Abstract
Postoperative efficacy of thoracic epidural analgesia (TEA) following thoracic surgery may vary in patients with different body mass index (BMI) values, regardless of the success of the method. This study aimed to investigate the effects of BMI on postoperative pain scores in patients who underwent thoracotomy with TEA.
After obtaining the ethical committee approval (Date: May 11, 2021, Number: 2012-KEAK-15/2305) the data of 1326 patients, who underwent elective thoracic surgery in high volume tertiary thoracic surgery center between January 2017 and January 2021, were analyzed retrospectively. Patients between the age of 18 and 80 years, who underwent thoracotomy and thoracic epidural catheterization (TEC), and who were assigned American Society of Anesthesiologists I to III physical status were included to the study. Of the 406 patients, who underwent a successful TEC, 378 received postoperative analgesia for 72 hours. Visual analog scale (VAS) scores of these patients were evaluated statistically. Based on BMI, patients were categorized into the following 5 groups: Group I: BMI < 20 kg/m2, Group II: BMI = 20 to 24.9 kg/m2, Group III: BMI = 25 to 29.9 kg/m2, Group IV: BMI = 30 to 34.9 kg/m2, and Group V: BMI ≥ 35 kg/m2.
There were no statistically significant differences in TEC success across different BMI groups (P > .05). Catheter problems and VAS scores significantly increased with higher BMI values in the postoperative 72-hours period (P < .05). Rates of rescue analgesic use were higher in BMI groups of 30 toto 34.9 kg/m2 and ≥35 kg/m2 compared to the other BMI groups.
This study revealed that higher BMI in patients may increase VAS scores, who administered TEA for pain management following thoracotomy. This correlation was supported by the increased need for additional analgesics in patients with high BMI. Therefore, patients with high BMI values would require close monitoring and follow-up.
Keywords: body mass index, thoracic epidural analgesia, thoracic epidural catheterization, thoracotomy, visual analog scale
1. Introduction
Thoracotomy is a frequently employed procedure in thoracic surgery, although it causes severe postoperative pain.[1] Thoracic epidural analgesia (TEA) is accepted as the gold standard to alleviate postoperative pain after thoracotomies.[2–5] However, performing thoracic epidural catheterization (TEC) requires substantial clinical experience and many factors affect the success of TEC.[5,6] It is known that technical difficulties may occur in obese patients undergoing regional analgesia methods such as epidural analgesia.[7,8] Difficulties in determining the anatomical landmark in the presence of a high body fat ratio is a major factor associated with low success rates.[7] Even though the epidural catheter is successfully applied, the epidural fat ratio and body mass index (BMI) are included among several factors acting on the distribution of local anesthetics and other agents in the epidural area.[9–12] Therefore, the postoperative efficacy of TEA following thoracic surgery may vary in patients with different BMI values, regardless of the success of the method applied.
This study aimed to investigate the effects of BMI on TEC success rate, TEC-related troubles, postoperative pain severity, additional analgesic requirement, and satisfaction in patients who were administered TEA for the treatment of postoperative pain following thoracotomies.
2. Materials and methods
After obtaining the ethical committee approval (Date: May 11, 2021, Number: 2012-KEAK-15/2305) the data of 1326 patients, who underwent elective thoracic surgery in University of Health Sciences, Ankara Atatürk Chest Diseases and Chest Surgery Training and Research Hospital which is a high-volume tertiary thoracic surgery center between January 2017 and January 2021, were analyzed retrospectively.
The study included 437 patients between the age of 18 and 80 years who underwent thoracotomy and TEC, and who were assigned American Society of Anesthesiologists I to III physical status. Patients below 18 or above 80 years, who underwent video-assisted thoracoscopic surgery and emergency surgery, and who suffered from chronic pain and a history of opioids therapy before surgery were excluded from the study.
Of the 406 patients, who underwent a successful TEC, 378 received postoperative epidural analgesia for 72 hours. Visual analog scale (VAS) scores of these patients were evaluated statistically. All patients were informed about the procedure and consent was obtained.
