Abstract
Pannexin 3 (PANX3) is a member of the pannexin family of single membrane channel-forming glycoproteins. Originally thought to have a limited localization in cartilage, bone, and skin, PANX3 has now been detected in a variety of other tissues including skeletal muscle, mammary glands, the male reproductive tract, the cochlea, blood vessels, small intestines, teeth, and the vomeronasal organ. In many cell types of the musculoskeletal system, such as osteoblasts, chondrocytes, and odontoblasts, PANX3 has been shown to regulate the balance of proliferation and differentiation. PANX3 can be induced during progenitor cell differentiation, functioning at the cell surface as a conduit for ATP and/or in the endoplasmic reticulum as a calcium leak channel. Evidence in osteoblasts and monocytes also highlight a role for PANX3 in purinergic signalling through its function as an ATP release channel. PANX3 is critical in the development and ageing of bone and cartilage, with its levels temporally regulated in other tissues such as skeletal muscle, skin, and the cochlea. In diseases such as osteoarthritis and intervertebral disc degeneration, PANX3 can have either protective or detrimental roles depending on if the disease is age-related or injury-induced. This review will discuss PANX3 function in tissue growth and regeneration, its role in cellular differentiation, and how it becomes dysregulated in disease conditions such as obesity, Duchenne’s muscular dystrophy, osteosarcoma, and non-melanoma skin cancer, where most of the findings on PANX3 function can be attributed to the characterization of Panx3 KO mouse models.
Keywords: Pannexin, PANX3, Purinergic signalling, ATP
Pannexins
Pannexin 1, 2, and 3 (PANX1, PANX2, PANX3) make up the family of integral membrane, channel-forming glycoproteins discovered due to their homology to the invertebrate gap junction proteins innexins [1]. This review will focus on the less extensively studied family member PANX3, covering its channel function and signalling, tissue distribution and roles in development and repair, knockout (KO) mouse models, and dysregulation in disease. PANX3 channels function to facilitate the passage of signalling molecules such as ATP at the cell surface and Ca2+ intracellularly (Fig. 1), contributing to cellular processes such as differentiation [2, 3]. Therefore, PANX3 needs to be considered as an additional channel that contributes to purinergic signalling [2, 4].
Fig. 1.
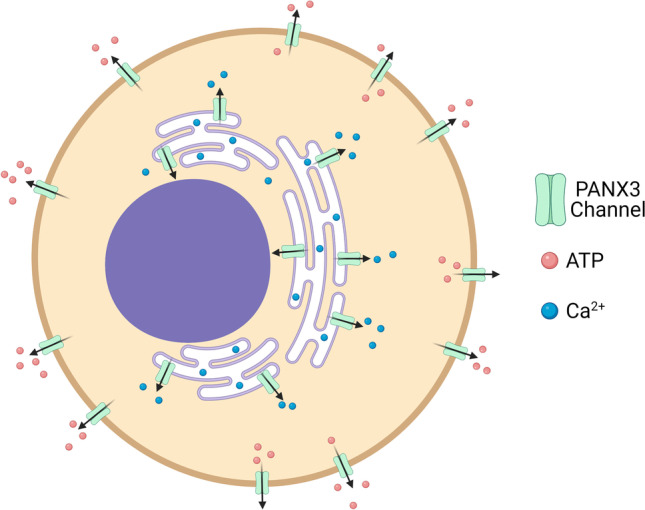
Schematic of PANX3 localization and channel function. PANX3 can oligomerize to form single membrane channels (green channel) that have been shown to localize at both the cell surface and endoplasmic reticulum in many cell types. Important in cellular communication, PANX3 channels allow the passage of metabolites such as ATP (pink sphere) into the extracellular space and Ca2+ (blue sphere) into the cytoplasm. Created with BioRender.com
Pannexin 3 overview
The Panx3 gene is located on chromosome 9 in mice [5] and 11 (11q24.2) in humans [6]. In both species, it consists of 4 exons and 3 introns [5, 6] and encodes the PANX3 protein that has 93% conservation between mouse and human [1, 6–8]. As the smallest member of the family, PANX3 has 392 amino acids with a molecular weight of ~ 43 kDa and is N-linked glycosylated at asparagine residue 71 in the first extracellular loop [9]. Both intracellularly and at the cell surface, PANX3 proteins oligomerize to form large-pore, single-membrane channels [2, 3], but PANX3 channel stoichiometry is currently unknown. The life cycle of PANX3, including protein trafficking, glycosylation, and other post-translational modifications, has been reviewed previously [10–12].
PANX3 tissue distribution
PANX3 is predominantly found in the musculoskeletal system [2, 3, 5, 9, 13–19], but through subsequent studies its expression pattern has broadened to include the reproductive [20, 21], circulatory [22], digestive [20], and sensory nervous systems [23–25]. Table 1 outlines PANX3 tissue distribution and cell type localization. Four global Panx3 KO mouse models currently exist and much of what is known about PANX3 function in development and pathologies is attributed to studies using these mice. The mouse models, described in Table 2, were developed chronologically by our group (Moon, Penuela et al.), Oh et al., Ishikawa et al., and Yorgan et al. and use various Panx3 ablation tactics [16, 17, 26, 27].
Table 1.
PANX3 tissue distribution and cellular localization
| Tissue | Location/cell types | Localization |
|---|---|---|
| Blood Vessels [22] | Alveoli | Peri-nuclear |
| Juxtaglomerular apparatus | Punctate staining throughout | |
| Endothelium of cortical arterioles, first-order pulmonary artery | ||
| Endothelium and smooth muscle cells of small coronary arteries (luminal diameter < 100 µM), pulmonary arteries of the distal lung | ||
| Male Reproductive Tract [21] | Testis, efferent ducts, epididymis | |
| Leydig cells (testis) | ||
| Ciliated cells (efferent ducts) | Throughout | |
| Non-ciliated cells (efferent ducts) | Lateral plasma membrane and basal region | |
| Principal cells (epididymis) | Apical membrane | |
| Lactating Mammary Gland [20] | ||
| Bone [2, 14, 26, 28] | Osteoblasts and osteoprogenitor cells | Intracellular, cell surface |
| Osteocytes | ||
| Cartilage [3, 16, 20] | Pre-hypertrophic chondrocytes | |
| Hypertrophic chondrocytes | Intracellular, cell surface | |
| Intervertebral Discs [18, 19] | Annulus Fibrosus | |
| Nucleus Pulposus | ||
| Teeth [13, 29–31] | Human dental pulp cells | |
| Human odontoblast-like cells | Cytosolic, cell surface | |
| Mouse P1 molars (dental mesenchyme) and incisors | ||
| Early and late pre-secretory stages of mouse preodontoblasts | Cell surface | |
| Ear [24, 25] | Cochlear bone and modiolus of inner ear | |
| Cochlea | ||
| Skin [4, 9, 32–34] | All layers of the epidermis (keratinocytes) | Intracellular |
| Juvenile thin and thick skin, but not newborn mouse dorsal skin; E13.5 dorsal skin | ||
| Sebaceous glands, blood vessels, eccrine glands | Intracellular | |
| Human dermal fibroblasts | ||
| Vomeronasal Organ (VNO) [23] | Autonomous sensory nerve, basal and ciliated epithelium of the non-sensory epithelium and cartilaginous section of the VNO in juvenile mice | |
| Small Intestine [20] | Epithelium | |
| Skeletal Muscle [4, 15, 35] | Adult skeletal muscle tissue, absent in human fetal tissue | Diffuse staining throughout muscle fibres, striations |
| Human skeletal muscle satellite cells | ||
| Embryonic to adult mouse skeletal muscle, more abundant in fast-twitch than slow-twitch muscles | Punctate structures throughout myofibers and cell surface | |
| Rat skeletal muscle |
Table 2.
