PURPOSE:
COVID-19 cancer patients (C19-CP) represent a population at high risk for mortality, whose clinical characteristics are still unknown in the second SARS-CoV-2 wave. The aim of this retrospective study was to compare epidemiology and clinical presentation of C19-CP referring to the emergency department (ED) of our institution (San Luigi Gonzaga University Hospital, Orbassano, Turin, Italy), in a 3-week observation period of the first and second COVID-19 waves, starting from the introduction of the corresponding national lockdowns.
METHODS:
We retrieved ED admissions from March 9 to 29, 2020, for the first wave, and from October 24 to November 13, 2020, for the second wave. We collected clinical characteristics of consecutive patients with molecularly confirmed SARS-CoV-2 infection. We also considered untested or SARS-CoV-2–negative cancer patients referring to the ED in the reference time frames.
RESULTS:
C19-CP in the second wave exceeded those in the first wave despite the nonsignificant difference (39 of 576 v 8 of 163; P = .5). Compared with nononcological patients, C19-CP were older (median age 70 years [interquartile range 61-77] v 60 years [interquartile range 45-73]; P = .02) and presented more often with ≥ 2 comorbidities (40.4% v 24.3%; P = .02). Compared with nononcological patients, in C19-CP, respiratory failure (29 of 47 v 321 of 692; P = .049) and hospitalization (37 of 47 v 363 of 692; P = .0004) were higher, with comparable frequencies across the waves. Five of 24 and 10 of 27 hospitalized cancer patients in the first and second waves developed SARS-CoV-2 infection during hospitalization.
CONCLUSION:
C19-CP were a vulnerable population, irrespective of the COVID-19 waves. This highlights the need to prioritize vaccinations in oncological patients to safeguard and guarantee optimal anticancer care.
INTRODUCTION
By the end of March 2020, Italy registered the largest number of COVID-19 cases worldwide after China.1 Similar to other countries, COVID-19 cases progressively decreased following the first national lockdown,2 reaching their nadir at the end of July.3 Nevertheless, since the end of summer, we have witnessed a second pandemic wave characterized by a novel exponential growth of infected cases and subsequent deaths, which lead to novel restrictive measures.3,4
COVID-19 cancer patients (C19-CP) represent a vulnerable population, who suffered from high mortality rate during the first pandemic wave.5-8 However, epidemiology and clinical presentation of this special population remain still largely unknown in the second wave, where overall COVID-19 incidence has increased, but the case mortality rate has dropped.3,9
In a 3-week observation period of the first and second COVID-19 waves, we compared epidemiological features and clinical presentation of C19-CP referring to the emergency department (ED) of an Italian University Hospital located in northern Italy (Turin), which was heavily hit by the SARS-CoV-2 pandemic.10
METHODS
We retrieved medical charts of patients referring to the ED of the San Luigi Gonzaga University Hospital (Orbassano-Turin, Italy) in a 3-week time frame during the first and second COVID-19 waves.
As an observation starting point, we considered the coming into effect of the restrictive measures undertaken under the Decree of the President of the Council of Ministers.2,4 Thus, we considered ED admissions from March 9 to March 29, 2020, for the first wave, and from October 24 to November 13, 2020, for the second wave.
We collected clinical characteristics of patients with a molecular diagnosis of SARS-CoV-2 during admission to the ED. General clinical characteristics included demographics and comorbidities. These were dichotomized in ≥ 2 and < 2 and considered in five groups: cerebro- and cardiovascular (CCV) disease, which included arterial hypertension; respiratory disease; metabolic disease, represented by obesity and/or diabetes mellitus; immunologic conditions including autoimmune, inflammatory, or rheumatologic diseases requiring immunosuppressive treatment; and others.
We considered C19-CP as patients affected from histologically confirmed active solid or hematologic malignancies, including all patients with cancer at advanced disease stage, and those at early stages who underwent treatment with radical intent up to 12 months before ED admission. Patients with cancer who were radically treated and disease-free for at least 12 months were excluded.
