann, 1804) | INTR | 8 | 10 | 4 | 22 | |
| Arachnida | Pseudoscorpiones | Chthoniidae | Ephippiochthoniustetrachelatus (Preyssler, 1790) | INTR | 18 | 9 | 27 | ||
| Arachnida | Pseudoscorpiones | Neobisiidae | Neobisiummaroccanum Beier, 1930 | INTR | 1 | 2 | 3 | ||
| Chilopoda | Geophilomorpha | Linotaeniidae | Strigamiacrassipes (C.L. Koch, 1835) | NAT | 2 | 2 | |||
| Chilopoda | Lithobiomorpha | Lithobiidae | Lithobiuspilicornispilicornis Newport, 1844 | NAT | 15 | 4 | 1 | 1 | 21 |
| Chilopoda | Scolopendromorpha | Cryptopidae | Cryptopshortensis (Donovan, 1810) | NAT | 6 | 1 | 2 | 9 | |
| Chilopoda | Scutigeromorpha | Scutigeridae | Scutigeracoleoptrata (Linnaeus, 1758) | INTR | 34 | 205 | 171 | 27 | 437 |
| Diplopoda | Chordeumatida | Haplobainosomatidae | Haplobainosomalusitanum Verhoeff, 1900 | INTR | 6 | 6 | |||
| Diplopoda | Julida | Blaniulidae | Blaniulusguttulatus (Fabricius, 1798) | INTR | 1 | 1 | |||
| Diplopoda | Julida | Blaniulidae | Nopoiuluskochii (Gervais, 1847) | INTR | 3 | 3 | |||
| Diplopoda | Julida | Blaniulidae | Proteroiulusfuscus (Am Stein, 1857) | INTR | 3 | 3 | |||
| Diplopoda | Julida | Julidae | Brachyiuluspusillus (Leach, 1814) | INTR | 138 | 138 | |||
| Diplopoda | Julida | Julidae | Cylindroiuluslatestriatus (Curtis, 1845) | INTR | 1 | 1 | |||
| Diplopoda | Julida | Julidae | Cylindroiuluspropinquus (Porat, 1870) | INTR | 4 | 14 | 18 | ||
| Diplopoda | Julida | Julidae | Ommatoiulusmoreleti (Lucas, 1860) | INTR | 221 | 1740 | 35 | 217 | 2213 |
| Diplopoda | Polydesmida | Polydesmidae | Brachydesmussuperus Latzel, 1884 | INTR | 1 | 1 | |||
| Diplopoda | Polydesmida | Polydesmidae | Polydesmuscoriaceus Porat, 1870 | INTR | 8 | 470 | 12 | 53 | 543 |
| Insecta | Archaeognatha | Machilidae | Diltasaxicola (Womersley, 1930) | NAT | 3 | 4 | 7 | ||
| Insecta | Coleoptera | Anthicidae | Hirticollisquadriguttatus (Rossi, 1792) | NAT | 1 | 166 | 176 | 343 | |
| Insecta | Coleoptera | Apionidae | Aspidapionradiolus (Marsham, 1802) | NAT | 1 | 1 | 2 | ||
| Insecta | Coleoptera | Apionidae | Ischnopterapionvirens (Herbst, 1797) | INTR | 6 | 2 | 8 | ||
| Insecta | Coleoptera | Carabidae | Acupalpusdubius Schilsky, 1888 | NAT | 37 | 8 | 45 | ||
| Insecta | Coleoptera | Carabidae | Acupalpusflavicollis (Sturm, 1825) | NAT | 47 | 1 | 48 | ||
| Insecta | Coleoptera | Carabidae | Agonummuellerimuelleri (Herbst, 1784) | INTR | 38 | 38 | |||
| Insecta | Coleoptera | Carabidae | Amaraaenea (De Geer, 1774) | INTR | 1 | 6 | 15 | 22 | |
| Insecta | Coleoptera | Carabidae | Anisodactylusbinotatus (Fabricius, 1787) | INTR | 1 | 3 | 65 | 69 | |
| Insecta | Coleoptera | Carabidae | Calosomaolivieri Dejean, 1831 | NAT | 14 | 41 | 55 | ||
| Insecta | Coleoptera | Carabidae | Harpalusdistinguendusdistinguendus (Duftschmid, 1812) | INTR | 1 | 3 | 40 | 44 | |
| Insecta | Coleoptera | Carabidae | Laemostenuscomplanatus (Dejean, 1828) | INTR | 5 | 41 | 1 | 47 | |
| Insecta | Coleoptera | Carabidae | Microlestesnegritanegrita (Wollaston, 1854) | NAT | 6 | 6 | |||
| Insecta | Coleoptera | Carabidae | Notiophilusquadripunctatus Dejean, 1826 | NAT | 1 | 1 | |||
| Insecta | Coleoptera | Carabidae | Ocysharpaloides (Audinet-Serville, 1821) | NAT | 5 | 5 | |||
| Insecta | Coleoptera | Carabidae | Paranchusalbipes (Fabricius, 1796) | INTR | 1 | 16 | 17 | ||
| Insecta | Coleoptera | Carabidae | Pseudoophonusrufipes (De Geer, 1774) | INTR | 7 | 74 | 55 | 6995 | 7131 |
| Insecta | Coleoptera | Carabidae | Pterostichusvernalis (Panzer, 1796) | INTR | 25 | 25 | |||
| Insecta | Coleoptera | Chrysomelidae | Chaetocnemahortensis (Fourcroy, 1785) | INTR | 1 | 2 | 3 | ||
| Insecta | Coleoptera | Chrysomelidae | Chrysolinabankii (Fabricius, 1775) | NAT | 10 | 10 | |||
| Insecta | Coleoptera | Chrysomelidae | Epitrixcucumeris (Harris, 1851) | INTR | 53 | 4 | 57 | ||
| Insecta | Coleoptera | Chrysomelidae | Longitarsuskutscherai (Rye, 1872) | INTR | 1 | 1 | |||
| Insecta | Coleoptera | Coccinellidae | Scymniscushelgae (Fürsch, 1965) | INTR | 1 | 1 | |||
| Insecta | Coleoptera | Corylophidae | Sericoderuslateralis (Gyllenhal, 1827) | INTR | 15 | 61 | 268 | 96 | 440 |
| Insecta | Coleoptera | Curculionidae | Calacallessubcarinatus (Israelson, 1984) | END | 1 | 1 | |||