Global Panx3 knockout (KO) mouse model generation and general properties
| Mouse model | Background | Exon deleted | Model generation | Viability and fertility |
|---|---|---|---|---|
|
Moon, Penuela [16] |
C57BL/6 N | 2 | Embryonic stem cells containing a Panx3 targeting vector (“Knockout-First” reporter tagged insertion promoter-driven cassette; KnockOut Mouse Project Repository) were used to create Panx3-floxed allele (Panx3fl/fl) mice. Panx3fl/fl mice were crossed with C57BL/6 J CMV-Cre-deleter mice (Cre under the control of the cytomegalovirus minimal promoter). Cre recombinase and any C57BL/6 J markers were out-bred after multiple crosses to ensure the mice were congenic to C57BL/6 N WT controls | Reduced litter sizes compared to WT. Heterozygous (Panx3±) crosses produced fewer KO pups than predicted by Mendelian ratios (8% instead of 25%). No difference in weight or size, body composition in aged mice, or increased mortality with ageing compared to WT |
| Oh [17] | C57BL/6 N | 2 | The same Panx3 targeting vector used by Moon, Penuela et al. was used to create Panx3fl/fl mice. Panx3fl/fl mice were then crossed with EIIa-Cre transgenic mice, where Cre recombinase is under the control of the adenovirus EIIa promoter | Normal |
| Ishikawa [26] | C57BL/6 | 1 | Panx3 deletion resulted from the insertion of a PMK3 Panx3-targeting vector (Invitrogen) into W4 embryonic stem cells. The targeting vector consisted of a 10-kb EcoR1 genomic fragment containing Panx3 exon 1 from bac clone RPCI22-47H21. Once heterozygotes (Panx3±) were developed, they were backcrossed for five generations to C57BL/6 mice before full KO mice were bred | None mentioned other than smaller body size |
| Yorgan [27] | C57BL/6 J | 2 | Panx3fl/fl mice (Panx3tm1a(KOMP)Wtsi, created using the same targeting vector as Moon, Penuela et al.) were obtained from the Mouse Biology Program (University of California, Davis) and mated with CMV-Cre transgenic mice (B6·C-Tg(CMV-cre)1Cgn/J) to achieve ubiquitous Panx3 deletion | Panx3 KO embryos followed Mendelian ratios just before birth at embryonic day 19.5, but 30% of Panx3 KO pups died shortly after birth |
PANX3 immunoreactive species
A 70 kDa immunoreactive form of PANX3 has also been reported, originally thought to result from PANX3 dimerization, glycosylation, or alternative splicing [9, 32, 33]. Others have postulated that this species is a glycosylated, phosphorylated, and sialylated form of the 43 kDa PANX3 species [15]. These beliefs arose because the 70 kDa species is recognizable by multiple PANX3 antibodies generated against distinct PANX3 epitopes (PANX3 CT-379, IL-169, and EL1-84) and its signal can be ablated with peptide preadsorption [9, 33]. However, these polyclonal antibodies were generated before the creation of the first global Panx3 KO mouse model—the foremost assessment of antibody specificity. Western blots using the PANX3 CT-379 antibody show that the 70 kDa species as well as a 50 kDa species are present in both wildtype (WT) and Panx3 KO hindlimb lysates, indicating the 50 kDa and 70 kDa bands most likely result from unspecific antibody binding (Fig. 2). These results reflect previous Western blots using the same antibody on global Panx1/Panx3 KO (dKO) mouse hindlimb and skin, where both immunoreactive species are detectable in dKO and control tissue [36]. Additionally, other studies which use either laboratory generated or commercially available antibodies from Invitrogen or Santa Cruz do not report any 50 kDa or 70 kDa immunoreactive species [3, 29, 37], highlighting the need to develop more specific monoclonal antibodies for PANX3 detection. This review will only focus on the 43 kDa PANX3 species. However, it should be noted that the results from previous studies using the PANX3 CT-379, IL-169, and EL1-84 antibodies have also been confirmed by other groups using different KO mouse models and other custom-made and commercially available antibodies, so the presence of additional immunoreactive species in some tissues does not fundamentally change the findings previously reported.
Fig. 2.
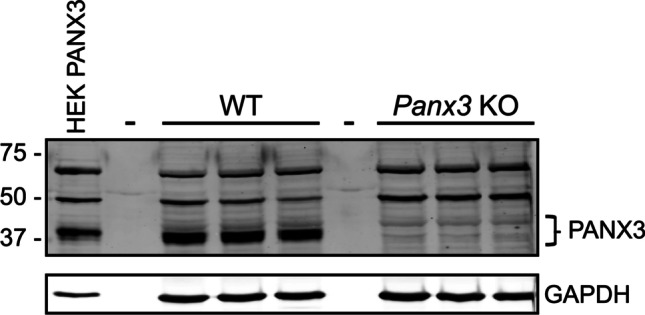
Both 50 kDa and 70 kDa immunoreactive species are seen in both WT and Panx3 KO mouse tissue when immunoblotting with the anti-PANX3 CT-379 polyclonal antibody. Western blots using WT and Panx3 KO postnatal day 5 hindlimb protein lysates were probed with a 1:1000 dilution of anti-PANX3 CT-379 antibody. The 43 kDa PANX3 species is only seen in WT tissue but multiple immunoreactive species at 50 kDa and 70 kDa are seen in hindlimbs of both genotypes. Dashes represent empty lanes. WT, wildtype. KO, knockout. HEK PANX3, human embryonic kidney cells (HEK-293 T), ectopically expressing a mouse PANX3 plasmid. N = 3 per genotype. GAPDH, glyceraldehyde 3 phosphate dehydrogenase was used as a protein loading control. Protein sizes in kDa
Channel function and purinergic signalling
Membrane depolarization has been demonstrated to open PANX3 plasma membrane channels in chondrocytes, osteoblasts, and odontoblasts [2, 3, 30]. During chondrocyte differentiation, ATP released through cell surface PANX3 channels reduces intracellular cyclic adenosine monophosphate (cAMP) levels and subsequent cAMP response element-binding protein (CREB) signalling; ultimately inhibiting parathyroid hormone-induced chondrocyte proliferation and promoting differentiation [3]. At the plasma membrane of osteoblasts, PANX3 channel opening facilitates ATP release which acts on purinergic receptors (P2R) in an autocrine and paracrine manner [2], but the specific ionotropic or metabotropic receptor involved is yet to be determined. P2R activates phosphatidylinositol-3-kinase and protein kinase B (Akt), leading to endoplasmic reticulum (ER) PANX3 phosphorylation at Ser68 and channel opening [2], but the specific kinase responsible is not known. The subsequent cytoplasmic Ca2+ increase drives calmodulin (CaM) and Smad1/5 signalling, promoting osteogenic differentiation [2, 28]. PANX3 activation of Akt signalling also promotes p53 degradation, lifting the negative regulation of osteogenic differentiation driven by p53 [2]. Furthermore, osteoprogenitor cell growth is suppressed through PANX3-mediated ATP release, which reduces cAMP/CREB pathway activity (similar to chondrocytes), induces osterix expression and subsequently inhibits Wnt/β-catenin signalling through decreased β-catenin levels and activity [14, 26]. Lastly in odontoblasts, ATP released from PANX3 surface channels reduces intracellular ATP which triggers adenosine monophosphate-activated protein kinase (AMPK) signalling to inhibit proliferation, while Ca2+ released from PANX3 ER channels stimulates CaM-Smad signalling to induce differentiation concomitantly [30]. In each case, ATP release and differentiation could be blocked by an anti-PANX3 antibody specific to the first extracellular loop of the protein or the PANX3 inhibitory peptide (‘I-peptide’) [2, 3, 30]. It should be noted that most of the cells and tissues that express PANX3 are also known to express PANX1, and therefore, any purinergic signalling involved in their function could be the effect of endogenous PANX1 present in those cells.