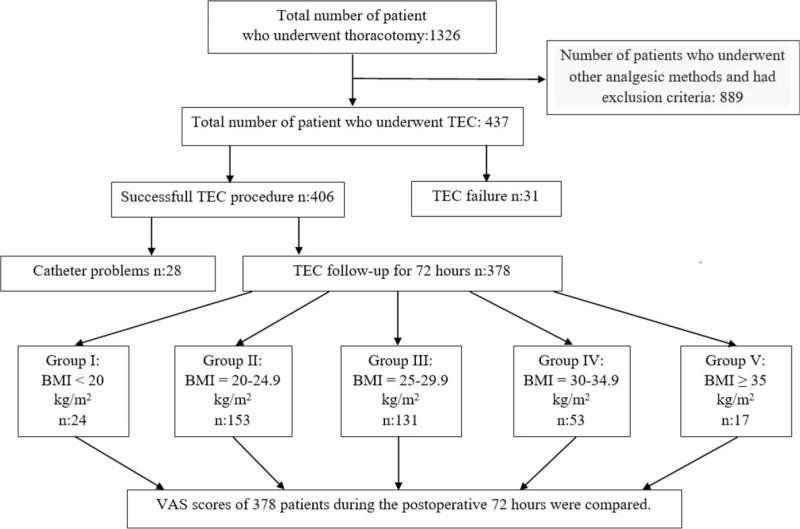
Data recorded include patients’ age, BMI, gender, the type of surgery, the success of TEC, perioperative complications associated with TEC, VAS scores during the postoperative 72 hours, rescue analgesia, and patient satisfaction. Kinking of the catheter in the postoperative 72 hours, dislocation of the catheter, and catheter occlusion were described as “catheter problems”. Patient satisfaction was categorized as “I am satisfied”, “I am moderately satisfied”, and “I am not satisfied”. Based on BMI, patients were categorized into the following 5 groups: Group I: BMI < 20 kg/m2, Group II: BMI = 20 to 24.9 kg/m2, Group III: BMI = 25 to 29.9 kg/m2, Group IV: BMI = 30 to 34.9 kg/m2, and Group V: BMI ≥ 35 kg/m2 as shown in Figure 1.
Figure 1.
Patient flowchart. BMI = body mass index, TEC = thoracic epidural catheterization, VAS = visual analog scale.
2.1. TEC and TEA protocols
The routine procedure in our hospital is to administer an intravenous (iv) injection of 25 mcg fentanyl before catheter insertion to avoid any potential occurrence of anxiety and periprocedural pain. The skin is cleansed in strict compliance with the principles of antisepsis while the patient is in the sitting position. After draping the patient, the skin is anesthetized by administering 3 ml of 2% prilocaine. Using an 18 G Tuohy needle, the epidural space is entered from the thoracic (T) 5 toto T6 or T6 to T7 intervertebral space using the median approach and the hanging drop method. A 4 to 5 cm portion of the catheter is left in the epidural space. In order to avoid a vascular or intrathecal injection, a test dose [5 μg/ml (1/200,000) adrenaline and 3 ml of 2% lidocaine] is administered via the catheter. The patients are then placed in the supine position. The following are considered TEC failure: performing more than 2 attempts, duration of TEC procedure longer than 10 minutes, difficulty advancing the catheter, blood coming out of the catheter, and a dural puncture.
Induction of general anesthesia was performed by using an iv injection of 2 mg/kg propofol, 0.1 mg/kg vecuronium, and 1 μg/kg fentanyl are performed. Maintenance of general anesthesia was performed with the use of 2% to 2.5% sevoflurane in an O2/air mixture and iv infusion of remifentanil (0.01 to 0.20 mcg/kg/min). Vecuronium 0.03 mg/kg is intravenously administered as needed in order to maintain the neuromuscular blockade. At the end of the surgical procedure, the patients are given iv injections of 50 mg dexketoprofen and 100 mg tramadol to provide analgesia and 10 mg iv metoclopramide to obtain an antiemetic effect. After the operation is concluded and prior to awakening the patient, an epidural administration of 0.125% bupivacaine as a mixture of 67.5 ml bupivacaine 0.5%, 201.5 ml normal saline, and 10 mg morphine is performed via a 270 ml-elastomeric infusion pump at a rate of 4 ml/h to be continued for 3 days postoperatively. After surgery, patients were kept in the postoperative intensive care unit for 1 day, and given paracetamol 1 gm intravenously every 8 hours for multimodal analgesia. Patients with VAS scores of ≥4 were received 50 mg tramadol intravenously. After postoperative intensive care unit, patients were received dexketoprofen 25 mg tablet every 12 hours, paracetamol 500 mg every 8 hours, and tramadol 50 mg capsule every 12 hours.