| Insecta | Coleoptera | Curculionidae | Cathormioceruscurvipes (Wollaston, 1854) | NAT | 18 | 18 | |||
| Insecta | Coleoptera | Curculionidae | Coccotrypescarpophagus (Hornung, 1842) | INTR | 71 | 3 | 2 | 76 | |
| Insecta | Coleoptera | Curculionidae | Naupactuscervinus (Boheman, 1840) | INTR | 4 | 4 | |||
| Insecta | Coleoptera | Curculionidae | Orthochaetesinsignis (Aubé, 1863) | NAT | 1 | 21 | 22 | ||
| Insecta | Coleoptera | Curculionidae | Otiorhynchuscribricollis Gyllenhal, 1834 | INTR | 5 | 5 | |||
| Insecta | Coleoptera | Curculionidae | Otiorhynchusrugosostriatus (Goeze, 1777) | INTR | 4 | 1 | 5 | ||
| Insecta | Coleoptera | Curculionidae | Pseudophloeophagustenax Wollaston, 1854 | NAT | 2 | 2 | |||
| Insecta | Coleoptera | Curculionidae | Xyleborinusalni Nijima, 1909 | INTR | 1 | 1 | |||
| Insecta | Coleoptera | Dryophthoridae | Cosmopolitessordidus (Germar, 1824) | INTR | 1 | 1 | |||
| Insecta | Coleoptera | Dryophthoridae | Sphenophorusabbreviatus (Fabricius, 1787) | INTR | 4 | 2 | 51 | 57 | |
| Insecta | Coleoptera | Elateridae | Aeolusmelliculusmoreleti Tarnier, 1860 | INTR | 8 | 8 | |||
| Insecta | Coleoptera | Elateridae | Heteroderesazoricus (Tarnier, 1860) | END | 2 | 1 | 3 | ||
| Insecta | Coleoptera | Elateridae | Heteroderesvagus Candèze, 1893 | INTR | 3 | 13 | 16 | ||
| Insecta | Coleoptera | Elateridae | Melanotusdichrous (Erichson, 1841) | INTR | 14 | 14 | |||
| Insecta | Coleoptera | Histeridae | Carcinopspumilio (Erichson, 1834) | INTR | 1 | 1 | |||
| Insecta | Coleoptera | Hydrophilidae | Sphaeridiumbipustulatum Fabricius, 1781 | INTR | 1 | 1 | 2 | ||
| Insecta | Coleoptera | Latridiidae | Cartoderenodifer (Westwood, 1839) | INTR | 2 | 1 | 3 | ||
| Insecta | Coleoptera | Leiodidae | Catopscoracinus Kellner, 1846 | NAT | 1 | 1 | |||
| Insecta | Coleoptera | Malachiidae | Attaluslusitanicuslusitanicus Erichson, 1840 | NAT | 2 | 2 | |||
| Insecta | Coleoptera | Mycetophagidae | Litargusbalteatus Le Conte, 1856 | INTR | 1 | 1 | 2 | ||
| Insecta | Coleoptera | Mycetophagidae | Typhaeastercorea (Linnaeus, 1758) | INTR | 1 | 642 | 5 | 648 | |
| Insecta | Coleoptera | Nitidulidae | Carpophilusfumatus Boheman, 1851 | INTR | 1 | 1 | |||
| Insecta | Coleoptera | Nitidulidae | Epuraeabiguttata (Thunberg, 1784) | INTR | 49 | 22 | 1 | 72 | |
| Insecta | Coleoptera | Nitidulidae | Phenolialimbatatibialis (Boheman, 1851) | INTR | 15 | 6 | 1 | 1 | 23 |
| Insecta | Coleoptera | Nitidulidae | Stelidotageminata (Say, 1825) | INTR | 128 | 18 | 146 | ||
| Insecta | Coleoptera | Phalacridae | Stilbustestaceus (Panzer, 1797) | NAT | 1 | 24 | 1 | 26 | |
| Insecta | Coleoptera | Ptiliidae | Ptenidiumpusillum (Gyllenhal, 1808) | INTR | 4 | 6 | 2 | 12 | |
| Insecta | Coleoptera | Scarabaeidae | Calamosternusgranarius (Linnaeus, 1767) | INTR | 7 | 7 | |||
| Insecta | Coleoptera | Scarabaeidae | Onthophagusvacca (Linnaeus, 1767) | INTR | 6 | 6 | |||
| Insecta | Coleoptera | Scarabaeidae | Popilliajaponica Newman, 1838 | INTR | 4 | 4 | |||
| Insecta | Coleoptera | Silvanidae | Cryptamorphadesjardinsii (Guérin-Méneville, 1844) | INTR | 3 | 3 | |||
| Insecta | Coleoptera | Staphylinidae | Aleocharabipustulata (Linnaeus, 1760) | INTR | 1 | 1 | 4 | 6 | |
| Insecta | Coleoptera | Staphylinidae | Aloconotasulcifrons (Stephens, 1832) | NAT | 11 | 11 | |||
| Insecta | Coleoptera | Staphylinidae | Amischaanalis (Gravenhorst, 1802) | INTR | 1 | 8 | 48 | 1321 | 1378 |
| Insecta | Coleoptera | Staphylinidae | Anotylusnitidifrons (Wollaston, 1871) | INTR | 10 | 377 | 4 | 8 | 399 |
| Insecta | Coleoptera | Staphylinidae | Anotylusnitidulus (Gravenhorst, 1802) | INTR | 2 | 2 | |||
| Insecta | Coleoptera | Staphylinidae | Astenuslyonessius (Joy, 1908) | NAT | 10 | 10 | |||
| Insecta | Coleoptera | Staphylinidae | Athetaaeneicollis (Sharp, 1869) | INTR | 1 | 2 | 3 | ||
| Insecta | Coleoptera | Staphylinidae | Athetafungi (Gravenhorst, 1806) | INTR | 1 | 76 | 66 | 49 | 192 |
| Insecta | Coleoptera | Staphylinidae | Carpelimuscorticinus (Gravenhorst, 1806) | NAT | 1 | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Coproporuspulchellus (Erichson, 1839) | INTR | 6 | 6 | |||
| Insecta | Coleoptera | Staphylinidae | Cordaliaobscura (Gravenhorst, 1802) | INTR | 20 | 17 | 256 | 316 | 609 |