Small molecule and mechanical stimulation of PANX3 channels
In addition to membrane depolarization, PANX3 channels can also be activated by extracellular molecules and mechanical stimulation. For instance, challenging myotubes with palmitate stimulates PANX3 channel opening and ATP release where ATP acts as a monocyte chemoattractant in vitro. This process requires the toll-like receptor 4-myeloid differentiation factor-88/nuclear factor-κB (NF-κB) pathway in myotubes and the P2R family in monocytes [4], but the specific receptors involved in the monocyte response were not identified and the mechanism behind this process is still unknown. This is the only instance of endogenous expression systems demonstrating PANX3 channel activation by stimulant treatment.
In PANX3-overexpressing HaCaTs, transforming growth factor beta (TGF-β) and tumour necrosis factor alpha (TNF-α) stimulation increased ATP release compared to vector controls. Additionally, when these cells were treated with exogenous ATP, they exhibited increased intracellular Ca2+ levels not seen in control HaCaTs. Thus, in keratinocytes, PANX3 may act at both the cell surface and ER as a conduit for ATP and Ca2+ respectively, upon TGF-β stimulation [37]. Subsequent studies using this system determined that p-Akt and unphosphorylated nuclear factor of activated T-cells 1 (NFATc1; active form) were increased compared to controls. This effect, along with the corresponding increase in the keratinocyte differentiation markers Epiprofin (Epfn) and Notch1, could be blocked by PANX3 antibody inhibition which targets ATP release through surface PANX3 channels. Interestingly, disrupting PANX3 Ser68 phosphorylation only affected NFATc1 activation and prevented the increase in Epfn, but did not change p-Akt levels [38]. Since Ser68 phosphorylated PANX3 was localized to the ER membrane in osteoblasts [28], the authors speculated that NFATc1 activation must be downstream of ER PANX3 channel function. Ultimately, ATP released from cell surface PANX3 channels activates Akt signalling, which triggers ER PANX3 channels, followed by NFATc1 activation, Epfn expression and keratinocyte differentiation—a very similar mechanism of action to PANX3 in osteoblast differentiation [2, 38].
Both PANX3-expressing rat epidermal keratinocytes (REKs) and human embryonic kidney 293 T cells showed PANX3 cell surface localization and were capable of sulforhodamine B dye uptake after mechanical stimulation, implicating PANX3 as a mechanosensitive channel [9, 32]. However, overexpression systems may not be representative of the endogenous signalling through this channel and may force the trafficking of PANX3 to the cell surface more than in endogenous conditions.
PANX3 metabolome
Apart from ATP, Ca2+, and experimental dyes [2, 3, 9, 32], very little is known about metabolites that can move through intracellular and cell surface PANX3 channels. Furthermore, despite evidence of plasma membrane channels and ATP release, there are currently no reports of electrophysiological measurements from endogenous or PANX3 overexpression systems. This may be due to the predominantly intracellular localization of PANX3 in cells other than chondrocytes and osteoblasts, and it is possible that the channel possesses a different current signature than PANX1 [39].
Cell proliferation and differentiation
Cartilage
Studies using chondrocyte cell lines have demonstrated that PANX3 functions in the transition between proliferative, pre-hypertrophic, and terminally differentiated hypertrophic chondrocytes [3]. PANX3 was found to be expressed in pre-hypertrophic chondrocytes and induced during chondrocyte differentiation, localizing to the ER and plasma membrane [3, 20]. When ectopically expressed, PANX3 increased the expression of chondrocyte markers such as aggrecan, collagen type X α1 and II α1, and cartilage proteoglycans. However, these cells were also subjected to an insulin differentiation protocol, indicating PANX3 induction alone is not sufficient to initialize chondrocyte differentiation. As mentioned, the role of PANX3 in chondrocyte differentiation was attributed to its action as an ATP release channel [3]. Interestingly, Panx3 ablation in mice did not disrupt the onset of hypertrophic chondrocyte differentiation, but rather the normal progression of chondrogenesis which resulted in elongated proliferative and pre-hypertrophic zones, with thinner and disorganized hypertrophic and terminal chondrocyte domains [17, 26].
Conflicting results were seen in a study that utilized the chicken embryo model to analyse PANX3 in skeletal development. PANX3 overexpression did not affect chondrocyte arrangement in the avian growth plate and with a 3.6-fold knockdown of PANX3 levels, the only differences seen were a 20% reduction in forelimb bone volumes and slightly smaller ossification centres. There were no differences in chondrocyte density, proliferation or hypertrophy markers, or cartilage histology, suggesting terminal chondrocyte differentiation is unaffected by PANX3 knockdown [40]. However, it is difficult to compare in vitro findings from cell lines and KO mouse models to avian models using knockdown or ectopic expression techniques.
Bone
Like chondrocytes, PANX3 also acts in osteoblast differentiation. Studies using C2C12 and primary calvarial cells demonstrated that PANX3 is induced during osteogenic differentiation where it acts as both an Akt-sensitive Ca2+ leak channel and ATP release channel [2, 14]. Similarly, in MC3T3-E1 pre-osteoblast cells subjected to an osteogenic differentiation programme, PANX3 levels were increased sevenfold compared to baseline [20]. However, since these cells were also subjected to a bone morphogenic protein 2 (BMP2) or β-glycerol phosphate and ascorbate differentiation protocol respectively [2, 20], PANX3 induction alone is not sufficient to initialize osteoblast differentiation.
PANX3 plays a similar role in the transition from osteoprogenitor proliferation to differentiation using multiple signalling pathways. PANX3 promotes cell cycle arrest at the gap 0/gap 1 (G0/G1) phase by inhibiting cell cycle molecules involved in the G1 to synthesis phase transition such as cyclin D1 and the retinoblastoma protein [14]. As mentioned, cell growth is further suppressed through PANX3-mediated ATP release and Wnt/β-catenin inhibition [14, 26], and PANX3 stimulation of the Akt pathway activates Smad1/5 signalling and increases levels of the cell cycle inhibitor p21 [14]. Interestingly, Ser68 phosphorylation of PANX3 does not affect osteoprogenitor proliferation, only differentiation, but disrupting the putative phosphorylation site Ser303 inhibits both proliferation and differentiation [28]. Together, these signalling cascades enable osteoprogenitors to exit the cell cycle and differentiate into osteoblasts [14].