2.2. Statistical analysis of data
Data were analyzed using IBM SPSS 25.0 (Armonk, NY: IBM Corp.) statistical package software. Frequency, percentage, mean, standard deviation, median, and interquartile range were used for descriptive statistics. Qualitative data were compared using the chi-square (χ2) test. The Kolmogorov-Smirnov test, skewness-kurtosis tests, and graphical methods (histogram, Q-Q Plot, steam-and-leaf plots, and Box plots) were used to test the conformity of the data to a normal distribution. Intergroup comparisons of quantitative data conforming to a normal distribution were performed using one-way ANOVA. When a difference was detected, the post-hoc Tukey HSD test was used to identify the origin of the difference. Statistical significance was accepted at α = 0.05.
3. Results
The data of 1326 patients, who underwent elective thoracic surgery were evaluated. Similarly, the data of a total of 437 patients who underwent elective thoracic surgery and TEC was examined (Fig. 1).
Demographic data of patients are presented in Table 1. There were no statistically significant differences in TEC success across different BMI groups (P > .05) (Table 2).
Table 1.
Demographic characteristics of patients.
| n = 437 Mean ± SD | % Median (IQR) | |
| ASA | ||
| II | 239 | 54.7 |
| III | 198 | 45.3 |
| Age (yrs) | 53.3 ± 14.4 | 56.0 (45.5-64.0) |
| Gender | ||
| Women | 102 | 23.3 |
| Men | 335 | 76.7 |
| BMI | 25.8 ± 4.5 | 26.0 (23.0-28.0) |
| Group I: <20 kg/m2 | 26 | 6 |
| Group II: 20-24.9 kg/m2 | 171 | 39.1 |
| Group III: 25-29.9 kg/m2 | 154 | 35.2 |
| Group IV: 30-34.9 kg/m2 | 64 | 14.7 |
| Group V: ≥35 kg/m2 | 22 | 5.0 |
| Type of surgery | ||
| Segmentectomy, lobectomy | 334 | 76.4 |
| Pneumonectomy | 57 | 13.0 |
| Total decortication | 30 | 6.9 |
| Hydatid cyst, cystectomy | 16 | 3.7 |
| Duration of surgery (min) | 240.4 ± 32.3 | 235.0 (215.0-255.0) |
Data were presented as mean ± standard deviation and percentage/median interquartile range.
ASA = American Society of Anesthesiologists, BMI = body mass index, IQR = interquartile range, TEC = thoracic epidural catheterization.
Table 2.
Thoracic epidural catheterization success by body mass index groups.
| TEC | Group I (n = 26) | Group II (n = 171) | Group III (n = 154) | Group IV (n = 64) | Group V (n = 22) | P ∗ |
| Success | 25 (96.2%) | 160 (93.6%) | 139 (90.3%) | 61 (95.3%) | 21 (95.5%) | .567 |
| Failure | 1 (3.8%) | 11 (6.4%) | 15 (9.7%) | 3 (4.7%) | 1 (4.5%) |
TEC = thoracic epidural catheterization.
Chi-Square test (n/%).
It was found that the occurrence of catheter problems within the postoperative 72-hour period was statistically significant across the groups (P < .05) and that such problems increased along with increasing BMI values (Table 3).
Table 3.
Distribution of catheter problems across body mass index groups within the postoperative 72-h period.
| TEC was performed | Group I (n = 25) | Group II (n = 160) | Group III (n = 139) | Group IV (n = 61) | Group V (n = 21) | P ∗ | Difference |
| Catheter problems | 1 (4%) | 7 (4.4%) | 8 (5.8%) | 8 (13.1%) | 4 (19.0%) | .030 | † |
TEC = Thoracic epidural catheterization.
Chi-Square test (n/%).
IV-V vs I-II-III.
