PURPOSE
A previous cancer diagnosis is a negative consideration in evaluating patients for possible solid organ transplantation. Statistical models may improve selection of patients with cancer evaluated for transplantation.
METHODS
We fitted statistical cure models for patients with cancer in the US general population using data from 13 cancer registries. Patients subsequently undergoing solid organ transplantation were identified through the Scientific Registry of Transplant Recipients. We estimated cure probabilities at diagnosis (for all patients with cancer) and transplantation (transplanted patients). We used Cox regression to assess associations of cure probability at transplantation with subsequent cancer-specific mortality.
RESULTS
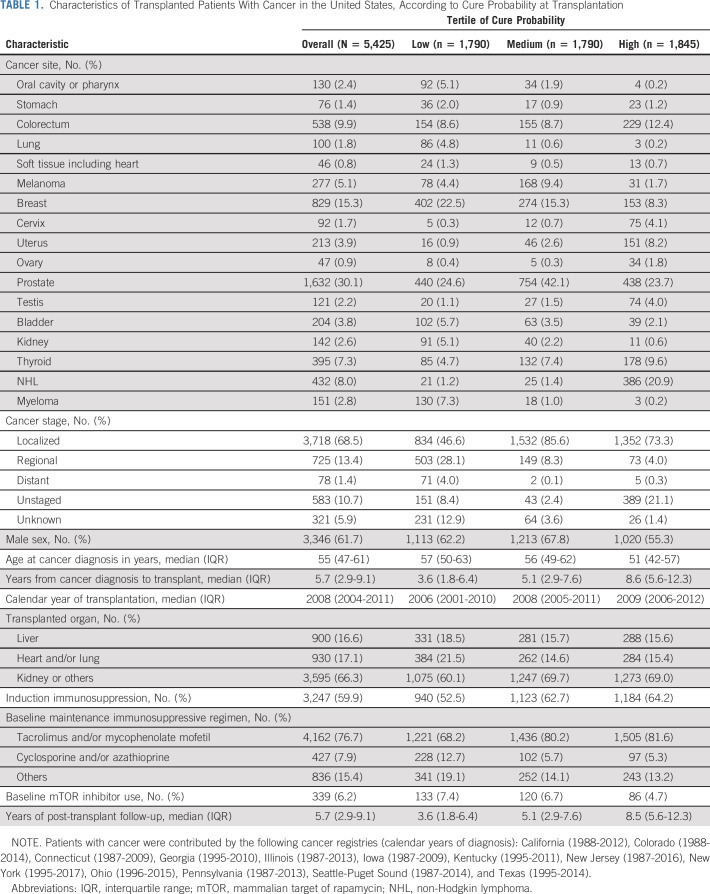
Among 10,524,326 patients with 17 cancer types in the general population, the median cure probability at diagnosis was 62%. Of these patients, 5,425 (0.05%) subsequently underwent solid organ transplantation and their median cure probability at transplantation was 94% (interquartile range, 86%-98%). Compared with the tertile of transplanted patients with highest cure probability, those in the lowest tertile more frequently had lung or breast cancers and less frequently colorectal, testicular, or thyroid cancers; more frequently had advanced-stage cancer; were older (median 57 v 51 years); and were transplanted sooner after cancer diagnosis (median 3.6 v 8.6 years). Patients in the low-cure probability tertile had increased cancer-specific mortality after transplantation (adjusted hazard ratio, 2.08; 95% CI, 1.48 to 2.93; v the high tertile), whereas those in the middle tertile did not differ.
CONCLUSION
Patients with cancer who underwent solid organ transplantation exhibited high cure probabilities, reflecting selection on the basis of existing guidelines and clinical judgment. Nonetheless, there was a range of cure probabilities among transplanted patients and low probability predicted increased cancer-specific mortality after transplantation. Cure probabilities may facilitate guideline development and evaluating individual patients for transplantation.
INTRODUCTION
Solid organ transplantation provides lifesaving treatment for patients with end-stage organ disease. However, transplant recipients must be administered immunosuppressive medications that target T-cell function to prevent organ rejection, which results in an increased incidence of cancer.1,2 In addition, there is a concern that transplant-associated immunosuppression may increase the risk of cancer recurrence among patients with a previous cancer diagnosis. Indeed, the recent success of immunotherapy for some advanced cancers highlights the possibility that the immune system may likewise help control cancer during remission for patients with cancer treated with other modalities.3
CONTEXT
Key Objective
Solid organ transplantation requires long-term immunosuppression, which may increase recurrence in patients with previous cancer. We sought to determine whether statistical models that predict a patient's probability of being cured of their cancer could inform evaluation of patients with cancer for solid organ transplantation.