PANX3 action in osteoblast differentiation is further supported by mesenchymal cell models of osteogenic differentiation, but the pathways involved seem to be cell-type specific. PANX3 is induced in human dental pulp mesenchymal-derived stromal cells subjected to osteogenic differentiation, and PANX3 overexpression decreased proliferation and promoted differentiation, with the opposite effects seen with PANX3 knockdown [2, 41]. In this case, PANX3 promotes osteogenic differentiation via mitogen-activated protein kinase signalling. Wnt/β-catenin signalling was also shown to positively regulate PANX3 expression, but the feedback effects of PANX3 induction of the Wnt/β-catenin pathway does not support the role of PANX3 in osteogenic differentiation, which requires Wnt inhibition. PANX3 induction is most likely a direct effect of Wnt signalling since binding sites for the Wnt pathway transcription factors T-cell factor/lymphoid enhancer-binding factor were found within the PANX3 promoter [41].
The differentiation of other resident bone cell types may also be influenced by PANX3 channel function. One study illustrated that Panx3 KO bones have reduced numbers of osteoclasts and osteoclast differentiation markers. However, based on studies that show that osteoblasts help to regulate osteoclast differentiation, and the fact that PANX3 was not detected in osteoclasts, it seems the reduced osteoclast differentiation is most likely due to the role of PANX3 in mediating osteoblast differentiation [26].
Teeth
When a dental mesenchymal cell line was differentiated into odontoblasts, PANX3 levels were induced along with p21. PANX3 overexpression inhibited cell proliferation, increased p21 levels, and triggered an early onset of odontoblast differentiation after BMP2 treatment through its action as an ATP and Ca2+ release channel [30]. The opposite effects were seen with PANX3 knockdown [30] and this may be reflected in the Panx3 KO mouse model where mice have delayed tooth eruption and smaller teeth compared to WT mice [31].
Muscle and skin
Ectopic expression of PANX3 in human skeletal muscle myoblasts inhibited proliferation and induced myoblast differentiation. These findings suggest that in skeletal muscle, PANX3 may function to maintain myoblasts in their terminally differentiated, post-mitotic state [15].
In keratinocytes, overexpression of PANX3 in REKs significantly reduced cell proliferation compared to controls. PANX3 exhibited both a cell surface and an intracellular localization in PANX3-expressing REK monolayer culture, but when these cells were differentiated into an organotypic epidermis their localization pattern changed, with PANX3 now showing a predominantly cytoplasmic localization. Unlike ectopic PANX1 expression in REKs, which caused organotypic epidermis disorganization and disrupted keratinocyte differentiation, PANX3 overexpression maintained proper organotypic epidermis morphology [32]. This suggests the presence of PANX3 may be important throughout ageing as the epidermis continuously turns over. In contrast, PANX1 levels are highly expressed in neonatal dorsal skin but become downregulated with age and must be decreased to maintain epidermal structure [42]. These differences may reflect the unique functions of PANX1 and PANX3 in skin tissue.
Using a different approach, Zhang et al. found that PANX3 decreases keratinocyte proliferation and stimulates differentiation. They discovered that Notch1 and Epfn were virtually undetectable in neonatal Panx3 KO dorsal skin compared to controls. To investigate this further, they used a HaCaT PANX3 overexpression system, finding that increased PANX3 levels induced terminal differentiation. PANX3 overexpression also decreased cell growth and Ki67 levels, with cells arrested in the G0/G1 phase of the cell cycle. In this case, the mechanism of PANX3 action is similar to osteoblasts, where channel function stimulates the Akt/NFAT pathway, ultimately driving Epfn and Notch1 expression to promote keratinocyte differentiation [38]. Future studies should investigate keratinocyte differentiation using primary keratinocytes isolated from WT and Panx3 KO mice to determine the effects of endogenous PANX3 in keratinocytes.
Tissue development and ageing
Bone and cartilage
PANX3 has been most extensively studied in the context of cartilage and bone development and ageing. During embryonic development, PANX3 is present in both murine and zebrafish intramembranous and endochondral bone [17, 20]. More specifically, high levels of PANX3 are localized to osteoblasts of maturing membranous bones, in interface regions of terminally differentiated chondrocytes and mineralized matrix (pre-hypertrophic and hypertrophic zones), and the chondrogenic layer of the perichondrium in cartilaginous bones [2, 3, 16, 20, 40]. Ser68 phosphorylated PANX3 is found in pre-hypertrophic and hypertrophic chondrocytes and cells of the perichondrium, periosteum, and bone areas, but is highest in the pre-hypertrophic zone. This indicates that phosphorylated PANX3 is induced at this transitional stage of bone development, where it is higher in early stages but decreases with bone maturation [28]. Furthermore, in a chicken embryo model, Panx3 mRNA was detected in cartilage condensations and the growth plate, specifically in the pre-hypertrophic zone and perichondrium. However, much higher levels were found in nascent trabecular bone, suggesting PANX3 may function more in osteogenesis rather than cartilage formation in this model [40].
In developing bone and cartilage, PANX3 expression is induced in part by runt-related transcription factor 2 (RUNX2). In silico analysis of the PANX3 promoter revealed conserved binding sites for RUNX2 and other bone development-associated transcription factors such as BarH-like homeobox 2, vitamin D receptor/retinoid-X receptor heterodimer, and msh homeobox 1/2. RUNX2 binds directly to the functional enhancer element in the PANX3 promoter, but other cofactors are likely involved. Interestingly, many of the aforementioned transcription factors are also upregulated in hypertrophic chondrocytes to promote mineralization during endochondral ossification [20]. Based on multiple studies, it is likely that in bone, BMP2 upregulates RUNX2 expression which then induces PANX3 [2, 14].
Despite complementary evidence of PANX3 function in osteoblasts and osteoprogenitor cells in vitro, there are some inconsistencies regarding the role of PANX3 in bone in vivo. Investigations into the effects of PANX3 on bone development were conducted using knockdown or knockout animal models, often with conflicting findings (outlined in Table 3). Generally, some global KO mouse models show much more severe phenotypes with impacts lasting into adulthood, whereas others see more mild phenotypes in young mice that are lost with age. Despite these inconsistencies, all models point to a clear role for PANX3 in bone, and the phenotypic differences seen between each global Panx3 KO model may be attributed to diverse background mouse strains or Cre recombination events. Additionally, sex differences could account for some discrepancies since earlier studies were conducted using predominantly male mice, but the later study by Yorgan et al. used only female mice [16, 17, 26, 27].
Table 3.
