PURPOSE:
There are numerous barriers to cancer clinical trial participation in the United States. This paper describes the approach and outcomes of The Leukemia & Lymphoma Society's Clinical Trial Support Center (CTSC), whose nurse navigators assist patients with a blood cancer and their oncologists by identifying all appropriate trials based on clinical data and patient preference, facilitating informed and shared decision making, and minimizing enrollment barriers.
METHODS:
Data on patients served from October 2017 to October 2019 were analyzed using bivariate and multivariate analyses to determine demographic and clinical characteristics associated with enrollment. Reasons for nonenrollment were examined.
RESULTS:
The CTSC opened 906 patient cases during this time frame. Among all US patients with a closed case (n = 750), the clinical trial enrollment rate was 16.1%. Among those with a known enrollment outcome after a trial search (n = 537), the enrollment rate was 22.5%. Multivariate analysis controlling for variables significant in bivariate analyses (insurance, treatment status, Eastern Cooperative Oncology Group performance status, and urban or rural residence) revealed that patients with Medicaid were less likely to enroll than those with private or commercial insurance (adjusted odds ratio, 0.054; CI, 0.003 to 0.899), and patients in treatment or maintenance were less likely to enroll than those relapsed or refractory to most recent therapy (adjusted odds ratio, 0.312; CI, 0.139 to 0.702). Primary reasons for nonenrollment were preference for standard of care (66.3%) and patient passed away (16.1%).
CONCLUSION:
The CTSC is an effective, replicable model for addressing multilevel barriers to clinical trial participation. The findings highlight the need to increase opportunities for trial participation sooner after diagnosis and among patients with Medicaid.
INTRODUCTION
Addressing barriers to clinical trial participation is critical to accelerating progress toward more effective and less toxic cancer treatments1 and to providing patients with access to novel therapies and treatment approaches. Approximately 20% of cancer clinical trials fail because of insufficient patient enrollment,2 which hinders progress to improve cancer care. Numerous barriers to cancer clinical trial participation in the United States have been documented3,4; participation rates have remained low for many years, hovering at 8%2 or less.5 These barriers include institutional and provider-related barriers such as trial availability, staff and infrastructure capacity and capability, the quality and variability of provider communication, and ineffective patient identification and enrollment practices6-12; barriers related to trial design such as restrictive inclusion or exclusion criteria and lack of patient-centeredness5,11,12; and patient-level barriers, including awareness, self-efficacy, fear and mistrust, a preference to not lose control of treatment decision making, cost, and logistical concerns.2,13-20 Barriers are even more pronounced—and participation rates are particularly low—for subgroups of patients of certain races and ethnicities, who live in rural areas, who are older or young adults, who are uninsured, and/or with low income.2,10,14,21-29 Underrepresentation in trials may perpetuate disparities in outcomes and lead to limited generalizability in practice.21,30-33
Finding an appropriate clinical trial can be overwhelming for patients, and time and resource intensive for physicians. Clinical trial matching services are designed to help patients find suitable clinical trials. Patients provide information about their health status and diagnosis, which is compared with the eligibility criteria of open trials from a public or private database.2 However, matching services can themselves present barriers2; to use these matching services effectively, patients must understand (1) the medical terminology related to their diagnosis and treatment; (2) the clinical research process; (3) specific trial attributes to determine their potential interest and eligibility; and (4) how to sort or refine search results.34,35 Search results may inadvertently include inaccurate information36 or may be limited in scope. They require significant health literacy skills37-39 and self-efficacy6,15,16 to initiate action around clinical trials, and they typically do not address logistical barriers or cost concerns.
Clinical trial patient navigation is a more patient-centered service that goes beyond matching. Over the past 10 years, some academic research centers have created internal navigation services that may focus primarily on trials within their institution but can extend beyond that institution as well.40-43 Service scope tends to focus on increasing awareness, knowledge, and access to appropriately matched clinical trials, facilitating access to community resources such as connection to a care coordination nurse or social worker within the health care system, and improving communication between the patient and treatment team.40 Studies have shown that these programs can increase patient awareness and knowledge about clinical trials36,44 and participation in trials.44 Several programs have also shown increased trial accrual and retention rates among underrepresented populations.40,42,45-48 However, many patients do not have access to clinical trial navigation services at their site of care.
The Leukemia & Lymphoma Society's Clinical Trial Support Center
To address the need for better access to highly personalized clinical trial information among patients with a blood cancer, The Leukemia & Lymphoma Society (LLS) developed its Clinical Trial Support Center (CTSC) in 2016. It is a free, national, telephone-based, nurse-led navigation service for patients with leukemia, lymphoma, myeloma, myeloproliferative neoplasms, and myelodysplastic syndromes. The goal of the CTSC is not to have every patient served enroll into a clinical trial, but to increase awareness of opportunities to receive treatment within a clinical trial, facilitate informed and shared decision making with their oncologist about participating, and minimize barriers to enrollment if the patient decides that a clinical trial is right for him or her in collaboration with his or her health care team. The CTSC's patient-centered approach aims to provide care that is respectful of and responsive to individual patient preferences, needs, and ensures that patients' values guide all clinical decisions.