Knowledge Generated
Among transplanted patients with cancer, cure probabilities at the time of transplantation were typically high but lower for patients with adverse characteristics (eg, older age, advanced cancer stage, and shorter interval between cancer diagnosis and transplantation). Patients with low cure probabilities had increased post-transplant mortality because of cancer and, consequently, overall mortality.
Relevance
Organ allocation decisions are complex, but it is reasonable to offer transplantation to patients who can be predicted to have a high likelihood of being cured of their cancer. Cure models may provide a useful common currency for assessing patients with previous cancer and thereby increase safety and population-level benefit of transplantation.
A previous cancer diagnosis is thus an important negative consideration in evaluating patients with end-stage organ disease for possible transplantation.4 The decision regarding whether a patient is considered eligible for organ transplantation and placed on the waitlist takes account of comorbid medical conditions that would make transplantation high-risk or of limited benefit to the patient.5,6 Because many cancers recur in the first few years after diagnosis, current guidelines for evaluating transplant candidates with a history of cancer typically recommend a waiting period of several years before a patient is listed, depending on the type of cancer (ie, site) and stage at diagnosis.7-11 Additionally, such individuals should be carefully evaluated to confirm that there are no signs of residual cancer. However, current guidelines are largely based on informal synthesis of clinical experience regarding patients with cancer in the general population and these guidelines have not used a systematic framework for assessing evidence or incorporated data on patients who subsequently receive an organ transplant.
Two features of providing solid organ transplantation for individuals with a previous cancer diagnosis (referred to as patients with cancer in this article, regardless of the time since diagnosis) pose unique challenges. First, limited availability of donor organs implies that an organ given to one patient with end-stage organ disease will result in another patient being denied that organ. There is thus a strong rationale for transplant providers to avoid futile transplants and provide transplantation to individuals who will most benefit.5,6 For patients with a history of cancer, this approach would correspond to selecting individuals for transplantation who have an acceptably low risk of cancer recurrence and death from their cancer (ie, cancer-specific mortality). Second, for patients with a previous cancer, the immunosuppressive medications administered during transplantation could increase their risk of cancer recurrence. This effect would occur if a patient with cancer in remission harbored small undetected foci of residual disease held in control by their immune system.3
One way to meet these considerations is to select for transplantation those patients who not only have a good prognosis but also have a high predicted probability of being cured from their cancer. It is difficult to determine for an individual patient whether they can be considered cured. However, for a population of patients with cancer, the probability of cure can be modeled statistically as the proportion of people who will not die from their cancer or, equivalently, who have the same overall mortality as the general population.12-14 Statistical cure models derived for a population can then be applied to an individual patient to estimate the probability of that being patient cured of their cancer, on the basis of the patient's demographic characteristics, features of their tumor, and cancer treatment. The cure probabilities from these models can be updated over time to capture the increasing cure probability as the patient survives for a longer period.12-14
For these reasons, cure probabilities may be useful for evaluating which patients with a previous cancer diagnosis could safely be listed for solid organ transplantation. However, no previous study has derived and applied cure models for this purpose. In the present study, we used US general population cancer registry data to develop cure models for 17 common cancer types. We applied these models to individuals with a previous cancer who underwent solid organ transplantation to estimate the probability that those patients had been cured of their cancer at the time of transplantation. We then evaluated the extent to which the cure probabilities for these patients predicted cancer-specific mortality after transplantation. Finally, we assessed associations of several transplant-related factors, that is, the transplanted organ and use of specific immunosuppressive medications, with cancer-specific mortality among this population.
METHODS
Detailed methods are provided in the Data Supplement (online only). We used data from the Transplant Cancer Match Study, a linkage of the US solid organ transplant registry (Scientific Registry of Transplant Recipients) with population-based cancer registries.1,15 This study is considered nonhuman subjects research by the National Institutes of Health and was approved by participating cancer registries.
From participating cancer registries, we selected patients with a first cancer diagnosis during 1987-2017, with one of the 17 cancer types (Table 1).16 Using the linked transplant registry data, we identified those patients who received a transplant after their cancer diagnosis. We identified cancer-specific deaths among patients using the underlying cause of death provided by cancer registries.17
TABLE 1.
Characteristics of Transplanted Patients With Cancer in the United States, According to Cure Probability at Transplantation
We fitted cure models for patients with cancer in the general population using data from the cancer registries. These models consider the overall population to be a mixture of two groups: patients who are cured of their cancer and those who will eventually die from their cancer.18 The cure probability was modeled using logistic regression and the survival function among uncured patients as a Weibull function. Details are provided in the Data Supplement.
