Supplemental Digital Content is available in the text
Keywords: camera navigation, COVID-19, laparoscopic cholecystectomy, surgical skills, tube figure test, virtual reality simulation, visual spatial ability
Abstract
Introduction:
Due to the current COVID-19 pandemic, surgical training has become increasingly challenging due to required social distancing. Therefore, the use of virtual reality (VR)-simulation could be a helpful tool for imparting surgical skills, especially in minimally invasive environments. Visual spatial ability (VSA) might influence the learning curve for laparoscopic surgical skills. However, little is known about the influence of VSA for surgical novices on VR-simulator training regarding the complexity of different tasks over a long-term training period. Our study evaluated prior VSA and VSA development in surgical trainees during VR-simulator training, and its influence on surgical performance in simulator training.
Methods:
In our single-center prospective two-arm randomized trial, VSA was measured with a tube figure test before curriculum training. After 1:1 randomization, the training group (TG) participated in the entire curriculum training consisting of 48 different VR-simulator tasks with varying difficulty over a continuous nine-day training session. The control group (CG) performed two of these tasks on day 1 and 9. Correlation and regression analyses were used to assess the influence of VSA on VR-related surgical skills and to measure procedural abilities.
Results:
Sixty students (33 women) were included. Significant improvements in the TG in surgical performance and faster completion times were observed from days 1 to 9 for the scope orientation 30° right-handed (SOR), and cholecystectomy dissection tasks after the structured 9-day training program. After training, the TG with pre-existing low VSA scores achieved performance levels similar to those with pre-existing high VSA scores for the two VR simulator tasks. Significant correlations between VSA and surgical performance on complex laparoscopic camera navigation SOR tasks were found before training.
Conclusions:
Our study revealed that that all trainees improved their surgical skills irrespective of previous VSA during structured VR simulator training. An increase in VSA resulted in improvements in surgical performance and training progress, which was more distinct in complex simulator tasks. Further, we demonstrated a positive relationship between VSA and surgical performance of the TG, especially at the beginning of training. Our results identified pre-existing levels of VSA as a predictor of surgical performance.
1. Introduction
COVID-19 pandemic has globally impacted patient care as well as all forms of medical education, above all training involving manual techniques and surgical training programs.[1,2] The enforced interruption of elective surgical activities has further reduced the already limited valuable training time for surgical trainees and residents and affected the practice of basic surgical skills in the operating room (OR).[3–6] Over the past decade, minimally invasive surgery (MIS) has modernized surgical care and revolutionized the face of surgery.[7,8] However, learning to perform and manage laparoscopic procedures is associated with additional difficulties for trainees and requires a set of psychomotor abilities differing substantially from those needed for conventional open surgery (improved hand-eye coordination, change from three-dimensional to a two-dimensional view, altered tactile and haptic feedback, etc.).[9–16] The development of these skills requires extra training time and practice.[17] However, during residency training, limited surgical capacities and growing public concern for medical errors precludes many residents from practical exposure on actual patients, which has been further heightened during the COVID-19 pandemic, consequently reducing training opportunities.[18]
To address this problem MIS training centers offer young surgeons the opportunity to acquire basic surgical skills prior to their first laparoscopic surgical experience.[19] In this context, surgical simulation using virtual reality (VR) is a useful tool in surgical training programs which enable a safe, repeatable, patient saving and cost-effective approach outside the OR.[20–22]
In times, surgical techniques are becoming increasingly difficult the optimal training of medical novices and the selection of qualified candidates are relevant components in medical education.[23] To select surgical users and provide them with improved training conditions, research has focused on the identification and consideration of predictive ability parameters. Previous research showing correlations between innate cognitive and perceptual motoric abilities or aptitudes and surgical technical skills. In this context, VSA- the two-dimensional to three-dimensional conversion ability- seems to predict future surgical skills.[23–28] Concerning open surgery, VSA correlated positively with surgical performance, particularly during complex surgical tasks.[20]
Other studies have also reported a positive correlation between VSA and improved surgical performance in complex surgical tasks, such as knot tying or laparoscopic camera navigation (LCN).[17] However, evidence correlating the surrogate parameter VSA to surgical technical skills is limited and inconsistent, and the complexity of observed tasks has not been fully examined.[29]
The aim of our study was to evaluate the effects of VSA on skills and performance of surgical novices during a structured VR simulator training over 9 days, focusing on training process and VSA progression along the initial learning curve. A positive impact, even under the current pandemic situation, allowing safe and successful surgical simulator training, may improve surgical education in diverse circumstances.
2. Methods
2.1. Study design
This prospective single-center two-arm randomized trial was conducted over a 2-week period at the Surgical Department of the University Hospital of Leipzig.
2.2. Participants
A total of 60 medical students in clinical years (third to sixth year out of 6-year course in Germany) were invited by email for participation. Participants completed a demographical survey and a questionnaire quantifying their relevant previous surgical and non-surgical experiences. The participants were informed about the purpose of the study, gave their consent voluntarily before the randomization process, and were free to leave the study at any time. The local ethics committee of the first affiliated Hospital approved the study protocol before participant enrolment (AZ- Nr: 111–16–14032016).
2.3. Visual spatial ability testing
VSA was tested with a validated tube figure test (TFT) among all participants before and after the training period, which formed part of an assessment battery for medical students in Germany to measure their cognitive abilities. The results indicated acceptable internal consistency (α=0.78).[30–33] The used VSA test depicted pictures of two identical cubes with shaped tubes inside and in different positions. Participants were requested to name the side from which one had to look at the first cube to see the same image of the second cube. The test contained 24 items with five possible answers, each in multiple-choice format. The result was calculated as the sum of the correct answers.
2.4. Study setting
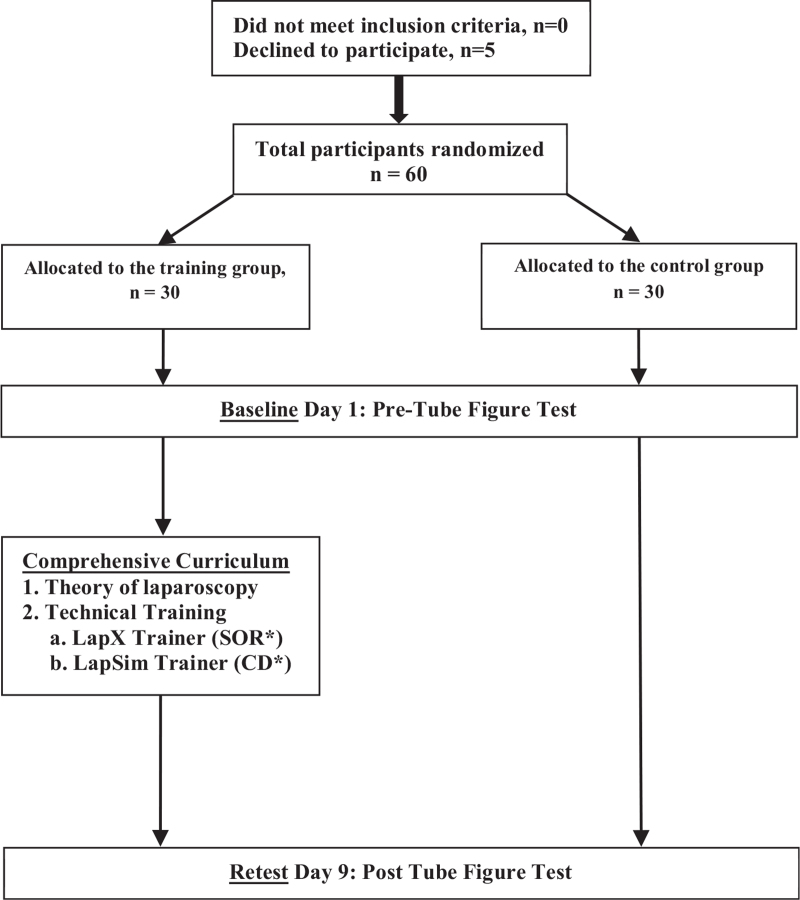
Participants were independently randomized by using a sealed envelope technique to the “Training Group” (TG; n = 30) or the “Control Group” (CG; n = 30) (Figure 1).
Figure 1.