This paper describes the CTSC's comprehensive approach and clinical trial enrollment outcomes among patients served and seeks to shed light on the demographic and clinical characteristics associated with enrollment. Secondarily, it describes the patient demographic and clinical characteristics of those who initially chose to undergo, or not undergo, a search for appropriate clinical trials with assistance from the CTSC, as well as the reasons that patients chose not to enroll after receiving the results of a trial search.
The CTSC's Characteristics and Processes
The CTSC employs nurse navigators who are oncology nurses.49 They are advanced practice nurses, nurse practitioners (both pediatric and adult), research nurses, and nurse educators, and they are supported by a coordinator. They undergo intensive and ongoing education in hematologic malignancy physiology, treatment methods, stem-cell transplantation, clinical trials, and genomics.
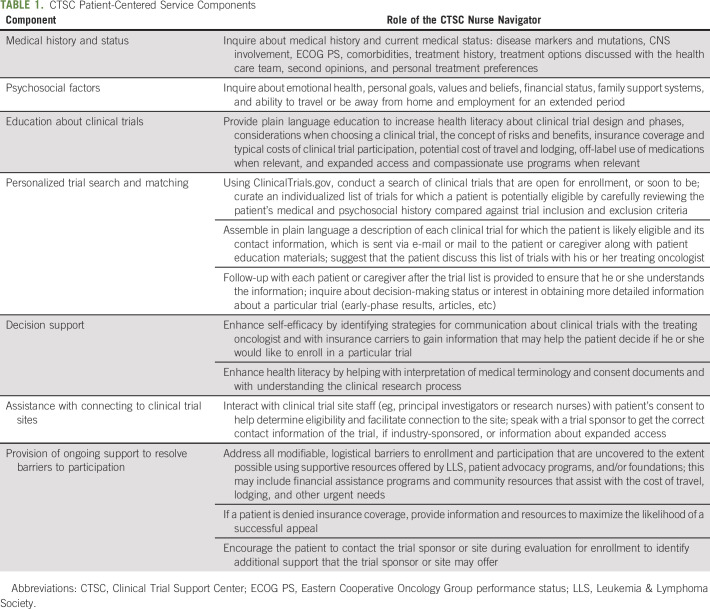
CTSC nurse navigators are assigned cases as they come in through LLS's website or Information Resource Center; patients self-refer or are referred by their oncology care team. The nurse navigators speak with a patient (or caregiver) to collect background information that will assist with conducting a targeted clinical trial search and to identify barriers to matching and enrollment. When working with a patient or caregiver, the nurse navigator focuses on seven comprehensive and essential service components described in Table 1, which were designed to surmount many of the multilevel barriers to enrollment described above. For example, increasing health literacy enhances patients' or caregivers' understanding of trial options and consent documents, and facilitates better communication with clinical trial investigators and staff. As needed, a language line is used to assist patients or caregivers who prefer to communicate in a language other than English.
TABLE 1.
CTSC Patient-Centered Service Components
METHODS
Retrospective analysis of secondary data was conducted with deidentified data from patient cases opened by the CTSC between October 2017 and October 2019. The study was reviewed by the Institutional Review Board at RTI International to ensure compliance with ethical principles (MOD00000870).
Data Collection
The CTSC routinely collected demographic and clinical information at the commencement of initial contact with the patient or caregiver, including insurance type, treatment status, and Eastern Cooperative Oncology Group performance status (ECOG PS).50 Among those for whom a clinical trial search was conducted, enrollment status (ie, whether the patient enrolled in a trial with the help of the CTSC) and the primary reason for deciding to not enroll were documented by the nurse navigator based on follow-up with the patient or caregiver. In some cases, after a search was conducted, the patient or caregiver did not respond to several follow-up attempts, so an enrollment outcome could not be determined. As there was no income or education data collected from patients, to supplement existing patient-reported data, socioeconomic status was classified using The Social Deprivation Index (SDI),51 a validated county-level measure based on the American Community Survey. Census data and Centers for Medicare and Medicaid Services classification guidance were also used to classify patient residency into rural or urban counties.52-54
Data Analysis
Bivariate analyses examined patient characteristics associated with the outcomes of search and enrollment using Pearson chi-square tests, Fisher exact tests, and Kruskal-Wallis tests for the SDI score (two-sided; α of .05). Multivariate logistic regression determined factors associated with enrollment using adjusted odds ratios (AORs) and 95% CI. Variables examined in bivariate analysis were initial contact (patient or caregiver), patient sex, patient age, patient ethnicity or race, insurance type, primary diagnosis, treatment status, CNS involvement, travel considerations, ECOG PS, SDI, and urban or rural county of residence. Covariates were included in the multivariate model only if bivariate analyses showed significance (α ≤ .05). A penalized maximum likelihood estimation technique (Firth correction) was used. Analyses were conducted using SAS version 9.4.