We used these models to calculate cure probabilities for individual patients with cancer at the time of cancer diagnosis and (for transplanted patients) at the time of transplantation. We assessed the calibration of these models by comparing the Kaplan-Meier estimate of cumulative cancer-specific mortality with 1 minus the cure probability.
We divided transplanted patients with cancer into three groups (ie, tertiles) according to their cure probability at the time of transplantation. We used descriptive statistics to compare patients in these tertiles according to demographic and tumor characteristics, transplanted organ, and immunosuppressive medications. Among transplanted patients with cancer, we used multivariate Cox regression to examine associations of cure probability at transplant, transplanted organ, and immunosuppressive medications with cancer-specific mortality. Similarly, we used Cox regression to examine associations of cure probability with noncancer and overall mortality.
RESULTS
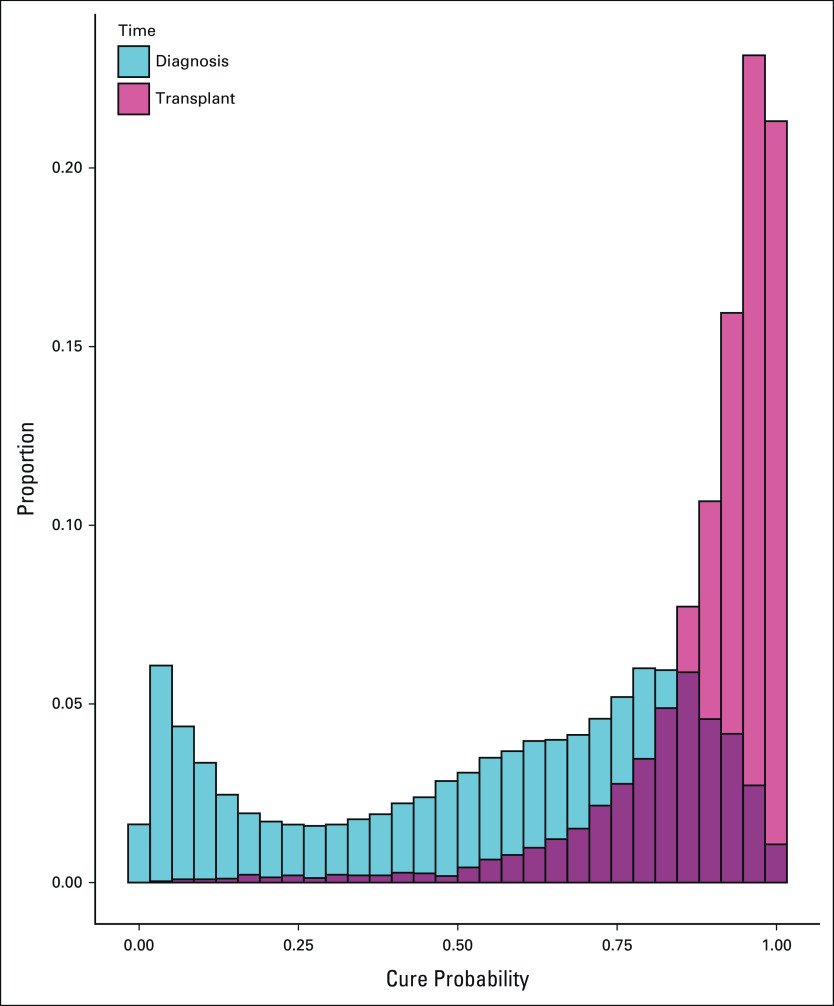
Using US general population cancer registry data, we estimated cure probabilities for 10,524,326 patients with cancer with 17 cancer types. At the time of cancer diagnosis, cure probabilities exhibited a bimodal distribution (Fig 1), with a median cure probability of 62% (interquartile range [IQR], 30%-81%). Cure probabilities at diagnosis were highest for testicular cancer, thyroid cancer, and melanoma (medians 87%-97%) and lowest for lung, stomach, and ovarian cancers and myeloma (7%-19%; Data Supplement). The cure models were well-calibrated for patients in the general population, with cumulative cancer-specific mortality approaching 1 minus the predicted cure fraction (Fig 2A).
FIG 1.
Cure probabilities of patients with cancer in the United States. The results are shown as overlapping histograms for (blue) all patients with cancer in the general population at the time of diagnosis and (red) the subset of transplanted patients with cancer at the time of solid organ transplantation.
FIG 2.
