Abstract
BACKGROUND:
Individuals who survive a cardiac arrest often sustain cognitive impairments due to ischemia-reperfusion injury. Mesenchymal stem cell (MSC) transplantation is used to reduce tissue damage, but exosomes are more stable and highly conserved than MSCs. This study was conducted to investigate the therapeutic effects of MSC-derived exosomes (MSC-Exo) on cerebral ischemia-reperfusion injury in an in vitro model of oxygen-glucose deprivation/reperfusion (OGD/R), and to explore the underlying mechanisms.
METHODS:
Primary hippocampal neurons obtained from 18-day Sprague-Dawley rat embryos were subjected to OGD/R treatment, with or without MSC-Exo treatment. Exosomal integration, cell viability, mitochondrial membrane potential, and generation of reactive oxygen species (ROS) were examined. Terminal deoxynucleotidyl transferase-mediated 2’-deoxyuridine 5’-triphosphate nick-end labeling (TUNEL) staining was performed to detect neuronal apoptosis. Moreover, mitochondrial function-associated gene expression, Nrf2 translocation, and expression of downstream antioxidant proteins were determined.
RESULTS:
MSC-Exo attenuated OGD/R-induced neuronal apoptosis and decreased ROS generation (P<0.05). The exosomes reduced OGD/R-induced Nrf2 translocation into the nucleus (2.14±0.65 vs. 5.48±1.09, P<0.01) and increased the intracellular expression of antioxidative proteins, including superoxide dismutase and glutathione peroxidase (17.18±0.97 vs. 14.40±0.62, and 20.65±2.23 vs. 16.44±2.05, respectively; P<0.05 for both). OGD/R significantly impaired the mitochondrial membrane potential and modulated the expression of mitochondrial function-associated genes, such as PINK, DJ1, LRRK2, Mfn-1, Mfn-2, and OPA1. The abovementioned changes were partially reversed by exosomal treatment of the hippocampal neurons.
CONCLUSIONS:
MSC-Exo treatment can alleviate OGD/R-induced oxidative stress and dysregulation of mitochondrial function-associated genes in hippocampal neurons. Therefore, MSC-Exo might be a potential therapeutic strategy to prevent OGD/R-induced neuronal injury.
Keywords: Mesenchymal stem cells, Exosomes, Oxygen-glucose deprivation/reperfusion, Reactive oxygen species, Mitochondria
INTRODUCTION
Cardiac arrest is a common critical condition, and survivors of cardiac arrest often suffer cognitive impairments or may even remain in a vegetative state.[1] The treatment of cardiac arrest involves the restoration of circulation as soon as possible, although this may induce secondary damage, which is called “ischemia-reperfusion injury” (IRI).[2] Therefore, efforts to mitigate the pathological responses and protect neurons from IRI have been continuously undertaken.
The use of mesenchymal stem cell (MSC) transplantation to reduce tissue damage and promote cerebral recovery[3,4] is based on the protective effects that MSCs exert by secreting paracrine mediators, such as DNA, messenger ribonucleic acid (mRNA), microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and proteins (i.e., exosomes).[5] Exosomes are more stable and conserved than MSCs, and exosome release from MSCs can be artificially monitored. Therefore, exosomes are valuable for several clinical applications.[6] However, how exosomes play positive effects on cerebral IRI is not clear. We used primary hippocampal cells and a model of oxygen-glucose deprivation/reperfusion (OGD/R) to mimic the IRI-associated conditions.[7] We aim to investigate the neuroprotective effects of MSC-derived exosomes (MSC-Exo) and to identify the associated signaling pathways.
METHODS
Primary hippocampal cell culture
Primary hippocampal neurons were obtained from the 18-day Sprague-Dawley (SD) rat embryos.[8] The hippocampi were triturated in D-Hanks solution (Gibco, USA) and incubated with trypsin (0.25%) at 37 °C for 20 minutes. The trypsin digestion was stopped by adding 5% fetal bovine serum albumin (BSA; Gibco, USA), and the mixture was centrifuged at 1,000×g for 10 minutes. The precipitated cell pellet was resuspended in a neurobasal medium (Gibco, USA) with B27 supplementation (Gibco, USA) and then seeded in poly-L-lysine-coated plates. The study-related experiments were performed following 8 days of cell culture.