Study flow diagram. Novice trainees were randomized to either the control group or the training group. All trainees had to pass the tube figure test on the first day. After nine days, both groups had to pass the tube figure test a second time. Only the laparoscopy training group underwent the comprehensive curriculum. Simulator tasks used in this study: CA = clip application; CD = cholecystectomy dissection; CU = cutting; FD = fine dissection; SOR = scope orientation 30° right-handed.
2.4.1. Training group
Participants in the TG underwent a two-week evidence-based, structured and validated curriculum training with different simulator tasks and increased levels of difficulty/and complexity.[34]
Before training, each participant received standardized information using the simulator as well as a curriculum handout with description of each exercise ensuring similar baseline. Furthermore, a member of the study team was present during each practice session to assist in handling the simulator. As a basic task “scope orientation 30° right-handed” (SOR) camera navigation using the LapX-Hybrid/VR (Epona Medical B.V., Rotterdam, Netherlands) was chosen for complex skill assessment “cholecystectomy dissection” (CD) using the LapSim simulator (Surgical Science, Gothenburg, Sweden). Regarding basic skills, all participants underwent a total of 48 simulator tasks. Each exercise was repeated two to eight times, depending on the need. To measure procedural complex skills, VR laparoscopic cholecystectomy was performed with three repetitions per participant.
The “SOR” task ensured that participants simultaneously used the right controller as a 30° scope and the left controller as a grasper. To perform the task, nine numbered boxes in different orientations had to be focused on and grasped in an artificial room in the correct order, from one to nine (Supplemental Digital Content 1, http://links.lww.com/MD/G498). The second indicator task was “CD” which took place in simulated gall bladder environment. The participant received optic and haptic feedback and could switch instruments. First, the cystic artery and cystic duct were prepared. Subsequently, the task was completed by clipping and cutting both structures. The measured outcome parameters were
-
1.
time for task completion (minutes),
-
2.
instrument movement (distance covered by the right/left hand in centimeters) and
-
3.
the number of missed clips (n) (Supplemental Digital Content 1, http://links.lww.com/MD/G498).
2.4.2. Control group
Participants of the CG didn’t undergo a complete curriculum training. In combination with VSA testing at day 1 and 9, they underwent SOR and CD exercises twice with a nine-days break interval.
2.5. Analysis of the visual spatial ability learning curves
The TG was subsequently divided into high score (Group A) and low score (Group B) subgroups on the basis of the median VSA in the pre-TFT to carry out an analysis of the learning curve development of spatial imagination between them. Group A was defined according to the median split (≥12 points) on a 24-point scale; whereas, Group B comprised participants with a score ≤12 points.
2.6. Statistical methods
All statistical analyses were performed using the SPSS statistical package (version 23.0, IBM). Values were presented as means unless stated otherwise. An intergroup analysis of the baseline characteristics between the two groups was conducted using the Mann–Whitney U test, Wilcoxon test, or the Student's t-test, where appropriate. The TFT score measuring VSA was considered an interval-scaled measure. The TFT-scores of the two groups were compared using the Mann–Whitney U test. Spearman's Rho correlation was used to examine the relationship between the VSA assessed by the performance on the VSA test and the performance on laparoscopic tasks. An r-value (r) between 0.00 and 0.25 were considered as no correlation, between 0.25 and 0.50 as small effect, between 0.50 and 0.75 as medium effect, and between 0.75 and 1.0 as strong effect.[32] Negative, values indicated an inverse correlation. Logarithmic regression was used to identify the predictors between the VSA skills and training progress concerning the surgical performance and completion time. Multivariate linear regression was used to analyze the relationship between VSA capabilities, demographic variables, training performance, and training progress. The following demographic variables were included: sex and experience with videogames, playing instruments, MIS, and using an LCN. The training progress was calculated as a mathematical difference between the performance values and the times between the individual training days of a laparoscopic training task. To assess the effect size in logarithmic regression, Cohen's classification was used, with f ≤ 0.10 depicting a poor effect, f ≤ 0.25 a medium effect, and f ≥ 0.40 a strong effect. Statistical significance was considered at P < .05.
3. Results
3.1. Demographics
Overall, 60 participants (27 male) were included in the study which completed the entire study protocol. Demographic data are presented in Table 1.
Table 1.
Demographics of training group and control group.
| TG | CG | ||
| N | N | P-value | |
| Sex (male/female) | 13/17 | 14/16 | NS |
| Handedness (right/left) | 24/6 | 23/7 | NS |
| Exposure to instrument (yes/no) | 19/11 | 3/7 | NS |
| Interest in MIS∗ (yes/no) | 7/23 | 5/5 | NS |
| Experience with video games (years/hours) | 15/15 | 6/4 | NS |
| Experience with playing billiards (a lot/little) | 9/21 | 5/5 | NS |
| Experience with laparoscopic simulator training (yes/no) | 19/11 | 0/30 | .001 |
| Assistant in laparoscopic surgery (yes/no) | 19/11 | 3/27 | .001 |
| Observing laparoscopic surgery (yes/no) | 28/2 | 13/13 | .001 |
| Age (years), median | 25.3 ± 2.8 | 23.8 ± 1.8 | .04 |
CG = control group; MIS = minimally invasive surgery; NS = not significant; TG = training group.
3.2. Visual spatial ability and learning curves
At the beginning of the training, the mean performance on the VSA test for all participants was 10.2 ± 4.1 points out of a maximum of 24 points. The initial day-1 VSA test scores were similar between the TG (10 ± 3.4) and CG (10 ± 4.7) (P = .41); whereas, significant differences were observed in the day-9 TG VSA test scores after curriculum training compared with that of the CG (day 9, TG: VSA score 13.1 ± 2.4 versus CG: VSA score 9.8 ± 3.3; P ≤ .001). Regarding the TG, pre- and post-VSA test scores were significantly different between male and female participants (males scoring higher), just like with participants with interest in MIS. No significant differences were observed in the VSA test scores for laparoscopic experience, handedness, or playing a musical instrument (Table 2).
Table 2.
Visual-spatial ability test score of training group and control group.
| VSA test before training | VSA test after nine days of training | |||||
| Participants (n) | Mean ± SD | P | Participants (n) | Mean ± SD | P | |
| Entire group | 60 | 10.2 ± 4.0 | 60 | 13.0 ± 2.4 | ||
| TG | ||||||
| Men∗ | 13 | 12.5 ± 3.3 | .007 | 13 | 14.5 ± 2.6 | .004 |
| Women | 17 | 9.1 ± 2.7 | 17 | 11.9 ± 1.6 | ||
| CG | ||||||
| Men | 14 | 9.8 ± 4.3 | NS | 14 | 9.0 ± 2.4 | NS |
| Women | 16 | 9.8 ± 5.0 | 16 | 10.6 ± 3.8 | ||
| TG | ||||||
| Laparoscopic experience | 19 | 10.1 ± 3.4 | NS | 19 | 13.1 ± 2.6 | NS |
| No laparoscopic experience | 11 | 11.1 ± 3.4 | 11 | 13.0 ± 2.4 | ||
| CG | ||||||
| Laparoscopic experience | 7 | 7.5 ± 4.0 | NS | 7 | 9.1 ± 3.9 | NS |
| No laparoscopic experience | 23 | 10.5 ± 4.6 | 23 | 10.0 ± 3.0 | ||
| TG | ||||||
| Exposure to video games | 15 | 12.0 ± 3.6 | .04 | 15 | 13.5 ± 2.8 | NS |
| No exposure to video games | 15 | 9.2 ± 2.6 | 15 | 12.6 ± 1.9 | ||
| CG | ||||||
| Exposure to video games | 12 | 12.0 ± 4.9 | NS | 12 | 11.0 ± 3.4 | NS |
| No exposure to video games | 18 | 8.3 ± 4.0 | 18 | 9.0 ± 2.9 | ||
| TG | ||||||
| Experience playing a musical instrument | 19 | 10.4 ± 3.3 | NS | 19 | 13.3 ± 2.7 | NS |
| No experience playing a musical instrument | 11 | 10.8 ± 3.8 | 11 | 12.5 ± 1.8 | ||
| CG | ||||||
| Experience playing a musical instrument | 14 | 11.5 ± 5.0 | NS | 14 | 11.1 ± 3.2 | NS |
| No experience playing a musical instrument | 16 | 8.4 ± 3.9 | 16 | 8.7 ± 3.1 | ||
| TG | ||||||
| Interest in MIS∗ | 23 | 11.3 ± 3.2 | .04 | 23 | 13.6 ± 2.5 | .02 |
| No interest in MIS | 7 | 8.2 ± 2.9 | 7 | 11.2 ± 1.2 | ||
| CG | ||||||
| Interest in MIS∗ | 21 | 11.0 ± 4.4 | .03 | 21 | 10.1 ± 3.2 | NS |
| No interest in MIS | 9 | 7.2 ± 4.3 | 9 | 9.2 ± 3.2 | ||
| TG | ||||||
| Right-handers | 24 | 10.7 ± 3.1 | NS | 24 | 13.3 ± 2.6 | NS |
| Left-handers | 6 | 10 ± 4.6 | 6 | 12 ± 0.89 | ||
| CG | ||||||
| Right-handers | 23 | 9.9 ± 3.4 | NS | 23 | 10.3 ± 4.7 | NS |
| Left-handers | 7 | 9.5 ± 3.0 | 7 | 8.14 ± 4.3 | ||
CG = control group; MIS = minimally invasive surgery; NS = not significant; SD = standard deviation; TG = training group; VSA = visual spatial ability.