Bone phenotypes of Panx3 KO or knockdown mouse, zebrafish and avian models
| Model | Age | Bone phenotypes |
|---|---|---|
| Global Panx3 KO Mouse Moon, Penuela [16, 43] | Neonatal | No growth plate abnormalities, deficits in bone mineral density or differences in the axial skeleton |
| Young Adult |
Shorter long bone diaphyses due to differences in allometric growth, but larger areas of muscle attachment and metaphyses/epiphyses in femora and humeri Femora and humeri are thicker and more robust, indicating they may have increased resistance to torsional and compressive forces |
|
| Aged |
Increased subchondral bone thickness in the medial compartment of knees and an altered subchondral collagen network with thicker collagen fibres Shortened appendicular skeleton |
|
| Panx3fl/fl:Col2cre Mouse (Cartilage-specific) [16] | No skeletal development or growth defects | |
|
Global Panx3 KO Mouse and panx3 knockdown zebrafish Oh [17] |
Newborn | Bone abnormalities such as atypical curvatures, shortened long bone lengths, delayed ossification and lower levels of mineralization in early stages of bone development via intramembranous and endochondral ossification |
| Young Adult | 5–10% shorter bone lengths | |
| Global Panx3 KO Mouse Ishikawa [26] | Newborn |
Reduced bone density, shorter appendicular and axial bones and smaller skull bones with mineralization defects in the cranial vaults Ossification delays and altered mineralization were attributed to the role of PANX3 in the onset and normal progression of osteogenesis Delayed development of secondary ossification centres, indicating a reduction in vascular invasion Reduced osteocyte markers |
| Global Panx3 KO Mouse Yorgan [27] | Newborn | Reduced long bone length and severe, but nonlethal, skeletal deformities |
| Adolescent and Adult |
No differences in bone mineral density, trabecular bone mass, nor any structural skeletal abnormalities apart from a moderately reduced femur length, suggesting that despite delays in the onset of KO mouse mineralization, subsequent mineralization processes are unaffected No differences in osteoblast, osteocyte or osteoclast numbers |
|
| Panx3fl/fl:Runx2cre Mouse (Osteoblast-Specific) [27] | Adult |
No trabecular abnormalities and normal bone formation rates Slightly decreased femoral cortical thickness |
| Avian Model [41] | Embryonic |
No major skeletal phenotypic defects or differences in any bone differentiation markers to control animals with PANX3 overexpression or knockdown Reduced long bone volumes and slightly smaller ossification centres with PANX3 knockdown |
Other tissues
PANX3 levels are developmentally and temporally regulated in multiple tissues. Within skeletal muscle, PANX3 is absent in human fetal tissue, but present in adult skeletal muscle [15]. In the skin, PANX3 is detectable in all epidermal layers of both thin and thick skin of 3-week-old mice and in the dorsal skin of embryonic day 13.5 mice, but not in newborn skin [32]. However, the regulation of PANX3 levels in skeletal muscle and skin throughout ageing remains unknown. In the cochlea, PANX3 is present in postnatal day 1 (P1) mice, its levels peak at P8 and then decrease at P16 and P60-P90 [24]. In intervertebral discs (IVD), high Panx3 transcript levels in 2-month-old mice become significantly decreased in mice 6–24 months of age [18]. Lastly, PANX3 levels in joints are also differentially regulated throughout ageing. At 3 months of age, PANX3 is present throughout all osteochondral and soft tissue, but is mostly seen in the meniscus and soft tissues and minimally in the articular cartilage of the joint by 12 months. However, by 18–24 months, PANX3 was observed again in joint articular cartilage and age-induced cartilage erosions [44].
Tissue regeneration
Bone remodelling and regeneration
Despite earlier beliefs that PANX3 may function in bone remodelling [43] due to its expression in bone-forming osteoblasts and osteocytes, Yorgan et al. determined that PANX3 is nonessential for postnatal bone remodelling regulation using both global and osteoblast-specific Panx3 KO mouse models [27].
In bone regeneration, one study used human dental pulp mesenchymal-derived stromal cells (hDPSCs), shown to differentiate into osteoblasts, to explore PANX3 in bone repair in vivo using a rat calvarial critical sized defect model. When control or ectopically expressing PANX3 hDPSCs on a β-tricalcium phosphate scaffold were inserted into the bone defects, PANX3 overexpression enhanced bone formation, with greater newly formed bone area and an increased number of osteocalcin positive cells [41]. Although only a small increase in bone formation was observed over an 8-week timeframe, these early results show promise for PANX3 application in situations where bone repair or regeneration is critical such as in fracture injuries or diseases like osteoporosis.
Wound healing
The use of punch wound biopsies in a global Panx3 KO mouse model [26] demonstrated that, similar to global Panx1 KO mice, global Panx3 KO mice exhibit delayed wound healing with compromised re-epithelialization, decreased inflammatory response, and reduced collagen remodelling abilities compared to controls [37]. In contrast, another study using primary human dermal fibroblasts to investigate the wound healing process in vitro showed that short interfering RNA (siRNA) knockdown of PANX3 increased the fibroblasts’ migratory index, which was maintained until wound closure [34]. However, scratch wound assays are only a 2D representation of wounding and is likely not representative of PANX3 action during wound healing in vivo.
With regard to skeletal muscle regeneration, in vivo testing using mice subjected to cardiotoxin-induced muscle degeneration illustrated that PANX3 levels were reduced 2 days post-injury, but expression steadily increased towards control levels as muscle regeneration occurred. This suggests that PANX3 functions in the muscle regeneration process, potentially as satellite cells differentiate to help repair the damaged skeletal muscle [35].
Inflammation
PANX3-driven ATP release from myotubes treated with the proinflammatory, saturated fatty acid palmitate, but not the unsaturated fatty acid palmitoleic acid, was found to be a chemoattractant for monocytes. Although other channel proteins are expressed in muscle cells, only Panx3 transcripts were induced by palmitate treatment where the increased expression was prevented with NF-κB inhibition. Additionally, only siRNA knockdown of PANX3 reduced palmitate-induced ATP release and monocyte migration [4].
Contrastingly, PANX3 levels were found to be reduced in both human and rat pulpitis tissues compared to normal dental pulp, and treatment of human dental pulp cells with the proinflammatory TNF-α led to a dose-dependent decrease in PANX3. Concomitantly, NF-κB counteracts the TNF-α mediated reduction of PANX3, where this induction most likely occurs through a transcriptional mechanism since three putative NK-κB binding sites were found in the PANX3 promoter. Short hairpin RNA knockdown of PANX3 increased interleukin 6 (IL-6) and IL-1β inflammatory cytokines and the phosphorylation, nuclear localization, and activity of NF-κB in human dental pulp cells stimulated with TNF-α [29]. Therefore, PANX3 may play more of a protective, anti-inflammatory role in dental pulp, unlike in myotubes.
Pannexin3 in disease
Obesity
Using a large cohort of male and female mice with varying tumour-susceptibility and body mass index (BMI), Panx3 was shown to be genetically linked to BMI and tumorigenesis. A strong correlation was also found between (mRNA) Panx3 and lipid metabolism genes involved in long-chain fatty acid transport and lipid storage via network analysis of both male and female murine tails. However, Panx3 mRNA levels only correlated with BMI in males, not females, which was the first account of PANX3 sex differences [45]. Interestingly, three nonsynonymous single nucleotide polymorphisms in Panx3 were present in the parental Mus spretus and Mus musculus strains, and although none were predicted to functionally impact the protein, they were close to or at the site of predicted phosphorylation [45]. Since Ser68 phosphorylation of PANX3 contributes to osteoblast differentiation [28], it is possible that PANX3 phosphorylation at other candidate sites could function in adipocyte differentiation and/or fat storage and be partly responsible for the difference in the BMI phenotypes of each mouse strain. Further investigation into fat accumulation and inflammation associated with obesity are warranted to confirm these assertions.