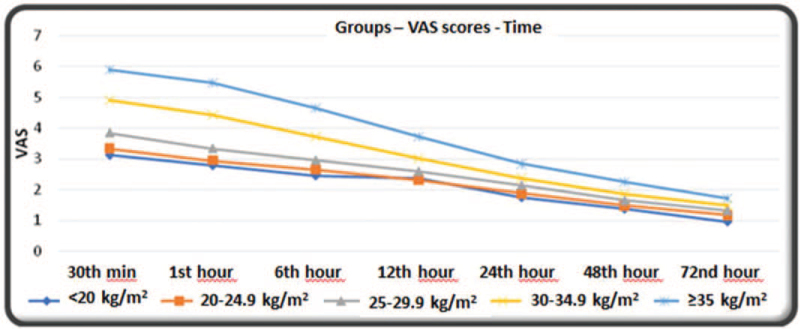
VAS scores at all matched time points were statistically significant across the groups (P < .05) and increased as the BMI value increased (Table 4, Fig. 2).
Table 4.
Visual analog scale scores of patients by body mass index.
| VAS | Group I (n = 24) | Group II (n = 153) | Group III (n = 131) | Group IV (n = 53) | Group V (n = 17) | P ∗ | Difference |
| 30th min | 3.1 ± 0.9 | 3.3 ± 1.1 | 3.8 ± 1.3 | 4.9 ± 1.0 | 5.9 ± 1.1 | <.001 | † |
| 1st h | 2.8 ± 0.7 | 2.9 ± 0.9 | 3.3 ± 1.2 | 4.4 ± 1.1 | 5.5 ± 1.1 | <.001 | ‡ , § , || |
| 6th h | 2.5 ± 0.5 | 2.6 ± 0.8 | 3.0 ± 1.1 | 3.7 ± 1.1 | 4.6 ± 1.1 | <.001 | § , || |
| 12th h | 2.4 ± 0.5 | 2.3 ± 0.8 | 2.6 ± 1.0 | 3.0 ± 1.0 | 3.7 ± 0.8 | <.001 | § , || |
| 24th h | 1.8 ± 0.6 | 1.9 ± 0.7 | 2.1 ± 1.0 | 2.4 ± 1.1 | 2.8 ± 0.9 | <.001 | ¶ , # |
| 48th h | 1.4 ± 0.5 | 1.5 ± 0.6 | 1.6 ± 0.9 | 1.9 ± 1.0 | 2.2 ± 0.7 | <.001 | # , ∗∗ |
| 72nd h | 1.0 ± 0.4 | 1.2 ± 0.6 | 1.3 ± 0.8 | 1.5 ± 1.0 | 1.7 ± 0.6 | <.001 | †† |
VAS = visual analog scale.
One-Way ANOVA (Mean ± SD).
All but I-II.
II vs III.
IV vs others.
V vs others.
IV vs I-II.
V vs I-II-III.
IV vs II.
I-II vs IV-V.
Figure 2.
VAS scores of patients in the postoperative 72-h period, VAS = visual analog scale.
Rates of additional analgesic use were statistically significant across the groups (P < .05). The rates were higher in BMI groups of 30 toto 34.9 kg/m2 and ≥ 35 kg/m2 compared to the other BMI groups. Additional analgesic use was also higher in the BMI group of 25 toto 29.9 kg/m2 compared to BMI groups of < 20 kg/m2 and 20 tototo 24.9 kg/m2. Increasing values of BMI were found to be associated with higher rates of additional analgesic use (Table 5).
Table 5.
Patients’ need for rescue analgesia by body mass index.
| Group I (n = 24) | Group II (n = 153) | Group III (n = 131) | Group IV (n = 53) | Group V (n = 17) | P ∗ | Difference | ||
| Rescue Analgesia | No | 22 (91.7%) | 137 (89.5%) | 97 (74.0%) | 17 (32.1%) | 1 (5.9%) | <.001 | † , ‡ |
| Yes | 2 (8.3%) | 16 (10.5%) | 34 (26.0%) | 36 (67.9%) | 16 (94.1%) |
Chi-Square Test (n/%).
III vs I-II.
IV-V vs I-II-III.
Patient satisfaction was statistically significant across groups (P < .05). Except for the lack of a difference between BMI groups of <20 kg/m2 and 20 to 24.9 kg/m2, patient satisfaction was different between all groups. Patient satisfaction rates were found to be poorer in association with increasing BMI values (Table 6).
Table 6.