Indicates statistical significance with a P < .05.
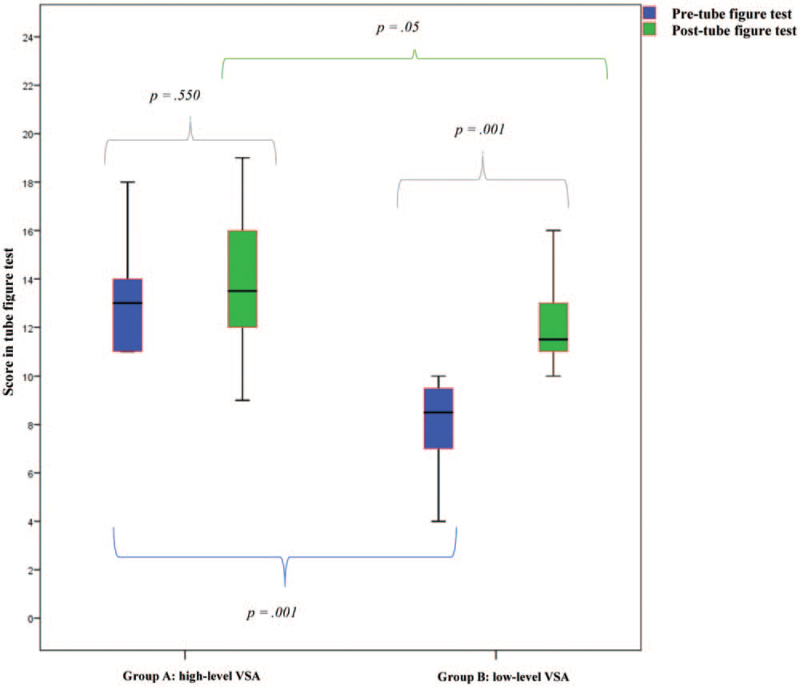
Subgroup analysis of the TG for VSA learning curve development revealed significant differences in day-1 pre-VSA scores (High-level Group (A): 13 ± 2.5 versus Low-level Group (B): 8.50 ± 1.746; P = .001). The group A did not improve their abilities significantly during the 9-day VR curriculum training whereas group B members significantly increased their abilities in VSA scores after simulator training (P = .001), but did not reach the VSA scores of Group A (P = .05) (Figure 2).
Figure 2.
Development of visual spatial ability during the VR simulator curriculum training. VSA = visual spatial ability.
3.3. Virtual reality simulator tasks
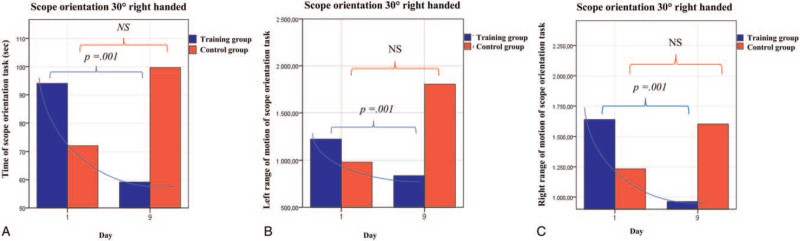
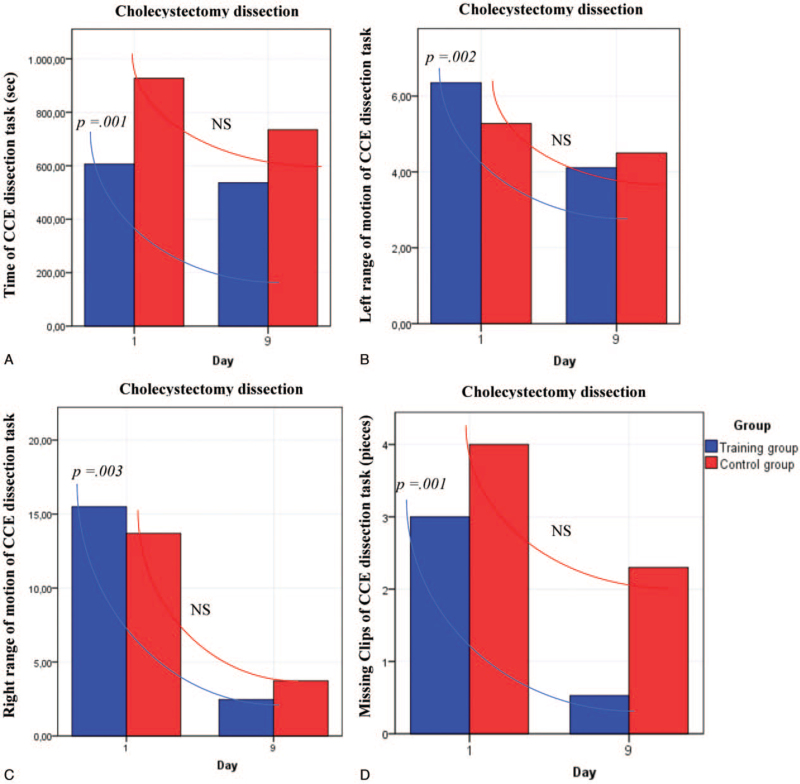
After VR curriculum training, significant improvements in surgical performance in five of the seven analyzed parameters were observed in the TG compared to the CG (Figures 3 and 4, Supplemental Digital Content 2, http://links.lww.com/MD/G498). Subgroup analysis of participants with interest in MIS revealed significant differences in simulator training parameters before the start of the training. Male participants depicted significantly better results in the performance of hand movement and reduced completion time on day 1 than women in the basic simulator task “SOR”, but not for the complex CD task. During the training process no significant differences regarding surgical performance and times required to complete the exercises between men and women in the TG were observed (Table 3). However, significant improvements in all evaluated parameters for the SOR task from day 1 to 9 for both sexes were observed (time, right- and left-hand movement: P = .001 for men and P = .001 for women) (Supplemental Digital Content 3, http://links.lww.com/MD/G498).
Figure 3.
Improvement of camera navigation skills for the task “scope orientation 30° right-handed”. (A) Time of the scope orientation task, (B) Left-handed range of motion of the scope orientation task. (C) Right-handed range of motion of the scope orientation task. Statistical analysis with the Mann–Whitney U test.
Figure 4.
Improvement of surgical performance for the task “cholecystectomy dissection”. (A) Time of cholecystectomy dissection task. (B) Left-handed range of motion of the cholecystectomy task. (C) Right-handed rand of motion of the cholecystectomy task. (D) Missing clips in the cholecystectomy task. Analysis with Mann–Whitney U test. CCE = cholecystectomy; NS = not significant.
Table 3.