Osteoarthritis
PANX3 has been implicated in both primary and secondary osteoarthritis (OA). Previously, a study looking at surgically induced OA in rats showed that (mRNA) Panx3 is markedly increased in osteoarthritic cartilage compared to sham surgery controls [46]. Our group followed up on this using global and chondrocyte-specific Panx3 KO mouse models to study the role of PANX3 in post-traumatic secondary OA. We found that destabilization of the medial meniscus in 20-week-old WT mice resulted in both matrix metalloprotease 13 (MMP13) and PANX3 upregulation in areas of cartilage degeneration not seen in the healthy cartilage of sham surgery controls. PANX3 was also upregulated in human osteoarthritic cartilage in comparison to non-weightbearing control knee regions [16]. Both global and chondrocyte-specific Panx3 KO mice were resistant to surgically-induced OA compared to controls, showing mostly healthy joints with minimal proteoglycan loss and cartilage degeneration and indicating a potential catabolic role of PANX3 in articular cartilage [16, 43]. Interestingly, in our more recent study, Panx3 deletion was shown to have an opposite, more detrimental effect in primary OA during ageing. In this case, we discovered that aged (18–24 months old) Panx3 KO knees had full thickness articular cartilage erosion, with increased osteophyte size, prevalence of low-grade synovitis, and MMP13 staining along the cartilage lesions, whereas WT knees showed minimal cartilage degeneration, low-grade synovitis, and paracellular MMP13 [44]. Therefore, a lack of Panx3 accelerates OA progression in ageing, but has a chondroprotective effect in post-traumatic OA in young mice, outlining the diverse molecular mechanisms contributing to the pathobiology of primary and secondary OA development [16, 44]. Unfortunately, all studies were completed using male mice, despite evidence that women are at a higher risk to develop OA and experience relatively more severe arthritis of the knee [47]. Future studies investigating both male and female mice are warranted to further investigate the context-dependent, mechanistic action of PANX3 in OA onset and progression, and assess the potential therapeutic benefit of PANX3 in this disease.
Intervertebral disc degeneration
Similar to the context-dependent action of PANX3 in OA [16, 44], PANX3 seems to vary in age-related and injury-induced IVD degeneration. With normal ageing of mice, Panx3 ablation did not alter age-associated IVD degeneration as evidenced by similar histopathological scoring and features of WT and Panx3 KO lumbar IVDs, as well as the lack of differences in chondrocyte hypertrophy and the extracellular matrix. The only notable difference between the lumbar IVDs of 19-month-old WT and Panx3 KO mice was the abundance and localization of type X collagen, where levels were decreased and localized to the outer annulus fibrosus (AF) in the KO. Contrarily, in an injury-induced IVD degeneration model, PANX3 seems to play more of a detrimental role, evidenced by increased AF structural integrity, decreased hypertrophic cells, and average lamellar thickness of the AF in Panx3 KO mice [18]. However, the authors noted that in Panx3 KO mice, uninjured IVDs adjacent to the needle puncture site had evidence of accelerated nucleus pulposus degeneration, even though adjacent IVDs in WT mice were completely healthy. This suggests that the mechanosensitive PANX3 channels may be involved in the homeostatic mechanism to adapt to the altered biomechanics of adjacent healthy joints.
Duchenne’s muscular dystrophy
Since a potential role for PANX3 was discovered in muscle regeneration, PANX3 was investigated in the context of Duchenne’s muscular dystrophy where this process is disrupted. In this study, both mild (dystrophin-deficient) and severe (dystrophin/utrophin double KO) Duchenne’s muscular dystrophy mouse models were used. PANX3 levels were significantly reduced in all dystrophic muscles investigated, apart from the soleus in dystrophin-deficient mice, showing that PANX3 levels are dysregulated in muscular dystrophy [35]. However, it remains to be determined whether PANX3 dysregulation is a driver of muscle wasting or is just a result of the exacerbated degeneration-regeneration cycles and disease progression.
Non-melanoma skin cancer
An early study analysing a small cohort of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) patient-derived tumours by immunofluorescence indicated that PANX3 was reduced in each tumour core compared to normal human epidermis. Additionally, PANX3 levels in the BCC periphery were similar to those found in the normal epidermis [33]. However, more investigation is required to confirm these early findings.
In the previously mentioned study, which uncovered Panx3 as the genetic link between BMI and tumorigenesis, a 7,12-dimethylbenz(a)anthracene/12-Otetradecanoylphorbol-13-acetate cutaneous carcinoma model was used to investigate PANX3 in cancer progression [45]. In this model, Panx3 mRNA levels were markedly reduced in papillomas and carcinomas compared to normal tail tissue [45], similar to human BCC and SCC samples [33]. However, the study showed contradicting evidence where (mRNA) Panx3 expression in pre-treated mouse tail skin was positively associated with tumour risk, more specifically with both early- (papilloma) and late-stage (carcinoma) tumour development, which the authors attributed to the influence of PANX3 on tumour susceptibility rather than tumour maintenance [45]. In both analyses, untreated dorsal skin should have been used as the control tissue instead of tail skin, since dorsal and tail skin have inherent differences in gene expression networks which contribute to varying skin tumour susceptibility [48].
Osteosarcoma
In a published case report, a 68-year-old man had an acute enlargement of a nodule in his left axilla excised and analysed for diagnosis. The initial biopsy analysis identified the tumour as a high-grade osteosarcoma with 96% certainty, but later reclassified it as a rare case of primary cutaneous sweat gland carcinoma with osteosarcomatous transformation. Interestingly, Panx3 mRNA was shown to be very highly expressed in the tumour and, based on the molecular analysis algorithm used in the study, was strongly predictive of osteosarcoma when highly expressed [49]. Furthermore, multiple studies using cell lines and patient tumours illustrated that (mRNA) Panx3 is markedly increased in osteosarcoma patient tumours and cell lines compared to control tissue and osteoblast cell lines [50, 51]. In the same tumour samples, microRNA 431-5p (miR 431-5p) was downregulated in osteosarcoma tumours compared to adjacent tissue. Further analysis demonstrated the tumour-suppressive role of miR 431-5p which is partially enacted through the direct targeting and downregulation of (mRNA) Panx3 [51]. With the outcome of osteosarcoma metastasis patients remaining poor and better treatment options needed, PANX3 targeted therapies should be explored.
Conclusions and future perspectives
Despite PANX1 being the primary focus of the pannexin field, recent interest in PANX3 in the development and pathologies of the musculoskeletal system and other tissues has propelled PANX3 research to gain traction, especially with its ability to compensate for the absence of PANX1 in the vomeronasal organ, skin, and vasculature [22, 23, 42]. Most PANX3 studies focus on its role in bone, teeth, and cartilage, but others such as skin, adipose, and vasculature are emerging as tissues of interest. However, it is evident from this review that there are still many unanswered questions regarding PANX3 function in tissue homeostasis and its dysregulation in disease.
The extent of the PANX3 interactome includes only PANX1, resulting in pannexin intermixing, but does PANX3 interact with any other proteins? Is the interaction direct, or part of a complex? More specifically, with the findings of the involvement of PANX3 in purinergic signalling in osteoblasts [2] and monocytes [4], does PANX3 interact with any purinergic receptors similar to PANX1 in the inflammasome [52–56]? The current understanding of PANX3 in reproduction is also very limited. Thus far, PANX3 has only been detected in lactating mammary glands and the male reproductive tract, but why are global Panx3 KO mice litters smaller than controls, with pups dying shortly after birth? This phenotype is also seen in dKO litters, suggesting a dominant effect of Panx3 ablation since no fertility issues are seen in global Panx1 KO mice [36, 57].
Given its role in chondrocyte [3] and osteoblast [2, 14] differentiation, further investigation into the therapeutic potential of PANX3 in bone and cartilage associated pathologies such as osteosarcoma, osteoarthritis, osteoporosis, and arthritis is warranted. There are also no reports of disease-causing germline PANX3 mutations in humans, which could give insights into the mechanism of action of PANX3 channel function and signalling. Moreover, future investigations into PANX3 in cell specialization will determine if its role in differentiation is broad or cell-type specific. Lastly, most PANX3 findings have resulted from studying global Panx3 KO mouse models, which come with the caveat of compensation and contradictory evidence depending on the model used. In the future, cell-type specific, conditional Panx3 KO mouse models should be used to identify PANX3 action more distinctly in healthy tissue and disease. In conclusion, despite a surge in efforts to understand the biology of PANX3 within the mammalian context and its role in communication, in part through purinergic signalling, much about PANX3 is still unknown, offering the possibility of many future studies within this field.