Patient satisfaction by body mass index.
| Group I (n = 24) | Group II (n = 153) | Group III (n = 131) | Group IV (n = 53) | Group V (n = 17) | P ∗ | Difference | |
| Not satisfied† | – | 1 (0.7%) | – | – | – | <.001 | ‡ |
| Moderately satisfied | 1 (4.2%) | 9 (5.9%) | 32 (24.4%) | 30 (56.6%) | 14 (82.4%) | ||
| Satisfied | 23 (95.8%) | 143 (93.4%) | 99 (75.6%) | 23 (43.4%) | 3 (17.6%) |
Chi-Square test (n/%).
The groups “I am not satisfied” and “I am moderately satisfied” were combined for intergroup comparisons.
All but I-II.
The distribution of complications was not statistically significant across the BMI groups (P > .05), (Table 7).
Table 7.
Distribution of complications by body mass index.
| Group I (n = 24) | Group II (n = 153) | Group III (n = 131) | Group IV (n = 53) | Group V (n = 17) | P ∗ | Difference | |
| Headache | |||||||
| No | 21 (87.5%) | 143 (93.5%) | 123 (93.9%) | 52 (98.1%) | 17 (100%) | .324 | – |
| Yes | 3 (12.5%) | 10 (6.5%) | 8 (6.1%) | 1 (1.9%) | – | ||
| Nausea–vomiting | |||||||
| No | 20 (83.3%) | 135 (88.2%) | 108 (82.4%) | 46 (86.8%) | 16 (94.1%) | .540 | – |
| Yes | 4 (16.7%) | 18 (11.8%) | 23 (17.6%) | 7 (13.2%) | 1 (5.9%) | ||
| Hypotension | |||||||
| No | 20 (83.3%) | 122 (79.7%) | 109 (83.2%) | 46 (86.8%) | 15 (88.2%) | .740 | – |
| Yes | 4 (16.7%) | 31 (20.3%) | 22 (16.8%) | 7 (13.2%) | 2 (11.8%) | ||
| Pruritus | |||||||
| No | 23 (95.8%) | 140 (91.5%) | 125 (95.4%) | 47 (88.7%) | 17 (100%) | .301 | – |
| Yes | 1 (4.2%) | 13 (8.5%) | 6 (4.6%) | 6 (11.3%) | – | ||
| Sweating | |||||||
| No | 24 (100%) | 151 (98.7%) | 126 (96.2%) | 51 (96.2%) | 17 (100%) | .499 | – |
| Yes | – | 2 (1.3%) | 5 (3.8%) | 2 (3.8%) | – | ||
| Bradycardia | |||||||
| No | 24 (100%) | 150 (98.0%) | 128 (97.7%) | 53 (100%) | 17 (100%) | .723 | – |
| Yes | – | 3 (2.0%) | 3 (2.3%) | – | – | ||
| Respiratory arrest | |||||||
| No | 24 (100%) | 153 (100%) | 131 (100%) | 53 (100%) | 17 (100%) | 1.000 | – |
| Yes | (0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | ||
Chi-Square test (n/%).
4. Discussion
Our study has shown that increase in BMI negatively affect the pain scores in the patients that underwent TEC for pain management following thoracotomy. We have demonstrated that patients’ postoperative pain scores increased in association with increasing BMI. Consequently, high BMI values have been associated with additional analgesic needs and poor patients’ satisfaction. TEC success and rates of TEC-related complications were similar across all groups.