Comparison of subgroup analysis for virtual reality simulator tasks (scope orientation 30° right handed and cholecystectomy dissection).
| Day 1 | Day 9 | Men | Women | |||||||
| Men | Women | Men | Women | Day 1 | Day 9 | Day 1 | Day 9 | |||
| Mean ± SD | Mean ± SD | P | Mean ± SD | Mean ± SD | P | P | P | |||
| Scope orientation 30° right-handed | ||||||||||
| Time (sec) | 133 ± 62 | 171 ± 137 | .01 | 44 ± 12.6 | 56 ± 55.3 | NS | .01 | .02 | ||
| Left-handed movement (degrees) | 855 ± 1223 | 1438 ± 1576 | .04 | 626 ± 238.5 | 704 ± 744.0 | NS | .02 | .01 | ||
| Right-handed movement (degrees) | 1327 ± 3198 | 2735 ± 1635 | .04 | 833 ± 240.8 | 940 ± 569.6 | NS | .01 | .01 | ||
| CD | .01 | .01 | ||||||||
| Time (Sec) | 683 ± 215 | 699 ± 385 | NS | 532 ± 108 | 541 ± 137 | NS | .03 | .02 | ||
| Left-handed movement (degrees) | 4.14 ± 3.4 | 7.9 ± 4.1 | NS | 2.6 ± 1.8 | 2.7 ± 2.9 | NS | .01 | .01 | ||
| Right-handed movement (degrees) | 13.9 ± 8.8 | 16.7 ± 10.8 | NS | 10.1 ± 3.9 | 11.0 ± 4.1 | NS | .02 | .01 | ||
| Missed clips (n) | 2 ± 1.0 | 3 ± 1.5 | NS | 0 ± .6 | 0 ± .8 | NS | .01 | .01 | ||
| Day 1 | Day 9 | |||||
| Interest in MIS | No Interest in MIS | Interest in MIS | No Interest in MIS | |||
| Mean ± SD | Mean ± SD | P | Mean ± SD | Mean ± SD | P | |
| Scope orientation 30° right-handed | ||||||
| Time (sec) | 89 ± 91 | 155 ± 125 | .042 | 58 ± 77.6 | 44 ± 16.5 | NS |
| Left-handed movement (degrees) | 1020 ± 1020 | 1562 ± 2121 | .003 | 621 ± 370 | 768 ± 916 | NS |
| Right-handed movement (degrees) | 1407 ± 2419 | 2290 ± 2241 | .037 | 835 ± 247 | 956 ± 783 | NS |
| CD | ||||||
| Time (sec) | 713 ± 414 | 606 ± 317 | NS | 495 ± 147 | 517 ± 165 | NS |
| Left-handed movement (degrees) | 9.5 ± 5.5 | 4.5 ± 3.1 | .028 | 4.9 ± 2.0 | 3.3 ± 2.0 | NS |
| Right-handed movement (degrees) | 21.8 ± 9.9 | 13.4 ± 6.4 | NS | 10.9 ± 4.4 | 10.9 ± 4.4 | NS |
| Missed clips (n) | 2.5 ± .5 | 3 ± 1.6 | NS | 0 ± 0.3 | 0 ± .8 | NS |
The results are analyzed with the Mann–Whitney U test results are means ± standard deviations (SD).
CD = cholecystectomy dissection; CG = control group; MIS = minimally invasive surgery; NS = not significant; TG = testing group.
Concerning high- and low-level VSA subgroups, both groups in each case significantly improved their skills and performances in all VR simulator tasks over 9-day training.
Interestingly, participants of Group B reached similar scores on day 9 after training in the basic task “SOR”, as well as in 2 of the 4 analyzed parameters of the complex CD task (Table 4).
Table 4.
Comparison of subgroup analysis of visual spatial ability (high- and low-level visual spatial ability groups) for virtual reality simulator tasks (scope orientation 30° right-handed and cholecystectomy dissection).
| Day 1 | Day 9 | Low VSA (B) | High VSA (A) | |||||||
| Low VSA (B) Mean ± SD | High VSA (A) Mean ± SD | P | Low VSA (B) Mean ± SD | High VSA (A) Mean ± SD | P | Day 1 | Day 9 | Day 1 | Day 9 | |
| P | P | |||||||||
| Scope orientation 30° right-handed | ||||||||||
| Time (sec) | 125.5 ± 91.1 | 76 ± 118.2 | .04 | 59 ± 57.17 | 42 ± 10.83 | .06 | .005 | .001 | ||
| Left-handed movement (degrees) | 1400 ± 1381 | 878.5 ± 1600 | .06 | 761 ± 715 | 614.5 ± 175 | .19 | .02 | .002 | ||
| Right-handed movement (degrees) | 1803.5 ± 1272 | 1326.5 ± 3281 | .12 | 920 ± 585 | 878.0 ± 245 | .33 | .005 | .002 | ||
| CD | ||||||||||
| Time (sec) | 654 ± 412.1 | 715 ± 219.7 | .33 | 592 ± 152.3 | 470 ± 146.4 | .04 | .001 | .001 | ||
| Left-handed movement (degrees) | 8.68 ± 4.87 | 2.94 ± 1.69 | .005 | 4.52 ± 2.12 | 2.82 ± 1.68 | .08 | .002 | .42 | ||
| Right-handed movement (degrees) | 17.99 ± 9.33 | 12.41 ± 4.19 | .110 | 13.37 ± 4.69 | 9.63 ± 2.73 | .03 | .001 | .001 | ||
| Missing clips (n) | 3 ± 1.59 | 3 ± 1.55 | .600 | 0 ± .96 | 0 ± .86 | .75 | .01 | .007 | ||
Analysis with Mann–Whitney U test. Results are indicated as mean ± standard deviation (SD).
CD = cholecystectomy dissection; VSA = visual spatial ability.
3.4. Impact of visual spatial ability on surgical performance in virtual reality simulator tasks
The analysis revealed significant correlations between higher VSA scores and better performance (degree of left-hand movement (r = -0.51, P = .005) as well as shorter times (r = -0.47, P = .009) to complete VR exercises for the basic “SOR” task on day 1 (Table 5). The results were consistent with significant results considering pre- and post-TFT on day 9. In contrast, when considering the more complex CD task, the significant correlation found in relation to the variables completion time (r = -0.419, P = .007), movement of the left hand (r = -0.439, P = .02), and missing clips (r = -0.491, P = .006) on day 1 were not detectable on day 9.
Table 5.
Correlation between visual-spatial ability and virtual reality simulator tasks.
| Pre tube figure test | Pre tube figure test | Post tube figure test | |
| (Day 1) | (Day 9) | (Day 9) | |
| r (P value) | r (P value) | r (P value) | |
| Scope orientation 30° right-handed | |||
| Time (sec) | −.468 (.009) | −.444 (.01) | −.385 (.01) |
| Left-handed movement (degrees) | −.505 (.005) | −.420 (.02) | −.525 (.03) |
| Right-handed movement (degrees) | NC (.067) | NC (.118) | NC (.132) |
| CD | |||
| Time (sec) | −.419 (.007) | NC (.40) | NC (.24) |
| Left-handed movement (degrees) | −.439 (.02) | NC (.12) | NC (.10) |
| Right-handed movement (degrees) | NC (.18) | NC (.22) | NC (.09) |
| Missed clips (n) | -0.491 (.006) | NC (.90) | NC (.19) |
Data analyzed by Spearman's rho correlation.
CD = cholecystectomy dissection; NC = no significant correlations; VR = virtual reality.
No significant differences were observed in the subgroup sex-based analysis relative to the impact of VSA on surgical performance or time for completion of the basic and complex VR-simulator task (SOR and CD).
Concerning VSA subgroup analysis, significant negative correlations were observed in Group B exclusively on day 1 and in the basic SOR task for the parameter completion time (r = -0.580; P = .02) such as movement of the left (r = -0.564; P = .02), and right hand (r = -0.525; P = .04). Whereas the group A could statistically improve their surgical abilities after curriculum training and significant correlations between higher VSA scores and surgical performance, as well shorter times to complete task were observed, especially for the complex CD task (Supplemental Digital Content 4, http://links.lww.com/MD/G498).
