PURPOSE
The primary aim of this phase III randomized trial was to test whether the addition of vincristine, topotecan, and cyclophosphamide (VTC) to interval compressed chemotherapy improved survival outcomes for patients with previously untreated nonmetastatic Ewing sarcoma.
METHODS
Patients were randomly assigned to receive standard five-drug interval compressed chemotherapy (regimen A) for 17 cycles or experimental therapy with five cycles of VTC within the 17 cycles (regimen B). Patients were stratified by age at diagnosis (< 18 years and ≥18 years) and tumor site (pelvic bone, nonpelvic bone, and extraosseous). Tumor volume at diagnosis was categorized as < 200 mL or ≥ 200 mL. Local control occurred following six cycles. Histologic response was categorized as no viable or any viable tumor. Event-free survival (EFS) and overall survival (OS) were compared between randomized groups with stratified log-rank tests.
RESULTS
Of 642 enrolled patients, 309 eligible patients received standard and 320 received experimental therapy. The 5-year EFS and OS were 78% and 87%, respectively. There was no difference in survival outcomes between randomized groups (5-year EFS regimen A v regimen B, 78% v 79%; P = .192; 5-year OS 86% v 88%; P = .159). Age and primary site did not affect the risk of an EFS event. However, age ≥ 18 years was associated with an increased risk of death at 5 years (hazard ratio 1.84; 95% CI, 1.15 to 2.96; P = .009). The 5-year EFS rates for patients with pelvic, nonpelvic bone, and extraosseous primary tumors were 75%, 78%, and 85%, respectively. Tumor volume ≥ 200 mL was significantly associated with lower EFS.
CONCLUSION
While VTC added to five-drug interval compressed chemotherapy did not improve survival, these outcomes represent the best survival estimates to date for patients with previously untreated nonmetastatic Ewing sarcoma.
INTRODUCTION
Ewing sarcoma (EWS) represents a rare childhood malignancy for which continued revision of conventional chemotherapy has led to progressively improved outcomes. Most notably, improved patient survival was achieved when the combination of etoposide and ifosfamide was included in a regimen that contained 17 cycles of chemotherapy,1 and once again achieved with dose intensification through the compression of cycle intervals to 2 weeks in a 14-cycle regimen.2 Despite these advances, a substantial proportion of patients with nonmetastatic EWS experience disease recurrence, with low likelihood of cure postrelapse.3
CONTEXT
Key Objective
To evaluate the impact of the addition of vincristine, topotecan, and cyclophosphamide to interval compressed chemotherapy on the survival of patients with previously untreated nonmetastatic Ewing sarcoma.
Knowledge Generated
Among 642 patients enrolled, of whom 629 were randomly assigned, the experimental therapy did not significantly improve survival outcomes, but the 78% and 87% 5-year event-free and overall survival rates, respectively, are the best reported estimates to date.
Relevance
Clinical and response biomarkers identify patients at risk for an event-free survival event within the context of interval compressed chemotherapy, which is identified as the optimal systemic therapy for previously untreated patients with nonmetastatic Ewing sarcoma.
Topotecan with cyclophosphamide (TC) is an effective chemotherapy combination for children with recurrent or metastatic EWS. TC resulted in a sustained 2- to 21-month complete response or partial response in 35% of heavily pretreated patients with relapsed refractory EWS4 and a 33% partial response rate in registry data for an additional n = 54 recurrent or refractory patients.5 In the frontline setting, TC was also effective for patients with newly diagnosed metastatic EWS, 57% of whom experienced a partial response to TC.6 An evaluation of the addition of TC to therapy in the newly diagnosed localized setting was considered a critical question and necessitated 17 chemotherapy cycles in a randomized study maintaining delivery of doxorubicin, ifosfamide, and etoposide consistent with previous phase III trials.1,2,7,8 Vincristine was added to TC based on single-agent activity of vincristine in EWS9 and synergistic activity with topotecan.10 The Children's Oncology Group (COG) demonstrated the feasibility of delivering vincristine, topotecan, and cyclophosphamide (VTC) in the context of interval compressed chemotherapy, with no unanticipated toxicity.11
COG conducted AEWS1031 to investigate the effect of adding VTC to five-drug interval compressed chemotherapy for newly diagnosed patients with nonmetastatic EWS of bone and soft tissue. This report provides the primary outcome of this randomized phase III trial, as well as survival outcomes by stratified parameters of age and primary tumor site, and survival outcomes by local control, primary tumor volume (TV), and histologic response to induction therapy.
METHODS
AEWS1031 was a phase III randomized trial of the addition of VTC to five-drug interval compressed chemotherapy in the treatment of patients with newly diagnosed nonmetastatic EWS (ClinicalTrials.gov identifier: NCT01231906).
Eligibility
Patients were eligible if they were younger than 50 years old at the time of a biopsy-confirmed new diagnosis of extracranial nonmetastatic EWS or primitive neuroectodermal tumor of bone or soft tissue. The diagnosis of EWS was established by an institutional pathologist, and central review was not required. Molecular confirmation of EWSR1 gene rearrangement was recommended, but not required. Primary site required three-dimensional radiographic imaging. Staging studies included chest computed tomography, whole-body bone scan, and bilateral bone marrow aspirate and biopsies. Computed tomography with positron emission tomography was recommended. Eligibility necessitated protocol-defined adequate renal, liver, and cardiac function. No prior chemotherapy or radiation therapy was permitted. Patients with a chest wall primary and ipsilateral pleural effusions, ipsilateral positive pleural cytology, or ipsilateral pleural-based secondary tumor nodules were eligible, as were patients with regional lymph node involvement. Exclusion criteria included distant metastases including solitary pulmonary nodules > 0.5 cm, or multiple pulmonary nodules > 0.3 cm unless biopsy-proven otherwise; EWS as a second malignant neoplasm (SNM); patients with dural or intradural tumors; and pregnancy or breastfeeding. All patients and or their parents or legal guardians signed a written informed consent approved by institutional review board, and all institutional, US Food and Drug Administration, and National Cancer Institute requirements for human studies were met.