| Insecta | Coleoptera | Staphylinidae | Euplectusinfirmus Raffray, 1910 | INTR | 1 | 2 | 3 | ||
| Insecta | Coleoptera | Staphylinidae | Gabriusnigritulus (Gravenhorst, 1802) | INTR | 2 | 3 | 5 | ||
| Insecta | Coleoptera | Staphylinidae | Medonapicalis (Kraatz, 1857) | NAT | 1 | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Ocypusaethiops (Waltl, 1835) | NAT | 308 | 1 | 309 | ||
| Insecta | Coleoptera | Staphylinidae | Ocypusolens (Müller, 1764) | NAT | 59 | 45 | 104 | ||
| Insecta | Coleoptera | Staphylinidae | Oligotapumilio Kiesenwetter, 1858 | NAT | 7 | 70 | 178 | 12 | 267 |
| Insecta | Coleoptera | Staphylinidae | Phloeonomuspunctipennis Thomson, 1867 | NAT | 1 | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Proteinusatomarius Erichson, 1840 | NAT | 10 | 10 | |||
| Insecta | Coleoptera | Staphylinidae | Pseudoplectusperplexus (Jacquelin du Val, 1854) | NAT | 22 | 4 | 41 | 67 | |
| Insecta | Coleoptera | Staphylinidae | Quediuscurtipennis Bernhauer, 1908 | NAT | 1 | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Rugilusorbiculatus (Paykull, 1789) | NAT | 2 | 365 | 757 | 1124 | |
| Insecta | Coleoptera | Staphylinidae | Sepedophiluslusitanicus Hammond, 1973 | NAT | 4 | 4 | |||
| Insecta | Coleoptera | Staphylinidae | Stenomastaxmaderae Assing, 2003 | NAT | 127 | 127 | |||
| Insecta | Coleoptera | Staphylinidae | Tachyporuschrysomelinus (Linnaeus, 1758) | INTR | 1 | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Tachyporusnitidulus (Fabricius, 1781) | INTR | 1 | 2 | 5 | 3 | 11 |
| Insecta | Coleoptera | Staphylinidae | Trichiusaimmigrata Lohse, 1984 | INTR | 3 | 3 | |||
| Insecta | Coleoptera | Staphylinidae | Xantholinuslongiventris Heer, 1839 | INTR | 3 | 1 | 4 | ||
| Insecta | Coleoptera | Tenebrionidae | Blaps lethifera Marsham, 1802 | INTR | 1 | 1 | |||
| Insecta | Coleoptera | Tenebrionidae | Lagriahirta (Linnaeus, 1758)* | INTR | 1 | 1 | |||
| Insecta | Dermaptera | Anisolabididae | Euborelliaannulipes (Lucas, 1847) | INTR | 2 | 116 | 26 | 144 | |
| Insecta | Dermaptera | Forficulidae | Forficulaauricularia Linnaeus, 1758 | INTR | 2 | 155 | 232 | 389 | |
| Insecta | Hemiptera | Anthocoridae | Anthocorisnemoralis (Fabricius, 1794) | NAT | 1 | 1 | |||
| Insecta | Hemiptera | Anthocoridae | Oriuslaevigatuslaevigatus (Fieber, 1860) | NAT | 1 | 1 | |||
| Insecta | Hemiptera | Aphididae | Rhopalosiphoninuslatysiphon (Davidson, 1912) | INTR | 6 | 43 | 49 | ||
| Insecta | Hemiptera | Cicadellidae | Anoscopusalbifrons (Linnaeus, 1758) | NAT | 1 | 3 | 6 | 10 | |
| Insecta | Hemiptera | Cicadellidae | Cicadellaviridis (Linnaeus, 1758) | INTR | 3 | 3 | |||
| Insecta | Hemiptera | Cicadellidae | Euscelidiusvariegatus (Kirschbaum, 1858) | NAT | 72 | 10 | 82 | ||
| Insecta | Hemiptera | Cicadellidae | Sophoniaorientalis (Matsumura, 1912) | INTR | 1 | 1 | |||
| Insecta | Hemiptera | Cydnidae | Geotomuspunctulatus (A. Costa, 1847) | NAT | 33 | 3 | 3 | 1 | 40 |
| Insecta | Hemiptera | Delphacidae | Kelisiaribauti Wagner, 1938 | NAT | 8 | 41 | 116 | 165 | |
| Insecta | Hemiptera | Delphacidae | Megamelodesquadrimaculatus (Signoret, 1865) | NAT | 1 | 1 | |||
| Insecta | Hemiptera | Lygaeidae | Aphanusrolandri (Linnaeus, 1758) | NAT | 7 | 3 | 10 | ||
| Insecta | Hemiptera | Lygaeidae | Heterogasterurticae (Fabricius, 1775) | NAT | 1 | 1 | |||
| Insecta | Hemiptera | Lygaeidae | Kleidocerysericae (Horváth, 1909) | NAT | 1 | 1 | |||
| Insecta | Hemiptera | Lygaeidae | Oxycarenuslavaterae (Fabricius, 1787) | INTR | 1 | 1 | |||
| Insecta | Hemiptera | Lygaeidae | Scolopostethusdecoratus (Hahn, 1833) | NAT | 6 | 33 | 1 | 1 | 41 |
| Insecta | Hemiptera | Microphysidae | Loriculaelegantula (Bärensprung, 1858) | NAT | 1 | 1 | |||
| Insecta | Hemiptera | Miridae | Campyloneuravirgula (Herrich-Schaeffer, 1835) | NAT | 1 | 1 | |||
| Insecta | Hemiptera | Miridae | Heterotomaplanicornis (Pallas, 1772) | NAT | 4 | 4 | |||
| Insecta | Hemiptera | Miridae | Pilophorusconfusus (Kirschbaum, 1856) | NAT | 1 | 1 | |||
| Insecta | Hemiptera | Miridae | Trigonotyluscaelestialium (Kirkaldy, 1902) | NAT | 493 | 231 | 724 | ||
| Insecta | Hemiptera | Nabidae | Nabispseudoferusibericus Remane, 1962 | NAT | 7 | 46 | 53 | ||
| Insecta | Hemiptera | Pentatomidae | Nezaraviridula (Linnaeus, 1758) | INTR | 5 | 6 | 11 | ||