Regarding cancer-related characteristics, we retrieved tumor type, Eastern Cooperative Oncology Group performance status; advanced versus early disease stage in case of solid tumors (advanced stage: unresectable III-IV according to the TNM classification), including lymphomas (advanced stage: III-IV according to Ann Arbor classification); and active systemic oncological treatment grouped in chemotherapy, immunotherapy, tyrosine-kinase inhibitors, hormonal therapy, and others.
At ED admission, data related to COVID-19 were collected, including presence of COVID-19 criteria at ED triage (high-risk contact with known SARS-CoV-2 cases; the presence, alone or in combination, of fever, dyspnea, dry cough, and diarrhea); type of symptoms; presence of respiratory failure, on the basis of the results of arterial blood gas test; and hospital discharge and type of unit in case of hospitalization (intensive care, high-dependency and other lower-dependency units).
C19-CP were compared with nononcological COVID-19 patients, overall and per pandemic wave. Additionally, we described epidemiology and hospitalization rate of oncological patients without COVID-19 (untested or SARS-CoV-2–negative), referring to the ED during the corresponding time frames. In case of hospitalization, these patients were followed up for eventual subsequent SARS-CoV-2 infection.
For descriptive statistics, we used medians with interquartile range (IQR). The t-test and Fisher's exact test were used to assess differences between oncological and nononcological patients across the two SARS-CoV-2 waves. Cochran-Mantel-Haenszel test was used to estimate the association of oncological status with respiratory failure, adjusted for pandemic wave, considering the latter as a possible confounder. A P value of .05 or less was considered statistically significant. Analyses were done with GraphPad-Prism (version 8.0) or SPSS (version 26.0).
RESULTS
COVID-19 Epidemiology
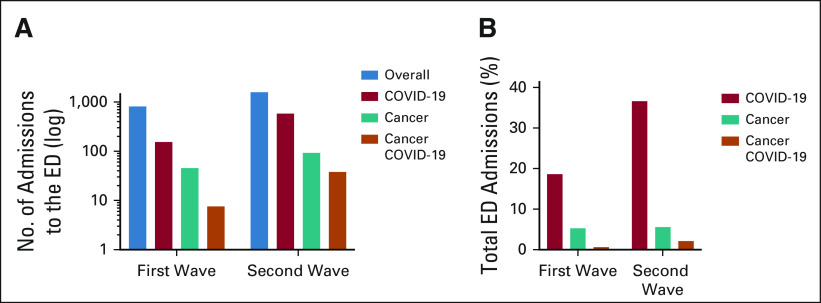
During the first observation period, of 859 ED admissions, 163 (19%) patients were found to be infected by SARS-CoV-2. Among these, eight (4.9% of total COVID-19 patients) had an active malignancy.
During the second observation period, ED admissions were 1,662. SARS-CoV-2 cases were 576 (34.7%), including 39 patients with cancer (6.8% of total COVID-19 patients). Figures 1A and 1B show the absolute number and frequency of ED admissions considered per pandemic wave and oncological status. In addition to the higher absolute numbers of COVID-19 patients in the second wave, we observed a significant difference also comparing the frequencies of COVID-19 patients by total ED admissions (163 of 859 and 576 of 1,662; P < .0001). However, in case of C19-CP, despite the higher absolute numbers in the second wave, the relative frequency of C19-CP of total COVID-19 cases was comparable across the two observation periods (8 of 163 and 39 of 576; P = .5).
FIG 1.
Admissions to the ED of the San Luigi Gonzaga University Hospital during the observation period of the first wave (March 9-23, 2020) and the second wave (October 24-November 6, 2020). (A) Data are shown as total numbers (log) and (B) data are shown as percentages of total ED admissions. ED, emergency department.
During the first wave, almost all SARS-CoV-2 cases were detected at ED admission (161 of 163, 98.8%), where only two patients had been tested 1 and 2 days before ED admission. In the second wave, SARS-CoV-2 diagnosis preceded admission in 114 of 576 cases (19.8%). Of note, during the reference time frame of the second wave, 36 of 576 (6.2%) COVID-19 patients referred to our ED twice and one patient referred three times because of symptoms worsening or planned medical control. Of those, one was a C19-CP. Thus, when examining the percentage of ED admissions for COVID-19 of total ED admissions, in the second wave, this got almost doubled (18.9% of 859 in the first wave v 36.9% of 1,662 in the second wave).