Study Design and Treatment
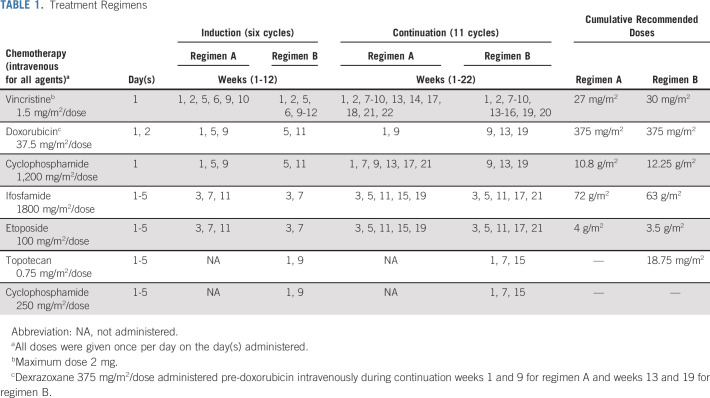
Patients were randomly assigned at the time of study entry to standard five-drug interval compressed chemotherapy (regimen A [the standard arm]) for 17 cycles or to experimental therapy with five cycles of VTC within the 17 cycles (regimen B [the experimental arm]). All patients received six cycles of induction therapy (12 weeks) and 11 cycles of consolidation therapy (22 weeks). Patients assigned to regimen A received five cycles of vincristine, doxorubicin, and cyclophosphamide, four cycles of vincristine and cyclophosphamide, and eight cycles of ifosfamide and etoposide. Patients assigned to regimen B received five cycles of VTC, five cycles of vincristine, doxorubicin, and cyclophosphamide, and seven cycles of ifosfamide and etoposide. Chemotherapy schedule and dose information are listed in Table 1.
TABLE 1.
Treatment Regimens
Dexrazoxane was administered only during the last two doxorubicin-containing cycles. All patients received myeloid growth factor support. Although granulocyte macrophage colony-stimulating factor was not permitted, the selection of myeloid growth factor was otherwise at the treating physicians' discretion.
Local tumor control was to take place upon recovery from induction chemotherapy typically at chronologic protocol week 13, whereas the mode of local control was determined by treating physicians. If surgery was performed for local control, consolidation therapy was to begin 1-2 weeks postoperatively allowing time for wound healing. For patients who received definitive radiation alone for local control, or who received preoperative radiation or as adjuvant for positive surgical margins, radiation and chemotherapy were given concurrently. If a combined modality approach was planned, preoperative radiation was recommended during weeks 1-4 of consolidation, with surgery as soon as possible thereafter. Further local control guidelines for rare tumor sites are included in the Data Supplement (online only).
Statistical Design
Outcome measures.
The primary outcome measure was event-free survival (EFS) defined as time from enrollment to (1) disease progression; (2) diagnosis of SMN; (3) death regardless of cause; or (4) date of last contact, whichever came first. Patients whose EFS follow-up was terminated because of reasons (1)-(3) were considered to have experienced an event; otherwise, the patient was censored at the time of last contact. Overall survival (OS) was defined as time from enrollment to death regardless of cause or date of last patient contact, whichever came first. Patients whose OS follow-up was terminated because of death were considered to have experienced an event; otherwise, the patient was censored at last contact.
Secondary objectives reported in this manuscript include survival outcomes by stratified parameters, by local control type, by TV, and response to chemotherapy at local control. Patients who completed induction according to protocol therapy and subsequently received surgery or radiation to the site of the primary tumor were considered for the analysis of the types and outcomes of local control modalities. EFS or OS after local control was calculated as EFS or OS as defined above minus the number of days from enrollment to local control surgery or the start of radiation therapy, whichever was later. The definitions of EFS event and OS event after local control were as defined above for EFS and OS. The analytical methodology used was identical to that used for EFS and OS.
Patients considered for the histologic assessment of tumor response (assessed as amount of viable tumor in the resection specimen) were those included in the analysis of local control modality who had surgery only or who had surgery followed by radiation therapy. EFS after surgery was calculated as above for EFS after local control, whereas the definitions of EFS event after surgery were as defined above for EFS. The analytical methodology used was identical to that used for EFS.
Study design.
The study was designed to enroll 630 eligible patients, which was estimated to take over 4.5 years. The first analysis was to occur 12 months after the last patient was enrolled. Random assignment was stratified according to age at enrollment (< 18 years v ≥ 18 years) and site of tumor (pelvic bone v nonpelvic bone v extraosseous). The study was designed to have 80% power to detect a relative hazard rate (RHR) of 0.63 associated with regimen B, using a 1-sided test of size 0.05. Detailed statistical properties of the design, interim monitoring, and analytical methods are described in the Data Supplement.
All statistical analyses were performed using SAS 9.4 (Cary, NC) or STATA 16.1 (College Station, TX). Except as specified by the study design, two-sided P values of .05 or less were considered statistically significant.
RESULTS
The study activated November 22, 2010, and was closed to patient accrual January 4, 2016. Data were released by the Data Safety and Monitoring Committee in March 2019, and data current to September 30, 2020, were used for this analysis.
Patient Characteristics
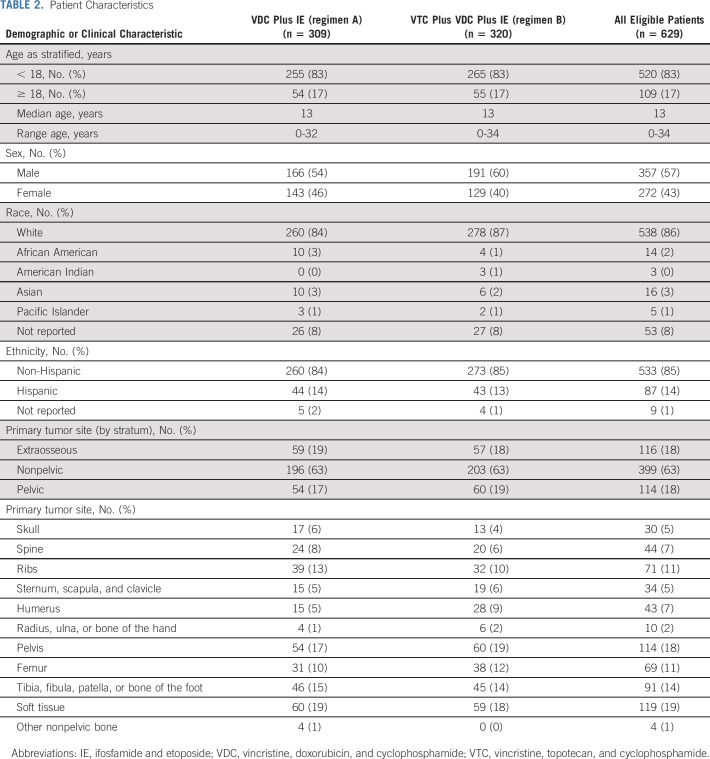
A total of 642 patients were enrolled, of whom 13 were considered ineligible (Fig 1). Of 629 eligible patients, 309 were randomly assigned to regimen A and 320 were randomly assigned to regimen B. Three patients did not receive any protocol therapy.