RESULTS
Characteristics of Patients and Caregivers Served by the CTSC
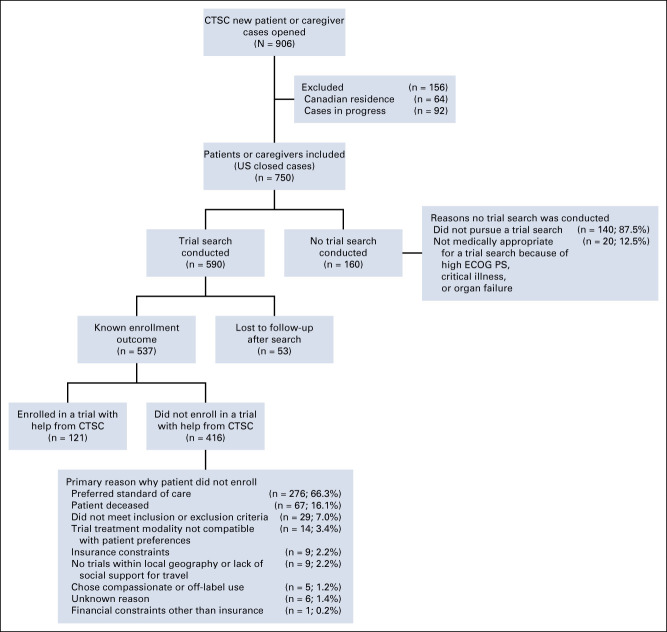
From October 2017 to October 2019, 906 patient cases were opened (Fig 1). Among these, 92 cases (10.2%) were excluded because the case was still in progress at the time of analysis and 64 cases (7.1%) were excluded because the patient resided outside of the United States.
FIG 1.
Characteristics of new referrals to the CTSC from October 2017 to October 2019. CTSC, Clinical Trial Support Center; ECOG PS, Eastern Cooperative Oncology Group performance status.
Of the remaining cases (n = 750), 590 patients or caregivers had a clinical trial search conducted and 160 did not. Of the 590 cases with a search conducted, 53 patients or caregivers were lost to follow-up, which leaves 537 cases with a known enrollment outcome. English was the primary language for all but three of the 537 cases, whose primary language was Spanish.
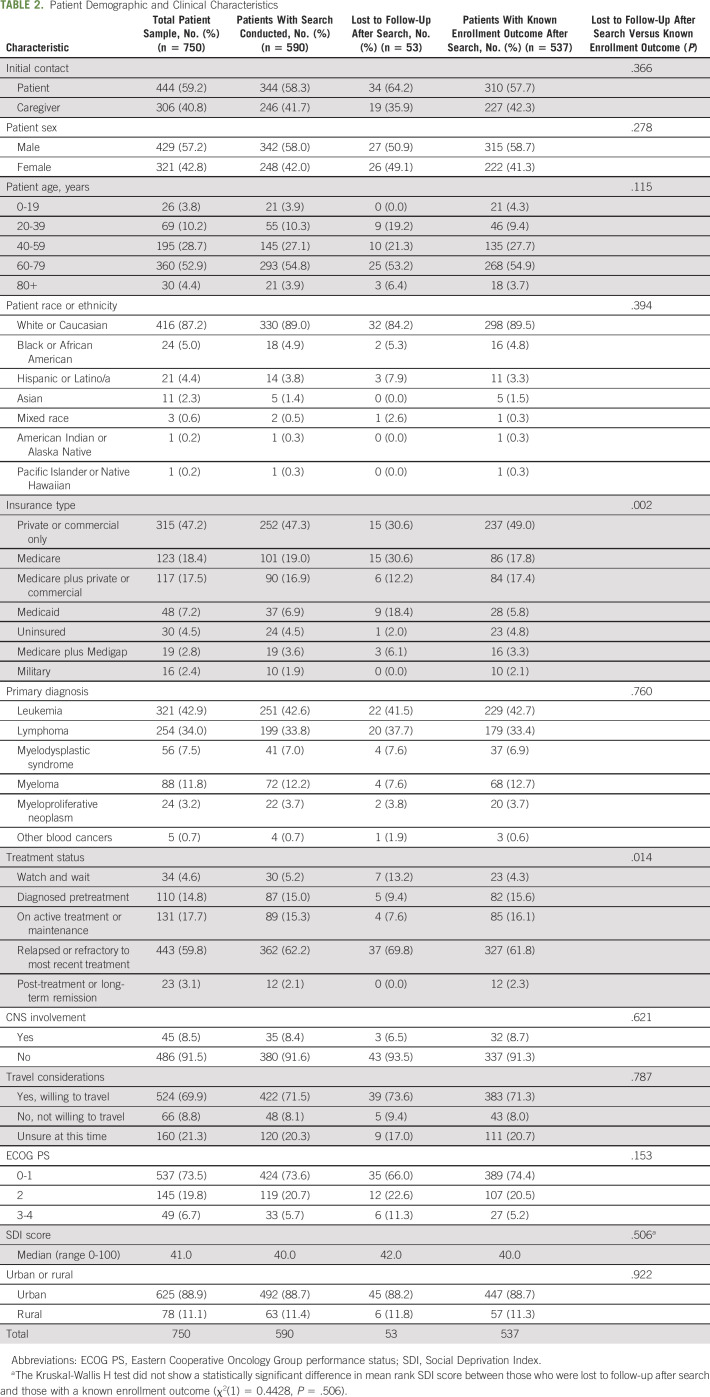
Table 2 shows the patient demographic and clinical characteristics for the following samples: total sample, those with a search, those lost to follow-up after search, and those with a search and known enrollment outcome. After having a trial search conducted, those who were lost to follow-up differed from those who had a known enrollment outcome by insurance type (P = .002) and treatment status (P = .014) (Table 2).