General Clinical Characteristics
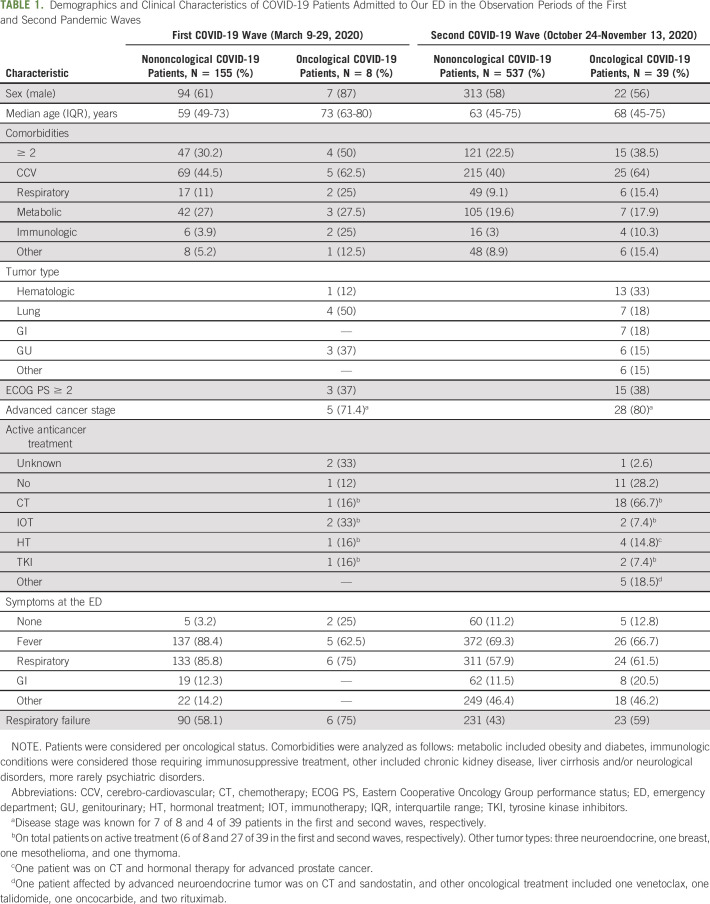
Clinical characteristics of COVID-19 patients are shown in Table 1. Irrespective of the oncological status and the pandemic wave, patients were more often male (59% of all 739 COVID-19 patients). C19-CP were significantly older than the nononcological patients (median age 71 years [IQR 63-80] in C19-CP and 62 years [IQR 47-74] in nononcological patients; P = .0005).
TABLE 1.
Demographics and Clinical Characteristics of COVID-19 Patients Admitted to Our ED in the Observation Periods of the First and Second Pandemic Waves
With regard to comorbidities, compared with nononcological patients, a higher number of C19-CP had at least two associated comorbidities (40.4% of 47 C19-CP v 24.3% of 692 nononcological patients; P = .02). Across the COVID-19 waves, the frequency of C19-CP and nononcological patients with over two comorbidities was not significantly different (for C19-CP: 50% of eight in the first wave v 38.5% of 39 in the second wave, P = .7; for nononcological patients: 30.3% of 155 in the first wave v 22.5% of 537 in the second wave, P = .05). As shown in Table 1, when considered per category, the most common comorbidity was CCV disease, whose frequency was higher in C19-CP than in the nononcological counterpart (63.8% of 47 C19-CP v 41.2% of 692 nononcological patients; P = .003). There was no significant difference observed between the first and second waves in C19-CP (62.5% of eight in the first wave v 64.1% of 39 in the second wave; P = .99), but in the nononcological counterpart, the frequency of CCV disease was higher during the first observation period compared with the second (60% of 155 v 40% of 537; P = .0001).