Brooke O’Donnell
graduated with a BMSc (Hons) in Physiology from the University of Western Ontario where she transitioned into the PhD program at Western in the Department of Anatomy and Cell Biology under the supervision of Dr. Silvia Penuela. Her current research focuses on the function of the channel protein Pannexin 3 in skin health and aging as well as its dysregulation in keratinocytic skin cancers. She is passionate about the cellular mechanisms of keratinocyte transformation in nonmelanoma skin cancers and in improving treatment options for skin cancer patients with advanced disease.
Funding
Western University and Petro-Canada Young Innovator award to SP.
Data availability
Raw data available upon request.
Declarations
Ethical approval
Experiments performed on animals were approved by the Animal Care Committee of the University Council on Animal Care at the University of Western Ontario, London ON, Canada (UWO # 2019–069), and in accordance with relevant guidelines and regulations.
Informed consent
Not applicable.
Conflicts of interest
No conflict of interest of any kind.
Footnotes
This article is part of the Topical Collection on The contribution of pannexin-1, connexins and CALHM ATP-release channels to purinergic signalling
Guest Editor: Charles Kennedy
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10(13):473–474. doi: 10.1016/S0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca 2+ channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol. 2011;193(7):1257–1274. doi: 10.1083/jcb.201101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwamoto T, Nakamura T, Doyle A, Ishikawa M, De Vega S, Fukumoto S, Yamada Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285:18948–18958. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillon N, Li Y, Fink L, Brozinick J, Nikolayev J, Kuo M, Bilan P, Klip A. Nucleotides released from palmitate-challenged muscle cells through pannexin-3 attract monocytes. Diabetes. 2014;63:3815–3826. doi: 10.2337/db14-0150. [DOI] [PubMed] [Google Scholar]
- 5.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100(23):13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83(4):706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Yen MR, Saier Jr, Milton H (2007) Gap junctional proteins of animals: the innexin/pannexin superfamily. Prog Biophys Mol Biol 94:5-14 [DOI] [PMC free article] [PubMed]
- 8.Penuela S, Bhalla R, Nag K, Laird D. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–4323. doi: 10.1091/mbc.e09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penuela S, Bhalla R, Gong X-Q, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120(21):3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 10.Boyce AKJ, Epp AL, Nagarajan A. Swayne LA (2018) Transcriptional and post-translational regulation of pannexins. Biochim Biophys Acta Biomembr. 1860;1:72–82. doi: 10.1016/j.bbamem.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Penuela S, Gehi R. Laird DW (2013) The biochemistry and function of pannexin channels. Biochim Biophys Acta. 1828;1:15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Penuela S, Laird DW. The cellular life of pannexins. WIREs Membr Transp Signal. 2012;1:621–632. doi: 10.1002/wmts.63. [DOI] [Google Scholar]
- 13.Fu D, Song F, Sun H, Pei D, Wang Y, Lei J, Huang C. Expression of pannexin3 in human odontoblast-like cells and its hemichannel function in mediating ATP release. Arch Oral Biol. 2015;60(10):1510–1516. doi: 10.1016/j.archoralbio.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa M, Iwamoto T, Fukumoto S, Yamada Y. Pannexin 3 inhibits proliferation of osteoprogenitor cells by regulating Wnt and p21 signaling. J Biol Chem. 2014;289(5):2839–2851. doi: 10.1074/jbc.M113.523241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langlois S, Xiang X, Young K, Cowan BJ, Penuela S, Cowan KN. Pannexin 1 and pannexin 3 channels regulate skeletal muscle myoblast proliferation and differentiation. J Biol Chem. 2014;289(44):30717–30731. doi: 10.1074/jbc.M114.572131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon PM, Penuela S, Barr K, Khan S, Pin CL, Welch I, Attur M, Abramson SB, Laird DW, Beier F. Deletion of Panx3 prevents the development of surgically induced osteoarthritis. J Mol Med. 2015;93(8):845–856. doi: 10.1007/s00109-015-1311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh SK, Shin JO, Baek JI, Lee J, Bae JW, Ankamerddy H, Kim MJ, Huh TL, Ryoo ZY, Kim UK, Bok J, Lee KY. Pannexin 3 is required for normal progression of skeletal development in vertebrates. FASEB J. 2015;29(11):4473–4484. doi: 10.1096/fj.15-273722. [DOI] [PubMed] [Google Scholar]
- 18.Serjeant M, Moon PM, Quinonez D, Penuela S, Beier F, Séguin CA. The role of Panx3 in age-associated and injury-induced intervertebral disc degeneration. International Journal of Molecular Sciences. 2021;22(3):1080. doi: 10.3390/ijms22031080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veras MA, McCann MR, Tenn NA, Seguin CA. Transcriptional profiling of the murine intervertebral disc and age-associated changes in the nucleus pulposus. Connect Tissue Res. 2020;61(1):63–81. doi: 10.1080/03008207.2019.1665034. [DOI] [PubMed] [Google Scholar]
- 20.Bond SR, Lau A, Penuela S, Sampaio AV, Underhill TM, Laird DW, Naus CC. Pannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytes. J Bone Miner Res. 2011;26(12):2911–2922. doi: 10.1002/jbmr.509. [DOI] [PubMed] [Google Scholar]
- 21.Turmel P, Dufresne J, Hermo L, Smith CE, Penuela S, Laird DW, Cyr DG. Characterization of pannexin1 and pannexin3 and their regulation by androgens in the male reproductive tract of the adult rat. Mol Reprod Dev. 2011;78(2):124–138. doi: 10.1002/mrd.21280. [DOI] [PubMed] [Google Scholar]
- 22.Lohman AW, Billaud M, C SA, Johnstone SR, Best A, Lee M, Barr K, Penuela S, Laird DW, Isakson BE, Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res. 2012;49:405–416. doi: 10.1159/000338758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whyte-Fagundes P, Kurtenbach S, Zoidl C, Shestopalov VI, Carlen PL, Zoidl G. A potential compensatory role of Panx3 in the VNO of a Panx1 knock out mouse model. Front Mol Neurosci. 2018;11:135. doi: 10.3389/fnmol.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abitbol J, Kelly J, Barr K, Schormans A, Laird D, Allman B. Differential effects of pannexins on noise-induced hearing loss. Biochem J. 2016;473:4665–4680. doi: 10.1042/BCJ20160668. [DOI] [PubMed] [Google Scholar]
