Abstract
Thermodynamic investigations provide information about the solute-solvent interactions in the selection of the proper solvent for different fields of pharmaceutical sciences. Especially, the study of antiepileptic drugs in solutions (ethanol/co-solvent) has been a subject of interest owing to their effect in the systems using interaction with a number of important biological membranes. This work focuses on the measurement of density and speed of sound of the phenytoin (PTH) in ethanol/deep eutectic solvents (choline chloride:ethylene glycol, and choline chloride:glycerol) solutions as the innovative class of green solvents at temperature range (288.15 to 318.15) K. It was determined Hansen solubility parameters for assessment of PTH interactions in the solvent media. Some thermophysical parameters including apparent molar volumes Vϕ, apparent molar isobaric expansion , and Hepler’s constant, apparent molar isentropic compressibility κφ were obtained and calculated using these data. To correlate the Vϕ and κφ values, the Redlich-Meyer equation was used to calculate the number of quantities containing standard partial molar volume and partial molar isentropic compressibility. Finally, values showed a strong interaction between PTH and solvent (ethanol/DES (ChCl:EG)). The thermodynamic analysis of the studied system also plays a crucial role in the pharmaceutical industry.
Subject terms: Pharmaceutics, Thermodynamics
Introduction
Extraction and recrystallization of pharmaceutical compounds are, by far, the most important step in the drug manufacturing processes. Poor solubility is a chief limitation to oral delivery of numerous emerging drugs and bioavailability is significantly affected by the drug solubility1,2. Phenytoin (PHT, Fig. 1) is an anti-epileptic drug, which is applied in the therapeutics. Phenytoin (PHT) is introduced as an anti-seizure drug as well as is proper for the snub of focal seizures and, tonic–clonic seizures but not absence seizures. It can also be utilized for some neuropathic pain or heart arrhythmias. It can be used mouth or intravenously3. The intravenous form generally begins within 30 min and is operational for 24 h. Blood levels can be measured to distinguish the appropriate dose4. This drug is categorized as a hydantoin derivative and despite its narrow therapeutic index, it is one of the most commonly used anticonvulsants. In addition, its applications are numerous such as an effective anti-epileptic, bipolar disorder, retina protection, and wound healing5. Low solubility of PHT has always presented major obstacle towards the development of extraction, re-crystallization and so drug delivery systems and the low solubility of PHT indicated the need of use the other solvents in these steps6,7.
Figure 1.
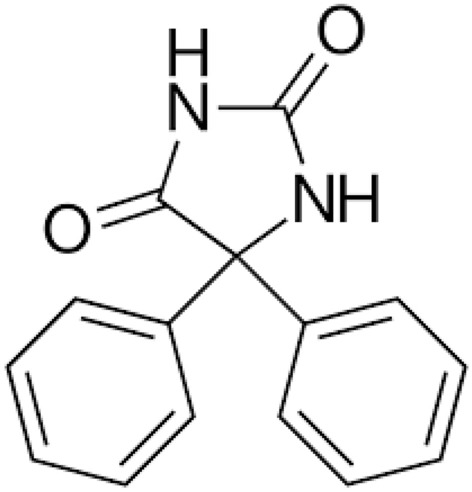
Molecular structure of Phenytoin (PTH).
The most common co-solvents for pharmaceutical compounds are organic solvents. However, the organic solvents applied in various sciences are usually flammable, toxic and volatile8,9. In contrast to conventional organic solvents, ionic liquids (ILs) and deep eutectic solvents (DESs) are considered environmentally benign “green and sustainable” solvents10. However, DESs exhibit similar physical and chemical properties of ILs and mostly DESs, especially the DESs used in this work are inexpensive to prepare, much less toxic, and are biocompatible and biodegradable. These green solvents were introduced and defined in 2003 and have many attractive potential applications in several fields11,12.
Solubility of several drugs in the presence of various DESs has been reported in our previous works, and the results show a significant increase in the solubility of drugs.
On the other hand, understanding the interactions of drugs in the solvent mixtures has been a topic of research to extract them from the basic media. Physicochemical and thermodynamic studies also attract researchers owing to the significant performance of drugs. The nature and the extent of the patterns of molecular interactions that exist in mixtures can be studied via physicochemical and thermodynamic investigations13,14. Thus, this research was aimed to represent the continuation of a systematic investigation of the volumetric properties of PHT in solvent mixtures at various temperatures T = (288.15, 298.15, 308.15 and 318.15) K. The derived thermophysical parameters including the apparent molar volume, , standard partial molar volume,, apparent molar isentropic compressibility, κφ, and infinite dilution apparent molar isentropic compressibility, values. Finally, Hansen solubility parameters for assessment of PTH interactions were used in the solvent media. These parameters can help to predict the solvent performance during the manufacturing processes and will be useful in explanation of solvent behavior in many other fields. The obtained parameters were used to survey the impact of the DES on the solute–solvent interactions in the systems of PHT.
Results and discussion
Density and speed of sound results
The apparent molar volumes Vϕ of binary PTH/ethanol and ternary PTH/ethanol/DESs (ChCl:Gly, and ChCl:EG) in diverse DES molalities (0.5, 1, and 1.5 mol kg-1) were calculated using the measurements of density d data. In the studied systems, the PTH is defined as a solute, and DESs are introduced as co-solvent. From the data in Table 1, it can be seen that the densities decrease with increasing temperature. The Eq. (1) was used to calculate the apparent molar volumes Vϕ:
| 1 |
where M (kg mol-1), and m (mol kg-1) are the molar mass and the molality of the PTH. The d0 (kg m-3) and d (kg m-3) are also density of solvent (ethanol and DESs + ethanol) and density of the solutions. The values of for the mentioned systems at all worked temperatures are given in Table 1. For the binary PTH + ethanol and ternary PTH/ethanol/DESs solutions, the values have a downward trend at all temperatures. Figure 2 indicate the values for binary PTH + ethanol and ternary PTH/ethanol/DESs (with molalities 0.5 and 1.5 mol kg-1) solutions at T = 298.15 K. The positive values of decreased with rising of the PTH molalities. The reduction in the values of with increasing temperature causes more attraction for DESs, which is evidence of strong interactions between PTH and solvent. According to the calculated results, it is clear that the values of also decreased with increasing DES amount. This behavior may be due to the attenuation of the interactions between PTH and the ethanol molecule that occur by increasing the concentrations of DESs. The intermolecular forces between PTH and ethanol are reinforced due to functional groups and various ionic groups in DESs.
Table 1.
The density (d) data and apparent molar volume () values for PTH molalities mPTH (mole of PTH per 1 kg of ethanol for binary system and mole of PTH per 1 kg of DESs/ethanol solutions for ternary system) in binary PTH/ethanol and ternary PTH/DESs (ChCl:Gly and ChCl:EG)/ethanol solutions at T = (288.15 to 318.15) K and ambient pressure (P = 871 hPa).