| Insecta | Hemiptera | Reduviidae | Empicorisrubromaculatus (Blackburn, 1889) | INTR | 10 | 1 | 11 | ||
| Insecta | Hemiptera | Reduviidae | Ploiariadomestica Scopoli, 1786 | INTR | 1 | 1 | |||
| Insecta | Hemiptera | Saldidae | Saldulapalustris (Douglas, 1874) | NAT | 1 | 1 | |||
| Insecta | Hemiptera | Tingidae | Acalyptaparvula (Fallén, 1807) | NAT | 5 | 4 | 9 | ||
| Insecta | Hymenoptera | Apidae | Bombusterrestris (Linnaeus, 1758) | INTR | 1 | 1 | 2 | ||
| Insecta | Hymenoptera | Formicidae | Hypoponeraeduardi (Forel, 1894) | NAT | 12 | 32 | 37 | 99 | 180 |
| Insecta | Hymenoptera | Formicidae | Lasiusgrandis Forel, 1909 | NAT | 10283 | 3058 | 1444 | 1091 | 15876 |
| Insecta | Hymenoptera | Formicidae | Linepithemahumile (Mayr, 1868) | INTR | 2 | 2 | |||
| Insecta | Hymenoptera | Formicidae | Monomoriumcarbonarium (Smith, 1858) | NAT | 272 | 367 | 1 | 640 | |
| Insecta | Hymenoptera | Formicidae | Tetramoriumcaespitum (Linnaeus, 1758) | NAT | 327 | 1329 | 1202 | 451 | 3309 |
| Insecta | Hymenoptera | Formicidae | Tetramoriumcaldarium (Roger, 1857) | INTR | 215 | 135 | 1 | 351 | |
| Insecta | Lepidoptera | Noctuidae | Mythimnaunipuncta (Haworth, 1809) | NAT | 1 | 1 | |||
| Insecta | Orthoptera | Gryllidae | Eumodicogryllusbordigalensis (Latreille, 1804) | INTR | 1 | 1 | 1559 | 1561 | |
| Insecta | Orthoptera | Gryllidae | Gryllusbimaculatus De Geer, 1773 | INTR | 10 | 10 | |||
| Insecta | Orthoptera | Phaneropteridae | Phaneropteranana Fieber, 1853 | NAT | 2 | 2 | |||
| Insecta | Psocoptera | Caeciliusidae | Valenzuelaflavidus (Stephens, 1836) | NAT | 1 | 27 | 1 | 29 | |
| Insecta | Psocoptera | Ectopsocidae | Ectopsocusbriggsi McLachlan, 1899 | INTR | 1 | 28 | 18 | 47 | |
| Insecta | Psocoptera | Ectopsocidae | Ectopsocusstrauchi Enderlein, 1906 | NAT | 1 | 1 | |||
| Insecta | Psocoptera | Trichopsocidae | Trichopsocusclarus (Banks, 1908) | NAT | 2 | 2 | |||
| Insecta | Thysanoptera | Thripidae | Hercinothripsbicinctus (Bagnall, 1919) | INTR | 3 | 1 | 4 | ||
| Grand Total | 12763 | 10062 | 7622 | 16390 | 46837 |
The five most abundant species account for 64% of all identified specimens and include two ant species: Lasiusgrandis Forel, 1909 (Hymenoptera: Formicidae) (n = 15876) and Tetramoriumcaespitum (Linnaeus, 1758) (Hymenoptera: Formicidae) (n = 3309), the ground-beetle Pseudoophonusrufipes (De Geer, 1774) (Coleoptera, Carabidae (n = 7131), the millipede (Diplopoda: Julida) Ommatoiulusmoreleti (Lucas, 1860) (n = 2213) and the cricket (Orthoptera: Gryllidae) Eumodicogryllusbordigalensis (Latreille, 1804) (n = 1561).
Within the non-identified morphospecies, the most abundant taxa was a millipede (MF 1006) with 1959 specimens mostly sampled in high elevation maize fields (see Suppl. material 1).
Considering only identified species, a total of 10062 (21.48%), 7622 (16.27%), 16390 (34.99%) and 12763 (27.27%) specimens were collected and identified at species level in citrus orchards, low elevation maize fields, high elevation maize fields and vineyards, respectively (Table 2).
The most abundant species in vineyards were the native ant Lasiusgrandis (n = 10283), the introduced spider Zodarionatlanticum Pekár & Cardoso, 2005 (n = 934) and the native ant Tetramoriumcaespitum (n = 327) (Table 2).
The most abundant species in citrus orchards were the native ant L.grandis (n = 3058), the introduced millipede Ommatoiulusmoreleti (n = 1740) and the native ant T.caespitum (n = 1329) (Table 2).
The most abundant species in low elevation maize fields were also ants, L.grandis (n = 1444) and T.caespitum (n = 1202), followed by the exotic beetle Typhaeastercorea (Linnaeus, 1758) (n = 642) and the mirid bug Trigonotyluscaelestialium (Kirkaldy, 1902) (n = 493) (Table 2).
Finally, the most abundant species in high elevation maize fields were the introduced ground-beetle Pseudoophonusrufipes (n = 6995), the introduced cricket Eumodicogryllusbordigalensis (n = 1559), the two rove-beetles Amischaanalis (Gravenhorst, 1802) (n = 1321) and Rugilusorbiculatus (Paykull, 1789) (757) and also the ant L.grandis (n =1091). Two spiders usually very abundant in intensive pastures are also relatively abundant, Oedothoraxfuscus (Blackwall, 1834) (n = 577) and Erigonedentipalpis (Wider, 1834) (n = 484) (Table 2).