MSC culture
The MSCs were harvested from the tibial and femoral bone marrow of 3-week-old male SD rats by using a previously described procedure,[8] wherein the animals were euthanized by anesthesia and the marrow was flushed out and suspended in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, USA). The MSCs were harvested after 3−4 passages for further experiments. An osteogenic differentiation kit and adipogenic differentiation medium (both from Cyagen, China) were used to assess the differentiation potential of the MSCs.
Isolation and identification of MSC-Exo
At 80% confluence, MSCs were incubated in low-glucose DMEM (L-DMEM) containing 10% exosome-depleted fetal bovine serum (System Biosciences, USA) for 48 hours, and the culture medium was collected and centrifuged at 3,000×g for 15 minutes. The supernatant was mixed (5:1) with the ExoQuick PLUS kit (System Biosciences, USA) at 4 °C for 12 hours and centrifuged at 1,500×g for 30 minutes. Thereafter, the supernatant was discarded and the pellets were resuspended in 100 μL phosphate buffered saline (PBS) and stored at −80 °C until analysis. The markers Alix and CD63 (Santa Cruz Biotechnology, USA) were examined using Western blotting. The electron microscopy was used to detect the diameter of exosomes.
MSC-Exo labeling and uptake
To facilitate exosome tracking, the exosomes were labeled with a fluorescent dye, by incubating the MSC-Exo with PKH26 (Sigma-Aldrich, USA) for 5 minutes at 37 °C in the dark; the cells were washed with PBS, and centrifuged at 110,000×g at 4 °C for 2 hours to remove any unbound PKH26, and subsequently incubated with triturated hippocampal neurons for 12 hours. Then the neurons were fixed with 4% paraformaldehyde and counterstained with 4’,6-diamidino-2-phenylindole (DAPI; Abcam, USA) staining solution, mounted on slides, and observed using a fluorescence microscope (Olympus, Japan).
OGD/R model
The neurons were randomly divided into three groups (control, OGD/R, and OGD/R+Exo) seeded in a glucose-free medium (Gibco, USA), and incubated for 2 hours at 95% N2 and 5% CO2 in an incubator (Longfujia, China). Then in the OGD/R and OGD/R+Exo groups, the neurons were incubated in the normal medium under normoxic conditions, and co-incubated with PBS or 20 µg/mL exosomes at the beginning of reoxygenation, respectively, for 12 hours,[8,9] whereas the cells in the control group were not exposed to OGD/R or exosomes.
Cell viability assay
To evaluate the cell viability of neurons using the cell counting kit-8 (CCK-8) (Dojindo Laboratories, Japan). The 10 μL CCK-8 solution was added to the triturated hippocampal neurons, the plate was incubated at 37 °C for 3 hours, and the optical density was measured at 450 nm using a microplate reader (Biotek, USA). The results were expressed as a relative percentage of the values obtained for the control group.
Intracellular reactive oxygen species (ROS) assay
To quantify the ROS in the neurons, the suspension containing triturated neurons was treated sequentially with 2’,7’-dichlorodihydrofluorescein diacetate solution (30 μmol/L; Sigma-Aldrich, USA) and Hoechst solution (Beyotime, China) and incubated at 37 °C for 30 minutes for each treatment. Subsequently, the ROS-related fluorescence intensity was measured using a confocal microscope (Zeiss, Germany), and the data were shown as the mean intensities in the entire fields of view in five random graphs and expressed as the fold intensity relative to that in the control group.
Terminal deoxynucleotidyl transferase-mediated 2’-deoxyuridine 5’-triphosphate nick-end labeling (TUNEL) staining
TUNEL staining using an in situ cell death detection kit (Roche, Switzerland) was performed by fixing the neurons with 4% paraformaldehyde for 1 hour, followed by permeabilization with 0.2% Triton X-100 for 20 minutes on ice and the addition of 50 μL TUNEL reaction solution and incubation for 2 hours. The cells were counterstained with DAPI, and examined using fluorescence microscopy. Hippocampal neuronal apoptosis was quantified as the ratio of TUNEL-positive cells to the total number of cells counted within five randomly chosen fields.