| m / mol kg-1 | 10–3 d / kg m-3 | 106/ m3 mol-1 | ||||||
|---|---|---|---|---|---|---|---|---|
| T / K | 288.15 | 298.15 | 308.15 | 318.15 | 288.15 | 298.15 | 308.15 | 318.15 |
| PTH in Ethanol | ||||||||
| 0.0206 | 0.795161 | 0.786702 | 0.777995 | 0.769197 | 200.21 | 198.89 | 197.64 | 196.37 |
| 0.0307 | 0.795919 | 0.787475 | 0.778778 | 0.769989 | 199.24 | 197.82 | 196.70 | 195.59 |
| 0.0411 | 0.796717 | 0.788287 | 0.779602 | 0.770821 | 198.23 | 196.81 | 195.67 | 194.69 |
| 0.0525 | 0.797591 | 0.789176 | 0.780517 | 0.771746 | 197.48 | 196.07 | 194.52 | 193.56 |
| 0.0606 | 0.798223 | 0.789823 | 0.781167 | 0.772410 | 196.63 | 195.11 | 193.76 | 192.62 |
| 0.0697 | 0.798931 | 0.790544 | 0.781894 | 0.773154 | 196.09 | 194.55 | 193.33 | 192.01 |
| PTH in ternary ethanol solution of ChCl:Gly (0.5 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0201 | 0.810656 | 0.802444 | 0.793945 | 0.785270 | 195.39 | 193.32 | 191.31 | 189.82 |
| 0.0293 | 0.811379 | 0.803183 | 0.794700 | 0.786041 | 193.85 | 191.80 | 189.75 | 188.02 |
| 0.0391 | 0.812170 | 0.804000 | 0.795532 | 0.786895 | 192.15 | 189.73 | 187.72 | 185.63 |
| 0.0516 | 0.813203 | 0.805059 | 0.796636 | 0.788012 | 190.16 | 187.68 | 184.89 | 182.99 |
| 0.0585 | 0.813801 | 0.805670 | 0.797236 | 0.788647 | 188.63 | 186.15 | 184.03 | 181.42 |
| 0.0697 | 0.814791 | 0.806701 | 0.798281 | 0.789699 | 186.43 | 183.49 | 181.44 | 179.12 |
| PTH in ternary ethanol solution of ChCl:Gly (1 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0202 | 0.824831 | 0.816802 | 0.808351 | 0.799892 | 192.34 | 190.05 | 188.65 | 187.00 |
| 0.0306 | 0.825657 | 0.817652 | 0.809218 | 0.800772 | 190.89 | 188.43 | 186.86 | 185.30 |
| 0.0423 | 0.826626 | 0.818637 | 0.810225 | 0.801790 | 188.66 | 186.47 | 184.69 | 183.28 |
| 0.0519 | 0.827441 | 0.819465 | 0.811072 | 0.802655 | 186.91 | 184.84 | 182.92 | 181.29 |
| 0.0596 | 0.828109 | 0.820158 | 0.811775 | 0.803356 | 185.59 | 183.21 | 181.32 | 180.00 |
| 0.0697 | 0.829002 | 0.821081 | 0.812688 | 0.804292 | 183.86 | 181.23 | 179.89 | 178.28 |
| PTH in ternary ethanol solution of ChCl:Gly (1.5 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0213 | 0.838607 | 0.830687 | 0.822476 | 0.814247 | 188.23 | 186.68 | 184.50 | 183.05 |
| 0.0302 | 0.839322 | 0.831420 | 0.823224 | 0.815011 | 187.47 | 185.70 | 183.60 | 181.94 |
| 0.0387 | 0.840032 | 0.832140 | 0.823956 | 0.815749 | 186.27 | 184.65 | 182.68 | 181.26 |
| 0.0494 | 0.840936 | 0.833053 | 0.824888 | 0.816713 | 184.76 | 183.34 | 181.35 | 179.35 |
| 0.0614 | 0.841944 | 0.834084 | 0.825932 | 0.817771 | 183.62 | 182.04 | 180.21 | 178.34 |
| 0.0700 | 0.842721 | 0.834849 | 0.826702 | 0.818554 | 182.02 | 180.93 | 179.27 | 177.40 |
| PTH in ternary ethanol solution of ChCl:EG (0.5 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0202 | 0.806469 | 0.797874 | 0.789627 | 0.780944 | 194.09 | 192.31 | 190.14 | 188.51 |
| 0.0306 | 0.807303 | 0.798724 | 0.790495 | 0.781826 | 192.19 | 190.42 | 188.23 | 186.59 |
| 0.0424 | 0.808281 | 0.799718 | 0.791511 | 0.782850 | 189.84 | 188.13 | 185.85 | 184.47 |
| 0.0520 | 0.809094 | 0.800553 | 0.792364 | 0.783728 | 188.42 | 186.45 | 184.12 | 182.27 |
| 0.0609 | 0.809858 | 0.801335 | 0.793149 | 0.784543 | 186.82 | 184.74 | 182.71 | 180.37 |
| 0.0691 | 0.810615 | 0.802094 | 0.793906 | 0.785305 | 184.68 | 182.83 | 181.12 | 178.95 |
| PTH in ternary ethanol solution of ChCl:EG (1 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0202 | 0.816446 | 0.808535 | 0.800168 | 0.791795 | 190.60 | 188.49 | 186.83 | 185.05 |
| 0.0306 | 0.817297 | 0.809394 | 0.801044 | 0.792680 | 188.75 | 187.15 | 185.38 | 183.91 |
| 0.0423 | 0.818276 | 0.810391 | 0.802044 | 0.793700 | 186.93 | 185.26 | 184.00 | 182.31 |
| 0.0519 | 0.819095 | 0.811217 | 0.802890 | 0.794563 | 185.48 | 184.00 | 182.46 | 180.62 |
| 0.0596 | 0.819768 | 0.811901 | 0.803584 | 0.795257 | 184.28 | 182.76 | 181.20 | 179.64 |
| 0.0697 | 0.820683 | 0.812810 | 0.804493 | 0.796180 | 182.32 | 181.20 | 179.92 | 178.31 |
| PTH in ternary ethanol solution of ChCl:EG (1.5 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0198 | 0.825866 | 0.818110 | 0.809971 | 0.801254 | 187.79 | 185.48 | 183.62 | 181.83 |
| 0.0313 | 0.826822 | 0.819067 | 0.810956 | 0.802249 | 185.98 | 184.68 | 182.34 | 180.91 |
| 0.0436 | 0.827856 | 0.820121 | 0.812015 | 0.803330 | 184.64 | 183.17 | 181.46 | 179.78 |
| 0.0539 | 0.828730 | 0.821015 | 0.812920 | 0.804255 | 183.70 | 182.05 | 180.44 | 178.58 |
| 0.0599 | 0.829255 | 0.821540 | 0.813449 | 0.804792 | 182.86 | 181.42 | 179.91 | 178.07 |
| 0.0683 | 0.829980 | 0.822265 | 0.814194 | 0.805547 | 182.09 | 180.88 | 179.17 | 177.37 |
Standard uncertainties (u) for each variable are u (T) = 0.001 K; u (m) = 0.0005 mol kg-1; u (p) = 10 hPa,
u (ρ) = 0.015 kg m-3.
Figure 2.