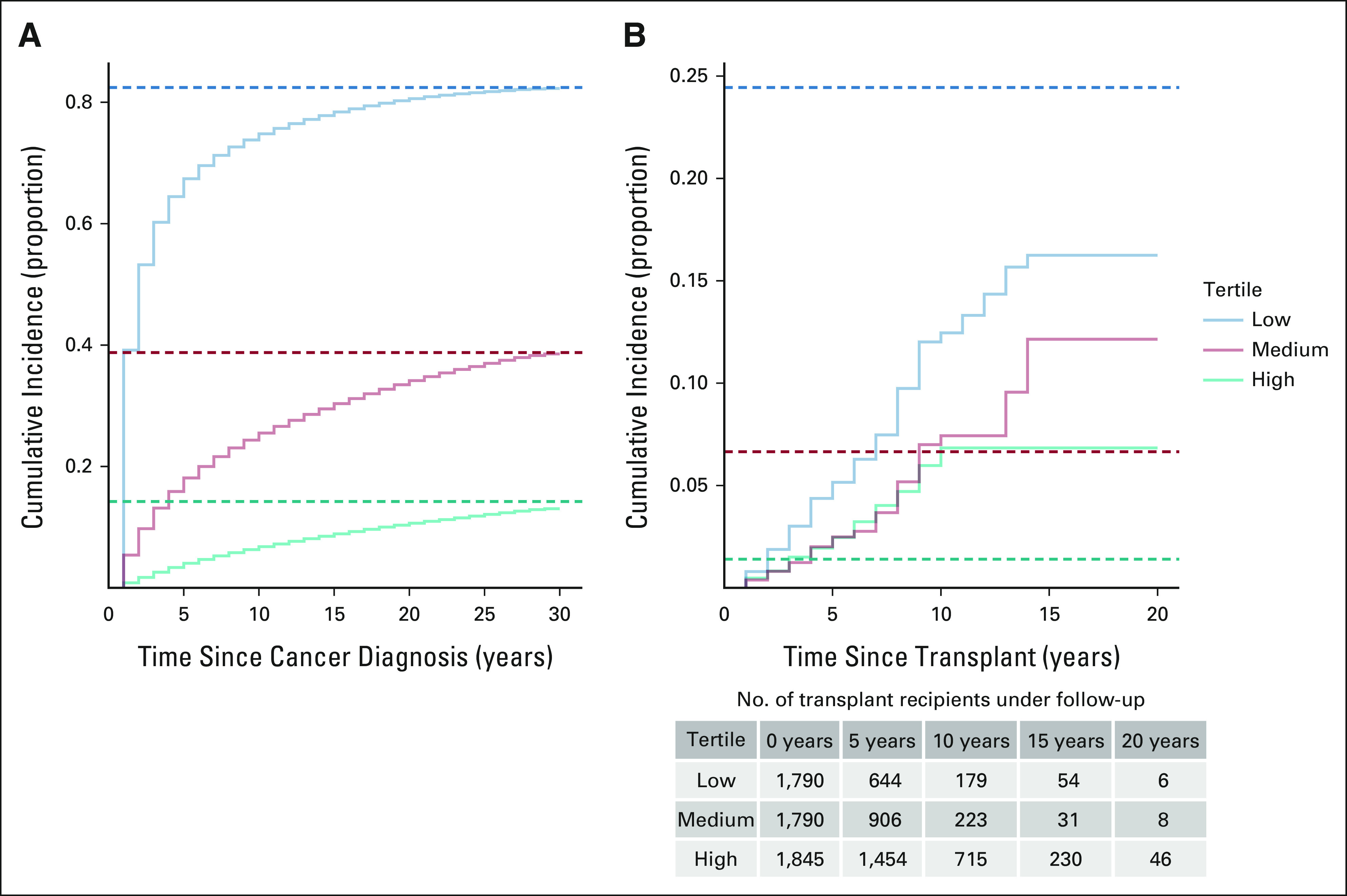
Observed and predicted cumulative incidence of cancer-specific mortality among patients with cancer. The observed results are shown as Kaplan-Meier curves, and the predicted results are shown as horizontal dashed lines calculated as 1 minus the mean cure probability from the cure models. (A) The results are shown for patients with cancer in the general population for tertiles on the basis of the predicted cure probability at cancer diagnosis. (B) The results are shown for transplanted patients with cancer on the basis of the predicted cure probability at the time of transplantation. Note that the vertical axes differ in the two panels.
A total of 5,425 patients with cancer (0.05%) subsequently underwent solid organ transplantation at a median of 5.7 years after cancer diagnosis (Table 1). The most common malignancies among transplanted patients were cancers of the prostate (30.1% of cases), breast (15.3%), colorectum (9.9%), and thyroid (7.3%) as well as non-Hodgkin lymphoma (NHL, 8.0%). Most transplanted patients were male (61.7%), and the median age at cancer diagnosis was 55 years. The most commonly transplanted organs were kidney (63.8%) and liver (16.6%).