Although the introduced species potentially have the ability to colonise and spread in human-disturbed habitats (e.g. Rigal et al. 2017), our results showed that Azorean agroecosystems represent habitat opportunities for native arthropods. Some of the most abundant species are generalist predators with omnivorous behaviour, like the ants and the ground-beetle P.rufipes. Remarkable was the high abundance of the predatory spider Z.atlanticum in vineyards that feed on ants and may act as an ED provider. Most other predators potentially provide an ES to the Azorean agroecosystem habitats, particularly in maize fields and vineyards, through biological control of pests (e.g. Heimpel and Mills 2017). Introduced species can also affect native species of arthropods, for example, through opportunistic predation. However, introduced species may also supplement the functional traits lost after the decline of native species in these habitats (e.g. Stavert et al. 2018).
Five species are new records for Terceira Island: three beetles (Coleoptera), one millipede (Diplopoda: Julida) and one true bug (Hemiptera). The new beetle records included one specimen sampled of Lagriahirta (Linnaeus, 1758), eight of Ischnopterapionvirens (Herbst, 1797) and six of Microlestesnegritanegrita (Wollaston, 1854). All these individuals were collected in maize fields. The new millipede record included three specimens of Nopoiuluskochii (Gervais, 1847), also collected in maize fields, but at low elevation. Finally, the new hemipteran record included three specimens of Cicadellaviridis (Linnaeus, 1758) from a citrus orchard. All new records belong to introduced species, with the exception of M.negritanegrita, which is native to the Azores.
Lagriahirta (Coleoptera, Tenebrionidae) is a new record for Azores. We have also recently sampled this species in the Island of Santa Maria. This seems to be a recent introduction in Azores, being still rare in Terceira, but already widespread in Santa Maria.
Future perspectives
Importantly, the EU Biodiversity Strategy 2020 lists, as a priority, the mapping and assessment of the state of biodiversity, ecosystems and their services in all EU member states (Maes et al. 2016). Azores are part of Europe’s nine Outermost Regions (ORs) for which there is a general lack of ES mapping and assessment as compared with mainland Europe (Sieber et al. 2018).
By focusing on Azorean Island agroecosystems (e.g. maize fields, vineyards, citrus orchards) and having the current baseline monitoring data, we aim to develop in the near future a multifaceted approach to gain more insight to evaluate the relative importance of native and exotic arthropod organisms as ecosystem services (ES)/ ecosystem disservices (ED) providers. In this way, it will be possible to understand the ecosystem processes and functions and the goods and services arthropods provide for improving the resilience of Azorean agro-ecosystems, as well as human well-being.
Supplementary Material
Complete list of sampled species and mophospecies
Paulo A. V. Borges
Data type
Occurrences
Brief description
Detailed complete list of sampled species and mophospecies with indication of the morphospecies codes in the column (Identification Remarks)
File: oo_611247.xlsx
Acknowledgements
We thank all the farmers and landowners who permitted us to work on their properties: Adega Simas, Eleutério Nunes, Evangelho, Francisco Helvideo Barcelos, Marcelino Faria, Mozart Macedo Ávila, Narciso Borba, Paulo Ferreira, Ruben Barcelos, José Baldaya and Márcio.
This work was financed by FEDER (European Regional Development Fund) in 85% and by Azorean Public funds by 15% through the Operational Program Azores 2020, under the project AGRO-ECOSERVICES (ACORES-01-0145-FEDER-000073).
The Darwin-Core database was prepared within the scope of the project AZORESBIOPORTAL –PORBIOTA (ACORES-01-0145-FEDER-000072).
Funding Statement
FEDER (European Regional Development Fund) in 85% and by Azorean Public funds by 15% through Operational Program Azores 2020, under the project AGRO-ECOSERVICES (ACORES-01-0145-FEDER-000073)
Author contributions
PAVB, PM, DHL, AOS, AG, FR, GL and MF contributed to study conceptualisation. PAVB, LLL, RN, PM, DHL and MF performed the fieldwork. PAVB, RN and RC performed the species sorting and identification. PAVB, EP and LLL contributed to dataset preparation and data analysis. All authors contributed to manuscript writing.
References
- Allan Eric, Manning Pete, Alt Fabian, Binkenstein Julia, Blaser Stefan, Blüthgen Nico, Böhm Stefan, Grassein Fabrice, Hölzel Norbert, Klaus Valentin H., Kleinebecker Till, Morris E. Kathryn, Oelmann Yvonne, Prati Daniel, Renner Swen C., Rillig Matthias C., Schaefer Martin, Schloter Michael, Schmitt Barbara, Schöning Ingo, Schrumpf Marion, Solly Emily, Sorkau Elisabeth, Steckel Juliane, Steffen‐Dewenter Ingolf, Stempfhuber Barbara, Tschapka Marco, Weiner Christiane N., Weisser Wolfgang W., Werner Michael, Westphal Catrin, Wilcke Wolfgang, Fischer Markus. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecology Letters. 2015;18(8):834–843. doi: 10.1111/ele.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameixa O. M. C. C., Soares A. O., Soares A. M. V. M., Lillebø A. I. In: Selected studies in biodiversity. Şen B., Grillo O., editors. IntechOpen; 2018. Ecosystem services provided by the little things that run the world.267-302. [DOI] [Google Scholar]