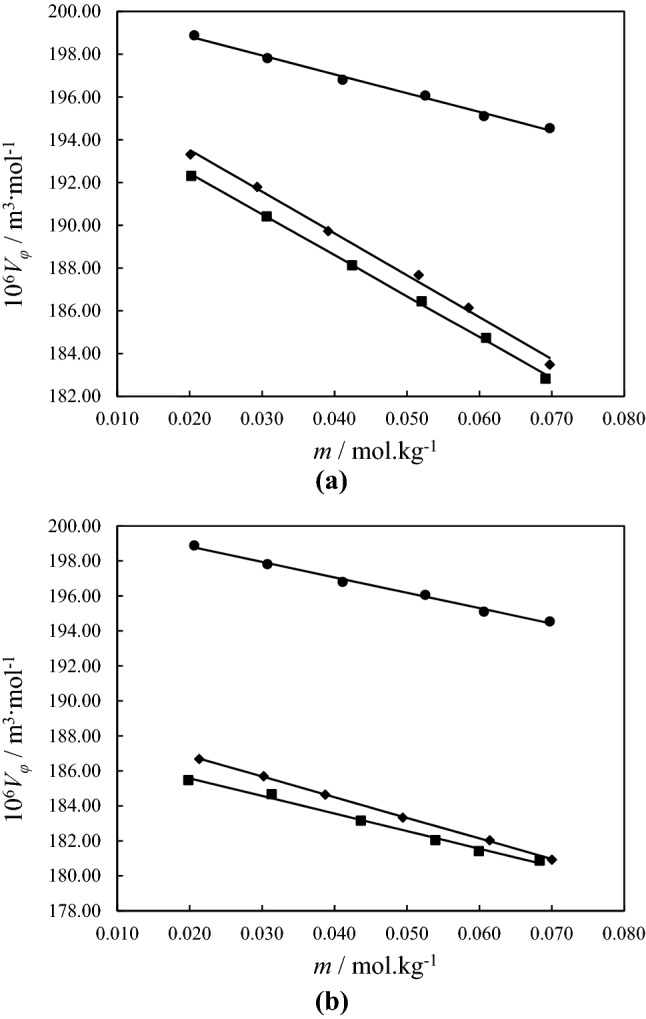
Apparent molar volumes,, of PTH in binary PTH/ethanol solutions and ternary PTH/DESs/ethanol solutions at T = 298.15 K; (a), ternary PTH/DESs/ethanol solutions with DESs molality = 0.5 mol/kg; (b), ternary PTH/DESs/ethanol solutions with DESs molality = 1.5 mol/kg; (filled black circle), binary PTH/ethanol solution; (filled black square), ternary PTH/ChCl:EG/ethanol; (filled black diamond), ternary PTH/ChCl:Gly/ethanol
The following relation, known as the Redlich-Meyer equation, is used to determine the standard partial molar volume for PTH15:
| 2 |
where Bv is the empirical parameter of the equation. The least-squares analysis was used to obtain the and Bv parameters, which were presented in Table 2. The obtained values of represent the solute–solvent interactions. In Fig. 3, variations of are demonstrated for each system at DESs molality m = 1 mol kg-1 versus the worked temperature. The obtained parameters show that the values are similar to the values decreasing with increasing temperature and decreasing with increasing DES molalities.
Table 2.
The parameters, , Bv, ∆trV0φ along standard deviations for the binary PTH/ethanol and ternary PTH/DESs/ethanol solutions at T = (288.15 to 318.15) K and at ambient pressure (P = 871 hPa).
| T / K | 106 V0φ / m3 mol-1 | 106 Bv / m3 kg mol-2 | 106 ∆trV0φ / m3 mol-1 | σ (Vφ) |
|---|---|---|---|---|
| PTH in ethanol | ||||
| 288.15 | 201.84 | − 84.14 | – | 0.13 |
| 298.15 | 200.58 | − 88.03 | – | 0.15 |
| 308.15 | 199.45 | − 91.11 | – | 0.17 |
| 318.15 | 198.35 | − 91.79 | – | 0.12 |
| PTH in ternary ethanol solution of ChCl:Gly (0.5 mol kg-1) | ||||
| 288.15 | 199.11 | − 178.97 | − 2.73 | 0.21 |
| 298.15 | 197.44 | − 195.52 | − 3.14 | 0.30 |
| 308.15 | 195.46 | − 199.76 | − 3.99 | 0.25 |
| 318.15 | 194.26 | − 218.22 | − 4.09 | 0.13 |
| PTH in ternary ethanol solution of ChCl:Gly (1 mol kg-1) | ||||
| 288.15 | 196.02 | − 174.54 | − 5.82 | 0.13 |
| 298.15 | 193.84 | − 177.88 | − 6.74 | 0.20 |
| 308.15 | 192.33 | − 180.89 | − 7.12 | 0.14 |
| 318.15 | 190.69 | − 178.60 | − 7.66 | 0.11 |
| PTH in ternary ethanol solution of ChCl:Gly (1.5 mol kg-1) | ||||
| 288.15 | 191.11 | − 126.55 | − 10.73 | 0.23 |
| 298.15 | 189.21 | − 117.86 | − 11.37 | 0.05 |
| 308.15 | 186.82 | − 108.20 | − 12.63 | 0.07 |
| 318.15 | 185.54 | − 117.68 | − 12.81 | 0.24 |
| PTH in ternary ethanol solution of ChCl:EG (0.5 mol kg-1) | ||||
| 288.15 | 197.91 | − 186.91 | − 3.93 | 0.26 |
| 298.15 | 196.25 | − 191.37 | − 4.33 | 0.15 |
| 308.15 | 193.80 | − 184.08 | − 5.65 | 0.12 |
| 318.15 | 192.65 | − 198.87 | − 5.70 | 0.16 |
| PTH in ternary ethanol solution of ChCl:EG (1 mol kg-1) | ||||
| 288.15 | 193.87 | − 163.62 | − 7.97 | 0.13 |
| 298.15 | 191.57 | − 147.81 | − 9.01 | 0.10 |
| 308.15 | 189.75 | − 141.07 | − 9.70 | 0.14 |
| 318.15 | 188.05 | − 140.08 | − 10.3 | 0.17 |
| PTH in ternary ethanol solution of ChCl:EG (1.5 mol kg-1) | ||||
| 288.15 | 189.81 | − 114.9 | − 12.03 | 0.21 |
| 298.15 | 187.58 | − 100.29 | − 13.00 | 0.18 |
| 308.15 | 185.32 | − 90.18 | − 14.13 | 0.10 |
| 318.15 | 183.78 | − 94.31 | − 14.57 | 0.10 |
Figure 3.
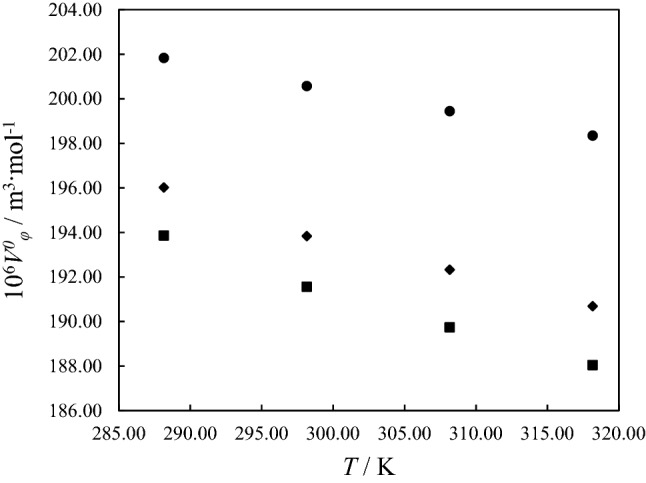
The comparison of the standard partial molar volumes,, of PTH in binary PTH/ethanol solutions and ternary PTH/DESs/ethanol solutions with DESs molality = 1 mol/kg at different temperatures: (filled black circle), binary PTH/ethanol solution; (filled black square), ternary PTH/ChCl:EG/ethanol; (filled black diamond), ternary PTH/ChCl:Gly/ethanol.
The partial molar transfer is another essential quantity to express useful information about interactions. The for PTH in the studied systems has been evaluated as follow:
| 3 |
The partial molar transfer volumes are listed in Table 2. Based on the developed model by Friedman and Krishnan16,17, the hydration cospheres overlap in the polar-nonpolar and nonpolar—nonpolar groups decreases the volume while the hydration cospheres overlap between polar groups or two ionic groups enhances volume. The obtained values for the systems studied in this work are negative and decrease with increasing in DESs molalities, which explains the superiority of nonpolar–nonpolar and polar—nonpolar interactions over the rest.
The polynomial equation was applied for the temperature dependence values as follow18:
| 4 |
where A, B and C are the parameters of the Eq. (4), which were given in Table 3. The apparent molar isobaric expansion was calculated using the derivative relative to the temperature of Eq. (4)19:
| 5 |
Table 3.