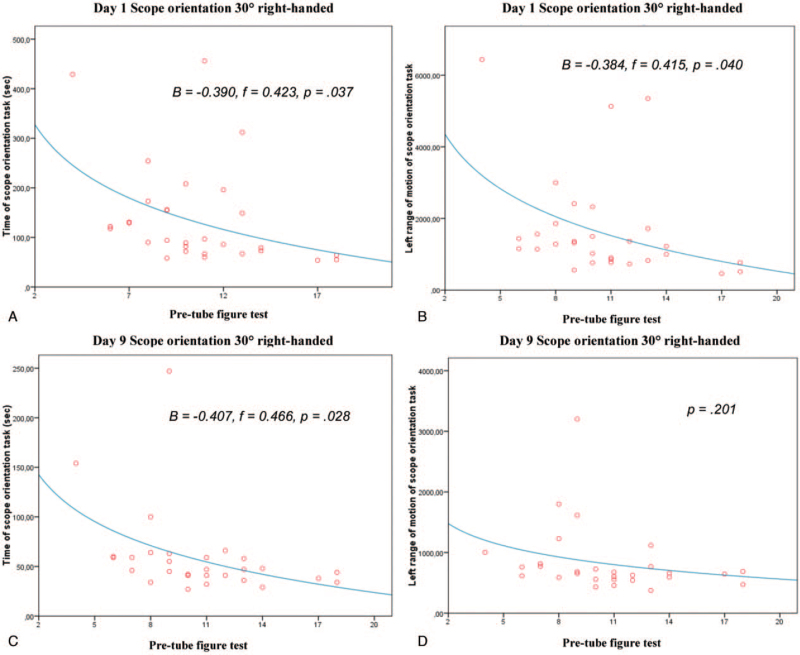
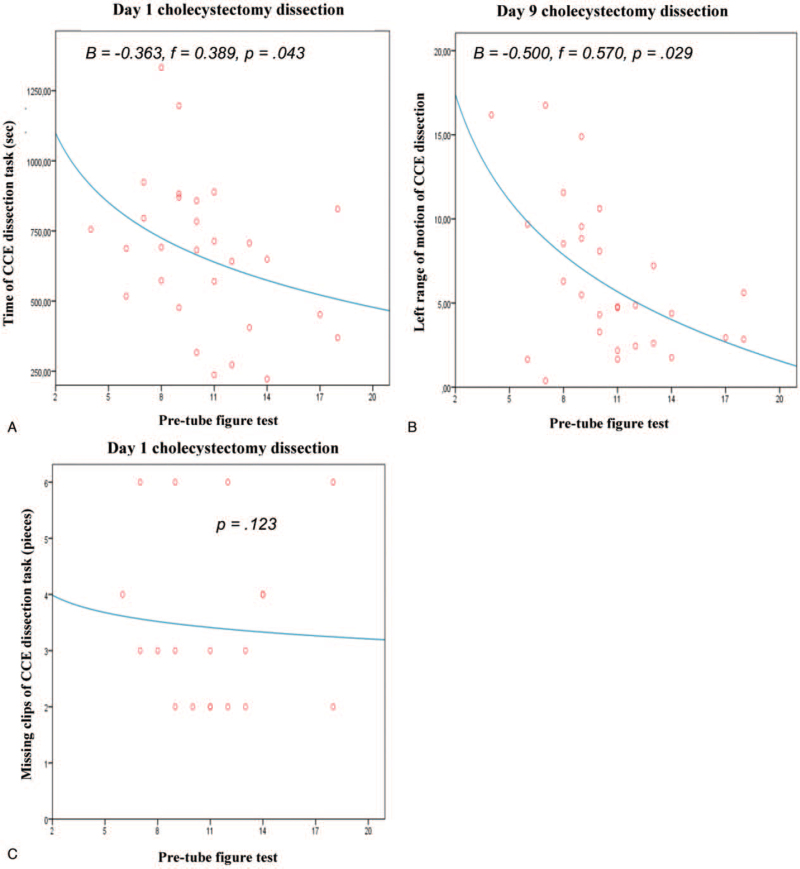
Logarithmic regression analysis revealed significant predictions between VSA and the training progress concerning the reduction of required time to complete the task (B = -0.390, f = 0.423, P = .04) and a lower degree of left-hand movement (B = -0.384, f = 0.415, P = .040) in the SOR task on day 1. Day-9 values for the required time for exercise completion (B = -0.407, f = 0.466, P = .03), but not the degree of left-hand movement, were identified as significantly predictive (Figure 5). Further for the complex CD task, completion time (day 1: B = -.363, f = .389, P = .04) and the degree of left-hand movement (day 9: B = -.500, f = .570, P = .03) were identified as significant predictors of surgical performance (Figure 6).
Figure 5.
Logarithmic regression analysis between visual spatial ability skill and training progress concerning surgical performance in the virtual reality simulator task “scope orientation 30° right-handed”. (A&C) Time of scope orientation task at days 1 and 9, respectively. (B&D) Left-handed range of motion of the scope orientation task at days 1 and 9, respectively. Each point in the scatter plots represents one or more of the 30 participants.
Figure 6.
Logarithmic regression between VSA skill and training progress concerning surgical performance in the virtual reality simulator task “Cholecystectomy dissection”. (A) Time of CCE dissection task (sec) at day 1. (B) Left-handed range of motion of CCE dissection at day 9. (C) Missing clips at CCE dissection task at day 1. Each point in the scatter plots represents one or more of the 30 participants. CCE = cholecystectomy.
Multivariate regression analysis revealed a constant influence of VSA on training progress from day 1 to day 9 in the SOR task for the time to complete the task (B = -0.672, f = 0.824, P = .001) and the degree of left-hand movement (B = -0.801, f = 0.642, P = .001).
Concerning the impact of further demographic characteristics on surgical performance and training progress in VR simulator tasks, significant relationships could be observed for participants with interest in MIS, playing a musical instrument or previous experience in simulator training (Supplemental Digital Content 5, http://links.lww.com/MD/G498).
4. Discussion
This study revealed that surgical simulation using VR could be a helpful tool in a surgical structured curriculum training program in the light of the challenges of the COVID-19 pandemic. Therefore, training in surgical VR simulators has become an increasingly applied approach for the training of future surgical staff.[35–39] We observed that students were able to significantly improve their visual spatial imagination as well as their surgical performance and completion time for simulator tasks during a structured VR-simulator curriculum training. Furthermore, our study established a positive impact of VSA on surgical performance and completion time in basic and especially complex tasks. Regarding subgroup analysis, participants with initial low-level VSA scores could significantly increase their surgical abilities, including their spatial imagination, with practice. Our findings are in line with previous studies which reported an important role of VSA for surgical performance and training progress, particularly for complex simulator tasks.[28,40]
Interestingly, significant correlations between VSA and surgical training performance were particularly notable at the beginning of training.
These results are also consistent with the findings of skill acquisition studies, showing that cognitive abilities, such as VSA, are important during the initial phase of learning a new skill, but are interestingly less important in later phase of learning in which skills become increasingly procedural.[35,40,41] Thus, Wanzel et al suggested that VSA is stronger related to initial competency in a spatially complex procedure compared to less complex tasks such as the two-flap plastics task.[41] Depending on diverse pre-existing abilities, psychomotor skills, three-dimensional spatial sense, and faculty for learning those skills varied significantly between individuals.[42–47] Individuals who perform better and faster in spatially complex tasks usually show a higher pre-existing spatial ability.[43–47] This inter-group influence of VSA on surgical performance in complex simulator tasks and better training progress is supported by the findings of this study. Regression analyses indicate that an increased VSA led to improved training progress in surgical performance and task completion time, particularly at the beginning of the learning phase.; whereas, participants with lower innate aptitudes (lower VSA scores) had slower learning curves. The higher relevance of VSA in this initial learning phase may be due to the use of different memory structures and habits as our results have supported.[35,41] Our regression analyses reveal that the VSA test is also able to predict performance. Predictions can be made especially at the beginning of the training. This can also be observed in other spatial performance tests.[17,25]
However, whether future performance in a residency-training program can be predicted based on this parameter remains unclear. As already known, long-term development of VSA occurs mainly during childhood and adolescence.[48–50] It remains unclear whether it is possible to train, develop, and improve VSAs of adults during short-term practice, though results of this and previous studies have led to the conclusion that innate abilities, such as higher VSA scores, are important qualities for future surgeons and influence a trainee's ability to handle numerous specific laparoscopic operative techniques and solve defined laparoscopic spatial problems. The influence of pre-existing advantages can be outweighed by other factors such as training and experience. This study is the first to illustrate that structured (short-term) curriculum training using a VR simulator significantly increases the VSA of trained medical novices compared to participants without any training. This can be confirmed by a more detailed investigation. This study revealed that participants with lower pre-VSA levels were able to significantly increase their surgical abilities and VSA scores after a structured 9-day VR-training curriculum. These participants could achieve levels of spatial surgical competence equal to pre-existing high-level VSAs. This conforms with previous studies reporting that training intervention eliminates novice differences in surgical performance related to VSA and that even minimal practice and training is sufficient for significant VSA progress.[41] This implicated that VSA may be a fluid and trainable characteristic with potential to be acquired throughout training and practice. Identifying VSA levels in surgical trainees allows training needs analysis at individual level.