The distribution of metabolic conditions was comparable in the oncological and nononcological population (21.4% of 47 C19-CP and 21.3% of 692; P = .9); however, nononcological patients presented more often with metabolic conditions in the first observation than in the second period (27% of 155 in the first wave v 19.5% of 537 in the second wave; P = .04), as opposed to C19-CP where no difference across the waves was observed (37.5% of eight in the first wave v 17.9% of 39 in the second wave; P = .3). The frequency of respiratory diseases was not significantly different in C19-CP and nononcological patients (14.9% of 47 C19-CP v 9.5% of 692 nononcological patients; P = .2).
Although rare, immunologic conditions requiring immunosuppressive treatment were more common in C19-CP than in the nononcological population (12.7% of 47 v 3.2% of 692; P = .006), without differences across the pandemic waves (P = .3 for C19-CP, and P = .6 in nononcological patients; refer to Table 1 for %).
C19-CP Characteristics
Lung cancer, and in particular non–small-cell lung cancer, was the most common type of cancer in the first observation period (4 of 8, 50%). The remaining four C19-CP were affected by kidney and prostate cancer, bladder cancer, prostate cancer, and chronic lymphocytic leukemia. In the second observation period, most C19-CP presented with hematologic malignancies (13 of 39, 33%): six B cell lymphomas, one ocular B cell lymphoma, two multiple myeloma, one acute myeloid leukemia, one chronic myeloid leukemia, and two myelodysplastic syndromes. The remaining C19-CP were affected by lung cancer (6 non–small-cell and one small-cell histology), gastrointestinal malignancies (one hepatocellular carcinoma, one colon cancer, three gastric adenocarcinomas, one cholangiocarcinoma, and one pancreatic adenocarcinoma), by genitourinary malignancies (four prostate cancer and two urothelial cancer), three gastro-entero-pancreatic neuroendocrine tumors, one breast cancer, one mesothelioma, and one thymoma.
During both the observation periods, the majority of C19-CP at our ED were in good Eastern Cooperative Oncology Group performance status (0-1; 62.5% of eight and 61.5% of 39 in the first and second waves, respectively, P = .9), despite mostly at advanced cancer stages (71.4% of seven and 80% of 35 evaluable patients in the first and the second periods, respectively, P = .6). The majority were on active systemic oncological treatment (5 of 6, 83.3%, and 27 of 38, 71%, in the first and second waves, respectively, P = .9).
COVID-19 Presentation
As shown in Table 1, the rate of respiratory failure in C19-CP was higher than in nononcological patients, reaching a borderline statistical significance (61.7% of 47 C19-CP v 46.4% of 692 nononcological patients; P = .049). In the whole population of the first observation period, the rate of respiratory failure was higher than in the second wave (58.9% of 163 in the first wave v 44.1% of 576 in the second wave; P = .001). However, the higher frequency of respiratory failure in C19-CP was significant even when stratifying for pandemic wave (Mantel-Haenszel test P = .045).
In both the pandemic waves, most C19-CP were hospitalized (87.5% of 8 and 76.9% of 39 in the first and second waves, respectively, P = .6), with a frequency significantly higher compared with nononcological patients (78.7% of 47 v 52.6% of 692; P = .0004). In contrast, nononcological patients were hospitalized more often in the first observation period compared with the second period (76.1% of 155 and 45.8% of 537; P < .0001). Of note, 3 of 39 (7.7%) C19-CP in the second observation period and 1 of 155 (0.6%) nononcological patient during the first observation period deceased at the ED, as shown in Figure 2.
FIG 2.