The parameters A, B, C and correlation coefficient for the temperature dependence of the values.
| Systems | Parameters | |||
|---|---|---|---|---|
| A | B | 102 C | R2 ()a | |
| PTH in ethanol | 271.93 | − 0.36 | 0.04 | 0.999 |
| PTH in ternary ethanol solution of ChCl:Gly (0.5 mol kg-1) | 354.51 | − 0.88 | 0.12 | 0.996 |
| PTH in ternary ethanol solution of ChCl:Gly (1 mol kg-1) | 370.17 | − 0.99 | 0.13 | 0.998 |
| PTH in ternary ethanol solution of ChCl:Gly (1.5 mol kg-1) | 385.93 | − 1.12 | 0.15 | 0.993 |
| PTH in ternary ethanol solution of ChCl:EG (0.5 mol kg-1) | 367.43 | − 0.96 | 0.13 | 0.987 |
| PTH in ternary ethanol solution of ChCl:EG (1 mol kg-1) | 386.92 | − 1.10 | 0.15 | 0.999 |
| PTH in ternary ethanol solution of ChCl:EG (1.5 mol kg-1) | 406.63 | − 1.25 | 0.17 | 0.998 |
Standard uncertainty (u) for DESs composition was estimated to be less than 5·10–2 mol ratio.
a Correlation coefficient for values.
The obtained values are reported in Table 4. The structure breaking or making behaviors of the various solutes can be interpreted with the values of that directly related to interactions20. The all binary PTH/ethanol and ternary PTH/ethanol/DESs have negative values. The obtained values for the PTH in the mentioned systems have been increased with rising temperatures.
Table 4.
The apparent molar isobaric expansions () and Hepler’s constants for binary PTH/ethanol and ternary PTH/ethanol/DESs solutions at T = (288.15 to 318.15) K and at ambient pressure (P = 871 hPa).
| Systems | 106 (m3.mol-1.K-1) | 102 | |||
|---|---|---|---|---|---|
| 288.15 K | 298.15 K | 308.15 K | 318.15 K | (m6.mol-2.K-2) | |
| PTH in ethanol | − 0.128 | − 0.120 | − 0.112 | − 0.104 | 0.08 |
| PTH in ternary ethanol solution of ChCl:Gly (0.5 mol kg-1) | − 0.2006 | − 0.1771 | − 0.1536 | − 0.1301 | 0.24 |
| PTH in ternary ethanol solution of ChCl:Gly (1 mol kg-1) | − 0.2155 | − 0.1885 | − 0.1615 | − 0.1345 | 0.27 |
| PTH in ternary ethanol solution of ChCl:Gly (1.5 mol kg-1) | − 0.2364 | − 0.2059 | − 0.1755 | − 0.1450 | 0.30 |
| PTH in ternary ethanol solution of ChCl:EG (0.5 mol kg-1) | − 0.2206 | − 0.1951 | − 0.1695 | − 0.1440 | 0.26 |
| PTH in ternary ethanol solution of ChCl:EG (1 mol kg-1) | − 0.2378 | − 0.2078 | − 0.1778 | − 0.1478 | 0.30 |
| PTH in ternary ethanol solution of ChCl:EG (1.5 mol kg-1) | − 0.2553 | − 0.2208 | − 0.1862 | − 0.1517 | 0.35 |
Standard uncertainty (u) for DESs composition was estimated to be less than 5·10–2 mol ratio.
The second derivative of relative to temperature is an important quantity to explain the structure breaking or making properties that developed by Hepler as follow21:
| 6 |
Table 4 reports the obtained values of for studied systems. The values of this constant for the all systems are positive that indicates the performance of PTH is as structure making in the presence of ethanol and DESs. The trend for PTH in the presence of DESs is as follows; ChCl:EG ˃ ChCl:Gly.
The experimental density and speed of sound data were used to calculate the isentropic compressibility, κs (Pa-1). This quantity is due to the resistance of the fluid to changes in pressure and consequently to changes in density and volume. Laplace-Newton’s equation was applied to compute κs as follow22:
| 7 |
where, the speed of sound is indicated by u. The partial molar isentropic compressibilities κφ, for binary PTH/ethanol and ternary PTH/ethanol/DESs solutions, are calculated as follow23:
| 8 |
where, κs0 is the isentropic compressibility of solvent. The calculated values of κφ were reported in Table 5. According to the results in Table 5, it can be seen that the κφ values decreased with increasing PTH molalities and also with increasing temperature. The interactions for PTH and solvent can also be explained using these values. Finally, the κφ values were correlated using the Redlich-Meyer equation as follow24.
| 9 |
where, κ0φ and Bκ are the partial isentropic compressibility and equation parameter, respectively. The obtained parameters are given in Table 6. Figure 4, shows the values of κ0φ versus the temperature. This quantity, like the expresses PTH-solvent interactions. The values are decreased with increasing temperature in the all studied systems.
Table 5.
Experimental speed of sounds u data and partial molar isentropic compressibility, values for PTH molalities mPTH (mole of PTH per 1 kg of ethanol for binary system and mole of PTH per 1 kg of DESs/ethanol solutions for ternary system) in binary PTH/ethanol and ternary PTH/DESs (ChCl:Gly and ChCl:EG)/ethanol solutions at T = (288.15 to 318.15) K and ambient pressure (P = 871 hPa).
| m / mol kg-1 | u / m s-1 | 1014 κφ / m3 mol-1 Pa-1 | ||||||
|---|---|---|---|---|---|---|---|---|
| T / K | 288.15 | 298.15 | 308.15 | 318.15 | 288.15 | 298.15 | 308.15 | 318.15 |
| PTH in Ethanol | ||||||||
| 0.0206 | 1178.32 | 1144.13 | 1110.23 | 1077.00 | − 2.58 | − 4.01 | − 5.11 | − 6.37 |
| 0.0307 | 1178.93 | 1144.73 | 1110.83 | 1077.55 | − 3.30 | − 4.68 | − 5.94 | − 6.95 |
| 0.0411 | 1179.60 | 1145.40 | 1111.50 | 1078.17 | − 3.93 | − 5.36 | − 6.76 | − 7.67 |
| 0.0525 | 1180.54 | 1146.27 | 1112.28 | 1078.92 | − 5.11 | − 6.37 | − 7.58 | − 8.61 |
| 0.0606 | 1181.12 | 1146.79 | 1112.88 | 1079.45 | − 5.49 | − 6.63 | − 8.22 | − 9.15 |
| 0.0697 | 1181.90 | 1147.49 | 1113.50 | 1080.11 | − 6.12 | − 7.14 | − 8.50 | − 9.79 |
| PTH in ternary ethanol solution of ChCl:Gly (0.5 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0201 | 1196.63 | 1163.50 | 1130.27 | 1096.99 | − 3.54 | − 4.50 | − 5.59 | − 6.12 |
| 0.0293 | 1197.19 | 1164.03 | 1130.75 | 1097.46 | − 3.96 | − 4.87 | − 5.76 | − 6.61 |
| 0.0391 | 1197.90 | 1164.71 | 1131.40 | 1098.03 | − 4.84 | − 5.87 | − 6.85 | − 7.59 |
| 0.0516 | 1198.89 | 1165.61 | 1132.23 | 1098.76 | − 5.89 | − 6.81 | − 7.89 | − 8.49 |