- 25.Wang XH, Streeter M, Liu YP, Zhao HB. Identification and characterization of pannexin expression in the mammalian cochlea. J Comp Neurol. 2009;512(3):336–346. doi: 10.1002/cne.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa M, Williams GL, Ikeuchi T, Sakai K, Fukumoto S, Yamada Y. Pannexin 3 and connexin 43 modulate skeletal development through their distinct functions and expression patterns. J Cell Sci. 2016;129(5):1018–1030. doi: 10.1242/jcs.176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yorgan TA, Peters S, Amling M, Schinke T. Osteoblast-specific expression of Panx3 is dispensable for postnatal bone remodeling. Bone. 2019;127:155–163. doi: 10.1016/j.bone.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa M, Williams G, Forcinito P, Ishikawa M, Petrie RJ, Saito K, Fukumoto S, Yamada Y. Pannexin 3 ER Ca(2+) channel gating is regulated by phosphorylation at the Serine 68 residue in osteoblast differentiation. Sci Rep. 2019;9(1):18759. doi: 10.1038/s41598-019-55371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song F, Sun H, Wang Y, Yang H, Huang L, Fu D, Gan J, Huang C. Pannexin3 inhibits TNF-alpha-induced inflammatory response by suppressing NF-kappaB signalling pathway in human dental pulp cells. J Cell Mol Med. 2017;21(3):444–455. doi: 10.1111/jcmm.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwamoto T, Nakamura T, Ishikawa M, Yoshizaki K, Sugimoto A, Ida-Yonemochi H, Ohshima H, Saito M, Yamada Y, Fukumoto S. Pannexin 3 regulates proliferation and differentiation of odontoblasts via its hemichannel activities. PLoS One. 2017;12(5):e0177557. doi: 10.1371/journal.pone.0177557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa M, Yamada Y. The role of pannexin 3 in bone biology. J Dent Res. 2017;96(4):372–379. doi: 10.1177/0022034516678203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celetti SJ, Cowan KN, Penuela S, Shao Q, Churko J, Laird DW. Implications of pannexin 1 and pannexin 3 for keratinocyte differentiation. J Cell Sci. 2010;123(8):1363–1372. doi: 10.1242/jcs.056093. [DOI] [PubMed] [Google Scholar]
- 33.Cowan KN, Langlois S, Penuela S, Cowan BJ, Laird DW. Pannexin1 and pannexin3 exhibit distinct localization patterns in human skin appendages and are regulated during keratinocyte differentiation and carcinogenesis. Cell Commun Adhes. 2012;19(3–4):45–53. doi: 10.3109/15419061.2012.712575. [DOI] [PubMed] [Google Scholar]
- 34.Flores-Muñoz C, Maripillán J, Vásquez-Navarrete J, Novoa-Molina J, Ceriani R, Sánchez HA, Abbott AC, Weinstein-Oppenheimer C, Brown DI, Cárdenas AM, García IE, Martínez AD. Restraint of human skin fibroblast motility, migration, and cell surface actin dynamics, by pannexin 1 and P2X7 receptor signaling. International Journal of Molecular Sciences. 2021;22(3):1069. doi: 10.3390/ijms22031069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham TL, St-Pierre ME, Ravel-Chapuis A, Parks TEC, Langlois S, Penuela S, Jasmin BJ, Cowan KN. Expression of Pannexin 1 and Pannexin 3 during skeletal muscle development, regeneration, and Duchenne muscular dystrophy. J Cell Physiol. 2018;233(10):7057–7070. doi: 10.1002/jcp.26629. [DOI] [PubMed] [Google Scholar]
- 36.Abitbol J, O’Donnell B, CB W, Jewlal E, Kelly J, Barr K, Willmore K, Allman B, Penuela S, Double deletion of Panx1 and Panx3 affects skin and bone but not hearing. J Mol Med. 2019;97(5):723–726. doi: 10.1007/s00109-019-01779-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhang P, Ishikawa M, Rhodes C, Doyle A, Ikeuchi T, Nakamura K, Chiba Y, He B, Yamada Y. Pannexin-3 deficiency delays skin wound healing in mice due to defects in channel functionality. J Invest Dermatol. 2019;139(4):909–918. doi: 10.1016/j.jid.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 38.Zhang P, Ishikawa M, Doyle A, Nakamura T, He B, Yamada Y. Pannexin 3 regulates skin development via Epiprofin. Sci Rep. 2021;11(1):1779. doi: 10.1038/s41598-021-81074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312(5775):924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 40.Bond SR, Abramyan J, Fu K, Naus CC, Richman JM. Pannexin 3 is required for late stage bone growth but not for initiation of ossification in avian embryos. Dev Dyn. 2016;245(9):913–924. doi: 10.1002/dvdy.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song F, Sun H, Huang L, Fu D, Huang C. The role of pannexin3-modified human dental pulp-derived mesenchymal stromal cells in repairing rat cranial critical-sized bone defects. Cell Physiol Biochem. 2017;44(6):2174–2188. doi: 10.1159/000486023. [DOI] [PubMed] [Google Scholar]
- 42.Penuela S, Kelly JJ, Churko JM, Barr KJ, Berger AC, Laird DW. Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J Invest Dermatol. 2014;134(7):2026–2035. doi: 10.1038/jid.2014.86. [DOI] [PubMed] [Google Scholar]
- 43.Caskenette D, Penuela S, Lee V, Barr K, Beier F, Laird D, Willmore K (2016) Global deletion of Panx3 produces multiple phenotypic effects in mouse humeri and femora. J Anat 228 (746–756) [DOI] [PMC free article] [PubMed]
- 44.Moon PM, Shao ZY, Wambiekele G, Appleton C, Laird DW, Penuela S, Beier F. Global deletion of pannexin 3 accelerates development of aging-induced osteoarthritis in mice. Arthritis Rheumatol. 2021 doi: 10.1002/art.41651. [DOI] [PubMed] [Google Scholar]
- 45.Halliwill K, Quigley D, Chung Kang H, Del Rosario R, Ginzinger D, Balmain A. Panx3 links body mass index and tumorigenesis in a genetically heterogeneous mouse model of carcinogen-induced cancer. Genome Med. 2016;8:83–100. doi: 10.1186/s13073-016-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appleton CT, Pitelka V, Henry J, Beier F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56(6):1854–1868. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- 47.O'Connor M. Sex differences in osteoarthritis of the hip and knee. J Am Acad Orthop Surg. 2007;15:S22–S25. doi: 10.5435/00124635-200700001-00007. [DOI] [PubMed] [Google Scholar]
- 48.Quigley DA, Kandyba E, Huang P, Halliwill KD, Sjolund J, Pelorosso F, Wong CE, Hirst GL, Wu D, Delrosario R, Kumar A, Balmain A. Gene expression architecture of mouse dorsal and tail skin reveals functional differences in inflammation and cancer. Cell Rep. 2016;16(4):1153–1165. doi: 10.1016/j.celrep.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romano RC, Gardner JM, Shalin SC, Ram R, Govindarajan R, Montgomery CO, Gilley JH, Nicholas RW. High relative expression of Pannexin 3 (PANX3) in an axillary sweat gland carcinoma with osteosarcomatous transformation. Am J Dermatopathol. 2016;38(11):846–851. doi: 10.1097/DAD.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 50.Ho XD, Phung P, V QL, V HN, Reimann E, Prans E, Koks G, Maasalu K, Le NT, L HT, H GN, Martson A, Koks S, Whole transcriptome analysis identifies differentially regulated networks between osteosarcoma and normal bone samples. Exp Biol Med (Maywood) 2017;242(18):1802–1811. doi: 10.1177/1535370217736512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun S, Fu L, Wang G, Wang J, Xu L. MicroRNA-431-5p Inhibits the tumorigenesis of osteosarcoma through targeting PANX3. Cancer Manag Res. 2020;12:8159–8169. doi: 10.2147/CMAR.S260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buvinic S, Almarza G, Bustamante M, Casas M, Lopez J, Riquelme M, Saez JC, Huidobro-Toro JP, Jaimovich E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem. 2009;284(50):34490–34505. doi: 10.1074/jbc.M109.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295(3):C752–760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581(3):483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284(27):18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol. 2011;186(11):6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data available upon request.