FIG 1.
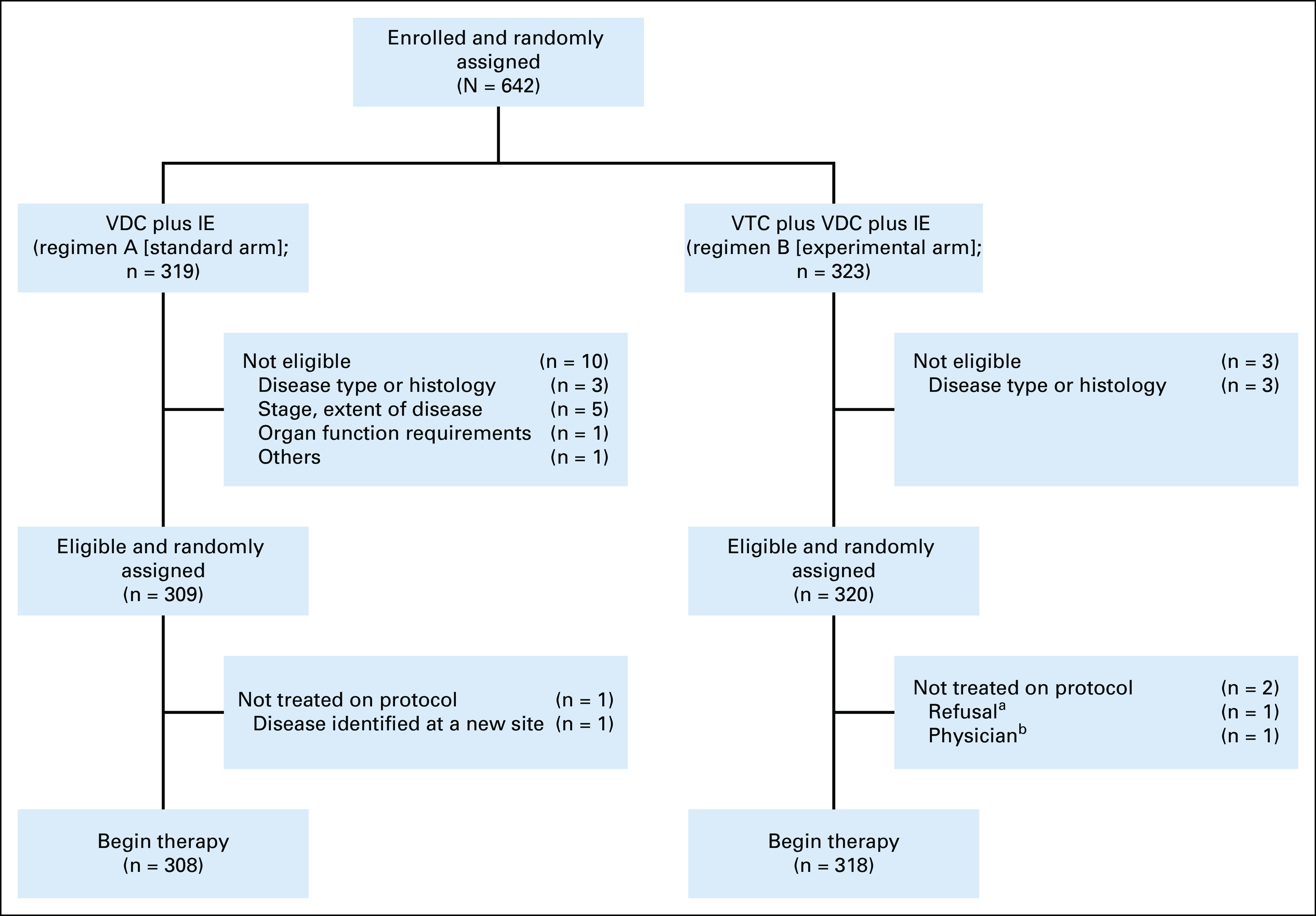
AEWS1031 CONSORT diagram. aRefusal of protocol therapy by patient, parent, or guardian. bPhysician considered administration of protocol therapy to not be in the patient's best interests. IE, ifosfamide and etoposide; VDC, vincristine, doxorubicin, and cyclophosphamide; VTC, vincristine, topotecan, and cyclophosphamide.
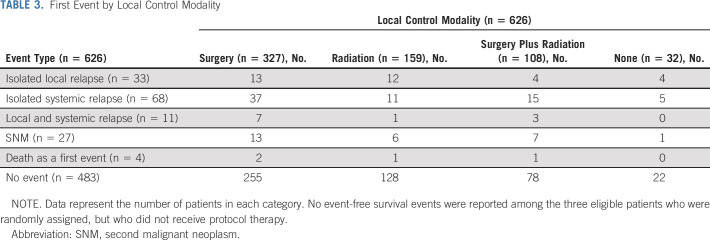
Patient characteristics were well balanced between randomized groups (Table 2). Eighty-three percent of patients were age < 18 years, 18% had pelvic primary tumors, and 18% had extraosseous primary tumors. Three hundred twenty-seven patients underwent surgery alone for local control, 159 patients received definitive radiation, and 108 patients had both (Table 3).
TABLE 2.
Patient Characteristics
TABLE 3.
First Event by Local Control Modality
Survival Outcomes
One hundred forty-three events were reported: 112 recurrences, 27 SNMs, and four deaths as a first event (Table 3). Estimated EFS and OS rates at 5 years for all eligible patients were 78% (95% CI, 75 to 81) and 87% (95% CI, 84 to 90), respectively (Data Supplement).