| 0.0585 | 1199.43 | 1166.08 | 1132.70 | 1099.20 | − 6.39 | − 7.20 | − 8.29 | − 9.10 |
| 0.0697 | 1200.35 | 1166.97 | 1133.44 | 1099.90 | − 7.15 | − 8.16 | − 8.97 | − 9.85 |
| PTH in ternary ethanol solution of ChCl:Gly (1 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0202 | 1213.28 | 1180.84 | 1147.98 | 1115.37 | − 4.40 | − 5.80 | − 7.27 | − 8.92 |
| 0.0306 | 1214.00 | 1181.60 | 1148.70 | 1116.05 | − 4.83 | − 6.53 | − 7.84 | − 9.26 |
| 0.0423 | 1214.82 | 1182.41 | 1149.50 | 1116.90 | − 5.33 | − 6.91 | − 8.31 | − 10.10 |
| 0.0519 | 1215.53 | 1183.14 | 1150.18 | 1117.55 | − 5.79 | − 7.44 | − 8.76 | − 10.50 |
| 0.0596 | 1216.05 | 1183.71 | 1150.75 | 1118.14 | − 5.97 | − 7.80 | − 9.19 | − 11.00 |
| 0.0697 | 1216.87 | 1184.53 | 1151.57 | 1118.90 | − 6.53 | − 8.41 | − 9.80 | − 11.53 |
| PTH in ternary ethanol solution of ChCl:Gly (1.5 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0213 | 1228.92 | 1196.85 | 1164.46 | 1132.73 | − 5.13 | − 6.80 | − 8.44 | − 10.16 |
| 0.0302 | 1229.58 | 1197.54 | 1165.15 | 1133.42 | − 5.35 | − 7.12 | − 8.77 | − 10.56 |
| 0.0387 | 1230.21 | 1198.25 | 1165.82 | 1134.09 | − 5.55 | − 7.56 | − 9.03 | − 10.80 |
| 0.0494 | 1231.05 | 1199.15 | 1166.71 | 1134.93 | − 5.97 | − 8.03 | − 9.54 | − 11.25 |
| 0.0614 | 1232.05 | 1200.16 | 1167.73 | 1135.93 | − 6.42 | − 8.43 | − 10.01 | − 11.71 |
| 0.0700 | 1232.69 | 1200.94 | 1168.45 | 1136.59 | − 6.62 | − 8.82 | − 10.26 | − 11.83 |
| PTH in ternary ethanol solution of ChCl:EG (0.5 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0202 | 1193.79 | 1159.65 | 1126.97 | 1093.46 | − 4.89 | − 5.27 | − 6.12 | − 6.61 |
| 0.0306 | 1194.49 | 1160.30 | 1127.55 | 1093.99 | − 5.48 | − 5.97 | − 6.63 | − 7.17 |
| 0.0424 | 1195.37 | 1161.06 | 1128.26 | 1094.64 | − 6.40 | − 6.68 | − 7.44 | − 7.99 |
| 0.0520 | 1196.14 | 1161.74 | 1128.85 | 1095.16 | − 7.04 | − 7.33 | − 7.96 | − 8.55 |
| 0.0609 | 1196.84 | 1162.43 | 1129.43 | 1095.67 | − 7.56 | − 8.10 | − 8.51 | − 9.16 |
| 0.0691 | 1197.58 | 1163.01 | 1130.00 | 1096.21 | − 8.32 | − 8.57 | − 9.10 | − 9.84 |
| PTH in ternary ethanol solution of ChCl:EG (1 mol kg-1) | ||||||||
| 0.0202 | 1207.46 | 1174.89 | 1142.08 | 1109.49 | − 5.72 | − 7.14 | − 8.92 | − 10.65 |
| 0.0306 | 1208.18 | 1175.62 | 1142.80 | 1110.23 | − 6.06 | − 7.50 | − 9.23 | − 11.15 |
| 0.0423 | 1208.98 | 1176.42 | 1143.60 | 1111.03 | − 6.33 | − 7.79 | − 9.44 | − 11.42 |
| 0.0519 | 1209.67 | 1177.11 | 1144.29 | 1111.68 | − 6.66 | − 8.11 | − 9.84 | − 11.70 |
| 0.0596 | 1210.18 | 1177.62 | 1144.81 | 1112.23 | − 6.77 | − 8.24 | − 10.01 | − 11.96 |
| 0.0697 | 1210.86 | 1178.3 | 1145.48 | 1112.91 | − 7.04 | − 8.45 | − 10.14 | − 12.15 |
| PTH in ternary ethanol solution of ChCl:EG (1.5 mol kg-1) | ||||||||
| 0.0000 | ||||||||
| 0.0198 | 1220.60 | 1188.97 | 1157.02 | 1123.70 | − 6.71 | − 8.27 | − 10.01 | − 12.08 |
| 0.0313 | 1221.53 | 1189.9 | 1157.95 | 1124.63 | − 7.25 | − 8.68 | − 10.54 | − 12.59 |
| 0.0436 | 1222.58 | 1190.95 | 1159 | 1125.68 | − 7.77 | − 9.28 | − 11.07 | − 13.23 |
| 0.0539 | 1223.44 | 1191.81 | 1159.86 | 1126.54 | − 8.01 | − 9.56 | − 11.36 | − 13.57 |
| 0.0599 | 1223.92 | 1192.29 | 1160.34 | 1127.02 | − 8.12 | − 9.64 | − 11.43 | − 13.63 |
| 0.0683 | 1224.71 | 1193.08 | 1161.13 | 1127.81 | − 8.50 | − 10.01 | − 11.87 | − 14.11 |
Standard uncertainties (u) for each variable are u (T) = 0.001 K; u (m) = 0.0005 mol kg-1; u (p) = 10 hPa.
The combined standard uncertainty for the average of n speed of sound measurements u (u) = 1 m s-1.
Standard uncertainty (u) for DESs composition was estimated to be less than 5·10–2 mol ratio.
am is the molality of PTH, mole of PTH per 1 kg of solvents.
Table 6.
The obtained partial molar isentropic compressibility κ0φ, experimental parameters Bk, and ∆trκ0φ along standard deviations σ (κφ) for binary PTH/ethanol and ternary PTH/ethanol/DESs solutions at T = (288.15 to 318.15) K and at ambient pressure (P = 871 hPa).
| T / K | 1014 κ0φ / m3 mol-1 Pa-1 | 1014 Bk / kg m3 mol-2 Pa-1 | 1014 ∆trκ0φ / m3 mol-1 Pa-1 | σ (κφ) |
|---|---|---|---|---|
| PTH in ethanol | ||||
| 288.15 | − 1.05 | − 73.63 | – | 0.12 |
| 298.15 | − 2.71 | − 65.09 | – | 0.13 |
| 308.15 | − 3.76 | − 71.08 | – | 0.16 |
| 318.15 | − 4.83 | − 71.05 | – | 0.07 |
| PTH in ternary ethanol solution of ChCl:Gly (0.5 mol kg-1) | ||||
| 288.15 | − 1.90 | − 75.83 | − 0.85 | 0.11 |
| 298.15 | − 2.86 | − 75.57 | − 0.15 | 0.13 |
| 308.15 | − 3.94 | − 73.47 | − 0.18 | 0.21 |
| 318.15 | − 4.49 | − 77.56 | 0.34 | 0.10 |
| PTH in ternary ethanol solution of ChCl:Gly (1 mol kg-1) | ||||
| 288.15 | − 3.54 | − 42.28 | − 2.49 | 0.06 |
| 298.15 | − 4.84 | − 50.47 | − 2.13 | 0.09 |
| 308.15 | − 6.26 | − 49.74 | − 2.50 | 0.07 |
| 318.15 | − 7.73 | − 54.31 | − 2.90 | 0.09 |
| PTH in ternary ethanol solution of ChCl:Gly (1.5 mol kg-1) | ||||
| 288.15 | − 4.40 | − 32.00 | − 3.35 | 0.06 |
| 298.15 | − 5.92 | − 41.48 | − 3.21 | 0.05 |
| 308.15 | − 7.60 | − 38.55 | − 3.84 | 0.05 |
| 318.15 | − 9.45 | − 35.34 | − 4.62 | 0.08 |
| PTH in ternary ethanol solution of ChCl:EG (0.5 mol kg-1) | ||||
| 288.15 | − 3.42 | − 69.69 | − 2.37 | 0.08 |
| 298.15 | − 3.87 | − 68.05 | − 1.16 | 0.07 |
| 308.15 | − 4.83 | − 60.95 | − 1.07 | 0.06 |
| 318.15 | − 5.22 | − 65.56 | − 0.39 | 0.08 |
| PTH in ternary ethanol solution of ChCl:EG (1 mol kg-1) | ||||
| 288.15 | − 5.22 | − 26.42 | − 4.17 | 0.04 |
| 298.15 | − 6.66 | − 26.60 | − 3.95 | 0.06 |
| 308.15 | − 8.42 | − 25.73 | − 4.66 | 0.07 |
| 318.15 | − 10.15 | − 29.64 | − 5.32 | 0.08 |
| PTH in ternary ethanol solution of ChCl:EG (1.5 mol kg-1) | ||||
| 288.15 | − 6.11 | − 35.13 | − 5.06 | 0.10 |
| 298.15 | − 7.61 | − 35.35 | − 4.90 | 0.08 |
| 308.15 | − 9.37 | − 36.41 | − 5.61 | 0.10 |
| 318.15 | − 11.33 | − 40.50 | − 6.50 | 0.10 |
Standard uncertainty (u) for DESs composition was estimated to be less than 5·10–2 mol ratio.