TABLE 2.
Patient Demographic and Clinical Characteristics
Among the 537 patients with a known enrollment outcome after a search, the average number of phone and e-mail interactions between a CTSC nurse navigator and any party (eg, patient, family member, treating health care provider, or clinical trial research staff) was 18.1. The majority of these interactions were with patients or caregivers (80.9%), followed by trial research staff (16.3%) and treating health care providers (2.3%). The average number of interactions for those who enrolled in a clinical trial was 25.0, and the average number of interactions for those who did not enroll was 16.0. These interactions include follow-up support by phone or e-mail after a patient enrolled or did not enroll.
Likelihood of Clinical Trial Search
Among the 750 US patients and caregivers with cases not in progress at the time of analyses (ie, closed cases), 160 (21.3%) had no clinical trial search conducted. The most common reason for a nurse navigator not conducting a search (87.5%) was because the patient or caregiver chose not to proceed with one. For 12.5%, a search was not conducted because the patient was determined by the nurse navigator as not medically appropriate for a trial search because of high ECOG PS, critical illness, or organ failure.
Bivariate analyses were conducted to identify patient demographic and clinical characteristics associated with a clinical trial search. Patients who were on active treatment or maintenance at the time of referral were less likely to have a trial search than all other treatment statuses (P = .001). Those who were relapsed or refractory to their most recent treatment were more likely to have a trial search conducted than all other treatment statuses (P = .004). There were no other characteristics significantly associated with the likelihood of a trial search being conducted (Data Supplement, online only).
Likelihood of Clinical Trial Enrollment Among Those for Whom a Search Was Conducted
Among patients or caregivers who had a trial search with a known enrollment outcome (n = 537), 22.5% of patients enrolled in a trial with help from the CTSC (n = 121). Among all US patients or caregivers with a closed case (n = 750; Fig 1), 16.1% enrolled in a trial with help from the CTSC.
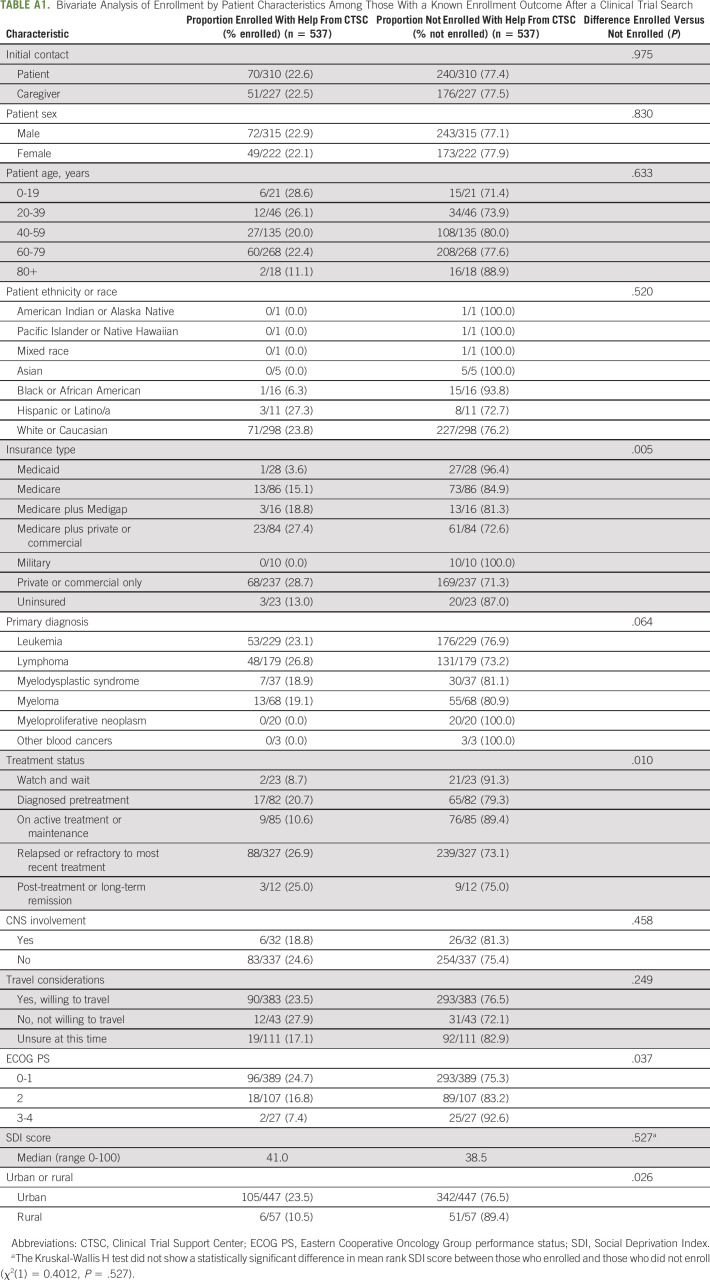
Bivariate analyses were conducted to determine patient demographic and clinical characteristics associated with clinical trial enrollment. There were statistically significant differences in clinical trial enrollment rates by insurance type (P = .005), treatment status (P = .010), ECOG PS (P = .037), and urban or rural residence (P = .026; Appendix Table A1, online only). There were no significant differences in rates of enrollment by other characteristics. The primary reasons why patients did not enroll in a clinical trial with assistance from the CTSC are presented in Figure 1.