At the time of transplantation, the median cure probability among transplanted patients with cancer was 94% (IQR, 86%-98%). Thus, cure probabilities were substantially higher in the transplanted patients at the time of transplantation, compared with the general population of patients with cancer at the time of diagnosis (Fig 1). A similar pattern was seen for each cancer site separately, except for testicular and thyroid cancers, where the cure probabilities were uniformly high (Data Supplement). Similarly, cure probabilities at diagnosis were higher for transplanted than untransplanted patients although the difference was less pronounced than at transplantation (Data Supplement).
Among transplanted patients with cancer, the tertiles of cure probabilities at the time of transplantation were divided at 89% and 96% (Table 1). Compared with transplanted patients in the high-cure probability tertile, those in the low tertile more frequently had previous diagnoses of oral cavity or pharyngeal, lung, breast, bladder, or kidney cancers or myeloma and less frequently had colorectal, cervical, uterine, testicular, or thyroid cancers or NHL. Compared with transplanted patients in the high-cure probability tertile, those in the low tertile more frequently had been diagnosed with advanced-stage cancer (28.1% regional and 4.0% distant stage, v 4.0% and 0.3%), had shorter intervals between cancer diagnosis and transplantation (median 3.6 v 8.6 years), and were older at cancer diagnosis (median age 57 v 51 years). In addition, patients with low cure probability were transplanted in earlier calendar years than those with high probability. Patients in the low tertile more frequently received a liver, heart, or lung transplant; were less likely to receive induction immunosuppression or maintenance immunosuppression limited to tacrolimus and/or mycophenolate mofetil; and were more likely to receive a mammalian target of rapamycin inhibitor as part of their maintenance regimen (Table 1).
During a median follow-up of 5.7 years (IQR, 2.9-9.1), 1,871 transplanted patients with cancer died, including 227 who died from their cancer. Patients in the low tertile of cure probability at transplantation had greater cancer-specific mortality compared with those in the middle and high tertiles (Fig 2B). However, for the medium- and high-cure probability tertiles, the cumulative cancer-specific mortality was higher than that predicted by the cure models. By contrast, the cumulative cancer-specific mortality in the low-cure probability tertile remained lower than that predicted by the cure models.
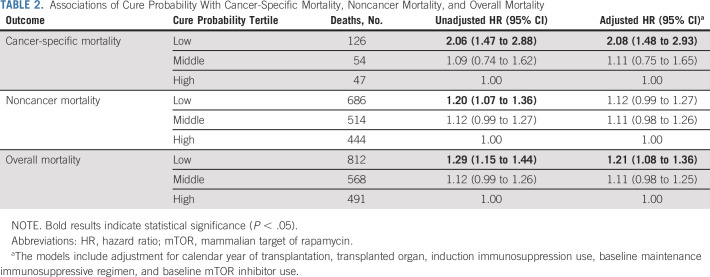
In a Cox regression model, patients with cancer in the low-cure probability tertile had significantly increased cancer-specific mortality compared with those in the high tertile (unadjusted hazard ratio [HR], 2.06; 95% CI, 1.47 to 2.88), whereas those in the middle tertile did not differ (1.09; 0.74 to 1.62; Table 2). The results were similar in the fully adjusted model, with patients in the low-cure probability tertile having significantly elevated cancer-specific mortality (adjusted HR, 2.08; 95% CI, 1.48 to 2.93). In comparison, cure probability was not predictive of mortality from noncancer causes (Table 2). The low-cure probability tertile had elevated overall mortality compared with the high tertile (adjusted HR, 1.21; 95% CI, 1.08 to 1.36) as a result of the association for cancer-specific mortality.
TABLE 2.
Associations of Cure Probability With Cancer-Specific Mortality, Noncancer Mortality, and Overall Mortality
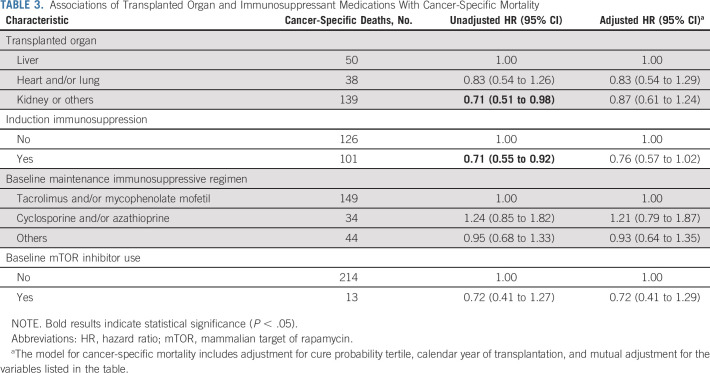
As shown in Table 3, we did not see clear associations between transplanted organ or immunosuppressive medications and cancer-specific mortality. In unadjusted analyses, kidney recipients had lower cancer-specific mortality than liver recipients and induction immunosuppression was inversely associated with cancer-specific mortality, but these associations were no longer significant after adjustment.