Immunofluorescence staining
The neuronal cells were fixed with 4% paraformaldehyde, permeabilized with 1% Triton X-100 for 10 minutes on ice, treated with 10% goat serum for 30 minutes, and incubated with rabbit anti-Nrf2 primary antibody and rabbit anti-Keap1 primary antibody (ab31163 and ab139729, respectively; 1:200; Abcam, USA) in PBS-BSA (1% BSA) at 4 °C overnight. Thereafter, the neuronal cells were incubated with A488 anti-rabbit IgG (1:500; Thermo Fisher Scientific, USA) for 2 hours, counterstained with DAPI, mounted on slides, and observed using a fluorescence microscopy.
Measurement of superoxide dismutase and glutathione peroxidase activity
According to the manufacturer’s instructions, the superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities (U/mg protein) were measured using the corresponding assay kits (Jiancheng and Beyotime, respectively, China).
Analysis of mitochondrial membrane potential
Following the manufacturer’s recommendations, the neurons were incubated with 1 mL 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetramethylbenzimidazolylcarbocyanine iodide (JC-1) solution (10 μg/mL; Beyotime, China) at 37 °C for 30 minutes, washed twice with the JC-1 staining buffer, and the fluorescence was ascertained using fluorescence microscopy. Image J (NIH, USA) was used to calculate the ratio of JC-1 aggregates (red fluorescence) to monomers (green fluorescence), wherein a decreased ratio of red to green fluorescence intensity indicated the loss of mitochondrial function.
Reverse transcription-polymerase chain reaction (RT-PCR)
We used the RNA extraction kit (Takara, Japan) to extract total RNA from the neuronal cells following the manufacturer’s instructions. First-strand complementary DNA (cDNA) was synthesized by reverse transcription of 2 μg of RNA using the PrimeScript® RT Master Mix Kit (Takara, Japan). The RT-PCR was performed using 10 μL aliquots containing SYBR® Premix Ex Taq™ II (Takara, Japan) on a real-time PCR detection system (Life Technologies, UK). The PCR primers glyceraldehyde-3-phosphate dehydrogenase (GAPDH)(forward 5’-GGGTGTGAACCACGAGAAATA-3’, reverse 5’-AGTTGTCATGGATGACCTTG-3’), OPA1 (forward 5’-CAGTTCAGAAGACCTCGCCA-3’, reverse 5’-CAGGTGTACCCG CAGTGAAG-3’), PINK (forward 5’-GTATGAAGCCACCATGCCCA-3’, reverse 5’-ACGACATCTGGGCCTTTTCC-3’), Mfn-1 (forward 5’-AAGAGAGGG AAGACCAAATC-3’, reverse 5’-AAACAGACAGGCGACAAA-3’), Mfn-2 (forward 5’-AGCGGGTTTATTGTCTTG-3’, reverse 5’-TTCCACTTCCTCTGTCATCT-3’), LRRK2 (forward 5’-TAAGACTTCAGAGCCCACCAG-3’, reverse 5’-ACTACG CCCAAACCGAATGTA-3’), and DJ1 (forward 5’-GGAGCTGAGGTACAGAGGTCA-3’, reverse 5’-AGTCACTGGTTCACATGGTGG-3’) were mixed with the SYBR® Green Master Mix (Takara, Japan) under the following conditions: denaturation at 95 °C for 30 seconds, annealing at 95 °C for 5 seconds, and elongation at 60 °C for 30 seconds for 40 PCR cycles. The relative mRNA values were estimated using the 2-ΔΔCt method, and the results were expressed as a relative percentage for the values in the control group.
Statistical analysis
One-way ANOVA followed by Tukey post-hoc test was used for multi-sample comparisons, and the results were presented as mean±standard error of the mean (SEM). P<0.05 was considered statistically significant. The statistical graphs were obtained using GraphPad Prism 6 (CA, USA).
RESULTS
Identification of MSCs and MSC-Exo
The cultured MSCs appeared as fibroblast-like cells under phase-contrast microscopy (Figure 1A). Alizarin red staining and Oil Red O staining showed the osteogenic and adipocytic differentiation abilities of the MSCs, respectively (Figures 1 B and 1C). Electron microscopy revealed that the exosomes had a concave hemisphere structure, a double-layered membrane, and a diameter of 40–50 nm (Figure 1D), which were typical characteristics of an exosome. We used Western blotting to detect the expression of CD63 (a member of the tetraspanin family) and Alix, two representative exosomal markers (Figure 1E). Fluorescence micrography showed the intracytoplasmic localization of PKH26-labeled exosomes in the neuronal cells (Figure 1F).