Multivariate Analysis
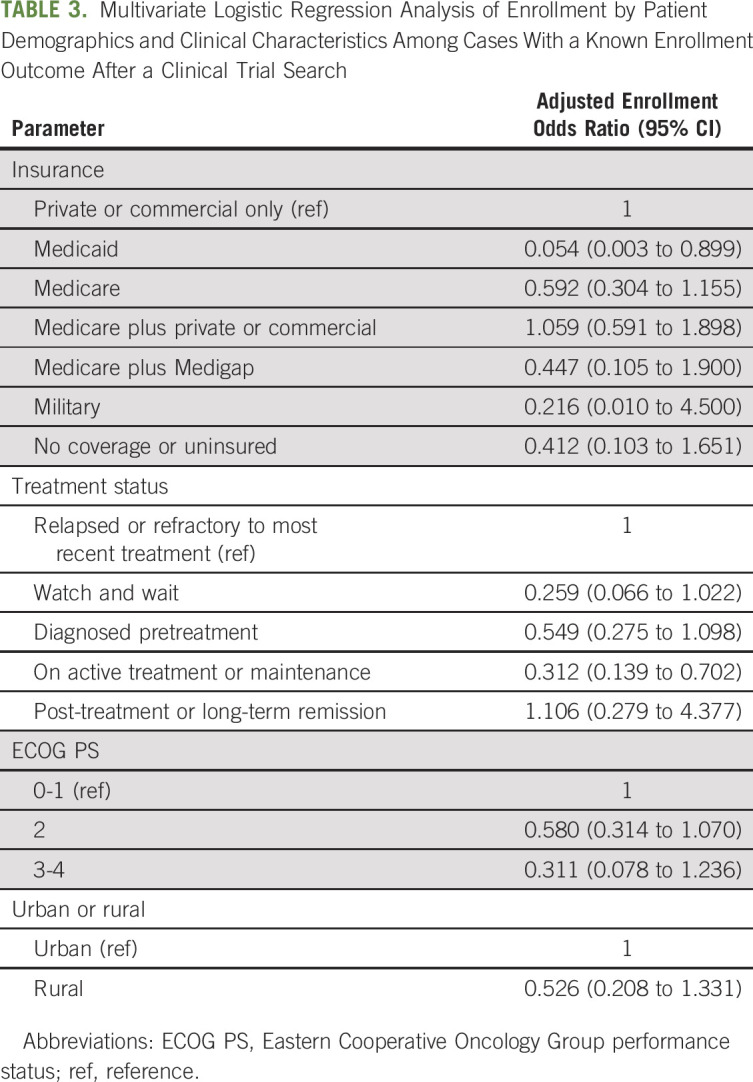
Multivariate logistic regression was conducted among patients who had a trial search with a known enrollment outcome. Controlling for factors significantly associated with enrollment in the bivariate analyses (treatment status, ECOG PS, and urban or rural residence), analysis results showed that compared to those with private or commercial insurance, patients with Medicaid were significantly less likely to enroll in a clinical trial with help from the CTSC (AOR, 0.054; CI, 0.003 to 0.899; Table 3). Additionally, patients who were on active treatment or maintenance at the time of referral were significantly less likely to enroll than those who were relapsed or refractory to their most recent treatment (AOR, 0.312; CI, 0.139 to 0.702).
TABLE 3.
Multivariate Logistic Regression Analysis of Enrollment by Patient Demographics and Clinical Characteristics Among Cases With a Known Enrollment Outcome After a Clinical Trial Search
DISCUSSION
This paper details the approach of a national, telephone-based nurse navigator-led service model that aims to reduce barriers to clinical trial participation among patients with a blood cancer and identifies patient demographic and clinical characteristics associated with enrollment. The findings suggest that this navigation service is effective at mitigating modifiable barriers to clinical trial enrollment. Among patients served in the United States with closed cases, the clinical trial enrollment rate was 16.1%. Among those for whom a search was conducted with a known enrollment outcome, the enrollment rate was 22.5%. While patients or caregivers assisted by the CTSC may be more open to clinical trial participation than the general population of patients with a blood cancer, these data do demonstrate the value of a comprehensive navigation program.