The findings of this study on demographic data and its possible influence as potential predictors on spatial surgical abilities differs somewhat from the existing literature. The correlation between surgical performance on VR simulator and extracurricular activities and motivation, were investigated according to previously reported associations (Supplemental Digital Content 6). For instance, recent studies on the potential predictors of surgical skills and former studies on a VR laparoscopy simulator illustrated no clearly correlation between a declared interest in a surgical career and the weighted composite training time to reach proficiency.[28,51,52] We observed significant correlations both in participants with declared interest in a surgical career and experience in video gaming and aptitude for surgical performance, especially at the beginning of training. Whereas, other surrogate markers, such as playing musical instruments or having previous laparoscopic experience, did not reveal any association with surgical performance, and the latter not surprising considered participants with limited laparoscopic experience.
The present study demonstrated better performance and shorter completion times for men compared with women for the complex LCN task on day-1 training. However, our study did not depict any difference concerning the impact of VSA on surgical performance between both sexes during further training processes without a larger extension of the learning curve. Sex differences in VSAs conform with earlier studies reporting better performance for men at the beginning of training with equal results in the end.[53,54] A possible explanation for better laparoscopic skills in males could be the difficulty females encounter when handling laparoscopic instruments that are generally designed for male surgeons. With increasing number of female surgeons, redesigning of laparoscopic instrument handles and operating rooms with optimal table height and monitor placement could compromise these ergonomic challenges.[55]
Due to the COVID 19 restrictions, fewer options to support learning and provide practicing surgeons with the means to improve technical skills are available. As already known, an operating surgeon's technical skill is directly associated with postoperative outcomes. In this context, surgical coaching, consisting of debriefing, video review, and feedback has emerged as a beneficial tool to improve technical skills and address changes in clinical outcomes, both in fellows and in skilled doctors.[56,57] To further simulate the real-world surgical environment, written or verbal feedback can be provided remotely, either through faculty review of uploaded recordings or hosted video chat sessions, in which experienced surgeons review operative techniques and discuss procedural nuances in surgical videos. Other teaching methods include residents solving different tasks during individual simulator training for faculty feedback in real-time. Residents can capitalize on near-peer learning by receiving feedback from their peers.[4] With the use of virtual didactics (e.g., podcasts, webinars) such as video- and phone-based conferencing platforms (e.g., Zoom, Skype) instructors can utilize screen sharing options to walk students through relevant surgical topics or can intensify online discussions and surgical operative steps.[4,58] In this context, virtual libraries, didactics, online platforms, or virtual surgical simulators (such as take-home box trainers or a safe environment in the clinic), as well as interactive virtual mentoring modules could be valuable tools to address the loss of learning environment, discuss trainees performance, and to ensure training opportunities during these difficult times.[4,59,60]
The question whether simulator performance translates to actual surgical skills in the OR is important. A recent review identified 34 studies (27 randomized and seven non-randomized studies) that explored VR simulator-related surgical performance and skill transferability to the OR.[61] These studies provided strong evidence that participants who reached proficiency in simulation-based training performed better and faster in the patient-based setting than their counterparts without simulation-based training. This strengthens the evidence that simulation-based training, as part of a structured program and incorporating predetermined proficiency levels, results in transfer of certain surgical skills to the operative and clinical setting. These findings could be supported by a recent meta-analysis by Schmidt et al[62] exploring skill transferability acquired via robotic VR simulation. Further, performance on robotic simulators seems to predict current technical performance in the OR. The extent, to which this may find application, remains unclear. However, assessing skill transfer in relation to clinical operative performance is complex. Practice in a training center, regardless of how real the VR of the training appears to be, will always be different from the actual experience performing surgery with an actual patient in a real-life operating room, given the procedural difficulties related to patient-specific characteristics, such as anatomy, general condition, previous surgical and medical history, and co-morbidities. The extent of pre-training, the nature and type of feedback, the types of task in relation to the operative performance assessed, and the amount of training and assessment tools used to evaluate surgical skill are other important variables.
This study had some limitations. This was a small, single-center prospective randomized controlled study. In the demographic data, the TG members showed more pre-existing interest in MIS procedures due to their voluntary participation in an optional subject during medical training. The VSA scores, however, were similar between the groups, which allowed for a correct statistical comparison. Further, we only used the well-established cube comparison test to assess VSA. Other studies, such as Louridas et al[29] and Hedman et al[35] used several tests to assess VSA. However, majority of studies observed relevant correlations concerning laparoscopic simulator tasks and skills using the cube comparison test.[12,25,29] Therefore, our choice for the applied cube test was deliberate and allowed our participants to focus on the baseline and complex laparoscopic simulator skills.[29,63,64] Third, only medical students with no regular operative practice and experience in image-guided surgery were included and not residents or surgeons with greater previous surgical or laparoscopic experience. Thus, conclusions about the influence of VSA and skill development depending on different levels of surgical experience and expertise should be interpreted with caution. Nevertheless, our findings based on a small sample size between and intra groups at a single German center does not allow adequate and homogenous statistical analyses regarding subgroup analysis of under- or postgraduate year status.
Nonetheless, according to the results of our study and the existing literature, VSA and VSA-guided practice play a significant role in the initial learning of manual spatial tasks and first contact with complex spatial problems. Thus, surgical trainees served as an optimal test group for this study. Future studies are warranted to investigate VSAs and VSA training of more experienced surgical trainees and skilled surgeons for further interesting insights into the possible values of VR simulator training programs for continuous improvement and further development of laparoscopic techniques.
5. Conclusion
The unprecedented COVID-19 times will change our approach on training young medical academics and force surgical instructors to re-organize surgical training curriculums for at least the coming months, if not for longer. In this context, the pandemic may also provide an important opportunity and a stimulus for the evolution and improvement of surgical teaching and training programs. Innovation and alternative approaches to ensure rigorous standards of education and surgical training are critical. In this context, VR-simulator training could change the landscape of training and education of next-generation surgeons. Assessing VSA with a simple test is feasible and high VSA scores indicate superior surgical spatial performance in VR simulator tasks. Moreover, VSA can be improved and trained by short-term structured training programs, thereby enhancing surgical spatial abilities in high-level and low-level VSA participants.
Acknowledgments
We thank Epona Medical B.V., Rotterdam, Netherlands and Surgical Science, Gothenburg, Sweden for the generous support and providing laparoscopic simulators. We also thank the ITB-Consulting company and its founder, Prof. G Trost, for handling the validation data for the tube figure test and for approving the use of the test sheets.
Author contributions
Conceptualization: Guillermo Marcos Sommer, Johannes Broschewitz, Sabine Huppert, Christina Gesine Sommer, Ines Gockel, Hans-Michael Hau.
Data curation: Guillermo Marcos Sommer, Johannes Broschewitz, Sabine Huppert, Christina Gesine Sommer, Ines Gockel, Hans-Michael Hau.
Formal analysis: Guillermo Marcos Sommer, Sabine Huppert, Christina Gesine Sommer, Ines Gockel, Hans-Michael Hau.
Funding acquisition: Guillermo Marcos Sommer, Johannes Broschewitz, Sabine Huppert, Christina Gesine Sommer, Nora Jahn, Ines Gockel, Hans-Michael Hau.
Investigation: Guillermo Marcos Sommer, Johannes Broschewitz, Sabine Huppert, Christina Gesine Sommer, Nora Jahn, Ines Gockel, Hans-Michael Hau.
Methodology: Guillermo Marcos Sommer, Sabine Huppert, Christina Gesine Sommer, Ines Gockel, Hans-Michael Hau.
Project administration: Guillermo Marcos Sommer, Johannes Broschewitz, Christina Gesine Sommer, Nora Jahn, Ines Gockel, Hans-Michael Hau.
Resources: Guillermo Marcos Sommer, Johannes Broschewitz, Christina Gesine Sommer, Ines Gockel, Hans-Michael Hau.