The 5-year EFS for patients randomly assigned to regimen A was 78% (95% CI, 72 to 82) compared with 79% (95% CI, 74 to 83) for regimen B (Fig 2A). The EFS treatment effect of the experimental arm (regimen B) compared with the standard arm (regimen A) was estimated as an RHR 0.86 (95% CI, 0.62 to 1.2; one-sided P value = .192). The 5-year OS for patients randomly assigned to regimen A was 86% (95% CI, 82 to 90) compared with 88% (95% CI, 84 to 91) for regimen B (Fig 2B). The OS treatment effect of the experimental arm compared with the standard arm was estimated as an RHR of 0.81 (95% CI, 0.54 to 1.2; one-sided P value = .159).
FIG 2.
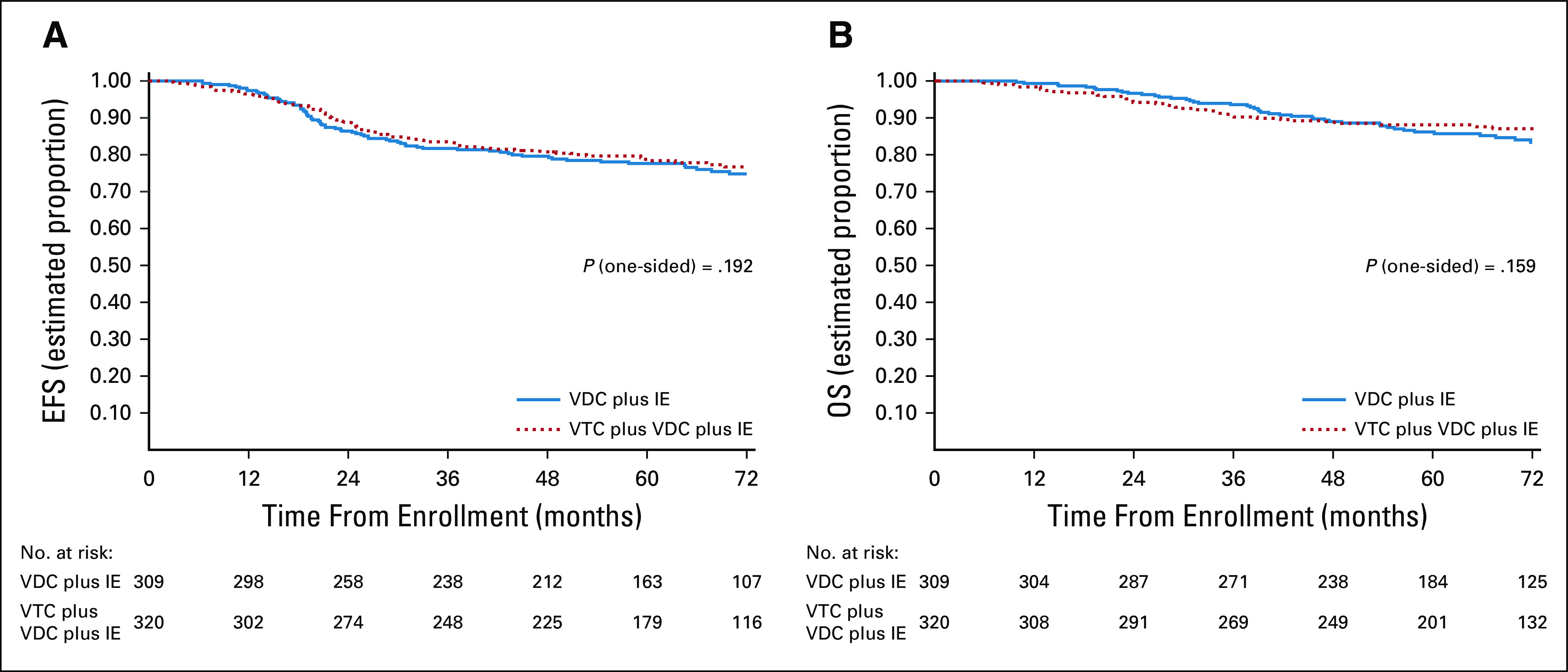
(A) EFS and (B) OS according to randomized treatment arm. Regimen A = VDC plus IE; regimen B = VTC plus VDC plus IE. EFS, event-free survival; IE, ifosfamide and etoposide; OS, overall survival; VDC, vincristine, doxorubicin, and cyclophosphamide; VTC, vincristine, topotecan, and cyclophosphamide.
Adverse Events
There were no differences in toxicities observed between treatment regimens (data not shown). Four patients experienced death as a first event (two each on regimens A and B). Fourteen SMN events occurred in patients on regimen A, whereas 13 SMN events occurred in patients treated on regimen B (Data Supplement).
Impact of Stratification Factors and Local Control
The 5-year EFS rates for patients < 18 years versus ≥ 18 years were 79% (95% CI, 75 to 82) and 75% (95% CI, 65 to 82), respectively (Fig 3A). Age ≥ 18 years was associated with an RHR for EFS event of 1.25 (95% CI, 0.82 to 1.9; P = .294). The 5-year OS rates for patients < 18 years versus 18+ years were 89% (95% CI, 86 to 91) and 78% (95% CI, 68 to 85), respectively (Fig 3B). Age ≥ 18 years was associated with an increased risk of death at 5-years with an RHR of 1.84 (95% CI, 1.15 to 2.96; P = .00993).
FIG 3.