The CTSC predominately provided services to and conducted searches for patients relapsed or refractory to their most recent treatment, and the findings demonstrated that this group of patients was more likely to enroll onto a trial than those in active treatment or maintenance. Yet, there was no significant difference in rate of enrollment between those seeking a first-line treatment option (ie, in the diagnosed pretreatment category) and those relapsed or refractory to their most recent treatment after search. Collectively, these findings point to the importance of offering clinical trial options earlier in the disease spectrum for those who may be interested.36,38,55
This study found that after controlling for other factors that were significant in the bivariate analysis (urban or rural residence, treatment status, and ECOG PS), patients with Medicaid insurance were significantly less likely to enroll in a clinical trial than those with private or commercial insurance. Similar results were found in another national navigation study.38 Factors contributing to this are likely complex; during the study period, Medicaid did not have a federal requirement to cover the cost of routine care within clinical trials, and clinical trial coverage depends on the state of residence.56 For the minority of states that do provide coverage, state-based Medicaid insurance may limit the clinical trials a patient may be eligible for trials occurring within their state, although some patients are able to successfully appeal. The effect of Medicaid coverage may evolve in 2022, when a recently enacted federal law, The Clinical Treatment Act, requiring Medicaid to cover the cost of routine care within trials, will take effect. Some researchers have highlighted the need to better understand how outcomes among clinical trial participants with Medicaid are affected by external factors associated with insurance (such as quality of survivorship care).57
Intensive navigation was provided by CTSC nurses to help eliminate modifiable barriers to enrollment (an average of 25 phone or e-mail interactions for those enrolled). CTSC nurse navigators reduce patient-level barriers by providing education that addresses fears and dispels myths. They supplement site-of-care capacity by providing detailed information about trial status, eligibility, and the trial referral process beyond what may be available on ClinicalTrials.gov. They encourage the patient to take search results to their oncologist for conversation and help the patient create a list of questions to ask, which facilitates patient-provider communication and shared decision making. They connect patients to resources that assist with travel, food, and lodging costs, and insurance coverage and appeal. Even after addressing these modifiable barriers, preference for standard of care was the primary driver of nonenrollment; based on nurse navigators' experience, this was often because of provider recommendation and/or the known effectiveness of an approved therapy.
A disproportionate percentage of patients served by the CTSC identified as White or Caucasian, as compared to the general population. This further highlights the need for expanded and more effective outreach efforts to increase opportunities for clinical trial participation among other racial and ethnic groups, to help reduce disparities in access to novel therapies and treatment response.2,21,30-33,45,58 Key lessons learned from the implementation of the CTSC include: the importance of having highly skilled nurses provide this service; maintaining deliberate focus on addressing multilevel barriers to accrual; leveraging technology to maximize comprehensiveness and relevance of trial searches; and giving patients or caregivers the tools they need to effectively communicate with their oncology care team about clinical trial options that are appropriate for them. Since the time the analysis was conducted, the CTSC has not only increased its service capacity by hiring more nurse navigators and enhancing the technological infrastructure, but also heightened outreach to underrepresented groups.
The population served by the CTSC was a group of individuals who have reached out to LLS for assistance. The findings may not be generalizable to other populations less engaged or among those with other cancer types. Moreover, the population served was primarily White or Caucasian. The results may not represent the experiences of subgroups whose primary language is not English and who have varying cultural preferences and circumstances. Although bivariate analysis did not reveal significant differences in search and enrollment rates by race and ethnicity, this may be due both to lack of variability within the sample and a high degree of missing data. At the time of data collection, race or ethnicity data were not systematically collected. Finally, this study was not able to control for comorbidities or individually reported socioeconomic indicators, which may differ from county-level data used in the analyses.
In conclusion, this paper describes the approach and outcomes of a free, national clinical trial matching and nurse navigation service for patients with a blood cancer, complementing the work of oncology care providers. Among US patients with closed cases, the clinical trial enrollment rate was 16.1%. Among those who had a trial search conducted and a known enrollment outcome, the enrollment rate was 22.5%. Given that clinical trial navigation services at sites of care are limited, the findings capture the value of this service in helping to mitigate clinical trial participation barriers that patients and providers face and demonstrate the potential benefits of replicating this model for patients with other cancer types. There remains a clear need to increase opportunities for clinical trial participation earlier in the cancer continuum, and the findings further support the importance of policies that foster clinical trial access among patients with Medicaid.
ACKNOWLEDGMENT
The authors are grateful to Kerry Lee, Coordinator of the CTSC, for contributing to data management.
Appendix
TABLE A1.
Bivariate Analysis of Enrollment by Patient Characteristics Among Those With a Known Enrollment Outcome After a Clinical Trial Search
Maria Sae-Hau
Stock and Other Ownership Interests: Moderna Inc. (I), Acceleron Pharma (I), argenx (I), Beam Therapeutics (I), BridgeBio Pharma (I), Schrodinger (I), Applied DNA Sciences (I)
Research Funding: Bristol Myers Squibb/Celgene (Inst), AstraZeneca (Inst), Amgen (Inst), AbbVie (Inst)
Elisa S. Weiss
Research Funding: AbbVie (Inst), AstraZeneca (Inst), Amgen (Inst), Bristol Myers Squibb/Celgene (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by The Leukemia & Lymphoma Society.