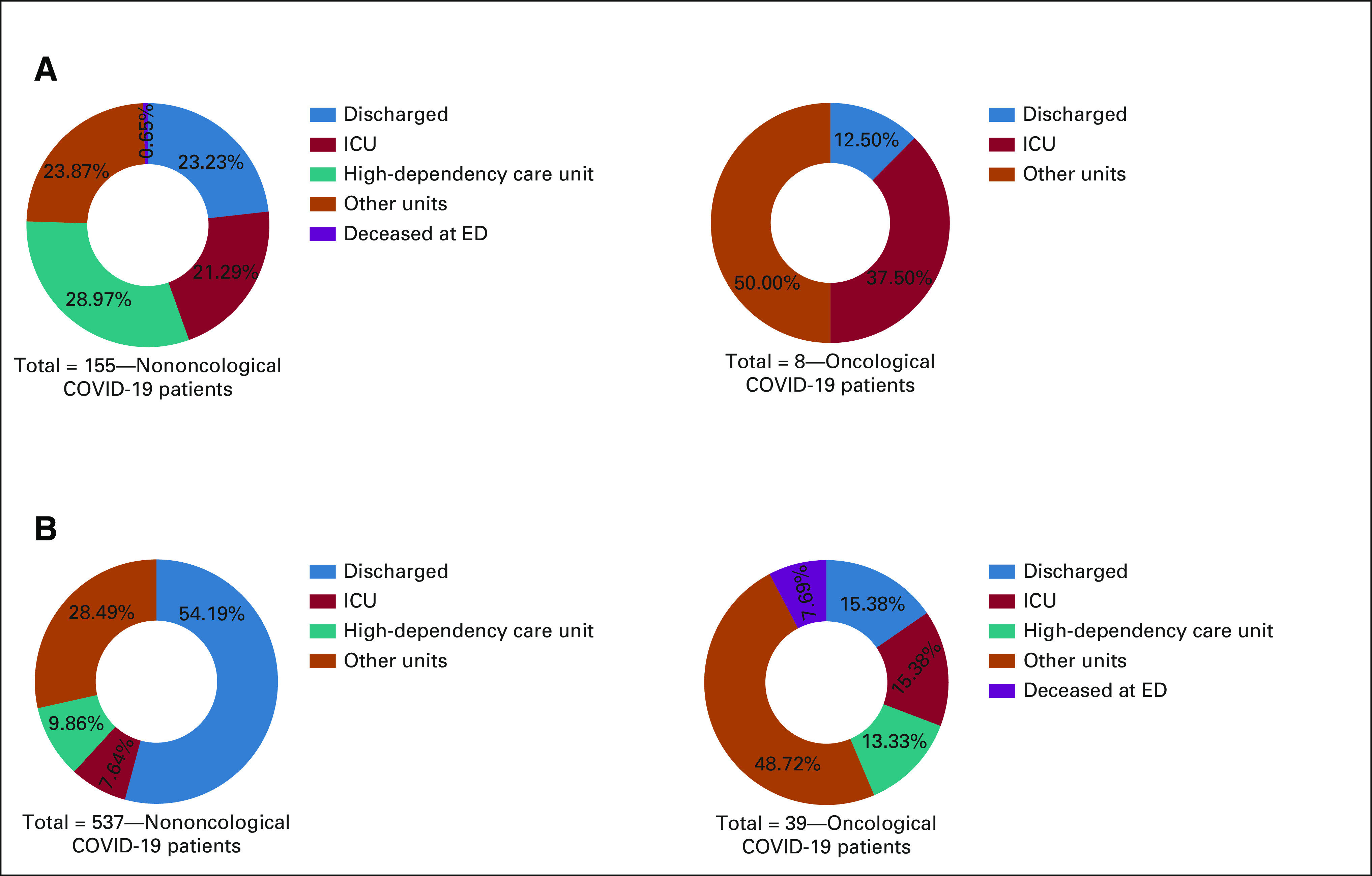
Discharge or hospitalization of COVID-19 patients admitted to the ED of the San Luigi Gonzaga University Hospital in the 3-week time frames of the first and second pandemic waves. Patients were considered per oncological status. In case of hospitalization, type of units where the patients were admitted are described. (A) Data for the first COVID-19 wave (observation period: March 9-29, 2020) and (B) data for the second COVID-19 wave (observation period: October 24-November 13, 2020). ED, emergency department; ICU, intensive care unit.
Among nononcological patients, 1 of 155 (0.6%) patient during the first wave and 31 of 537 (5.8%) during the second wave were completely asymptomatic and inappropriately referred to the ED for SARS-CoV-2 testing after prolonged exposure to COVID-19 cases. None of these instances occurred in C19-CP.