- Aparício Bruno A., Cascalho José, Cruz Maria J., Borges P. A. V., Azevedo E. B., Elias R. B., Ascensão Fernando. Assessing the landscape functional connectivity using movement maps: a case study with endemic Azorean insects. Journal of Insect Conservation. 2018;22(2):257–265. doi: 10.1007/s10841-018-0059-7. [DOI] [Google Scholar]
- Barnosky Anthony D., Matzke Nicholas, Tomiya Susumu, Wogan Guinevere O. U., Swartz Brian, Quental Tiago B., Marshall Charles, McGuire Jenny L., Lindsey Emily L., Maguire Kaitlin C., Mersey Ben, Ferrer Elizabeth A. Has the Earth’s sixth mass extinction already arrived? Nature. 2011;471(7336):51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- Bennett Elena M, Cramer Wolfgang, Begossi Alpina, Cundill Georgina, Díaz Sandra, Egoh Benis N, Geijzendorffer Ilse R, Krug Cornelia B, Lavorel Sandra, Lazos Elena, Lebel Louis, Martín-López Berta, Meyfroidt Patrick, Mooney Harold A, Nel Jeanne L, Pascual Unai, Payet Karine, Harguindeguy Natalia Pérez, Peterson Garry D, Prieur-Richard Anne-Hélène, Reyers Belinda, Roebeling Peter, Seppelt Ralf, Solan Martin, Tschakert Petra, Tscharntke Teja, Turner BL, Verburg Peter H, Viglizzo Ernesto F, White Piran CL, Woodward Guy. Linking biodiversity, ecosystem services, and human well-being: three challenges for designing research for sustainability. Current Opinion in Environmental Sustainability. 2015;14:76–85. doi: 10.1016/j.cosust.2015.03.007. [DOI] [Google Scholar]
- Blackburn Tim M., Cassey Phillip, Duncan Richard P., Evans Karl L., Gaston Kevin J. Avian extinction and mammalian introductions on oceanic islands. Science. 2004;305(5692):1955–1958. doi: 10.1126/science.1101617. [DOI] [PubMed] [Google Scholar]
- Borges P. A. V., Aguiar C., Amaral J., Amorim I. R., André G., Arraiol A., Baz A., Dinis F., Enghoff H., Gaspar C., Ilharco F., Mahnert V., Melo C., Pereira F., Quartau J. A., Ribeiro S. P., Ribes J., Serrano A. R.M., Sousa A. B., Strassen R. Z., Vieira L., Vieira V., Vitorino A., Wunderlich J. Ranking protected areas in the Azores using standardised sampling of soil epigean arthropods. Biodiversity and Conservation. 2005;14(9):2029–2060. doi: 10.1007/s10531-004-4283-y. [DOI] [Google Scholar]
- Borges P. A. V., Lobo J. M., Azevedo E. B., Gaspar C. S., Melo C., Nunes L. V. Invasibility and species richness of island endemic arthropods: a general model of endemic vs. exotic species. Journal of Biogeography. 2006;33:169–187. doi: 10.1111/j.1365-2699.2005.01324.x. [DOI] [Google Scholar]
- Borges P. A. V., Vieira V., Amorim I. R, Bicudo N., et al. In: A list of the terrestrial and marine biota from the Azores. Borges P. A. V., Costa A., Cunha R., Gabriel R., et al., editors. Princípia; Cascais: 2010. List of arthropods (Arthropoda)179-246. English. [Google Scholar]
- Borges P. A. V., Gabriel R., Fattorini S. In: Life on land. Encyclopedia of the UN sustainable development goals. Leal-Filho W., Azul A., Brandli L., Özuyar P., Wall T., editors. The Springer Nature; Switzerland: 2019. Biodiversity erosion: causes and consequences.1-10. [DOI] [Google Scholar]
- Borges P. A. V., Santos A. M. C., Elias R. B., Gabriel R. In: Encyclopedia of the World's biomes-Earth systems and environmental sciences. , editor. Elsevier; Amsterdam, Netherlands: 2019. The Azores archipelago: biodiversity erosion and conservation biogeography.1-18. [DOI] [Google Scholar]
- Borges P. A. V., Lamelas-López L., Nunes R., Monjardino P., Lopes D. H., Soares A. O., Ferrante M. GBIF; 2021. Monitoring arthropods in Azorean agroecosystems: the project AGRO-ECOSERVICES. v.1.10. Universidade dos Açores. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso Pedro, Aranda S. C., Lobo J. M., Dinis Francisco, Gaspar Clara, Borges P. A. V. A spatial scale assessment of habitat effects on arthropod communities of an oceanic island. Acta Oecologica. 2009;35(5):590–597. doi: 10.1016/j.actao.2009.05.005. [DOI] [Google Scholar]
- Costanza Robert, d'Arge Ralph, de Groot Rudolf, Farber Stephen, Grasso Monica, Hannon Bruce, Limburg Karin, Naeem Shahid, O'Neill Robert V., Paruelo Jose, Raskin Robert G., Sutton Paul, van den Belt Marjan. The value of the world's ecosystem services and natural capital. Nature. 1997;387(6630):253–260. doi: 10.1038/387253a0. [DOI] [Google Scholar]
- Cruz J. V., Pereira R., Moreira A. Secretaria Regional do Ambiente. Direcção Regional do Ordenamento do território e dos Recursos Hídricos; 2007. Carta de ocupação do solo da região Autónoma dos Açores—projecto SUEMAC. [Google Scholar]
- Ecosystem Services Intergovernmental Science-Policy Platform On Biodiversity And. Summary for policymakers of the global assessment report on biodiversity and ecosystem services. Zenodo. 2019 doi: 10.5281/zenodo.3553458. [DOI]
- Ferreira M. T., Cardoso Pedro, Borges P. A. V., Gabriel Rosalina, de Azevedo E. B., Reis Francisco, Araújo M. B., Elias R. B. Effects of climate change on the distribution of indigenous species in oceanic islands (Azores) Climatic Change. 2016;138:603–615. doi: 10.1007/s10584-016-1754-6. [DOI] [Google Scholar]