TABLE 3.
Associations of Transplanted Organ and Immunosuppressant Medications With Cancer-Specific Mortality
DISCUSSION
With improvements in cancer survival, the prevalence of cancer has increased among people evaluated for organ transplantation and among those who eventually receive a transplant.19 Because of the medical complexity of transplantation (including the need to administer lifelong immunosuppression), transplant programs must make complex decisions that weigh benefits and risks in determining who should be referred for transplantation.5,6
To our knowledge, the present study is the first to apply a formal statistical framework to inform the evaluation of transplant candidates with a previous cancer diagnosis. We used general population cancer registry data on 10.5 million patients with cancer to model statistical cure and applied these models to calculate individual patients' cure probabilities. We demonstrated that these cure probabilities offer prognostic information: After transplantation, patients in the low-cure probability tertile had twice the mortality from cancer as patients in the other two tertiles, which translated into an increase in overall mortality. By contrast, and as expected, cure probability was not predictive of noncancer mortality among transplanted patients.
Clinicians have used existing guidelines and informal clinical judgment to select patients with a previous cancer diagnosis to list for transplantation. Reflecting this selection process, the cure probabilities that we calculated were much higher in transplanted patients than for patients in the general population. Some characteristics of the transplanted group reflected their good prognosis, for example, patients with lung cancer were under-represented and those with testicular or thyroid cancers were over-represented, and most transplanted patients had localized stage cancer. A typical patient with cancer had more than 5 years elapse between cancer diagnosis and transplantation.
Importantly, however, there was a range in prognosis among transplanted patients, as manifested by the long left tail in cure probabilities at the time of transplantation (Fig 1). Among transplanted patients with low cure probability, there were relatively large fractions with adverse characteristics, including advanced cancer stage, older age, and shorter time between cancer diagnosis and transplantation (Table 1). The low-cure probability tertile had relatively large proportions of patients receiving organs other than a kidney, which may reflect greater urgency in providing transplantation to such individuals, whereas patients with cancer with end-stage kidney disease have an option of maintenance dialysis. There was also a trend for later calendar year of transplantation with increasing cure probability, which may reflect an increase in stringency of selection for transplantation over time.
The elevated cancer-specific mortality among patients in the low-cure tertile suggests that this group had a relatively high prevalence of undetected residual cancer at the time of transplantation. Notably, however, we did not see any associations of transplanted organ or immunosuppressive medications with cancer-specific mortality. Compared with kidney transplantation, heart and lung transplantation requires more intensive immunosuppression and liver transplantation requires less. Induction immunosuppression causes substantial immunosuppression in the period immediately after transplantation. The lack of association of these factors with cancer-specific mortality argues against a strong effect of immunosuppression in increasing recurrence among transplanted patients with cancer who have achieved an initial remission. Mammalian target of rapamycin inhibitors are believed to have anticancer properties, but this protection may be limited to selected malignancies,20,21 and we did not observe any reduction in cancer-specific mortality.
We propose that cure probability may be useful for evaluating potential transplant candidates with a previous cancer because it is reasonable to offer transplantation to individuals whose cancer, in the absence of transplantation, would be predicted not to have a fatal recurrence. Several features of cure models support such an application. First, cure probability models can be readily estimated from publicly available data from population-based cancer registries.12-14 Second, as noted above, these estimated cure probabilities predict cancer-specific mortality after transplantation. Third, cure probabilities plausibly provide a common currency for equivalently assessing diverse patients with different cancer types and clinical features.
Nonetheless, the decision to offer transplantation is complex and must incorporate many considerations. Cancer is only one of many comorbid medical conditions that affects patients with end-stage organ disease, but we did not compare post-transplant outcomes in recipients with and without a pretransplant cancer. Furthermore, because waitlist mortality is very high, many end-stage organ disease patients with cancer might benefit from transplantation even if their predicted cure probability is low. If cure models were adopted, the cure probability threshold at which end-stage organ disease patients with cancer would be considered eligible for transplantation would need to be determined through consensus discussion among transplantation stakeholders. One direct application could be to inform guidelines regarding transplant waiting periods. For example, guidelines might specify waiting periods for various categories of patients with cancer on the basis of the minimum elapsed interval after diagnosis when the estimated cure probability first exceeds the accepted threshold. Cure probabilities increase as patients survive for longer time after diagnosis, which is the mathematical justification for the common clinical approach of wait-and-see to select patients with good prognosis.