Figure 1.
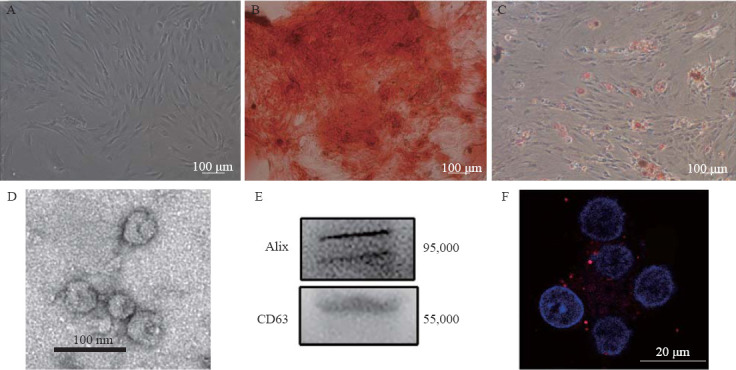
Identification of MSCs and MSC-Exo. A: the morphology of the MSCs; B: differentiation of MSCs into osteoblasts; C: differentiation of MSCs into adipocytes; D: electron microscopic image of exosomes; E: Western blotting luminograms showing exosomal expressions of CD63 and Alix; F: PKH26-tagged (red fluorescence) exosomes in the neuronal cytoplasm. MSCs: mesenchymal stem cells; MSC-Exo: MSC-derived exosomes.
Protection of hippocampal neurons by MSC-Exo against OGD/R-induced injury
Compared with the control group, OGD/R treatment increased the number of apoptotic cells, and this effect was reversed by treatment with exosomes (Table 1, Figures 2 A and C). Compared with the control group, the ROS production increased in the OGD/R group, and exosomes inhibited ROS overproduction (Table 1, Figures 2 B and D). The cell viability in the OGD/R group was lower than that in the control and OGD/R+Exo groups (Table 1).
Table 1.
The comparison between groups
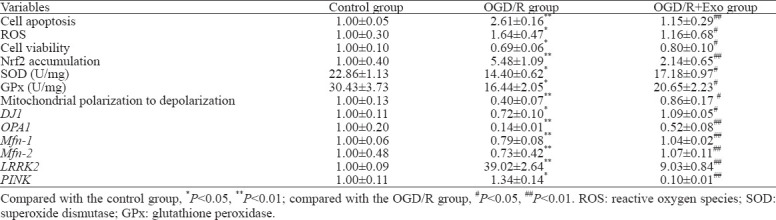
Figure 2.
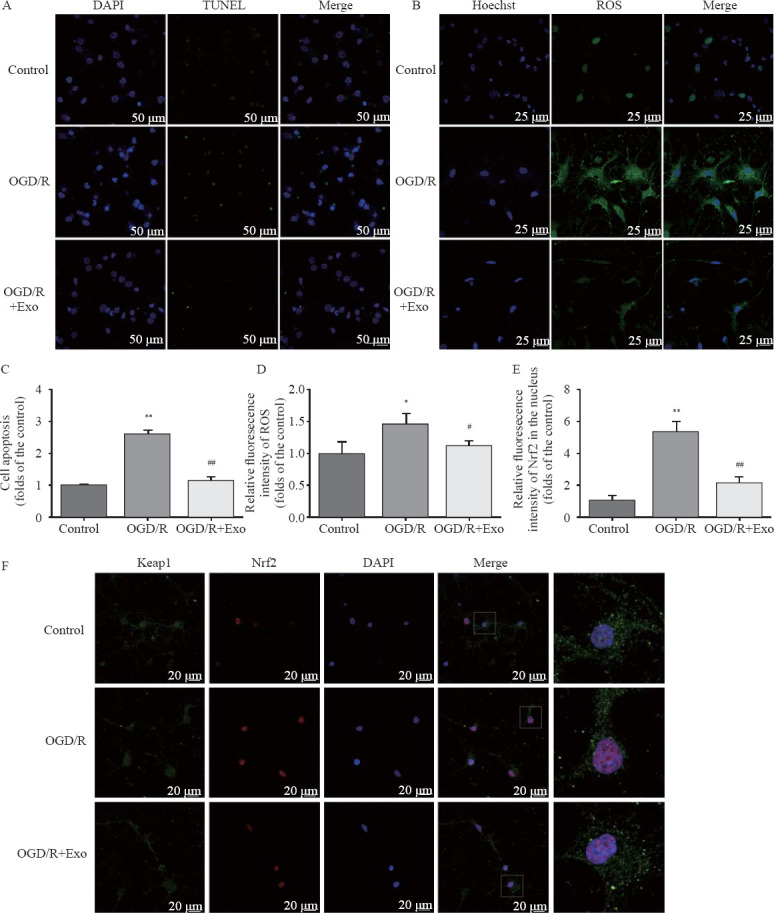
Protective effects of MSC-Exo against OGD/R-induced injury in rat hippocampal neurons. A, C: the antiapoptotic activity of exosomes based on TUNEL staining; the apoptosis in hippocampal neurons was assessed as the ratio of TUNEL-positive cells to the total number of cells counted within five randomly chosen fields; B, D: intracellular ROS generation; the data were shown as the mean intensities in the entire fields of view in five random graphs and were expressed as the fold intensity relative to the intensity in the control group; E, F: representative images and quantitative analysis indicating the nuclear translocation of Nrf2. Compared with the control group, *P<0.05, **P<0.01; compared with the OGD/R group, #P<0.05, ##P<0.01. MSCs: mesenchymal stem cells; MSC-Exo: MSC-derived exosomes; OGD/R: oxygen-glucose deprivation/reperfusion; ROS: reactive oxygen species.