- Florencio Margarita, Lobo J. M., Cardoso Pedro, Almeida-Neto Mário, Borges P. A. V. The colonisation of exotic species does not have to trigger faunal homogenisation: lessons from the assembly patterns of arthropods on oceanic islands. PLOS One. 2015;10(5):e0128276. doi: 10.1371/journal.pone.0128276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florencio Margarita, Patiño Jairo, Nogué Sandra, Traveset Anna, Borges P. A. V., Schaefer Hanno, Amorim I. R., Arnedo Miquel, Ávila S. P., Cardoso Pedro, de Nascimento Lea, Fernández-Palacios J. M., Gabriel S. I., Gil Artur, Gonçalves Vítor, Haroun Ricardo, Illera J. C., López-Darias Marta, Martínez Alejandro, Martins G. M., Neto A. I., Nogales Manuel, Oromí Pedro, Rando J. C., Raposeiro P. M., Rigal François, Romeiras M. M., Silva Luís, Valido Alfredo, Vanderpoorten Alain, Vasconcelos Raquel, Santos A. M. C. Macaronesia as a fruitful arena for ecology, evolution, and conservation biology. Frontiers in Ecology and Evolution. 2021;9(Article 718169) doi: 10.3389/fevo.2021.718169. [DOI] [Google Scholar]
- Frutuoso G. Saudades da terra. Published in 6 volumes from 1978 to 1983. Instituto Cultural de Ponta Delgada; Ponta Delgada: 2011. [Google Scholar]
- Gaspar C, Borges P. A. V., Gaston K. J. Diversity and distribution of arthropods in native forests of the Azores archipelago. https://repositorio.uac.pt/bitstream/10400.3/249/1/pp_1_30_Gaspar_etal_25.pdf Arquipelago Life and Marine Sciences. 2008;25:1–30. [Google Scholar]
- Gil Artur, Fonseca Catarina, Benedicto-Royuela José. Land cover trade-offs in small oceanic islands: a temporal analysis of Pico Island, Azores. Land Degradation & Development. 2017;29(2):349–360. doi: 10.1002/ldr.2770. [DOI] [Google Scholar]
- Harvey Jeffrey A., Heinen Robin, Armbrecht Inge, Basset Yves, Baxter-Gilbert James H., Bezemer T. Martijn, Böhm Monika, Bommarco Riccardo, Borges P. A. V., Cardoso Pedro, Clausnitzer Viola, Cornelisse Tara, Crone Elizabeth E., Dicke Marcel, Dijkstra Klaas-Douwe B., Dyer Lee, Ellers Jacintha, Fartmann Thomas, Forister Mathew L., Furlong Michael J., Garcia-Aguayo Andres, Gerlach Justin, Gols Rieta, Goulson Dave, Habel Jan-Christian, Haddad Nick M., Hallmann Caspar A., Henriques Sérgio, Herberstein Marie E., Hochkirch Axel, Hughes Alice C., Jepsen Sarina, Jones T. Hefin, Kaydan Bora M., Kleijn David, Klein Alexandra-Maria, Latty Tanya, Leather Simon R., Lewis Sara M., Lister Bradford C., Losey John E., Lowe Elizabeth C., Macadam Craig R., Montoya-Lerma James, Nagano Christopher D., Ogan Sophie, Orr Michael C., Painting Christina J., Pham Thai-Hong, Potts Simon G., Rauf Aunu, Roslin Tomas L., Samways Michael J., Sanchez-Bayo Francisco, Sar Sim A., Schultz Cheryl B., Soares António O., Thancharoen Anchana, Tscharntke Teja, Tylianakis Jason M., Umbers Kate D. L., Vet Louise E. M., Visser Marcel E., Vujic Ante, Wagner David L., WallisDeVries Michiel F., Westphal Catrin, White Thomas E., Wilkins Vicky L., Williams Paul H., Wyckhuys Kris A. G., Zhu Zeng-Rong, de Kroon Hans. International scientists formulate a roadmap for insect conservation and recovery. Nature Ecology & Evolution. 2020;4(2):174–176. doi: 10.1038/s41559-019-1079-8. [DOI] [PubMed] [Google Scholar]
- Heimpel G. E., Mills N. J. Biological control: ecology and applications. Cambridge University Press; 2017. 386. [DOI] [Google Scholar]
- Kier G., Kreft H., Lee T. M., Jetz W., Ibisch P. L., Nowicki C., Mutke J., Barthlott W. A global assessment of endemism and species richness across island and mainland regions. Proceedings of the National Academy of Sciences. 2009;106(23):9322–9327. doi: 10.1073/pnas.0810306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes D. J. H., Cabrera P. R., Borges P. A. V., Aguin-Pombo D., Pereira A. M. N., Mumford J. D., Mexia A. M. M. Folhas Divulgativas. 1st Editio. Centro de Biotecnologia dos Açores; Angra do Heroísmo: 2009. 192. [Google Scholar]
- Lövei Gábor L., Ferrante Marco. A review of the sentinel prey method as a way of quantifying invertebrate predation under field conditions. Insect Science. 2017;24(4):528–542. doi: 10.1111/1744-7917.12405. [DOI] [PubMed] [Google Scholar]
- Maes Joachim, Liquete Camino, Teller Anne, Erhard Markus, Paracchini M. L., Barredo J. I., Grizzetti Bruna, Cardoso Ana, Somma Francesca, Petersen Jan-Erik, Meiner Andrus, Gelabert E. R., Zal N., Kristensen P., Bastrup-Birk A., Biala K., Piroddi C., Egoh B., Degeorges P., Fiorina C., Santos-Martín F., Naruševičius V., Verboven Jan, Pereira H. M., Bengtsson Jan, Gocheva Kremena, Marta-Pedroso Cristina, Snäll Tord, Estreguil Christine, San-Miguel-Ayanz Jesus, Pérez-Soba Marta, Grêt-Regamey Adrienne, Lillebø A. I., Malak D. A., Condé Sophie, Moen Jon, Czúcz Bálint, Drakou E. G., Zulian Grazia, Lavalle Carlo. An indicator framework for assessing ecosystem services in support of the EU Biodiversity Strategy to 2020. Ecosystem Services. 2016;17:14–23. doi: 10.1016/j.ecoser.2015.10.023. [DOI] [Google Scholar]
- Marcelino José, Borges P. A. V., Borges Isabel, Pereira Enésima, Santos Vasco, Soares A. O. Standardised arthropod (Arthropoda) inventory across natural and anthropogenic impacted habitats in the Azores archipelago. Biodiversity Data Journal. 2021;9:e62157. doi: 10.3897/bdj.9.e62157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney Michael L, Lockwood Julie L. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends in Ecology & Evolution. 1999;14(11):450–453. doi: 10.1016/s0169-5347(99)01679-1. [DOI] [PubMed] [Google Scholar]