Importantly, there are disparities related to race or ethnicity and socioeconomic status in both cancer and organ transplantation, which have multiple causes.22,23 We did not address these factors in our analyses. Of interest, our statistical models (which do not include terms for race or ethnicity) yield different average cure probabilities across racial or ethnic groups, but these differences appear small, and the pattern varies among cancer sites (Data Supplement). It is possible that the use of cure probabilities from models such as ours could lead to differences across racial or ethnic groups in the proportions of patients with cancer referred to transplantation. The extent to which this assessment would unfairly limit access to transplantation for disadvantaged groups is difficult to determine,24 and further consideration of this issue is beyond the scope of the present study.
Several study limitations should be noted. First, we used a simplified system to classify cancers as localized, regional, or distant stage at diagnosis, and we lacked staging for NHL and myeloma. We also lacked additional important prognostic information on cancers (eg, molecular tumor characteristics) and treatment details, including the use of new targeted medications and immunotherapies. Second, our models relied only on information available at cancer diagnosis. By contrast, it is likely that clinicians' selection of some patients for transplantation incorporated data from follow-up imaging or circulating tumor markers to rule out residual disease. The additional reassurance provided by such testing may explain, for example, why the cancer-specific mortality among low-cure probability patients was less than predicted (Fig 2B). Third, we relied on causes of death obtained by cancer registries from death certificate records. The algorithm that we used to classify deaths as cancer-related, on the basis of the cause of death, has been validated against measures of relative survival for patients with cancer in the general population.17 Nonetheless, some deaths might have been incorrectly specified among transplanted patients with cancer, who have more complex medical problems than patients in the general population and are also at increased risk of developing new cancers after transplantation (Data Supplement). Misclassification of causes of deaths among transplant recipients may be another reason for the lack of calibration of the cure models with respect to the observed cancer-specific mortality in this population (Fig 2B).
In conclusion, statistical cure probability may provide a useful framework to inform transplant guidelines and evaluate individual patients. Before application of this approach in a real-world clinical setting, our results should be replicated using additional data, ideally incorporating detailed tumor and treatment information. If validated, cure models could be readily translated into the form of an application program for use in clinical settings and a patient's updated cure probability and its trajectory could then be calculated and assessed in real time. Furthermore, although these probabilities can serve as an appropriate benchmark, it would be reasonable for clinicians to use additional patient data, including tests regarding the presence or absence of residual disease, to modify their estimate of a patient's cure probability. This approach might result in some low-risk patients being offered transplantation earlier than under the current approach, whereas other high-risk patients who are currently offered a transplant would be deferred. Our results suggest that evaluation and referral of patients with cancer on the basis of cure probability could help increase the safety and overall population-level benefit of transplantation.
ACKNOWLEDGMENT
The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration, the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, and Jon Snyder), and the following cancer registries: the states of California (Cyllene Morris), Colorado (Jack Finch), Connecticut (Lou Gonsalves), Florida (Brad Wohler), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa, Illinois (Lori Koch), Kentucky (Jaclyn Nee), Michigan (Georgetta Alverson), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Ohio (Roberta Slocumb), Pennsylvania (Jim Rubertone), Texas (Leticia Nogueria), Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons, and Bill Wheeler).
The SRTR is currently operated under contract No. HHSH250201500009C by the Hennepin Healthcare Research Institute, Minneapolis, MN. Previously, the SRTR was managed under contract Nos. HHSH250201000018C and HHSH234200537009C.
Gregory Haber
Employment: Asklepion Pharmaceuticals (I)
Allyson Hart
Research Funding: CSL Behring (Inst)
Charles F. Lynch
Research Funding: Pancreatic Cancer Action Network (Inst), Novo Nordisk (Inst), Lilly (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The views expressed in this article are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors.
This is a US Government work. There are no restrictions on its use.
SUPPORT
Supported in part by the Intramural Research Program of the National Cancer Institute. The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201800002I), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (75N91021D00009), New York (75N91018D00005 [Task Order 75N91018F00001]), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN2612013000171). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5U58DP003883-03), Maryland (U58DP12-1205 3919-03), Michigan (5U58DP003921-03), New Jersey (5NU58DP006279-02-00), New York (6NU58DP006309), North Carolina (U58DP003933), and Texas (5U58DP000824-04). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, Massachusetts (Massachusetts Cancer Prevention and Control Cooperative Agreement 5458DP003920), New Jersey, New York (including the Cancer Surveillance Improvement Initiative), Texas, Utah, and Washington, as well as the University of Utah and Fred Hutchinson Cancer Research Center in Seattle, WA.