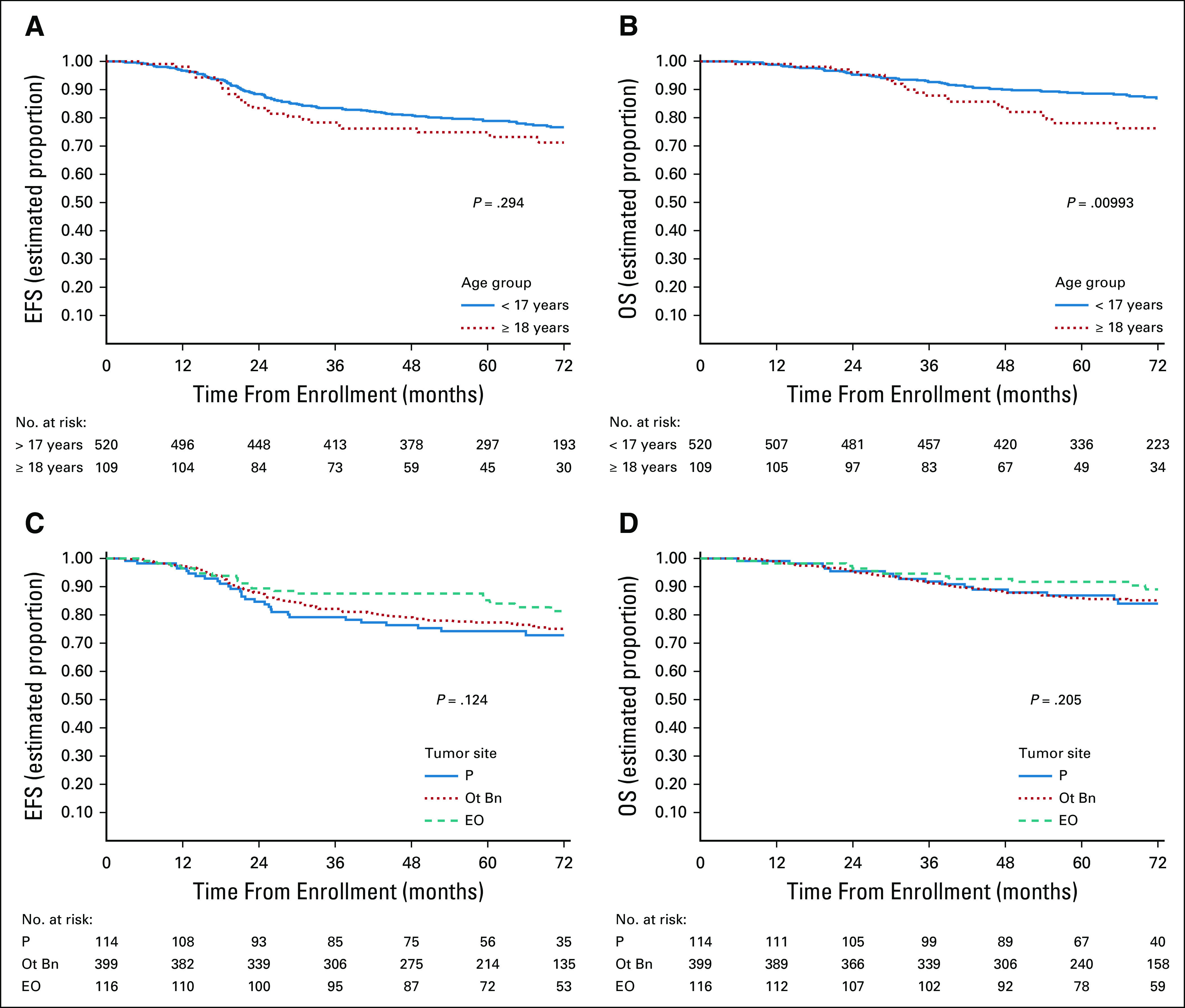
(A) EFS and (B) OS according to age; (C) EFS and (D) OS by primary site. EFS, event-free survival; EO, extraosseous; Ot Bn, nonpelvic bone; OS, overall survival; P, pelvic bone.
The 5-year EFS rates for patients with pelvic bone, nonpelvic bone, and extraosseous primary sites were 75% (95% CI, 65 to 82), 78% (95% CI, 73 to 81), and 85% (95% CI, 76 to 90), respectively (Fig 3C). Relative to pelvic bone primary tumors, the RHR for an EFS event associated with bone nonpelvic sites was 0.84 (95% CI, 0.56 to 1.26) and associated with extraosseous sites was 0.58 (95% CI, 0.33 to 1.03), with a global P = .124. The 5-year OS rates for patients with pelvic, nonpelvic bone, and extraosseous primary sites were 87% (95% CI, 79 to 92), 85% (95% CI, 82 to 89), and 92% (95% CI, 84 to 96), respectively (Fig 3D). Relative to pelvic bone primary tumors, the RHR for death for bone nonpelvic sites was 1.03 (95% CI, 0.61 to 1.73) and for extraosseous was 0.61 (95% CI, 0.29 to 1.29), with a global P = .205.
Local control was completed according to protocol recommendations for 594 patients (Table 3). No significant differences in postinduction survival were identified based on local control modality (Data Supplement). The 5-year EFS rates for patients who had surgery, radiation, or combined local control were 79% (95% CI, 74 to 83), 82% (95% CI, 75 to 87), and 74% (95% CI, 60 to 78), respectively (P = .3). Event types by local control are shown in Table 3.
Tumor Biomarkers
Institutional investigators reported three tumor dimensions in 587 of the 629 eligible patients who were considered in the analysis of TV. The 5-year EFS rates for patients with TV < 200 mL and ≥ 200 mL were 81% (95% CI, 2 to 85) and 71% (95% CI, 4 to 77), respectively (Fig 4A). TV ≥ 200 mL was associated with an RHR for an EFS event of 1.63 (95% CI, 1.16 to 2.31; P = .005).
FIG 4.