As for admissions to intensive care unit (ICU)/high-dependency units, no significant difference was observed between oncological and nononcological patients (29.8% of 47 C19-CP and 25.1% of 692 nononcological patients, P = .5). As shown in Figure 2, 3 of 8 (37.5%) C19-CP in the first and 11 of 39 (28.2%) in the second were admitted to ICUs or high-dependency units (shown in Fig 2), with a comparable admission rate (P = .7). In the nononcological population, admissions to ICU/high-dependency units were significantly more common in the first observation period compared with the second period (52.6% of 155 in the first wave v 17.3% of 537 in the second wave, P < .0001). Other details on hospitalization unit or discharge, per oncological status, and pandemic wave are shown in Figure 2.
When considering comorbidities, irrespective of the oncological status, over half COVID-19 patients with CCV disease were admitted to ICU/high-dependency units (46 of 75 [61.3%]) in the first observation period, whereas in the second one, only 26.2% (63 of 240) of the patients with CCV disease were admitted to ICU/high-dependency units (P < .0001). In addition, in the first wave, the majority of patients with metabolic conditions were admitted to ICU/high-dependency unit (69.6% [32 of 46]), while in the second wave, the number of COVID-19 patients with metabolic conditions admitted to ICU/high-dependency unit was much lower (25% [28 of 112]; P < .0001).
Non–COVID-19 Cancer Patients
We also considered untested or SARS-CoV-2–negative cancer patients entering the ED during the observation periods of the two pandemic waves (Figs 1A and 1B). In case of hospitalization, in the second observation period, all patients underwent SARS-CoV-2 molecular testing before admission to inward units. Although, during the first pandemic wave, especially in the first weeks, SARS-CoV-2 molecular testing was not routinely performed before hospitalization because of diagnostic kits shortage. Patients underwent SARS-CoV-2 testing only in case of COVID-19 criteria at ED admission (see Methods).
During the 3-week reference period of the first wave, patients with cancer referring to our ED were 47, for a total of 48 ED accesses (5.6% of a total of 859 ED accesses). Of those patients, 17% (8 of 47) were C19-CP. In the second observation period, patients with cancer referring to the ED were 94, for a total of 98 admissions (5.9% of 1,662 total ED accesses). When considering the total patients with cancer entering ED, C19-CP frequency was higher in the second observation period (41.5% [39 of 94]; P = .004). Among non–COVID-19 cancer patients (SARS-CoV-2 untested or negative), the hospitalization rate was 61.5% (24 of 39) in the first wave and 49% (27 of 55) in the second wave.
Five of 24 patients (20.8%) in the first wave and 10 of 27 patients (37%) in the second wave developed SARS-CoV-2 infection during hospitalization (P = .2). Median of days from ED admission to infection was 10 days (IQR 6-12.5) in the first wave and 7 days (IQR 5-10.2) in the second wave.
Of note, during the first wave, SARS-CoV-2 testing before admission in COVID-19–free units was performed in 10 of 24 hospitalized patients (42%), including the five who developed SARS-CoV-2 during hospitalization.
DISCUSSION
The results of this retrospective study showed that number and frequency of COVID-19 cases during the second observation period exceeded those registered within the first one (P < .0001).
In this context, the relative frequency of C19-CP of total COVID-19 patients was comparable across the two observation periods. However, absolute number and the relative frequency of C19-CP of total cancer patients at the ED were significantly higher in the second wave than in the first wave.
In addition to the inherent frailty related to cancer, our data suggest that C19-CP presented with further conditions predisposing to COVID-19 complications, in terms of older age and higher number of comorbidities and of CCV disease, compared with the nononcological population. Moreover, in both the observation periods of the pandemic waves, the majority of C19-CP were at advanced stage and were on active anticancer systemic treatment.
Respiratory failure and hospitalization, in particular admission to ICU/high-dependency unit, are generally considered as the main indicators of COVID-19 severity. In our study, the frequency of respiratory failure was significantly increased in C19-CP compared with the nononcological counterpart, even when adjusted for pandemic wave. Indeed, when major differences in COVID-19 presentation did not emerge across the pandemic waves in C19-CP, nononcological patients reported more often respiratory failure, hospitalization, and admission to ICU/high-dependency unit in the first wave. Interestingly, also the number of comorbidities, as well as in particular CCV and metabolic diseases, was higher in the first wave compared with the second wave and associated with increased admissions to ICU/high-dependency unit only in the first observation period.