The postoperative analgesic efficacy of TEA is quite high. However, many factors affect analgesic success after TEC.[1,3,6] Several conditions such as type of anesthesia, method of administration of local anesthetics, level of TEC, the patient's height and age, type of the operation, and obesity can be listed as factors that act on the efficacy of the procedure.[6,13–15] Obesity which is one of the most important of these factors, may adversely affect the success of the TEC, the pharmacokinetics of the drugs used, and the anatomy of the epidural space.[9,14,15] Although the spread of local anesthetic given to the epidural space has not been fully depicted yet, it has been shown that solutions injected into the epidural space generally disseminate freely and cover the cylindrical dural sac while partially passing through the neural foramina.[15,16] Inter-individual variability in epidural adipose tissue may act on the pharmacokinetics of drugs injected into the epidural space based on whether the given local anesthetic has a significant affinity for epidural fat. Consequently, the transport of drugs to target tissues and the duration of anesthesia are affected as potential mechanisms that may be responsible for the inter-individual variability in the spread and the intensity of epidural anesthesia.[16] Studies have shown that dural surface area affects the spread of epidural anesthesia and that posterior epidural fat volume affects the duration of epidural anesthesia. The velocity of the flow in the epidural venous plexus may affect the duration of epidural anesthesia.[17] Wu et al[18] examined the association of the amount of epidural fat with body weight, height, BMI, and obesity. That study reported that the amount of epidural adipose tissue was not associated with BMI and obesity but posterior epidural fat deposition was correlated with the body weight. In another study, Alicioglu et al[19] found that the amount of lumbar epidural adipose tissue was not clearly correlated with age, gender, or BMI. However, those studies have examined only lumbar epidural procedures. The literature indicates only a few studies are available that demonstrate the association between BMI and the spread of local anesthetics in the thoracic epidural space. Although we may not provide a direct interpretation based on these results, the increase in pain scores along with increases in BMI in our study suggests that BMI may affect the spread of the local anesthetic in the epidural space. In addition, albeit limited, high amounts of posterior epidural adipose tissue may be associated with a high rate of local anesthetic absorption. Future large case series and studies on cadavers may clarify the role of BMI in the spread of local anesthetics.
The considerable increase in the prevalence of obesity has become a threat to public health in recent years,[20] bringing along many complications and failures to the regional analgesia practice. Studies relating the association of obesity and regional anesthesia have usually been performed about the subarachnoid block and the lumbar epidural block in the field of obstetric anesthesia. Studies have reported that, in obese pregnant women, the cephalad spread of the local anesthetic becomes fast and the efficacy of the local anesthetic increases because of the increased abdominal pressure and engorged epidural veins, leading to potential untoward effects including hypotension and bradycardia. Therefore, it has been suggested that the dose of the local anesthetic should be lowered to reduce complications.[21,22] However, we believe that the aforementioned explanation does not depict the proposed mechanism of the efficacy of epidural anesthesia occurring via the spread of the local anesthetic to the subarachnoid area through relevant foramina. In our study, we performed a retrospective data analysis on patients, who underwent continuous local anesthetic infusion through the thoracic epidural catheter. Considering local anesthetics were not administered at high dosages or volumes, rates of hypotension and other potentially related complications were similar across all groups. The occurrence of only a few hemodynamic side effects in our study may be associated with the administration of the local anesthetics at a low dosage and a small volume.
Besides being associated with high rates of postoperative complications, obesity acts unfavorably on success rates of interventions.[7,13,14] These effects become even more prominent in interventions, such as TEC, requiring substantial experience. Failure rates of TEC may be as high as 30% when applied by trainees.[23,24] Moreover, the procedure may even become more complicated when the deposition of subcutaneous fat at the planned site of the thoracic epidural intervention in obese patients makes the identification of relevant anatomical landmarks difficult.[11] In our study, BMI and TEC failure were not correlated and failure rates were quite low. We suggest that this result may be associated with the fact that experienced anesthesiologists performed the TEC in thoracic anesthesia in our study.
One of the major problems in obese patients is the high rates of postoperative epidural catheter problems resulting in the interruption of continuous and effective pain management.[8] Catheter securement failures due to sweating, edema, and other similar reasons at the catheterization site, or the catheter's occlusion, kinking, or displacement due to difficulties in the appropriate patient positioning are listed as potential extrinsic problems.[25–27] Changes in the patient's position and the position of catheter insertion may cause catheter displacements in the postoperative period.[27] Although not well-defined, Hamza et al[28] have reported that the epidural distance is longer in the lateral decubitus position compared to the sitting position as a finding more commonly seen in obese patients. We excluded patients with catheter problems in our study but catheter problems were more common in the obese patients with BMI values of 30 kg/m2 and above. Therefore, close follow-up of the catheter is crucial for effective pain management, especially in obese patients.