Software: Guillermo Marcos Sommer, Christina Gesine Sommer, Ines Gockel, Hans-Michael Hau.
Supervision: Guillermo Marcos Sommer, Sabine Huppert, Christina Gesine Sommer, Ines Gockel, Hans-Michael Hau.
Validation: Guillermo Marcos Sommer, Johannes Broschewitz, Sabine Huppert, Christina Gesine Sommer, Nora Jahn, Ines Gockel, Hans-Michael Hau.
Visualization: Guillermo Marcos Sommer, Christina Gesine Sommer, Nora Jahn, Ines Gockel, Hans-Michael Hau.
Writing – original draft: Guillermo Marcos Sommer, Christina Gesine Sommer, Nora Jahn, Ines Gockel, Hans-Michael Hau.
Writing – review & editing: Nora Jahn, Boris Jansen-Winkeln, Ines Gockel, Hans-Michael Hau.
Footnotes
Abbreviations: CA = Clip applying, CCE = Cholecystectomy, CD = Cholecystectomy dissection, CG = Control group, CU = Cutting, FD = Fine dissection, GRASP = Gain, Recognize, Analyze, Simulate, and Perform, LCN = Laparoscopic camera navigation, MIS = Minimally invasive surgery, NS = Not significant, SOR = Scope orientation 30° right-handed, TFT = Tube figure test = VSA test = cube comparison test, TG = Training group, VISTA = Video-Based Feedback for the Improvement of Surgical Technique, VR = Virtual reality, VSA = Visual spatial ability.
How to cite this article: Sommer GM, Broschewitz J, Huppert S, Sommer CG, Jahn N, ansen-Winkeln B, Gockel I, Hau HM. The role of virtual reality simulation in surgical training in the light of COVID-19 pandemic: Visual spatial ability as a predictor for improved surgical performance: a randomized trial. Medicine. 2021;100:50(e27844).
This work was supported by the German Research Foundation (DFG) and Dresden University within the program of Open Access Publishing. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate: The study was approved by the local ethical commission board of Leipzig University (AZ- Nr: 111–16–14032016). Participation took place voluntarily after written agreement. No money or other benefits were granted.
Consent for publication: Not applicable.
Competing interests: The authors declare that they have no competing interests.
The datasets generated during and/or analyzed during the present study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Jupraset JM, Gray KD, Moore MD, et al. Restructuring of a general surgery residency program in an epicenter of the Coronavirus Disease 2019 pandemic: lessons from New York City. JAMA Surg 2020;155:870–5. [DOI] [PubMed] [Google Scholar]
- [2].Sabharwal S, Ficke JR, Laporte DM. How we do it: modified residency programming and adoption of remote didactic curriculum during the COVID-19 pandemic. J Surg Educ 2020;77:1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhi VF, Motaz Q, Ross MK, et al. Practical Implications of novel Coronavirus COVID-19 on hospital operations, board certification, and medical education in surgery in the USA. J Gastrointest Surg 2020;24:1232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hau HM, Weitz J, Bork U. Impact of the COVID-19 pandemic on student and resident teaching and training in surgical oncology. J Clin Med 2020;9:3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hassan I, Gerdes B, Koller M, et al. Spatial perception predicts laparoscopic skills on virtual reality laparoscopy simulator. Childs Nerv Syst 2007;23:685–9. [DOI] [PubMed] [Google Scholar]
- [6].Nilsson C, Sorensen JL, Konge L, et al. Simulation-based camera navigation training in laparoscopy-a randomized trial. Surg Endosc 2017;31:2131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Klein J, Frie KG, Blum K, Knesebeck OVD. Psychosocial stress at work and perceived quality of care among clinicians in surgery. BMC Health Serv Res 2011;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scheppokat KD, Neu J. Medical data and quality management. Dtsch Arztebl 2007;104:0A–3172. [Google Scholar]
- [9].Abe T, Raison N, Shinohara N, Shamim Khan M, Ahmed K, Dasgupta P. The effect of visual-spatial ability on the learning of robot-assisted surgical skills. J Surg Educ 2018;75:458–64. [DOI] [PubMed] [Google Scholar]
- [10].Moglia A, Morelli L, Ferrari V, Ferrari M, Mosca F, Cuschieri A. Distribution of innate psychomotor skills recognized as important for surgical specialization in unconditioned medical undergraduates. Surg Endosc 2018;32:4087–95. [DOI] [PubMed] [Google Scholar]
- [11].Maan ZN, Maan IN, Darzi AW, Aggarwal R. Systematic review of predictors of surgical performance. Br J Surg 2012;99:1610–21. [DOI] [PubMed] [Google Scholar]
- [12].Schlickum M, Hedman L, Felländer-Tsai L. Visual-spatial ability is more important than motivation for novices in surgical simulator training: a preliminary study. Int J Med Educ 2016;7:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sørensen SM, Savran MM, Konge L, Bjerrum F. Three-dimensional versus two dimensional vision in laparoscopy: a systematic review. Surg Endosc 2016;30:11–23. [DOI] [PubMed] [Google Scholar]
- [14].Crothers IR, Gallagher AG, McClure N, James DT, McGuigan J. Experienced laparoscopic surgeons are automated to the “fulcrum effect”: an ergonomic demonstration. Endoscopy 1999;31:365–9. [DOI] [PubMed] [Google Scholar]
- [15].Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg 1993;165:09–14. [DOI] [PubMed] [Google Scholar]
- [16].Nasca TJ, Day SH. Amis ES Jr; ACGME Duty Hour Task Force. The new recommendations on duty hours from the ACGME task force. N Engl J Med 2010;363:e3. [DOI] [PubMed] [Google Scholar]
- [17].Jungmann F, Gockel I, Hecht H, et al. Impact of perceptual ability and mental imagery training on simulated laparoscopic knot-tying in surgical novices using a Nissen fundoplication model. Scand J Surg 2011;100:78–85. [DOI] [PubMed] [Google Scholar]
- [18].Britt LD, Sachdeva AK, Healy GB, Whalen TV, Blair PG. Members of ACS Task Force on Resident Duty Hours. Resident duty hours in surgery for ensuring patient safety, providing optimum resident education and training, and promoting resident well-being: a response from the American College of Surgeons to the Report of the Institute of Medicine, “Resident Duty Hours: enhancing sleep, supervision, and safety”. Surgery 2009;146:398–409. [DOI] [PubMed] [Google Scholar]
- [19].Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg 2002;236:458–63. discussion 463-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wanzel KR, Hamstra SJ, Caminiti MF, Anastakis DJ, Grober ED, Reznick RK. Visual-spatial ability correlates with efficiency of hand motion and successful surgical performance. Surgery 2003;134:750–7. [DOI] [PubMed] [Google Scholar]
- [21].Celentano V, Smart N, McGrath J, et al. LAP-VEGaS practice guidelines for reporting of educational videos in laparoscopic surgery: a joint trainers and trainees consensus statement. Ann Surg 2018;268:920–6. [DOI] [PubMed] [Google Scholar]
- [22].Zevin B, Dedy NJ, Bonrath EM, Grantcharov TP. Comprehensive simulation-enhanced training curriculum for an advanced minimally invasive procedure: a randomized controlled trial. Surg Obes Relat Dis 2017;13:815–24. [DOI] [PubMed] [Google Scholar]
- [23].Buckley CE, Kavanagh DO, Nugent E, Ryan D, Traynor OJ, Neary PC. The impact of aptitude on the learning curve for laparoscopic suturing. Am J Surg 2014;207:263–70. [DOI] [PubMed] [Google Scholar]
- [24].Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach 2010;32:638–45. [DOI] [PubMed] [Google Scholar]
- [25].Kramp KH, van Det MJ, Hoff C<ET AL>. The predictive value of aptitude assessment in laparoscopic surgery: a meta-analysis. Med Educ 2016;50:409–27. [DOI] [PubMed] [Google Scholar]