Figure 4.
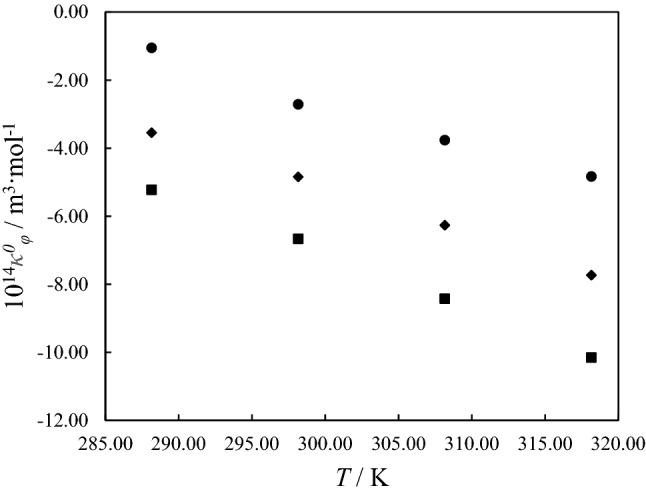
The comparison of the partial molar isentropic compressibility κ0φ, of PTH in binary PTH/ethanol solutions and ternary PTH/DESs/ethanol solutions with DESs molality = 1 mol/kg at different temperatures: (filled black circle), binary PTH/ethanol solution; (filled black square), ternary PTH/ChCl:EG/ethanol; (filled black diamond), ternary PTH/ChCl:Gly/ethanol.
The partial molar transfer isentropic compressibility for PTH in the systems is obtained as follow:
| 10 |
These values are listed in Table 8.
Table 8.
The calculated ∆δ for PTH drug and solvents (ethanol and ethanol/DESs).
| Systems solute | ethanol | ethanol/ChCl:Gly | ethanol/ChCl:EG |
|---|---|---|---|
| Phenytoin (PTH) | 19.832 | 19.363 | 19.066 |
The standard deviation (σ) is applied to check the adaptability of the experimental values by the obtained values with the Redlich-Meyer equation using the subsequent equation:
| 11 |
where , , n, and N are introduced as the experimental and calculated values of and values, the number of parameters and experimental points, respectively. The values of σ for the all studied systems are given in Tables 2 and 6.
Hansen solubility parameters results
Hansen solubility parameters are one of the most important methods for investigation of solute interaction in the presence of solvent. With these parameters, the appropriate solvent can be selected. Hildebrand first introduced solubility parameters that "similar solves similar"25. This parameter is modified by Hansen26 and is used as the Hildebrand-Hansen parameter. Solubility parameters are determined experimentally or by calculations as follow:
| 12 |
where ΔHvap, Vm, and Ecoh are the evaporation enthalpy, the molar volume and the intermolecular forces (adhesion energy), respectively. Also, R and T are the general constant of the gases and the temperature.
The introduced solubility parameter is expressed as follow; failure of hydrogen bonds between molecules (δh), adjacent intermolecular forces (bipolar interactions) (δp), and adhesion energy density, from the sum of energies required to overcome scattering forces (δd):
| 13 |
The mutual solubility between solute i and solvent j is calculated as follow:
| 14 |
to determine δh, δp, and δd, methods based on structural contributions of functional groups are used. Thus, δd is estimated from the following relation:
| 15 |
where Fd is the constant dispersion component of molar adsorption. The interactions of polar groups are also expressed by using the following equation:
| 16 |
where, Fp is the constant polar component of molar adsorption. δh can also be determined as follow:
| 17 |
where Eh is the hydrogen bond adhesion energy per structural group. Using the literature27, we can estimate the solubility parameters for DESs (ChCl:EG and ChCl:Gly), PTH and ethanol.
In this study, the parameters δd, δp and δh were estimated from sources and some were obtained using the Krevelen and Hoftyzer method28,29 for PTH drug, DESs and ethanol, which are collected in the Table 7. Differences between drug solubility parameter and solvents (ethanol and ethanol/DESs) are calculated from Eq. (14) and are reported in the Table 8. As can be seen from the results in Table 8, values indicating a strong interaction between PTH and solvent (ethanol/DES (ChCl:EG)) relative to others systems.
Table 7.
The calculated Hildebrand-Hansen solubility parameters for the materials used by Hoftyzer and Van Krevelen method29.
| Systems | δd | δp | δh | δt |
|---|---|---|---|---|
| PTH | 23.93 | 7.39 | 8.133 | 26.332 |
| Ethanol | 15.80 | 8.80 | 19.40 | 26.522 |
| Ethanol/DES (ChCl:Gly) | 17.31 | 5.05 | 22.07 | 28.496 |
| Ethanol/DES (ChCl:EG) | 16.47 | 4.86 | 19.74 | 26.169 |
Experimental
Chemicals
Choline chloride (GR, 0.998), ethylene glycol (GR, 0.999), glycerol (GR, 0.998), and ethanol (GR, 0.998) were purchused from Merck Co. Phenytoin (PTH) in mass fraction (> 0.99) is purchased from Daana Pharm. Co. (Tabriz, Iran). All chemicals used are reagent grade without further purification. Table 1 summarized the information of the chemicals applied in this work. It should be mentioned that the purity of the all chemicals is provided by the suppliers (Table 9).
Table 9.
A summary of the used chemicals.
| Chemical name | Abbreviation | Supplier | CAS No | Mass fraction (purity) | Structure |
|---|---|---|---|---|---|
| Phenytoin | PTH | Daana Pharm. Co. Iran | 57–41-0 | > 0.99 |
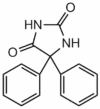
|
| Choline Chloride | ChCl | Merck | 67–48-1 | > 0.99 |
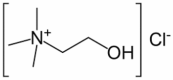
|
| Ethylene Glycol | EG | Merck | 107–21-1 | > 0.99 |
|
| Glycerol | Gly | Merck | 56–81-5 | > 0.99 |
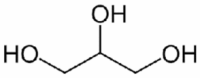
|
| Ethanol | − | Merck | 64–17-5 | > 0.99 |
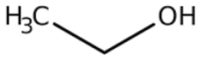
|
The suppliers were provided the purities of the used components.