Even if the higher rate of admissions to ICU/high-dependency unit in C19-CP was not significant compared with the nononcological population, potential confounders should be considered.
Indeed, three of 47 C19-CP deceased at the ED due to severe respiratory failure COVID-19-related. In addition, because of the ICU overflow, it is likely that younger and fitter patients were preferred for invasive ventilation in ICU, whereas C19-CP were mostly admitted in lower-dependency units, even in case of serious respiratory failure. The higher frequency of hospitalization in C19-CP observed in our study may support this hypothesis, at least in part.
Our data from the observation periods of the two SARS-CoV-2 waves are in line with national comparative analyses. It has been reported that, compared with the second wave, overall COVID-19 incidence was lower, but disease severity was higher in the first pandemic wave, likely because of testing challenges and underestimation of subsequent cases.9,10 Considering a regional perspective, the average SARS-CoV-2 positivity rate in our region was 35% and 20.5% during the observation periods of the first and second wave, respectively, with 14.3-fold higher number of SARS-CoV-2 tests performed during the second wave (22,422 and 319,965 SARS-CoV-2 tests in the first and second observation periods, respectively, in the Piedmont region).11
The lower ED admissions and the trend for higher disease severity observed in the first wave may also reflect a different social attitude during the first months of pandemic, when Italy was one of the world epicenter and people were more scared of contagion and thus more reluctant to hospital referral. On the other hand, the reduced hospitalization in the second wave, observed in nononcological patients, may be explained by a change in physicians' attitude in reducing hospital admission and encouraging outpatient management, which improved as experience with COVID-19 increased.
In C19-CP, the rate of SARS-CoV-2 infections developed during hospitalization in this study was high. The median of days to positive SARS-CoV-2 testing suggests that infection was likely due to intrahospital spread despite the widely adopted preventive measures.
As a single institution experience, the numbers observed are low, and this represents the major limitation of this study, preventing a fair comparison of additional tumor characteristics. Also, COVID-19 patients were not followed up during hospitalization, thus missing case fatality rate.
Observation periods for the pandemic waves were arbitrary in this study. Since official starting dates for both SARS-CoV-2 waves in Italy are not available, we deemed the coming into effect of the national lockdown as the most appropriate starting points of observation.2,4 About the duration of the observation periods, it has been proposed that COVID-19 cases start to decrease after 10 days of lockdown and continue up to 20 days thereafter.12 Using a 3-week time frame, we captured the steeper rising phases of both the pandemic curves,11 when hospital stress and health care management were likely at the most critical point.
Our comparative analysis remarks that C19-CP represent a vulnerable population for COVID-19 severity, irrespective of the pandemic waves. Patients with cancer were often hospitalized with subsequent increased exposure to hospital-acquired SARS-CoV-2 infection. As the pandemic continues to expand worldwide, our findings highlight the need to prioritize an efficient vaccine distribution in oncological patients to safeguard this special population and thus guarantee optimal anticancer care.