- Norder S. J., de Lima R. F., de Nascimento Lea, Lim J. Y., Fernández-Palacios J. M., Romeiras M. M., Elias R. B., Cabezas F. J., Catarino Luís, Ceríaco L. M. P., Castilla-Beltrán Alvaro, Gabriel Rosalina, de Sequeira M. M., Rijsdijk K. F., Nogué Sandra, Kissling W. D., van Loon E. E., Hall Marcus, Matos Margarida, Borges P. A. V. Global change in microcosms: environmental and societal predictors of land cover change on the Atlantic ocean islands. Anthropocene. 2020;30:10042. doi: 10.1016/j.ancene.2020.100242. [DOI] [Google Scholar]
- Noriega Jorge Ari, Hortal Joaquín, Azcárate Francisco M., Berg Matty P., Bonada Núria, Briones Maria J. I., Del Toro Israel, Goulson Dave, Ibanez Sébastien, Landis Douglas A., Moretti Marco, Potts Simon G., Slade Eleanor M., Stout Jane C., Ulyshen Michael D., Wackers Felix L., Woodcock Ben A., Santos Ana M. C. Research trends in ecosystem services provided by insects. Basic and Applied Ecology. 2018;26:8–23. doi: 10.1016/j.baae.2017.09.006. [DOI] [Google Scholar]
- Ribeiro S. P., Borges P. A. V., Gaspar Clara, Melo Catarina, Serrano A. R. M., Amaral João, Aguiar Carlos, André Genage, Quartau J. A. Canopy insect herbivores in the Azorean Laurisilva forests: key host plant species in a highly generalist insect community. Ecography. 2005;28(3):315–330. doi: 10.1111/j.0906-7590.2005.04104.x. [DOI] [Google Scholar]
- Rigal François, Cardoso Pedro, Lobo J. M., Triantis K. A., Whittaker R. J., Amorim I. R., Borges P. A. V. Functional traits of indigenous and exotic ground-dwelling arthropods show contrasting responses to land-use change in an oceanic island, Terceira, Azores. Diversity and Distributions. 2017;24(1):36–47. doi: 10.1111/ddi.12655. [DOI] [Google Scholar]
- Sax D. F. Latitudinal gradients and geographic ranges of exotic species: implications for biogeography. Journal of Biogeography. 2008;28(1):139–150. doi: 10.1046/j.1365-2699.2001.00536.x. [DOI] [Google Scholar]
- Shapiro Julie, Báldi András. Accurate accounting: How to balance ecosystem services and disservices. Ecosystem Services. 2014;7:201–202. doi: 10.1016/j.ecoser.2014.01.002. [DOI] [Google Scholar]
- Sieber I. M., Borges P. A. V., Burkhard Benjamin. Hotspots of biodiversity and ecosystem services: the Outermost Regions and Overseas Countries and Territories of the European Union. One Ecosystem. 2018;3 doi: 10.3897/oneeco.3.e24719. [DOI] [Google Scholar]
- Simberloff Daniel, Martin Jean-Louis, Genovesi Piero, Maris Virginie, Wardle David A., Aronson James, Courchamp Franck, Galil Bella, García-Berthou Emili, Pascal Michel, Pyšek Petr, Sousa Ronaldo, Tabacchi Eric, Vilà Montserrat. Impacts of biological invasions: what's what and the way forward. Trends in Ecology & Evolution. 2013;28(1):58–66. doi: 10.1016/j.tree.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Stachowicz J. J, Tilman D. In: Species invasions: insights into ecology, evolution, and biogeography. Sax D. F., Stachowicz J. J., Gaines S. D., editors. Sinauer Associates, Inc; Sunderland, Massachusetts: 2005. Species invasions and the relationships between species diversity, community saturation, and ecosystem functioning.41-64. [Google Scholar]
- Stavert Jamie R., Pattemore David E., Bartomeus Ignasi, Gaskett Anne C., Beggs Jacqueline R. Exotic flies maintain pollination services as native pollinators decline with agricultural expansion. Journal of Applied Ecology. 2018;55(4):1737–1746. doi: 10.1111/1365-2664.13103. [DOI] [Google Scholar]
- Triantis K. A., Borges P. A. V., Ladle R. J., Hortal Joaquín, Cardoso Pedro, Gaspar Clara, Dinis Francisco, Mendonça Enésima, Silveira L. M. A., Gabriel Rosalina, Melo Catarina, Santos A. M. C., Amorim I. R., Ribeiro S. P., Serrano A. R. M., Quartau J. A., Whittaker R. J. Extinction debt on oceanic islands. Ecography. 2010;33:285–294. doi: 10.1111/j.1600-0587.2010.06203.x. [DOI] [Google Scholar]
- Tsafack Noelline, Fattorini Simone, Boieiro Mário, Rigal François, Ros-Prieto Alejandra, Ferreira M. T., Borges P. A. V. The role of small lowland patches of exotic forests as refuges of rare endemic Azorean arthropods. Diversity. 2021;13(9):443. doi: 10.3390/d13090443. [DOI] [Google Scholar]
- Vilà Montserrat, Espinar J L., Hejda Martin, Hulme P. E., Jarošík Vojtěch, Maron J. L., Pergl Jan, Schaffner Urs, Sun Yan, Pyšek Petr. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecology Letters. 2011;14(7):702–708. doi: 10.1111/j.1461-0248.2011.01628.x. [DOI] [PubMed] [Google Scholar]
- Vitousek Peter M., Mooney Harold A., Lubchenco Jane, Melillo Jerry M. Human domination of Earth's ecosystems. Science. 1997;277(5325):494–499. doi: 10.1126/science.277.5325.494. [DOI] [Google Scholar]
- Zhang Wei, Ricketts Taylor H., Kremen Claire, Carney Karen, Swinton Scott M. Ecosystem services and dis-services to agriculture. Ecological Economics. 2007;64(2):253–260. doi: 10.1016/j.ecolecon.2007.02.024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete list of sampled species and mophospecies
Paulo A. V. Borges
Data type
Occurrences
Brief description
Detailed complete list of sampled species and mophospecies with indication of the morphospecies codes in the column (Identification Remarks)
File: oo_611247.xlsx