MSC-Exo regulation of intranuclear Nrf2 accumulation
Compared with the control group, OGD/R promoted the nuclear accumulation of Nrf2. Exosomal treatment inhibited OGD/R-induced Nrf2 accumulation (Table 1, Figures 2 E and F). Furthermore, OGD/R significantly decreased SOD and GPx activities in the OGD/R group. However, the exosomal treatment significantly reversed OGD/R-induced changes in SOD and GPx activities (Table 1).
Amelioration of mitochondrial dysfunction and modulation of mitochondrial function-associated gene expression by MSC-Exo
Compared with the control group, OGD/R hastened the transition from mitochondrial polarization to depolarization (Table 1, Figures 3 A and B). However, exosomal treatment markedly improved OGD/R-induced mitochondrial dysfunction. Furthermore, OGD/R significantly downregulated the mRNA expression of DJ1, OPA1, Mfn-1, and Mfn-2 in the control group, and this downregulation was reversed by exosomal treatment. In addition, OGD/R upregulated mRNA expression of LRRK2 and PINK in the control group, which was markedly downregulated by exosomal treatment (Table 1, Figures 3 C-H).
Figure 3.
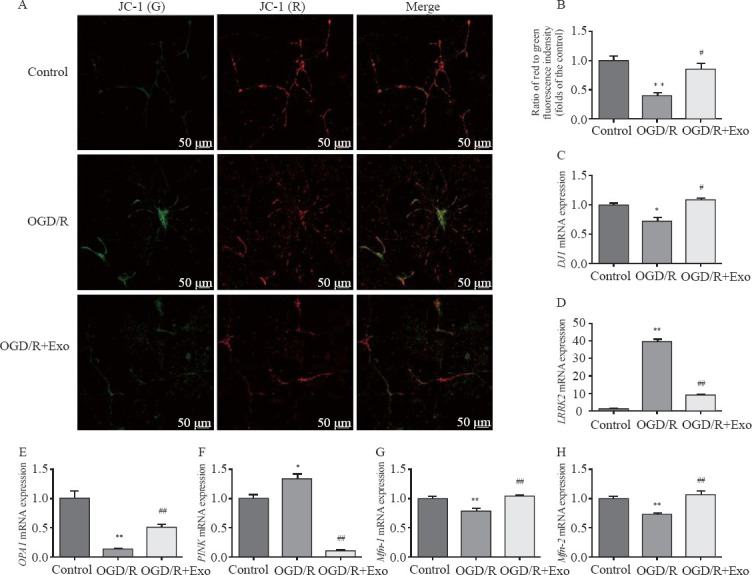
Effects of exosomes on mitochondrial membrane potential and expression of mitochondrial function-associated genes. A, B: the mitochondrial membrane potential was described as the ratio of red to green fluorescence; C-K: effects of exosomes on the expression of mitochondrial function-associated genes in hippocampal neurons after oxygen-glucose deprivation/reperfusion (OGD/R)-induced injury. Compared with the control group, *P<0.05, **P<0.01; compared with the OGD/R group, #P<0.05, ##P<0.01.