AUTHOR CONTRIBUTIONS
Conception and design: Maria Sae-Hau, Kate Disare, Margo Michaels, Alissa Gentile, Leah Szumita, Elisa S. Weiss
Collection and assembly of data: Kate Disare, Leah Szumita
Data analysis and interpretation: Maria Sae-Hau, Kate Disare, Margo Michaels, Leah Szumita, Katherine Treiman, Elisa S. Weiss
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Overcoming Barriers to Clinical Trial Participation: Outcomes of a National Clinical Trial Matching and Navigation Service for Patients With a Blood Cancer
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Maria Sae-Hau
Stock and Other Ownership Interests: Moderna Inc. (I), Acceleron Pharma (I), argenx (I), Beam Therapeutics (I), BridgeBio Pharma (I), Schrodinger (I), Applied DNA Sciences (I)
Research Funding: Bristol Myers Squibb/Celgene (Inst), AstraZeneca (Inst), Amgen (Inst), AbbVie (Inst)
Elisa S. Weiss
Research Funding: AbbVie (Inst), AstraZeneca (Inst), Amgen (Inst), Bristol Myers Squibb/Celgene (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Stensland KD, McBride RB, Latif A, et al. : Adult cancer clinical trials that fail to complete: An epidemic? J Natl Cancer Inst 106:dju229, 2014 [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Cancer Action Network : Barriers to Patient Enrollment in Therapeutic Clinical Trials for Cancer—A Landscape Report 2018. https://www.fightcancer.org/sites/default/files/National%20Documents/Clinical-Trials-Landscape-Report.pdf
- 3.Unger JM, Vaidya R, Hershman DL, et al. : Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst 111:245-255, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford JG, Howerton MW, Lai GY, et al. : Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer 112:228-242, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Unger JM, Cook E, Tai E, et al. : The role of clinical trial participation in cancer research: Barriers, evidence, and strategies. Am Soc Clin Oncol Ed Book 35:185-198, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comis RL, Miller JD, Colaizzi DD, et al. : Physician-related factors involved in patient decisions to enroll onto cancer clinical trials. J Oncol Pract 5:50-56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown R, Albrecht T: Enrollment in clinical trials, in Kissane D, Bultz B, Butow P. (eds): Handbook of Communication in Oncology and Palliative Care. Oxford, United Kingdom, Oxford University Press, 2010 [Google Scholar]
- 8.Grand MM, O'Brien PC: Obstacles to participation in randomised cancer clinical trials: A systematic review of the literature. J Med Imaging Radiat Oncol 56:31-39, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Somkin CP, Altschuler A, Ackerson L, et al. : Organizational barriers to physician participation in cancer clinical trials. Am J Manag Care 11:413-421, 2015 [PubMed] [Google Scholar]
- 10.Hamel LM, Penner LA, Albrecht TL, et al. : Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control 23:327-337, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Go RS, Frisby KA, Lee JA, et al. : Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer 106:426-433, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Guarino M, Masters G, Schneider C, et al. : Barriers exist to patient participation in clinical trials. J Clin Oncol 23:6015, 2005 [Google Scholar]
- 13.Jones JM, Nyhof-Young J, Moric J, et al. : Identifying motivations and barriers to patient participation in clinical trials. J Cancer Educ 21:237-242, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Pinto HA, McCaskill-Stevens W, Wolfe P, et al. : Physician perspectives on increasing minorities in cancer clinical trials: An Eastern Cooperative Oncology Group (ECOG) initiative. Ann Epidemiol 10:S78-S84, 2010. (8 suppl) [DOI] [PubMed] [Google Scholar]
- 15.Meropol NJ, Buzaglo JS, Millard J, et al. : Barriers to clinical trial participation as perceived by oncologists and patients. J Natl Compr Canc Netw 5:655-664, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Melisko ME, Hassin F, Metzroth L, et al. : Patient and physician attitudes toward breast cancer clinical trials: Developing interventions based on understanding barriers. Clin Breast Cancer 6:45-54, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Wong YN, Schluchter MD, Albrecht TL, et al. : Financial concerns about participation in clinical trials among patients with cancer. J Clin Oncol 34:479-487, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avis NE, Smith KW, Link CL, et al. : Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol 24:1860-1867, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Nipp RD, Hong K, Paskett ED: Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Ed Book 39:105-114, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Lara PN, Jr, Higdon R, Lim N, et al. : Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol 19:1728-1733, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Regnante JM, Richie NA, Fashoyin-Aje L, et al. : US cancer centers of excellence strategies for increased inclusion of racial and ethnic minorities in clinical trials. J Oncol Pract 15:e289-e299, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Townsley CA, Selby R, Siu LL: Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 23:3112-3124, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Roth ME, O'Mara AM, Seibel NL, et al. : Low enrollment of adolescents and young adults onto cancer trials: Insights from the community clinical oncology program. J Oncol Pract 12:e388-e395, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unger JM, Hershman DL, Albain KS, et al. : Patient income level and cancer clinical trial participation. J Clin Oncol 31:536-542, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unger JM, Gralow JR, Albain KS, et al. : Patient income level and cancer clinical trial participation: A prospective survey study. JAMA Oncol 2:137-139, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sateren WB, Trimble EL, Abrams J, et al. : How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol 20:2109-2117, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Murthy VH, Krumholz HM, Gross CP: Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 