Paolo Bironzo
Honoraria: AstraZeneca, Bristol Myers Squibb, MSD Oncology, BeiGene, Roche, Takeda
Speakers' Bureau: AstraZeneca, Roche
Research Funding: Roche
Travel, Accommodations, Expenses: Amgen
Enrica Capelletto
Consulting or Advisory Role: AstraZeneca, MSD, Boehringer Ingelheim
Francesco Passiglia
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Amgen
Travel, Accommodations, Expenses: MSD Oncology, Roche
Massimo Di Maio
Honoraria: Pfizer, Takeda, AstraZeneca, Janssen, Eisai, Novartis, Roche, Astellas Pharma
Consulting or Advisory Role: AstraZeneca, Pfizer, Takeda, Janssen, Eisai, Novartis, Roche
Research Funding: Tesaro
Silvia Novello
Consulting or Advisory Role: Sanofi
Speakers' Bureau: AstraZeneca, MSD, Bristol Myers Squibb, Roche, Pfizer, Lilly, Takeda, AbbVie, Boehringer Ingelheim, Bayer, Amgen, Beigene
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Annapaola Mariniello, Paolo Bironzo, Chiara Pisano, Valeria Caramello, Enrica Capelletto, Silvia Novello
Provision of study materials or patients: Silvia Novello
Collection and assembly of data: Annapaola Mariniello, Paolo Bironzo, Chiara Pisano, Marco De Filippis, Irene Persano, Adriana Boccuzzi, Enrica Capelletto
Data analysis and interpretation: Annapaola Mariniello, Paolo Bironzo, Marco De Filippis, Emanuela Olmetto, Enrica Capelletto, Francesco Passiglia, Massimo Di Maio, Silvia Novello
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Descriptive Comparative Analysis of Patients With Cancer Referring to the Emergency Department of an Italian University Hospital Across the SARS-CoV-2 Waves
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Paolo Bironzo
Honoraria: AstraZeneca, Bristol Myers Squibb, MSD Oncology, BeiGene, Roche, Takeda
Speakers' Bureau: AstraZeneca, Roche
Research Funding: Roche
Travel, Accommodations, Expenses: Amgen
Enrica Capelletto
Consulting or Advisory Role: AstraZeneca, MSD, Boehringer Ingelheim
Francesco Passiglia
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Amgen
Travel, Accommodations, Expenses: MSD Oncology, Roche
Massimo Di Maio
Honoraria: Pfizer, Takeda, AstraZeneca, Janssen, Eisai, Novartis, Roche, Astellas Pharma
Consulting or Advisory Role: AstraZeneca, Pfizer, Takeda, Janssen, Eisai, Novartis, Roche
Research Funding: Tesaro
Silvia Novello
Consulting or Advisory Role: Sanofi
Speakers' Bureau: AstraZeneca, MSD, Bristol Myers Squibb, Roche, Pfizer, Lilly, Takeda, AbbVie, Boehringer Ingelheim, Bayer, Amgen, Beigene
No other potential conflicts of interest were reported.
REFERENCES
- 1.WHO : Coronavirus Disease 2019 (COVID-19) Situation Report–67. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200327-sitrep-67-covid-19.pdf?sfvrsn=b65f68eb_4 [Google Scholar]
- 2.Government of Italy : Decree of the President of the Council of Ministers 9th of March 2020. https://www.gazzettaufficiale.it/eli/id/2020/03/08/20A01522/sg [Google Scholar]
- 3.WHO : COVID-19. https://covid19.who.int/region/euro/country/it [Google Scholar]
- 4.Government of Italy : Decree of the President of the Council of Ministers 24th of October 2020. https://www.gazzettaufficiale.it/eli/id/2020/10/25/20A05861/sg [Google Scholar]
- 5.Kuderer NM, Choueiri TK, Shah DP, et al. : Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 395:1907-1918, 2020. [Erratum: Lancet 396:758, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee LY, Cazier JB, Angelis V, et al. : COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 395:1919-1926, 2020. [Erratum: Lancet 396:534, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee LYW, Cazier JB, Starkey T, et al. : COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol 21:1309-1316, 2020. [Erratum: Lancet Oncol 21:e462, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reale ML, Bironzo P, Bertaglia V, et al. : SARS-CoV-2 infection in cancer patients: A picture of an Italian Onco-Covid Unit. Front Oncol 10:1722, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coccia M: Comparative Analysis of the First and Second Wave of the COVID- 19: Is the On-Going Impact of Second Wave on Public Health Stronger than First One? Working Paper CocciaLab n. 57/2020. CNR—National Research Council of Italy, 2020. https://ssrn.com/abstract=3731416 [Google Scholar]
- 10.Sartor G, Del Riccio M, Dal Poz I, et al. : COVID-19 in Italy: Considerations on official data. Int J Infect Dis 98:188-190, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.COVID-19 Situation in Italy. Department of Civil Protection. https://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1 [Google Scholar]
- 12.Alfano V, Ercolano S: The efficacy of lockdown against COVID-19: A cross-country panel analysis. Appl Health Econ Health Policy 18:509-517, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]