DISCUSSION
Oxidative stress is the main factor that aggravates OGD/R injury, which contributes to cognitive impairment or brain damage. The Keap1-Nrf2 signaling pathway, which is a crucial antioxidant pathway, plays a key role in protecting against ROS-induced cell injury. Under homeostatic conditions, Nrf2 combines with Keap1 and co-localizes in the cytoplasm, in a functionally inactivated form. However, with continued oxidative stress and increased ROS generation, Nrf2 dissociates from the Keap1-Nrf2 complex and enters the nucleus to combine with downstream elements and exert antioxidative effects. MSC-exosomal treatment ameliorated lipopolysaccharide-stimulated mitochondrial dysfunction and inflammatory responses, which involved Nrf2-NF-κB signaling.[9] Contrary to the observations that mitochondrial ROS activates Nrf2,[10-12] Nrf2 activity was inhibited during mitochondrial dysfunction.[13] In Trypanosoma cruzi infection, Nrf2 activity was repressed as the mitochondrial function diminishes, whereas SOD restores the decrease in Nrf2 activity.[14] Xiao et al[15] showed that Nrf2 activity was repressed by ROS overproduction or mitochondrial dysfunction in kidney epithelial cells exposed to high glucose concentrations. In the present and a previous study, exosomal pretreatment potently alleviated oxidative stress, which potentially resulted in diminished Nrf2 responses[16] and less intranuclear translocation of Nrf2 in the MSC-Exo group than in the OGD/R group. Moreover, Nrf2 activation is affected by the site and duration of mitochondrial ROS production;[17] thus, potent ROS inhibition by exosomes may elicit fewer Nrf2 nuclear aggregation.
Mitochondria are the main source of ROS and are sensitive targets in oxidative stress conditions. Excessive mitochondrial oxidative stress contributes to the disruption of ROS homeostasis and subsequent mitochondrial membrane damage and a resultant decrease in the mitochondrial membrane potential.[18] A decrease in the mitochondrial membrane potential may induce cytochrome C expression and activate apoptosis.[19] This study showed that the mitochondrial membrane potential of OGD/R-induced neuronal cells was significantly increased after treatment with MSC-Exo. This suggests that exosomes could confer neuronal protection by increasing the mitochondrial membrane potential.
As mitochondria play a crucial role in maintaining ROS homeostasis and cell apoptosis, the expression profiles of genes related to mitochondrial activity were ascertained. DJ1, an antioxidant-related gene, that regulates mitochondrial functions, is mainly localized in the cytoplasm under physiological conditions; however, when exposed to oxidative stress, DJ1 is translocated to the mitochondria and interacts with mitochondrial complex I to maintain mitochondrial homeostasis and exert cytoprotective effects.[20] Thus, downregulation of DJ1 could induce dysfunctions, such as mitochondrial swelling, mitochondrial complex I inactivation, or mass production of ROS, in the PC-12 neuronal cell line,[21] but was significantly reversed by exosomal treatment in the present study. The mRNA expression of DJ1 was significantly decreased after OGD/R induction, whereas exosomal treatment markedly reversed this change. Therefore, our results support the known protective effects of DJ1[21] and confirm the beneficial effect of exosomes in OGD/R-induced neuronal damage.