- [26].Gallagher AG, Cowie R, Crothers I, Jordan-Black JA, Satava RM. PicSOr: an objective test of perceptual skill that predicts laparoscopic technical skill in three initial studies of laparoscopic performance. Surg Endosc 2003;17:1468–71. [DOI] [PubMed] [Google Scholar]
- [27].Stefanidis D, Korndorffer JR, Black FW, et al. Psychomotor testing predicts rate of skill acquisition for proficiency-based laparoscopic skills training. Surgery 2006;140:252–62. [DOI] [PubMed] [Google Scholar]
- [28].Louridas M, Quinn LE, Grantcharov TP. Predictive value of background experiences and visual spatial ability testing on laparoscopic baseline performance among residents entering postgraduate surgical training. Surg Endosc 2016;30:1126–33. [DOI] [PubMed] [Google Scholar]
- [29].Louridas M, Szasz P, de Montbrun S, Harris KA, Grantcharov TP. Can we predict technical aptitude? A systematic review. Ann Surg 2016;263:673–91. [DOI] [PubMed] [Google Scholar]
- [30].Stumpf H, Fay E. VSA test. A test to assess the spatial imagination [German] Publisher of Psychology, Hogrefe 1983. [Google Scholar]
- [31].ITB-Consulting GmbH (1977): TMS. Medical study test. TMS coordination office. Heidelberg. [Google Scholar]
- [32].Stumpf H, Fay E. Tube figures. A test for the evaluation of the spatial imagination [German] Vol 1 Publisher of Psychology Hogrefe, Göttingen 1983. [Google Scholar]
- [33].Trost G, Blum F, Fay F, Klieme F, Maichle U, Meyer M. Evaluation of the medical study program (TMS): synopsis of the results [German]. Institute for Test and Gifted Research. Bonn: ITB; 1998. [Google Scholar]
- [34].Ericsson KA, Krampe RT, Tesch-Römer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev 1993;100:363–406. [Google Scholar]
- [35].Hedman L, Ström P, Andersson P, Kjellin A, Wredmark T, Felländer-Tsai L. High-level visual-spatial ability for novices correlates with performance in a visual-spatial complex surgical simulator task. Surg Endosc 2006;20:1275–80. [DOI] [PubMed] [Google Scholar]
- [36].Grantcharov TP, Funch-Jensen P. Can everyone achieve proficiency with the laparoscopic technique? Learning curve patterns in technical skills acquisition. Am J Surg 2009;197:447–9. [DOI] [PubMed] [Google Scholar]
- [37].Van Herzeele I, O’Donoghue KG, Aggarwal R, Vermassen F, Darzi A, Cheshire NJ. Visuospatial and psychomotor aptitude predicts endovascular performance of inexperienced individuals on a virtual reality simulator. J Vasc Surg 2010;51:1035–42. [DOI] [PubMed] [Google Scholar]
- [38].Satava RM, Gallagher AG, Pellegrini CA. Surgical competence and surgical proficiency: definitions, taxonomy, and metrics. J Am Coll Surg 2003;196:933–7. [DOI] [PubMed] [Google Scholar]
- [39].Satava RM. Surgeon responsibility in the era of change. JSLS 2003;7:293–4. [PMC free article] [PubMed] [Google Scholar]
- [40].Roch PJ, Rangnick HM, Brzoska JA, et al. Impact of visual-spatial ability on laparoscopic camera navigation training. Surg Endosc 2018;32:1174–83. [DOI] [PubMed] [Google Scholar]
- [41].Wanzel KR, Hamstra SJ, Anastakis DJ, Matsumoto ED, Cusimano MD. Effect of visual-spatial ability on learning of spatially-complex surgical skills. Lancet 2002;359:230–1. [DOI] [PubMed] [Google Scholar]
- [42].Siska VB, Ann L, Gunter de W, et al. Surgical skill: trick or trait? J Surg Educ 2015;72:1247–53. [DOI] [PubMed] [Google Scholar]
- [43].Henn P, Gallagher AG, Nugent E, et al. Visual spatial ability for surgical trainees: implications for learning endoscopic, laparoscopic surgery and other image-guided procedures. Surg Endosc 2018;32:3634–9. [DOI] [PubMed] [Google Scholar]
- [44].Wanzel KR, Anastakis DJ, McAndrews MP, et al. Visual-spatial ability and fMRI cortical activation in surgery residents. Am J Surg 2007;193:507–10. [DOI] [PubMed] [Google Scholar]
- [45].White C, Rodger MWM, Tang T. Current understanding of learning psychomotor skills and the impact on teaching laparoscopic surgical skills. Obstet Gynaecol 2016;18:53–63. [Google Scholar]
- [46].Gallagher AG, Richie K, McClure N, McGuigan J. Objective psychomotor skills assessment of experienced, junior, and novice laparoscopists with virtual reality. World J Surg 2001;25:1478–83. [DOI] [PubMed] [Google Scholar]
- [47].Gallagher AG, Satava RM. Virtual reality as a metric for the assessment of laparoscopic psychomotor skills. Learning curves and reliability measures. Surg Endosc 2002;16:1746–52. [DOI] [PubMed] [Google Scholar]
- [48].Brandt MG, Davies ET. Visual-spatial ability, learning modality and surgical knot tying. Can J Surg 2006;49:412–6. [PMC free article] [PubMed] [Google Scholar]
- [49].Arsalidou M, Im-Bolter N. Why parametric measures are critical for understanding typical and atypical cognitive development. Brain Imaging Behav 2017;11:1214–24. [DOI] [PubMed] [Google Scholar]
- [50].Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci 2002;14:01–10. [DOI] [PubMed] [Google Scholar]
- [51].Cope DH, Fenton-Lee D. Assessment of laparoscopic psychomotor skills in interns using the MIST virtual reality simulator: a prerequisite for those considering surgical training? ANZ J Surg 2008;78:291–6. [DOI] [PubMed] [Google Scholar]
- [52].Moglia A, Sinceri S, Ferrari V, Ferrari M, Mosca F, Morelli L. Proficiency-based training of medical students using virtual simulators for laparoscopy and robot-assisted surgery: results of a pilot study. Updates Surg 2018;70:401–5. [DOI] [PubMed] [Google Scholar]
- [53].Palter VN, Orzech N, Reznick RK, Grantcharov TP. Validation of a structured training and assessment curriculum for technical skill acquisition in minimally invasive surgery: a randomized controlled trial. Ann Surg 2013;257:224–30. [DOI] [PubMed] [Google Scholar]
- [54].White MT, Welch K. Does gender predict performance of novices undergoing Fundamentals of Laparoscopic Surgery (FLS) training? Am J Surg 2012;203:397–400. [DOI] [PubMed] [Google Scholar]
- [55].Sutton E, Irvin M, Zeigler C, Lee G, Park A. The ergonomics of women in surgery. Surg Endosc 2014;28:1051–5. [DOI] [PubMed] [Google Scholar]
- [56].Greenberg CC, Ghousseini HN, Pavuluri Quamme SR, et al. Wisconsin surgical coaching program. a statewide surgical coaching program provides opportunity for continuous professional development. Ann Surg 2018;267:868–73. [DOI] [PubMed] [Google Scholar]
- [57].Coe TM, Jogerst KM, Sell NM, et al. Practical techniques to adapt surgical resident education to the COVID-19 era. Ann Surg 2020;272:e139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med 2004;79:S70–81. [DOI] [PubMed] [Google Scholar]
- [59].Agrawal V, Yadav SK, Agarwal P, Sharma D. “GRASP” module of self-assessment with virtual mentoring for uninterrupted surgical training during COVID-19 pandemic. Indian J Surg 2020. 01–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schlick CJR, Bilimoria KY, Stulberg JJ. Video- based feedback for the improvement of surgical technique: a platform for remote review and improvement of surgical technique. JAMA Surg 2020;155:1078–9. [DOI] [PubMed] [Google Scholar]
- [61].Dawe SR, Pena GN, Windsor AJ, et al. Systematic review of skills transfer after surgical simulation-based training. Br J Surg 2014;101:1063–76. [DOI] [PubMed] [Google Scholar]
- [62].Schmidt MW, Köppinger KF, Fan C, et al. Virtual reality simulation in robot-assisted surgery: meta-analysis of skill transfer and predictability of skill. BJS Open 2021;5:zraa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dawson B, Trapp R. Basic & clinical biostatistics. 4th ed.New York: McGraw-Hill; 2004. [Google Scholar]
- [64].Ruth B. Ekstrom RB, French JW, Harman HH, Dermen D. Manual for kit of factor-referenced cognitive tests. Educational testing service, Princeton, New Jersey. August 1976. [Google Scholar]