291:2720-2726, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Baquet CR, Ellison GL, Mishra SI: Analysis of Maryland cancer patient participation in National Cancer Institute-supported cancer treatment clinical trials. J Health Care Poor Underserved 20:120-134, 2009. (2 suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Advani AS, Atkeson B, Brown CL, et al. : Barriers to the participation of African-American patients with cancer in clinical trials: A pilot study. Cancer 97:1499-1506, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kirtane K, Lee SJ: Racial and ethnic disparities in hematologic malignancies. Blood 130:1699-1705, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazha B, Mishra M, Pentz R, et al. : Enrollment of racial minorities in clinical trials: Old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Ed Book 39:3-10, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Marinac CR, Ghobrial IM, Birmann BM, et al. : Dissecting racial disparities in multiple myeloma. Blood Cancer J 10:19, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duma N, Vera Aguilera J, Paludo J, et al. : Representation of minorities and women in oncology clinical trials: Review of the past 14 years. J Oncol Pract 14:e1-e10, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Atkinson NL, Massett HA, Mylks C, et al. : User-centered research on breast cancer patient needs and preferences of an internet-based clinical trial matching system. J Med Internet Res 9:e13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson NL, Saperstein SL, Massett HA, et al. : Using the internet to search for cancer clinical trials: A comparative audit of clinical trial search tools. Contemp Clin Trials 29:555-564, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffitt K, Brogan F, Brown C, et al. : Statewide cancer clinical trial navigation service. J Oncol Pract 6:127-132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Utami D, Bickmore TW, Barry B, et al. : Health literacy and usability of clinical trial search engines. J Health Commun 19:190-204, 2014. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 38.Gansler T, Jin M, Bauer J, et al. : Outcomes of a cancer clinical trial matching service. J Cancer Educ 27:11-20, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Wu DT, Hanauer DA, Mei Q, et al. : Assessing the readability of ClinicalTrials.gov. J Am Med Inform Assoc 23:269-275, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghebre RG, Jones LA, Wenzel JA, et al. : State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual. Cancer 120:1122-1130, 2014. (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St Germain D, Dimond E, Olesen K, et al. : The NCCCP patient navigation project. Oncology Issues 29:45-53, 2014 [Google Scholar]
- 42.Holmes DR, Major J, Lyonga DE, et al. : Increasing minority patient participation in cancer clinical trials using oncology nurse navigation. Am J Surg 203:415-422, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Steinberg ML, Fremont A, Khan DC, et al. : Lay patient navigator program implementation for equal access to cancer care and clinical trials. Cancer 107:2669-2677, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Cartmell KB, Bonilha HS, Matson T, et al. : Patient participation in cancer clinical trials: A pilot test of lay navigation. Contemp Clin Trials Commun 3:86-93, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fouad MN, Acemgil A, Bae S, et al. : Patient navigation as a model to increase participation of African Americans in cancer clinical trials. J Oncol Pract 12:556-563, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wujcik D, Wolff SN: Recruitment of African Americans to national oncology clinical trials through a clinical trial shared resource. J Health Care Poor Underserved 21:38-50, 2010. (1 suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guadagnolo BA, Petereit DG, Helbig P, et al. : Involving American Indians and medically underserved rural populations in cancer clinical trials. Clin Trials 6:610-617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proctor JW, Martz E, Schenken LL, et al. : A screening tool to enhance clinical trial participation at a community center involved in a radiation oncology disparities program. J Oncol Pract 7:161-164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oncology Nursing Society : Oncology Nurse Navigator Core Competencies. Pittsburgh, PA, Oncology Nursing Society, 2017 [Google Scholar]
- 50.Oken MM, Creech RH, Tormey DC, et al. : Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649-656, 1982 [PubMed] [Google Scholar]
- 51.Butler DC, Petterson S, Phillips RL, et al. : Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res 48:539-559, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.United States Census Bureau : 2010 Census urban and rural classification and urban area criteria. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- 53.Centers for Medicare & Medicaid Services : Medicare advantage network adequacy criteria guidance. https://www.cms.gov/Medicare/Medicare-Advantage/MedicareAdvantageApps/Downloads/MA_Network_Adequacy_Criteria_Guidance_Document_1-10-17.pdf
- 54.United States Census Bureau : https://www2.census.gov/programs-surveys/popest/datasets/2010-2017/counties/totals/
- 55.Zaorsky NG, Zhang Y, Walter V, et al. : Clinical trial accrual at initial course of therapy for cancer and its impact on survival. J Natl Compr Canc Netw 17:1309-1316, 2019 [DOI] [PubMed] [Google Scholar]
- 56.Preussler JM, Farnia SH, Denzen EM, et al. : Variation in medicaid coverage for hematopoietic cell transplantation. J Oncol Pract 10:e196-e200, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Unger JM, Blanke CD, LeBlanc M, et al. : Association of patient demographic characteristics and insurance status with survival in cancer randomized clinical trials with positive findings. JAMA Netw Open 3:e203842, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicholas GZ, Ying Z, Vonn W, et al. : Clinical trial accrual at initial course of therapy for cancer and Its impact on survival. J Natl Compr Canc Netw 17:1309-1316, 2019 [DOI] [PubMed] [Google Scholar]