Neurons have an abundance of mitochondria, and mitochondrial dynamics, including fusion and fission, play pivotal roles in ROS generation. OPA1, Mfn-1, and Mfn-2, which are located on the mitochondrial membrane, are crucial for mitochondrial fusion. In a heart failure model, OPA1, Mfn-1, Mfn-2, and other genes that promote mitochondrial fusion, were downregulated.[22] The present study revealed that OGD/R affected mitochondrial dynamics and reduced the expression of OPA1, Mfn-1, and Mfn-2. Exosomes significantly improved the expression of mitochondrial fusion-related genes in hippocampal neurons with OGD/R injury, which suggests that the abovementioned genes may be potential targets of MSC-Exo treatment to mitigate OGD/R injury. Moreover, the exosomal treatment decreased LRRK2 expression, which was associated with acute neurological diseases that were induced by oxidative stress.[23]
Following a decrease in outer membrane potential, mitophagy is induced in damaged mitochondria through the aggregation of the PINK protein, and mitochondrial autophagy ensures the replenishment of essential substances to enable cell survival under oxidative stress. The abovementioned findings were confirmed with a previous study of PINK-mediated mitochondrial autophagy.[24] As a downstream target gene in the ROS-mediated pathway and as a marker of autophagy, PINK was highly expressed in hippocampal neurons with OGD/R injury, and the increase was significantly attenuated by exosomal treatment. The findings of this study indicated that, as a main source of ROS, the mitochondria of hippocampal neurons displayed significantly dysregulated expression of several genes after OGD/R injury, which resulted in decreased mRNA expression of genes that promoted mitochondrial fission and mitophagy. Notably, exosomes can reverse these changes by regulating the mRNA expression of mitochondrial function-associated genes.
This study has some limitations that need to be mentioned. Firstly, the dramatic inhibition of ROS by exosomal treatment may elicit insufficient Nrf2 nuclear aggregation. We attempted small interfering RNA (siRNA)-induced inhibition or knockdown of Nrf2, but the low transfection ratio prevented a clear elucidation of the role of Nrf2 in ROS generation; thus, we hoped to undertake adenovirus-coated Nrf2 RNA interference to clarify these details in further research. Secondly, after exosomal treatment, only the expression profiles of mitochondrial function-associated genes were investigated; therefore, the expression/activity of the corresponding proteins that are involved in the mitochondrial activity needs to be determined. Thirdly, further research is required to verify the possibility of a direct relationship between exosomal treatment and mitochondrial activities, ROS, and neuronal damage in OGD/R.
CONCLUSIONS
MSC-Exo exerts protective effects against OGD/R-induced injury in hippocampal neurons through a mechanism that partially involves the inhibition of oxidative stress and the regulation of aberrant mitochondrial activity. Therefore, our findings indicate the beneficial effects of MSC-Exo, which might provide novel insights for the development of potential therapies against OGD/R-induced neuronal injury.
Footnotes
Funding: This study was supported by a grant from the National Natural Science Foundation of China (81701872).
Ethical approval: All animal experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (1801008).
Conflicts of interests: The authors have no conflict of interest to declare.
Contributors: XFG and SSG contributed equally to this study. JSZ, JW, and LJ conceived and designed the experiments, and modified the manuscript; SSG, YJZ, XFG, and LJ performed the experiments; SSG, XFG, and PFY analyzed the data; XFG wrote the paper.
REFERENCES
- 1.Lilja G, Nielsen N, Friberg H, Horn J, Kjaergaard J, Nilsson F, et al. Cognitive function in survivors of out-of-hospital cardiac arrest after target temperature management at 33 ? versus 36 ? Circulation. 2015;131(15):1340–9. doi: 10.1161/CIRCULATIONAHA.114.014414. [DOI] [PubMed] [Google Scholar]
- 2.Puyal J, Ginet V, Clarke PGH. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage:a challenge for neuroprotection. Prog Neurobiol. 2013;105:24–48. doi: 10.1016/j.pneurobio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Liao LY, Lau BWM, Sánchez-Vidaña DI, Gao Q. Exogenous neural stem cell transplantation for cerebral ischemia. Neural Regen Res. 2019;14(7):1129–37. doi: 10.4103/1673-5374.251188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu B, Sekine H, Homma J, Kobayashi T, Kobayashi E, Kawamata T, et al. Allogeneic adipose-derived mesenchymal stem cell sheet that produces neurological improvement with angiogenesis and neurogenesis in a rat stroke model. J Neurosurg. 2020;132(2):442–55. doi: 10.3171/2018.11.JNS182331. [DOI] [PubMed] [Google Scholar]