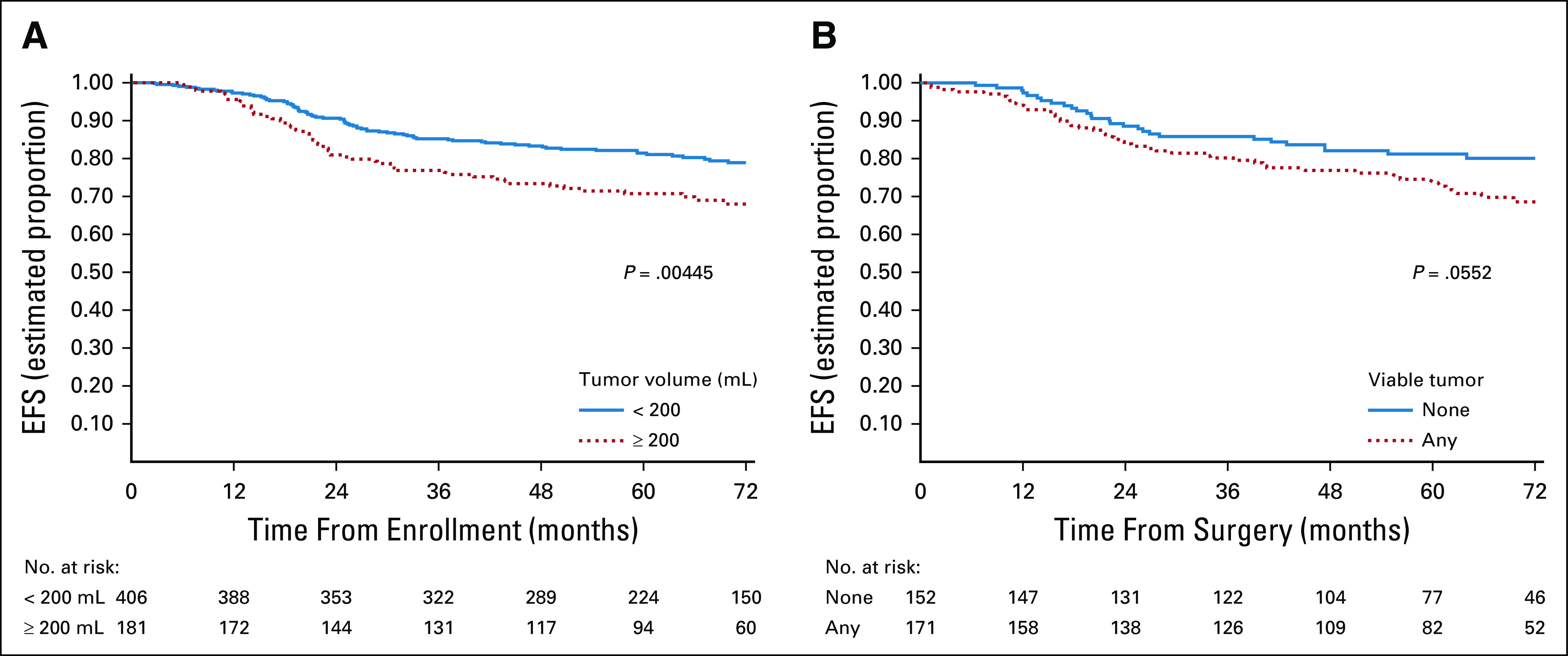
(A) EFS by tumor volume and (B) EFS by amount of viable tumor at time of local control surgery. EFS, event-free survival.
Three hundred twenty-three (323) patients contributed to an analysis of impact of viable tumor at local control. Two-hundred fifty patients were not evaluable for this analysis because: (1) 34 patients experienced an EFS event before any local control intervention; (2) 209 patients did not have surgery as a local control modality; and (3) seven patients had radiation therapy before surgical resection as local control. Data on the amount of viable tumor in the resection specimen were not available for an additional 56 patients. The 5-year EFS rates for patients with no viable tumor versus any viable tumor at the time of local control were 81% (95% CI, 3 to 87) and 75% (95% CI, 4 to 81), respectively (Fig 4B). Presence of any viable tumor was associated with an RHR for an EFS event of 1.57 (95% CI, 0.99 to 2.51; P = .055).
DISCUSSION
The addition of VTC to five-drug interval compressed chemotherapy did not improve EFS or OS for the study population. However, the 5-year EFS and OS in this trial represent the best reported outcomes for patients with previously untreated nonmetastatic EWS to date.
This trial further validates excellent patient outcomes with interval compressed chemotherapy, a finding also seen in preliminary results comparing interval compressed therapy with a prior European standard combining vincristine, ifosfamide, doxorubicin and etoposide.7,12 This study achieved further dose intensification to interval compression by the addition of a novel chemotherapy cassette in the experimental arm, which has recently been identified as one of the two more effective regimens in a randomized trial in recurrent or refractory EWS.13 Intensification was also achieved by increasing vincristine exposure and by administration of 17 cycles of chemotherapy.1,8 These results compare favorably to the 73% and 83% EFS and OS rates, respectively, with 14 cycles of interval compressed chemotherapy reported by Womer et al.2 Direct comparison of these sequential trials is limited by possible differences in patient populations, approaches to local control, and supportive care. Consequently, there remains an opportunity to determine whether interval compressed 17 cycles with higher cumulative vincristine doses are superior to 14 cycles.
Older patients (≥ 18 years) experienced a significantly worse OS in this study but age was not a significant stratification parameter for EFS. We enrolled more patients ≥ 18 years compared with each of three sequential preceding COG cooperative group trials (17% of patients v 10%,1 14%,8 and 11%2) and report the highest EFS for this age group, 75% at 5 years compared with 47% and 63% in two of the aforementioned preceding studies.2,8 The improvement in EFS in this group is considered to reflect the numerical overall improvement in EFS for all patients in this study and appears consistent with observations in Euro-Ewing studies.14,15 The inferior OS in older patients may relate to differences in access to off-study health care for older patients versus younger patients remains largely unexplained.
We report a 75% 5-year EFS for patients with pelvic bone primary site, but we identified no significant association between risk for EFS event or death and primary tumor site. The association between tumor site and survival outcome, specifically pelvic primary and unfavorable outcome, is inconsistent in reported studies.2,8,14-17 In this study, 18% of patients had pelvic tumors, for whom local control was surgery in 32%, radiation in 56%, and the combination in 12%. The absence of an outcome effect of primary tumor site as a stratified parameter in our data remains unexplained but may reflect the numerical improvement in EFS for all patients or changes in local control, given nominal differences in local control therapy reported by Womer et al2 for the same cohort of patients (27%, 45%, and 28% surgery, radiation, and combination, respectively).
We also examined the influence of TV and histologic response to induction chemotherapy on EFS, previously unexplored prognostic factors in the context of interval compressed chemotherapy. Consistent with prior reports in patients treated with other chemotherapy regimens, patients with a TV ≥ 200 mL experienced a greater risk of an EFS event.18 Patients with any viable tumor had an increased risk of an EFS event (RHR 1.57) that did not meet statistical significance (P = .055). Notably, histologic response was unavailable for 15% of surgical patients, and the lack of central review for histologic response likely impedes a uniform assessment of this biomarker.