There are few limitations in this study. Firstly, the study has a retrospective and single-center design. Secondly, TEC success was achieved in all patients in general but data by dermatomal levels were not recorded. If data about dermatomal levels were available, we could have a clue about dermatomal blockade levels and might conclude about how the spread of the local anesthetic was affected by obesity. In addition, we did not evaluate postoperative failures due to catheter dysfunction because our primary aim was to investigate the relationship between VAS scores and obesity. Further comprehensive and prospective studies may provide precise results about the relationship between BMI and VAS scores in patients undergoing TEC.
5. Conclusion
There is a positive correlation between BMI and VAS scores in patients, who underwent TEC for pain management following thoracotomy. This correlation was supported by the increased need for additional analgesics in patients with high BMI. Therefore, patients with high BMI values should be followed up closely. However, this finding has not been reflected in complication rates across groups. Furthermore, no significant correlations were observed between BMI and TEC success rates.
Author contributions
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conceptualization: MZ, AA.
Data curation: MZ, RB, AA.
Formal analysis: GU, RB, HS.
Investigation: GU, RB, HS.
Methodology: MZ, GU, HS, AA.
Project administration: MZ, HS, AA.
Resources: MZ, GU, RB.
Supervision: MZ, HS, AA.
Visualization: MZ, HS, AA.
Writing – original draft: MZ, AA.
Writing – review & editing: HS, AA.
Footnotes
Abbreviations: BMI = body mass index, IQR = interquartile range, iv = intravenous, T = thoracic, TEA = thoracic epidural analgesia, TEC = thoracic epidural catheterization, VAS = visual analog scale.
How to cite this article: Zengin M, Ulger G, Baldemir R, Sazak H, Alagoz A. Is there a relationship between body mass index and postoperative pain scores in thoracotomy patients with thoracic epidural analgesia?. Medicine. 2021;100:50(e28010).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration (as revised in 2013) and its later amendments or comparable ethical standards.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin 2008;26:355–67. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reyad RM, Shaker EH, Ghobrial HZ, et al. The impact of ultrasound-guided continuous serratus anterior plane block versus intravenous patient-controlled analgesia on the incidence and severity of post-thoracotomy pain syndrome: a randomized, controlled study. Eur J Pain 2020;24:159–70. Epub 2019 Sep 6. [DOI] [PubMed] [Google Scholar]
- [3].Sztain JF, Gabriel RA, Said ET. Thoracic epidurals are associated with decreased opioid consumption compared to surgical infiltration of liposomal bupivacaine following video-assisted thoracoscopic surgery for lobectomy: a retrospective cohort analysis. J Cardiothorac Vasc Anesth 2019;33:694–8. Epub 2018 Jun 23. [DOI] [PubMed] [Google Scholar]
- [4].Ciftci B, Ekinci M, Celik EC, Tukac IC, Bayrak Y, Atalay YO. Efficacy of an ultrasound-guided erector spinae plane block for postoperative analgesia management after video-assisted thoracic surgery: a prospective randomized study. J Cardiothorac Vasc Anesth 2020;34:444–9. Epub 2019 Apr 30. [DOI] [PubMed] [Google Scholar]
- [5].Osman YMM, El-Gamal N. Acoustic puncture assist device versus ultrasound imaging technique for thoracic epidural space identification in obese patients. Res Opin Anesth Intensive Care 2018;5:141.doi: 10.4103/roaic.roaic_49_17. [Google Scholar]
- [6].Rawal N. Epidural technique for postoperative pain: gold standard no more? Reg Anesth Pain Med 2012;37:310–7. [DOI] [PubMed] [Google Scholar]
- [7].Ingrande J, Brodsky JB, Lemmens HJ. Regional anesthesia and obesity. Curr Opin Anaesthesiol 2009;22:683–6. [DOI] [PubMed] [Google Scholar]