- 5.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles:toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–23. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Jiang N, Zhang L, Meng C, Zhao J, Wu J. NLRP6 expressed in astrocytes aggravates neurons injury after OGD/R through activating the inflammasome and inducing pyroptosis. Int Immunopharmacol. 2020;80:106183. doi: 10.1016/j.intimp.2019.106183. [DOI] [PubMed] [Google Scholar]
- 8.Gu SS, Kang XW, Wang J, Guo XF, Sun H, Jiang L, et al. Effects of extracellular vesicles from mesenchymal stem cells on oxygen-glucose deprivation/reperfusion-induced neuronal injury. World J Emerg Med. 2021;12(1):61–7. doi: 10.5847/wjem.j.1920-8642.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xian PP, Hei Y, Wang R, Wang T, Yang JL, Li JY, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956–75. doi: 10.7150/thno.33872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X. Nrf2—a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol. 2017;54(8):6006–17. doi: 10.1007/s12035-016-0111-0. [DOI] [PubMed] [Google Scholar]
- 11.Strom J, Xu B, Tian X, Chen QM. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016;30(1):66–80. doi: 10.1096/fj.14-268904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rushworth SA, Shah S, MacEwan DJ. TNF mediates the sustained activation of Nrf2 in human monocytes. J Immunol. 2011;187(2):702–7. doi: 10.4049/jimmunol.1004117. [DOI] [PubMed] [Google Scholar]
- 13.Itoh K, Ye P, Matsumiya T, Tanji K, Ozaki T. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J Clin Biochem Nutr. 2015;56(2):91–7. doi: 10.3164/jcbn.14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen JJ, Porter C, Garg NJ. Inhibition of NFE2L2-antioxidant response element pathway by mitochondrial reactive oxygen species contributes to development of cardiomyopathy and left ventricular dysfunction in chagas disease. Antioxid Redox Signal. 2017;27(9):550–66. doi: 10.1089/ars.2016.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao L, Xu XX, Zhang F, Wang M, Xu Y, Tang D, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Shi Z, Wang X, Mu S, Xu X, Shen L, et al. Protective effects of bovine milk exosomes against oxidative stress in IEC-6 cells. Eur J Nutr. 2021;60(1):317–27. doi: 10.1007/s00394-020-02242-z. [DOI] [PubMed] [Google Scholar]
- 17.Ricart KC, Bolisetty S, Johnson MS, Perez J, Agarwal A, Murphy MP, et al. The permissive role of mitochondria in the induction of haem oxygenase-1 in endothelial cells. Biochem J. 2009;419(2):427–36. doi: 10.1042/BJ20081350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan Y, Zhang YJ, Zhao SW, Chen J, Yang JL, Wang T, et al. Cadmium-induced apoptosis in neuronal cells is mediated by Fas/FasL-mediated mitochondrial apoptotic signaling pathway. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-27106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo Y, Hu J, Xu X, Gao X, Wang Y, Zhu S. Sodium azide induces mitochondria-mediated apoptosis in PC12 cells through Pgc-1α-associated signaling pathway. Mol Med Rep. 2019;19(3):2211–9. doi: 10.3892/mmr.2019.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maita C, Maita H, Iguchi-Ariga SM, Ariga H. Monomer DJ-1 and its N-terminal sequence are necessary for mitochondrial localization of DJ-1 mutants. PLoS One. 2013;8(1):e54087. doi: 10.1371/journal.pone.0054087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang XL, Wang ZZ, Shao QH, Zhang Z, Li L, Guo ZY, et al. RNAi-mediated knockdown of DJ-1 leads to mitochondrial dysfunction via Akt/GSK-3ßand JNK signaling pathways in dopaminergic neuron-like cells. Brain Res Bull. 2019;146:228–36. doi: 10.1016/j.brainresbull.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Riba A, Deres L, Eros K, Szabo A, Magyar K, Sumegi B, et al. Doxycycline protects against ROS-induced mitochondrial fragmentation and ISO-induced heart failure. PLoS One. 2017;12(4):e0175195. doi: 10.1371/journal.pone.0175195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maksoud E, Liao EH, Haghighi AP. A neuron-glial trans-signaling cascade mediates LRRK2-induced neurodegeneration. Cell Rep. 2019;26(7):1774–86.e4. doi: 10.1016/j.celrep.2019.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu XW, Li XM, Liu Y, Yuan NN, Li CW, Kang ZM, et al. Hydrogen exerts neuroprotective effects on OGD/R damaged neurons in rat hippocampal by protecting mitochondrial function via regulating mitophagy mediated by PINK1/Parkin signaling pathway. Brain Res. 2018;1698:89–98. doi: 10.1016/j.brainres.2018.06.028. [DOI] [PubMed] [Google Scholar]