The purified compounds of EG or Gly as HBDs and ChCl as HBA were mixed with the molar ratio 1:2 in the water bath at temperature about 333 K for 4 h until a colorless and homogeneous liquid formed11. For the prepared DESs composition, the uncertainty of less than 5·10–2 mol was estimated. Using the Karl − Fisher titration technique (method TitroLine KF), the water content was measured for the prepared DESs. Eventually, a vacuum pump was used to remove moisture and excess impurities of the DESs. Some of the properties of DESs (ChCl:Gly and ChCl:EG) are listed in Table 10.
Table 10.
Some of the physical properties of DESs (binary mixtures) used in the work at 298.15 K and pressure (p = 871 hPa).
| Molar ratio | Melting Point (K) | Water content (w%) | Molar mass (g mol-1)a | T / K | ρ / g cm-3 (Exp) | ρ / g cm-3 (Lit) | u / m s-1 (Exp) | u / m s-1 (Lit) | |
|---|---|---|---|---|---|---|---|---|---|
| ChCl:EG | 1:2 | 207.1530 | < 0.01% | 87.921 | 298.15 | 1.115551 | 1.11561631 | 1909.20 | 1909.6531 |
| 1.13832 | 1911.0430 | ||||||||
| 1905.133 | |||||||||
| 303.15 | 1.112750 | 1.11271531 | 1897.43 | 1897.4831 | |||||
| 1894.033 | |||||||||
| 1885.63 | 191432 | ||||||||
| 308.15 | 1.109927 | 1.10992731 | 1886.0831 | ||||||
| 1882.833 | |||||||||
| 313.15 | 1.107151 | 1.108434 | 1873.86 | 188232 | |||||
| 1.105735 | 1871.833 | ||||||||
| 318.15 | 1.104361 | 1.1052936 | 1861.58 | 1860.733 | |||||
| ChCl:Gly | 1:2 | 233.1530 | 0.06% | 107.937 | 298.15 | 1.186358 | 1.192037 | 2012.42 | 2012.5930 |
| 1.1908538 | |||||||||
| 1.18132 | |||||||||
| 1.1957539 | 2001.2939 | ||||||||
| 303.15 | 1.183556 | 1.189537 | 2001.05 | 208032 | |||||
| 1.1880738 | |||||||||
| 1.1929039 | 1990.2339 | ||||||||
| 308.15 | 1.180849 | 1.186737 | 1989.90 | ||||||
| 1.1852838 | |||||||||
| 1.1901539 | 1979.2439 | ||||||||
| 313.15 | 1.178128 | 1.183837 | 1978.98 | 197632 | |||||
| 1.1824938 | |||||||||
| 1.1874039 | 1968.3039 | ||||||||
| 318.15 | 1.175437 | 1.181437 | 1967.90 | ||||||
| 1.1797038 | |||||||||
| 1.1846539 | 1957.3839 |
Standard uncertainties (u) for each variable are u (T) = 0.001 K; u (p) = 10 hPa.
The combined standard uncertainty for the average of n density measurements u (ρ) = 0.015 kg m-3 and speed of sound u (u) = 1 m s-1.
Standard uncertainty (u) for DESs composition was estimated to be less than 5·10–2 mol ratio.
a Molar mass of DESs = x1 M1 + x2 M2.
x1 and M1; mole fraction and molar mass of ChCl.
x2 and M2; mole fraction and molar mass of HBD.
The melting point is expressed for the solidus (formation of the first liquid) or liquids (disappearance of last crystals).
The density and speed of sound were measured for the liquid state of the prepared DESs.
Apparatus and procedure
All solutions were prepared by filling tight glass vials, which are containing different amounts of the PTH in the water and ternary DESs solutions. In this regard, an analytical balance with precision 10–4 g (AW 220, GR220, Shimadzu, Japan) was used.
The molality of PTH was introduced as follows; mole of PTH per kg of solvent (binary in ethanol and ternary in DESs/ethanol solutions). For all of the prepared solutions, the uncertainty was estimated to be less than 5·10–4 mol·kg-1.
Density and speed of sound measuring device of Anton Paar Co. (with model DSA 5000, Austria) at the frequency (approximately 3 MHz) was utilized for all the binary (PTH/ethanol) and ternary (PTH/DESs/ethanol) solutions. After washing the device with deionized water and ethanol and drying with air, the device was calibrated using degassed and deionized water at the T = 293.15 K and atmospheric pressure. A Peltier device embedded inside the apparatus has been utilized to keep the temperature of the samples with an accuracy of 0.001 K. The standard uncertainties for density and speed of sound measurements were estimated to be 0.015 kg m-3 and 1 m s-1, respectively20. The measured data for the DESs used in this work were compared with the data reported in the literature and are given in Table 10. The data are well matched and in an acceptable range. Uncertainties are also given for the data reported in the relevant tables.
Conclusions
The most important part of drug preparation and production is the investigation of the interactions that occur between the drug and the solvent. In this regard, the volumetric and compressibility properties were applied to describe these interactions. As can be understood from the results of and values, the interaction between PTH and ethanol molecules has increased with increasing DESs molality and temperature. The results represent stronger interactions for DES (ChCl:EG). The Hepler values for the systems are positive that indicating the performance of PTH is studied as structure making in ethanol and in the presence of DESs solutions. The trend of this behavior for the PTH in presence of DESs as follows: ChCl:EG ˃ ChCl:Gly. The experimental results and the Hansen solubility parameters are very well compatible. Experimental and calculations results indicating a strong interaction between PTH and solvent (ethanol:DES (ChCl:EG m = 1.5 mol kg-1)) than the other systems.
Acknowledgements
We are grateful to University of Tabriz research council (project S/ 860) for the financial support of this research.
Author contributions
All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muñoz, M. M., Jouyban, A. & Martínez, F. Solubility and preferential solvation of acetaminophen in methanol+ water mixtures at 298.15 K. Phys. Chem. Liq.54, 515–528 (2016).
- 2.Shekaari, H., Zafarani-Moattar, M. T., Shayanfar, A. & Mokhtarpour, M. Effect of choline chloride/ethylene glycol or glycerol as deep eutectic solvents on the solubility and thermodynamic properties of acetaminophen. J. Mol. Liq. (2017).
- 3.Rao, R. & Bal, S. Int. J. Curr. Adv. (2017).
- 4.Marx J, Hockberger R, Walls R. Rosen's Emergency Medicine-Concepts and Clinical Practice E-Book: 2-Volume Set. Elsevier; 2013. [Google Scholar]
- 5.Thorn CF, Whirl-Carrillo M, Leeder JS, Klein TE, Altman RB. Pharm GKB summary: phenytoin pathway. Pharmacogenet. Genom. 2012;22:466. doi: 10.1097/FPC.0b013e32834aeedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouyban, A., Panahi-Aza, V., AA Fakhree, M. & Ahmadian, S. Solubility of phenytoin in aqueous mixtures of ethanol and sodium dodecyl sulfate at 298 K. Rev. Colomb. Cienc. Quim. Farm.43, 153–161 (2014).
- 7.Dagenais R, Wilby KJ, Elewa H, Ensom MH. Impact of genetic polymorphisms on phenytoin pharmacokinetics and clinical outcomes in the Middle East and North Africa Region. Drugs R&D. 2017;17:341–361. doi: 10.1007/s40268-017-0195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai Y, Rozema E, Verpoorte R, Choi YH. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A. 2016;1434:50–56. doi: 10.1016/j.chroma.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Alonso DA, et al. Deep eutectic solvents: the organic reaction medium of the century. Eur. J. Org. Chem. 2016;2016:612–632. doi: 10.1002/ejoc.201501197. [DOI] [Google Scholar]
- 10.Troter DZ, Todorović ZB, Đokić-Stojanović DR, Stamenković OS, Veljković VB. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016;61:473–500. doi: 10.1016/j.rser.2016.04.011. [DOI] [Google Scholar]
- 11.Abbott AP, et al. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011;13:82–90. doi: 10.1039/C0GC00395F. [DOI] [Google Scholar]
- 12.Smith EL, Abbott AP, Ryder KS. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014;114:11060–11082. doi: 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- 13.Dhondge SS, Zodape SP, Parwate DV. Volumetric and viscometric studies of some drugs in aqueous solutions at different temperatures. J. Chem. Thermodyn. 2012;48:207–212. doi: 10.1016/j.jct.2011.12.022. [DOI] [Google Scholar]
- 14.Pal, A. & Soni, S. Volumetric properties of glycine in aqueous solutions of some sulfa drugs at (288.15, 298.15, and 308.15) K. J. Chem. Eng. Data58, 18–23 (2013).