Patients enrolled on this study experienced 27 (4.3%) SNM events. This is numerically higher than previous North American cooperative group studies with similar patient accrual (7 [1.4%] SMN events reported by Grier et al,1 19 [4.0%] SMN events reported by Granowetter et al,8 and 16 [2.8%] SMN events reported by Womer et al2). No significant difference was noted in SMN events between the randomized arms of the current study, suggesting that VTC did not influence SMN risk. Alkylator equivalent dosing on the standard arm of this study was the same as that in two prior studies,1,8 yet the predominance of hematopoietic malignancies draws attention to systemic therapy as opposed to radiation therapy as the main etiology for these cases.
In conclusion, although the addition of VTC to interval compressed chemotherapy did not improve EFS in patients with previously untreated nonmetastatic EWS, this study reports the best outcomes to date. Furthermore, we report the first prospective evaluation of age, tumor site and volume, and response to therapy as potentially important prognostic factors in the context of interval compressed therapy. Newer approaches are needed to further improve outcome by decreasing reliance on cytotoxic agents and relying on international collaboration, while incorporating novel agents in biomarker or risk-stratified patients with newly diagnosed EWS.
ACKNOWLEDGMENT
Dr Mason Bond spearheaded the development of AEWS1031. His tireless perseverance and dedication to our patients was abruptly cut short by his untimely passing in 2013. He is remembered fondly as a friend and colleague.
Patrick J. Leavey
Research Funding: Eleison Pharmaceuticals
Nadia N. Laack
Research Funding: Bristol Myers Squib
Mark D. Krailo
Consulting or Advisory Role: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
R. Lor Randall
Honoraria: Daiichi Sankyo, Onkos Surgical, Onclive
Travel, Accommodations, Expenses: Biomet, Daiichi Sankyo/Lilly
Steven G. DuBois
Research Funding: Merck (Inst), Roche/Genentech (Inst), Lilly (Inst), Curis (Inst), Loxo (Inst), BMS (Inst), Eisai (Inst), Pfizer (Inst), Turning Point Therapeutics (Inst), Bayer (Inst), Salarius Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Salarius Pharmaceuticals
Uncompensated Relationships: Y-mAbs Therapeutics Inc
Damon R. Reed
Consulting or Advisory Role: Eisai, Pfizer
Travel, Accommodations, Expenses: Salarius Pharmaceuticals
Douglas S. Hawkins
Research Funding: Loxo (Inst), Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Bayer (Inst), Lilly (Inst), Eisai (Inst), Amgen (Inst), Seattle Genetics (Inst), Incyte (Inst), Jazz Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Bayer, AstraZeneca
Helen Nadel
Consulting or Advisory Role: ICON Clinical Research
G. Douglas Letson
Employment: Stryker
Consulting or Advisory Role: Stryker
Travel, Accommodations, Expenses: Stryker
Mark Bernstein
Research Funding: Merck
Paul Chuba
Employment: Radiation Oncology Specialists
Neyssa Marina
Employment: Five Prime Therapeutics, Genentech/Roche (I), Synthorx, Sanofi Pasteur
Stock and Other Ownership Interests: Five Prime Therapeutics, Genentech/Roche (I), Synthorx, Sanofi Pasteur
Travel, Accommodations, Expenses: Five Prime Therapeutics, Synthorx
Uncompensated Relationships: Stanford University School of Medicine
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1210881
Richard Gorlick
Research Funding: Eisai
Katherine A. Janeway
Honoraria: Foundation Medicine
Consulting or Advisory Role: Bayer, Ipsen
Travel, Accommodations, Expenses: Bayer
Leo Mascarenhas
Consulting or Advisory Role: Bayer
Research Funding: AstraZeneca/MedImmune, Lilly, Bayer, Salarius Pharmaceuticals, Turning Point Therapeutics, Pfizer, Incyte, Amgen, E.R. Squibb Sons, LLC, Jazz Pharmaceuticals, Merck
Travel, Accommodations, Expenses: Bayer, Lilly, Thermo Fisher Scientific, Salarius Pharmaceuticals
Uncompensated Relationships: Children's Oncology Group Foundation, The Pablove Foundation, American Society of Pediatric Hematology/Oncology
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented at the Connective Tissue Oncology Society annual meeting, Tokyo, Japan, November 16, 2019; the American Society of Clinical Oncology virtual meeting, 2020; and the General Session of the Children's Oncology Group Annual Meeting (virtual), September 16, 2020.
SUPPORT
Supported by the Children's Oncology Group Grants U10CA180886, U10CA180899, U10CA098543, and U10CA098413 and St Baldrick's Foundation.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Nadia N. Laack, Mark D. Krailo, R. Lor Randall, Holcombe E. Grier, Douglas S. Hawkins, Bruce Pawel, Helen Nadel, Richard B. Womer, Kenneth Brown, Alexis Maciej, Atif A. Ahmed, Dian Wang, Neyssa Marina, Richard Gorlick, Katherine A. Janeway, Leo Mascarenhas
Financial support: Katherine A. Janeway
Administrative support: Katherine A. Janeway
Provision of study materials or patients: Patrick J. Leavey, Nadia N. Laack, Steven G. DuBois, Damon R. Reed, G. Douglas Letson, Dian Wang, Katherine A. Janeway, Leo Mascarenhas
Collection and assembly of data: Patrick J. Leavey, Mark D. Krailo, Allen Buxton, R. Lor Randall, Steven G. DuBois, Damon R. Reed, Helen Nadel, Kenneth Brown, Paul Chuba, Daniel J. Indelicato, Katherine A. Janeway, Leo Mascarenhas
Data analysis and interpretation: Patrick J. Leavey, Nadia N. Laack, Mark D. Krailo, Allen Buxton, R. Lor Randall, Steven G. DuBois, Damon R. Reed, Douglas S. Hawkins, Helen Nadel, Richard B. Womer, G. Douglas Letson, Mark Bernstein, Paul Chuba, Daniel J. Indelicato, Dian Wang, Katherine A. Janeway, Leo Mascarenhas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III Trial Adding Vincristine-Topotecan-Cyclophosphamide to the Initial Treatment of Patients With Nonmetastatic Ewing Sarcoma: A Children's Oncology Group Report
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Patrick J. Leavey
Research Funding: Eleison Pharmaceuticals
Nadia N. Laack
Research Funding: Bristol Myers Squib
Mark D. Krailo
Consulting or Advisory Role: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
R. Lor Randall
Honoraria: Daiichi Sankyo, Onkos Surgical, Onclive
Travel, Accommodations, Expenses: Biomet, Daiichi Sankyo/Lilly
Steven G. DuBois