- [8].Motamed C, Farhat F, Rémérand F, Stéphanazzi J, Laplanche A, Jayr C. An analysis of postoperative epidural analgesia failure by computed tomography epidurography. Anesth Analg 2006;103:1026–32. [DOI] [PubMed] [Google Scholar]
- [9].Saryazdi HHG, khalili G, Talakoub R, Shahbazi M, Abbasi S. Distribution of bupivacaine in epidural space. J Cell Mol Anesth 2018;3:60–5. [Google Scholar]
- [10].Lee I, Yamagishi N, Oboshi K, Yamada H. Distribution of new methylene blue injected into the lumbosacral epidural space in cats. Vet Anaesth Analg 2004;31:190–4. [DOI] [PubMed] [Google Scholar]
- [11].Lee I, Yamagishi N, Oboshi K, Yamada H. Eliminating the effect of epidural fat during dorsolumbar epidural analgesia in cattle. Vet Anaesth Analg 2004;31:86–9. [DOI] [PubMed] [Google Scholar]
- [12].Gorgi AA, Hofmeister EH, Higginbotham MJ, Kent M. Effect of body position on cranial migration of epidurally injected methylene blue in recumbent dogs. Am J Vet Res 2006;67:219–21. [DOI] [PubMed] [Google Scholar]
- [13].von Ungern-Sternberg BS, Regli A, Reber A, Schneider MC. Effect of obesity and thoracic epidural analgesia on perioperative spirometry. Br J Anaesth 2005;94:121–7. Epub 2004 Oct 14. [DOI] [PubMed] [Google Scholar]
- [14].Alagoz A, Sazak H, Tunc M, et al. Teaching practices of thoracic epidural catheterizations in different grade of anesthesia residents. Braz J Anesthesiol 2016;66:01–6. Epub 2014 Oct 27. [DOI] [PubMed] [Google Scholar]
- [15].Bromage PR. Spread of analgesic solutions in the epidural space and their site of action: a statistical study. Br J Anaesth 1962;34:161–78. [DOI] [PubMed] [Google Scholar]
- [16].Hogan QH. Lumbar epidural anatomy. A new look by cryomicrotome section. Anesthesiology 1991;75:767–75. [DOI] [PubMed] [Google Scholar]
- [17].Higuchi H, Adachi Y, Kazama T. Factors affecting the spread and duration of epidural anesthesia with ropivacaine. Anesthesiology 2004;101:451–60. [DOI] [PubMed] [Google Scholar]
- [18].Wu HT, Schweitzer ME, Parker L. Is epidural fat associated with body habitus? J Comput Assist Tomogr 2005;29:99–102. [DOI] [PubMed] [Google Scholar]
- [19].Alicioglu B, Sarac A, Tokuc B. Does abdominal obesity cause increase in the amount of epidural fat? Eur Spine J 2008;17:1324–8. Epub 2008 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Biener A, Cawley J, Meyerhoefer C. The high and rising costs of obesity to the US health care system. J Gen Intern Med 2017;32: (Suppl 1): 06–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hodgkinson R, Husain FJ. Obesity and the cephalad spread of analgesia following epidural administration of bupivacaine for Cesarean section. Anesth Analg 1980;59:89–92. [PubMed] [Google Scholar]
- [22].Vricella LK, Louis JM, Mercer BM, Bolden N. Impact of morbid obesity on epidural anesthesia complications in labor. Am J Obstet Gynecol 2011;205:370.e1-6. Epub 2011 Jun 29. [DOI] [PubMed] [Google Scholar]
- [23].Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: causes and management. Br J Anaesth 2012;109:144–54. Epub 2012 Jun 26. [DOI] [PubMed] [Google Scholar]
- [24].Yeap YL, Randolph T, Lemmon AJ, Mann MD, Stewart J, Wolfe JW. Effect of prior formal education on successful thoracic epidural placement by anesthesia residents. J Cardiothorac Vasc Anesth 2020;34:3044–8. Epub 2020 Jun 12. [DOI] [PubMed] [Google Scholar]
- [25].McLeod G, Davies H, Munnoch N, Bannister J, MacRae W. Postoperative pain relief using thoracic epidural analgesia: outstanding success and disappointing failures. Anaesthesia 2001;56:75–81. [DOI] [PubMed] [Google Scholar]
- [26].Gartrell P. Disappearing epidural catheters. Anaesth Intensive Care 1992;20:121–2. [PubMed] [Google Scholar]
- [27].Hamilton CL, Riley ET, Cohen SE. Changes in the position of epidural catheters associated with patient movement. Anesthesiology 1997;86:778–84. discussion 29A. [DOI] [PubMed] [Google Scholar]
- [28].Hamza J, Smida M, Benhamou D, Cohen SE. Parturient's posture during epidural puncture affects the distance from skin to epidural space. J Clin Anesth 1995;7:01–4. [DOI] [PubMed] [Google Scholar]