- 15.Redlich O, Meyer DM. The molal volumes of electrolytes. Chem. Rev. 1964;64:221–227. doi: 10.1021/cr60229a001. [DOI] [Google Scholar]
- 16.Friedman H, Krishnan C. & Franks, F. Plenum Press; 1973. [Google Scholar]
- 17.Shekaari, H. & Kazempour, A. Effect of ionic liquid, 1-octyl-3-methylimidazolium bromide on the thermophysical properties of aqueous d-glucose solutions at 298.15 K. Fluid Phase Equilib.309, 1–7 (2011).
- 18.Zafarani-Moattar, M. T. & Shekaari, H. Apparent molar volume and isentropic compressibility of ionic liquid 1-butyl-3-methylimidazolium bromide in water, methanol, and ethanol at T=(298.15 to 318.15) K. J. Chem. Thermodyn.37, 1029–1035 (2005).
- 19.Shekaari, H. & Armanfar, E. Apparent molar volumes and expansivities of aqueous solutions of ionic liquids, l-alkyl-3-methylimidazolium alkyl sulfate at T=(298.15–328.15) K. Fluid Phase Equilib.303, 120–125 (2011).
- 20.Shekaari, H., Zafarani-Moattar, M. T. & Mirheydari, S. N. Thermodynamic study of aspirin in the presence of ionic liquid, 1-hexyl-3-methylimidazolium bromide in acetonitrile at T=(288.15 to 318.15) K. J. Mol. Liq.209, 138–148 (2015).
- 21.Hepler LG. Thermal expansion and structure in water and aqueous solutions. Canad. J. Chem. 1969;47:4613–4617. doi: 10.1139/v69-762. [DOI] [Google Scholar]
- 22.Majdan-Cegincara R, Zafarani-Moattar MT, Shekaari H. The study of solute–solvent interactions in 1-butyl-1-methylpyrrolidinium trifluoromethanesulfonate+ acetonitrile from solvent activity, density, speed of sound, viscosity, electrical conductivity and refractive index measurements. J. Mol. Liq. 2015;203:198–203. doi: 10.1016/j.molliq.2014.12.048. [DOI] [Google Scholar]
- 23.Kumar H, Singla M, Katal A. Effect of N-acetylglycine on volumetric, acoustic and viscometric behavior of aqueous amoxicillin solutions. Thermochim. Acta. 2014;583:49–58. doi: 10.1016/j.tca.2014.03.013. [DOI] [Google Scholar]
- 24.Dey NC, Bhuyan J, Haque I. Partial molar volumes and partial molar adiabatic compressibilities of Fe (III) tetrafluoroborate complexes with DMSO, pyridine, and pyridine derivatives. J. Solution Chem. 2003;32:547–558. doi: 10.1023/A:1025318017188. [DOI] [Google Scholar]
- 25.Hildebrand J, Scott R. Solutions of nonelectrolytes. Ann. Rev. Phys. Chem. 1950;1:75–92. doi: 10.1146/annurev.pc.01.100150.000451. [DOI] [Google Scholar]
- 26.Charles, M. (CRC Press: New York, 1998).
- 27.Just S, Sievert F, Thommes M, Breitkreutz J. Improved group contribution parameter set for the application of solubility parameters to melt extrusion. Eur. J. Pharm. Biopharm. 2013;85:1191–1199. doi: 10.1016/j.ejpb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Van Krevelen DW, Te Nijenhuis K. Properties of polymers: their correlation with chemical structure; their numerical estimation and prediction from additive group contributions. Elsevier; 2009. [Google Scholar]
- 29.Van Krevelen D. & Hoftyzer, P. Elsevier; 1976. [Google Scholar]
- 30.Shekaari H, Zafarani-Moattar MT, Shayanfar A, Mokhtarpour M. Effect of choline chloride/ethylene glycol or glycerol as deep eutectic solvents on the solubility and thermodynamic properties of acetaminophen. J. Mol. Liq. 2018;249:1222–1235. doi: 10.1016/j.molliq.2017.11.057. [DOI] [Google Scholar]
- 31.Shekaari, H., Zafarani-Moattar, M. T., Mokhtarpour, M. & Faraji, S. Volumetric and compressibility properties for aqueous solutions of choline chloride based deep eutectic solvents and Prigogine–Flory–Patterson theory to correlate of excess molar volumes at T=(293.15 to 308.15) K. J. Mol. Liq. 111077 (2019).
- 32.Mjalli FS, Jabbar NMA. Acoustic investigation of choline chloride based ionic liquids analogs. Fluid Phase Equilib. 2014;381:71–76. doi: 10.1016/j.fluid.2014.08.017. [DOI] [Google Scholar]
- 33.Lapeña D, Lomba L, Artal M, Lafuente C, Giner B. Thermophysical characterization of the deep eutectic solvent choline chloride: ethylene glycol and one of its mixtures with water. Fluid Phase Equilib. 2019;492:1–9. doi: 10.1016/j.fluid.2019.03.018. [DOI] [Google Scholar]
- 34.Leron, R. B., Soriano, A. N. & Li, M.-H. Densities and refractive indices of the deep eutectic solvents (choline chloride+ ethylene glycol or glycerol) and their aqueous mixtures at the temperature ranging from 298.15 to 333.15 K. J. Taiwan Inst. Chem. Eng.43, 551–557 (2012).
- 35.Yadav, A., Kar, J. R., Verma, M., Naqvi, S. & Pandey, S. Densities of aqueous mixtures of (choline chloride+ ethylene glycol) and (choline chloride+ malonic acid) deep eutectic solvents in temperature range 283.15–363.15 K. Thermochim. Acta600, 95–101 (2015).
- 36.Harifi-Mood AR, Buchner R. Density, viscosity, and conductivity of choline chloride+ ethylene glycol as a deep eutectic solvent and its binary mixtures with dimethyl sulfoxide. J. Mol. Liq. 2017;225:689–695. doi: 10.1016/j.molliq.2016.10.115. [DOI] [Google Scholar]
- 37.Shahbaz K, Baroutian S, Mjalli F, Hashim M, AlNashef I. Densities of ammonium and phosphonium based deep eutectic solvents: Prediction using artificial intelligence and group contribution techniques. Thermochim. Acta. 2012;527:59–66. doi: 10.1016/j.tca.2011.10.010. [DOI] [Google Scholar]
- 38.Chemat F, You HJ, Muthukumar K, Murugesan T. Effect of l-arginine on the physical properties of choline chloride and glycerol based deep eutectic solvents. J. Mol. Liq. 2015;212:605–611. doi: 10.1016/j.molliq.2015.10.016. [DOI] [Google Scholar]
- 39.Lapeña D, Lomba L, Artal M, Lafuente C, Giner B. The NADES glyceline as a potential Green Solvent: A comprehensive study of its thermophysical properties and effect of water inclusion. J. Chem. Thermodyn. 2019;128:164–172. doi: 10.1016/j.jct.2018.07.031. [DOI] [Google Scholar]