Research Funding: Merck (Inst), Roche/Genentech (Inst), Lilly (Inst), Curis (Inst), Loxo (Inst), BMS (Inst), Eisai (Inst), Pfizer (Inst), Turning Point Therapeutics (Inst), Bayer (Inst), Salarius Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Salarius Pharmaceuticals
Uncompensated Relationships: Y-mAbs Therapeutics Inc
Damon R. Reed
Consulting or Advisory Role: Eisai, Pfizer
Travel, Accommodations, Expenses: Salarius Pharmaceuticals
Douglas S. Hawkins
Research Funding: Loxo (Inst), Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Bayer (Inst), Lilly (Inst), Eisai (Inst), Amgen (Inst), Seattle Genetics (Inst), Incyte (Inst), Jazz Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Bayer, AstraZeneca
Helen Nadel
Consulting or Advisory Role: ICON Clinical Research
G. Douglas Letson
Employment: Stryker
Consulting or Advisory Role: Stryker
Travel, Accommodations, Expenses: Stryker
Mark Bernstein
Research Funding: Merck
Paul Chuba
Employment: Radiation Oncology Specialists
Neyssa Marina
Employment: Five Prime Therapeutics, Genentech/Roche (I), Synthorx, Sanofi Pasteur
Stock and Other Ownership Interests: Five Prime Therapeutics, Genentech/Roche (I), Synthorx, Sanofi Pasteur
Travel, Accommodations, Expenses: Five Prime Therapeutics, Synthorx
Uncompensated Relationships: Stanford University School of Medicine
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1210881
Richard Gorlick
Research Funding: Eisai
Katherine A. Janeway
Honoraria: Foundation Medicine
Consulting or Advisory Role: Bayer, Ipsen
Travel, Accommodations, Expenses: Bayer
Leo Mascarenhas
Consulting or Advisory Role: Bayer
Research Funding: AstraZeneca/MedImmune, Lilly, Bayer, Salarius Pharmaceuticals, Turning Point Therapeutics, Pfizer, Incyte, Amgen, E.R. Squibb Sons, LLC, Jazz Pharmaceuticals, Merck
Travel, Accommodations, Expenses: Bayer, Lilly, Thermo Fisher Scientific, Salarius Pharmaceuticals
Uncompensated Relationships: Children's Oncology Group Foundation, The Pablove Foundation, American Society of Pediatric Hematology/Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Grier HE, Krailo MD, Tarbell NJ, et al. : Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 348:694-701, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Womer RB, West DC, Krailo MD, et al. : Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children's Oncology Group. J Clin Oncol 30:4148-4154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leavey PJ, Mascarenhas L, Marina N, et al. : Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: A report from the Children's Oncology Group. Pediatr Blood Cancer 51:334-338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saylors RL III, Stine KC, Sullivan J, et al. : Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: A Pediatric Oncology Group phase II study. J Clin Oncol 19:3463-3469, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Hunold A, Weddeling N, Paulussen M, et al. : Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer 47:795-800, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bernstein ML, Devidas M, Lafreniere D, et al. : Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children's Cancer Group phase II study 9457—A report from the Children's Oncology Group. J Clin Oncol 24:152-159, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Brennan B, Kirton L, Marec-Berard P, et al. : Comparison of two chemotherapy regimens in Ewing sarcoma (ES): Overall and subgroup results of the Euro Ewing 2012 randomized trial (EE2012). J Clin Oncol 38, 2020. (15_suppl; abstr 11500) [Google Scholar]
- 8.Granowetter L, Womer R, Devidas M, et al. : Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: A Children's Oncology Group study. J Clin Oncol 27:2536-2541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gidding CE, Kellie SJ, Kamps WA, et al. : Vincristine revisited. Crit Rev Oncol Hematol 29:267-287, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Thompson J, George EO, Poquette CA, et al. : Synergy of topotecan in combination with vincristine for treatment of pediatric solid tumor xenografts. Clin Cancer Res 5:3617-3631, 1999 [PubMed] [Google Scholar]
- 11.Mascarenhas L, Felgenhauer JL, Bond MC, et al. : Pilot study of adding vincristine, topotecan, and cyclophosphamide to interval-compressed chemotherapy in newly diagnosed patients with localized Ewing sarcoma: A report from the Children's Oncology Group. Pediatr Blood Cancer 63:493-498, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderton J, Moroz V, Marec-Berard P, et al. : International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours—EURO EWING 2012 Protocol. Trials 21:96, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCabe MG, Kirton L, Khan M, et al. : Results of the second interim assessment of rEECur, an international randomized controlled trial of chemotherapy for the treatment of recurrent and primary refractory Ewing sarcoma (RR-ES). J Clin Oncol 38, 2020. (suppl; abstr 11502) [Google Scholar]
- 14.Le Deley MC, Paulussen M, Lewis I, et al. : Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: Results of the randomized noninferiority Euro-EWING99-R1 trial. J Clin Oncol 32:2440-2448, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Whelan J, Le Deley MC, Dirksen U, et al. : High-dose chemotherapy and blood autologous stem-cell rescue compared with standard chemotherapy in localized high-risk Ewing sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008. J Clin Oncol 36:JCO2018782516, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacci G, Ferrari S, Mercuri M, et al. : Multimodal therapy for the treatment of nonmetastatic Ewing sarcoma of pelvis. J Pediatr Hematol Oncol 25:118-124, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Cotterill SJ, Ahrens S, Paulussen M, et al. : Prognostic factors in Ewing's tumor of bone: Analysis of 975 patients from the European Intergroup cooperative Ewing's Sarcoma Study Group. J Clin Oncol 18:3108-3114, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Bacci G, Longhi A, Ferrari S, et al. : Prognostic factors in non-metastatic Ewing's sarcoma tumor of bone: An analysis of 579 patients treated at a single institution with adjuvant or neoadjuvant chemotherapy between 1972 and 1998. Acta Oncol 45:469-475, 2006 [DOI] [PubMed] [Google Scholar]