AUTHOR CONTRIBUTIONS
Conception and design: Eric A. Engels, Gregory Haber, Ruth M. Pfeiffer
Financial support: Eric A. Engels
Administrative support: Eric A. Engels, Kelly J. Yu
Provision of study materials or patients: Charles F. Lynch, Jie Li, Karen S. Pawlish, Baozhen Qiao
Collection and assembly of data: Eric A. Engels, Charles F. Lynch, Jie Li, Karen S. Pawlish, Baozhen Qiao, Kelly J. Yu
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Predicted Cure and Survival Among Transplant Recipients With a Previous Cancer Diagnosis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Gregory Haber
Employment: Asklepion Pharmaceuticals (I)
Allyson Hart
Research Funding: CSL Behring (Inst)
Charles F. Lynch
Research Funding: Pancreatic Cancer Action Network (Inst), Novo Nordisk (Inst), Lilly (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. : Spectrum of cancer risk among US solid organ transplant recipients. JAMA 306:1891-1901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grulich AE, van Leeuwen MT, Falster MO, et al. : Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 370:59-67, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Engels EA: Epidemiologic perspectives on immunosuppressed populations and the immunosurveillance and immunocontainment of cancer. Am J Transplant 19:3223-3232, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart A, Engels EA: Balancing uncertain risks in candidates for solid organ transplantation with a history of malignancy: Who is safe to transplant? Am J Transplant 21:447-448, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Faitot F, Michard B, Artzner T: Organ allocation in the age of the algorithm: Avoiding futile transplantation—Utility in allocation. Curr Opin Organ Transplant 25:305-309, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Petrini C: Ethical models in bioethics: Theory and application in organ allocation policies. Minerva Med 101:445-456, 2010 [PubMed] [Google Scholar]
- 7.Chadban SJ, Ahn C, Axelrod DA, et al. : KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation 104:S11-S103, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Weill D, Benden C, Corris PA, et al. : A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 34:1-15, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Martin P, DiMartini A, Feng S, et al. : Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 59:1144-1165, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Al-Adra DP, Hammel L, Roberts J, et al. : Preexisting melanoma and hematological malignancies, prognosis, and timing to solid organ transplantation: A consensus expert opinion statement. Am J Transplant 21:475-483, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Adra DP, Hammel L, Roberts J, et al. : Pre-transplant solid organ malignancy and organ transplant candidacy: A consensus expert opinion statement. Am J Transplant 21:460-474, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal Maso L, Panato C, Tavilla A, et al. : Cancer cure for 32 cancer types: Results from the EUROCARE-5 study. Int J Epidemiol 49:1517-1525, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Othus M, Barlogie B, Leblanc ML, et al. : Cure models as a useful statistical tool for analyzing survival. Clin Cancer Res 18:3731-3736, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu XQ, De Angelis R, Andersson TM, et al. : Estimating the proportion cured of cancer: Some practical advice for users. Cancer Epidemiol 37:836-842, 2013 [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute: Transplant cancer match study . https://transplantmatch.cancer.gov/
- 16.Engels EA, Haber G, Hart A, et al. : Solid organ transplantation and survival among individuals with a history of cancer. Cancer Epidemiol Biomark Prev 30:1312-1319, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howlader N, Ries LA, Mariotto AB, et al. : Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 102:1584-1598, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farewell VT: The use of mixture models for the analysis of survival data with long-term survivors. Biometrics 38:1041-1046, 1982 [PubMed] [Google Scholar]
- 19.Livingston-Rosanoff D, Foley DP, Leverson G, et al. : Impact of pre-transplant malignancy on outcomes after kidney transplantation: United Network for Organ Sharing database analysis. J Am Coll Surg 229:568-579, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stallone G, Schena A, Infante B, et al. : Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med 352:1317-1323, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Euvrard S, Morelon E, Rostaing L, et al. : Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 367:329-339, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Ward E, Jemal A, Cokkinides V, et al. : Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 54:78-93, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Malek SK, Keys BJ, Kumar S, et al. : Racial and ethnic disparities in kidney transplantation. Transpl Int 24:419-424, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Vyas DA, Eisenstein LG, Jones DS: Hidden in plain sight—Reconsidering the use of race correction in clinical algorithms. N Engl J Med 383:874-882, 2020 [DOI] [PubMed] [Google Scholar]